Abstract
This study explored the functions of the signal transducers and activators of transcription 5a and 5b (referred to as Stat5 here) during different stages of mouse mammary gland development by using conditional gene inactivation. Mammary gland morphogenesis includes cell specification, proliferation and differentiation during pregnancy, cell survival and maintenance of differentiation throughout lactation, and cell death during involution. Stat5 is activated by prolactin, and its presence is mandatory for the proliferation and differentiation of mammary epithelium during pregnancy. To address the question of whether Stat5 is also necessary for the maintenance and survival of the differentiated epithelium, the two genes were deleted at different time points. The 110-kb Stat5 locus in the mouse was bracketed with loxP sites, and its deletion was accomplished by using two Cre-expressing transgenic lines. Loss of Stat5 prior to pregnancy prevented epithelial proliferation and differentiation. Deletion of Stat5 during pregnancy, after mammary epithelium had entered Stat5-mediated differentiation, resulted in premature cell death, indicating that at this stage epithelial cell proliferation, differentiation, and survival require Stat5.
The signal transducer and activator of transcription (STAT) family of transcription factors conveys cytokine signals from the respective membrane receptors to the nucleus, where they activate diverse genetic programs (11, 12). The two highly conserved Stat5 proteins (Stat5a and Stat5b) are activated by many cytokines, including interleukins, erythropoietin, and prolactin, as well as growth hormone. Mice in which either one or both Stat5 genes were inactivated have revealed unique and redundant roles of the two Stat5 isoforms. Stat5a deficiency results in the loss of prolactin-dependent mammary gland development (15) but does not affect body growth. In contrast, inactivation of Stat5b does not adversely affect mammary development and function but leads to severe growth retardation (34). Mice lacking Stat5a and -5b show defects in multiple organs (33). T-cell proliferation is severely compromised in the absence of Stat5 (22, 33), and mammary alveolar epithelium fails to develop during pregnancy (20). Moreover, the multilineage reconstitution potential of hematopoietic stem cells is highly dependent on Stat5 (2).
Expansion and differentiation of the mouse mammary alveolar compartment during pregnancy are controlled through the prolactin receptor (23), Jak2 (29), and Stat5 (15, 20). Inactivation of the Stat5 locus by using conventional gene targeting resulted in the complete lack of mammary alveolar epithelium, suggesting that the progenitor cells were unable to proliferate. However, those studies could not address the potential roles of Stat5 in the physiology of the epithelial cell beyond its initial proliferation, i.e., whether Stat5 is required for epithelial proliferation, differentiation, and survival throughout pregnancy and for maintenance of epithelial function during lactation. These questions were now addressed with mice in which both Stat5 genes could be deleted in mammary epithelium at distinct time points in development. Specifically, the role of Stat5 in epithelium prior to and after its proliferation phase and upon prolactin-induced differentiation was investigated.
MATERIALS AND METHODS
Construction of targeting vectors.
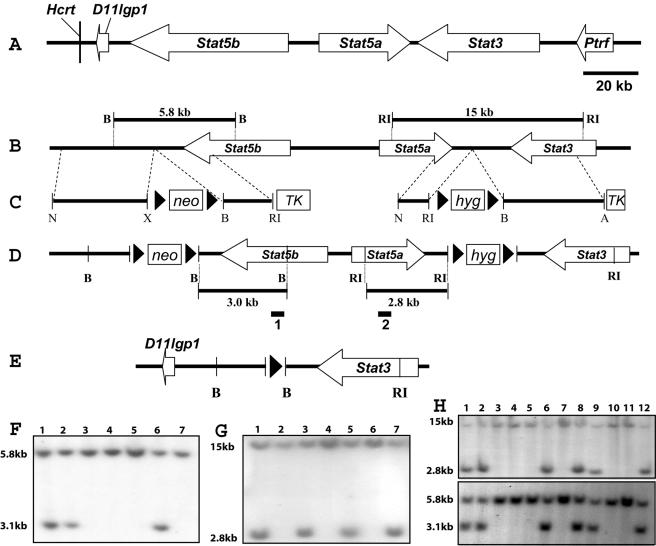
Four bacterial artificial chromosomes (BACs) and two recombinant phages containing 500 kbp of genomic DNA of the Stat3/5a/5b locus were isolated from a 129 mouse library (Stratagene) by using full-length Stat5a, Stat5b, and Stat3 cDNAs (GenBank accession numbers NM_011488, NM_011489, and NM_011486, respectively) as probes. We cloned two new genes and annotated eight known genes in this locus (Fig. 1C) (4, 19). To construct the Stat5b-targeting vector, a 2.5-kb EcoRI fragment, which contained part of the 3′end of Stat5b and flanking sequence of the Stat5b gene, was subcloned into the EcoRI site of the pLoxPneo vector. The resulting construct was cleaved with XhoI and NotI, followed by insertion of a 4.7-kb XhoI-NotI fragment, which mapped downstream of the Stat5b gene. The finished targeting construct was designated pLoxpneoStat5b (Fig. 1B).
FIG. 1.
Introduction of loxP sites into the Stat5a/b locus. (A) The BAC clone spans approximately 210 kb and encompasses five known genes and one new gene. The Stat5a gene is 30 kb, Stat5b is more than 54 kb, and Stat3 is more than 37 kb. The Hcrt and D11lgp1 genes are downstream of the Stat5b gene, and the Ptrf gene is upstream of the Stat3 gene. The gene sizes and intergenic sequences are drawn to scale. (B) Restriction map of the wild-type allele. B, BamHI; RI, EcoRI. (C) The Stat5b-targeting construct contains a 7.2-kb genomic sequence with a pLoxpneo cassette, and the Stat5a-targeting vector contains an 8.6-kb genomic sequence with a pLoxphyg cassette. Neomycin (neo), hygromycin (hyg), and thymidine kinase (TK) are positive and negative selection cassettes, respectively. Prior to electroporation, each targeting vector was linearized at the unique NotI site (N) indicated. X, XbaI; A, Asp718. (D) Restriction map of the targeted allele. Two loxP sites and the neomycin cassette were introduced downstream of Stat5b, and two loxP sites with the hygromycin cassette were inserted downstream of Stat5a. The indicated probes were used to assess recombination events. (E) Structure of the deleted Stat5a/b allele. D11lgp1 and Stat3 are next to each other, with one loxP site between them. (F) Southern blot analysis of homologous recombination in ES cells electroporated with the Stat5b-targeting vector. The BamHI (lanes 1, 2, and 6) restriction fragment size changes from 5.8 to 3.0 kb, as seen in the targeted cells with the 32P-labeled 500-bp external probe 1. (G) ES cell clone 1 was chosen to be electroporated with the Stat5a-targeting vector. The EcoRI (lanes 1, 3, 5, and 7) restriction fragment size changes from 15 to 2.8 kb, as seen in the targeted cells with the 32P-labeled 500-bp external probe 2. (H) Southern blot analysis of tail DNA from chimeric males (lanes 1 and 2) and their agouti pups (lanes 3 to 12). Both Stat5a- and Stat5b-targeted bands were detected in the male chimeras (lanes 1 and 2) and in four agouti pups (lanes 6, 8, 9, and 12).
To construct the Stat5a-targeting vector, a 1.4-kb XbaI-SacI fragment, which matched to part of the 3′ end and flanking sequence of the Stat5a gene, was subcloned into the XbaI-SacI sites of pBluescript vector. The pBluescript vector was digested with NotI and XhoI, and then the 1.4-kb NotI-XhoI fragment was subcloned into the NotI-XhoI sites of the pLoxPhygro vector. The resulting construct was cleaved with BamHI and Asp718, followed by insertion of a 7.2-kb BamHI-Asp718 fragment, which contained sequence downstream of Stat5a and part of the 5′ end of the Stat3 gene. The finished targeting construct was designated pLoxphygroStat5a (Fig. 1C).
Homologous recombination in ES cells and generation of germ line chimeras.
TC1 embryonic stem (ES) cells (5) were transfected with NotI-digested pLoxpneoStat5b and selected with G418 and 1(1-2-deoxy-2-fluoro-β-d-arabino-furanosyl)-5-iodouracil (FIAU) as described previously (6). ES cell colonies that were resistant to both G418 and FIAU were picked and analyzed for homologous recombination events within the Stat5b locus by Southern blotting. Genomic DNAs from these clones and the parental TC1 cell line were digested with BamHI, followed by Southern blotting with a 500-bp external probe that is 3′ to the targeting vector (Fig. 1D). ES cell lines showed a 5.8-kb fragment diagnostic of the wild-type allele, but in the targeted cell lines this fragment was shifted to 3.0 kb as the result of the introduction of a BamHI site (Fig. 1F). Four out of 114 G418-resistant ES clones were pLoxpneoStat5b targeted. These four ES cell clones were karyotyped, and all had normal chromosome numbers.
The successive transfection conditions for the pLoxphygroStat5a vector were identical to those for the pLoxpneoStat5b vector, except for selection with hygromycin B. ES cells resistant to hygromycin were analyzed for homologous recombination events by Southern blotting (digestion was with EcoRI) with a 664-bp external probe specific for the Stat5a gene. DNAs from the parental ES cell lines showed a 15-kb fragment diagnostic of the wild-type allele, but in the targeted cell lines this fragment was shifted to 2.8 kb as the result of the introduction of an EcoRI site (Fig. 1G). Of a total of 65 G418- and hygromycin-resistant colonies analyzed, 19 contained the targeted allele.
The 19 double-positive ES cells were transfected with the pic-Cre vector by using Lipofectin reagent (Invitrogen). We harvested the transfected ES cells 48 h after transfection, extracted genomic DNA, and detected the recombination allele by PCR to determine whether the two targeting events occurred on the same allele. The primers were Stat5b-3′ (5′-AGC AGC AAC CAG AGG ACT AC-3′) and Stat5a-3′ (5′-CCC ATT ATC ACC TTC TTT ACA G-3′). The recombination band was 500 bp. Four ES cell clones were confirmed as having four loxP sites on the same allele.
ES cells heterozygous for the targeted insertions were microinjected into C57BL/6 blastocysts to obtain germ line transmission. The injected blastocysts were implanted into the uteri of pseudopregnant FVB (Taconic) foster mothers and allowed to develop to term. Male chimeras (identified by the presence of agouti coat color) were mated with NIH Black Swiss females (Taconic). Germ line transmission was confirmed by agouti coat color in F1 animals, and all agouti offspring were tested for the presence of the inserted loxP sites by Southern analysis, with the same conditions for detection of the homologous recombination event as in the ES cells (Fig. 1H).
Breeding and genotype analysis.
Genotypes of Stat5 floxed mice were determined by PCR. For PCR analysis, the wild-type Stat5 allele was detected by using primer 1 (5′-GAA AGC ATG AAA GGG TTG GAG-3′) and primer 2 (5′-AGC AGC AAC CAG AGG ACT AC-3′), located at either side of the insertion. This primer pair amplifies a fragment of 450 bp from wild-type mice but not from Stat5fl/fl mice. DNA was also amplified by using primers 2 and 3; the latter is located in the pLoxpneo vector (5′-AAG TTA TCT CGA GTT AGT CAG G-3′) to detect the Stat5 floxed allele. In this case, a 200-bp fragment was detected in mice heterozygous or homozygous for the Stat5 floxed allele, while no signal was detected in wild-type mice.
The Stat5 floxed mice were mated with mouse mammary tumor virus (MMTV)-Cre and whey acidic protein (WAP)-Cre transgenic mice (14, 36). The genotypes of the mice were determined by PCR analysis. Primers for the Cre transgenes were 5′-TAG AGC TGT GCC AGC CTC TTC C-3′, which binds in the WAP gene promoter; 5′-GGT TCT GAT CTG AGC TCT GAG TG-3′, which binds in the MMTV long terminal repeat; and 5′-CAT CAC TCG TTG CAT CGA CCG G-3′, which binds in the Cre sequence. The WAP-Cre transgene produced a 240-bp fragment, and the MMTV-Cre transgene yielded a 280-bp fragment. All products were separated in 2% agarose-Tris-acetate gels. All of the control mice were Stat5fl/fl littermates.
Antibodies.
The rabbit polyclonal anti-phospho-histone H3 (H3P) was obtained from Upstate Ltd. (Charlottesville, Va.). The rabbit polyclonal anti-Stat5a (L-20) and anti-Stat3 (C-20) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Mouse monoclonal E-cadherin antibody was obtained from Transduction Laboratories, and smooth muscle actin antibody was from Sigma (St. Louis, Mo.). The rabbit polyclonal anti-Cre antibody was purchased from Novagen (EMD Biosciences, Inc.). The rabbit polyclonal anti-NKCC1 antibody (21) was a gift from Jim Turner (National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Md.). The rabbit polyclonal anti-Npt2b antibody (9) was a gift from Jurg Biber (Department of Physiology, University of Zurich, Zurich, Switzerland).
Analysis of cellular proliferation.
Five-month-old Stat5fl/fl/MC and Stat5fl/fl mice were treated by interscapular subcutaneous injection with 1 μg of β-estradiol and 1 mg progesterone (both from Sigma) in 100 μl of sesame oil at 24-h intervals. After 48 h, the number 4 mammary glands were removed. The tissue was fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight at 4°C. Immunofluorescent and H3P-positive cells were counted. Five mice per genotype were used for each experiment. Cells from 20 fields at a magnification of ×400 were counted for each sample. The number of H3P-positive cells in a given field was expressed as a percentage of the total number of DAPI (4′,6′-diamidino-2-phenylindole)-stained cells. Statistical significance was determined by two-tailed Student t tests.
Whole-mount analysis and histological analysis.
Mammary glands were fixed overnight at room temperature in Carnoy's fixative (6 parts 100% ethanol, 3 parts chloroform, 1 part glacial acetic acid) and stained in carmine alum (0.2% carmine alum, 0.5% aluminum potassium sulfate, 1 crystal of thymol). After dehydration and clearing in Histoclear (National Diagnostics), the samples were permanently mounted (Permount; Fischer Scientific). For histological analyses, tissues were fixed in 4% paraformaldehyde overnight at 4°C. Following fixation, tissues were placed in 70% ethanol, dehydrated, cleared in xylene, embedded in paraffin, and sectioned at 5 μm. Hematoxylin and eosin (H&E) staining was performed by standard procedures.
Immunofluorescence.
After fixation in 4% paraformaldehyde overnight at 4°C, tissues were embedded in paraffin and sectioned at 5 μm. Sections were cleared in xylene and rehydrated. Antigen retrieval was performed by heat treatment with an antigen unmasking solution (Vector Laboratories Inc.), and tissue sections were blocked for 30 min in PBS-Tween (PBST) containing 3% goat serum. Sections were incubated with E-cadherin (1:200) and Npt2b (1:100) antibodies, smooth muscle actin (1:1,000) and NKCC1 (1:1,000) antibodies, E-cadherin (1:200) and Stat5a (1:100) antibodies, H3P (1:200) and E-cadherin (1:200) antibodies, or Cre (1:500) and E-cadherin (1:200) antibodies. The primary antibodies were allowed to bind for 60 min at 37°C, except for Stat5a-E-cadherin (4°C, overnight). Nonspecifically bound antibody was removed by rinsing in PBST before the addition of both anti-mouse fluorescein isothiocyanate (FITC)-conjugated (1:400) and anti-rabbit Texas red-conjugated (1:400) secondary antibodies. Sections were incubated in the dark for 30 min, washed twice in PBST, and mounted with Vectashield (Vector Laboratories Inc.). Immunofluorescence was viewed under an Axioscop microscope (Carl Zeiss, Inc., Thornwood, N.Y.) equipped with filters for FITC, tetramethyl rhodamine isothiocyanate, and FITC-tetramethyl rhodamine isothiocyanate. Images were captured with a Sony DKC5000 digital camera.
RESULTS
Targeting of the mouse Stat5 locus.
The genes encoding mouse Stat5a and Stat5b (referred to as Stat5 here) are located within a stretch of 110 kb on chromosome 11 in a head-to-head orientation with no other genes between them (Fig. 1A) (4, 19). A BAC clone that contained both genes was isolated, and loxP sites were introduced successively at their 3′ ends by using a double-targeting homologous recombination approach (Fig. 1). After electroporation with the Stat5b-targeting vector, 4 out of 114 neomycin-resistant ES clones had undergone homologous recombination (Fig. 1F). These ES cell clones were karyotyped and shown to contain normal chromosome numbers. One of these clones was electroporated with the Stat5a-targeting vector, and out of the 65 neomycin- and hygromycin-resistant colonies analyzed, 19 contained the double-targeted allele (Fig. 1G). To identify ES cells that carried both targeted Stat5 alleles on the same chromosome, cells were transfected with a pic-Cre vector and the recombination event was verified by PCR. Four ES cell clones carried the four loxP sites on the same allele. Subsequently, mice that carried one or two copies of the targeted Stat5 locus (Stat5fl/fl mice) (Fig. 1H) were generated. Stat5fl/fl mice developed normally, and adults were indistinguishable from their control littermates. Both males and females were fertile, and dams could nurse their pups, thus demonstrating that the presence of the neomycin and hygromycin selection markers had no adverse effect.
Germ line deletion of the Stat5 locus.
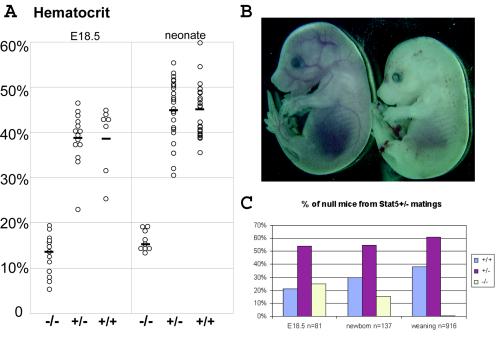
Cre-mediated recombination of the Stat5 locus in oocytes was achieved with a specific line of transgenic mice carrying the Cre gene under control of the MMTV long terminal repeat (36). Females that carried one Stat5 floxed allele and the MMTV-Cre transgene passed on a Stat5-null allele to their offspring. Females and males hemizygous for Stat5 (Stat5+/− mice) were mated, and a total of 916 pups were weaned. From these matings only 5 (0.5%) Stat5-null mice were obtained instead of the 229 (25%) expected according to the Mendelian ratio. These five mice were weak and had only one-third of the body weight of control littermates. To assess when lethality occurred, Stat5 hemizygous mice were mated, and fetuses at embryonic day 18.5 (E18.5) and neonates were genotyped. While at E18.5 Stat5-null fetuses were present at the expected Mendelian ratio of 25% (n = 81), only 15% (n = 137) of the neonates (litters were evaluated within 12 h after delivery) were Stat5 null (Fig. 2C). Both Stat5-null embryos and neonates appeared very pale compared to their wild-type or hemizygote littermates (Fig. 2B), indicating severe anemia. The hematocrit of E18.5 Stat5-null fetuses was 10.7% ± 4.3% (mean ± standard error of the mean [SEM]), compared to 39.5% ± 7.4% in wild-type littermates. Stat5-null neonates had a hematocrit of 16.1% ± 2.5%, compared to 45.2% ± 6.1% in wild-type littermates (Fig. 2A). The perinatal lethality of Stat5-null mice could be the result of the severe anemia combined with other physiological defects, which remain to be identified.
FIG. 2.
Stat5-null embryos are perinatal lethal. (A) Hematocrits of Stat5−/− (n = 13 and 9), Stat5+/− (n = 15 and 28), or Stat5+/+ (n = 7 and 25) E18.5 fetuses and newborn littermates. Differences between Stat5+/+ and Stat5−/− animals were highly significant (P < 0.0001 and P < 10−18 by the two-tailed Student t test). There was no difference between wild-type and hemizygous animals (P = 0.913 and P = 0.676). (B) An E14.5 Stat5−/− embryo (right) is paler and smaller than its wild-type littermate (left). (C) The Mendelian ratio (25%; n = 81) was observed in E18.5 Stat5−/− fetuses; it dropped to 15% (n = 137) in the neonates, and only 0.5% (n = 916) were Stat5 null at weaning.
Stat5-null mammary epithelium retains virgin-like ductal characteristics during pregnancy.
Through breeding, mice that carried two Stat5 floxed alleles and a well-characterized MMTV-Cre transgene (Stat5fl/fl/MC mice), which expresses Cre recombinase in mammary epithelium already prior to pregnancy (36), were generated. These mice developed normally, with body sizes comparable to those of wild-type or Stat5fl/fl controls, and they exhibited normal fertility. Although Stat5fl/fl/MC females delivered litters of normal sizes, most of the dams were unable to nurse their pups. Only 2 out of 12 dams could secrete small amounts of milk, and the malnourished pups died within 10 days.
Most mammary epithelial cells in Stat5fl/fl/MC virgin mice were devoid of Stat5, as demonstrated by immunostaining (Fig. 3C and D). Due to the mosaic expression of Cre recombinase (Fig. 3H), occasional Stat5-expressing cells were observed (Fig. 3D). While the loss of Stat5 did not affect ductal outgrowth and primary and secondary branching, there was a clear paucity of tertiary branches in mature Stat5fl/fl/MC mice. Lobular units were absent in Stat5fl/fl/MC mature virgins compared with their Stat5fl/fl littermates, and at parturition there was a complete absence of the alveolar compartment (data not shown). The Na-K-Cl cotransporter, NKCC1, is present at high levels on the basolateral membrane of ductal epithelial cells in virgin mammary tissue and declines in alveolar epithelium during pregnancy (27-29). At parturition, high levels of NKCC1 were observed in Stat5fl/fl/MC epithelia, while a reduction in NKCC1 occurred in control epithelium (data not shown). The presence of the sodium phosphate cotransporter Npt2b on the apical membrane of mammary alveolar cells is indicative of functional secreting cells (20, 27-29). In contrast to the case for wild-type epithelium at parturition, apical Npt2b was not detectable in Stat5fl/fl/MC epithelium (data not shown). The histological lesions were similar to those observed in the previously reported conventional Stat5-null mice (20). These experiments demonstrate that Stat5 is absolutely required for the proliferation of mammary secretory epithelium during pregnancy and confirm earlier conclusions derived from the conventional Stat5-targeted mice.
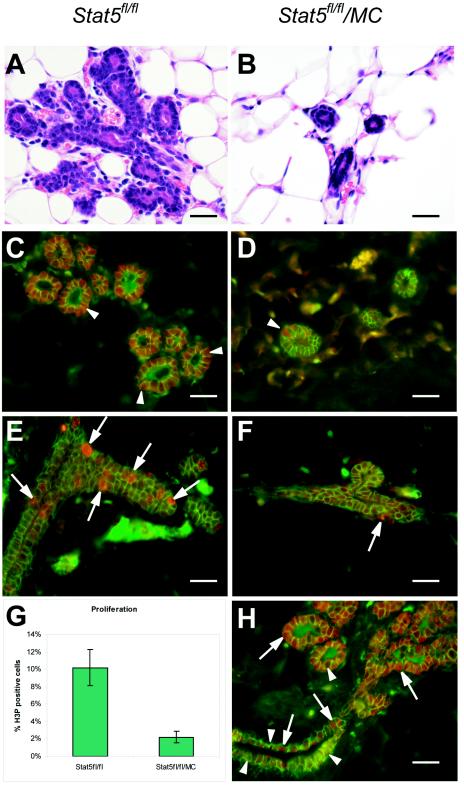
FIG. 3.
Reduced proliferation of epithelial cells in the absence of Stat5. Stat5fl/fl and Stat5fl/fl/MC virgin mice (12 weeks) were injected with estrogen and progesterone. Forty-eight hours after treatment, mammary glands were harvested and fixed. (A and B) H&E-stained sections showed sparse branching in the Stat5fl/fl/MC glands compared to Stat5fl/fl littermate controls after acute estrogen-progesterone treatment. (C and D) Most mammary epithelial cells in estrogen-progesterone-treated Stat5fl/fl/MC virgin mice were devoid of Stat5 as demonstrated by immunofluorescence staining with Stat5a (red) and E-cadherin (green) antibodies. Occasional Stat5-expressing cells were observed (D) (arrowhead) in Stat5fl/fl/MC epithelia due to the mosaic expression of Cre recombinase. (E and F) Immunofluorescence staining showed abundant H3P (a proliferation marker, in red) in estrogen-progesterone-treated Stat5fl/fl mammary tissues (E) (arrows), it but was found only in few cells in Stat5fl/fl/MC epithelia(F) (arrow). E-cadherin (green) was used as a cell membrane stain. (G) H3P staining demonstrated that 2.1% ± 0.67% (mean ± SEM) of mammary cells in Stat5fl/fl/MC mice were positive for H3P, while 10.2% ± 2.2% of cells were stained in control mice. The difference was statistically significant (P < 0.0001 by the two-tailed Student t test). (H) Immunofluorescence staining showed Cre recombinase (red, arrows) expression in the mammary tissue of a Stat5fl/fl/MC virgin animal. E-cadherin is in green. MMTV-Cre expression was mosaic in ductal mammary epithelium (arrows and arrowheads), and only a few cells expressed Cre recombinase (arrows). Bars, 50 μm.
Reduced proliferation of Stat5-null epithelium.
Extensive proliferation of mammary alveolar epithelium occurs throughout pregnancy. The acute treatment of virgin mice with estrogen and progesterone also stimulates epithelial cells to enter the cell cycle (26). This experimental approach is considered to mimic some hormonal effects of early pregnancy on alveolar development. Five Stat5fl/fl/MC and five Stat5fl/fl mice were injected with estrogen and progesterone for 2 days. After this treatment, extensive side branching and alveolar budding were observed in the control mice (Fig. 3A and C). In contrast, only very limited side branching was induced in Stat5fl/fl/MC mammary epithelia (Fig. 3B and D). Immunofluorescence staining with a proliferation marker (anti-H3P) demonstrated that 2.1% ± 0.67% (mean ± SEM) of mammary cells in Stat5fl/fl/MC mice were positive for H3P, while 10.2% ± 2.2% of cells were stained in control mice (Fig. 3E, F, and G). The difference was statistically significant (P < 0.0001 by the two-tailed Student t test). MMTV-Cre expression was mosaic in ductal mammary epithelium (Fig. 3H), and only few cells expressed Cre recombinase (Fig. 3H). We suggest that acute hormone treatment elicits proliferation only of ductal epithelium, which has retained a Stat5 gene. These findings establish that Stat5 is required for an estrogen- and progesterone-induced proliferative response in mammary epithelial cells.
Stat5 is required for the survival of differentiated mammary epithelium during pregnancy.
To address the question of whether Stat5 is needed for the maintenance of differentiated function and survival of mammary epithelium after it has undergone pregnancy-induced proliferation and differentiation, the Stat5 locus was deleted in a second line of mice expressing a Cre transgene (Stat5fl/fl/WC mice) (36). In this line the Cre transgene is under control of the WAP gene promoter, which itself is activated by prolactin and Stat5 in late pregnancy (3, 13). WAP is one of the late proteins to be produced in differentiating mammary tissue, and its presence heralds the appearance of functional alveolar epithelial cells that secrete milk (24). Although Stat5fl/fl/WC dams could support their litters during lactation, histological sections revealed that their alveoli were sparser than those of Stat5fl/fl littermates (Fig. 4A and B).
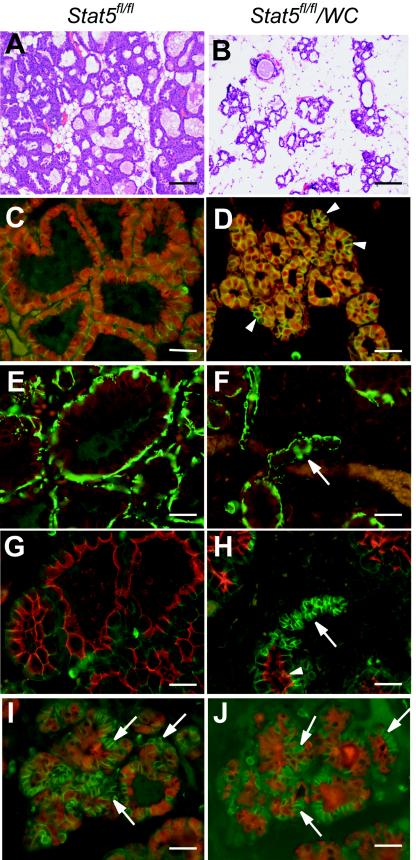
FIG.4.
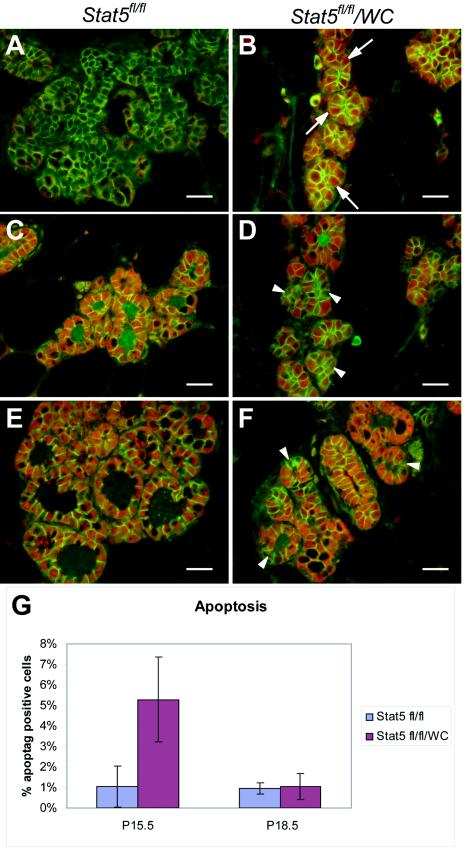
Stat5 is required for functional differentiation of the mammary epithelium. Inguinal mammary glands were collected from Stat5fl/fl (A, C, E, and G) and Stat5fl/fl/WC (B, D, F, H, I, and J) mice at lactation. (A and B) H&E staining showed sparser alveoli in Stat5fl/fl/WC mammary gland at lactation than in the Stat5fl/fl littermate. (C to J) Mammary tissues were harvested at parturition and stained with antibodies that characterize the differentiation state of epithelial cells. Uniform nuclear Stat5 (red) staining was seen in the Stat5fl/fl gland (C). However, a conspicuous nonuniform staining of Stat5 was observed in most alveoli in Stat5fl/fl/WC tissue (D) (arrowhead). NKCC1 (red) expression was undetectable in Stat5fl/fl/WC and Stat5fl/fl epithelia during lactation (E and F) (arrow); smooth muscle actin (green) stained the myoepithelial cells. Alveoli in Stat5fl/fl/WC displayed heterogeneous Npt2b staining (red) at parturition (H) (arrow). While this marker for secretory activity was seen on the apical membrane in expanded alveoli, it was absent in alveoli with a small lumen (G and H) (arrowhead). E-cadherin (green) was used to stain the cell membrane. (I and J) Serial sections from a Stat5fl/fl/WC gland stained with Stat5 (red) (I) and WAP (red) (J); E-cadherin (green) was applied in both sections. Note that cells without Stat5a (I) (arrows) do not express WAP at parturition (J) (arrows). Bars, 250 μm (A and B) and 50 μm (C to J).
The use of the WAP-Cre transgene, which is activated itself by Stat5, permitted the deletion of the Stat5 locus only in those cells that had activated the differentiation program. Thus, it was possible to explore the fate of a cell that had lost Stat5 after it had gone through differentiation. Mammary tissue harvested during lactation displayed distended alveoli, with a conspicuous nonuniform staining of Stat5 (Fig. 4D). NKCC1 expression was not detected in either Stat5fl/fl/WC or Stat5fl/fl epithelia during lactation (Fig. 4E and F). Whereas in Stat5fl/fl/WC mice no Npt2b was detected in the small alveolar structures that lacked Stat5 (Fig. 4H), Npt2b was present in the apical membrane in mature alveoli (Fig. 4H). Furthermore, cells that had lost Stat5 failed to express the WAP gene (Fig. 4I and J). This suggests that the loss of Stat5 from a differentiated cell results in a loss of the differentiated status.
To further explore the fate of cells that had lost Stat5 after the pregnancy-induced differentiation program had been activated, we harvested the Stat5fl/fl/WC or Stat5fl/fl epithelia at day 15.5 (p15.5) and at day 18.5 (p18.5) of pregnancy. At p15.5, Cre recombinase was detected in a mosaic pattern in Stat5fl/fl/WC mammary tissue (Fig. 5B), while it was not detected in control Stat5fl/fl epithelium (Fig. 5A). While uniform Stat5 expression was observed in Stat5fl/fl epithelium at p15.5, about half of the epithelial cells were devoid of Stat5 in Stat5fl/fl/WC mice (Fig. 5C and D). At p18.5, only a few mammary epithelial cells in Stat5fl/fl/WC mice were negative for Stat5 (Fig. 5E and F). To investigate whether mammary epithelial cells in Stat5fl/fl/WC mice had a higher apoptotic rate, terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assays were performed. The inguinal mammary glands were isolated at p15.5 and p18.5 from three Stat5fl/fl and six Stat5fl/fl/WC mice and stained. At p15.5, 5.29% ± 2.07% (mean ± SEM) TUNEL-positive cells were observed in Stat5fl/fl/WC epithelium, compared with 1.02% ± 1.0% in Stat5fl/fl tissue. In contrast, at p18.5 the number of apoptotic cells decreased to 1.03% ± 0.64% in Stat5fl/fl/WC mammary gland, which was comparable to the percentage of positive cells (0.97% ± 0.27%) in Stat5fl/fl mice (Fig. 5G). Taken together, these data suggest that loss of Stat5 during late pregnancy after mammary epithelia have undergone differentiation resulted in an inability to maintain differentiation and caused elimination of these cells by apoptosis. The observation that only a few Stat5-null cells were observed just prior to parturition suggests that cells that had failed to undergo Cre-mediated deletion of the Stat5 locus had a growth and survival advantage. A similar observation has been made after the deletion of the Jak2 gene in mammary epithelium (35).
FIG.5.
Stat5 is required for survival of the differentiated mammary epithelium. (A to D) Inguinal mammary glands were collected from Stat5fl/fl (A and C) and Stat5fl/fl/WC (B and D) mice at p15.5. (E and F) Mammary tissues harvested at p18.5. (A and B) Immunofluorescence staining of Cre recombinase (red) and E-cadherin (green) showed a mosaic pattern of WAP-Cre expression in p15.5 Stat5fl/fl/WC mammary tissue (B) (arrows), and no WAP-Cre was detected in Stat5fl/fl mice (A). (C and D) Uniform Stat5a (red) expression was observed in Stat5fl/fl epithelium at p15.5, while about one-half of the epithelial cells had lost Stat5a expression in Stat5fl/fl/WC mice (D) (arrowheads). E-cadherin (green) was used to stain cell membranes. (E and F) At p18.5 only a few mammary epithelial cells in Stat5fl/fl/WC mice were negative for Stat5a (F) (arrowheads). E-cadherin was stained as green. (G) TUNEL assays were performed with the inguinal mammary glands at p15.5 and p18.5 from three Stat5fl/fl and six Stat5fl/fl/WC mice. At p15.5, 5.29% ± 2.07% (mean ± SEM) TUNEL-positive cells were observed in Stat5fl/fl/WC epithelium, compared with 1.02% ± 1.0% in Stat5fl/fl tissue. The difference was highly significant (P = 0.0046 by the two-tailed Student t test). In contrast, at p18.5 the number of apoptotic cells decreased to 1.03% ± 0.64% in the Stat5fl/fl/WC mammary gland, which was comparable to the percentage of positive cells (0.97% ± 0.27%) in Stat5fl/fl mice (P = 0.798).
DISCUSSION
Stat5a and Stat5b are critical transcription factors that initiate many cytokine-induced genetic programs, as demonstrated in mice which carry mutations in either gene alone or in both genes (33). Mammary epithelium cannot differentiate during pregnancy in the absence of Stat5a (15) and fails to proliferate and differentiate during pregnancy when both the Stat5a and Stat5b genes are mutated (20). To explore whether Stat5 exhibits unique functions at various stages of mammary development, we have now deleted the entire 110-kb Stat5 locus at different time points specifically in mammary epithelium by using Cre-loxP-mediated recombination. This study demonstrates that Stat5 is essential not only for pregnancy-mediated cell proliferation and differentiation but also for the survival of mammary epithelium and the maintenance of differentiation (Fig. 6).
FIG. 6.
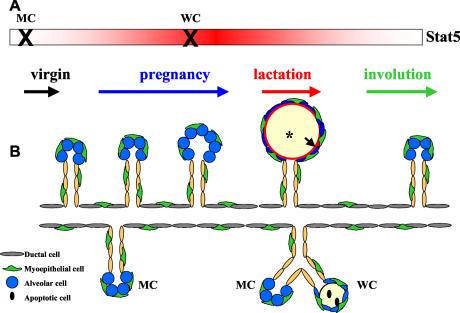
Stat5 controls proliferation, differentiation, and survival of mammary alveolar epithelium. Wild-type mammary epithelium differentiates into functional alveoli during pregnancy. When Stat5 deletion occurs prior to pregnancy, the mammary epithelium loses the ability to respond to proliferative signals at the onset of pregnancy; it retains ductal characteristics and fails to acquire a marker indicative of secretory function. When the loss of Stat5 occurs late in pregnancy after mammary epithelia have entered differentiation, differentiation is stalled and premature cell death takes place. (A) The rectangle indicates that Stat5 is expressed throughout mammary gland development, and the red gradient reflects Stat5 activity during pregnancy and lactation. The X refers to the time when Stat5 is inactivated. MC, Stat5 deletion occurs before puberty in Stat5fl/fl/MC mouse. WC, Stat5 deletion takes place during late pregnancy. (B) Schematic presentation of mammary epithelial development in the presence and absence of Stat5. Ducts and alveoli in the upper part depict mammary epithelial development in wild-type mice. Ducts and alveoli in the lower part illustrate the abnormal mammary epithelial development in Stat5fl/fl/MC or Stat5fl/fl/WC mice during pregnancy and lactation. *, milk secretion in the alveoli. The arrow points to the Npt2b localization in the functional alveoli.
Stat5 is critical for cell proliferation and differentiation during pregnancy.
Mammary epithelium in mice does not develop during pregnancy in the absence of the prolactin receptor (23), Jak2 (29), or Stat5 (20). There are two scenarios to explain these results. It is possible that the presence of Stat5 is required to establish alveolar stem cells or progenitor cells, leading to a failure to develop differentiated cells. Alternatively, the stem cells and progenitor cells are established but fail to proliferate in the absence of Stat5. Deletion of the Stat5 genes in mammary epithelia of mice that carry the targeted Stat5 locus and the MMTV-Cre transgene occurs perinatally (36). Loss of Stat5 during this time inhibited mammary epithelial proliferation during pregnancy, and the histological lesion was similar to that observed in tissue from conventional Stat5-targeted mice (20). Moreover, cell proliferation induced by estrogen and progesterone was severely impaired in the absence of Stat5. Since the ductal tree developed normally during puberty, mammary stem cells are present. The absence of secretory alveoli, combined with the fact that epithelial cells retained ductal characteristics and lacked secretory features, may indicate a paucity of alveolar progenitors. However, it is also possible that these cells fail to recognize and accurately execute prolactin signals, leading to the expansion of this cell population. The latter scenario is supported by the finding that cell cycle-related genes, such as those encoding cyclins D1 and D2 and c-myc, are considered to be Stat5 target genes (7, 16, 18, 38). We therefore suggest that in mammary epithelium, Stat5 is a bona fide activator of cell proliferation.
Stat5 is a survival factor of differentiated mammary alveolar epithelium.
Upon their differentiation during pregnancy, mammary alveolar epithelial cells are functional throughout the lactation period, which is followed by extensive cell death and tissue remodeling during involution. The signals required for secretory epithelial cells to survive a period of several weeks during pregnancy and lactation remain to be defined. This study now identifies Stat5 as a key for the survival of mammary epithelium during the second part of pregnancy after the cells have entered a differentiation program. Increased apoptosis was observed at day 15 of pregnancy after the Stat5 genes had been deleted, strongly suggesting that the presence of Stat5 late in pregnancy and during lactation is required for cell survival. While on day 15 of pregnancy one-half of the epithelial cells have lost Stat5 expression, this percentage is considerably lower just prior to parturition and during lactation. Given the notion that Stat5-null cells undergo premature cell death during pregnancy, those cells that fail to undergo Cre-mediated deletion of the Stat5 locus will eventually populate the entire gland. An analogous observation, using the same strain of Cre expressing mice, has been made in mice in which the Jak2 gene was inactivated during pregnancy (35).
Stat5 also controls cell survival in the hematopoietic lineage (30), and a unifying concept is emerging that a key aspect of cytokine signaling through type I receptors is the prevention of unscheduled cell death. However, the means by which cells achieve this goal and translate Stat5 signals into survival cues might be different between cell types. Fetal liver erythroid progenitors from Stat5-deficient mice give rise to fewer erythroid colonies and exhibit increased apoptosis (32). Notably, the defective erythropoiesis was correlated with decreased expression of Bcl-x in early erythroblasts, and the bcl-x gene promoter was shown to be regulated by Stat5 (32). However, since the Stat5-null mice described in this study are different from the ones generated by using conventional gene targeting, it will be necessary to revisit this issue. This is particularly important in light of the fact that the vast majority of Stat5-null mice described here die perinatally. Although Bcl-x is the most abundant member of the Bcl-2 family throughout mammary development (8), the deletion of the bcl-x gene from mammary tissue did not impair the function and survival of mammary epithelium during pregnancy and lactation (37). In addition, the phosphatidylinositol 3-kinase-Akt pathway can be activated by prolactin (1, 17), and it possibly synergizes with Stat5 in mammary epithelium. This is supported by findings that the expression of an activated Akt leads to increased cell survival in mammary tissue and precocious Stat5-mediated differentiation (25).
Stat5 is required for the maintenance of mammary epithelial differentiation.
Since Stat5 is required for the establishment of mammary alveolar epithelium during pregnancy, it is not possible to study its function in the maintenance of cellular differentiation by using conventional gene targeting. The use of a Cre transgene that was under direct control of Stat5 permitted us to address this question. Like the endogenous WAP gene promoter, the WAP-based transgenes are activated by prolactin and Stat5 (3, 13). Therefore, at the time of Cre activation, alveoli have already formed and caseins and lipid droplets are secreted into the lumen. Loss of Stat5 in differentiated mammary epithelium resulted in the loss of differentiation, as evidenced by the absence of endogenous WAP and Npt2b. Since the WAP gene promoter is a direct target of Stat5 (13), the lack of WAP in Stat5-null cells demonstrates that transcription of this gene cannot be maintained in the absence of Stat5. These results support the notion that the presence of Stat5 is required for maintenance of the differentiated function of mammary epithelium. Interestingly, the function of Stat5 as a multifunctional regulator of mammary cell proliferation, differentiation, and survival was confirmed in a recent study. Overexpression and forced activation of Stat5 in mammary glands of transgenic mice led to high degree of cellular proliferation, enhanced differentiation, and delayed involution (10).
A role for Stat5 in the maintenance of differentiation is also seen in other cell types. Differentiation of cytotoxic T lymphocytes is regulated through interleukin-2 receptor signaling and accompanied by the induction of perforin, which is regulated by Stat5 (40). Moreover, interleukin-2 receptor-Stat5 signaling is required for the maintenance of the regulatory T-cell population, as this cell population is reduced due to a higher rate of apoptosis in Stat5-null mice (31). We therefore propose that the differentiation status of these cells can be maintained only in the continuous presence of Stat5. Rapid loss of Stat5 will result in a loss of transcription of target genes. This suggests that the purpose of Stat5 is not only to establish transcription, possibly by opening chromatin, but also to maintain an active transcription complex (39).
REFERENCES
- 1.Acosta, J. J., R. M. Munoz, L. Gonzalez, A. Subtil-Rodriguez, M. A. Dominguez-Caceres, J. M. Garcia-Martinez, A. Calcabrini, I. Lazaro-Trueba, and J. Martin-Perez. 2003. Src mediates prolactin-dependent proliferation of T47D and MCF7 cells via the activation of focal adhesion kinase/Erk1/2 and phosphatidylinositol 3-kinase pathways. Mol. Endocrinol. 17:2268-2282. [DOI] [PubMed] [Google Scholar]
- 2.Bunting, K. D., H. L. Bradley, T. S. Hawley, R. Moriggl, B. P. Sorrentino, and J. N. Ihle. 2002. Reduced lymphomyeloid repopulating activity from adult bone marrow and fetal liver of mice lacking expression of STAT5. Blood 99:479-487. [DOI] [PubMed] [Google Scholar]
- 3.Burdon, T., L. Sankaran, R. J. Wall, M. Spencer, and L. Hennighausen. 1991. Expression of a whey acidic protein transgene during mammary development. Evidence for different mechanisms of regulation during pregnancy and lactation. J. Biol. Chem. 266:6909-6914. [PubMed] [Google Scholar]
- 4.Cui, Y., M. Li, K. D. Walton, K. Sun, J. A. Hanover, P. A. Furth, and L. Hennighausen. 2001. The Stat3/5 locus encodes novel endoplasmic reticulum and helicase-like proteins that are preferentially expressed in normal and neoplastic mammary tissue. Genomics 78:129-134. [DOI] [PubMed] [Google Scholar]
- 5.Deng, C., A. Wynshaw-Boris, F. Zhou, A. Kuo, and P. Leder. 1996. Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911-921. [DOI] [PubMed] [Google Scholar]
- 6.Deng, C. X., A. Wynshaw-Boris, M. M. Shen, C. Daugherty, D. M. Ornitz, and P. Leder. 1994. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 8:3045-3057. [DOI] [PubMed] [Google Scholar]
- 7.Friedrichsen, B. N., E. D. Galsgaard, J. H. Nielsen, and A. Moldrup. 2001. Growth hormone- and prolactin-induced proliferation of insulinoma cells, INS-1, depends on activation of STAT5 (signal transducer and activator of transcription 5). Mol. Endocrinol. 15:136-148. [DOI] [PubMed] [Google Scholar]
- 8.Heermeier, K., M. Benedict, M. Li, P. Furth, G. Nunez, and L. Hennighausen. 1996. Bax and Bcl-xs are induced at the onset of apoptosis in involuting mammary epithelial cells. Mech. Dev. 56:197-207. [DOI] [PubMed] [Google Scholar]
- 9.Hilfiker, H., O. Hattenhauer, M. Traebert, I. Forster, H. Murer, and J. Biber. 1998. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc. Natl. Acad. Sci. USA 95:14564-14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iavnilovitch, E., B. Groner, and I. Barash. 2002. Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol. Cancer Res. 1:32-47. [PubMed] [Google Scholar]
- 11.Ihle, J. N. 2001. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 13:211-217. [DOI] [PubMed] [Google Scholar]
- 12.Levy, D. E., and J. E. Darnell, Jr. 2002. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3:651-662. [DOI] [PubMed] [Google Scholar]
- 13.Li, S., and J. M. Rosen. 1994. Distal regulatory elements required for rat whey acidic protein gene expression in transgenic mice. J. Biol. Chem. 269:14235-14243. [PubMed] [Google Scholar]
- 14.Liu, X., G. W. Robinson, and L. Hennighausen. 1996. Activation of Stat5a and Stat5b by tyrosine phosphorylation is tightly linked to mammary gland differentiation. Mol. Endocrinol. 10:1496-1506. [DOI] [PubMed] [Google Scholar]
- 15.Liu, X., G. W. Robinson, K. U. Wagner, L. Garrett, A. Wynshaw-Boris, and L. Hennighausen. 1997. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11:179-186. [DOI] [PubMed] [Google Scholar]
- 16.Lord, J. D., B. C. McIntosh, P. D. Greenberg, and B. H. Nelson. 2000. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J. Immunol. 164:2533-2541. [DOI] [PubMed] [Google Scholar]
- 17.Martin, A. G., B. San-Antonio, and M. Fresno. 2001. Regulation of nuclear factor kappa B transactivation. Implication of phosphatidylinositol 3-kinase and protein kinase C zeta in c-Rel activation by tumor necrosis factor alpha. J. Biol. Chem. 276:15840-15849. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura, I., T. Kitamura, H. Wakao, H. Tanaka, K. Hashimoto, C. Albanese, J. Downward, R. G. Pestell, and Y. Kanakura. 1999. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 18:1367-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyoshi, K., Y. Cui, G. Riedlinger, P. Robinson, J. Lehoczky, L. Zon, T. Oka, K. Dewar, and L. Hennighausen. 2001. Structure of the mouse Stat 3/5 locus: evolution from Drosophila to zebrafish to mouse. Genomics 71:150-155. [DOI] [PubMed] [Google Scholar]
- 20.Miyoshi, K., J. M. Shillingford, G. H. Smith, S. L. Grimm, K. U. Wagner, T. Oka, J. M. Rosen, G. W. Robinson, and L. Hennighausen. 2001. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J. Cell Biol. 155:531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore-Hoon, M. L., and R. J. Turner. 1998. Molecular and topological characterization of the rat parotid Na+-K+-2Cl− cotransporter 1. Biochim. Biophys. Acta 1373:261-269. [DOI] [PubMed] [Google Scholar]
- 22.Moriggl, R., D. J. Topham, S. Teglund, V. Sexl, C. McKay, D. Wang, A. Hoffmeyer, J. van Deursen, M. Y. Sangster, K. D. Bunting, G. C. Grosveld, and J. N. Ihle. 1999. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity 10:249-259. [DOI] [PubMed] [Google Scholar]
- 23.Ormandy, C. J., N. Binart, and P. A. Kelly. 1997. Mammary gland development in prolactin receptor knockout mice. J. Mammary Gland Biol. Neoplasia 2:355-364. [DOI] [PubMed] [Google Scholar]
- 24.Robinson, G. W., R. A. McKnight, G. H. Smith, and L. Hennighausen. 1995. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development 121:2079-2090. [DOI] [PubMed] [Google Scholar]
- 25.Schwertfeger, K. L., M. M. Richert, and S. M. Anderson. 2001. Mammary gland involution is delayed by activated Akt in transgenic mice. Mol. Endocrinol. 15:867-881. [DOI] [PubMed] [Google Scholar]
- 26.Seagroves, T. N., J. P. Lydon, R. C. Hovey, B. K. Vonderhaar, and J. M. Rosen. 2000. C/EBPbeta (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol. Endocrinol. 14:359-368. [DOI] [PubMed] [Google Scholar]
- 27.Shillingford, J. M., K. Miyoshi, M. Flagella, G. E. Shull, and L. Hennighausen. 2002. Mouse mammary epithelial cells express the Na-K-Cl cotransporter, NKCC1: characterization, localization, and involvement in ductal development and morphogenesis. Mol. Endocrinol. 16:1309-1321. [DOI] [PubMed] [Google Scholar]
- 28.Shillingford, J. M., K. Miyoshi, G. W. Robinson, B. Bierie, Y. Cao, M. Karin, and L. Hennighausen. 2003. Proteotyping of mammary tissue from transgenic and gene knockout mice with immunohistochemical markers: a tool to define developmental lesions. J. Histochem. Cytochem. 51:555-565. [DOI] [PubMed] [Google Scholar]
- 29.Shillingford, J. M., K. Miyoshi, G. W. Robinson, S. L. Grimm, J. M. Rosen, H. Neubauer, K. Pfeffer, and L. Hennighausen. 2002. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol. Endocrinol. 16:563-570. [DOI] [PubMed] [Google Scholar]
- 30.Snow, J. W., N. Abraham, M. C. Ma, N. W. Abbey, B. Herndier, and M. A. Goldsmith. 2002. STAT5 promotes multilineage hematolymphoid development in vivo through effects on early hematopoietic progenitor cells. Blood 99:95-101. [DOI] [PubMed] [Google Scholar]
- 31.Snow, J. W., N. Abraham, M. C. Ma, B. G. Herndier, A. W. Pastuszak, and M. A. Goldsmith. 2003. Loss of tolerance and autoimmunity affecting multiple organs in STAT5A/5B-deficient mice. J. Immunol. 171:5042-5050. [DOI] [PubMed] [Google Scholar]
- 32.Socolovsky, M., H. Nam, M. D. Fleming, V. H. Haase, C. Brugnara, and H. F. Lodish. 2001. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood 98:3261-3273. [DOI] [PubMed] [Google Scholar]
- 33.Teglund, S., C. McKay, E. Schuetz, J. M. van Deursen, D. Stravopodis, D. Wang, M. Brown, S. Bodner, G. Grosveld, and J. N. Ihle. 1998. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell 93:841-850. [DOI] [PubMed] [Google Scholar]
- 34.Udy, G. B., R. P. Towers, R. G. Snell, R. J. Wilkins, S. H. Park, P. A. Ram, D. J. Waxman, and H. W. Davey. 1997. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc. Natl. Acad. Sci. USA 94:7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner, K. U., A. Krempler, A. A. Triplett, Y. Qi, N. M. George, J. Zhu, and H. Rui. 2004. Impaired alveologenesis and maintenance of secretory mammary epithelial cells in jak2 conditional knockout mice. Mol. Cell. Biol. 24:5510-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner, K. U., R. J. Wall, L. St.-Onge, P. Gruss, A. Wynshaw-Boris, L. Garrett, M. Li, P. A. Furth, and L. Hennighausen. 1997. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 25:4323-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walton, K. D., K. U. Wagner, E. B. Rucker III, J. M. Shillingford, K. Miyoshi, and L. Hennighausen. 2001. Conditional deletion of the bcl-x gene from mouse mammary epithelium results in accelerated apoptosis during involution but does not compromise cell function during lactation. Mech. Dev. 109:281-293. [DOI] [PubMed] [Google Scholar]
- 38.Wen, X., H. H. Lin, H. M. Shih, H. J. Kung, and D. K. Ann. 1999. Kinase activation of the non-receptor tyrosine kinase Etk/BMX alone is sufficient to transactivate STAT-mediated gene expression in salivary and lung epithelial cells. J. Biol. Chem. 274:38204-38210. [DOI] [PubMed] [Google Scholar]
- 39.Ye, S. K., Y. Agata, H. C. Lee, H. Kurooka, T. Kitamura, A. Shimizu, T. Honjo, and K. Ikuta. 2001. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity 15:813-823. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, J., I. Scordi, M. J. Smyth, and M. G. Lichtenheld. 1999. Interleukin 2 receptor signaling regulates the perforin gene through signal transducer and activator of transcription (Stat)5 activation of two enhancers. J. Exp. Med. 190:1297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]