Significance
The use of methyl-α-cyclodextrin to fully replace endogenous sphingolipids and phospholipids in plasma membrane outer leaflets of living mammalian cells with exogenous natural and unnatural lipids is introduced in this paper. Outer leaflet lipid species were evaluated, with lipids exchanged out of the plasma membrane outer leaflet matching expected lipid asymmetry. The method will make possible a range of membrane structure/function studies.
Keywords: lipid exchange, plasma membrane outer leaflet, lipid asymmetry, methyl-alpha-cyclodextrin, mass spectrometry
Abstract
Our understanding of membranes and membrane lipid function has lagged far behind that of nucleic acids and proteins, largely because it is difficult to manipulate cellular membrane lipid composition. To help solve this problem, we show that methyl-α-cyclodextrin (MαCD)-catalyzed lipid exchange can be used to maximally replace the sphingolipids and phospholipids in the outer leaflet of the plasma membrane of living mammalian cells with exogenous lipids, including unnatural lipids. In addition, lipid exchange experiments revealed that 70–80% of cell sphingomyelin resided in the plasma membrane outer leaflet; the asymmetry of metabolically active cells was similar to that previously defined for erythrocytes, as judged by outer leaflet lipid composition; and plasma membrane outer leaflet phosphatidylcholine had a significantly lower level of unsaturation than phosphatidylcholine in the remainder of the cell. The data also provided a rough estimate for the total cellular lipids residing in the plasma membrane (about half). In addition to such lipidomics applications, the exchange method should have wide potential for investigations of lipid function and modification of cellular behavior by modification of lipids.
In the last half century, our understanding of cells and biological processes at the cellular level has undergone a revolution based on advances in our ability to control the structure and composition of cellular nucleic acids and proteins. In contrast, our understanding of the function of the lipids in the membranes surrounding cells and their internal compartments has seen much slower progress. The basic problem is the inability to manipulate membrane lipid composition as easily as other cellular molecules. This problem is a complex one, complicated by the fact that eukaryotic plasma membranes exhibit asymmetry (i.e., a difference in inner membrane leaflet and outer membrane leaflet lipid composition) with respect to their lipid distribution across the lipid bilayer. In general, the outer membrane leaflet in mammalian cells is composed primarily of sphingomyelin (SM) and phosphatidyl choline (PC). In contrast, the inner or cytoplasmic leaflet consists mostly of aminophospholipids [e.g., phosphatidylethanolamine (PE) and phosphatidylserine (PS)] (1, 2). The asymmetric arrangement of lipids in the cellular membrane affects various biological properties, such as membrane permeability, membrane potential, surface charge, the mechanical stability of membranes, and membrane shape (3–5). Therefore, the ability to manipulate the lipid composition of living cell membrane would provide a useful tool for use in the research and development of cell membrane-mediated pathological disease. At present, there are limited approaches to exploring lipid function by altering lipid composition, using synthesis inhibitors and metabolic engineering (6–8). However, inhibitor molecules are slow acting, limited to certain classes of lipids, and not always sufficiently specific. Metabolic engineering is a powerful, but laborious, method that has been effectively applied only to bacteria to date. Such methods do not permit efficient introduction of a wide variety of exogenous lipids with specific headgroups and acyl chain compositions, or the introduction of unnatural lipids. In this regard, changing lipid composition by lipid exchange, using cyclodextrins (CDs), is an attractive alternative. CDs, cyclic oligomers of glucose with a hydrophobic cavity, have been long used to transfer and modify cell sterols (9), but their use to exchange sphingolipids and phospholipids in cells has been little explored. Prior studies have shown that phospholipids can be delivered into cells by methyl-β-cyclodextrin (MβCD), and that delivered lipids are not rapidly turned over (10, 11). However, the amount of exchange was low, except for lipids with short acyl chains. In addition, to overcome the effects of cholesterol extraction by MβCD, cholesterol had to be added back to the cells.
We previously developed use of CDs to carry out efficient outer leaflet lipid exchange in model membrane vesicles, defining conditions such that the entire outer lipid monolayer (leaflet) of a membrane lipid bilayer can be replaced with exogenous lipids in situ, using MβCD or hydroxypropyl (HP)αCD without disturbing the lipid composition of the inner leaflet (12–18). This allowed preparation of artificial vesicles that mimic natural membranes by having both natural lipid composition and asymmetry. The approach is beginning to see applications by other laboratories (19–22).
In this study, we establish a procedure to efficiently exchange cellular phospholipids and sphingolipids from cell plasma membrane outer leaflet of living mammalian cells with exogenous lipids, using lipid-loaded MαCD. Alpha CDs have the advantage of having a cavity too small to interact with sterols (15). TLC and lipid MS was carried out to assay the efficiency of exchange and the asymmetry of cellular lipids. Conditions giving efficient exchange were defined and revealed important basic lipidomic information concerning the plasma membrane lipid outer leaflet lipid composition. This method should enable a range of membrane structure and function studies in the future.
Results
MαCD Binds a Variety of Phospholipids and Sphingolipids and Can Carry Out Efficient Lipid Exchange in Model Membranes.
Previous studies have shown that MβCD interacts strongly with phospholipids and cholesterol, whereas HPαCD interacts specifically with phospholipids, but only weakly (15, 23). We reasoned that MαCD, which, similar to HPαCD, has a cavity too small to bind cholesterol, should, similar to MβCD, interact strongly with phospholipids. To confirm this, MαCD was titrated into lipid vesicles. Solubilization of vesicles, observed when CD binds lipids efficiently, was induced by MαCD at concentrations somewhat lower than needed for MβCD (SI Appendix, Fig. S1). The dependence of solubilization on phospholipid structure was modest, and was similar for MαCD and MβCD. To confirm a lack of phospholipid specificity during exchange, a protocol for lipid exchange in model membrane vesicles analogous to that developed previously for MβCD (12) was devised. Efficient exchange was observed using egg SM (eSM) to donate the lipid exchanged into the outer leaflet of acceptor vesicles composed of 1:1:1 (mol:mol) 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE)/1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS)/1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (POPC) with 40 mol% cholesterol (SI Appendix, Fig. S2). POPC, POPE, and POPS were replaced by eSM to a similar extent, with perhaps slightly lesser exchange of POPS. Because cyclodextrins catalyze exchange of lipids into the outer leaflet of membranes, after exchange, the vesicles should have an asymmetric distribution of lipids. Labeling of POPE with the membrane-impermeable probe trinitrobenzenesulfonate labeling confirmed the expected lipid asymmetry (SI Appendix, Fig. S2C).
MαCD-Mediated Exchange Procedure for Mammalian Cells.
A protocol for lipid exchange experiments using A549 lung carcinoma cells grown on cell culture plates was next devised (Fig. 1). Cells were sensitive to treatment with MαCD alone, rounding up within 15 min (SI Appendix, Table S1). To avoid this, MαCD was preincubated with lipid vesicles before being added to cells. After incubation of cells with the lipid-MαCD mixture, the solution containing MαCD and exchanged cellular lipids was removed. Incubating cells with MαCD premixed with sphingolipid or phospholipid maintained normal cell morphology for up to and beyond 6 h (SI Appendix, Table S1). After exchange under optimal conditions, <1% of cells stained with trypan blue in the cytoplasm (i.e., >99% of cells) survived. Note that maintenance of normal morphology is very sensitive to both lipid and MαCD concentrations. Maintenance of normal cell behavior after incubation with lipid–MαCD mixtures was also seen by measuring clathrin-mediated endocytosis of transferrin. Normal endocytosis levels were observed after incubation of cells with brain SM (bSM)/POPC-loaded MαCD (SI Appendix, Fig. S3). In contrast, endocytosis was strongly inhibited after partial extraction of cellular cholesterol with MβCD.
Fig. 1.
Schematic illustration of lipid exchange in cells. MαCD is indicated by closed hexagon. Exogenous lipids introduced into cells are shown in red. Endogenous lipids are shown in blue.
Kinetics of MαCD-Mediated Exchange of Plasma Membrane Outer Leaflet Lipids with Exogenous Lipids.
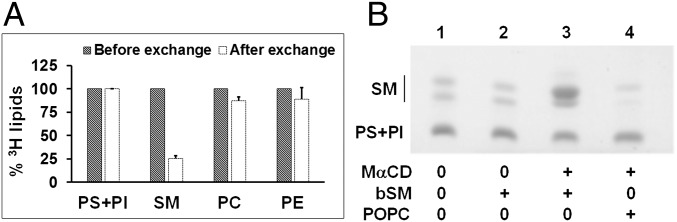
Exchange levels using bSM as a donor were assayed under conditions avoiding changes in cell morphology. To do this, endogenous cell lipids were metabolically labeled with 3H acetate, and cellular lipids before and after exchange were then quantified by extraction, followed by TLC and measurement of radioactivity in each lipid band. SI Appendix, Fig. S4A shows that the predominant cell phospholipids were PC, SM, PS+phosphatidylinositol (PI), and PE with only small amounts of phosphatidylglycerol (PG) and cardiolipin (CL). Their relative level of radiolabeling (Table 1) was roughly consistent with their bulk concentration in cells, as assessed by charring of HP-TLC plates (SI Appendix, Fig. S4B) and MS (Table 1).
Table 1.
Effect of lipid exchange on cell phospholipid and sphingomyelin content
| Lipids | Percentage total (endogenous) lipids* in cell before exchange | Percentage of total endogenous lipids* in cells after exchange† | ||
| MS | Radioactivity | MS¶ | Radioactivity | |
| PS | 11.5 ± 0.8 | 14.3 ± 1.3‡ | 9.9 ± 0.2 | 17.7 ± 2.3‡ |
| PI | 10.5 ± 0.6 | 13.7 ± 0.9 | ||
| SM | 17.7 ± 0.5 | 13.0 ± 0.7 | 3.3 ± 0.0 | 4.1 ± 0.6 |
| PC | 50.7 ± 2.0 | 57.4 ± 2.4 | 60.1 ± 0.2 | 61.6 ± 3.3 |
| PE | 9.6 ± 0.1 | 15.3 ± 1.6 | 11.9 ± 0.4 | 16.7 ± 2.2 |
For the MS data, the average from duplicate experiments and the 1/2 range is shown. For the radiolabeling +TLC analysis, average and SD from three independent experiments are shown. From MS data, SM exchange efficiency = (17.7 − 3.3)/17.7 × 100% = 81%. From the radioactivity data, SM exchange efficiency = (13 − 4.1)/13 × 100% = 70%. Assuming this efficiency corresponds to 100% exchange of outer leaflet SM, then outer leaflet SM is 17.7% − 3.3% = 14.4% from the MS data, or 13% − 4.1% = 8.9% from the radioactivity data.
Total lipids = total (phospholipids + sphingomyelin). Not corrected for small amounts of PG and CL.
Values shown after exchange are percentage of endogenous lipids (i.e., do not include exogenous lipid introduced into the cells).
Radioactivity is shown for the sum of PS+PI, which have a similar mobility on TLC.
Exogenous C17:0 SM was 37.9 ± 0.4% of the total lipid after exchange.
Exchange was monitored from the introduction of exogenous fluorescently labeled lipid 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2–1,3-benzoxadiazol-4-yl) (NBD-DPPE) into cells, and from the extent of MαCD-catalyzed removal of endogenous lipid. Lipid levels after exchange were then compared with those before exchange. Delivery of NBD-DPPE and removal of endogenous SM, a lipid predominantly located in plasma membrane (PM) outer leaflets (2), followed similar kinetics, with a half-time on the order of 15–20 min at 37 °C (Fig. 2A). Exchange at 15 °C was about twofold less after 1 h, perhaps because of slower exchange (SI Appendix, Fig. S5 A and B). Association of exogenous NBD-DPPE with cells in the absence of MαCD was negligible, showing that MαCD was necessary for lipid exchange. Experiments also showed very little or no delivery of exogenous bSM in the absence of MαCD (see following).
Fig. 2.
Efficiency and kinetics of lipid exchange between lipid-loaded MαCD and A549 cells at 37 °C. (A) Kinetics of lipid exchange using 1.5 mM bSM (or 1:9 mol:mol NBD-DPPE/bSM) and 40 mM MαCD. Removal of endogenous SM was measured by 3H SM remaining in 3H labeled A549 cells after lipid exchange; 10% of the samples were analyzed. Delivery of NBD-labeled fluorescent lipid was assayed from the level of cell-associated NBD fluorescence. Fluorescence units are arbitrary. (B) Effect of MαCD concentration on the residual percentage 3H SM = [(cpm SM after exchange)/(cpm SM before exchange)] × 100% remaining in A549 cells after lipid exchange. Percentage exchanged endogenous SM = 100% − percentage residual 3H SM. The exogenous lipid was 1.5 mM bSM. (C) Effect of the concentration of lipid mixed with MαCD on the residual percentage 3H SM remaining in A549 cells after lipid exchange. MαCD concentration was 40 mM. Some cells rounded up when exogenous bSM concentration was 0.2 mM or less. (D) Different lipid combinations resulted in similar levels of percentage 3H SM exchange. The lipids were composed of 1.5 mM bSM, 3 mM POPC, 3 mM 1:1 (mol:mol) bSM/POPC, or bSM/1,2-dioleoyl-sn-glycero-3-phosphocholine. MαCD concentration was 40 mM. For B–D, data were normalized to the level of radioactive PS+PI in the same sample, using the equation percentage 3H SM = (cpm SM after exchange/cpm SM before exchange) × (cpm PS+PI before exchange/cpm PS+PI after exchange) × 100%. PS and PI, which comigrate on TLC, are inner leaflet lipids inaccessible to exchange (as confirmed in Fig. 3A). For A–D, average values and SDs from three experiments are shown. cpm, counts per minute.
MαCD-Mediated Replacement of Plasma Membrane Outer Leaflet Lipids with Exogenous Lipids Is Efficient.
The maximal extent of radiolabeled SM exchange out of cells was about 70–75% (Fig. 2 A–D). This maximum value was not affected when MαCD concentration or exogenous lipid concentrations were varied (Fig. 2 B and C). A slightly higher level of endogenous SM removal by exchange (∼80%) was measured using MS (Table 1). Levels of endogenous SM exchange were similar with different cell types (SI Appendix, Fig. S6) and when the exogenous lipid used was bSM, POPC, a bSM/POPC, or an eSM/1,2-dioleoyl-sn-glycero-3-phosphocholine mixture (Fig. 2D). Trypsinization of cells before exchange did not increase exchange levels, indicating exchange was not limited by cell-plate contacts. After a 30-min exchange with bSM and MαCD, adding a fresh mixture of bSM and MαCD only exchanged out a small additional amount of endogenous SM, and the total exchange was no more than for a single 1-h round of exchange (SI Appendix, Fig. S5C). Similarly, measuring the amount of extracted radioactivity transferred from cells to the supernatant on exchange showed that the vast majority of exchangeable endogenous SM (85%) and PC (80%) was removed after a 30 min round of exchange. The small fraction of SM (15% of the total extracted after two rounds of exchange) and PC (20% of the total extracted after two rounds of exchange) appearing in the supernatant after a second round of exchange may mainly represent a background resulting from cell detachment or cell debris.
The most likely explanation for all these results is that 70–80% SM exchange represents complete exchange of PM outer leaflet SM, with the unexchangable pool of SM being inaccessible to exchange because of the location in the cytosolic leaflet of PM or internal membranes, consistent with prior studies (24).
The Exchange of Lipids Other than SM Is Consistent with Exchange Being Restricted to Lipids in the Outer Leaflet of the PM.
As shown in Fig. 3A, under conditions of maximal (∼75%) SM exchange, percentage exchange of PC and PE was very low (10–15%), and there was virtually no exchange of PS+PI. (MS indicates PS and PI are present in a near 1:1 ratio; Table 1.) These lipids all either are found in high amounts in internal membranes and/or are located in the cytosolic leaflet, inaccessible to MαCD. These conclusions were not affected by how radioactivity values were normalized (Fig. 3A vs. SI Appendix, Fig. S7). Exchange had very little, if any, effect on cholesterol or triglyceride levels (SI Appendix, Fig. S8).
Fig. 3.
Lipid composition and localization of cellular and exogenous lipid after exchange at 37 °C. (A) Percentage residual endogenous 3H labeled lipids in A549 cells after 1 h exchange with 1.5 mM bSM and 40 mM MαCD. (Radioactivity after exchange/radioactivity before exchange) × 100% was calculated for each lipid as shown, with values before exchange normalized to 100% for each lipid. Average values of three experiments and SDs are shown. (B) TLC of A549 lipids after 1 h exchange. Exogenous lipids were loaded onto 40 mM MαCD. (Lane 1) Control samples with no MαCD or exogenous lipids. (Lane 2) Treatment with 1.5 mM exogenous bSM but no MαCD. (Lane 3) Exchange with 1.5 mM exogenous bSM and MαCD. (Lane 4) Exchange with 3 mM exogenous POPC and MαCD. The low mobility section of a TLC plate onto which 1/10 of total sample was chromatographed is shown. Notice that SM runs as multiple bands.
Analogous experiments with unlabeled bSM in which lipid levels were estimated by charring of lipid bands on TLC gave similar results as those obtained with radiolabeled lipids. In agreement with radioactivity measurements, most endogenous SM was removed on exchange with exogenous POPC (Fig. 3B, lane 4), whereas PS+PI levels were not affected by exchange (Fig. 3B, lanes 3 and 4). There was very little or no lipid delivery in the absence of MαCD (Fig. 3B, lane 2).
Because endogenous SM is ∼40% total outer leaflet lipid (see following), complete 1:1 exchange of outer leaflet lipid with exogenous SM should increase SM concentration in the plasma membrane outer leaflet ∼2.5-fold (if outer leaflet SM is 80% of total SM, total SM should increase by 2.2-fold). This is roughly what was observed, as judged by staining intensity, using charring (Fig. 3B, lanes 2 and 3). MS experiments using exchange of the unnatural lipid N-hepadecanoyl-d-erythro-sphingosylphosphorylcholine (C17:0 SM) into cells gave similar results. The amount of exogenous C17:0 SM in cells after exchange was 2.1-fold higher than the amount of total endogenous cell SM before exchange (Table 1), indicative of complete replacement of the PM outer leaflet phospholipid and sphingolipid in a roughly 1:1 exchange process. In general, we checked the extent of exchange not only by radiolabeling but also by the charring of lipids on TLC and/or by MS. Wherever the methods could be compared, they gave similar answers.
Finally, the amount of the PM outer leaflet lipid ganglioside GM1 exchanged out of cells was also consistent with efficient exchange of PM outer leaflet lipids. As assayed by the binding of fluorescently labeled cholera toxin B subunit, ∼90% of cholera toxin B binding was abolished (SI Appendix, Fig. S9), although this might not correspond to exactly 90% removal of GM1 (25).
Interestingly, combining the observation that SM comprises 40% of outer leaflet lipid with the observation that 9–14% of all cell lipid is outer leaflet SM (Table 1) indicates that in these cells, the outer leaflet contains ∼25–35% of total phospholipid and sphingolipid (assuming glycosphingolipids are minor lipid components). Thus, it can be estimated that the plasma membrane contains roughly ≥50% of total phospholipid and sphingolipid.
Outer Leaflet Lipid Asymmetry and Distinct Acyl Chain Composition.
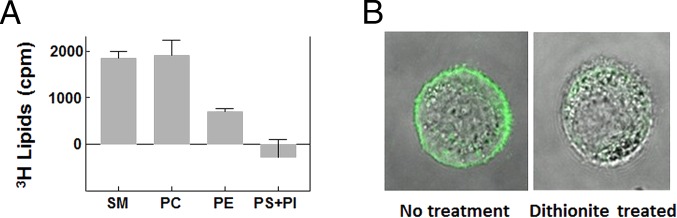
The composition of the cellular lipid extracted by the MαCD was as expected for its origin in the plasma membrane outer leaflet. It contained a high level of PC and SM, a much lower amount of PE, and little or no PS+PI (Fig. 4A). Overall, SM was ∼40% of the extracted lipids. A similar overall composition of outer leaflet lipids was previously found for the plasma membrane of erythrocytes (2). MS experiments using exchange of exogenous C17:0 SM into cells provided additional information concerning the molecular composition of the lipid species exchanged out of cells; in particular, in terms of acyl chain composition (SI Appendix, Tables S2 and S3). In the case of PC, the residual species in the cells after exchange showed that the ratio of highly unsaturated species (with three or more double bonds) to relatively less unsaturated species (with one or two double bonds) increases by [0.99/0.74 − 1] × 100% = 34% (SI Appendix, Table S2). This suggests the PM outer leaflet was enriched in the species that are not highly unsaturated, as they were preferentially removed on exchange. This is consistent with and extends a previous MS study that compared unsaturation in whole PM and whole cells and found enrichment of less highly unsaturated species in whole PM (26).
Fig. 4.
Endogenous lipids extracted from cells and localization of exogenous lipids. (A) Endogenous lipids extracted from A549 cells on exchange with 1.5 mM bSM and 40 mM MαCD. Radioactivity from endogenous lipids extracted from A549 cells on treatment with 1.5 mM bSM, but without MαCD, was used as background and was subtracted. Average values of five experiments and SDs are shown. All sample data were calculated based on chromatography data of either 10% or 30% of sample, scaled appropriately. (B) Localization of exogenous lipid after exchange. Micrographs of single confocal slices show representative cell after 1 h exchange with 1.5 mM 1:9 (mol:mol) NBD-DPPE/bSM loaded onto 40 mM MαCD (Left, nontreatment), or after exchange and a 5-min incubation with dithionite (Right, dithionite treated).
In the case of endogenous SM, although all species showed a high level of exchange (SI Appendix, Table S3, Right), we found the ratio for exchange for long-chain SM (with no fewer than 36 carbons) relative to short-chain SM (with fewer than 36 carbons) increases. The ratio of long to short acyl chain SM species increases by fourfold. These results demonstrate that shorter acyl chain SM species were exchanged to a much greater degree than the longer acyl chain species, so that the residual endogenous SM in cells after exchange was enriched in long-chain species (SI Appendix, Table S3, Left). This strongly suggests that a larger fraction of long-chain SM species than of the short-chain species is located elsewhere than the PM outer leaflet. The alternate possibility, selective extraction of shorter-chain SM by MαCD, is less likely because the exchange of outer leaflet lipids appears to be nearly complete. For the other phospholipid species, only a small fraction of the species reside in the PM outer leaflet (Fig. 3A), so it is not surprising that no obvious effect of exchange on acyl chain composition of residual lipids versus those present before exchange was noted (SI Appendix, Table S2).
Exchanged NBD–Lipid Mainly Resides in the Plasma Membrane.
Fluorescence micrographs showed a plasma membrane localization of NBD–lipid exchanged into cells (Fig. 4B, Left). An outer leaflet location was shown from accessibility of the NBD group to destruction by the relatively membrane-impermeable reducing agent sodium dithionite (Fig. 4B, Right). Most of the fluorescence was lost rapidly on dithionite treatment, with the weak residual fluorescence associated with the cell interior. This interpretation was confirmed by spectroscopic measurements of the decrease in NBD fluorescence on dithionite treatment, which showed that 70–80% of NBD-lipid exchanged into cells was accessible to dithionite (SI Appendix, Fig. S10). Accessibility to dithionite was not strongly temperature-dependent.
In addition, an experiment was carried out in which the distribution of exogenous radiolabeled SM introduced into cells after exchange was evaluated. As in the case of the endogenous SM, 80% of the exogenous 14C-SM delivered into cells could be removed by a second exchange, showing its distribution was similar to that of the endogenous lipid (SI Appendix, Fig. S11). This also indicates that SM asymmetry remained stable at least 1 h after the initial exchange. Another experiment was carried out to measure SM content in cells after replacement of outer leaflet lipids with exogenous SM. Elevated SM levels persisted for many hours (SI Appendix, Fig. S12).
Discussion
This study shows that facile and efficient replacement of PM outer leaflet lipid with exogenous lipids can be achieved in living cells. This process gives important information about the fraction of various lipid species in the PM outer leaflet. Lipid headgroup composition for cultured cells was very similar to that previously established using erythrocytes (2). This is interesting because, although relatively simple to study, as they largely lack internal membranes, erythrocytes do not contain or perform the same complex metabolic functions as most other cells, and this might have altered outer leaflet composition. In addition, erythrocyte leaflet composition was largely solved using phospholipases (2), which could perturb membrane structure, especially if lipid hydrolysis alters lipid flip-flop between the inner and outer leaflet. Our exchange method should be much less perturbing, so it should be widely applicable to asymmetry analysis, which has seen little progress since the 1970s (2).
We also checked the extent of lipid exchange not only by radiolabeling but also by the total lipid levels, as detected by charring of lipids on TLC, which reflects total lipid, and by MS. Each method could potentially give slightly different answers. Radiolabeling could be influenced if radioactivity were distributed differently in different lipids. Charring depends to some degree on lipid acyl chain and headgroup structure (although we mainly used it for evaluating changes in the level of a single headgroup type). MS assumes sensitivity is not affected by whether the standards have different acyl chain structures than the natural species. The observation that when these diverse methods could be compared they gave very similar answers indicates that all of them were giving reliable results, despite these issues.
The exchange studies also indicate that SM is predominantly a lipid of the PM outer leaflet in cultured cells. Unless metabolic processes such as transverse diffusion are unusually active, PM outer leaflet SM content must greatly exceed that in internal membranes plus cytosolic membrane leaflets to yield the high level of SM exchange seen in our experiments.
An issue that should not be ignored is if lipid flip-flop (transverse diffusion between leaflets) increases during or after lipid exchange. If flip-flop had increased, and the lipid distribution became random during exchange, the pattern of lipids extracted would have been very different from what we observed. We observed that the extracted lipids (PC and SM, little PE, no PS+PI) correspond to what is known for the outer leaflet from the red blood cell studies of asymmetry. In addition, the distribution of exogenous radiolabeled SM introduced into cells by exchange was shown to be similar to that of endogenous SM (∼80% of SM being in the plasma membrane outer leaflet). This would not have been observed if SM had distributed equally between the inner and outer leaflet (i.e., 50% or less exchange). However, one could imagine with some exogenous lipids not tested in this report (e.g., PE) that there might be enhanced flip-flop.
The observation that the conditions that preserve normal cell morphology were different for egg, brain, and milk SM (SI Appendix, Table S1) shows that exchange conditions are sensitive to acyl chain structure. We do not yet understand the origin for this effect, but it shows that for future applications, optimal conditions for exchange have to be experimentally determined, and will not necessarily be identical to those in this report.
The exchange method should have important biological applications beyond those described here involving lipidomic analysis. This includes studies of how altering lipid composition influences biological functions. In this regard, exchange should greatly extend what can be achieved by modifying lipids with inhibitors or engineering modified lipid synthesis pathways (6, 7). Exchange may be useful to introduce a very wide variety of lipids, lipids with defined headgroup and single acyl chain composition, and as shown here, unnatural lipids. Introduction of nonhydrolyzable lipid analogs would allow studies of phospholipase functions. It may also be possible to target lipid exchange to remove specific lipid species. To do this, outer leaflet lipids would first be isolated from PM. After isolation of different lipid species, for example, using TLC or HPLC, a PM lipid mixture could be reconstituted with one specific lipid species removed. Exchange using this reconstituted mixture would result in selective removal of the targeted lipid lacking in the reconstituted mixture from the cells.
It should also be noted that exchange could also allow studies of how lipids influence PM proteins in a much less perturbing fashion than when proteins must first be isolated and then reconstituted in artificial membranes with different lipids. Finally, altering cell properties favorably by altering lipids may have practical applications in biotechnology.
Materials and Methods
Materials.
Breast cancer cell line MDA-MB-231, COS 7 kidney cells, A549 lung carcinoma, and BxPC-3 pancreatic cells are from ATCC. bSM, eSM, milk sphingomyelin, C17:0 SM, POPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine, and NBD-DPPE, POPE, POPS, and cholesterol were from Avanti Polar Lipids. 3H acetate was from PerkinElmer, Inc. MαCD was purchased from AraChem. MαCD dissolved in distilled water was slightly turbid. Turbidity was removed by filtration through a 0.2-μm filter or by centrifugation. To calculate concentration, the refractive index of MαCD solutions in water were measured on a Bausch and Lomb refractometer. The refractive index values were compared with a standard curve generated using a series of solutions of clarified MαCD, the concentration of which had been measured by dry weight. 14C-SM was from American Radiolabeled Chemicals (St. Louis). It was repurified by TLC prior to use. MβCD, FITC-conjugated cholera toxin B and paraformaldehyde powder were purchased from Sigma Aldrich. RPMI medium 1640, DMEM, FBS, HBSS, Dulbecco's PBS, 200 mg/L KCl, 200 mg/L KH2PO4, 8 g/L NaCl, and 2.16 g/L Na2HPO4 (DPBS) were Gibco brand and purchased from Life Technologies. Transferrin conjugated with Alexa Fluor 488 (TF-AF488) and CellMask Deep Red (plasma membrane staining solution) were Molecular Probes brand and obtained from Life Technologies. VECTASHIELD mounting medium was bought from Vector Laboratories, Inc. BSA was obtained from Millipore. The 10× PBS at pH 7.8 ± 0.2 when diluted to 1× was bought from Bio-Rad Laboratories Inc. Citric acid and sodium chloride were purchased through Fisher Scientific. Distilled and de-ionized water was used. Other chemicals were reagent grade.
Cell Culture.
A549 and BxPC-3 cells were cultured in RPMI medium 1640. DMEM was used to culture MDA-MB-231 and COS 7 cells. All media were supplemented with 10% FBS. All cells were cultured in an incubator at 37 °C with 5% CO2.
High-Performance TLC.
The samples and pure lipid standards were dissolved in 1:1 (v:v) chloroform/methanol. In some cases, lipids from the asymmetric vesicle samples were first extracted using a 2:2:1 (v:v) chloroform/methanol/water and dried under a nitrogen stream, but this did not affect the results. The dissolved lipids were then applied to HP-TLC (Silica Gel 60) plates (Merck) or Uniplate Silica Gel G plates (Analtech, Inc.) and chromatographed in 65:25:5 (v:v) chloroform/methanol/28.0–30.0% (vol/vol) ammonium hydroxide. After chromatography, the TLC plates were air-dried and saturated with 3% (wt/vol) cupric-acetate-8% (vol/vol) phosphoric acid by spraying, and then air-dried again. Plates were then charred at 180 °C to develop lipid bands. Lipid band intensity was measured using ImageJ software (National Institutes of Health). Lipids in samples were quantified by comparing background-subtracted band intensity with that of a curve composed of various standard amounts of each lipid chromatographed on the same TLC plate. The intensity in the standard bands was fit to a linear intensity vs. lipid quantity curve.
Preparation of Lipid-Loaded MαCD for Lipid Exchange Experiments in Cells.
The desired amount of lipids dissolved in organic solvent was introduced into glass tubes and dried by nitrogen and then high vacuum for 1 h. Multilamellar vesicles were prepared from the dried lipids at 70 °C by adding prewarmed RPMI medium 1640. The desired amount of MαCD (from a 400-mM stock solution of MαCD dissolved in DPBS) was added to the multilamellar vesicles. The concentration of lipids and MαCD depended on experimental requirements, but the most usual conditions were that after mixing lipid and MαCD, the lipid concentration was 1.5 mM and MαCD concentration was 40 mM. The mixture of MαCD and lipid was then incubated at 37 °C for 30 min. The lipid-loaded MαCD mixture was then used for exchange of lipids into cells.
Exchange of Lipids Between Cells and Lipid-Loaded MαCD.
Unless otherwise noted, the cells were cultured in 10-cm plates with 10 mL medium. After removing the growth medium and washing three times with 10 mL DPBS, a 1,500-μL aliquot of lipid-loaded MαCD with 1.5 mM lipid and 40 mM MαCD was added onto ∼90% confluent mammalian cells cultured in 100-mm dishes (Corning Incorporated). The cells were incubated with the lipid-loaded MαCD for 1 h at 37 °C, unless otherwise noted. After removal of the lipid-loaded MαCD and three washes with 10 mL DPBS, the cells were removed from the plate by scraping in 5 mL DPBS. The cells were pelleted in glass tube by 3-min centrifugation at 300 × g in speed vac concentrator (Savant). For experiments in which there were two rounds of exchange, after removing the supernatant from the first round of exchange, a fresh lipid–MαCD mixture was added to the cell and a second round of lipid exchange was carried out.
3H Labeling Cells, Lipid Exchange, and Extraction of Lipids.
Unless otherwise noted, 11 µL 1.8 M sodium acetate and 10 µCi 3H acetate was added to 10-cm dishes with 70% confluent A549 cells in 10 mL RPMI medium 1640 supplemented with 10% FBS. Cells were incubated for 24 h at 37 °C. The medium was then removed and the cells washed three times with 10 mL DPBS supplemented with 2 mM sodium acetate. (The pH increased slightly from 7.4 to 7.5 after addition of sodium acetate.) For typical experiments, 1.5 mL lipid-loaded MαCD (40 mM MαCD and 1.5 mM bSM) was added to one plate, and as a control, 1.5 mL of 1.5 mM bSM multilamellar vesicles was added to another plate. The plates were incubated at 37 °C for 1 h in a 5% CO2 incubator. After incubation, the supernatant was removed for analysis of 3H-labeled lipids changed out from cells (described here). To analyze the residual radiolabeled lipids in the cells after exchange, the plates were washed three times with 10 mL DPBS supplemented with 2 mM sodium acetate. Cells were scraped off in 5 mL DPBS with supplemented 2 mM sodium acetate and pelleted in glass tubes by centrifugation for 3 min at 300 × g. After removing the supernatant, lipids were extracted from the cells with 3 mL 3:2 (v:v) hexane/isopropanol, incubating at RT for 30 min, with vortexing every 5 min, and the solvent was removed to a new glass tube and then dried with nitrogen gas while gently warming.
Separation and Analysis of 3H-Labeled Lipids from Cells.
Lipids extracted as described earlier were dissolved in 100 µL 1:1 (v:v) chloroform/ methanol, and a 10-µL aliquot was loaded onto a HP-TLC Silica gel 60 plates (Merck). The lipids were separated in 65:25:5 (v:v) chloroform/methanol/concentrated ammonium hydroxide. After the plate was air dried, lipids were stained in an iodine tank. Lipid bands were marked with pencil, and the plate was placed in a fume hood until the iodine staining disappeared. Silica gel corresponding to specific lipid bands, identified by their position relative to lipid standards, was scraped from the plates. As control, silica gel between the bands was also counted to confirm that there was no lipid between the iodine-stained bands. After addition of 2.5 mL ScintiVerse BD mixture (Fisher Scientific), radioactivity was counted using a Beckman LS6500 scintillation counter (Beckman Coulter, Inc.).
Separation and Analysis of 3H Labeled Lipids Exchanged out from Cells.
The lipid–MαCD mixture supernatant after lipid exchange was collected and centrifuged at 72,000 rpm (Beckman Coulter TLA 100.3 rotor) for 30 min in an Optima TL Ultracentrifuge (Beckman Coulter) to remove cells/cell debris. An equal volume of 3:2 (v:v) hexane/isopropanol was added to the supernatant after ultracentrifugation to extract the lipids. The same procedure was repeated for a second round of extraction from the aqueous phase from the first extractions. The extracted lipids were combined, dried with nitrogen, and then dissolved in 90 µL 1:1 (v:v) methanol/chloroform together with 1 μg/μL of each nonradioactive PS, SM, PC, and PE (the addition of unlabeled lipids allowed visualization of lipid positions upon iodine staining of TLC plate). Then 9- or 30-μL aliquots were loaded on a HP-TLC silica gel plate. The separation and analysis of 3H labeled lipids were carried out as described earlier.
Lipid Extraction and Analysis of Unlabeled A549 Cells.
Lipids were extracted and separated following the same procedure as radiolabeled cells. After chromatography, the TLC plates were air-dried and saturated with 3% (wt/vol) cupric-acetate-8% (vol/vol) phosphoric acid by spraying, and then air-dried again. Plates were charred at 180 °C to develop lipid bands.
Monitoring Kinetics of Exchange with Fluorescence and Radioactivity.
A mixture of 40 mM MαCD with 1.5 mM 1:9 (mol:mol) NBD-DPPE/bSM was prepared as described earlier. Then 500 μL of the lipid-loaded MαCD was added to 90% confluent A549 cells cultured in 6-well plates (Corning Inc.). The cells were incubated for different times at 37 °C, and then the lipid-loaded MαCD was removed. After three washes with 2 mL DPBS, the cells were removed from the plate by scraping in 1 mL DPBS and placed in 1.5-mL plastic centrifuge tubes. The cells were pelleted by 3-min centrifugation at 300 × g and resuspended in 100 μL DPBS. Then 900 μL ethanol was added. The NBD fluorescence intensity was measured in fluorescence cuvettes, using a Fluorolog 3 (Jobin Yvon Horiba). Fluorescence was measured with an excitation wavelength of 465 nm and emission wavelength of 534 nm. A control for nonspecific lipid sticking to cells was prepared in a similar fashion, but without MαCD, and used as the zero time point. In an analogous experiment, A549 cells were 3H labeled and subjected to lipid exchange, using a 1.5-mM bSM and 40-mM MαCD mixture, as described earlier. The cells were collected after different incubation times, and lipids were extracted and separated on HP-TLC plate, as described earlier. Radioactivity in the PS+PI and SM bands was then measured by scintillation counting, as described earlier. A control for nonspecific lipid sticking to cells was prepared in a similar fashion, but without MαCD, and used as the zero time point.
Effect of MαCD Concentration on SM Exchange Efficiency.
After 3H labeling, A549 cells were treated with lipid-loaded MαCD with 1.5 mM bSM combined with MαCD concentrations of 0, 2, 10, 40, or 80 mM at 37 °C for 1 h in the CO2 incubator. Cells were collected and radioactivity in the PS+PI and SM bands analyzed as earlier.
Effect of SM Concentration on SM Exchange Efficiency.
After 3H labeling, A549 cells were treated with 40 mM MαCD loaded with 0, 0.1, 0.2, 0.5, 1, 1.5, 2, or 3 mM bSM at 37 °C for 1 h in the CO2 incubator. Cells were collected and radioactivity in the PS+PI and SM bands analyzed as earlier.
Dithionite to Quench NBD-DPPE Fluorescence.
NBD-DPPE was exchanged into A549 cells as described earlier [except that lipid exchange step at 15 °C, room temperature (23 °C), or 37 °C]. The cells were suspended in 1 mL DPBS, and fluorescence was measured before and (as a function of time) after an addition of a 50-µL aliquot freshly prepared 1 M dithionite made in 1 M Tris buffer (pH 10) to give a final dithionite concentration of 50 mM. For microscopy experiments, exchange was carried out as earlier for 1 h at 37 °C: a 7-µL aliquot from cells suspended in 1 mL DPBS, before or 5 min after dithionite treatment, was loaded on microscope slides and covered with a coverslip. NBD fluorescence was then imaged by confocal laser scanning microscopy, using a Zeiss LSM 5 Pascal confocal laser scanning microscope system (Carl Zeiss AG) to visualize the fluorescence location in the cell.
Phospholipid Content by LC/MS/MS.
Phospholipids were extracted from provided samples, using the method of Bligh and Dyer. Extracts were diluted with internal standards (Avanti Polar Lipids) respective to the structure of phospholipid classes. The samples were prepared in silanized 500-µL injection inserts and vials for LC/MS/MS analysis. Each sample extract was assayed on a Waters’ Acquity ultraperformance liquid chromatograph (Waters Corporation)/AB Sciex 5500 MS system. The class specific phospholipid extracts for each sample were injected on an Agilent Eclipse XDB-C8 reversed phase column (4.6 × 50 mm, 1.8-µm particle size) for separation of molecular species within each class by gradient elution and detected by MS, using scheduled multiple reaction monitoring. In this manner, a peak with unique column elution time and mass-to-fragment profile was measured. The peaks of phospholipid species were integrated and normalized to the internal standard compounds within respective classes. This provided quantification using the compound/internal standard area ratio multiplied by the internal standard concentration or concentrations initially added to the sample extraction.
Supplementary Material
Acknowledgments
We thank Avanti Polar Lipids, Inc., and Jeff D. Moore for carrying out the MS studies reported here. This work was supported by National Science Foundation Grant DMR 1404985 and NIH Grant GM 112638.
Footnotes
Conflict of interest statement: A provisional patent application has been submitted on the technology used.
This article is a PNAS Direct Submission. W.D. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610705113/-/DCSupplemental.
References
- 1.Bretscher MS. Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972;236(61):11–12. doi: 10.1038/newbio236011a0. [DOI] [PubMed] [Google Scholar]
- 2.Verkleij AJ, et al. The asymmetric distribution of phospholipids in the human red cell membrane: A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973;323(2):178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- 3.Hill WG, Rivers RL, Zeidel ML. Role of leaflet asymmetry in the permeability of model biological membranes to protons, solutes, and gases. J Gen Physiol. 1999;114(3):405–414. doi: 10.1085/jgp.114.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill WG, Zeidel ML. Reconstituting the barrier properties of a water-tight epithelial membrane by design of leaflet-specific liposomes. J Biol Chem. 2000;275(39):30176–30185. doi: 10.1074/jbc.M003494200. [DOI] [PubMed] [Google Scholar]
- 5.Manno S, Takakuwa Y, Mohandas N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc Natl Acad Sci USA. 2002;99(4):1943–1948. doi: 10.1073/pnas.042688399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowhan W. Molecular genetic approaches to defining lipid function. J Lipid Res. 2009;50(Suppl):S305–S310. doi: 10.1194/jlr.R800041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adada M, Luberto C, Canals D. Inhibitors of the sphingomyelin cycle: Sphingomyelin synthases and sphingomyelinases. Chem Phys Lipids. 2016;197:45–59. doi: 10.1016/j.chemphyslip.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Delgado A, Casas J, Llebaria A, Abad JL, Fabrias G. Inhibitors of sphingolipid metabolism enzymes. Biochim Biophys Acta. 2006;1758(12):1957–1977. doi: 10.1016/j.bbamem.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768(6):1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kainu V, Hermansson M, Somerharju P. Introduction of phospholipids to cultured cells with cyclodextrin. J Lipid Res. 2010;51(12):3533–3541. doi: 10.1194/jlr.D009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanhuanpää K, Somerharju P. gamma-cyclodextrins greatly enhance translocation of hydrophobic fluorescent phospholipids from vesicles to cells in culture. Importance of molecular hydrophobicity in phospholipid trafficking studies. J Biol Chem. 1999;274(50):35359–35366. doi: 10.1074/jbc.274.50.35359. [DOI] [PubMed] [Google Scholar]
- 12.Cheng HT, London E. Preparation and properties of asymmetric large unilamellar vesicles: Interleaflet coupling in asymmetric vesicles is dependent on temperature but not curvature. Biophys J. 2011;100(11):2671–2678. doi: 10.1016/j.bpj.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HT, Megha, London E. Preparation and properties of asymmetric vesicles that mimic cell membranes: Effect upon lipid raft formation and transmembrane helix orientation. J Biol Chem. 2009;284(10):6079–6092. doi: 10.1074/jbc.M806077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiantia S, Schwille P, Klymchenko AS, London E. Asymmetric GUVs prepared by MβCD-mediated lipid exchange: An FCS study. Biophys J. 2011;100(1):L1–L3. doi: 10.1016/j.bpj.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Q, London E. Preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry. PLoS One. 2014;9(1):e87903. doi: 10.1371/journal.pone.0087903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Q, London E. Ordered raft domains induced by outer leaflet sphingomyelin in cholesterol-rich asymmetric vesicles. Biophys J. 2015;108(9):2212–2222. doi: 10.1016/j.bpj.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son M, London E. The dependence of lipid asymmetry upon phosphatidylcholine acyl chain structure. J Lipid Res. 2013;54(1):223–231. doi: 10.1194/jlr.M032722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son M, London E. The dependence of lipid asymmetry upon polar headgroup structure. J Lipid Res. 2013;54(12):3385–3393. doi: 10.1194/jlr.M041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitrac H, MacLean DM, Jayaraman V, Bogdanov M, Dowhan W. Dynamic membrane protein topological switching upon changes in phospholipid environment. Proc Natl Acad Sci USA. 2015;112(45):13874–13879. doi: 10.1073/pnas.1512994112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitrac H, Bogdanov M, Dowhan W. In vitro reconstitution of lipid-dependent dual topology and postassembly topological switching of a membrane protein. Proc Natl Acad Sci USA. 2013;110(23):9338–9343. doi: 10.1073/pnas.1304375110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perillo VL, Peñalva DA, Vitale AJ, Barrantes FJ, Antollini SS. Transbilayer asymmetry and sphingomyelin composition modulate the preferential membrane partitioning of the nicotinic acetylcholine receptor in Lo domains. Arch Biochem Biophys. 2016;591:76–86. doi: 10.1016/j.abb.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Heberle FA, et al. Subnanometer structure of an asymmetric model membrane: Interleaflet coupling influences domain properties. Langmuir. 2016;32(20):5195–5200. doi: 10.1021/acs.langmuir.5b04562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z, London E. Effect of cyclodextrin and membrane lipid structure upon cyclodextrin-lipid interaction. Langmuir. 2013;29(47):14631–14638. doi: 10.1021/la4031427. [DOI] [PubMed] [Google Scholar]
- 24.Koivusalo M, Jansen M, Somerharju P, Ikonen E. Endocytic trafficking of sphingomyelin depends on its acyl chain length. Mol Biol Cell. 2007;18(12):5113–5123. doi: 10.1091/mbc.E07-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmgren J, Lönnroth I, Månsson J, Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci USA. 1975;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fridriksson EK, et al. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38(25):8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.