Significance
Nuclear male sterility is common in flowering plants, but its application in hybrid breeding and seed production is limited because of the inability to propagate a pure male sterile line for commercial hybrid seed production. Here, we characterized a rice nuclear gene essential for sporophytic male fertility and constructed a male sterility system that can propagate the pure male sterile seeds on a large scale. This system is fundamentally advantageous over the current cytoplasmic male sterile and photoperiod/thermo-sensitive genic male sterile systems. Application of this technology will greatly enhance the effectiveness and efficiency in hybrid rice breeding and production.
Keywords: hybrid rice, male sterility, breeding, hybrid seed production, OsNP1
Abstract
The breeding and large-scale adoption of hybrid seeds is an important achievement in agriculture. Rice hybrid seed production uses cytoplasmic male sterile lines or photoperiod/thermo-sensitive genic male sterile lines (PTGMS) as female parent. Cytoplasmic male sterile lines are propagated via cross-pollination by corresponding maintainer lines, whereas PTGMS lines are propagated via self-pollination under environmental conditions restoring male fertility. Despite huge successes, both systems have their intrinsic drawbacks. Here, we constructed a rice male sterility system using a nuclear gene named Oryza sativa No Pollen 1 (OsNP1). OsNP1 encodes a putative glucose–methanol–choline oxidoreductase regulating tapetum degeneration and pollen exine formation; it is specifically expressed in the tapetum and miscrospores. The osnp1 mutant plant displays normal vegetative growth but complete male sterility insensitive to environmental conditions. OsNP1 was coupled with an α-amylase gene to devitalize transgenic pollen and the red fluorescence protein (DsRed) gene to mark transgenic seed and transformed into the osnp1 mutant. Self-pollination of the transgenic plant carrying a single hemizygous transgene produced nontransgenic male sterile and transgenic fertile seeds in 1:1 ratio that can be sorted out based on the red fluorescence coded by DsRed. Cross-pollination of the fertile transgenic plants to the nontransgenic male sterile plants propagated the male sterile seeds of high purity. The male sterile line was crossed with ∼1,200 individual rice germplasms available. Approximately 85% of the F1s outperformed their parents in per plant yield, and 10% out-yielded the best local cultivars, indicating that the technology is promising in hybrid rice breeding and production.
The breeding and large-scale adoption of hybrid rice contributes significantly to the food supply worldwide. Currently, commercial hybrid rice production includes a cytoplasmic male sterile (CMS) line-based three-line system and a photoperiod/thermo-sensitive genic male sterile (PTGMS) line-based two-line system (1). The CMS line, the maintainer line, and the restorer line are required for the three-line system (1, 2). CMS is correlated to aberrant, often chimeric, mitochondrial genes absent in maintainer lines, and the male sterility can be suppressed by Rf genes in restorer lines (2). CMS hybrid varieties have been deployed for commercial production since the 1970s and covered ∼40% of rice growing areas in China in 2012 (3). Despite the wide application, CMS systems suffer from several intrinsic problems, including the narrow germplasm resources of restorer lines, the poor genetic diversity between the CMS lines and restorer lines, the instability of male sterility under certain weather conditions, the negative impact of aberrant mitochondrial genes on hybrid performance, and the difficulty to breed new traits into the parental lines (1, 3, 4). These problems limit further improvement in CMS hybrid breeding, which is believed to be the reason that CMS varieties reached a yield plateau in the last 20 y (1, 3).
Photoperiod-sensitive and thermo-sensitive lines are the two major types of rice PTGMS germplasm resources (5–8). Two widely used PTGMS genes have been cloned: one is a noncoding RNA gene (6, 7), and the other codes for RNase ZS1 (8). The male fertility of PTGMS lines is reversible in response to environmental conditions, which enables the PTGMS lines to propagate via self-pollination under environmental conditions restoring the male fertility and to outcross with restorer lines for hybrid seed production under conditions suppressing male fertility (5–8). Because PTGMS traits are controlled by nuclear recessive genes and male fertility can be restored by any normal rice cultivars (5–8), broader genetic resources can be explored for strong heterosis (1, 5). PTGMS rice was first adopted for farming in 1995, and its planting area quickly increased and almost covered 20% of rice fields in China by 2012 (1, 3). In fact, the highest yielding varieties currently cultivated in China are mostly PTGMS hybrids (1, 3). Nonetheless, the PTGMS system also has intrinsic problems primarily in that its fertility is regulated by environmental conditions (5). Thus, propagation of PTGMS seeds and production of hybrid seeds both require strict environmental conditions, and both are vulnerable to unpredictable environmental changes. In addition, the critical temperature for fertility transformation (CTFT) in PTGMS lines often shifts up after a few generations of propagation, and PTGMS individuals of suitable CTFT have to be reisolated repeatedly during production (5). Furthermore, the CTFT trait is influenced by genetic backgrounds, which significantly increases the difficulty and uncertainty to breed new practical PTGMS lines (5).
Plant male reproductive development involves a series of events, from stamen meristem specification to pollen grain formation and pollination. Defects in any of these events can lead to male sterility. More than 40 nuclear genes required for male fertility have been identified in rice (3, 9, 10), but these genes have not been tapped for hybrid production because of the inability to propagate pure male sterile seeds on a production scale. Here we isolated a nuclear gene, Oryza sativa No Pollen 1 (OsNP1), essential for pollen development and male fertility in rice. By transforming the osnp1 mutant with OsNP1 coupled with a gene to deactivate the transgenic pollen and a gene to mark the transgenic seed, we constructed a male sterility system that can overcome the intrinsic problems of both CMS and PTGMS systems. The male sterile line generated by this system was tested for hybrid breeding, and the results show great practical potential of the system in hybrid rice breeding and production.
Results
osnp1-1 Mutant Exhibits Male Sterile Phenotype.
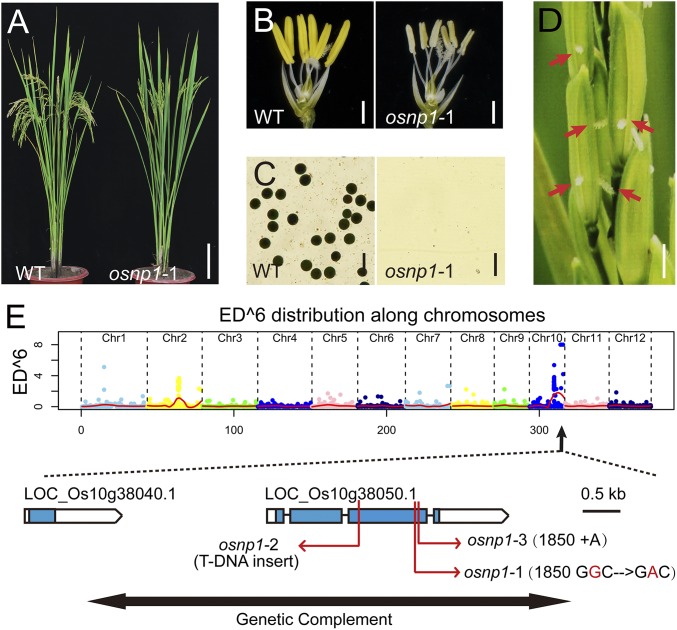
To identify new rice genes regulating male fertility, we generated an ethyl methanesulfonate-induced mutant library in Huanghuazhan (HHZ), an elite indica cultivar in China, and screened for male sterile mutants. One mutant, named osnp1-1, displayed complete male sterility but normal vegetative growth, inflorescence, and flower morphology (Fig. 1A). The mutant anther was small and whitish (Fig. 1B), lacking pollen grains (Fig. 1C), and this phenotype was stable under variable day length (10–16 h) and temperature range (20–38 °C). Approximately 87–92% of spikelets displayed extrusion of one or both stigmas (Fig. 1D), and cross-pollination by wild-type (WT) HHZ under natural field conditions resulted in 40% or higher seed set. All of these traits indicated that osnp1-1 could be a promising male sterile line for hybrid rice technology. When back-crossed with the WT HHZ, all F1 progeny were fertile, and the F2 population displayed 3:1 segregation of fertile to sterile plants (216:68), indicating that osnp1-1 is a single recessive mutation.
Fig. 1.
Phenotypes of osnp1-1 and molecular identification of OsNP1. (A) A WT plant and osnp1-1 mutant plant after bolting. (Scale bar, 10 cm.) (B) Spikelets of WT and osnp1-1 with the palea and lemma removed. (Scale bar, 1 mm.) (C) I2-KI staining of the WT and osnp1-1 pollen grains. (Scale bar, 100 μm.) (D) Inflorescence of osnp1-1, showing stigma extrusion. (Scale bar, 2 mm.) (E) The identification of osnp1 alleles. The Top section showed distributions of Euclidean distance (ED) scores of SNP sites along chromosomes in osnp1-1. OsNP1 gene structure and mutation sites of osnp1-1, osnp1-2, and osnp1-3 are shown in the Bottom section. The genomic fragment for gene complementation is shown by the double-headed black arrow. Empty boxes represent 5′- and 3′-UTRs, blue boxes represent exons, and the lines between boxes represent introns.
OsNP1 Encodes a Putative Glucose–Methanol–Choline Oxidoreductase.
The osnp1-1 mutant gene was cloned via a modified MutMap method (11). The analyses identified a candidate region at the end of chromosome 10 (from 21.27 to 22.05 Mbp) harboring four candidate SNPs: two of which are in the intergenic regions, one in the intron of LOC_Os10g39044, and one (Chr10: 20,379,153) in the third exon of LOC_Os10g38050 (Fig. 1E) that causes amino acid substitution from Gly561 (GGC) to Asp (GAC) (Fig. S1). Phenotype association assay showed that all 68 F2 male sterile plants carried this mutation, whereas the 216 F2 fertile plants showed 2:1 ratio of heterozygous and homozygous WT genotypes (149:67), suggesting that LOC_Os10g38050 is OsNP1.
Fig. S1.
Alignment of the Nipponbare, WT HHZ, and osnp1-1 mutant OsNP1 protein sequences. Sequence codes for Nipponbare and HHZ OsNP1 sequences are KX066198 and KX066199, respectively. A20A21 and A151 in HHZ indicated by red letter and asterisks are polymorphic amino acids compared to Nipponbare. Amino acid D563 in osnp1-1 is the mutation site.
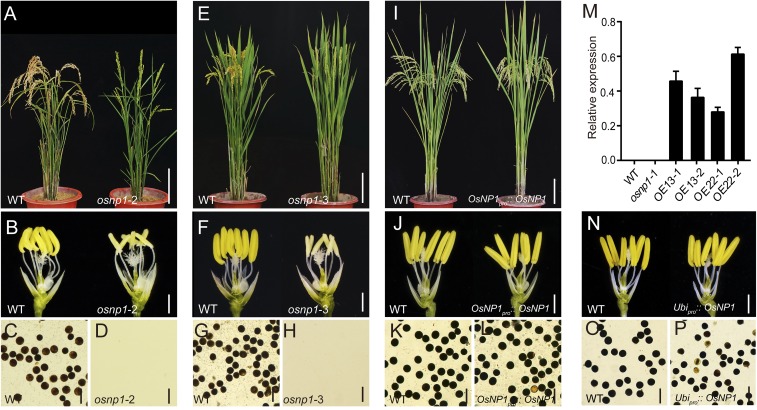
To confirm the prediction, we analyzed two other mutant alleles of LOC_Os10g38050. osnp1-2 was derived from japonica Zhonghua11, with a transfer DNA (T-DNA) insertion in the third exon, whereas osnp1-3 was created by CRISPR/Cas with a 1-bp insertion in the third exon (Fig. 1E). Both osnp1-2 and osnp1-3 exhibited normal plant growth but small whitish anthers lacking pollen grains and were completely male sterile (Fig. S2 A–H). When a genomic fragment covering LOC_Os10g38050 with 2.5-kb upstream and 1.4-kb downstream regions was introduced into the osnp1-1 mutant, the transgenic plants developed normal anthers and fertile pollens (Fig. S2 I–L).
Fig. S2.
Phenotype of other osnp1 alleles and transgenic lines. (A–D) Phenotype of WT and osnp1-2 in the Zhonghua11 background. (E–H) Phenotype of WT and osnp1-3 in the Wuyunjing7 background. (I–L) Transgenic complementation line in osnp1-1. Complementation line was in osnp1-1 background and transformed with OsNP1pro::OsNP1 construct. (M) qPCR analysis of OsNP1 expression in spikelets at anther developmental stage 12 of WT, osnp1-1, and OE transgenic lines. The OE transgenic line was in osnp1-1 background and transformed with Ubipro::OsNP1 construct. OsACTIN1 served as a control. Data were shown as means ± SD (n = 3). (N–P) Phenotype of WT and transgenic plant OE22-1. Plants after bolting (A, E, and I), spikelets with the palea and lemma removed (B, F, J, and N), and I2-KI staining of the pollen grains (C, D, G, H, K, L, O, and P) are shown. (Scale bars: A, E and I, 10 cm; B, F, J, and N, 1 mm; C, D, G, H, K, L, O, and P, 100 μm.)
LOC_Os10g38050 proteins in Nipponbare and HHZ are almost identical (Fig. S1). Pfam analysis showed two conserved domains of the glucose–methanol–choline oxidoreductase superfamily. This superfamily catalyzes the oxidation of an alcohol moiety to the corresponding aldehyde in diverse substrates (12).
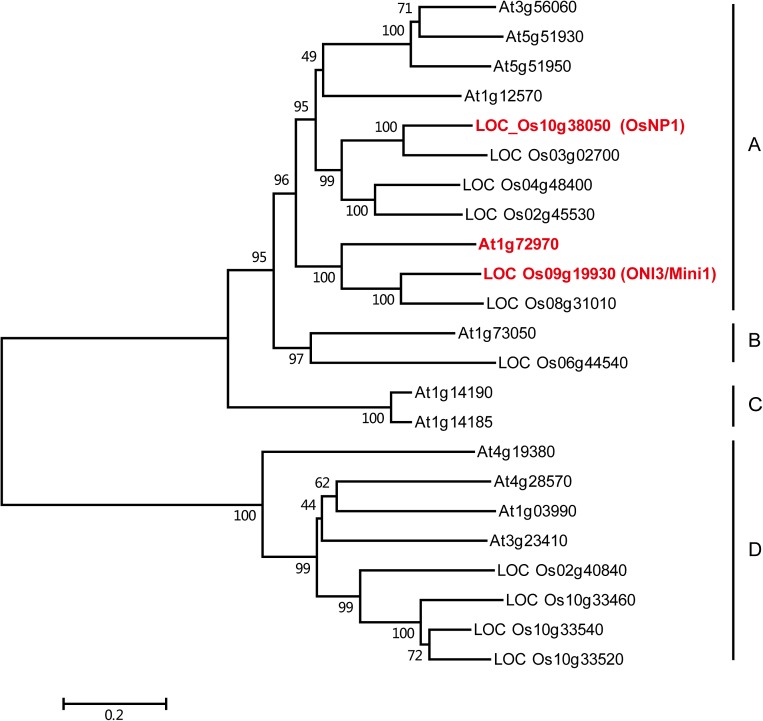
Both monocot and dicot species have genes highly homologous to OsNP1 (Fig. S3). There are six homologs in Nipponbare with >40% amino acid identity to OsNP1, of which ONI3 (Mini1, LOC_Os09g19930) has a role in preventing organ fusion during shoot development (13, 14). A phylogenetic tree constructed from alignment of OsNP1 and the highly similar sequences in rice and Arabidopsis defined four groups (Fig. S4). OsNP1 belongs to group A, represented by the Arabidopsis protein At1g72970, which was proposed to act as a long-chain fatty acid (LCFA) ω-alcohol dehydrogenase catalyzing the biosynthesis of long-chain α-,ω-dicarboxylic FAs (15). α-,ω-dicarboxylic FAs were proposed to participate in the cross-linking of surface cutin (15).
Fig. S3.
Protein sequence alignment of OsNP1 homologs in 15 species. Bases in red show the conserved amino acid residues.
Fig. S4.
A phylogenetic tree of OsNP1 and related proteins. The neighbor-joining tree was performed using MEGA6 based on the alignment of OsNP1 and the most similar sequences in rice and Arabidopsis. The numbers at the branches are bootstrap values provided as percent over 1,000 replications. Four groups of genes can be defined as follows: group A is represented by At1g72970, which is involved in the biosynthesis of long-chain α-,ω-dicarboxylic fatty acids; group B comprises putative mandelonitrile lyases; group C includes two proteins predicted to be aldehyde lyases; and group D comprises several putative LCFA alcohol dehydrogenases.
OsNP1 Is an Anther-Specific Gene.
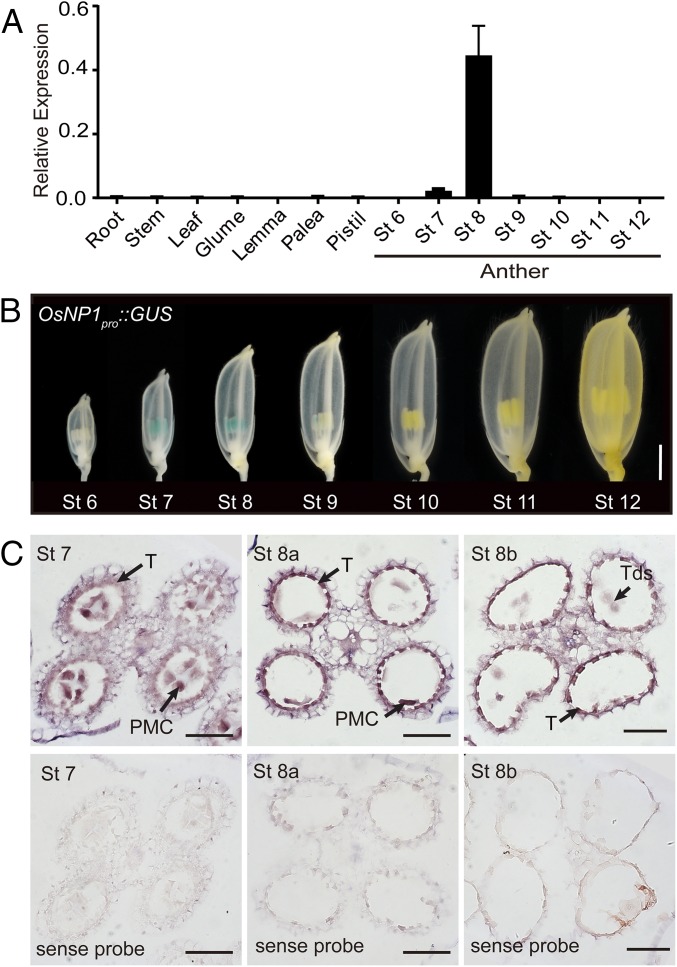
Quantitative real-time PCR (qPCR) revealed that OsNP1 was specifically expressed in anthers during meiosis (stages 7 and 8), but not in the root, stem, leaf, glume, palea, lemma, pistil, and anthers at other developmental stages (Fig. 2A). Accordingly, transgenic plants expressing the promoter reporter gene OsNP1pro::GUS showed β-glucuronidase (GUS) activity specifically in anthers at stages 7 and 8 (Fig. 2B), but not in other tissues. More precisely, OsNP1 transcription was detected in the tapetum and microspores by in situ hybridization (Fig. 2C). When a Ubipro::OsNP1 construct was introduced into the osnp1-1 mutant, the transgenic plants, which ectopically expressed OsNP1 (Fig. S2M), showed normal vegetative and reproductive growth (Fig. S2N). It is noteworthy that pollen fertility was restored to 60–90% (Fig. S2 O and P). Together, these results indicated that OsNP1 plays a specific role for the development of anther and microspore.
Fig. 2.
Expression pattern of OsNP1. (A) qPCR analysis of OsNP1 expression in different organs of WT. OsACTIN1 served as a control. Data are shown as means ± SD (n = 3). (B) GUS expression (blue staining) patterns of developmental spikelets on the OsNP1pro::GUS transgenic line. (Scale bar, 2 mm.) (C) In situ hybridization of OsNP1 in WT anthers from stages 7–8b. Anthers hybridized with OsNP1 sense probe served as controls. PMC, pollen mother cell; T, tapetal layer; Tds, tetrads. (Scale bars, 50 μm.)
OsNP1 Regulates the Tapetum Degeneration and Pollen Exine Formation.
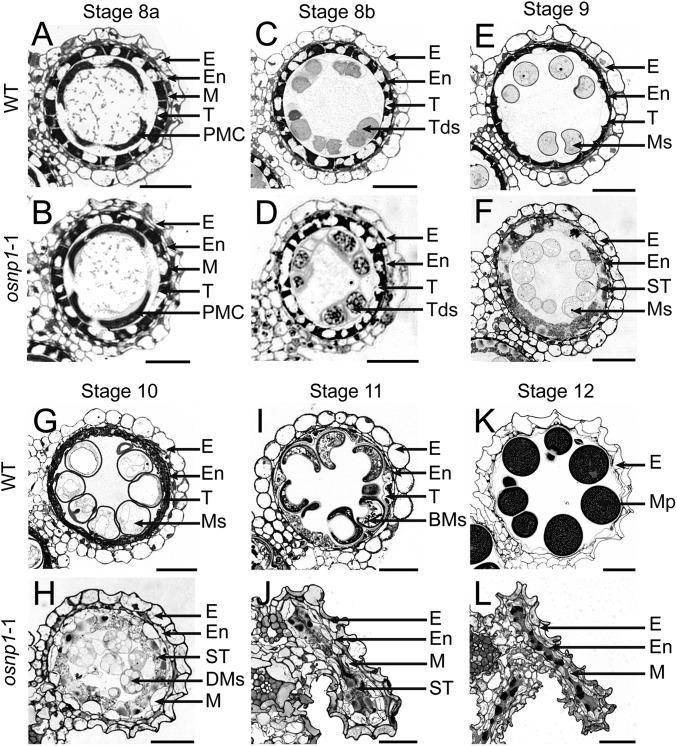
Microscopic analyses divide the rice anther development into 14 stages, from the formation of stamen primordium to the release of mature pollen during anther dehiscence (16). By stage 6, the WT anther primordia differentiate into a concentric structure, with pollen mother cells (PMCs) in the locule surrounded by a four-layered anther wall, from surface to interior, the epidermis, endothecium, middle layer, and tapetum. The PMC subsequently undergoes meiosis and generates a tetrad by the end of stage 8b. Meanwhile, tapetal cells initiate programmed cell death (PCD), and the middle layer becomes nearly invisible (16). No visible defect was observed in osnp1-1 anthers before tetrad formation (Fig. 3 A–D). However, both the anther wall and microspores displayed abnormal development after the microspores were released from the tetrads (stage 9). The WT tapetal cells gradually degenerate by PCD, becoming condensed and deeply stained (Fig. 3 E and G). The mutant tapetal cells were swollen and lightly stained (Fig. 3 F and H), indicating abnormal PCD. Different from the WT middle layer that vanishes after stage 9 (Fig. 3 E, G, and I), the mutant middle layer expanded at stage 10 (Fig. 3H). Unlike the WT microspores that complete pollen exine deposition and starch accumulation by stage 12 (Fig. 3K), the mutant microspores did not form exine, and they aborted by stage 11 (Fig. 3J), leaving debris in the locule (Fig. 3L).
Fig. 3.
Histological features of anther development in the WT and osnp1-1. Anther sections of WT (A, C, E, G, I, and K) and osnp1-1 (B, D, F, H, J, and L) plants from developmental stages 8a to 12 are shown. BMs, binuclear microspores; DMs, degenerated microspores; E, epidermis; En, endothecium; M, middle layer; Mp, mature pollen; Ms, microspores; PMC, pollen mother cell; ST, swollen tapetal layer; T, tapetal layer; Tds, tetrads. (Scale bars, 20 μm.)
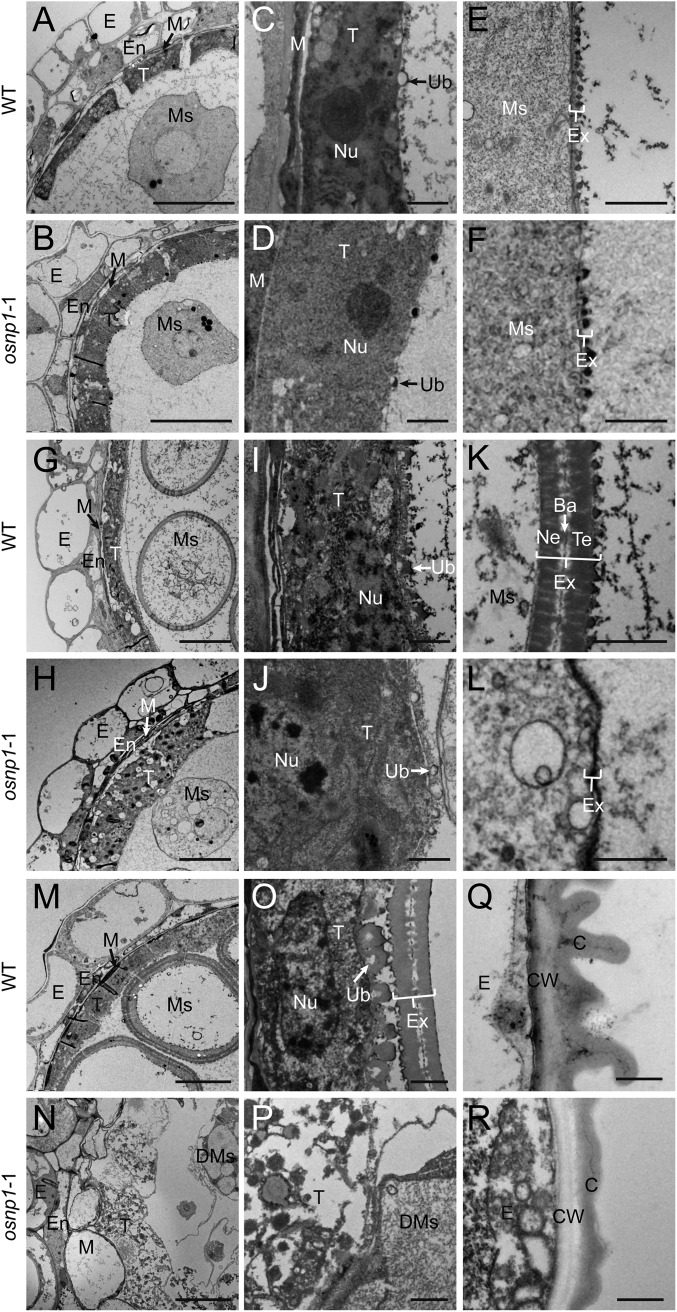
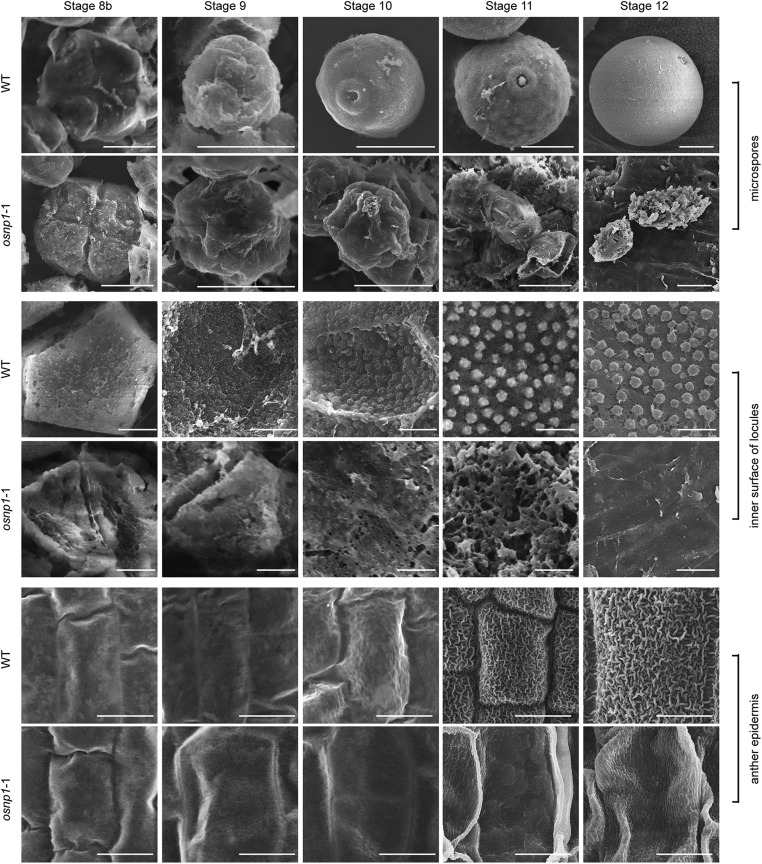
Transmission and scanning electron microscopic analyses show that, in the WT anthers at stage 9, the tapetum starts to produce Ubisch bodies that gradually grow into electron-dense orbicules facing the locule (Fig. 4 A, C, G, I, M, and O and Fig. S5). Ubisch bodies are believed to secrete tapetum-produced sporopollenin precursors for pollen exine formation (17). The sporopollenin is made up of complex biopolymers derived mainly from LCFAs, long aliphatic chains, and phenolic compounds (17). Accumulation of sporopollenin on the microspore cell wall in the WT was visible at stage 9 (Fig. 4E), which eventually formed thick exine with distinctive layers of tectum, bacula, and nexine (Fig. 4 G, K, M, and O). Although the mutant tapetum appeared to form pre-Ubisch bodies at stage 9, these structures did not grow into mature Ubisch bodies (Fig. 4 B, D, H, J, N, and P and Fig. S5). Accumulation of electron-dense materials occurred on the mutant microspore cell wall at stage 9 (Fig. 4 B and F), but it stayed thin and failed to form an exine structure (Fig. 4 H, L, N, and P). Unlike the WT microspores that eventually grew into spherical pollen grains, the mutant microspores aborted at stage 11, leaving remnants in the locule (Fig. 4 M–P and Fig. S5).
Fig. 4.
TEM analyses of the WT and osnp1-1 anthers from stages 9–12. The transverse sections of the WT (A, C, E, G, I, K, M, O, and Q) and osnp1-1 (B, D, F, H, J, L, N, P, and R) anthers at stage 9 (A–F), early stage 10 (G–L), late stage 10 (M–P), and stage 12 (Q and R) are compared. (C, D, I, and J) Higher magnification of the tapetum showing Ubisch body. (E, F, K, and L) Higher magnification showing deposition of sporopollenin in pollen exine. (O and P) Higher magnification of the tapetum and pollen exine. (Q and R) The outer anther epidermis. Ba, bacula; C, cuticle; CW, cell wall; DMs, degenerated microspores; E, epidermis; En, endothecium; Ex, exine; M, middle layer; Ms, microspores; Nu, nucleus; Ne, nexine; T, tapetum; Te, tectum; Ub, Ubisch body. (Scale bars: A, B, G, H, M, and N, 10 μm; C, D, I–L, O, and P, 1 μm; E, F, Q, and R, 500 nm.)
Fig. S5.
Observation of the anther and pollen grain in the WT and osnp1-1 by SEM. The microspores, inner surface of anther wall, and anther epidermis of WT and osnp1-1 from stages 8b to 12 are shown. (Scale bars: rows 1, 2, 5, and 6, 10 μm; rows 3 and 4, 2 μm.)
In addition, defects were observed on the exterior of osnp1-1 anther. The WT anther epidermis was covered with a smooth cuticle layer at early developmental stages, which gradually thickened and formed grid-like structure at stages 11 and 12 (Fig. 4Q and Fig. S5). However, the grid-like structure was not formed on the surface of osnp1-1 anther (Fig. 4R and Fig. S5).
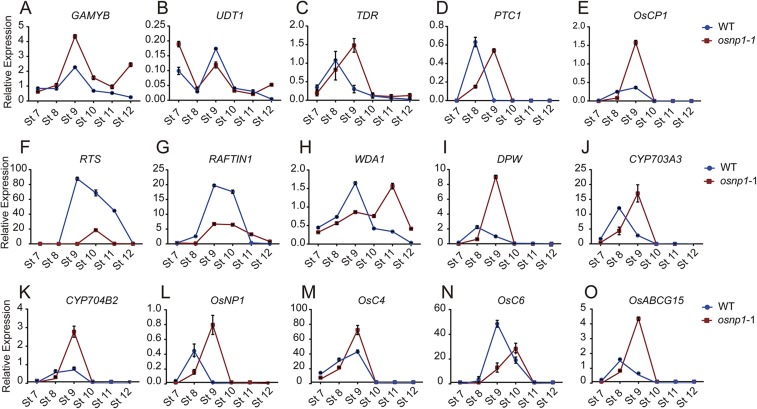
The anther surface cuticle consists of cutin and intracuticular and epicuticular waxes. Cutin is a polymer of ω-and midchain hydroxy and epoxy C16–C18 FAs, whereas cuticular waxes mainly comprise very LCFAs (18, 19). In rice, the lipid precursors for anther surface cuticle and sporopollenin are presumably produced in the tapetum, and then secreted via Ubisch bodies and transported to the anther and microspore surfaces (9, 17). Several rice male sterile mutants with defects in tapetum lipid metabolism or transportation also have defects in the development of Ubisch bodies, pollen exine, and anther surface cuticle (20–22). Moreover, the expression of several genes regulating tapetum PCD and synthesis/transportation of sporopollenin precursors displayed abnormal expression patterns in osnp1-1 (Fig. S6). Together, these results suggest that osnp1-1 has defects in the synthesis of lipophilic molecules for anther surface cuticle and sporopollenin formation, which is essential for pollen development.
Fig. S6.
Expression of genes involved in pollen development in the WT and osnp1-1. Expression of GAMYB (A), UDT1 (B), TDR (C), PTC1 (D), OsCP1 (E), RTS (F), RAFTIN1 (G), WAD1 (H), DPW (I), CYP703A3 (J), CYP704B2 (K), OsNP1 (L), OsC4 (M), OsC6 (N), and OsABCG15 (O) at stage 7–12 in the WT and osnp1-1 was analyzed using qPCR. OsACTIN1 served as a control. Data are shown as means ± SD (n = 3).
Development of a Male Sterility System Using OsNP1.
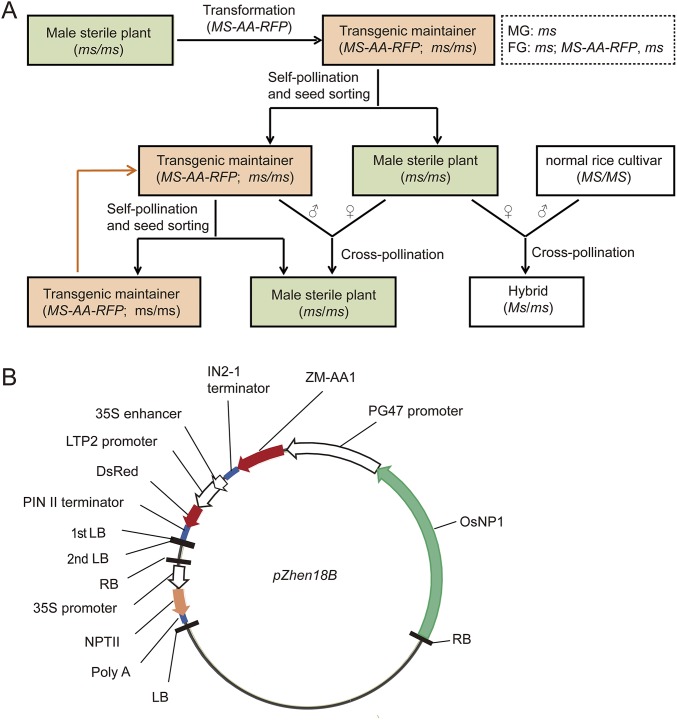
Because the osnp1-1 mutant exhibited traits desirable to the male sterile line, we sought to develop a nuclear male sterility system using the mutant plant and OsNP1 gene. The strategy is shown in Fig. S7A. We transformed osnp1-1 with a double T-DNA binary vector pZhen18B (Fig. S7B). The first T-DNA contained NPTII under the CaMV 35S promoter for transformation selection. The second T-DNA contained three functional modules: OsNP1 under its native promoter for restoration of male fertility, the maize α-amylase gene ZM-AA1 under the pollen-specific PG47 promoter to devitalize the transgenic pollen (23, 24), and the red fluorescence protein gene from Discosoma sp. (DsRed) (25) under the aleurone-specific LTP2 promoter (26) to mark the transgenic seed. Because OsNP1 is a sporophytic male fertility gene, a hemizygous OsNP1 transgene in the osnp1 mutant plant can fully restore the male fertility. Because ZM-AA1 driven by a PG47 promoter is a gametophytic factor that disrupts starch accumulation only in the transgenic pollen (23), only the transgenic pollen grains produced by the hemizygous transgenic plant are deactivated. The T0 transgenic plants were allowed to self-pollinate, and the T1 progeny was screened for the plant lacking the first T-DNA but carrying a single copy of the second T-DNA. The selected T1 plant was homozygous of the osnp1-1 locus but hemizygous of the second T-DNA, because pollen grains carrying the transgene were all defective, and the transgene was inherited only by the female gamete.
Fig. S7.
A nuclear male sterility system in rice via a transgenic approach. (A) Schematic diagram of the nuclear male sterility system. The male sterile mutant was transformed with a transgene containing three functional modules, including the following: (i) the WT fertility gene (MS) to restore the male fertility, (ii) the α-amylase gene (AA) to devitalize transgenic pollens, and (iii) the red fluorescence protein (RFP) gene to differentiate the transgenic seeds from the nontransgenic seeds. Because the male fertility gene is a recessive sporophytic gene, a hemizygous transgene in the male sterile mutant plant can fully restore the male fertility. Whereas the α-amylase gene driven by a pollen-specific promoter disrupts starch accumulation only in the transgenic pollen, only the transgenic pollen grains produced by the hemizygous transgenic plant are defective, and the nontransgenic pollen grains are viable for pollination. The resulting transgenic maintainer plant (MS-AA-RFP; ms/ms) produces male gametes (MG) of one genotype (ms), and female gametes (FG) of two genotypes (ms and MS-AA-RFP, ms). Self-pollination of the transgenic maintainer generates the transgenic seed (MS-AA-RFP; ms/ms) and the male sterile seed (ms/ms) in 1:1 ratio, and the seeds can be sorted based on red fluorescence. The male sterile seed can also be propagated via cross-pollination of the transgenic maintainer with the male sterile plant. The male sterile plants are pollinated by the paternal line for production of hybrid seeds. (B) The pZhen18B plasmid for transformation. The osnp1-1 mutant was transformed with a double T-DNA binary vector pZhen18B. The first T-DNA contained the NPTII gene under the 35S promoter for transformation selection. The second T-DNA contained three functional modules, including the following: (i) OsNP1 under its native promoter for restoration of male fertility, (ii) the maize α-amylase gene (ZM-AA1) under PG47 promoter to devitalize the transgenic pollens, and (iii) the DsRed gene under LTP2 promoter for detection of the transgenic seeds.
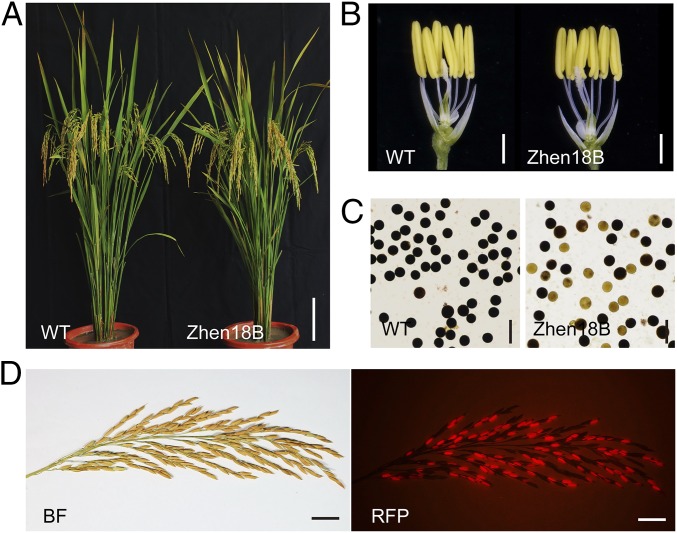
One T1 transgenic plant named Zhen18B was chosen as a representative for further study and presentation here. The plant exhibited normal vegetative and reproductive growth (Fig. 5A), normal male organ development (Fig. 5B), 1:1 ratio of fertile and defective pollen grains (Fig. 5C), and 1:1 (109:111) ratio of fluorescent seeds (with a hemizygous transgene) and nonfluorescent seeds (no transgene) on the panicle (Fig. 5D). The seeds were sorted out manually based on the fluorescence and cultivated for the next generation. All plants from the fluorescent seeds were fertile and genetically identical to the parent Zhen18B, and all plants from the nonfluorescent seeds (named Zhen18A) were male sterile. The fluorescence screening and fertility examination experiments were repeated for five generations, with a total of more than 15 million seeds, and all showed the same results, indicating the transgene was stable.
Fig. 5.
Phenotype of the nuclear male sterility system. (A) A WT plant and Zhen18B plant after bolting. (Scale bar, 10 cm.) (B) Spikelets of WT and Zhen18B with the palea and lemma removed. (Scale bars, 1 mm.) (C) I2-KI staining of the pollen grains in WT and Zhen18B. (Scale bars, 100 μm.) (D) Zhen18B panicle under bright field (BF) and a red fluorescence filter (RFP), respectively. (Scale bars, 2 cm.)
To determine the potential of Zhen18B as a maintainer, Zhen18A plants were cross-pollinated by Zhen18B plants. The Zhen18A plants exhibited 40% or higher seed setting, and one to three fluorescent seeds were found in 1 × 104 outcross seeds in each of five generations of the experiment, indicating the high stability and very low transgene transmission through pollen. Therefore, this system can efficiently propagate the male sterile seeds either by selfing or outcrossing the transgenics, and the maintainer and male sterile seeds can be sorted based on the fluorescence.
Zhen18A Is Promising for Commercial Hybrid Rice Breeding and Production.
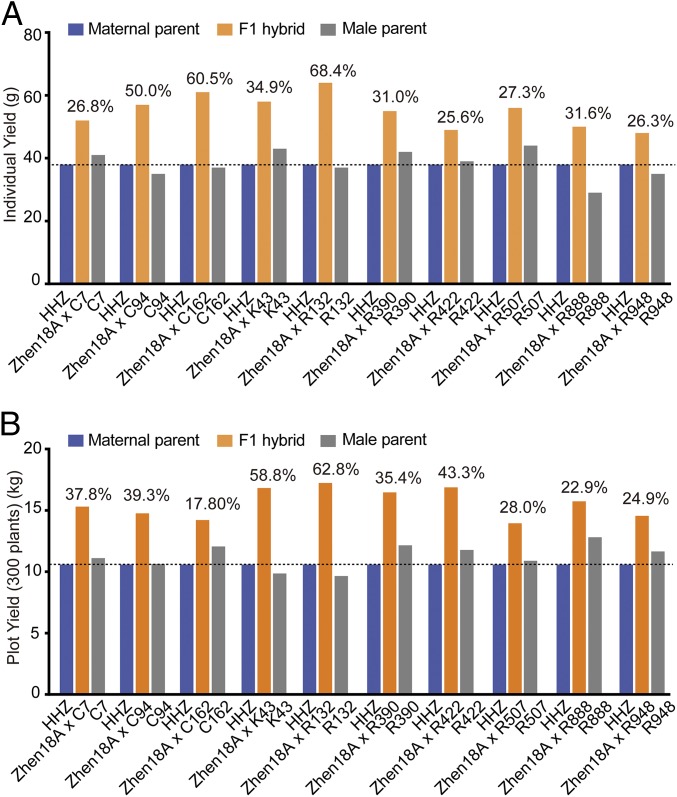
Propagation of pure Zhen18B seeds was achieved by Zhen18B self-pollination and mechanical sorting, whereas propagation of pure Zhen18A seeds was achieved by cross-pollination of Zhen18B to Zhen18A. With these prerequisites for commercial application fulfilled, we set to test the potential of Zhen18A in breeding of unique hybrid varieties. Zhen18A plants were pollinated with ∼1,200 individual rice germplasms available. Approximately 85% of the F1s outperformed their parents in per-plant yield, and 10% outyielded the local control cultivars (i.e., the best-yielding local varieties). A few representative F1s are shown in Fig. 6. These results indicated that our system is promising for hybrid breeding and production.
Fig. 6.
Heterosis of Zhen18A-derived F1 hybrids. Representative F1s from Zhen18A cross with various germplasms out-performed their parents in individual yield (A) and plot yield (300 plants) (B). The number above the column represents the yield increase of the F1 over the better yielding parent.
Zhen18B Provides a Platform for Breeding of Various Male Sterile Lines.
To test whether the osnp1-1 and transgene loci can be transferred into other germplasms to breed male sterile lines, Zhen18B (as maternal parent) was crossed with 10 different rice germplasms as recurrent male parent. Progeny was analyzed for the presence of the osnp1-1 locus and transgene and their associated phenotypes in two consecutive generations of back-cross and self-pollination that have been obtained so far. Both osnp1-1 and the transgene were accurately associated with their respective phenotypes in the segregated progeny. The results of F1 and F2 progeny from Zhen18B crossed with indica rice 9311 are shown in Table S1 as representative. These results indicated that both osnp1-1 and the transgene from Zhen18B maintain their functions when transferred into different genetic backgrounds, making it possible to breed male sterile lines via traditional crossing and selection.
Table S1.
Analysis of F1 and F2 populations from Zhen18B hybridization with indica rice 9311
| Population | Genotype | Fertility | Fluorescence | Unstained pollen |
| F1 (96) | MS/ms (46) | F (46) | No (46) | No (46) |
| MS/ms;MS-AA-RFP (50) | F (50) | Yes (50) | Yes (50) | |
| F2 (285) | MS/MS (35) | F (35) | No (35) | No (35) |
| MS/ms (70) | F (70) | No (70) | No (70) | |
| ms/ms (37) | S (37) | No (37) | No pollen (37) | |
| MS/MS;MS-AA-RFP (32) | F (32) | Yes (32) | Yes (32) | |
| MS/ms;MS-AA-RFP (74) | F (74) | Yes (74) | Yes (74) | |
| ms/ms;MS-AA-RFP (37) | F (37) | Yes (37) | Yes (37) |
F1s were generated from Zhen18B hybridization with indica cultivar 9311. The F1s carrying the transgene (MS-AA-RFP) were self-pollinated to generate the F2 progeny. A total of 96 F1 plants and 285 F2 plants were analyzed. The number in parenthesis following each genotype and phenotype represents the number of plants. F, fertile; MS, WT OsNP1 gene; ms, osnp1 mutant gene; S, male sterile.
Discussion
Nuclear male sterility caused by non–PTGMS genes is common in flowering plants. However, commercial application of these mutants is limited because of the difficulty to propagate a large quantity of pure male sterile lines. In 1993, a strategy was proposed by Williams and Leemans to obtain a transgenic maintainer by transforming recessive male sterile plant with a fertility-restoration gene linked with a pollen-lethality gene and a marker gene (27). Self-pollination of the maintainer would propagate the maintainer line and the male sterile line as well. Later in 2002, Perez-Prat and van Lookeren Campagne (28) proposed two strategies to obtain maintainer lines: one to transform the fertility-restoration gene linked with a seed-color gene into the male sterile plant, and the other to transform the fertility-restoration gene linked with a pollen-lethality gene into the male sterile plant. Cross-pollination of the color-maintainer to the male sterile line would generate 50% of male sterile seeds and 50% of color-maintainer seeds that can be separated based on the seed color. Cross-pollination of the pollen-lethality maintainer to the male sterile line would generate a pure male sterile line. Based on these ideas, DuPont-Pioneer devised seed production technology in maize by transformation of a male sterile mutant with a fertility-restoration gene linked with the α-amylase gene to disrupt the transgenic pollen and the DsRed gene to mark the transgenic seed (24, 29). Self-pollination of the resulting maintainer propagates 50% of maintainer seeds and 50% of male sterile seeds that can be color sorted. Cross-pollination of the maintainer to the male sterile line produces 100% male sterile seeds. Application of seed production technology in maize would save the costs for mechanical detasseling, which is the predominant method for commercial maize hybrid seed production. However, nuclear male sterility maintainers are fundamentally more useful for crops that have bisexual flowers not amenable to manual emasculation, including rice, wheat, and sorghum.
Construction of the nuclear male sterility maintainer in rice was made possible by the isolation of osnp1-1 mutant and the cloning of OsNP1, an anther-specific gene. osnp1-1 is derived from HHZ, an elite indica cultivar that is semidwarf, super high yield, good eating quality, and widely cultivated in diverse geographical regions in China (30). As the mutant displays numerous traits highly desirable to the male sterile line, we sought to transform it into a practical male sterile system. In this work, we deployed the maize α-amylase as the pollen-lethality gene and DsRed as the seed-marker gene. We are also testing whether cytotoxin Barnase (31) can be used to deactivate the transgenic pollen and whether herbicide resistance genes can replace DsRed for seed-sorting during the maintainer propagation.
A transgenic line, Zhen18B, with a hemizygous single insertion of the transgene was identified. As expected, self-pollination of Zhen18B propagated itself and the male sterile Zhen18A in 1:1 ratio, whereas cross-pollination of Zhen18B to Zhen18A propagated pure Zhen18A seeds to a large quantity. Thus far, the transgene in Zhen18B is highly stable in five generations of ∼10,000 plants tested. These results indicated that Zhen18B fulfills the role as a maintainer. The availability of a sorting machine makes it possible to separate the transgenic maintainer seeds from the nontransgenic male sterile seeds based on the presence of red fluorescence, which ensures high purity of both lines at a commercial scale. Hybridization of Zhen18A with other germplasms generated many hybrids outperforming the best local rice cultivars, indicating the system is promising in application.
This system is advantageous over CMS and PTGMS systems in several aspects. First, the male sterility is controlled by a single recessive nuclear gene; thus, any rice germplasms with the WT OsNP1 gene can complement the mutation. This approach provides broader choices of germplasms as paternal lines to breed hybrids of superior heterosis. Second, the male sterility is insensitive to photoperiod and environmental temperature; thus, both the male sterile seeds and the hybrid seeds can be propagated under regular farming conditions, which significantly lowers the demand on specific environmental conditions for seed production and reduces the risk induced by weather changes. Furthermore, the male sterility and fertility restoration are each controlled only by a single genetic locus and thus can be easily bred into other cultivars to generate new sets of maintainer and male sterile lines. Our initial breeding experiments showed that both loci can be stably transferred from Zhen18B into different genetic backgrounds, without altering the corresponding phenotypes. Finally, although the technology involves transgenics, only the maintainer line carries the transgenes. Both the male sterile seeds and hybrid seeds are nontransgenic. Thus, transgenic oversight is applicable only to the maintainer line cultivation, which requires only a small acreage, and production of hybrid seeds and hybrid cultivation do not require transgenic oversight. Application of this technology will greatly enhance hybrid rice breeding and production.
Materials and Methods
All of the plants (Oryza sativa) were grown in the paddy field in Shenzhen during the natural growing season and maintained regularly. The osnp1-1 mutant was derived from HHZ by ethyl methanesulfonate mutagenesis, as described previously (11). Details of experimental procedures, such as cloning of OsNP1, qPCR, GUS staining, in situ hybridization, microscopic analyses, and field test, are described in SI Materials and Methods. See Table S2 for the primers used in this study.
Table S2.
Primers used in this study
| Primer | Forward (5′ to 3′) | Reverse (5′ to 3′) | Purpose |
| OsNP1-HRM | CAGGGTGATCGACAGC | CCTGCCGAGCATCATG | HRM analysis |
| OsNP1-TDNA-P1 | CCGTCTCCCGTATCCTCTT | CAAGGGCATGACGAAGAAA | T-DNA insertion mutant |
| OsNP1-TDNA-P2 | AATCCAGATCCCCCGAATTA | CAAGGGCATGACGAAGAAA | T-DNA insertion mutant |
| OsNP1-CRISPR | GGCAGGCCTGAGGGTTGGTGCCGG | AAACCCGGCACCAACCCTCAGGCC | Vector, pCRISPR-OsNP1 |
| OsNP1-Co | GTTTAAACTTCTGACCAAAGAAGGGCG | GGATCCGCAACTCGTTTGCTTTACACTGT | Vector, pOsNP1pro::OsNP1 |
| OsNP1-Pro | GGATCCTTCTGACCAAAGAAGGGCG | GTCGACTTTCGCCGGGCAAATTC | Vector, pOsNP1pro::GUS |
| OsNP1-OE-P1 | AGCACGCGTAAGTTAGCGAATTTGCCCGGC | TCAGGATCCTCATTTCTTCCATCTCTCGG | Vector, pUbipro::OsNP1 |
| Zhen18B-P1 | AGTGTCGTGCTCCACCATGTTGGGCTTGGCGGGTAAACCTAAGAG | GAGTGTAGCCGAGTAATGGCCTCCTCCGAGAACGTGATCA | Vector, pZhen18B |
| Zhen18B-P2 | TACTCGGCTACACTCACACGCTCGC | AAACACTGATAGTTTAAACAGAAGCTTCTGGCGCGCCAACCGTCTCTTCGTGAGAATAACCGTGG | Vector, pZhen18B |
| Zhen18B -P3 | GAGACGGTTGGCGCGCCAATTCGAGCTCGGTACCCGGCCGCT | AAGGTCGTCCGGGCGGCCTGCGGCCTGGTCCAGGCACAAG | Vector, pZhen18B |
| Zhen18B-P4 | CGCCCGGACGACCTTGGGATCGG | ATGGCGGCGACAATGGCAGTGAC | Vector, pZhen18B |
| Zhen18B-P5 | CATTGTCGCCGCCATGGTGTCGTGATCGATGCTTTATTCG | GTTTAAACAGAAGCTTGCATGCCTGCAGGTCGACTCTAGAGGATCTGCACCGGACACTGTCTGGTGGCAT | Vector, pZhen18B |
| Zhen18B-P6 | GCAGGCATGCAAGCTTAATCCAGCCATATATATTTGTTGCA | CGTGCAGACGGCGTCCCATACCCG | Vector, pZhen18B |
| Zhen18B-P7 | GACGCCGTCTGCACGCCATGGCAA | AAACACTGATAGTTTAAACTCAATTGAAGAATTTACCATTTGTCA | Vector, pZhen18B |
| Zhen18B-P8 | GAGACGGTTGGCGCGCCATCACATCAATCCACTTGCTTTGAA | GCTCGAATTGGCGCGCCCGTCAACATGGTGGAGCACGACACG | Vector, pZhen18B |
| HPT-P1 | GTCTCCGACCTGATGCAGCTCTC | CCAAGCTCTGATAGAGTTGGTC | Transgene determination |
| HPT-P2 | AATTAATTCGGGGGATCTGG | CATTGGGGAGTTTAGCGAGA | Transgene determination |
| OsNP1-OE-P2 | TGCTGTGCGCCGAAGAAGCT | TCAGGATCCTCATTTCTTCCATCTCTCGG | Transgene determination |
| NPT-P1 | TTCTCGGCAGGAGCAAGGT | GGATGACGCACAATCCCACTA | Transgene determination of Zhen18B |
| DsRed-P1 | GGACTTGAACTCCACCAGG | CGAGAACGTGATCACCGAGT | Transgene determination of Zhen18B |
| LINK | GGATTGTGCGTCATCCCTTAC | AGATGCATTTCATTAACCAAATCC | Transgene determination of Zhen18B |
| NPT-P2 | TGCGTGCAATCCATCTTGTT | GGATGACGCACAATCCCACTA | Transgene copy number of Zhen18B |
| DsRed-P2 | GCCACTACCTGGTGGAGTTCAA | CCTCGTTGTGGGAGGTGATGT | Transgene copy number of Zhen18B |
| BJ | CACCTTCAAGTACTCCCCCGA | TGTGTTGTTACTCATGGCAATGC | Background genotype of Zhen18B |
| SPS1 | CCGCAGGCTGTTCGTCAT | CCCGGAGATCCTGGACATC | Endogenous reference gene |
| TAIL-1A | CCACTACCTGGTGGAGTTCAA | ACGATGGACTCCAGAGVNVNNNGGAA | TAIL-PCR for Zhen18B |
| TAIL-2A | GCCACCACCTGTTCCTGTAGTTC | ACGATGGACTCCAGAG | TAIL-PCR for Zhen18B |
| TAIL-3A | CCAGATGCATTTCATTAACCAAATCC | ACGATGGACTCCAGAG | TAIL-PCR for Zhen18B |
| OsNP1-qPCR | GCCTCACCGTCCTCCTCTAC | CGGGTCCGAGAACACCAC | qPCR |
| OsACT-qPCR | GCTATGTACGTCGCCATCCA | GGACAGTGTGGCTGACACCAT | qPCR |
| GAMYB-qPCR | GCGACGGTATCATGTTCAAT | GTCGCATAAGAGAACATCTG | qPCR |
| UDT1-qPCR | GAAGCACTCTGCAGCTAC | CTGCGTAGCCGTAGAAGG | qPCR |
| TDR-qPCR | TGCTCTGGGAGCACAAGCC | CTCGCTGTCCCTCACCATG | qPCR |
| PTC1-qPCR | CACCAGATCATGGACCTCTG | AGCAGCCTCAGCTCCATGTG | qPCR |
| OsCP1-qPCR | AGCAATGCGACCGGTACAG | TACGGGCTGTTCTTGCTCTT | qPCR |
| RTS-qPCR | ACATGTGGACTCGCTTGACT | CATGGCTGCATGCAGATTCAT | qPCR |
| RAFTIN1-qPCR | AGAGCTTACATGGTGGAGATGG | CGTCGAGCTCTTCACGTTCT | qPCR |
| WDA1-qPCR | ACCACTCTTCAATCGTCACTGAG | GCAGTTCCAGTCAAGGCACAG | qPCR |
| OsDPW-qPCR | ACCACAGGAGACACGGTGATG | CTTGGCCTGATGGTGACAA | qPCR |
| CYP703A3-qPCR | GAGTGCATCCCTTGATGATG | ACTCGTTGGTCACCGATGAT | qPCR |
| CYP704B2-qPCR | GCTGGTTGATGACTTCACCT | CGACAGTATGTCGTGCTTGAT | qPCR |
| OsC4-qPCR | TGCCTAAGACGAGACGAGAG | GCCAAAGGAGGTCATCGTTA | qPCR |
| OsC6-qPCR | CTCCATCTGCCTGAGTATAT | GTCCATGCATGTTGCAGAAT | qPCR |
| OsABCG15-qPCR | CATGTGTGGCCAACCAAAGA | GTGGTCTTGCCACTGCCAGA | qPCR |
| OsNP1-ISH-P1 | CCAAGCTTCTAATACGACTCACTATAGGGAGAATTCAAGACATCTACTCT | GATAAGCTTGAACCGCAGGTTCTCCAGTTGC | In situ hybridization |
| OsNP1-ISH-P2 | AGGGAATTCATTCAAGACATCTACTCTTG | CCAAGCTTCTAATACGACTCACTATAGGGAGAGAACCGCAGGTTCTCCAG | In situ hybridization |
SI Materials and Methods
Plant Materials and Growth Conditions.
All of the plants (Oryza sativa) were grown in the paddy field in Shenzhen during the natural growing season and maintained regularly. osnp1-1 mutant line was derived from the indica HHZ by ethyl methanesulfonate (EMS) mutagenesis. EMS treatment and mutant screening was as described previously (11).
Cloning of OsNP1.
An improved MutMap protocol (11) was applied to clone the osnp1-1 mutant gene. The osnp1-1 mutant was backcrossed with WT HHZ, and the resulting F1 was selfed to generate the F2 population. Thirty male sterile F2 plants were randomly selected for DNA extraction, and equal amount of DNA was pooled and sequenced to 29× of genome coverage using the Illumina Hiseq platform (genome resequencing data available at the Sequence Read Archive with accession no. SRP073226). After removal of adapter sequences and reads of low sequence quality, the clean reads were analyzed using an improved MutMap protocol (11), using the whole-genome sequences of seven other HHZ mutants (genome resequencing data available at the Sequence Read Archive with accession no. SRP058039) as references. Euclidean distance (ED) score was calculated for each SNP site. A local linear regression curve (Loess fit) was appended to show the distribution of ED values for each SNP. A cluster of SNPs of high ED scores is likely the candidate region harboring the causal mutation.
Phenotype Association Assay.
To verify the association between the candidate mutation site in LOC_Os10g38050 and the male sterile phenotype, 216 fertile plants and 68 male sterile plants in the F2 population were genotyped using the high-resolution melting (HRM) (32) assay with primer set OsNP1-HRM.
Identification of T-DNA Insertion Mutants.
The T-DNA insertion mutant osnp1-2 (RMD_04Z11DP96-2) derived from the japonica cv. Zhonghua11 was obtained from the Chinese Rice Mutant Database (rmd.ncpgr.cn). The T-DNA insertion in osnp1-2 was located in the third exon of LOC_Os10g38050 (OsNP1). The T-DNA insertion was verified by genomic DNA-PCR with primer sets OsNP1-TDNA-P1 and OsNP1-TDNA-P2.
CRISPR/Cas-Mediated Mutation.
The CRISPR/Cas-targeted genome editing tool was used to create mutant alleles of OsNP1 in japonica cv Wuyunjing7 (WYJ). The pCRISPR-OsNP1 plasmid was constructed as described previously (33), with minor modification. In brief, both the OsU3::gRNA and 35S::Cas9 fragments were cloned into a pCAMBIA1300 binary vector, followed by the introduction of the recognition sequence (GGCCTGAGGGTTGGTGCCGG, in the third exon of OsNP1) via the oligonucleotide pair OsNP1-CRISPR (Table S2). The construct was introduced in Agrobacterium tumefaciens strain AGL0 and transformed into rice WYJ, as described previously (34). Transgenic plants regenerated from hygromycin-resistant calli were first examined for the presence of transgene using primer set HPT-P1, and the transgenic plants were then subjected to HRM analysis using primer set OsNP1-HRM to evaluate whether mutation occurred. Mutations on OsNP1 were further confirmed by sequencing. The mutant line (termed osnp1-3) carrying a 1Nt (+A) insertion in the third exon of OsNP1 was male sterile. It was back-crossed with WT WYJ to generate the F1, and the resulting F1s were further selfed to generate an F2 population. The genotype of OsNP1 in the F2 population was analyzed using HRM (32).
Protein Alignment and Phylogenetic Analysis.
The full-length amino acid of OsNP1 in Nipponbare was used as a query to search for the homologs in the National Center for Biotechnology Information using BlastP. The homologs were retrieved and aligned using ClustalW in MEGA6 (35). The similar sequences in rice and Arabidopsis were aligned, and the phylogenetic tree was constructed with the neighbor-joining algorithm in MEGA6 (35), with the following parameters: Poisson model, pairwise deletion, and 1,000 bootstrap replicates.
Plasmids Construction, Transformation, and Transgene Determination.
For complementation, a 5.9-kb HHZ genomic DNA fragment containing the OsNP1 coding region, 2.5-kb upstream region, and 1.4-kb downstream region was amplified using primer set OsNP1-Co and inserted into the binary vector pCAMBIA2300 between PmeI and BamHI sites to generate the transformation construct pOsNP1pro:: OsNP1. For the GUS reporter gene construct, the 2.52-kb DNA fragment upstream of the start codon ATG of OsNP1 was amplified from Nipponbare using primer set OsNP1-Pro and cloned into the binary vector pHPG between SalI and BamHI sites, resulting in a pOsNP1pro::GUS construct. For overexpression (OE), HHZ OsNP1 full-length cDNA was amplified from reverse-transcription of total RNA extracted from the anther using primer set OsNP1-OE-P1. The PCR product was digested with MluI and BamHI and cloned into binary vector pUbi-intron between the maize Ubiquitin1 promoter and the Nos terminator, resulting in construct pUbipro::OsNP1. All of the constructs were sequence-confirmed before transformation into A. tumefaciens strain AGL0. The pOsNP1pro::OsNP1 and pUbipro::OsNP1 constructs were transformed into progeny of osnp1-1 heterozygous plants, respectively, and the pOsNP1pro::GUS construct was transformed into japonica cv. Zhonghua11. The transgenic plants of OsNP1pro::OsNP1 were screened by PCR amplification using primer set NPT-P1 for the presence of selection marker gene NPTII. Transgenic plants of Ubipro::OsNP1 and OsNP1pro::GUS were identified by PCR-amplification using primers HPT-P1 or HPT-P2 for the presence of selection marker gene HPTII. The genotypes of the pOsNP1pro::OsNP1 transgenic plants and their progeny were determined by relative qPCR with an Applied Biosystems 7500 Fast Real-Time PCR system, using primer sets BJ and SPS1. Primer set BJ was specific for the mutant allele of OsNP1. Primer set SPS1 was specific for the rice sucrose phosphate synthase (SPS) gene (36), which served as the endogenous reference gene. In addition, the nontransgenic osnp1-1 mutant was used as the reference sample. To genotype the Ubipro::OsNP1 transgenic plants, a fragment of OsNP1 was amplified from each transgenic plant using the OsNP1-OE-P2 primers, and then the PCR products were analyzed via HRM analysis (32).
To develop the male sterile system for hybrid rice production, a double T-DNA binary vector pZhen18B was constructed. The first T-DNA contained a NPTII gene driven by a CaMV 35S promoter for transformation selection. The second T-DNA contained three functional modules, including the following: (i) OsNP1 under its native promoter for restoration of male fertility; (ii) the maize α-amylase gene ZM-AA1 with the amyloplast-targeting signal peptide from the maize brittle-1 gene (Bt1) (37), under a PG47 promoter (a pollen-specific promoter) (23) to devitalize transgenic pollen; and (iii) the RFP gene DsRed (25) under 35S enhancer and LTP2 promoter (an aleurone-specific promoter) (26) for detection of the transgenic seeds. An In-Fusion PCR cloning kit (Clontech) was used for DNA ligation. First, the fragment containing the RB-LB2-LB1-PINII terminator-DsRed was synthesized and then amplified using primers Zhen18B-P1. The promoter of LTP2 was amplified from a barley (Hordeum vulgare L.) genome using primers Zhen18B-P2. The two fragments were fused together and then cloned into pCAMBIA2300 between BstXI and PmeI, resulting in construct pZhen18BM1. Second, the IN2-1 terminator–ZM-AA1 fragment was synthesized and then amplified with primers Zhen18B-P3; ZM-bt1 was amplified with primers Zhen18B-P4 using cDNA from maize (Zea mays) callus as a template; and the promoter of PG47 was amplified from maize genome using primer set Zhen18B-P5. The three fragments were fused together and cloned into pZhen18BM1 between AscI and HindIII, generating pZhen18BM2. Third, the OsNP1 fragment was amplified from the rice genome using two primer sets, Zhen18B-P6 and Zhen18B-P7. The two fragments were fused together and then cloned into pZhen18BM2 between HindIII and PmeI, generating pZhen18BM3. Finally, the 35S enhancer, which was synthesized and then amplified using the primer set Zhen18B-P8, was cloned into pZhen18BM3 in the AscI site, forming pZhen18B.
The pZhen18B construct was introduced into A. tumefaciens AGL0 and transformed into the progeny of osnp1-1 heterozygous plants. To confirm the existence of two T-DNA regions and ascertain their genetic linkage, transgenic plants were screened via PCR amplification using specific primer sets NPT-P1, DsRed-P1, and LINK. To determine the transgene copy number of each T-DNA and to genotype the OsNP1 background, genomic DNA of each transgenic plant was further subjected to relative ;qPCR analysis on an Applied Biosystems 7500 Fast Real-Time PCR system, using primer sets NPT-P2, DsRed-P2, BJ, and SPS1. Here, the rice SPS (36) was used as an endogenous reference gene, and a known two-copy transgenic plant was used as the reference sample. Insertion sites of the second T-DNA were determined by thermal assymetric interlaced (TAIL)-PCR (38), using primer sets TAIL-1A, TAIL-2A, and TAIL-3A.
Gene-Expression Analysis by qPCR.
The developmental stages of the anthers were determined as reported previously (16). For gene-expression analysis, rice tissues at the heading stage—including leaf, stem, root, glume, palea, lemma, pistil, and anthers at different developmental stages—were collected from HHZ. To confirm the results of overexpression, the spikelets at stage 12 of Ubipro::OsNP1 transgenic plants in homozygous mutant background were collected. Total RNA was isolated using TRIzol reagent (Invitrogen) and then reverse-transcribed with a PrimeScript RT reagent Kit containing gDNA Eraser (Takara), according to the manufacturer’s instructions. qPCR was performed with the Applied Biosystems 7500 Real-Time PCR System using SYBR Premix Ex Taq II (Takara). Each experiment was repeated biologically three times, each with three replicates. OsACTIN1 was used as the normalizing reference gene. The relative expression levels were measured using the 2−ΔCt analysis method, and the results were represented as the means ± SD. Primers for qPCR are listed in Table S2.
GUS Staining.
Histochemical GUS assays were performed as described previously (39), with the exception of the addition of 0.1% (vol/vol) Triton X-100 to the staining solution. After staining, samples were cleared with 70% (vol/vol) ethanol and photographed using Nikon AZ100 microscope.
In Situ Hybridization.
WT spikelets at different developmental stages were fixed for 16 h at 4 °C with solution containing 0.25% (vol/vol) glutaraldehyde, 4% (wt/vol) paraformaldehyde, 0.1% Tween-20 and 0.1% Triton X-100, dehydrated in a graded ethanol series followed by a xylene series, and then embedded in Paraplast Plus (Sigma-Aldrich), as described previously (40). Samples were sectioned by Leica RM2235 into 8-μm-thick and mounted on poly-l-lysine–coated slides. To generate the antisense and sense RNA probes, the 345-bp region on OsNP1 cDNA was amplified, adding a T7 promoter sequence to 5′ or 3′ end of fragment using primer sets OsNP1-ISH-P1 and OsNP1-ISH-P2, respectively, and the fragments were transcribed in vitro with T7 RNA polymerase using the DIG RNA labeling kit (Roche), according to the manufacturer’s instructions. Pretreatment of sections, hybridization, and immunological detection were performed as described previously (41). Photomicrographs were taken under brightfield microscopy with a Zeiss Axio Imager A1 microscope.
Microscopy and Histology.
Plants and floral organs were photographed with a FinePix HS11 digital camera and Nikon AZ100 microscope, respectively. To analyze pollen fertility, pollen grains at the anther dehiscence stage were released, and then stained with I2-KI solution before photography using Nikon AZ100 microscope.
For transverse section analysis of anthers, spikelets at different developmental stages were collected and embedded as described previously (42), with some modifications. Spikelets were prefixed overnight at 4 °C with 2.5% (vol/vol) glutaraldehyde and 2% (wt/vol) paraformaldehyde in 0.1 M PBS (pH 7.0) and postfixed in 1% osmium tetroxide in 0.1 M PBS (pH 7.0) for 1–2 h. Samples were further dehydrated with a series of ethanol and embedded in SPI-PON 812 resin (SPI-CHEM). Semithin sections (1 μm) were made using an ultramicrotome (Leica EM-UC6), stained with 0.5% toluidine blue, and photographed under brightfield microscopy with a Zeiss Axio Imager A1 microscope. Ultrathin sections (100 nm) were performed, stained with uranyl acetate and lead citrate, and then examined with a transmission electron microscope (JEOL JEM1400) at 120 kV. Histological analysis was performed with at least three biological section replicates.
For scanning electron microscopy, spikelets were fixed in FAA solution containing 5% (vol/vol) formaldehyde, 5% (vol/vol) acetic acid, and 50% (vol/vol) ethanol. After ethanol dehydration, samples were processed for critical point drying with liquid CO2. The dried samples were mounted on copper supports and gold-coated. Finally, samples were observed under scanning electron microscope (Hitachi S-3400N) with an acceleration voltage of 10 kV.
Rice Trait and Field Test.
Stigma extrusion of osnp1-1 was investigated in the afternoon when flowering was finished for the day. The longest panicles from 10 plants, one from each, were counted for the spikelets that had the stigma remaining outside. The counts then were divided by the total number of spikelets, resulting in the stigma extrusion rate. For seed setting rate of cross-pollination, osnp1-1 plants were cross-pollinated with WT HHZ. The filled and unfilled grains on the main panicle were sorted manually, and the filled-grain ratio was calculated.
The pollen fertility of transgenic plant Zhen18B was analyzed by I2-KI staining as described above. Zhen18B was self-fertilized, and the fluorescent and nonfluorescent T1 seeds were sorted manually using equipment illuminating the red florescence. T1 transgenic plants were further self-pollinated, and the ratio of fluorescent progeny was tracked. To evaluate the transgene transmission rate, the nontransgenic plants (Zhen18A) were cross-pollinated by Zhen18B transgenic plants, and the resulting seeds were collected and analyzed for the presence of fluorescent seeds. To evaluate the heterosis, Zhen18A plants were pollinated with ∼1,200 rice germplasms, and the yields of the F1s were compared with their parents and the local control cultivars (i.e., the best-yielding local varieties).
Acknowledgments
We thank Roger Thilmony for critical reading of the manuscript. This work was supported by Guangdong Innovative Research Team Program 201001S0104725509; Ministry of Agriculture Transgenic Program 2012ZX08001001; and Shenzhen Commission on Innovation and Technology Programs JCYJ20130402154520292, KQF201109160004A, and JSGG20150508105340526.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in the NCBI Sequence Read Archive, https://www.ncbi.nlm.nih.gov/sra (accession nos. SRP073226 and SRP058039 for genome resequencing data and KX066198 and KX066199 for OsNP1 nucleotide sequences).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613792113/-/DCSupplemental.
References
- 1.Cheng SH, Zhuang JY, Fan YY, Du JH, Cao LY. Progress in research and development on hybrid rice: A super-domesticate in China. Ann Bot (Lond) 2007;100(5):959–966. doi: 10.1093/aob/mcm121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Liu YG. Male sterility and fertility restoration in crops. Annu Rev Plant Biol. 2014;65:579–606. doi: 10.1146/annurev-arplant-050213-040119. [DOI] [PubMed] [Google Scholar]
- 3.Huang JZ, e ZG, Zhang HL, Shu QY. Workable male sterility systems for hybrid rice: Genetics, biochemistry, molecular biology, and utilization. Rice (N Y) 2014;7(1):13. doi: 10.1186/s12284-014-0013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X, Zhang S, Liang K. Progress and discussion in breeding of indica rice CMS lines in China. Chin Agr Sci Bul. 2007;3:176–180. [Google Scholar]
- 5.Chen L, Lei D, Tang W, Xiao Y. Thoughts and practice on some problems about research and application of two-line hybrid rice. Chin J Rice Sci. 2011;18(2):79–85. [Google Scholar]
- 6.Ding J, et al. A long noncoding RNA regulates photoperiod-sensitive male sterility, an essential component of hybrid rice. Proc Natl Acad Sci USA. 2012;109(7):2654–2659. doi: 10.1073/pnas.1121374109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, et al. Photoperiod- and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res. 2012;22(4):649–660. doi: 10.1038/cr.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou H, et al. RNase Z(S1) processes UbL40 mRNAs and controls thermosensitive genic male sterility in rice. Nat Commun. 2014;5:4884. doi: 10.1038/ncomms5884. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Cui M, Yang L, Kim YJ, Zhang D. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci. 2015;20(11):741–753. doi: 10.1016/j.tplants.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Guo JX, Liu YG. Molecular control of male reproductive development and pollen fertility in rice. J Integr Plant Biol. 2012;54(12):967–978, i. doi: 10.1111/j.1744-7909.2012.01172.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen Z, et al. [Cloning of a rice male sterility gene by a modified MutMap method] Yi Chuan. 2014;36(1):85–93. Chinese. doi: 10.3724/sp.j.1005.2014.00085. [DOI] [PubMed] [Google Scholar]
- 12.Wongnate T, Chaiyen P. The substrate oxidation mechanism of pyranose 2-oxidase and other related enzymes in the glucose-methanol-choline superfamily. FEBS J. 2013;280(13):3009–3027. doi: 10.1111/febs.12280. [DOI] [PubMed] [Google Scholar]
- 13.Akiba T, et al. Organ fusion and defective shoot development in oni3 mutants of rice. Plant Cell Physiol. 2014;55(1):42–51. doi: 10.1093/pcp/pct154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, et al. Identification and characterization of Mini1, a gene regulating rice shoot development. J Integr Plant Biol. 2015;57(2):151–161. doi: 10.1111/jipb.12230. [DOI] [PubMed] [Google Scholar]
- 15.Kurdyukov S, et al. Genetic and biochemical evidence for involvement of HOTHEAD in the biosynthesis of long-chain α-,ω-dicarboxylic fatty acids and formation of extracellular matrix. Planta. 2006;224(2):315–329. doi: 10.1007/s00425-005-0215-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Luo X, Zhu L. Cytological analysis and genetic control of rice anther development. J Genet Genomics. 2011;38(9):379–390. doi: 10.1016/j.jgg.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Ariizumi T, Toriyama K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol. 2011;62:437–460. doi: 10.1146/annurev-arplant-042809-112312. [DOI] [PubMed] [Google Scholar]
- 18.Lee SB, Suh MC. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015;34(4):557–572. doi: 10.1007/s00299-015-1772-2. [DOI] [PubMed] [Google Scholar]
- 19.Samuels L, Kunst L, Jetter R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu Rev Plant Biol. 2008;59:683–707. doi: 10.1146/annurev.arplant.59.103006.093219. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, et al. Defective pollen wall is required for anther and microspore development in rice and encodes a fatty acyl carrier protein reductase. Plant Cell. 2011;23(6):2225–2246. doi: 10.1105/tpc.111.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, et al. Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell. 2010;22(1):173–190. doi: 10.1105/tpc.109.070326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao G, et al. Two ATP binding cassette G transporters, rice ATP Binding Cassette G26 and ATP Binding Cassette G15, collaboratively regulate rice male reproduction. Plant Physiol. 2015;169(3):2064–2079. doi: 10.1104/pp.15.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen RL, Lonsdale DM. Molecular characterization of one of the maize polygalacturonase gene family members which are expressed during late pollen development. Plant J. 1993;3(2):261–271. doi: 10.1111/j.1365-313x.1993.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, et al. Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross-pollinating crops. Plant Biotechnol J. 2016;14(3):1046–1054. doi: 10.1111/pbi.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matz MV, et al. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol. 1999;17(10):969–973. doi: 10.1038/13657. [DOI] [PubMed] [Google Scholar]
- 26.Kalla R, et al. The promoter of the barley aleurone-specific gene encoding a putative 7 kDa lipid transfer protein confers aleurone cell-specific expression in transgenic rice. Plant J. 1994;6(6):849–860. doi: 10.1046/j.1365-313x.1994.6060849.x. [DOI] [PubMed] [Google Scholar]
- 27.Williams M, Leemans J. 1993. Maintenance of male-sterile plants. Patent No. US 5750867.
- 28.Perez-Prat E, van Lookeren Campagne MM. Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci. 2002;7(5):199–203. doi: 10.1016/s1360-1385(02)02252-5. [DOI] [PubMed] [Google Scholar]
- 29.Albertsen MC, Beach LR, Howard J, Huffman GA. 2006. Nucleotide sequences mediating plant male fertility and method of using same. US Patent WO2007002267.
- 30.Zhou D, et al. Pedigree-based analysis of derivation of genome segments of an elite rice reveals key regions during its breeding. Plant Biotechnol J. 2016;14(2):638–648. doi: 10.1111/pbi.12409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariani C, De Beuckeleer M, Truettner J, Leemans J, Goldberg RB. Induction of male sterility in plants by a chimaeric ribonuclease gene. Nature. 1990;374(6295):737–741. [Google Scholar]
- 32.Lochlainn SO, et al. High Resolution Melt (HRM) analysis is an efficient tool to genotype EMS mutants in complex crop genomes. Plant Methods. 2011;7(1):43. doi: 10.1186/1746-4811-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shan Q, et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol. 2013;31(8):686–688. doi: 10.1038/nbt.2650. [DOI] [PubMed] [Google Scholar]
- 34.Hiei Y, Ohta S, Komari T, Kumashiro T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994;6(2):271–282. doi: 10.1046/j.1365-313x.1994.6020271.x. [DOI] [PubMed] [Google Scholar]
- 35.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding J, et al. Validation of a rice specific gene, sucrose phosphate synthase, used as the endogenous reference gene for qualitative and real-time quantitative PCR detection of transgenes. J Agric Food Chem. 2004;52(11):3372–3377. doi: 10.1021/jf049915d. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan TD, Strelow LI, Illingworth CA, Phillips RL, Nelson OE., Jr Analysis of maize brittle-1 alleles and a defective Suppressor-mutator-induced mutable allele. Plant Cell. 1991;3(12):1337–1348. doi: 10.1105/tpc.3.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YG, Chen Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques. 2007;43(5):649–650, 652, 654 passim. doi: 10.2144/000112601. [DOI] [PubMed] [Google Scholar]
- 39.Aya K, et al. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell. 2009;21(5):1453–1472. doi: 10.1105/tpc.108.062935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang X, Gomes A, Bhatia A, Woodson WR. Pistil-specific and ethylene-regulated expression of 1-aminocyclopropane-1-carboxylate oxidase genes in petunia flowers. Plant Cell. 1994;6(9):1227–1239. doi: 10.1105/tpc.6.9.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hejátko J, et al. In situ hybridization technique for mRNA detection in whole mount Arabidopsis samples. Nat Protoc. 2006;1(4):1939–1946. doi: 10.1038/nprot.2006.333. [DOI] [PubMed] [Google Scholar]
- 42.Li N, et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell. 2006;18(11):2999–3014. doi: 10.1105/tpc.106.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]