Significance
Mushroom bodies (MBs) are higher brain structures of several invertebrate groups, vastly studied in insects as a key structure involved in memory processes. Moreover, MBs and the vertebrate pallium have been proposed to share an ancestral common origin. In crustaceans, the hemiellipsoid bodies (HBs) are proposed to be homologues of the insect MBs. However, functional evidence for the involvement of HBs in memory processes is lacking. Here, in the crab Neohelice, we show memory traces in the HBs that, as for MBs, reflect the context attribute of memory. These results extend the homology based on anatomy and gene expression to the functional level. Consequently, present data support the hypothesis of a common origin for the arthropods’ high-order memory centers.
Keywords: memory centers, evolution, homology, Arthropoda, in vivo Ca2+ imaging
Abstract
The hypothesis of a common origin for the high-order memory centers in bilateral animals is based on the evidence that several key features, including gene expression and neuronal network patterns, are shared across several phyla. Central to this hypothesis is the assumption that the arthropods’ higher order neuropils of the forebrain [the mushroom bodies (MBs) of insects and the hemiellipsoid bodies (HBs) of crustaceans] are homologous structures. However, even though involvement in memory processes has been repeatedly demonstrated for the MBs, direct proof of such a role in HBs is lacking. Here, through neuroanatomical and immunohistochemical analysis, we identified, in the crab Neohelice granulata, HBs that resemble the calyxless MBs found in several insects. Using in vivo calcium imaging, we revealed training-dependent changes in neuronal responses of vertical and medial lobes of the HBs. These changes were stimulus-specific, and, like in the hippocampus and MBs, the changes reflected the context attribute of the memory trace, which has been envisioned as an essential feature for the HBs. The present study constitutes functional evidence in favor of a role for the HBs in memory processes, and provides key physiological evidence supporting a common origin of the arthropods’ high-order memory centers.
Learning skills vary across species depending upon specific adaptations to environmental features (1). However, beyond such adaptations, different species share many of the basic mechanisms involved in learning and memory. Both the molecular machinery involved in neural plasticity and the dynamics of the memory processes are conserved throughout evolution (2–5). This characteristic is critical to the hypothesis of a common origin of the high-order memory centers in bilateral animals (6, 7), centers that play a fundamental role in learning and memory by orchestrating high-order sensory processing within contextual frameworks (8, 9). The idea that these centers evolved from the same structure in a common ancestor has been reborn after the remarkable study of Tomer et al. (7) indicating deep homology of mushroom bodies (MBs) and the vertebrate pallium that dates back the origin of higher brain centers to the protostome-deuterostome ancestor times. The vertebrate pallium and the annelid MBs have a conserved overall molecular brain topology and neuron types (7). Furthermore, MBs and the hippocampus’ dentate gyrus share the ability to generate new neurons during adult life (6, 10, 11). In this context, a recent study by Wolff and Strausfeld (6) has proposed that the similarities in both neuronal architectures and protein expression patterns between the mammalian hippocampus, the MBs, and the hemiellipsoid bodies (HBs) of crustaceans are important indicators of genealogical correspondence.
MBs are complex paired structures of the forebrain of invertebrate species and have been vastly studied in insects (12). Since their description in the mid-1850s, the MBs have been considered higher order brain centers involved in memory processes, “compared them with the convolutions of the human brain, and even thought them associated with hexapod intelligence” (13). Although a number of studies show that these centers have olfaction-based functions, MBs are also involved in several behavioral functions (14). Accordingly, insect MBs have been described as one of the key neural structures to encode and retain experiences not just in olfactory instances but also in spatial- and context-dependent memory paradigms (3, 8, 14–17). As for the hippocampus in the vertebrate brain (9), the MBs have been proposed to be involved in linking learned items within a contextual framework (8).
Supporting the hypothesis of a common origin for the high-order memory centers in bilateral animals are the studies showing evidence of structural homology between the MBs of insects and the HBs of crustaceans (12, 18). The similarities between MBs and HBs in regard to morphological and immunoreactive patterns [including the major catalytic subunit of protein kinase A and Ca2+/calmodulin-dependent protein kinase II (CaMKII), proteins required in memory processes] are relevant evidence of genealogical correspondence of these arthropod brains’ higher centers (12, 19). Moreover, HBs also generate new neurons during adult life (20, 21). The concordance among several types of data, including topology, topography, gene expression, and functions, are useful to support phylogenic and/or structural homologies in the central nervous system (6, 22–24). Based on solid evidence about the MB function, the HB has been largely predicted as a high-order memory center (6, 25, 26). However, to the extent of our knowledge, there is yet no direct evidence in favor of the occurrence of learning and memory processes in this neuropil. Here, we evaluated whether, as expected for high-order memory centers (17, 27), the HB’s activity reflects the link between a learned item and the context with which it has been associated.
In the memory paradigm developed in the crab Neohelice (Chasmagnathus) granulata (Fig. 1A), animals learn to associate the training context with a visual danger stimulus (VDS) passing overhead (28, 29). Several neuronal correlates of memory processes in this paradigm have been studied using biochemical, molecular, and electrophysiological approaches (29). A group of motion-sensitive neurons that project from the lobula (Fig. 1B) to the lateral protocerebrum reflects short- and long-term behavioral changes related to the VDS but not to the context specificity of the memory (29, 30). A high-order memory trace linking the VDS with the training context was envisioned to reside in the HBs (30).
Fig. 1.
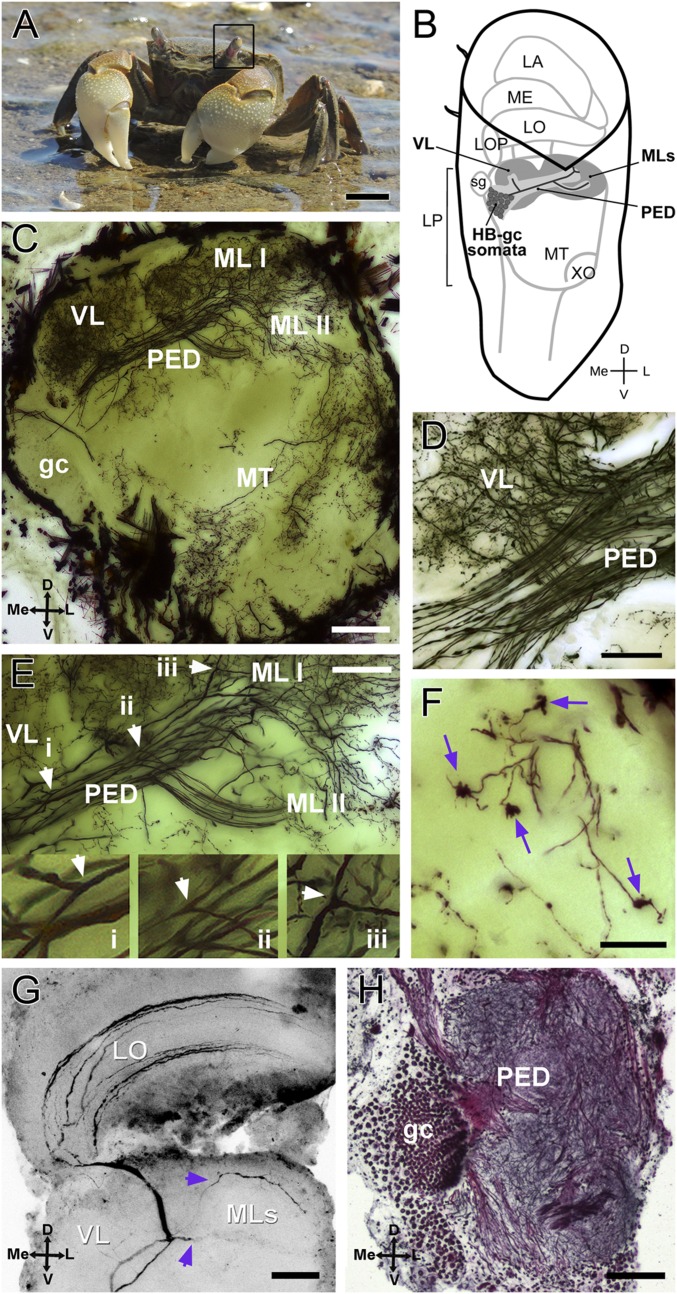
Crab N. granulata and the HB. (A) N. granulata crab. The black frame surrounds the left eyestalk. (B) Scheme showing the position and shape of the neuropils within the eyestalk. The HB is part of the lateral protocerebrum and is drawn in gray. Complete gc and some somata of the HB are drawn. (C–F) Golgi impregnation of the optic lobes (50-μm sections). (C) Overall structure of the HB seems mainly formed by intrinsic cells derived from the HB-gc cluster. (D) Detail of the bifurcation site that originates the vertical lobe. (E) Detail of the bifurcation sites that give rise to the vertical lobe and to both medial lobes. (Insets) Arrowheads show three amplified bifurcations (i, ii, and iii) in the same neuron. (F) Claw-like specializations are present in the medial lobes (arrows). (G) Intracellularly stained BLG2 neuron showing two branches that invade the two medial lobes of the HB (arrowheads). (H) Bodian-stained section (12 μm) of the lateral protocerebrum showing gc and the pedunculus. D, dorsal; L, lateral; LA, lamina; LO, lobula; LOP, lobula plate; LP, lateral protocerebrum; Me, medial; ME, medulla; MT, medulla terminalis; PED, pedunculus; MLs, medial lobes; sg, sinus gland; V, ventral; VL, vertical lobe; XO, X-organ. (Scale bars: A, 1 cm; C, G, and H, 100 μm; D and E, 50 μm; F, 25 μm.)
In the present study, we combine histological and immunohistochemical analyses, neuronal recordings, and behavioral experiments to examine putative neuronal correlates of memory in the HBs of the crab Neohelice. Neuroanatomical and immunohistochemical data revealed HBs whose intrinsic neurons [globuli cells (gc) in a cluster showing adult neurogenesis] form a parallel-projecting bundle of neurites (pedunculus) that then divide to outline two main lobes, resembling the anatomy of calyxless MBs observed in some insect species. In vivo calcium imaging showed that the neural activity of HBs, elicited by the training stimulus, is modified by training. Moreover, the data revealed a context-specific memory trace in the HBs.
Results
HBs of the Crab N. granulata, Histology, and Immunostaining.
In decapod crustaceans, the brain is formed by the proto-, deuto-, and tritocerebrum. The optic lobes, located within eyestalks and connected with the rest of the brain via the protocerebral tract, are part of the protocerebrum and comprise the lamina, medulla, lobula, lobula plate, and lateral protocerebrum (Fig. 1B). The lateral protocerebrum comprises, in turn, several discrete neuropils, including the medulla terminalis and the HB. The medulla terminalis is a generic name used for several closely packed neuropils that receive input from the olfactory globular tract, the ipsilateral and contralateral optic neuropils, and the HB (25, 31). In Neohelice, as in other crabs, a marked division between the HB and the medulla terminalis is not obvious (31–34). In the present study, the nomenclature proposed for the HB is based on the nomenclature used for calyxless MBs of insects (15).
The intrinsic components of the HB, as for the MB, derive from a cluster of minute basophilic gc (12, 15). Golgi impregnations, in which the HB-gc were revealed (Fig. 1C), show that the basic structure of the HB in Neohelice resembles the organization of the calyxless MB of insects: a cluster of small cell somata projecting parallel fibers constituting the pedunculus, which bifurcates twice. The first bifurcation, oriented dorsally, originates what we called the vertical lobe [in analogy to the insect’s MB (15); Fig. 1D]. The second bifurcation, oriented anterior-laterally, originates the medial lobes (constituted by two close structures, medial lobe I and medial lobe II), called neuropils I and II in crayfish and other Decapoda (26, 31). Fig. 1E shows a magnification where particular gc fibers can be seen bifurcating and ramifying in all of the lobes. In close parallel to Kenyon cells of insect MBs and gc from HBs of hermit crabs, intrinsic cells of the HBs in Neohelice show specializations similar to claw terminals (19, 35) (Fig. 1F). One possible input to the HB could be the bistratified lobula giant 2 (BLG2) neurons. Fig. 1G shows an intracellularly stained BLG2 that has processes entering the two HB medial lobes. Interestingly, the BLG2 neurons respond to visual and mechanical stimulation (36).
Intrinsic neurons (HB-gc) constituting the HB are numerous, near a thousand (Movies S1 and S2). Part of the HB-gc somata cluster and the fibers forming the pedunculus are shown in a Bodian-stained section of the lateral protocerebrum (Fig. 1H). HB-gc somata are easily distinguished from other surrounding somata by their size, smaller than neighboring cells.
Synapsins (SYNs), proteins associated with synaptic vesicles, are effective for revealing synaptic neuropils in arthropods because they are abundantly expressed in regions of high synaptic density. Fig. 2A shows a confocal section of the lateral protocerebrum for whole-mount immunohistochemistry against SYN. We found strong SYN immunoreactivity in regions that correspond to the arborization areas of the vertical and medial lobes of the HB, but not in the pedunculus. Both vertical and medial lobes show a SYN immunoreactivity texture consistent with a microglomeruli structure as described in HBs of other crustacean and in MBs (19, 31, 35). Fig. 2 B and C show details obtained by digital zoom of areas of the vertical (Fig. 2B) and medial (Fig. 2C) lobes, where traits compatible with microglomeruli structures can be recognized as rounded areas delimited by high SYN immunoreactivity.
Fig. 2.
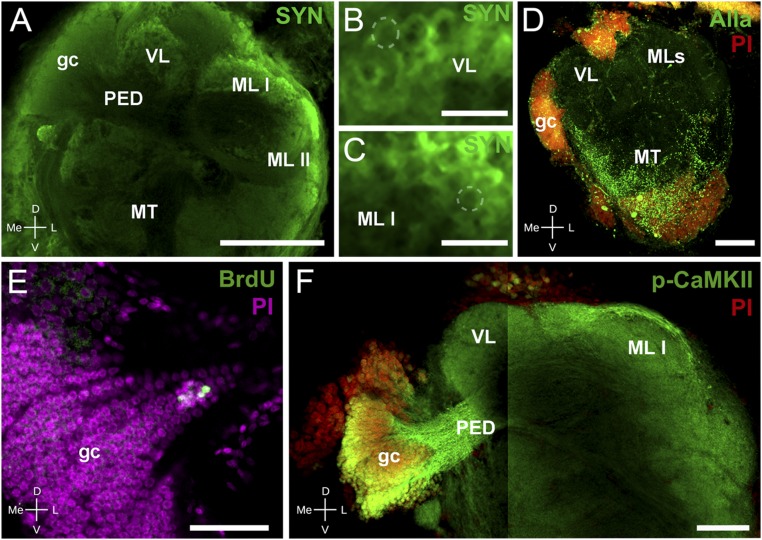
Immunohistochemistry and neurogenesis in Neohelice’s HBs. (A–C) Confocal laser scan of the lateral protocerebrum labeled with antibodies against SYN (green). (A) The gc somata cluster, the pedunculus, and both the vertical and medial lobes (medial lobes I and II) composing the HB can be distinguished. (B) Detail of the vertical lobe showing traits resembling microglomeruli (dashed line points out an example). (C) Same as B for the medial lobe I. (D) Maximum projection of confocal laser scan sections showing immunoreactivity against allatostatin (Alla, green) and nuclei stained with propidium iodide (PI, red). Alla-like immunoreactivity appears widely distributed in the medulla terminalis, whereas there is scarce immunoreaction in the area corresponding to the HB. (E) Confocal laser scan sections of the HB-gc somata labeled with antibody against BrdU (green) and with PI (magenta) visualizing newborn cells in the gc cluster. (F) Confocal laser scan of the HB labeled with antibodies against p-CAMKII-α (green) and with PI (red). The image is composed of two stacks taken at different focal planes. (Left) The gc, comarked p-CAMKII-α and PI, and the pedunculus that projects toward the vertical and medial lobes. (Right) Pedunculus projecting toward both medial lobes. Abbreviations are as in Fig. 1. (Scale bars: A, 200 μm; B and C, 20 μm; D and F, 100 μm; E, 50 μm.)
As in other crustaceans, we found a strong allatostatin-like immunoreactivity in the closely related medulla terminalis neuropils, but not in the HB (37) (Fig. 2D). Adult MB neurogenesis has been previously observed in some insect species (38) and in the HB somata cluster of other crustaceans (20, 21). In vivo 5-bromo-2′-deoxyuridine (BrdU) labeling stained HB-gc somata, suggesting neurogenesis in a zone of the somata cluster proximal to the neck of the pedunculus (Fig. 2E). The expression of proteins associated with mechanisms of memory processes, like p-CaMKII-α, is another characteristic of forebrain centers such as MBs and HBs (12). The p-CaMKII-α immunoreactivity reveals the medial and vertical lobes (Fig. 2F). Moreover, HB-gc somata show strong p-CaMKII-α immunoreactivity, in contrast to their bigger neighboring cells.
Memory-Related Neural Plasticity in the Crab’s HBs.
The memory paradigm used in Neohelice is based on the crab’s escape response elicited by a VDS resembling an aerial predator. Upon repeated presentations (15 spaced trials) of the VDS, the escape response declines over trials turning into a freezing behavior. The change in behavior persists in the long term, and is mediated by an association between a training context (a bowl that receives above-light illumination) and VDS (29, 39–41). The training protocol regularly used in Neohelice yields a decrease in escape response that, in the short-term, is not specific to the training context (42). However, the goal of this study is to evaluate whether the activity of HBs might reflect context specificity during imaging experiments, which are tested in the short term. Hence, in the present study, we switched to a slightly different version of the training protocol that enhances the associative strength between VDS and context (43). Here, we tested whether this protocol yields short-term memory that, like long-term memory, is context-specific. Fig. 3 A, i shows a scheme of the experimental procedure. The experimental device, the actometer (28, 29), consisted of a bowl-shaped opaque container where the crab was lodged during training and testing sessions. During each trial, a VDS (an opaque rectangular screen) was moved twice horizontally in a 90° clockwise and counterclockwise excursion from the starting position and back. The VDS induces an escape response in the crab, and consequent container vibrations are converted into electrical signals through microphones placed on the external wall of the container. The activity of the crab was recorded throughout each VDS presentation (9 s) in arbitrary units. The actometer was illuminated from above during the training trials and from below during intertrial intervals (153 s) (Fig. 3 A, i).
Fig. 3.
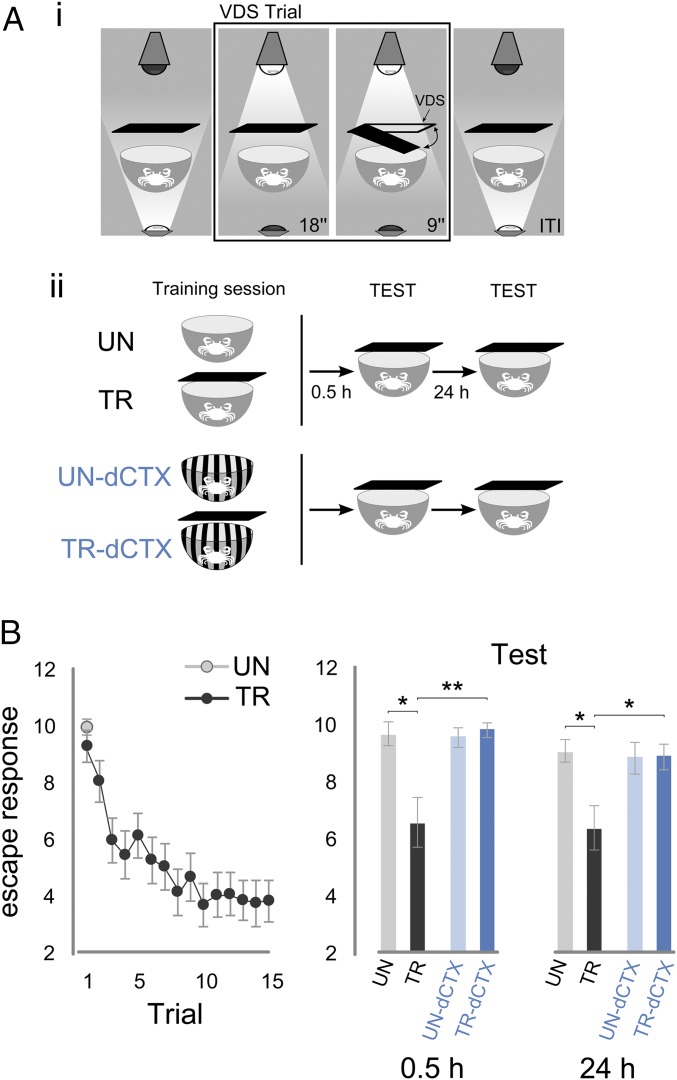
Behavioral experiments and memory in the crab Neohelice. (A, i) Scheme of a VDS trial. A crab is inside the actometer that is illuminated from below during the intertrial interval (ITI) for 153 s. During the VDS trial, the actometer is illuminated from above for 27 s. During the last 9 s, the VDS moves twice in a 90° clockwise and counterclockwise excursion from the starting position. (A, ii) Scheme showing the different groups of crabs. The TR receives 15 VDS trials, whereas the UN receives the VDS once, only in the first of the 15 trials. UN-dCTX and TR-dCTX are trained with the same protocol as UN-TR but in a different context (actometers with striped walls). In the TEST sessions, 0.5 and 24 h later, all four groups receive a VDS trial in the actometer without stripes. Thus, UN-TR crabs are tested in the same context in which they were trained, whereas UN-TR-dCTX crabs are tested in a different context from training. (B, Left) Escape response (log2-transformed) of crabs to the VDS throughout the 15 spaced trials of the training session. (B, Right) During the memory retention tests 0.5 and 24 h later, crabs exhibited memory when tested in the same training context (TR or UN), but not when the testing context was different from the one at training (TR-dCTX or UN-dCTX). For each group, n = 28. Games–Howell post hoc test: *P < 0.05, **P < 0.01. Plots show mean escape response (log2-transformed) ± SEM.
The experiment consisted of two pairs of groups, with each pair comprising an untrained group (UN) and a trained group (TR) of animals. During training session, the TR received 15 VDS trials, whereas the UN received only one VDS trial. Two test sessions were run with the same animals: short term at 0.5 h and long term at 24 h after training. To evaluate the memory’s context specificity, the UN-TR pair was trained and tested in the same context and the UN-TR tested in a different context (dCTX) pair was tested in a context different from the one used during training (Fig. 3 A, ii). No difference was found for the first training trial between TRs and UNs (Welch’s t test: UN vs. TR: t[39.89] = 1.04, P = 0.30, n = 28,28), and a significant trial effect was evidenced for the TR during training (Fig. 3B, Left) (repeated measures ANOVA: TR: F[7.60,205.31] = 9.55, P < 0.001, epsilon = 0.54, n = 28; Greenhouse–Geisser correction for sphericity violation). During the short-term memory test session (0.5 h), crabs expressed memory when the test was run in the training context (UN vs. TR) but not when the test was run in a different context from the one used during training (UN-dCTX vs. TR-dCTX) (Fig. 3B, Right) (one-way ANOVA Welch’s test: F[3, 57.23] = 4.13, P = 0.01; post hoc Games–Howell test: UN-TR: q[38.76] = 4.53, P < 0.016; UN-dCTX–TR-dCTX: q[49.71] = −0.86, P > 0.05; TR–TR-dCTX: q[31.62] = −5.02, P < 0.007; UN–UN-dCTX: q[48.84] = 0.54, P > 0.05). As expected (43), long-term memory (24 h) evaluated in the same groups of animals showed similar results (Fig. 3B, Right) (one-way ANOVA Welch’s test: F[3, 58.76] = 3.27, P = 0.027; post hoc Games–Howell test: UN-TR: q[40.27] = −4.38, P < 0.0195; UN-dCTX–TR-dCTX: q[51.69] = −0.086, P > 0.05; TR–TR-dCTX: q[43.30] = −3.91, P < 0.042; UN–UN-dCTX: q[52.23] = 0.39, P > 0.05). Therefore, the use of a training protocol in which a change in the illumination of the training context anticipates the VDS by a few seconds renders context-specific memory expression in the short term as in the long term.
We ran in vivo calcium imaging experiments to examine memory-related changes in neural activity of the crab’s HBs. For that aim, a small window was opened in the crab’s left eyestalk allowing visual access to the lateral protocerebrum. Small crystals of the calcium-sensitive dye Calcium Green-1 dextran were stabbed into the neck of the pedunculus of the HB. The dye was internalized by gc and transported along the neurons to the HB lobes. Fig. 4 A, i shows a diagram of the lateral protocerebrum, where the site of insertion of the dye is shown; a view of the preparation is shown in Fig. 4 A, ii. Fig. 4 A, iii shows an example of a confocal stack of the lateral protocerebrum incubated with antibodies against SYN. The intense green spot corresponds to the position where Calcium Green was inserted; multiple somata and some processes of HB neurons that were stained with Calcium Green can still be seen after tissue fixation.
Fig. 4.
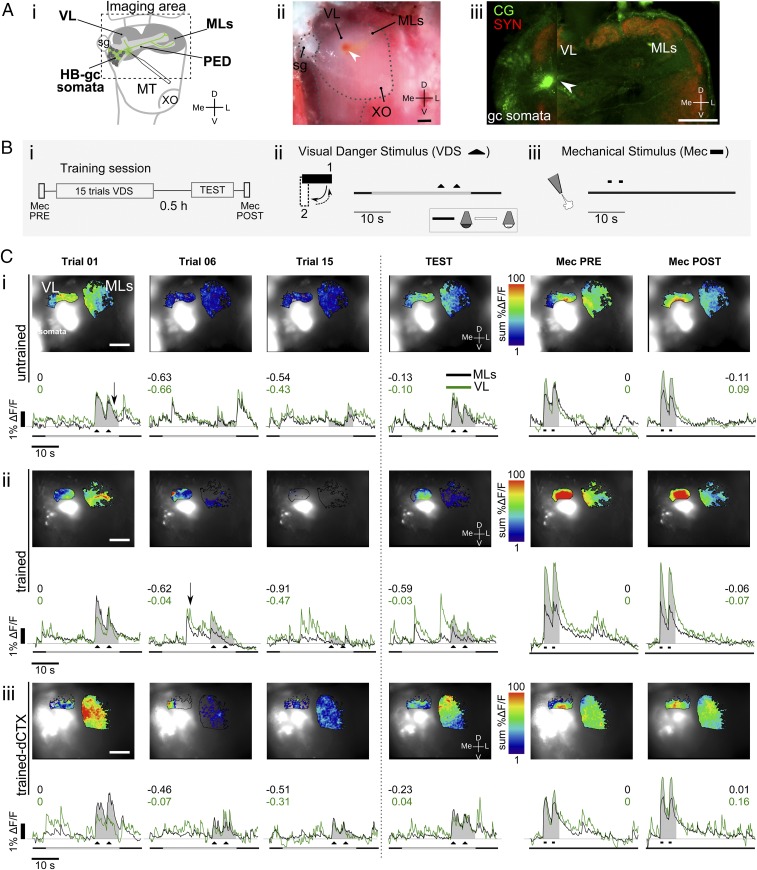
In vivo calcium imaging preparation and neural activity in the crab’s HB. (A) Preparation for calcium imaging experiments. (i) Diagram of the lateral protocerebrum showing the site where the crystals of calcium dye were stabbed with a glass capillary. A complete gc and some somata of the HB drawn in green represent cells filled with the dye. A dashed frame outlines the approximately recorded area during experiments. (ii) Picture of the preparation under the stereomicroscope. The red spot corresponds to the Calcium Green crystals, which look red under white light. (Scale bar: 100 μm.) (iii) Confocal stack showing immunoreaction against SYN and Calcium Green staining. (Scale bar: 200 μm.) (B) Training protocol and scheme of the stimulation trials. (i) Training protocol consists of 15 VDS trials followed by a short-term test 0.5 h later. A Mec is delivered before training (PRE) and after testing (POST) as a control stimulus. (ii) VDS consists of a screen moving back and forth 90° above the animal (black triangles). The trial consists of a delayed context-VDS presentation. The time line represents the whole extent of each recording event (40 s), including the 27 s in which illumination changes from below to above, and the VDS toward the end of this period. Dark line, below illumination; white line, above illumination. (iii) Mec consists of two 1-s pulses of nitrogen at the dorsal posterior part of the crab’s carapace (black rectangle denotes the Mec timing). (C) Examples of calcium dynamics. (Upper) Superimposed raw and false color-coded images for the ML and VL areas showing neuronal calcium responses as the summation of change in fluorescence (sum %ΔF/F) upon stimulation for training trials 1, 6, and 15 and the test, and Mec for the medial and vertical lobes. The white glow corresponds to intensity saturation, where the dye was stabbed. (Scale bar: 200 μm.) (Lower) Time course calcium dynamics as %ΔF/F during trials corresponding to the image in the upper row. The shaded area shows the time window considered for summation of the activity elicited by the stimulus and used for calculation of the CSR. Numbers in black and green show the CSR for each trial and each lobe. MecPRE/POST, Mec pretraining or posttesting. (i) UN crab example. (ii) TR crab example. (iii) TR-dCTX crab example.
During in vivo imaging experiments, calcium signals from the vertical and medial lobes were simultaneously registered while the crab was held in the imaging setup. All recordings were obtained from the HB of the left eyestalk, whereas a training protocol similar to the one described for behavioral experiments was run stimulating the animal through its right eye. It is well known that when the crabs are immobilized in a way that body movements related to escape response are impeded, learning and memory formation still occur (44). A short-term test trial was performed 0.5 h after the end of the training protocol. In addition, a stimulus of a different sensory modality that does not trigger the escape response (45), a mechanical stimulus (Mec), was used at the beginning (MecPRE) and at the end of the imaging experiments (MecPOST) in all experimental groups to control for unspecific changes that may happen to the preparation during the experiment. Fig. 4B shows the training protocol (i) and a scheme of the timing of the stimuli: VDS (ii) and Mec (iii) (VDS or Mec is applied twice in each trial). Recordings were made during every Mec, during every VDS, and during nonstimulated trials. Calcium signals were calculated as changes from the basal fluorescence as %ΔF/F (SI Materials and Methods, In Vivo Optical Imaging Experiments), where F represents the basal fluorescence and ΔF represents the difference between the basal fluorescence and the fluorescence measured during the frame of interest.
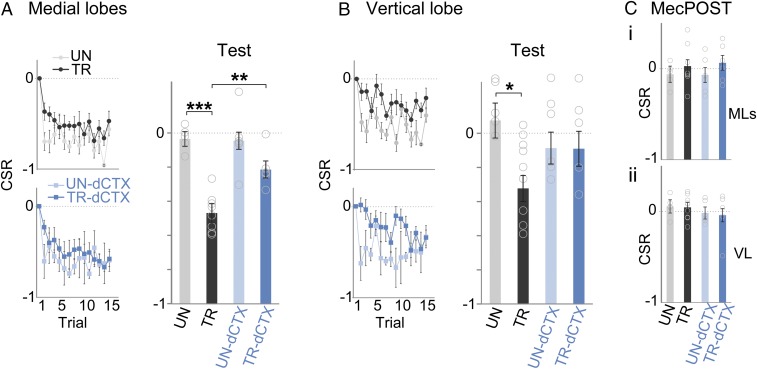
Both VDS and Mec triggered transient calcium signals in the vertical and medial lobes (Fig. 4C). For analysis, calcium responses were quantified as the summation of the %ΔF/F obtained for each frame during VDS or Mec (Fig. 4C, shaded area under the traces). A calcium signal rate (CSR) index (SI Materials and Methods, Statistical Analysis, Optical imaging) was calculated to analyze relative changes in comparison to the first VDS (trial 1) or Mec (MecPRE) trials: CSR = −1 corresponds to a complete reduction of the response, CSR = 0 corresponds to no change, and a positive CSR corresponds to an increase in the response. As for behavioral experiments, four groups were run in the imaging experiments: UN crabs (Fig. 4 C, i; representative example), TR crabs (Fig. 4 C, ii; representative example), UN-dCTX crabs, and TR-dCTX crabs (Fig. 4 C, ii; representative example). It is noteworthy that UNs show negative CSRs in trials 2–15 because they do not receive VDS stimulation along those trials.
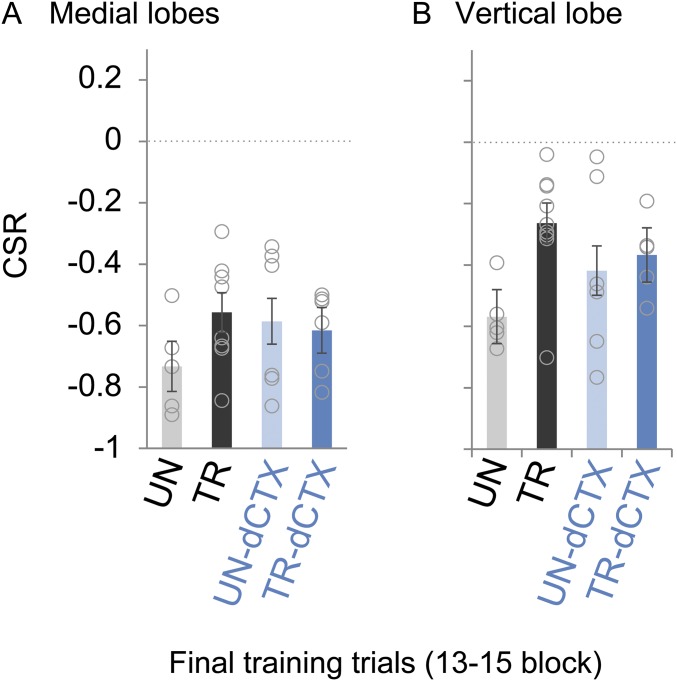
Both TRs, TR and TR-dCTX, exhibited responses in the medial and vertical lobes elicited by the VDS. Responses decreased with a similar profile throughout trials and resulted in negative CSRs by the end of the training sessions (Fig. 5 A and B, Left). Moreover, during the last trials of the training sessions, trained animals (TR and TR-dCTX) showed a CSR indistinguishable from the basal activity of unstimulated animals (UN and UN-dCTX) (Fig. S1; block of 13–15 trials, one-way ANOVA: medial lobes: F[3,21] = 1.01, P = 0.40; vertical lobe: F[3,21] = 2.64, P = 0.076).
Fig. 5.
Context-dependent learning plasticity in the HBs. (A) HB CSRs elicited with the VDS during training and testing corresponding to the medial lobes (UN, TR, UN-dCTX, and TR-dCTX: n = 5, n = 8, n = 6, and n = 6, respectively). (B) Same as A for the vertical lobe (UN, TR, UN-dCTX, and TR-dCTX: n = 5, n = 9, n = 6, and n = 5, respectively). (C) MecPOST. CSRs for medial and vertical lobes are shown. One-way ANOVA, Tukey HSD test: ***P < 0.001, **P < 0.01, *P < 0.05. Circles represent individual animals. CSR = −1 corresponds to a complete reduction of the response, CSR = 0 corresponds to no change, and a positive CSR corresponds to an increase in the response. Plots show mean CSR ± SEM.
Fig. S1.
HB calcium imaging activity during the final training trials block (related to Fig. 5). A CSR corresponding to the last three training trials for the four experimental groups is shown. Notice that UNs are not stimulated with the VDS. In analysis by one-way ANOVA, no differences between groups were found in the medial lobes (A) or in the vertical lobe (B). Circles represent individual animals.
At a short-term test performed 0.5 h after the end of training (Fig. 5A; Test), the medial lobes of the HB showed decreased responses to the VDS if the test was run in the training context (UN vs. TR), but not if the test was run in a different context from the one used during training (UN-dCTX vs. TR-dCTX) (one-way ANOVA: F[3,21] = 18.77, P = 0.000004; Tukey honest significance difference (HSD) test, same context groups: UN vs. TR: P = 0.00018, context shift groups: UN-dCTX vs. TR-dCTX: P = 0.112, UNs: UN vs. UN-dCTX: P = 0.99, TRs: TR vs. TR-dCTX: P = 0.005).
In regard to activity measured in the vertical lobe, the short-term test showed a similar profile (Fig. 5B; Test) (one-way ANOVA: F[3,21] = 3.58, P = 0.03). However, the decrease of the VDS-elicited activity in the TR was less pronounced (Tukey HSD: UN vs. TR: P = 0.025, UN-dCTX vs. TR-dCTX: P = 0.99, UN vs. UN-dCTX: P = 0.65, TR vs. TR-dCTX: P = 0.29).
Importantly, the training-dependent change in neuronal responses measured in the HBs was specific to the VDS because responses elicited by the Mec showed no differences in any of the groups before and after the training session (Fig. 5C): The CSR was not different from zero in any group, and there were no differences between groups (MecPOST t test single sample: medial lobes and vertical lobes for all groups: P > 0.32; one-way ANOVA, medial lobes: F[3,21] = 0.65, P = 0.59; vertical lobes: F[3,21] = 0.48, P = 0.70).
In summary, the calcium imaging experiments show changes in neural activity in the crab’s HBs in the form of a context stimulus-specific decrease in responses elicited by the VDS that correlates with the crab’s memory expression.
SI Materials and Methods
Animals.
Intermolt adult male crabs of the species Neohelice (Chasmagnathus) granulata measuring between 2.7 and 3.0 cm across the carapace were collected from the narrow coastal inlets of San Clemente del Tuyú, Buenos Aires Province, Argentina. In the laboratory, crabs were kept on a 12-h/12-h light/dark cycle in collective plastic tanks (20 animals each) filled up to a depth of 1 cm with brackish water prepared with hw-Marinex (Winex) salt (1.0%, pH 7.4–7.6). The holding and experimental rooms were kept at 22–24 °C and 80 ± 10% relative humidity. Experiments were carried out in the daytime within 15 d after the animals had been captured. All animals were injected systemically with 50 μL of saline solution 15 min before training protocols. Each crab was used in only one experiment. All efforts were made to minimize the number of animals. All of the research was conducted in accordance with the Ethical Reference Frame for Biomedical Investigations of the Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina, equivalent to the standard procedures for animal care and use of the US NIH.
Histology and Immunostaining.
Animals were submerged in ice-cold brackish water for 3–5 min until anesthetized. The dissection of the left optic lobe was carried out in 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and, later on, fixed overnight in the same solution at 4 °C with constant stirring. A modification of the procedure proposed by Ott (62) was used. The fixed whole-mount tissue sample was washed in PBS three consecutive times for 15 min at room temperature. Dent’s fixative was then applied for 2 h, and the sample was subsequently washed twice with 0.1 M Tris buffer (pH 7.4) and once in PBSd-normal goat serum (NGS) [5% (vol/vol) NGS, 1% DMSO, and 0.005% NaN3 in 0.1 M PBS] over 2 h. Afterward, tissue was incubated with anti-SYNORF1, anti-SYN antibody (3C11; Hybridoma Bank, University of Iowa), antiallatostatin antibody (5F10; Hybridoma Bank, University of Iowa), or anti–p-CAMKII-α sc-12886-R (Santa Cruz Biotechnology, Inc.) at a dilution of 1:100 or anti–alpha-tubulin (12G10; Hybridoma Bank, University of Iowa) at a dilution of 1:250 in PBSd-NGS for 3.5 d at 4 °C. Subsequently, tissues were washed several times at room temperature for 6 h in PBSd and incubated with an Alexa Fluor 488-labeled polyclonal goat anti-mouse antibody, IgG-H&L A11029 (Molecular Probes/Invitrogen) at a dilution of 1:1,000 for 2.5 d in the dark at 4 °C. The tissue was then washed in PBS three consecutive times for 15 min at room temperature. In cases where propidium iodide (PI) was used for nuclei staining, tissues were incubated in the dark in 1.5 μM PI-PBS, and then washed again with PBS. Tissues were then dehydrated in an increasing concentration of glycerol in PBS [1%, 2%, and 4% for 2 h each; 8%, 15%, 30%, 50%, 60%, 70%, and 80% (vol/vol) for 1 h each] and later submitted to several consecutive ethanol washes for 0.5 h and coverslipped with methylsalicylate (Sigma–Aldrich).
Golgi impregnation.
Optic lobes of N. granulata were impregnated using a combined Golgi Colonnier–Golgi rapid method that has been described in detail previously (63).
Bodian staining.
Optic lobe sections of N. granulata were stained adapting a Bodian staining original method that has been described in detailed previously (61).
Intracellular filling of lobula giant neurons (44, 64).
Borosilicate glass electrodes (WPI; outer diameter = 1.2 mm, inner diameter = 0.68 mm) were pulled with a horizontal puller. Electrodes were slowly advanced from the lobula to the lateral protocerebrum using a micromanipulator. The tips of the electrodes were filled with the calcium indicator Calcium Green-1 dextran, hexapotassium salt (Molecular Probes); 500 μg of Calcium Green was diluted in 17 μL of KOH (5 mmol⋅L−1 with 5 μL of Hepes 200 mmol⋅L−1, pH 7.5) and 83 μL of water for 100-μL stock solutions. Electrodes were backfilled with a drop of 3 mmol⋅L−1 KCl. Resistance in the tissue was generally 10–20 ΩM. Tissue was fixed as described above.
In vivo labeling with BrdU.
For in vivo labeling with BrdU, 5 mg of BrdU per 100 g of body weight was injected into the right side of the pericardial sac of crabs (BrdU labeling reagent, Cell Proliferation Kit RPN 20; Amersham). After 96 h, brains were dissected and fixed with Bodian 2 fixative [90 mL of 80% (vol/vol) ethanol, 5 mL of formol, and 5 mL of glacial acidic acid] for 4–5 h (65). Afterward, rehydration in several decreasing concentrations of ethanol was carried out, and the sample was subsequently fixed overnight in 4% (wt/vol) paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) at 4 °C with constant stirring. The whole-mount tissue was then washed in PBS, and progressive dehydration in increasing ethanol solutions and with Xylene [50%, 70%, 90%, 96%, and 100% (vol/vol) Xylene for 10 min each] was done. Later, rehydration was performed with the same decreasing solutions. Overnight incubation at 4 °C with constant stirring was carried out in a blocking solution [2% NGS, 1% Triton detergent, and 5% (vol/vol) Svelty skimmed milk in 0.1 M PBS]. Samples were incubated for 72 h with blocking solution and IgG2a, anti-BrdU at a dilution of 1:100 at 4 °C, and subsequently washed five times each for 20 min in PBS-1% Triton and incubated for 24 h with the secondary antibody Alexa Fluor 488 (Molecular Probes/Invitrogen) at a dilution of 1:1,000. The tissue was then washed in PBS three consecutive times for 15 min at room temperature, incubated in the dark in 1.5 μM PI-PBS, and then washed again with PBS. Tissues were then dehydrated in an increasing concentration of ethanol in PBS [50%, 70%, 90%, 96%, and 100% (vol/vol) for 20 min each] and coverslipped with methylsalicylate (Sigma–Aldrich).
Image processing.
Fluorescence in fixed tissue samples was visualized using a confocal microscope (Olympus FV300) with a 488-nm argon laser, 10-mW and 543-nm HeNe green laser at 1 mW, a UplanFl 20× objective (N.A. = 0.5), an SDM 570 dichroic cube to split the acquisition channel, and BA510 emission (Olympus Optical). The pixel size of images was 0.69 × 0.69 μm. Data were recorded using FluoView Software. Images were analyzed using ImageJ software (NIH; https://imagej.nih.gov/ij/), applying a Kalman filter to reduce background noises, and frame brightness and contrast were adjusted.
Behavioral Experiments.
Experimental device.
The experimental device, the actometer (28, 29), consisted of a bowl-shaped opaque container with a steep concave wall 12 cm high (23-cm top diameter and 9-cm floor diameter) filled with brackish water prepared with hw-Marinex (Winex) salt (1.0%, pH 7.4–7.6) to a depth of 0.5 cm, where the crab was lodged during the training and testing sessions of the behavioral experiments. During each trial, an opaque rectangular screen (25 × 7.5 cm), termed visual danger stimulus (VDS), was moved twice horizontally 12 cm over the actometer at a constant speed. The movement of the VDS was driven by a motor at a velocity of 82° per second. The motion cycle, which consisted of a 90° clockwise and counterclockwise excursion from the starting position and back, was completed in circa 2.2 s. Each recording trial was composed of two cycles separated by 2 s. The VDS presentation induces an escape response in the crab and consequent container vibrations that are converted into electrical signals through four microphones placed on the external wall of the container. The activity of the crab was recorded throughout each trial in arbitrary units. The experimental room has 40 actometers, separated from each other by partitions, so animals from different experimental groups can be treated simultaneously. During the experiment, the actometer could be illuminated using two different light sources. The actometer was illuminated from above during the training trials and from below during intertrial intervals (153 s) (Fig. 3 A, i). This alternation between two light sources provided a controlled phasic presentation of the training context (TC) during the experiments (43, 66).
Training and memory test protocol.
The experimental protocols involved paired groups of crabs. Each pair consisted of a TR and an UN. The UN remained in the actometer during the entire training session, but received only one trial with the VDS, which was simultaneous with the first training trial of the TR, and was used to control homogeneity in the escape response between groups at the beginning of the experiments.
Training session.
Training consisted of 15 VDS trials separated by 153-s intervals (total time = 42 min) after a 10-min adaptation period during which animals do not receive VDS and which was illuminated from below throughout. Each VDS training trial consisted of a period of 27 s of illumination from above in which the VDS was presented during the last 9 s (43) (Fig. 3 A, i). When the session was finished, TR and UN animals were immediately placed in individual resting containers until the test session.
Test.
To evaluate short-term memory, 25 min after the end of training, the animals were reinstalled in the actometer (below illumination) for 5 min and memory expression was then tested during a VDS trial (total time after end of training = 0.5 h). When the session was finished, animals were immediately placed in individual resting containers until the next test session 24 h later. Memory expression is revealed in the test session as a significantly lower escape response of the TR in comparison to the UN (29).
Context specificity.
To evaluate memory context specificity a modification in the training protocol was made. Basically, what was done was an ad hoc modification in the actometer container during the training session. In context A, the standard actometer container was an orange plastic container with a steep concave wall 12 cm high (23-cm top diameter and 9-cm floor diameter). In context B, a cylindrical (15-cm diameter and 15-cm height) plastic container with black and white striped walls was used. Context B was arranged to fit inside the actometer, thus vibrations provoked by the motor activity of the animal could not be registered properly. Consequently, context A is the only one in which the activity of the crab can be measured. Thus, experiments were designed in such a way that the test session occurs always in context A. This method imposes a limitation in experimental designs, because context presentation cannot be counterbalanced. However, this strategy had been successfully used in previous work, proving that animals recognize the containers as different contexts (42, 43, 67, 68).
In Vivo Optical Imaging Experiments.
Crab preparation procedures and staining for calcium imaging.
The in vivo optical imaging method is an adaptation of other methods that have been described in previous works (45, 69). The crabs were submerged in ice-cold brackish water for anesthesia before preparation. During preparation and measurements, the chelae were immobilized with a rubber band and the crabs were firmly held by their exoskeleton with an adjustable clamp that allowed free movements of the legs. The right eyestalk was covered with wet paper to avoid visual stimulation during the preparation. The left eyestalk was fixed with instant acrylic glue (La Gotita; Akapol S.A.) with an orientation that allowed access to the anteromedial side of the eyestalk. A small container was built with dental cement (Dycal) around the eyestalk to allow continuous flow of crab Ringer’s solution (468.00 mM NaCl, 9.46 mM KCl, 7.50 mM MgCl2, 12.53 mM CaCl2, 5.00 mM Hepes, 37.00 mM NaHCO, and 2 mM glucose at 1,045.00 mOsm) (70). A window (circa 3 × 2 mm) was opened in the cuticle of the eyestalk using a sharp scalpel. To allow visual access to the HB neuropil, the connective tissue was removed and the neuroepithelium was cut along the longest axis of the eyestalk with Vannas scissors (WPI 555640S) and attached to the edges of the window (Fig. 4 A, ii). Borosilicate glass electrodes (outer diameter = 1.2 mm, inner diameter = 0.69 mm, length = 100 mm; Sutter Instruments) were pulled with a horizontal puller (P97; Sutter Instruments), and a small crystal of calcium-sensitive dye (Calcium Green-1 dextran, Potassium Salt 3000 MW Anionic; Molecular Probes, Life Technologies) was loaded in its tip (71). The crystal was set in place by hand, letting it dissolve for a few seconds in the neck of the pedunculus of the HB. Successive washes with Ringer’s solution were carried out to clean the dye that remained outside the tissue.
Experimental procedures.
Optical imaging experiments started 2 h after dye application. Experiments were performed inside a cage completely covered with black cloth to prevent undesired visual stimulation. During calcium imaging recordings, the crabs were held with half of their body submerged in a container with brackish water and the recorded neuropils under continuous superfusion with crab Ringer’s solution. Recordings began after the animal had remained visually undisturbed for 15 min in the recording position. Similar to behavioral experiments, controlled phasic presentation of the TC during experiments was achieved by means of illumination from above or below the container. The crab and container were surrounded by white walls that provided the TC and that could be manually changed. The visual stimulus was an analog of the VDS used in behavioral experiments (size = 7.5–2.25 cm, distance to the crab was adjusted to present the same angular size to the animal as in the behavior experiments). VDS stimulation was carried out through the contralateral eye. In all animals, we stimulated the right eye and recorded signals in the left eyestalk’s HB. The Mec that served as a control stimulation, consisted of a pulse of nitrogen gas (10 psi) delivered using a PV830 Pneumatic Pico Pump (WPI) by positioning a tip (1-mm aperture) at ∼0.5 cm of the midright dorsal carapace backside. Each Mec trial consisted of two 1-s pulses separated by 2 s (Fig. 4 B, iii).
Stimulation protocol.
A training session was run under the imaging setup conditions. The training protocol consists of 15 VDS trials, each trial with the same structure described before in behavioral experiments (Fig. 4 B, ii). When the training session finished, the illumination inside the setup was turned off, aiming to mimic the removal of the animals from the container without affecting the preparation (72). For the test session, 25 min after the end of the training session, the illumination from below the container was turned on (mimicking the reinstallation of the animal in the container); 5 min later, a VDS trial was run as a test trial. Mec trials were run before training (MecPRE) and after testing (MecPOST). For context-specificity evaluation, the white walls surrounding the animal and container were changed to black and white striped walls during the 25-min intersession period. Illumination, Mec, and VDS stimulation were controlled with the acquisition software TillvisION v4.01 (TILL Photonics Imaging System Software; TILL Photonics, GmbH). It is worth mentioning that crabs receiving a training protocol while being physically restrained, as they were in the imaging experiments, are able to learn and exhibit normal long-term memory (29).
Acquisition and data analysis of calcium imaging.
Optical recordings were carried out using a Nikon E-600 microscope; a 10×, 0.30-N.A. water immersion objective (Nikon); and a calcium imaging system (TILL Photonics). Images were taken under 499 nm excitation light provided by Polychrome IV (TILL Photonics) at a rate of five images per second (5 Hz). Imaging measurements lasted 40 s (200 frames). Exposure times varied across preparations depending on the intensity of staining and aimed at getting signals within the linear range of the imaging system. The dichroic mirror was 525 nm, and the emission filter was 530 nm long pass. Images were taken with a CCD camera (12 bits, 640 × 480 pixels, Imago; TILL Photonics) and binned on chip to 320 × 240 pixels, resulting in a pixel size of 3 × 3 μm. Data acquisition was done using TillvisION v4.01 software. Imaging data analysis was done using custom-written macro routines in Fiji/ImageJ. Raw images were spatially filtered with a 3 × 3 median filter to remove “salt-and-pepper” noise. Displacements within and between measurements (trials) were corrected before analysis using the ImageJ Image Stabilizer plug-in (www.cs.cmu.edu/∼kangli/code/Image_Stabilizer.html). The analysis of the calcium signals was based on%ΔF/F calculated for each pixel. F represents the basal fluorescence, and ΔF represents the difference between the basal fluorescence and the fluorescence measured during the frame of interest. The basal fluorescence was calculated for each measurement by averaging five frames before stimulus onset, or before spontaneous activity in cases in which the stimulus onset was superimposed on noninduced activity. For visualization of the spatial pattern of the calcium signal, we calculated false color-coded images that represent the summation of the %ΔF/F inside the areas of interest (AOIs) along all frames during the VDS or Mec stimulation. Animals or trials were removed when movement could not be corrected in the videos.
AOIs corresponding to the vertical and medial lobes of the HB for each crab were semiautomatically created using Fiji. Median filtered images (5 × 5) representing the average %ΔF/F during the first 2 s after the stimulus onset were binarized by setting a threshold of 3–5 SDs above baseline. A median filter (radius = 2 pixels) was applied on the binarized image showing active pixels during stimulation. A mask was created, resulting from considering every pixel that was active in at least one trial. The two AOIs, vertical lobe, and medial lobes, were delimited inside the mask by hand based on morphological inspection of the raw fluorescence images. The change of activity across trials was calculated by averaging the %ΔF/F values of all pixels inside the AOIs. Dye bleaching during each measurement was corrected by subtracting a log curve [y = a × ln(bx), log-fitting option of Fiji] fitted to the brightness profile excluding the time window where the stimuli (or high spontaneous signals) were present.
Statistical Analysis.
For statistical tests, the Kolmogorov–Smirnov test for normality was done to check normal distributions. For all statistical tests, significance was measured against an α value of 0.05. No statistical methods were used to predetermine sample sizes; however, sample sizes are similar to the sample sizes reported in previous publications (29, 45, 69, 73). Data collection and analysis were not performed blinded to the conditions of the experiments. Crabs from the collective tanks were taken randomly without a defined method.
Behavioral experiments, memory retention criterion.
The escape response values resulting from the 9-s integration that lasted during each VDS stimulation (Fig. 3 A, i) were further transformed by log2 to get a normal data distribution. The training session was analyzed with a one-way repeated-measures ANOVA using trials as the within-subjects factor. A Greenhouse–Geisser correction was used for violation of sphericity. Animals that did not respond in the first VDS trial or died before the end of all experiment sessions were excluded from analysis. Statistical analysis of memory expression was assessed by focusing on test trial scores and looking for statistical differences between response levels of the TR and UN. In this memory model, the TR is said to express memory when its escape response during the test trial is lower than the escape response of its respective UN, which has had exactly the same manipulation and treatment, with the exception of the training itself (29, 73). When two UN-TR pairs were compared (i.e., four groups), we used a one-way ANOVA Welch test for unequal variances, followed by Games–Howell post hoc comparisons. The data shown in the figures correspond to mean ± SEM.
Optical imaging.
For calculation of the neuronal calcium responses of each AOI, the summation of positive %ΔF/F values during VDS (frames 116–161) or Mec (frames 26–55) was calculated for each trial. Similar to activity rates defined in other works (74), we defined the CSR as the change in calcium response from trial 1 to trial “n” toward the stimulus divided by total calcium response: CSR = (response trial n − response trial 1)/(response trial n + response trial 01). A negative CSR means a reduced response (−1 for a complete reduction of the response), a positive CSR means an increased response, and CSR = 0 means no change in the response from trial 1 to trial n. Animals that did not show calcium responses to both the first VDS and Mec were excluded. For CSR analysis, one-way ANOVAs were conducted and a Tukey HSD post hoc test was performed to compare between groups. Homogeneity was evaluated by Levene’s test. The final training trials block (13–15 trials) (Fig. S1) was calculated as the average of the CSR of the last three trials of the training that were available. The data shown in the figures correspond to CSR mean ± SEM.
For analysis of noninduced calcium responses, linear regression and covariance analysis (Fig. S2) were done in QtiPlot. According to QtiPlot, ; for a number of points N, the function was computed between −N/2 and N/2. The power spectrum was computed in Fiji. Analyses were done using Excel (Microsoft) with analysis plug-ins (Real Statistics Using Excel Resource Pack software, Release 4.3; www.real-statistics.com), Fiji, QtiPlot (www.qtiplot.com), and STATISTICA 8.0 (StatSoft, Inc.).
Fig. S2.
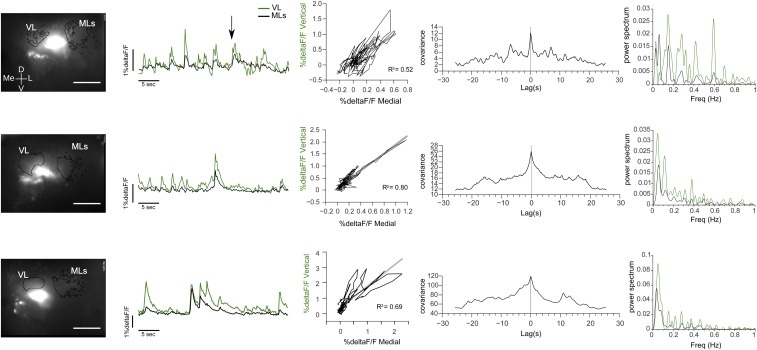
Spontaneous activity in the HBs. Examples of trained animals showing calcium transients in the absence of stimuli. (Left) Pictures of the registered area showing the AOI of the vertical lobe (VL) and medial lobes (MLs) from which the %ΔF/F was obtained. (Scale bar: 200 μm.) (Right) %DeltaF/F registered in the absence of explicit stimulation, correlation and cross-correlation, and power spectrum for the signals obtained in the vertical and medial lobes of the HBs. D, dorsal; Freq, frequency; L, lateral; Me, medial; V, ventral.
Code Availability.
Fiji/ImageJ macro code use for calcium imaging analyses and a brief user guide can be requested from the authors.
Data Availability.
The data that support the findings of this study are available from the corresponding author upon request.
Discussion
Like in other crustaceans, the basic structure of the Neohelice’s HBs and the immunoreactive pattern obtained based on p-CaMKII-α, SYN, and allatostatin resemble the profile of the MBs in insects. Moreover, adult neurogenesis among the intrinsic elements of the HBs further supports this interpretation. Understanding the morphology of Neohelice’s HBs allowed us to start uncovering its role in memory processes. Our in vivo calcium imaging results indicate that the HBs undergo stimulus-specific changes during training that last at least until a short-term test 0.5 h after training. Moreover, plasticity might support the context-specific association observed in the behavior.
HBs in Neohelice: Structure and Homology with Insect MBs.
Whether the crustacean HBs are homologous to the insect MBs has been a subject of long debate (25, 31). If demonstrated, the proposed homology would constitute important support in favor of the hypothesis for a common origin of the high-order memory centers in bilateral animals (6, 12). Strong evidence supporting the genealogical correspondence between MBs and HBs is the shared ground pattern of neuronal organization and the similar protein expression profile associated with memory processes that have been described recently (12, 19). The present study provides morphological data and immunoreactivity patterns that reveal the similarity of structures between insect MBs and Neohelice HBs.
Bilobed HBs with neuropil regions I and II formed by thousands of microglomeruli were described in crayfish and lobster (31, 32, 46). These lobes were also manifested in the crab by the immunoreactivity against SYN (medial lobe I and medial lobe II; Fig. 2A), showing structures compatible with spherical microglomeruli (19, 31, 35). The existence of bilobed HBs in the lateral protocerebrum of Neohelice has been previously suggested (33, 34). However, Golgi impregnations (Fig. 1C) unveiled the presence of another lobe, the vertical lobe, termed thus in correspondence to insect MBs (15). Consequently, the Golgi impregnations exposed HBs that closely resemble the calyxless MBs observed in some insects (15) (further discussion is provided in SI Comment About Nomenclature). The parallel fibers of gc constitute the pedunculus that bifurcates to give rise to the vertical lobe, medial lobe I, and medial lobe II (26, 31) (Fig. 1 D and E). The expression of p-CAMKII-α revealed also a view of the vertical and medial lobes, the HB small gc, and the pedunculus (Fig. 2F).
Neuronal elements previously described in crayfish HBs are parasol cells: approximately 200 neurons that are activated by olfactory, visual, and mechanical stimulation (26, 47). Parasol cells have been proposed as HBs’ extrinsic neurons, resembling the output channel of MBs that receive multimodal sensory information from different Kenyon cells (19, 26). The intrinsic elements of the HBs shown in the present work appear not to resemble parasol cells. Unlike parasol cells that exclusively arborize within only one lobe of the HB, most of the fibers of gc arborize in the vertical lobe, the medial lobe I, and the medial lobe II (Fig. 1 D and E). In addition, big somata, as expected for parasol cells (26), are found neighboring the HB-gc cluster and do not present p-CaMKII-α immunoreactivity (Fig. 2F).
The Golgi impregnations also revealed claw-like specializations indicative of postsynaptic sites in the medial lobes (Fig. 1F) resembling both clawed Kenyon cells of MBs and intrinsic elements of HBs in hermit crabs (12, 15, 19). MBs’ clawed Kenyon cells (also called class II or γ) are highly conserved across insect species and are proposed to represent an ancestral cell type (35). The presence of claw specializations in the medial lobes of Neohelice suggests input areas. Supporting the concept of multimodality of HBs, these lobes receive neuronal processes of the BLG2 neurons (Fig. 1G), which respond to mechanical and visual stimulation, integrate binocular visual information, and reduce their response to the VDS during training and testing sessions (44, 48). The interaction between these and intrinsic elements of HBs in the medial lobes may constitute a place where visual stimuli are integrated with other sensory modalities similar to what occurs in Drosophila MBs, where associative memories are processed in a centralized circuit that receives both visual and olfactory inputs (49–51).
Another concordance supporting homology is that adult neurogenesis occurs in both MBs and HBs (6, 38). The BrdU-positive labeling in the HB-gc cluster (Fig. 2E) is consistent with descriptions of adult neurogenesis in other crabs and in crayfish in a cluster that is called cluster A (20, 21, 31). Interestingly, a brain center that generates new neurons during adult life is just one of several similarities between the MBs and the hippocampus. In the vertebrate hippocampus, new granule cells add mossy fibers in parallel to the existing ensemble. In MBs and HBs, gc provide new parallel fibers to their neuropils (6, 20, 52). Several years after the seminal studies comparing MBs and vertebrate cortical structures on anatomical and physiological grounds (14), the remarkable work by Tomer et al. (7) added invaluable support for the hypotheses of a common origin of these higher brain centers. By the detailed comparison of the overall molecular topography of the brain anlage, the expression profile of several genes in forebrain-specific subregions during development, and the “molecular fingerprint” of vertebrate telencephalon and annelid MB neuron types, they showed that the vertebrate pallium and the annelid MBs most likely evolved from the same sensory associative brain center that was already present in their bilaterian ancestors (7). This conclusion is in accordance with the view that the ancient architecture of the paleo- and archipallium found in all vertebrates might have been present in the urbilaterian brain. The hippocampus’s dentate gyrus, consisting of only one layer of principal neurons, noticeably resembles MBs: densely packed, basophilic somata of the gc that send out parallel processes intersected by afferent and efferent networks (14). Accordingly, recent studies by Wolff and Strausfeld (6, 12, 19) sustain that several anatomical items also support a genealogical correspondence. These items include, for instance, an extension from each intrinsic cell body of a long process always bearing presynaptic and postsynaptic specializations along its length, the intersection of the ensemble of such processes by sequential domains of afferent terminals and efferent dendrites forming an approximately orthogonal matrix, and the presence of recurrent interneurons within the ensemble. According to the authors’ view, this ground pattern organization of HBs and MBs of arthropods is similar to the mammalian hippocampal structure, where the intrinsic cell bodies, the granule cell layer, send parallel axons that form an orthogonal matrix, interacting with local interneurons and afferent inputs from the entorhinal cortex, and projecting to the subiculum and back to the entorhinal cortex (6). Remarkably, in vertebrate archipallium structures, in MBs, and in HBs, several orthologous genes whose proteins play a critical role in the plasticity associated with memory processes (including p-CaMKII) show similar expression patterns (6). The present study tested the proposed similarity in functions (i.e., high-order memory processes) of MBs and HBs (13, 18, 25), a feature that adds functional evidence to the homology hypothesis (6, 22, 53).
Neural Activity in the HBs.
Ex vivo recordings and neuroanatomical studies made in other decapods substantiate that the HBs have multimodal sensory inputs (25, 54). In the present study, we found that, like in medulla terminalis (45), calcium signals in the HBs are elicited by both mechanical and visual stimulation. In the medulla terminalis, proposed also to function as a region of higher order multimodal integration (25, 31, 32), we recently found that training induces specific changes in the neuronal responses triggered by the learned VDS (45); these changes correlate with memory persistence but not with long-term memory expression (55). The specific neuronal inputs to HBs that lead to the responses of both Mec and visual stimuli still need to be explored. The lobula giant neurons, which innervate the medial lobes (Fig. 1G) and respond to mechanical and visual inputs, are promissory candidates for this role (25, 43). Accordingly, and like in MBs of some insects, postsynaptic sites on the intrinsic cells of the medial lobes were found (12, 15, 19) (Fig. 1F).
Spontaneous and stimuli-triggered calcium signals in vertical and medial lobes were synchronized and highly correlated (Fig. S2). This finding was expected based on the Golgi impregnations showing the bifurcated structure of the intrinsic elements of the HB (Fig. 1 C–E) and from functional imaging studies on the axonal branches of the MB neurons (56). However, we also found sporadic spontaneous calcium signal transients that took place separately in both HB lobes (Fig. 4C and Fig. S2; arrows over traces in both figures). This observation might represent distinct inputs in the different lobes. Differential participation of the vertical and medial lobes in the activity elicited by different stimuli is also expected because it happens in the MBs (8, 50, 57). Either two-photon microscopy or a higher definition in the recording activity within each lobe would be necessary to shed light on this attribute of Neohelice HBs. In addition, HB activity presented slow oscillation components (Fig. S2). Oscillations have been described in both MBs of several insects and in HBs (47, 58). Interestingly, we did not observe such oscillations in the other protocerebral neuropil recently evaluated, the medulla terminalis (45).
Memory-Related Neural Plasticity in the Neohelice’s HBs.
The HBs of crustaceans were long proposed to be centers of higher order integration that play a role in memory processes. The present study represents in vivo physiological evidence supporting that view (6, 19, 25, 26, 31). Here, we found that calcium signals triggered in the HB by the VDS decreased in both medial and vertical lobes during training. This decrease persisted at least until a short-term test 0.5 h after training. The decay of the CSR index was less pronounced in the vertical lobe than in the medial lobes, a difference that cannot be explained by disparity in the initial calcium signal to the training stimulus (all trained animals’ VDS trial 1 summation %ΔF/F; mean ± SEM: medial lobes 46.38 ± 21.21, vertical lobe 41.12 ± 17.60; t test for dependent samples: t[12] = 0.77, P = 0.45). The minor decay in the calcium signals of the vertical lobe could reflect, like in MBs (57), a differential involvement of the different input/output regions of the HB in this memory process.
The interpretation that the changes were induced by training rather than by a time-dependent decrease in the signals, such as modifications in dye distribution, photobleaching, or cytotoxicity, is supported by the fact that there were no changes between the responses elicited by the Mec before and after training. This view is also supported by the lack of changes in the UNs and by the recovery of the calcium signals elicited in response to the VDS when crabs were tested in a context different from the one used during training (Fig. 5). In addition, the changes in CSR are not likely explained by motion artifacts because escape responses are suppressed when the animals are held in the setup (44) and by the fact that the Mec triggered calcium signals but not escape responses (45).
MBs of insects are key structures for context-dependent memories. Their intrinsic neurons are required for visual memories, where their contribution to memory is proposed to involve linking items within a contextual framework (8, 15, 16, 51, 59). As previously mentioned, in Neohelice, the lobula giant neurons play a role in short- and long-term memory of the VDS stimulus; however, they show reduced responses to the learned VDS independent of a context-specific shift between training and testing (30). In this scenario, the HBs seem to be candidates for linking the VDS with the context, allowing memory expression when the VDS is presented in the learned context. In this study, we used a training protocol that yields context-specific memory expression in the short term (Fig. 3). Using the same training protocol for the calcium imaging experiments, we found that the Ca2+ signal in response to the VDS was recovered in trained animals when the context was modified between training and testing (TR-dCTX; Figs. 4 and 5).
The present results constitute functional evidence in favor of a role of the HBs in memory processes that, similar to MBs (8, 15, 16), show neuronal changes correlated with a context-stimulus–specific association of the acquired information. These findings are in agreement with the historically proposed function for the HBs (25).
The hippocampus and MBs are key structures in processes that form long-term internal representations in their intrinsic neurons and categorize stimuli with respect to their indicative functions as cue and context (8, 9). The present study adds functional data to endorse the incorporation of crustacean HBs into this group, thus supporting the hypothesis for a common origin of high-order memory centers in bilateral animals (6, 7).
SI Comment About Nomenclature
Based on the structure of the intrinsic elements of HBs described here (i.e., the presence of small basophilic cell bodies that project parallel fibers constituting the pedunculus with bifurcations that originate vertical and medial lobes), the proposed nomenclature is taken from the one used for calyxless MBs described in some insects. The nomenclature has also taken into account classical studies (25, 31) that described a bilobed HB, neuropil I and II (here, medial lobes I and II) and its associated cluster, A or HB. Hermit crabs’ HBs (Decapoda, Coenobita) are an example of the diverse organization of these neuropils in crustaceans, with cap-and-core structures that we cannot found in Neohelice (Decapoda, Brachyura), conceivably due to their different lifestyles (19, 32). In Neohelice, we were not able to stain (Alexa-dextrans) from olfactory lobes to lateral protocerebrum retrogradely, perhaps due to both the long distance and the very compact organization of the protocerebral tracts. The view of the vertical lobe as an integral part of the HB’s intrinsic elements, consistent with the finding of synchronized activity between HB lobes (Fig. S2), compels us to propose the present nomenclature. Certainly, the names vertical and medial lobes do not reflect the nature posture of the eyestalks in this semiterrestrial crab with movable eyes (61). The Neohelice HBs’ vertical and medial lobes appear to be the glomeruli pedunculares, medial and lateral groups, described in the true crab Carcinus maenas (Decapoda, Brachyura) by Hanström (18), neuropils proposed already at that time as homologs of the calyces of the MBs (18). Indeed, the lateral group (here, medial lobes) was considered in that seminal study as more intimately connected with the visual paths and, consequently, highly developed in decapods with normal eyes. It is noteworthy that MBs that lack calyces correlate with aquatic species secondarily lacking antennal lobes (15, 35), whereas decapods such as Neohelice have large olfactory lobes (25). In summary, even though we are still far from fully understanding the circuitry of inputs and outputs of the Neohelice HB, the present results allow us to compare its basic structure with the one described in other related species.
Experimental Procedures
Crabs used were N. granulata (previously C. granulata) collected in the field and kept in the laboratory as described in SI Materials and Methods. The VDS used in behavioral and imaging experiments consisted of the displacement of a black rectangle passing above the animal in a 90° clockwise and anticlockwise excursion that was completed in 2.2 s. For in vivo calcium imaging experiments, a crystal of Calcium Green-1 dextran (Molecular Probes, Life Technologies) was stabbed in the neck of the pedunculus of the HB within the left eyestalk of the crab. Visual stimulation was done through the contralateral eye. The Mec consisted of pulses of nitrogen gas in the midright backside of the dorsal carapace. In dCTXs, the walls surrounding the animal were changed before testing. Imaging was done with a conventional epifluorescence microscope. Fluorescence data were analyzed with custom-written macros in Fiji/ImageJ (60). Research was conducted in accordance with the Ethical Reference Frame for Biomedical Investigations of the Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina, equivalent to the standard procedures for animal care and use of the US NIH. Additional information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Professor G. Galizia for support in developing the calcium imaging preparation in Neohelice, Professor Haydée Viola for the p-CaMKII-α antibody, Dr. Sergio I. Nemirovsky for manuscript proofreading, and Angel Vidal and José Clemente for technical assistance. The constructive advice of the two referees is thankfully acknowledged. This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas of Argentina (CONICET) and Universidad de Buenos Aires (UBA), Argentina (UBA: 20020110100071, CONICET: PIP 11220120100170CO, and Agencia Nacional de Promoción Científica y Tecnológica: PICT-2013-1020).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1612418113/-/DCSupplemental.
References
- 1.Menzel R. Phylogeny and evolution: On comparing species at multiple levels. In: Roediger HL III, Dudai Y, Fitzpatrick SM, editors. Science of Memory: Concepts. Oxford Univ Press; New York: 2007. [Google Scholar]
- 2.Dudai Y, Morris RG. Memorable trends. Neuron. 2013;80(3):742–750. doi: 10.1016/j.neuron.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 3.Menzel R. Memory dynamics in the honeybee. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:323–340. [Google Scholar]
- 4.Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97(6):1520–1533. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- 5.Glanzman DL. Common mechanisms of synaptic plasticity in vertebrates and invertebrates. Curr Biol. 2010;20(1):R31–R36. doi: 10.1016/j.cub.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff GH, Strausfeld NJ. Genealogical correspondence of a forebrain centre implies an executive brain in the protostome-deuterostome bilaterian ancestor. Philos Trans R Soc Lond B Biol Sci. 2016;371(1685):20150055. doi: 10.1098/rstb.2015.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomer R, Denes AS, Tessmar-Raible K, Arendt D. Profiling by image registration reveals common origin of annelid mushroom bodies and vertebrate pallium. Cell. 2010;142(5):800–809. doi: 10.1016/j.cell.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 8.Menzel R. The insect mushroom body, an experience-dependent recoding device. J Physiol Paris. 2014;108(2-3):84–95. doi: 10.1016/j.jphysparis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie S, et al. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 2014;83(1):202–215. doi: 10.1016/j.neuron.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 11.Cayre M, et al. Neurogenesis in adult insect mushroom bodies. J Comp Neurol. 1996;371(2):300–310. doi: 10.1002/(SICI)1096-9861(19960722)371:2<300::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Wolff GH, Strausfeld NJ. Genealogical correspondence of mushroom bodies across invertebrate phyla. Curr Biol. 2015;25(1):38–44. doi: 10.1016/j.cub.2014.10.049. [DOI] [PubMed] [Google Scholar]
- 13.Kenyon FC. The meaning and structure of the so-called “mushroom bodies” of the hexapod Brain. Am Nat. 1896;30(356):643–650. [Google Scholar]
- 14.Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Mem. 1998;5(1-2):11–37. [PMC free article] [PubMed] [Google Scholar]
- 15.Strausfeld NJ, Sinakevitch I, Brown SM, Farris SM. Ground plan of the insect mushroom body: Functional and evolutionary implications. J Comp Neurol. 2009;513(3):265–291. doi: 10.1002/cne.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Wolf R, Ernst R, Heisenberg M. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature. 1999;400(6746):753–756. doi: 10.1038/23456. [DOI] [PubMed] [Google Scholar]
- 17.Mizunami M, Yokohari F, Takahata M. Further exploration into the adaptive design of the arthropod “microbrain”: I. Sensory and memory-processing systems. Zoolog Sci. 2004;21(12):1141–1151. doi: 10.2108/zsj.21.1141. [DOI] [PubMed] [Google Scholar]
- 18.Hanström B. The olfactory centers in Crustaceans. J Comp Neurol. 1925;38(3):221–250. [Google Scholar]
- 19.Wolff G, Harzsch S, Hansson BS, Brown S, Strausfeld N. Neuronal organization of the hemiellipsoid body of the land hermit crab, Coenobita clypeatus: Correspondence with the mushroom body ground pattern. J Comp Neurol. 2012;520(13):2824–2846. doi: 10.1002/cne.23059. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt M. The olfactory pathway of decapod crustaceans--an invertebrate model for life-long neurogenesis. Chem Senses. 2007;32(4):365–384. doi: 10.1093/chemse/bjm008. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan JM, Beltz BS. Adult neurogenesis in the central olfactory pathway in the absence of receptor neuron turnover in Libinia emarginata. Eur J Neurosci. 2005;22(10):2397–2402. doi: 10.1111/j.1460-9568.2005.04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papini MR. Pattern and process in the evolution of learning. Psychol Rev. 2002;109(1):186–201. doi: 10.1037/0033-295x.109.1.186. [DOI] [PubMed] [Google Scholar]
- 23.Campbell CB, Hodos W. The concept of homology and the evolution of the nervous system. Brain Behav Evol. 1970;3(5):353–367. doi: 10.1159/000125482. [DOI] [PubMed] [Google Scholar]
- 24.Butler AB. Homology and homoplasy. In: Squire L, editor. Encyclopedia of Neuroscience. Vol 4. Academic; Oxford: 2009. pp. 1195–1199. [Google Scholar]
- 25.Sandeman DC, Henning M, Harzsch S. Adaptive trends in Malacostracan brain form and function related to behavior. In: Derby CD, Thiel M, editors. Nervous Systems and Control of Behavior. Vol 3. Oxford Univ Press; Ney York: 2014. pp. 11–45. [Google Scholar]
- 26.McKinzie ME, Benton JL, Beltz BS, Mellon D. Parasol cells of the hemiellipsoid body in the crayfish Procambarus clarkii: Dendritic branching patterns and functional implications. J Comp Neurol. 2003;462(2):168–179. doi: 10.1002/cne.10716. [DOI] [PubMed] [Google Scholar]
- 27.Menzel R. The honeybee as a model for understanding the basis of cognition. Nat Rev Neurosci. 2012;13(11):758–768. doi: 10.1038/nrn3357. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado H. Crustacean as model to investigate memory illustrated by extensive behavioral and physiological studies in Chasmagnathus. In: Wiese K, editor. The Crustacean Nervous System. Springer; Berlin: 2002. pp. 314–327. [Google Scholar]
- 29.Tomsic D, Romano A. 2013. A multidisciplinary approach to learning and memory in the crab neohelice (Chasmagnathus) granulata. Invertebrate Learning and Memory, Handbooks of Behavioral Neurosciences, eds Menzel R, Benjamin PR (Academic, Amsterdam), pp 337–355.
- 30.Sztarker J, Tomsic D. Brain modularity in arthropods: Individual neurons that support “what” but not “where” memories. J Neurosci. 2011;31(22):8175–8180. doi: 10.1523/JNEUROSCI.6029-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaustein DN, Derby CD, Simmons RB, Beall AC. Structure of the brain and medulla terminalis of the spiny lobster Panulirus argus and the crayfish Procambarus clarkii, with an emphasis on olfactory centers. J Crustac Biol. 1988;8(4):493–519. [Google Scholar]
- 32.Sullivan JM, Beltz BS. Neural pathways connecting the deutocerebrum and lateral protocerebrum in the brains of decapod crustaceans. J Comp Neurol. 2001;441(1):9–22. doi: 10.1002/cne.1394. [DOI] [PubMed] [Google Scholar]
- 33.Frenkel L, et al. Neuroanatomical distribution of angiotensin-II-like neuropeptide within the central nervous system of the crab Chasmagnathus; physiological changes triggered by water deprivation. Cell Tissue Res. 2010;341(1):181–195. doi: 10.1007/s00441-010-0990-8. [DOI] [PubMed] [Google Scholar]
- 34.Hepp Y, Tano MC, Pedreira ME, Freudenthal RA. NMDA-like receptors in the nervous system of the crab Neohelice granulata: A neuroanatomical description. J Comp Neurol. 2013;521(10):2279–2297. doi: 10.1002/cne.23285. [DOI] [PubMed] [Google Scholar]
- 35.Farris SM. Evolution of insect mushroom bodies: Old clues, new insights. Arthropod Struct Dev. 2005;34(3):211–234. [Google Scholar]
- 36.Medan V, Oliva D, Tomsic D. Characterization of lobula giant neurons responsive to visual stimuli that elicit escape behaviors in the crab Chasmagnathus. J Neurophysiol. 2007;98(4):2414–2428. doi: 10.1152/jn.00803.2007. [DOI] [PubMed] [Google Scholar]
- 37.Krieger J, et al. Comparative brain architecture of the European shore crab Carcinus maenas (Brachyura) and the common hermit crab Pagurus bernhardus (Anomura) with notes on other marine hermit crabs. Cell Tissue Res. 2012;348(1):47–69. doi: 10.1007/s00441-012-1353-4. [DOI] [PubMed] [Google Scholar]
- 38.Cayre M, Scotto-Lomassese S, Malaterre J, Strambi C, Strambi A. Understanding the regulation and function of adult neurogenesis: Contribution from an insect model, the house cricket. Chem Senses. 2007;32(4):385–395. doi: 10.1093/chemse/bjm010. [DOI] [PubMed] [Google Scholar]
- 39.Romano A, et al. Lessons from a crab: Molecular mechanisms in different memory phases of Chasmagnathus. Biol Bull. 2006;210(3):280–288. doi: 10.2307/4134564. [DOI] [PubMed] [Google Scholar]
- 40.Maldonado H, Romano A, Tomsic D. Long-term habituation (LTH) in the crab Chasmagnathus: A model for behavioral and mechanistic studies of memory. Braz J Med Biol Res. 1997;30(7):813–826. doi: 10.1590/s0100-879x1997000700001. [DOI] [PubMed] [Google Scholar]
- 41.Pereyra P, González Portino E, Maldonado H. Long-lasting and context-specific freezing preference is acquired after spaced repeated presentations of a danger stimulus in the crab Chasmagnathus. Neurobiol Learn Mem. 2000;74(2):119–134. doi: 10.1006/nlme.1999.3945. [DOI] [PubMed] [Google Scholar]
- 42.Suárez LD, Smal L, Delorenzi A. Updating contextual information during consolidation as result of a new memory trace. Neurobiol Learn Mem. 2010;93(4):561–571. doi: 10.1016/j.nlm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Fustiñana MS, Carbó Tano M, Romano A, Pedreira ME. Contextual Pavlovian conditioning in the crab Chasmagnathus. Anim Cogn. 2013;16(2):255–272. doi: 10.1007/s10071-012-0570-2. [DOI] [PubMed] [Google Scholar]
- 44.Tomsic D, Berón de Astrada M, Sztarker J. Identification of individual neurons reflecting short- and long-term visual memory in an arthropodo. J Neurosci. 2003;23(24):8539–8546. doi: 10.1523/JNEUROSCI.23-24-08539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maza FJ, Locatelli FF, Delorenzi A. Neural correlates of expression-independent memories in the crab Neohelice. Neurobiol Learn Mem. 2016;131:61–75. doi: 10.1016/j.nlm.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Polanska MA, Yasuda A, Harzsch S. Immunolocalisation of crustacean-SIFamide in the median brain and eyestalk neuropils of the marbled crayfish. Cell Tissue Res. 2007;330(2):331–344. doi: 10.1007/s00441-007-0473-8. [DOI] [PubMed] [Google Scholar]
- 47.Mellon D., Jr Electrophysiological evidence for intrinsic pacemaker currents in crayfish parasol cells. PLoS One. 2016;11(1):e0146091. doi: 10.1371/journal.pone.0146091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sztarker J, Tomsic D. Binocular visual integration in the crustacean nervous system. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190(11):951–962. doi: 10.1007/s00359-004-0551-2. [DOI] [PubMed] [Google Scholar]
- 49.Farris SM, Van Dyke JW. Evolution and function of the insect mushroom bodies: Contributions from comparative and model systems studies. Curr Opin Insect Sci. 2015;12:19–25. [Google Scholar]
- 50.Vogt K, et al. Direct neural pathways convey distinct visual information to Drosophila mushroom bodies. eLife. 2016;5:e14009. doi: 10.7554/eLife.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogt K, et al. Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. eLife. 2014;3:e02395. doi: 10.7554/eLife.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan JM, Benton JL, Sandeman DC, Beltz BS. Adult neurogenesis: A common strategy across diverse species. J Comp Neurol. 2007;500(3):574–584. doi: 10.1002/cne.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strausfeld NJ, Hirth F. Homology versus convergence in resolving transphyletic correspondences of brain organization. Brain Behav Evol. 2013;82(4):215–219. doi: 10.1159/000356102. [DOI] [PubMed] [Google Scholar]
- 54.Yagodin S, Collin C, Alkon DL, Sheppard NF, Jr, Sattelle DB. Mapping membrane potential transients in crayfish (Procambarus clarkii) optic lobe neuropils with voltage-sensitive dyes. J Neurophysiol. 1999;81(1):334–344. doi: 10.1152/jn.1999.81.1.334. [DOI] [PubMed] [Google Scholar]
- 55.Delorenzi A, et al. Memory beyond expression. J Physiol Paris. 2014;108(4-6):307–322. doi: 10.1016/j.jphysparis.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008;28(17):4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis RL. Traces of Drosophila memory. Neuron. 2011;70(1):8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang SA, Campusano JM, Su H, O’Dowd DK. Drosophila mushroom body Kenyon cells generate spontaneous calcium transients mediated by PLTX-sensitive calcium channels. J Neurophysiol. 2005;94(1):491–500. doi: 10.1152/jn.00096.2005. [DOI] [PubMed] [Google Scholar]
- 59.Mizunami M, Weibrecht JM, Strausfeld NJ. Mushroom bodies of the cockroach: Their participation in place memory. J Comp Neurol. 1998;402(4):520–537. [PubMed] [Google Scholar]
- 60.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sztarker J, Strausfeld NJ, Tomsic D. Organization of optic lobes that support motion detection in a semiterrestrial crab. J Comp Neurol. 2005;493(3):396–411. doi: 10.1002/cne.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ott SR. Confocal microscopy in large insect brains: Zinc-formaldehyde fixation improves synapsin immunostaining and preservation of morphology in whole-mounts. J Neurosci Methods. 2008;172(2):220–230. doi: 10.1016/j.jneumeth.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 63.Sztarker J, Strausfeld N, Andrew D, Tomsic D. Neural organization of first optic neuropils in the littoral crab Hemigrapsus oregonensis and the semiterrestrial species Chasmagnathus granulatus. J Comp Neurol. 2009;513(2):129–150. doi: 10.1002/cne.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delorenzi A, et al. 2004. Visual learning and memory assessed by in vivo calcium imaging from individual interneurons in the crab Chasmagnathus. 2004 Neuroscience Meeting Planner (Society for Neuroscience, San Diego), Program No. 553.1.
- 65.Schmidt M, Harzsch S. Comparative analysis of neurogenesis in the central olfactory pathway of adult decapod crustaceans by in vivo BrdU labeling. Biol Bull. 1999;196(2):127–136. doi: 10.2307/1542558. [DOI] [PubMed] [Google Scholar]
- 66.Pérez-Cuesta LM, Hepp Y, Pedreira ME, Maldonado H. Memory is not extinguished along with CS presentation but within a few seconds after CS-offset. Learn Mem. 2007;14(1):101–108. doi: 10.1101/lm.413507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frenkel L, Maldonado H, Delorenzi A. Memory strengthening by a real-life episode during reconsolidation: An outcome of water deprivation via brain angiotensin II. Eur J Neurosci. 2005;22(7):1757–1766. doi: 10.1111/j.1460-9568.2005.04373.x. [DOI] [PubMed] [Google Scholar]
- 68.Pedreira ME, Maldonado H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron. 2003;38(6):863–869. doi: 10.1016/s0896-6273(03)00352-0. [DOI] [PubMed] [Google Scholar]
- 69.Berón de Astrada M, Bengochea M, Sztarker J, Delorenzi A, Tomsic D. Behaviorally related neural plasticity in the arthropod optic lobes. Curr Biol. 2013;23(15):1389–1398. doi: 10.1016/j.cub.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 70.Genovese G, et al. Dopaminergic regulation of ion transport in gills of the euryhaline semiterrestrial crab Chasmagnathus granulatus: Interaction between D1- and D2-like receptors. J Exp Biol. 2006;209(Pt 14):2785–2793. doi: 10.1242/jeb.02308. [DOI] [PubMed] [Google Scholar]
- 71.Galizia CG, Vetter RS. Optical methods for analyzing odor-evoked activity in the insect brain. In: Christensen TA, editor. Frontiers in Neuroscience: Methods in Insect Sensory Neuroscience. CRC Press; Boca Raton, FL: 2004. pp. 349–392. [Google Scholar]
- 72.Pérez-Cuesta LM, Maldonado H. Memory reconsolidation and extinction in the crab: Mutual exclusion or coexistence? Learn Mem. 2009;16(11):714–721. doi: 10.1101/lm.1544609. [DOI] [PubMed] [Google Scholar]
- 73.Pedreira ME, Romano A, Tomsic D, Lozada M, Maldonado H. Massed and spaced training build up different components of long-term habituation in the crab Chasmagnathus. Anim Learn Behav. 1998;26(1):34–45. [Google Scholar]
- 74.Hussaini SA, Menzel R. Mushroom body extrinsic neurons in the honeybee brain encode cues and contexts differently. J Neurosci. 2013;33(17):7154–7164. doi: 10.1523/JNEUROSCI.1331-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.