Significance
Fertilization mechanisms explain broad patterns in the taxonomic distribution of diversity in marine animals, as argued previously for land plants. We argue that fertilization via copulation (or some other significant interaction among adults) permits additional mechanisms of reproductive isolation and smaller population sizes relative to fertilization via the broadcasting of sperm into the water, thus enhancing diversification potential. Maximum-likelihood modeling of paleontological data indicates that the diversity of sperm broadcasters has been limited by diversity-dependent factors for the last 450 million years. In contrast, animals that copulate, etc., were also limited until the Cretaceous and then radiated dramatically, coincident with apparent major changes in marine productivity. Fertilization may have acted synergistically with ecological specialization in promoting diversification, particularly in predators.
Keywords: diversity dependence, Phanerozoic, Mesozoic marine revolution, fertilization, copulation
Abstract
The fossil record of marine animals suggests that diversity-dependent processes exerted strong control on biodiversification: after the Ordovician Radiation, genus richness did not trend for hundreds of millions of years. However, diversity subsequently rose dramatically in the Cretaceous and Cenozoic (145 million years ago–present), indicating that limits on diversification can be overcome by ecological or evolutionary change. Here, we show that the Cretaceous–Cenozoic radiation was driven by increased diversification in animals that transfer sperm between adults during fertilization, whereas animals that broadcast sperm into the water column have not changed significantly in richness since the Late Ordovician (∼450 million years ago). We argue that the former group radiated in part because directed sperm transfer permits smaller population sizes and additional modes of prezygotic isolation, as has been argued previously for the coincident radiation of angiosperms. Directed sperm transfer tends to co-occur with many ecological traits, such as a predatory lifestyle. Ecological specialization likely operated synergistically with mode of fertilization in driving the diversification that began during the Mesozoic marine revolution. Plausibly, the ultimate driver of diversification was an increase in food availability, but its effects on the fauna were regulated by fundamental reproductive and ecological traits.
The fossil record provides direct, historical evidence that can help constrain controls on biodiversification, such as the relative roles of diversity-dependent and diversity-independent processes (1–3). Statistical analyses of the well-preserved fossil record of marine animals support diversity dependence, albeit with a shifting value of equilibrium diversity (4-6, but see refs. 7 and 8 for alternate opinions). Recent analyses suggest that such shifts might be quite substantial: after not trending appreciably for 300 million years (Late Ordovician–Jurassic; ∼458–145 MA), global genus richness approximately doubled in the Cretaceous and Cenozoic (9) (Fig. 1A). Here, we probe the fossil record for insights into this massive shift in global diversity and present a theory on the underlying evolutionary and ecological dynamics. We show that the Cretaceous–Cenozoic (K-Cz) radiation was highly selective, with diversity increase concentrated in animals that copulate or otherwise transfer sperm between interacting adults. In contrast, the aggregate diversity of animals that broadcast sperm into the water column has not increased appreciably since the Ordovician. We argue that directed sperm transfer promoted diversification by permitting additional modes of prezygotic isolation, smaller population sizes, and greater ecological specialization. Previous work has cited increased productivity as a driver of the K-Cz radiation (10–12), but we argue that synergistic interactions between mating strategy and ecology modulated the effects of environmental change on biodiversification.
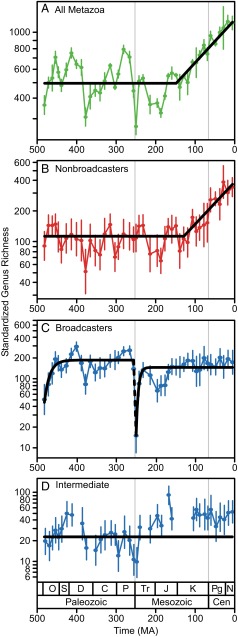
Fig. 1.
Sampling-standardized genus richness of marine animals on a logarithmic scale. (A) All animals, (B) nonbroadcasters (copulators, etc.), (C) broadcasters, and (D) intermediate taxa. Error bars mark ± one SE based on 1,000 SQS replicates. SQS quorum was 0.70 for A and 0.50 for B–D. Black lines mark the trajectory of expected genus richness according to the preferred model, as determined by maximum likelihood methods (A and B: stasis followed by directional trend; C: OU-OU; D: random walk). These data are plotted on arithmetic axes in SI Appendix, Fig. S1, and values are listed in SI Appendix, Tables S1 and S2. C, Carboniferous; Cen, Cenozoic; D, Devonian; J, Jurassic; K, Cretaceous; N, Neogene; O, Ordovician; P, Permian; Pg, Paleogene; S, Silurian; Tr, Triassic.
To characterize diversity history, we generated sampling-standardized genus richness curves from the Paleobiology Database (PBDB; paleobiodb.org) and used maximum likelihood methods (13) to model diversity history. Models of diversity change include random walk, directional change, and two models that are consistent with diversity dependence [stasis and Ornstein-Uhlenbeck (OU-OU), in which diversity approaches and then fluctuates around an equilibrium value]. We also tested two-phase models incorporating a shift between simple models. These methods can rigorously identify shifts in the pattern of diversity history that suggest changes in underlying dynamics.
Fertilization Mechanism
Fertilization mechanisms have never been considered explicitly in analyses of marine fossil biodiversity. However, they have been extensively discussed in the case of land plants, with animal pollination proposed as a key factor in the K-Cz radiation of angiosperms (14–18). Pollination of plants by animals, as opposed to wind, is postulated to permit additional modes of prezygotic reproductive isolation, which could enhance speciation in conjunction with other isolation mechanisms (19). This effect may be strongest when pollinator and plant species are closely coevolved, limiting gene flow among species, as in orchids (20, 21). Pollination could also permit increased rarity because uncommon, widely spaced individuals may still reproduce as long as pollinators are present (18). Interestingly, the greater diversity in terrestrial relative to aquatic habitats has also been attributed in part to differences related to fertilization. For example, motile marine animals do not commonly transmit the gametes of sessile species, as pollinators do on land (22–24). Also, chemical and visual signals are transmitted more effectively in air, which could enhance sexual selection and speciation; it could also enable animals to find mates at greater distances, which could permit greater rarity (22, 23).
Significantly, marine species also vary widely in fertilization strategy. At a broad scale, these strategies are phylogenetically conserved in most higher taxa of Metazoa that are abundant as fossils, so they can be inferred reliably based on taxonomic affiliation (SI Appendix, Reproductive Faunas). Our first group (broadcasters) includes animals that broadcast sperm into the water column. Some species broadcast eggs, whereas others retain them, but we include both in this group because sperm broadcasting is sufficient to limit interactions during mating. As in wind-pollinated plants, fertilization depends on the vicissitudes of transport in a fluid, and fertilization potential decreases dramatically over short distances (25, 26). Thus, individuals often produce copious gametes, and fertilization is enhanced in large, dense populations. Species recognition during reproduction occurs only between sperm (or spermatophore) and egg (or mother) (27), and adult–adult interactions are limited to factors such as timing of gamete release. Broadcasting animals include bivalves, brachiopods, bryozoans, cnidarians, sedentary echinoderms, and several less diverse groups (SI Appendix, Reproductive Faunas). Most broadcasters are nonmotile or not regularly motile and are suspension feeders, as a sedentary lifestyle precludes many other feeding modes. Most fossil cnidarians are corals, many of which capture small living organisms from the water as food (microcarnivory) and may house photosymbionts.
Other animals (nonbroadcasters) transfer sperm directly between adults via copulation or some other interaction (e.g., the male fertilizing eggs as the female releases them). Whether fertilization is internal or external, adults must recognize each other and interact directly, and reproductive isolation is driven not just by gametic incompatibility, habitat separation, and the like but also by behavioral and anatomical barriers at the adult level (e.g., courtship rituals, physical inability to copulate) (28–32). Behavioral barriers to gene flow and sexual selection are generally believed to facilitate speciation (15, 33–35), and reduced Allee effects may permit species to survive, even if rare. To the extent that animals are adept at recognizing and mating with members of their own species, they would be analogous to orchids, where pollen delivery is more species-specific than with many other plants (20, 21). Even if abundance or biomass is limited by resources, a change in the predominant reproductive strategy from broadcasting to nonbroadcasting could permit greater numbers of more specialized species characterized by smaller populations. Adults must find, recognize, and interact with each other directly, so nonbroadcasters tend to be motile, with well-developed senses. Feeding modes vary, but many species are predators, omnivores, or scavengers. Major taxa include arthropods, cephalopods, vertebrates, and some groups of gastropods (Caenogastropoda, Neritopsina, Cocculinoidea, and Heterobranchia) (36).
The final group (intermediate taxa) is composed of mobile animals that broadcast sperm or that belong to taxa in which broadcasting is plesiomorphic and common. Given their mobility, many of these species aggregate before spawning to increase fertilization success (37–39), potentially introducing adult–adult interactions unavailable to more sedentary broadcasters. Some living species exhibit interactions similar to nonbroadcasters, such as mounting during spawning (40–43), or even direct sperm transfer (43–47). Such variation would be impossible to identify in the fossil record, so this category is of necessity more variable than the other two, although most members do broadcast gametes. The intermediate group includes noncopulating gastropods, several minor molluscan taxa, and mobile echinoderms (SI Appendix, Reproductive Faunas). Feeding modes vary and include suspension feeding, deposit feeding, grazing, and predation, although the number of predators is small relative to the nonbroadcasters.
Results
As shown previously (9), sampling-standardized genus richness of the total marine fauna fluctuated without trending from the Ordovician to mid-Mesozoic, then increased (Fig. 1A, shown on log scale). Maximum likelihood modeling supports this interpretation; the best model was stasis with a shift to a directional, increasing trend (SI Appendix, Table S3). The most likely position for the shift in dynamics was Jurassic 6 (Tithonian; midpoint ∼149 Ma), although a shift in the next bin, Cretaceous 1 (Berriasian-Valanginian; ∼139 Ma), was nearly as good (SI Appendix, Fig. S2). There was also some support for a two-phase OU-OU model with an upward shift in equilibrium diversity, also in Jurassic 6, to a much higher equilibrium diversity (3.34 log units, or ∼2,200 genera) that is not yet approached by the end of the Cenozoic (SI Appendix, Fig. S3 and Table S3).
Major Faunas.
The diversity of nonbroadcasters was also described best by stasis followed by a directional trend (Fig. 1B and SI Appendix, Table S4). The timing of the shift was somewhat uncertain; the most likely shift point between the models was Cretaceous 2 (Hauterivian and Barremian; ∼129 Ma), but shifts as early as Jurassic 2 (Pliensbachian; ∼187 Ma) or as late as Cretaceous 4 (Albian; ∼107 Ma) were also within two log-likelihood units of the best solution (SI Appendix, Fig. S2).
The preferred model for the broadcasters was OU-OU (Fig. 1C and SI Appendix, Table S5). Diversity rose during the Ordovician to an equilibrium value that persisted for the rest of the Paleozoic, fell during the end-Permian mass extinction, and then rose to a similar (although slightly lower) equilibrium. The intermediate group was not diverse or well-sampled enough to produce a continuous standardized diversity curve (Fig. 1D). From the available data, their richness increased during the Triassic, but then flattened out, never significantly exceeding levels reached in the Paleozoic (Fig. 1D). The random walk model received the most support, although random walk–stasis and stasis–stasis (with the shift most likely occurring in Triassic 1 in both cases) also received some support, but were not favored by the likelihood ratio test (SI Appendix, Table S6).
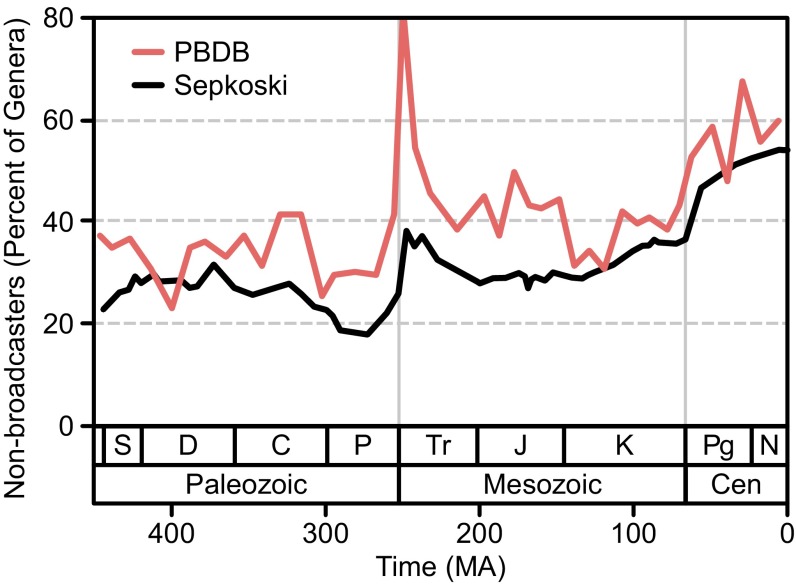
Relative richness trends in Sepkoski’s (48) compendium are similar to those in the PBDB (Fig. 2). Nonbroadcasters comprised about 20–40% of genera during the mid-Paleozoic to mid-Mesozoic, with some fluctuations. They climbed in proportional richness by about 20%, beginning in the Cretaceous and continuing in the Cenozoic, indicating they were diversifying at a greater rate than the other groups.
Fig. 2.
Percentage of animal genera classified as nonbroadcasters in the PBDB (red) and Sepkoski’s (48) compendium (black). For the PBDB, broadcasters and intermediate taxa were combined to get a continuous time series from SQS. The spike in the Early Triassic reflects the selective extinction of broadcasters in the end-Permian extinction, and their subsequent recovery. Nonbroadcasters radiate strongly in the K-Cz and increase permanently as a percentage of the fauna.
Individual Taxa.
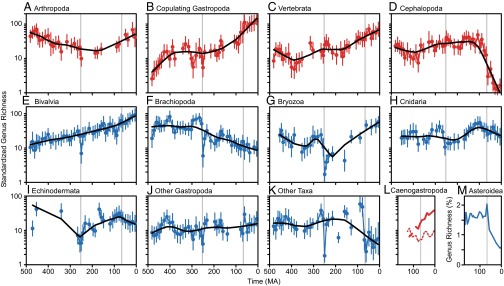
Most nonbroadcasting taxa have diversity histories that are consistent with the whole group: relatively stable richness in the late Paleozoic–early Mesozoic, followed by diversification in the late Mesozoic and Cenozoic (Fig. 3 A–C and SI Appendix, Table S7). The histories of these groups in the early Paleozoic differ, however; for example, arthropods declined as the trilobites dwindled. Cephalopods differ from the other nonbroadcasters because they lost most of their fossil diversity in the end-Cretaceous extinction (Fig. 3D).
Fig. 3.
Sampling-standardized genus richness of individual higher taxa or groups of taxa. (A–D) Nonbroadcasters (red). (E–K) Broadcasters and intermediate taxa (blue). Error bars mark ± one SE based on 1,000 replicates; SQS quorum = 0.40. Black lines mark lowess regressions (smoothing parameter = 0.4). (L) Caenogastropods, which are nonbroadcasters, split into carnivores (thick solid line) and noncarnivores (thin dashed line) (SQS quorum = 0.25). Values are shown for the K-Cz because dietary assignments are less clear-cut for some earlier genera. (M) Proportional richness of asteroids, which are broadcasting predators, in Sepkoski’s data (48).
The diversity curves of individual broadcasting and intermediate taxa vary widely (Fig. 3 E–K). Bivalves increased at a fairly constant rate from the Ordovician onward (Fig. 3E) (49), with perhaps a slight uptick after the end-Cretaceous extinction (50). Bryozoans declined in the end-Permian extinction, but recovered during the Mesozoic, although the details are poorly constrained in the PBDB data (Fig. 3G). Brachiopods declined after the Paleozoic (Fig. 3F), and cnidarians, echinoderms, and noncopulating gastropods fluctuated through time (Fig. 3 H–J). Several groups, mostly sponges and minor molluscan taxa (SI Appendix, Table S7), were combined because none were represented by sufficient data to generate reasonably complete individual curves (Fig. 3K). This polyphyletic group simply represents the remainder of the data, rather than an evolutionarily meaningful unit, but together these taxa do not increase in diversity in the Cenozoic.
The asteroids and caenogastropods were examined separately because they provide unusual combinations of fertilization and feeding method. The caenogastropods are nonbroadcasters, but include a wide range of feeding methods (tabulated from ref. 51). Although the carnivorous caenogastropods radiated strongly in the K-Cz, the noncarnivores did not (Fig. 3L). Asteroids, which are broadcasting predators, declined as a proportion of the total fauna during the Cenozoic in Sepkoski’s data (Fig. 3M), although their fossil record is poor and should be interpreted with some caution (52). The PBDB curve was extremely incomplete, but also showed no radiation (SI Appendix, Table S8).
Discussion
Several analyses of the marine animal fossil record have detected diversity dependence (4–6), but there is also strong evidence of a major K-Cz radiation (9, 53, 54). Our analyses provide statistical support for these patterns: the optimal model implies stasis in diversity from the Ordovician to the Jurassic, consistent with diversity dependence, followed by a directional increase in the Cretaceous and Cenozoic (Fig. 1A). The OU-OU model also received some support (SI Appendix, Table S3), which would suggest the K-Cz radiation might have slowed in the late Cenozoic (cf ref. 55).
The richness of nonbroadcasters was also in stasis from the Ordovician through Jurassic or Cretaceous, when it too shifted to a directional trend, leading to a tripling in richness (Fig. 1B). All nonbroadcasters except the cephalopods (shelled forms of which were largely eliminated by the end-Cretaceous extinction) contributed to the K-Cz radiation, transitioning from nontrending to increasing richness in the later Mesozoic (Fig. 3 A–D).
In contrast, broadcasters have essentially maintained a stable equilibrium since the Ordovician, despite fluctuations (Fig. 1C). Their richness was severely depressed by the end-Permian extinction, but they returned to a remarkably similar equilibrium afterward and did not radiate in the K-Cz. The intermediate fauna also did not radiate during this time (Fig. 1D). Individual broadcaster and intermediate taxa show a range of diversity trajectories (Fig. 3 E–K). The fact that some taxa increase in diversity does not obviate the point that broadcasters, in the aggregate, behaved differently than nonbroadcasters; because there is turnover among taxa within the broadcasters, some individual taxa must increase as others decrease. Critically, no broadcaster/intermediate taxon clearly shows a shift in diversification rate in the late Mesozoic similar to those observed for the nonbroadcasters.
Notably, the broadcaster and nonbroadcaster faunas do not correspond to Sepkoski’s evolutionary faunas (55), as members of his Paleozoic and Modern faunas belong to both categories. Sepkoski’s Paleozoic and Modern faunas largely correspond to those groups that fared poorly and well in the end-Permian extinction, whereas mass extinctions contributed to turnover within, rather than between, our groups.
Nonbroadcasters comprised 20–40% of genera from the mid-Paleozoic to mid-Mesozoic, then rose to 55–65% in the later Cenozoic (Fig. 2). In the living marine fauna, nonbroadcasters account for ∼73% of genera according to data from the World Registry of Marine Species (marinespecies.org) (SI Appendix, Table S9). Thus, the selective nature of the K-Cz radiation was a major determinant of the composition of the living marine biota.
Fertilization vs. Feeding.
Mode of fertilization tends to covary with a number of ecological traits, including feeding, motility, and cephalization, so it is possible that the radiation documented here in nonbroadcasters was causally related to one of these other variables. The most obvious candidate is feeding mechanism: broadcasters, especially sedentary broadcasters, are primarily suspension feeders, whereas the nonbroadcasters include many predators and scavengers. There is considerable evidence for increased diversity of predators and increased intensity of predation in the late Mesozoic and Cenozoic (which correspond to our nonbroadcaster radiations) (56–59), and these radiations have been linked to increased food supply to marine animal food webs (10–12).
To distinguish whether feeding or fertilization mechanisms more closely correlate to diversification, one can examine the diversity history of animals that are carnivorous broadcasters or noncarnivorous nonbroadcasters. Few such fossil taxa are preserved and studied sufficiently for analysis, but three examples are highly suggestive. Caenogastropods are nonbroadcasters that vary widely in diet; the noncarnivorous forms did not radiate in the K-Cz, whereas carnivorous forms diversified strongly (Fig. 3L). The Asteroidea are broadcasting predators, and they did not increase in relative richness in the K-Cz (Fig. 3M), although, again, their record should be viewed with caution. Finally, corals are broadcasters that exhibit carnivory or microcarnivory, although many also get nutrition from photosymbiosis, and they likewise did not radiate (Fig. 3H).
Together, these results suggest that diversification was concentrated in animals that combined direct sperm transfer with carnivory. Further testing would be useful; for example, arthropods and fish use a variety of feeding modes and could be analyzed after additional data collection and ecological analysis.
The Mesozoic Sexual Revolution.
Our results, in concert with previous demonstrations (4–6), suggest that diversification in marine animals was limited by diversity-dependent processes from the mid-Paleozoic to mid-Mesozoic, at which point the global equilibrium diversity increased dramatically (Fig. 1A). This radiation was driven by increased rates of diversification in nonbroadcasters (Figs. 1B and 3 A–C), particularly carnivorous forms (Fig. 3L). In theory, radiations in the arthropods, copulating gastropods, and vertebrates could have been driven by independent, unrelated factors, such as the evolution of key innovations. However, the coincident timing of these shifts in diversification suggests a common environmental driver. The evolution of key innovations may be related to the radiation of particular subgroups within these taxa, such as the teleost fish. However, we would argue that changing environmental conditions drove these radiations, with key innovations permitting particular subgroups to capitalize on the situation. A novel morphology only permits radiation if environmental conditions are permissive.
Food is often discussed as the limiting resource that drives diversity dependence (1), and indeed, numerous paleontologists have argued that increasing food and/or oxygen availability in the late Mesozoic permitted ecological reorganization, including diversification (10–12, 59–61). As animals diversified, they also became, on average, larger, fleshier, more motile, and more energetic (10, 58, 62, 63). Predation increased (57–60), and several groups of large phytoplankton radiated (64). All these lines of evidence suggest a widening of the base of the food chain during the Mesozoic, and the ultimate drivers of increased primary production have been discussed by numerous authors (10–12, 62, 64–66).
However, the effects of increased food and/or oxygen availability on diversity dynamics were strongly modulated by reproductive biology and ecology, with nonbroadcasters possessing several traits that predisposed them to radiation when conditions permitted. Direct sperm transfer permitted additional mechanisms for speciation to occur, or for incipient species to differentiate. It also permitted smaller viable population sizes (i.e., greater rarity), which permitted the co-occurrence of a greater number of species, given the available food resources (cf. ref. 8). The proliferation of a greater number of less abundant species was also enhanced because these animals (largely predators, scavengers, and omnivores) have many ways to specialize ecologically (e.g., different types of prey, feeding at different times of day). In contrast, most broadcasters are suspension feeders, which take small particles of food from the water and have fewer ways to specialize. We suggest that reproductive biology and ecology operated synergistically: motile predators had a greater ability to ecologically specialize, and direct sperm transfer permitted smaller population sizes that allowed specialized taxa to survive. Before the Cretaceous, there were presumably insufficient food resources to support large numbers of specialized predators, counteracting the inherent macroevolutionary potential of these nonbroadcasters to diversify.
Broadcasters, in contrast, lacked the traits that facilitated explosive diversification in the nonbroadcasters. Broadcasting fertilization favors large, denser populations because small populations may not be reproductively viable. Thus, available food resources may be consumed by a smaller number of more abundant species. Also, as noted, suspension feeders have fewer ways to specialize in diet and feeding than predators. The intermediate group, which is more variable ecologically, also did not diversify appreciably through time (Fig. 1C). Although they did not expand in diversity, these groups persisted as successful components of Cretaceous and Cenozoic ecosystems, maintaining the diversity they achieved in the Paleozoic.
These mechanisms are not inconsistent with other ideas on ecosystem evolution during the Mesozoic and Cenozoic. For example, an increase in primary production may have facilitated an increase in the average biomass of suspension feeders, which provided greater food resources for predators (10, 11). If so, however, this increased biomass did not translate into elevated diversity. Increased predation and seafloor disturbance may have helped drive down the diversity and abundance of some taxa, such as brachiopods (67–69), although competition may also have been important (70), consistent with diversity dependence.
Biodiversity dynamics are often studied through the lens of the living biota; for example, inferred from phylogenies generated from living organisms. However, living organisms represent a single time slice in the history of life, and they may have evolved under conditions not typical of its entirety. In the case of marine animals, the living fauna evolved largely during conditions of exceptional diversification, whereas much of the history of marine life was characterized by stasis and diversity limitation. Inferences based on living organisms may not reflect the full range of processes that have shaped the biosphere through time.
Conclusions
After not trending for hundreds of millions of years, marine animal diversity doubled in the K-Cz, but this radiation was highly selective. Animals that transfer sperm from adult to adult, whether internally or externally, tripled in genus richness, whereas animals that broadcast sperm did not radiate significantly. By analogy with similar arguments about land plants, we propose that adult–adult fertilization facilitated diversification by permitting smaller population sizes and/or allowing additional mechanisms of prezygotic isolation. In permitting diversification, fertilization mechanism may have operated synergistically with covarying ecological traits; many nonbroadcasting taxa are predators, which can specialize ecologically in ways not available to suspension-feeding, broadcasting taxa. The fossil record of marine animals indicates that long-standing limits to diversification can be overcome, quite possibly by changes in resource availability, and the effects of these shifts on the fauna are controlled by fundamental aspects of reproductive biology and ecology.
Methods
Data were downloaded from the PBDB following previous work (9) (SI Appendix, Paleobiology Database). Exceptional modes of preservation that are concentrated in particular time intervals were excluded (e.g., silicification, original aragonite; refs. 9 and 71). All marine animals were included except for annelids, which add little diversity in the fossil record, and for which functional assignments were uncertain in some cases.
Sampling was standardized using shareholder quorum subsampling (SQS), following previous works (9, 72), except that a single collection was drawn per literature reference, and the results were not smoothed using the 3T correction to maintain independence among data points. One thousand iterations were run per analysis, and the results from each iteration were logged before analysis (results were similar for raw values, but we concentrate on logged values because the slopes of the diversity curves are equivalent to rate of diversification, and changes in slope indicate changes in rate). Metazoa as a whole were analyzed at a sampling quorum of 0.70, and the reproductive faunas at a quorum of 0.50. These levels were chosen to balance the trade-off between sampling coverage and the temporal completeness of the results.
We analyzed the temporal pattern of change in the standardized diversity curves using the maximum likelihood methods of Hunt et al. (13). Models were fit that corresponded to stasis, directional change, unbiased random walk, an OU-OU process, as well as two-phase models incorporating a switch between simple models. The OU model conforms closely to the expectations of diversity dependence because diversity approaches and then fluctuates around a stable equilibrium value. Stasis is also consistent with diversity dependence, and it could represent the latter portion of an OU process (i.e., sampling begins after the shift to the stable value). A linear directional increase in logged data could correspond to exponential diversification, to the initial portion of an OU process (before the stable attractor is reached), or to some other pattern that is indistinguishable given the resolution of the data. Model support was evaluated initially using AICc (Akaike information criterion with correction for finite sample size). Hunt et al. (13) found that AICc tended to be overly liberal in favoring complex models and used parametric bootstrapping as a more conservative test of when complex models were favored over simple ones, an approach we follow here (SI Appendix, Maximum Likelihood Modeling).
We also generated standardized diversity curves for major taxonomic groups within the Metazoa. These groups largely correspond to phyla, although the mollusks were split because they vary in fertilization method and the data were sufficient for separate analyses. Other phyla are more homogeneous with respect to reproduction. Most of these curves had temporal gaps (time bins that did not have enough data to subsample at the given quorum), so we highlighted long-term patterns using lowess regression, rather than formal modeling.
We focus our analyses on the PBDB because diversity curves can be standardized for various biases, but we also tabulated the diversity of the fertilization faunas in Sepkoski’s compendium (48) to show that the results are consistent. However, we only examined proportional diversity for Sepkoski’s data because of concerns about heterogeneous sampling among time bins.
Supplementary Material
Acknowledgments
For helpful discussions and comments, thanks to C. Marshall, D. Jablonski, A. Knoll, J. Valentine, P. Getty, and two anonymous reviewers. Thanks to J. Alroy for providing SQS computer code. Thanks to PBDB data enterers, including W. Kiessling, A. Hendy, M. Clapham, A. Miller, M. Foote, J. Alroy, M. Aberhan, M. Kosnik, M. Patzkowsky, and P. Wagner. This is Paleobiology Database publication 272.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.F. is a Guest Editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610726113/-/DCSupplemental.
References
- 1.Rabosky DL, Hurlbert AH. Species richness at continental scales is dominated by ecological limits. Am Nat. 2015;185(5):572–583. doi: 10.1086/680850. [DOI] [PubMed] [Google Scholar]
- 2.Harmon LJ, Harrison S. Species diversity is dynamic and unbounded at local and continental scales. Am Nat. 2015;185(5):584–593. doi: 10.1086/680859. [DOI] [PubMed] [Google Scholar]
- 3.Marshall CR, Quental TB. The uncertain role of diversity dependence in species diversification and the need to incorporate time-varying carrying capacities. Philos Trans R Soc Lond B Biol Sci. 2016;371(1691):20150217. doi: 10.1098/rstb.2015.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alroy J. Colloquium paper: Dynamics of origination and extinction in the marine fossil record. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11536–11542. doi: 10.1073/pnas.0802597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alroy J. The shifting balance of diversity among major marine animal groups. Science. 2010;329(5996):1191–1194. doi: 10.1126/science.1189910. [DOI] [PubMed] [Google Scholar]
- 6.Foote M. The geological history of biodiversity. In: Bell MA, Futuyma DJ, Eanes WF, Levinton JS, editors. Evolution Since Darwin: The First 150 Years. Sinauer; Sunderland, Mass.: 2010. pp. 479–510. [Google Scholar]
- 7.Stanley SM. An analysis of the history of marine animal diversity. Paleobiology Memoirs. 2007;4:1–55. [Google Scholar]
- 8.Benton MJ, Emerson BC. How did life become so diverse? The dynamics of diversification according to the fossil record and molecular phylogenetics. Palaeontology. 2007;50(1):23–40. [Google Scholar]
- 9.Bush AM, Bambach RK. Sustained Mesozoic–Cenozoic diversification of marine Metazoa: A consistent signal from the fossil record. Geology. 2015;43(11):979–982. [Google Scholar]
- 10.Bambach RK. Seafood through time: Changes in biomass, energetics, and productivity in the marine ecosystem. Paleobiology. 1993;19(3):372–397. [Google Scholar]
- 11.Bambach RK. Energetics in the global marine fauna: A connection between terrestrial diversification and change in the marine biosphere. Geobios. 1999;32(2):131–144. [Google Scholar]
- 12.Allmon WD, Martin RE. Seafood through time revisited: The Phanerozoic increase in marine trophic resources and its macroevolutionary consequences. Paleobiology. 2014;40(2):256–287. [Google Scholar]
- 13.Hunt G, Hopkins MJ, Lidgard S. Simple versus complex models of trait evolution and stasis as a response to environmental change. Proc Natl Acad Sci USA. 2015;112(16):4885–4890. doi: 10.1073/pnas.1403662111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knoll AH. Patterns of change in plant communities through geologic time. In: Case TJ, Diamond J, editors. Community Ecology. Harper and Row; New York: 1986. pp. 126–141. [Google Scholar]
- 15.Coyne JA, Orr HA. Speciation. Sinauer; Sunderland, Mass.: 2004. p. 545. [Google Scholar]
- 16.Grant V. Pollination systems as isolating mechanisms in angiosperms. Evolution. 1949;3(1):82–97. doi: 10.1111/j.1558-5646.1949.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 17.van der Niet T, Johnson SD. Phylogenetic evidence for pollinator-driven diversification of angiosperms. Trends Ecol Evol. 2012;27(6):353–361. doi: 10.1016/j.tree.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Regal PJ. Ecology and evolution of flowering plant dominance. Science. 1977;196(4290):622–629. doi: 10.1126/science.196.4290.622. [DOI] [PubMed] [Google Scholar]
- 19.Kay KM, Sargent RD. The role of animal pollination in plant speciation: Integrating ecology, geography, and genetics. Annu Rev Ecol Evol Syst. 2009;40:637–656. [Google Scholar]
- 20.Armbruster WS, Muchhala N. Associations between floral specialization and species diversity: Cause, effect, or correlation? Evol Ecol. 2009;23:159–179. [Google Scholar]
- 21.Xu S, et al. Floral isolation is the main reproductive barrier among closely related sexually deceptive orchids. Evolution. 2011;65(9):2606–2620. doi: 10.1111/j.1558-5646.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- 22.Vermeij GJ, Grosberg RK. The great divergence: When did diversity on land exceed that in the sea? Integr Comp Biol. 2010;50(4):675–682. doi: 10.1093/icb/icq078. [DOI] [PubMed] [Google Scholar]
- 23.Grosberg RK, Vermeij GJ, Wainwright PC. Biodiversity in water and on land. Curr Biol. 2012;22(21):R900–R903. doi: 10.1016/j.cub.2012.09.050. [DOI] [PubMed] [Google Scholar]
- 24.Strathmann RR. Why life histories evolve differently in the sea. Am Zool. 1990;30:197–207. [Google Scholar]
- 25.Pennington JT. The ecology of fertilization of echinoid eggs: The consequences of sperm dilution, adult aggregation, and synchronous spawning. Biol Bull. 1985;169(2):417–430. doi: 10.2307/1541492. [DOI] [PubMed] [Google Scholar]
- 26.Babcock R, Keesing J. Fertilization biology of the abalone Haliotis laevigata: Laboratory and field studies. Can J Fish Aquat Sci. 1999;56(9):1668–1678. [Google Scholar]
- 27.Palumbi SR. Genetic divergence, reproductive isolation, and marine speciation. Annu Rev Ecol Syst. 1994;25:547–572. [Google Scholar]
- 28.Malay MCD, Paulay G. Peripatric speciation drives diversification and distributional pattern of reef hermit crabs (Decapoda: Diogenidae: Calcinus) Evolution. 2010;64(3):634–662. doi: 10.1111/j.1558-5646.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 29.Solignac M. Isolating mechanisms and modalities of speciation in the Jaera albifrons species complex (Crustacea, Isopoda) Syst Zool. 1981;30(4):387–405. [Google Scholar]
- 30.Cruz R, Carballo M, Conde-Padín P, Rolán-Alvarez E. Testing alternative models for sexual isolation in natural populations of Littorina saxatilis: Indirect support for by-product ecological speciation? J Evol Biol. 2004;17(2):288–293. doi: 10.1111/j.1420-9101.2003.00689.x. [DOI] [PubMed] [Google Scholar]
- 31.Bauer RT, Martin JW, editors. Crustacean Sexual Biology. Columbia University Press; New York: 1991. [Google Scholar]
- 32.Duffy JE, Thiel M. Evolutionary Ecology of Social and Sexual Systems: Crustaceans As Model Organisms. Oxford University Press; New York: 2007. [Google Scholar]
- 33.Boughman JW. Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature. 2001;411(6840):944–948. doi: 10.1038/35082064. [DOI] [PubMed] [Google Scholar]
- 34.Ellis EA, Oakley TH. High rates of species accumulation in animals with bioluminescent courtship displays. Curr Biol. 2016;26(14):1916–1921. doi: 10.1016/j.cub.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 35.West-Eberhard MJ. Sexual selection, social competition, and speciation. Q Rev Biol. 1983;58(2):155–183. [Google Scholar]
- 36.Ponder WF, Lindberg DR. Towards a phylogeny of gastropod molluscs: An analysis using morphological characters. Zool J Linn Soc. 1997;119(2):83–265. [Google Scholar]
- 37.Dix TG. Aggregating in the echinoid Evechinus chloroticus. Pac Sci. 1969;23(1):123–124. [Google Scholar]
- 38.Shepherd SA. Studies on southern Australian abalone (genus Haliotis) Mar Biol. 1986;90(2):231–236. [Google Scholar]
- 39.Young CM, Tyler PA, Cameron JL, Rumrill SG. Seasonal breeding aggregations in low-density populations of the bathyal echinoid Stylocidaris lineata. Mar Biol. 1992;113(4):603–612. [Google Scholar]
- 40.Run J-Q, Chen C-P, Chang K-H, Chia F-S. Mating behavior and reproductive cycle of Archaster typicus (Echinodermata: Asteroidea) Mar Biol. 1988;99(2):247–253. [Google Scholar]
- 41.Tominaga H, Nakamura S, Komatsu M. Reproduction and development of the conspicuously dimorphic brittle star Ophiodaphne formata (Ophiuroidea) Biol Bull. 2004;206(1):25–34. doi: 10.2307/1543195. [DOI] [PubMed] [Google Scholar]
- 42.Picken GB, Allan D. Unique spawning behaviour by the Antarctic limpet Nacella (Patinigera) concinna (Strebel, 1908) J Exp Mar Biol Ecol. 1983;71(3):283–287. [Google Scholar]
- 43.Young CM. The biology of external fertilization in deep-sea echinoderms. In: Young CM, Eckelbarger KJ, editors. Reproduction, Larval Biology, and Recruitment of the Deep-Sea Benthos. Columbia University Press; New York: 1994. pp. 179–200. [Google Scholar]
- 44.Warén A, Bouchet P. New records, species, genera, and a new family of gastropods from the hydrothermal vents and hydrocarbon seeps. Zool Scr. 1993;22(1):1–90. [Google Scholar]
- 45.Quinn JF. Systematic position of Basilissopsis and Guttula, and a discussion of the phylogeny of the Sequenzioidea (Gastropoda: Prosobranchia) Bull Mar Sci. 1991;49(1-2):575–598. [Google Scholar]
- 46.Golikov AN, Kussakin OG. Sur la biologie de la reproduction des patelles de la famille Tecturidae (Gastropoda: Docoglossa) et sur la position systématique des ses subdivisions. Malacologia. 1972;11:287–294. [Google Scholar]
- 47.Healy JM, Rowe FWE, Anderson DT. Spermatozoa and spermiogenesis in Xyloplax (Class Concentricycloidea): A new type of spermatozoon in the Echinodermata. Zool Scr. 1988;17(3):297–310. [Google Scholar]
- 48.Sepkoski JJ., Jr A compendium of fossil marine animal genera. Bull Am Paleontol. 2002;363:1–560. [Google Scholar]
- 49.Miller AI, Sepkoski JJ., Jr Modeling bivalve diversification: The effect of interaction on a macroevolutionary system. Paleobiology. 1988;14(4):364–369. doi: 10.1017/s0094837300012100. [DOI] [PubMed] [Google Scholar]
- 50.Krug AZ, Jablonski D, Valentine JW. Signature of the end-Cretaceous mass extinction in the modern biota. Science. 2009;323(5915):767–771. doi: 10.1126/science.1164905. [DOI] [PubMed] [Google Scholar]
- 51.Beesley PL, Ross GJB, Wells A, editors. Mollusca: The Southern Synthesis, Part B. Vol 5 CSIRO Publishing; Melbourne: 1998. [Google Scholar]
- 52.Mah CL, Blake DB. Global diversity and phylogeny of the Asteroidea (Echinodermata) PLoS One. 2012;7(4):e35644. doi: 10.1371/journal.pone.0035644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sepkoski JJ, Jr, Bambach RK, Raup DM, Valentine JW. Phanerozoic marine diversity: A strong signal from the fossil record. Nature. 1981;293:435–437. [Google Scholar]
- 54.Benton MJ. Diversification and extinction in the history of life. Science. 1995;268(5207):52–58. doi: 10.1126/science.7701342. [DOI] [PubMed] [Google Scholar]
- 55.Sepkoski JJ., Jr A kinetic model of Phanerozoic taxonomic diversity. III. Post-Paleozoic families and mass extinctions. Paleobiology. 1984;10(2):246–267. [Google Scholar]
- 56.Vermeij GJ. The Mesozoic marine revolution: Evidence from snails, predators, and grazers. Paleobiology. 1977;3(3):245–258. [Google Scholar]
- 57.Huntley JW, Kowalewski M. Strong coupling of predation intensity and diversity in the Phanerozoic fossil record. Proc Natl Acad Sci USA. 2007;104(38):15006–15010. doi: 10.1073/pnas.0704960104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bambach RK, Knoll AH, Sepkoski JJ., Jr Anatomical and ecological constraints on Phanerozoic animal diversity in the marine realm. Proc Natl Acad Sci USA. 2002;99(10):6854–6859. doi: 10.1073/pnas.092150999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bush AM, Bambach RK. Paleoecologic megatrends in marine metazoa. Annu Rev Earth Planet Sci. 2011;39:241–269. [Google Scholar]
- 60.Vermeij GJ. Evolution and Escalation: An Ecological History of Life. Princeton University Press; Princeton, NJ: 1987. [Google Scholar]
- 61.Martin R, Quigg A. Evolving phytoplankton stoichiometry fueled diversification of the marine biosphere. Geosciences (Basel) 2012;2(2):130–146. [Google Scholar]
- 62.Finnegan S, McClain CR, Kosnik MA, Payne JL. Escargots through time: An energetic comparison of marine gastropod assemblages before and after the Mesozoic marine revolution. Paleobiology. 2011;37(2):252–269. [Google Scholar]
- 63.Heim NA, Knope ML, Schaal EK, Wang SC, Payne JL. Animal evolution. Cope’s rule in the evolution of marine animals. Science. 2015;347(6224):867–870. doi: 10.1126/science.1260065. [DOI] [PubMed] [Google Scholar]
- 64.Falkowski PG, et al. The evolution of modern eukaryotic phytoplankton. Science. 2004;305(5682):354–360. doi: 10.1126/science.1095964. [DOI] [PubMed] [Google Scholar]
- 65.Falkowski P, Knoll AH, editors. Evolution of Primary Producers in the Sea. Academic Press; Amsterdam: 2007. [Google Scholar]
- 66.Meyer KM, Ridgwell A, Payne JL. The influence of the biological pump on ocean chemistry: Implications for long-term trends in marine redox chemistry, the global carbon cycle, and marine animal ecosystems. Geobiology. 2016;14(3):207–219. doi: 10.1111/gbi.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thayer CW. Sediment-mediated biological disturbance and the evolution of the marine benthos. In: Tevesz MJS, McCall PL, editors. Biotic Interactions in Recent and Fossil Benthic Communities. Plenum; New York: 1983. pp. 479–625. [Google Scholar]
- 68.Tomašových A. Substrate exploitation and resistance to biotic disturbance in the brachiopod Terebratalia transversa and the bivalve Pododesmus macrochisma. Mar Ecol Prog Ser. 2008;363:157–170. [Google Scholar]
- 69.Stanley SM. What has happened to the articulate brachiopods? GSA Abstracts with Programs. 1974;6:966–967. [Google Scholar]
- 70.Liow LH, Reitan T, Harnik PG. Ecological interactions on macroevolutionary time scales: Clams and brachiopods are more than ships that pass in the night. Ecol Lett. 2015;18(10):1030–1039. doi: 10.1111/ele.12485. [DOI] [PubMed] [Google Scholar]
- 71.Alroy J, et al. Phanerozoic trends in the global diversity of marine invertebrates. Science. 2008;321(5885):97–100. doi: 10.1126/science.1156963. [DOI] [PubMed] [Google Scholar]
- 72.Alroy J. Accurate and precise estimates of origination and extinction rates. Paleobiology. 2014;40(3):374–397. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.