Significance
Normal development of the visual cortex depends critically on early life visual experience. In humans, a disparity in the quality of vision between two eyes during infancy or early childhood leads to a visual impairment called amblyopia. In animal models, amblyopia can be induced early in life using a brief period of monocular deprivation via eyelid closure. Here we show in two evolutionarily divergent species that experimental amblyopia can be rapidly corrected if binocular visual experience is restored after temporarily silencing the retinas with a local anesthetic. These findings point the way to an approach for clinical management of amblyopia with advantages over the current standard of care.
Keywords: ocular dominance plasticity, metaplasticity, amblyopia, visual cortex, lateral geniculate nucleus
Abstract
A half-century of research on the consequences of monocular deprivation (MD) in animals has revealed a great deal about the pathophysiology of amblyopia. MD initiates synaptic changes in the visual cortex that reduce acuity and binocular vision by causing neurons to lose responsiveness to the deprived eye. However, much less is known about how deprivation-induced synaptic modifications can be reversed to restore normal visual function. One theoretically motivated hypothesis is that a period of inactivity can reduce the threshold for synaptic potentiation such that subsequent visual experience promotes synaptic strengthening and increased responsiveness in the visual cortex. Here we have reduced this idea to practice in two species. In young mice, we show that the otherwise stable loss of cortical responsiveness caused by MD is reversed when binocular visual experience follows temporary anesthetic inactivation of the retinas. In 3-mo-old kittens, we show that a severe impairment of visual acuity is also fully reversed by binocular experience following treatment and, further, that prolonged retinal inactivation alone can erase anatomical consequences of MD. We conclude that temporary retinal inactivation represents a highly efficacious means to promote recovery of function.
Amblyopia is a widespread form of human visual disability that arises from imbalanced visual experience in the two eyes during infancy or early childhood. The core pathophysiological process underlying amblyopia is ocular dominance plasticity in the primary visual cortex (V1). Ocular dominance plasticity, evolutionarily conserved in mammals with binocular vision, has become a classic paradigm for studying how brain development is influenced by experience and deprivation. A brief period of monocular deprivation (MD) during early postnatal life causes functional depression of synapses in V1 that serve the deprived eye (1–7). Consequently, early-life MD severely and persistently degrades visual acuity through the deprived eye, which typically fails to recover spontaneously when binocular vision is restored (8–10). A critical step in developing targeted interventions for treating amblyopia is to identify strategies for reversing deprivation-driven synaptic modifications in V1.
The traditional approach to promote recovery following MD has been to occlude the strong eye to force vision through the weak amblyopic eye. This “reverse occlusion” approach has been validated in animals (11, 12) and represents the cornerstone of current treatment (patching therapy) of human amblyopia (13–16). However, this treatment has well-known limitations that include poor compliance, potential loss of vision through the newly patched eye, failure to recover binocular vision, and a declining treatment efficacy with age (15, 17–21). Nevertheless, the success of reverse occlusion strategies demonstrates that severely weakened synaptic inputs in the brain can be rejuvenated under appropriate circumstances.
An insight into how reverse occlusion might promote recovery came from a study in cat V1, which showed that neurons lose responsiveness to the newly occluded eye days before gaining responses to the newly open (amblyopic) eye (22). It is now understood that the qualities of cortical synaptic plasticity depend on the recent history of cortical activity, a property called metaplasticity (23). After a period of attenuated cortical activity, the threshold for synaptic potentiation is reduced (24–26). Thus, the period of quiescence that immediately follows initiation of reverse occlusion lowers the threshold for synaptic potentiation, enabling subsequent visual experience to increase synaptic effectiveness when it would have otherwise been without effect (27). An intriguing possibility is that imposition of a period of binocular inactivity following early MD could be sufficient to promote visual recovery without forcing the eyes to compete.
In support of this concept, studies have shown that exposure of adult rats and kittens to a period of continuous darkness can prime the visual cortex for recovery from the effect of MD when vision is restored to the deprived eye (28–30). However, available data suggest that this priming effect requires ≥10 d of darkness, which cannot be interrupted, even briefly, by light exposure and cannot be substituted with binocular lid closure (31, 32). Further, work in kittens has shown that when initiated before 10 wk of age, exposure to total darkness itself can cause temporary visual impairment and even blindness before 7 wk of age (33). Thus, although these results show the potential of using insights gained from the study of synaptic plasticity to promote recovery of function (34), application of this approach to clinical practice presents many challenges.
In the current study, we set out to examine an alternative method involving temporary anesthetic inactivation of the retinas. For decades, researchers of visual system development have used tetrodotoxin (TTX) to investigate the consequences of blocking action potentials in retinal ganglion cells. A single dose, administered by microinjection into the vitreous humor, can locally block all impulse activity in the optic nerve for a day or two. Of significance, inactivation of one eye with TTX fails to trigger depression of deprived-eye responses in V1 that is observed after comparable periods of monocular deprivation by lid closure or image blurring (5, 7, 35–37). Thus, brief inactivation of both eyes with TTX could potentially augment recovery of function by lowering the plasticity threshold without the liability associated with other forms of deprivation. Furthermore, because the retinas are silent until the drug wears off, compliance is assured.
To test this hypothesis, we performed parallel studies in mice and kittens. Our interest in mice was driven by the utility of this species for mechanistic studies. Although the immediate effects of MD in mice have been well documented, less was known about the stability of these effects. Therefore, we first conducted a longitudinal electrophysiological study to track V1 responsiveness to stimulation of the two eyes in awake mice for several weeks following early-life MD. We found that 7 d of MD drove a profound reduction in visual cortical responsiveness to deprived eye vision that persisted through adulthood, validating this species as a model to study the pathophysiology of amblyopia. We then investigated the effect of bilateral retinal inactivation and observed a complete recovery from the effect of MD once binocular visual experience was restored. We also studied treatment effectiveness in the cat, which for decades has been the preferred species to study the effects of MD on visual system physiology, anatomy, and behavior. We found that bilateral retinal inactivation followed by a short period of binocular visual experience promoted fast recovery of the visual acuity of the deprived eye to normal levels. Further, we discovered that even in the absence of subsequent visual experience, prolonged TTX treatment led to recovery from the anatomical consequences of MD on the visual thalamus of kittens. Our experiments demonstrate, in two species, that temporary retinal inactivation is an effective strategy for promoting rapid recovery from MD-driven visual impairments.
Results
Monocular Deprivation Drives Lasting Visual Impairment in Mice.
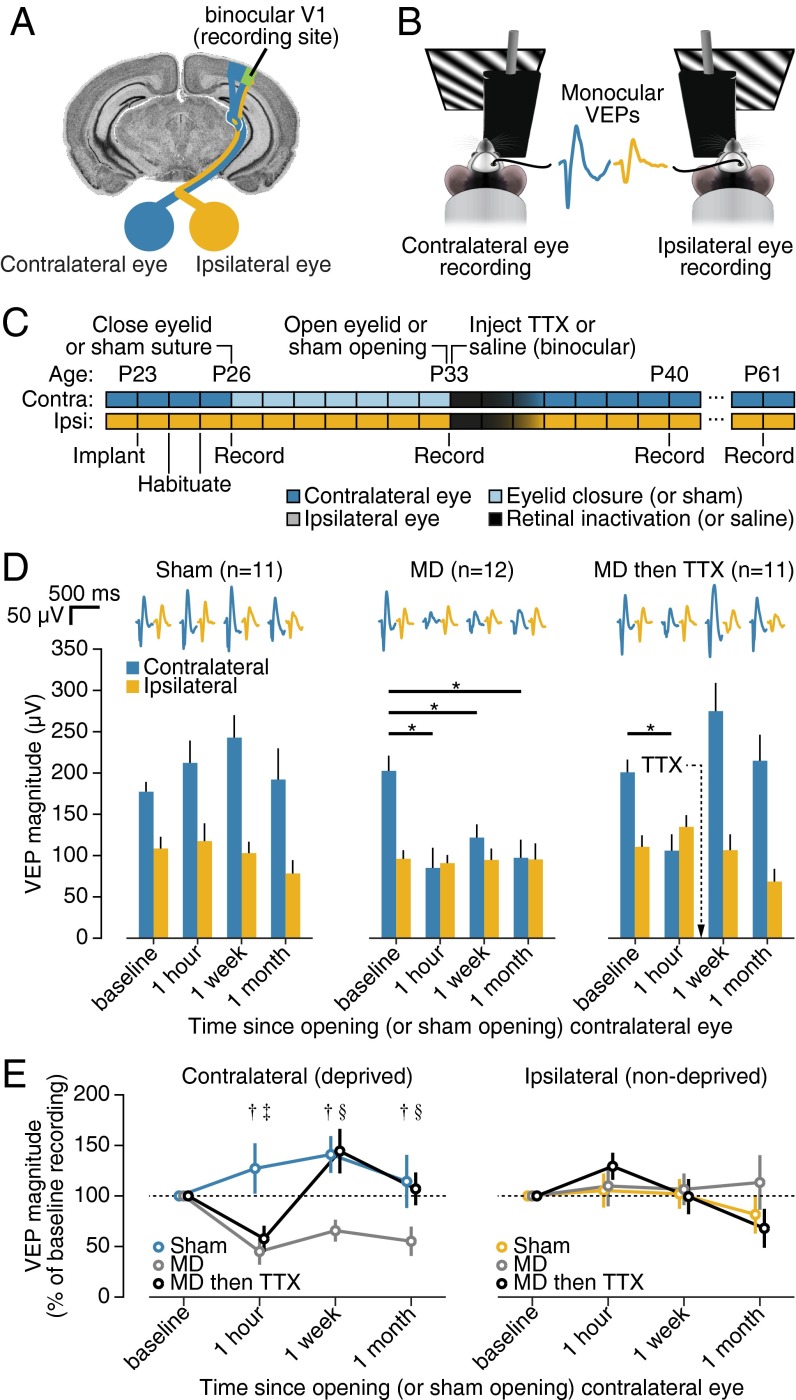
Mice are commonly used for mechanistic studies of ocular dominance plasticity in response to early-life MD (3, 5, 38). Recent investigations have focused on the mechanisms responsible for the loss of responsiveness to the deprived eye (39, 40), but very little is known about the stability of these changes or whether they can be reversed. To address these questions, we chronically recorded visual evoked potentials (VEPs) in thalamo-recipient layer 4 of binocular V1 over the course of several weeks in three cohorts of mice (Fig. 1). One group had normal visual experience; the second was monocularly deprived by eyelid closure from postnatal day (P) 26 to P33 followed by normal binocular visual experience; and the third group was treated as the second except that the period of MD was followed by a single dose of TTX injected into the vitreous humor of both eyes (Fig. 1 A–C). TTX is a long-lasting voltage-gated sodium channel blocker, and in agreement with previous studies (7, 41), we found that a single dose completely abolished visual responses for 24–48 h, followed by a gradual recovery. In all MD cases, VEPs were recorded from V1 contralateral to the deprived eye (Fig. 1A).
Fig. 1.
Binocular retinal inactivation following MD promotes recovery of visually evoked responses in mouse V1. (A) Coronal section of mouse brain showing recording site relative to eyes. (B) Cartoon showing VEPs measured during visual stimulus presentation to the contralateral or ipsilateral eyes. (C) Experimental timeline. Seven days of MD or sham MD of the contralateral eye were followed by intravitreal injections of TTX or saline into both eyes. (D) Mean monocular VEP magnitudes for littermates that underwent sham eyelid closure followed by saline injections (sham, n = 11), MD followed by saline injections (MD, n = 12), or MD followed by TTX injections (MD then TTX, n = 11). Deprived eye responses varied for each treatment group over time [time, F(3, 93) = 7.116, P < 10−3; treatment, F(2, 31) = 7.963, P < 10−2; and interaction, F(6, 93) = 5.166, P < 10−3], with MD significantly reducing responses to vision through the deprived, contralateral eye compared with its own baseline (Dunnett: MD, P = 0.0006; MD then TTX, P = 0.0099; and sham, P = 0.5524) and compared with sham controls (Tukey: sham vs. MD, P = 0.0011; sham vs. MD then TTX, P = 0.0093; and MD vs. MD then TTX, P = 0.82). Contralateral responses recovered following binocular retinal inactivation (Dunnett: baseline vs. 1 wk, P = 0.0562 and baseline vs. 1 mo, P = 0.9454) and were statistically indistinguishable from sham controls (Tukey: 1 wk, P = 0.9866 and 1 mo, P = 0.9347). Error bars, SEM. Asterisks denote statistically significant differences from baseline (Dunnett, adjusted P < 0.05). Stimulus spatial frequency: 0.2 cpd. (E) Mean monocular VEP magnitudes over time normalized to baseline values. Contralateral VEP magnitudes varied for each treatment group over time [time, F(3, 93) = 5.514, P < 10−2; treatment, F(2, 31) = 7.481, P < 10−2; and interaction, F(6, 93) = 4.225, P < 10−3]. Error bars, SEM. Statistical significance between treatment groups (Tukey, P < 0.05) is denoted by † (sham vs. MD), ‡ (sham vs. MD then TTX), or § (MD vs. MD then TTX). Ipsilateral VEP magnitudes were not significantly different across time or between treatment groups [time, F(3, 93) = 1.879, P = 0.1386; treatment, F(2, 31) = 0.3222, P = 0.7270; and interaction, F(6, 93) = 1.020, P = 0.4172].
In agreement with previous reports (5, 42), 7-d MD drove a profound reduction in the magnitude of VEPs during presentation of phase-reversing sinusoidal grating stimuli to the deprived eye [Fig. 1D, Center; P = 0.0006; 0.2 cycles per degree (cpd)]. Further, we found that deprived-eye VEP magnitude remained significantly reduced after 1 wk and 1 mo of binocular visual experience following MD, compared with pre-MD baseline values (Fig. 1D, Center; 1 wk: P = 0.0246; 1 mo: P = 0.0023) and to sham littermate controls (Fig. 1D, Left; 1 wk: P = 0.0020; 1 mo: P = 0.0204). The sustained depression of deprived-eye responses was also observed at lower and higher spatial frequencies (Fig. S1). These results demonstrate that the visual deficit observed after 7 d of MD is long lasting and further validate the mouse as a useful animal model of amblyopia.
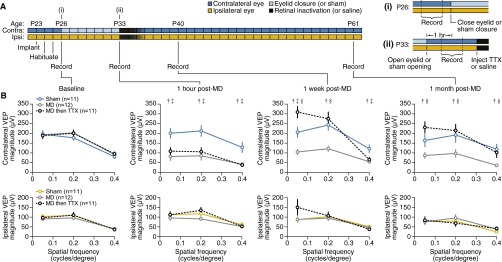
Fig. S1.
Rate of recovery of V1 responsiveness to deprived eye vision following retinal inactivation varies across spatial frequencies. (A) Experimental timeline showing recording schedule. Insets at right show time of recordings at (i) P26 and (ii) P33 relative to ocular manipulations performed on those days. (B) Mean monocular VEP magnitudes across varying spatial frequencies on each recording date. Error bars, SEM. At all spatial frequencies, contralateral VEP magnitudes showed significant effects of treatment [0.05 cpd, F(2, 31) = 12.68, P < 10−4; 0.2 cpd, F(2, 31) = 7.963, P < 10−2; 0.4 cpd, F(2, 31) = 7.485, P < 10−2]. Ipsilateral VEP magnitudes were not significantly different across treatment groups [0.05 cpd, F(2, 31) = 1.320, P = 0.1008; 0.2 cpd, F(2, 31) = 0.2740, P = 0.7622; 0.4 cpd, F(2, 31) = 0.05233, P = 0.9491]. Statistical significance (Tukey, P < 0.05) is denoted by † (sham vs. MD), ‡ (sham vs. MD then TTX), or § (MD vs. MD then TTX). At the lowest spatial frequency (0.05 cpd), monocular VEP magnitudes were transiently increased over sham controls following TTX injection (1 wk post-MD: contralateral, P = 0.0054 and ipsilateral, P = 0.0596). However, 1 mo after the injections, this trend was greatly attenuated for contralateral responses (P = 0.1071) and eliminated for ipsilateral responses (P = 0.9877). At the highest spatial frequency (0.4 cpd), deprived contralateral responses did not return to sham control levels immediately (1 wk post-MD: P = 0.0336), but recovery was observed 1 mo following injections (P = 0.7262).
Temporary Retinal Inactivation Promotes Electrophysiological Recovery in Amblyopic Mice.
After confirming depression of deprived-eye VEP magnitude immediately following 7-d MD (Fig. 1D, Right; P = 0.0099; 0.2 cpd), a cohort of mice received a binocular intravitreal TTX injection, eliminating retinal activity for ∼2 d, followed by normal binocular experience. Subsequent VEP recordings revealed responses to the deprived eye that actually exceeded baseline values 1 wk later (P = 0.0562) and were no different from baseline 1 mo later (P = 0.9454). Visually evoked responses in animals that experienced binocular inactivation following MD were significantly increased over littermates that experienced MD only (Fig. 1 D and E; 1 wk: P < 0.0001; 1 mo: P = 0.0028) and were statistically indistinguishable from sham littermate controls that had no MD (1 wk: P = 0.6442; 1 mo: P = 0.7981). Assessment of VEPs measured with lower and higher spatial frequency stimuli (0.05 and 0.4 cpd, respectively) revealed similar trends, although recovery for the higher spatial frequency was not observed immediately, but instead occurred between 1 wk and 1 mo following the injection (Fig. S1). Meanwhile, binocular inactivation had no lasting impact on nondeprived eye responses (Fig. 1E and Fig. S1). Thus, whereas early-life MD in mice can drive a sustained deficit in V1 responsiveness to vision through the deprived eye, this deficit can be eliminated following binocular retinal inactivation.
Temporary Retinal Inactivation Restores Visual Acuity in Amblyopic Kittens.
Although mice are useful for mechanistic studies of amblyopia, the species with the longest history and richest database is the cat. Advantages of the cat include visual pathways that share a similar organization to those of primates, good visual acuity, stereoscopic vision, and functional and morphological responses to early MD that are very large and similar to those observed in primates. The lineage of modern cats and mice diverged early in mammalian evolution, even before the divarication of rodents and primates (43). A similar response to treatment in cats and mice would thus be consistent with evolutionary conservation of a common mechanism that might apply generally to visual mammals, including humans.
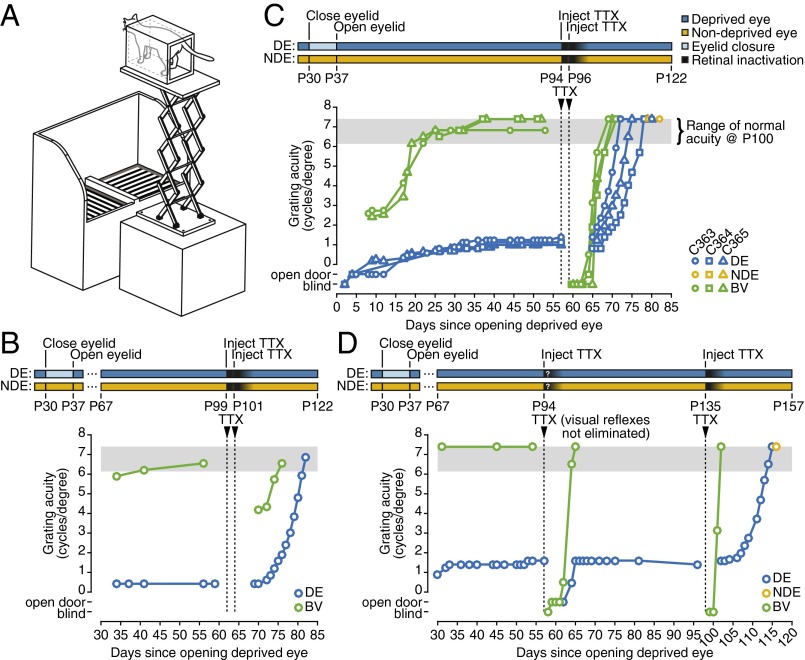
Visual acuity was measured in kittens using a two-alternative forced-choice discrimination task (30, 44) (Fig. 2A). Consistent with previous studies, we found that 7-d MD initiated at P30 induced a long-lasting deficit in deprived-eye visual acuity (Fig. 2 B–D). In our first pilot experiment (animal C349) we injected the eyes with two doses of TTX 2 d apart, starting on P99, ∼2 mo after the deprived eye had been opened (Fig. 2B). Remarkably, within 1 wk of the return of normal acuity in the nondeprived eye (reflecting clearance of the TTX), we observed a rapid and complete recovery from amblyopia in the fellow deprived eye. To confirm this encouraging result, we repeated the experiment in three littermates using a similar experimental design (Fig. 2C). Assessed with the pupillary light reflex and visual placement behavior, it appeared that blockade of retinal activity lasted ∼5 d, with full restoration of vision through the nondeprived eye apparent 10 d after the first TTX dose. Over the next 10 d, acuity in the deprived (amblyopic) eyes of all three additional kittens increased until it was indistinguishable from that of the nondeprived eye.
Fig. 2.
Binocular retinal inactivation following MD promotes recovery of visual acuity in behaving kittens. (A) The jumping stand used to test grating acuity. (B) Visual spatial acuity of the deprived eye (DE: blue) and nondeprived eye (NDE), assessed using binocular vision (BV: green) of a kitten (C349) that received two injections of TTX at P99 and P101. The period of complete and waning retinal inactivation that ensued is depicted by completeness of black shading. As with subsequent panels, the rearing history is displayed in schematic form above the graph. Gray shading shows the range of grating acuities measured in normal kittens by use of the same testing procedure. The designations “blind“ and “open door” refer, respectively, to an inability or ability to locate a closed door on the jumping stand by visual cues alone. (C) The visual acuity of the DE and NDE tested in three littermates (C363, C364, and C365) before and after two binocular intravitreal injections of TTX made at P94 and P96. All animals received a 7-d period of MD at P30 (NDE acuity assessed monocularly: yellow; other conventions as in A). (D) Results from C362 that received an initial binocular TTX injection at P94 that apparently did not achieve full retinal activity block as the pupils were not dilated fully and visual placing behavior was still evident. Although the animal appeared blind on the jumping stand, the acuity of the NDE recovered to normal in 8 d but the acuity of the DE recovered only to its preinjection level. Remarkably, following a second binocular intravitreal injection at P135 the acuity of this eye recovered to normal levels.
In a fifth kitten (C362) we attempted to investigate the effect of a single binocular dose of TTX at P94 (Fig. 2D). Unfortunately, although visuomotor behavior was affected by the treatment, the kitten showed incomplete pupil dilation as well as weak light reflexes and visual placing responses. The animal’s impairment on the behavioral task indicated that retinal activity was at least partially disrupted, although the sustained visual reflexes suggested that activity blockade was incomplete. Interestingly, we also failed to observe any recovery of acuity in the deprived eye, suggesting that a partial activity blockade is insufficient for promoting visual recovery. We attempted a second TTX injection, 1.5 mo after the initial injection. This time, the treatment successfully blocked visual reflexes (indicative of a complete retinal activity blockade) in both eyes, and we observed a full recovery of acuity in the deprived eye over the next 2 wk (Fig. 2D). Together, these data suggest that complete blockade of retinal spiking is required to promote visual recovery, and that a single administration of an inactivating agent can promote normal visual acuity even several months after MD.
Sustained Retinal Inactivation Drives Anatomical Recovery from Consequences of MD in Kittens.
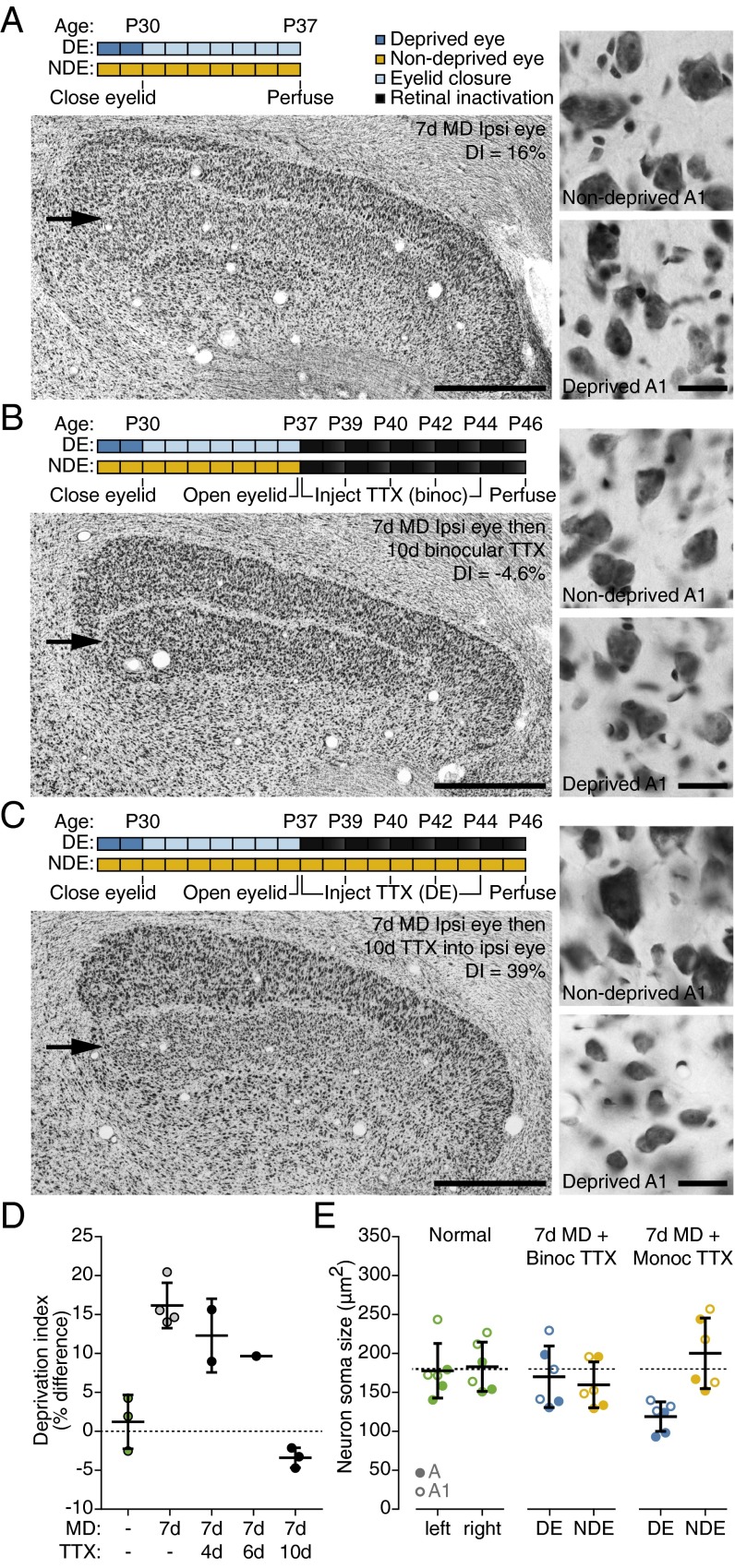
In cats as in monkeys, substantial shrinkage of neurons occurs in the layers of the lateral geniculate nucleus (LGN) that relay information to V1 from the deprived eye. Shrinkage of these neurons correlates well with the loss of their axonal arbors and synaptic influence in V1 (45–48). Thus, assessment of LGN cell-size changes after MD ± TTX has the potential to provide additional insight into how the treatment promotes recovery. We found that 7-d MD produced a clear deprivation effect (expressed as the percent difference in cell size between deprived and nondeprived LGN layers) (48) (Fig. 3 A and D), which was still apparent in animals receiving 2–3 doses of TTX every 2 d immediately following MD (Fig. 3D). This finding indicates that brief binocular activity blockade itself does not restore function to deprived-eye cortical inputs, but possibly primes the cortex for recovery when the TTX wears off and binocular vision is provided. However, we also found that with five binocular doses over 10 d, soma sizes in deprived layers of LGN returned to normal and the deprivation effect was eliminated (Fig. 3 B, D, and E). This striking finding contrasts with the effect of treating only the deprived eye with TTX, which further exacerbated the deprivation effect (Fig. 3 C and E). Thus, some aspects of recovery are experience independent, as they occur during the period of retinal inactivation and presumably provide a scaffold for visual recovery that ensues with subsequent binocular vision.
Fig. 3.
Recovery of neuron soma size in the kitten LGN following prolonged binocular retinal inactivation. (A, Top) Schematic depicting rearing history. Kittens received retinal TTX immediately after the period of MD to isolate the effect of inactivation on recovery and in the absence of any visual experience. (Left) Low magnification image of the LGN stained for Nissl substance after 7-d MD started at P30. Arrow indicates deprived eye (DE) layer (lamina A1, ipsilateral to the DE). (Scale bar, 1 mm.) (Right) High magnification images from NDE (Top) and DE (Bottom) A1 layers after 7-d MD. (Scale bar, 25 µm.) (B and C) Same as A except that MD was followed by 10 d of binocular retinal silencing (B) or monocular retinal silencing of the DE (C). (D) Stereological quantification of neuron soma size within DE and NDE A and A1 LGN layers (deprivation index, DI) revealed that after MD, DE neurons recovered progressively during the period of binocular inactivation so that DE and NDE neurons were of equal size by 10 d. Dashed line denotes DI observed in normal kittens. Colors denote normal visual experience (green), 7-d MD alone (gray), or 7-d MD followed by binocular TTX (black). Center values and error bars, mean ± SD. (E) Average left- and right-eye neuron soma size from age-matched normal controls (Left), and when MD was followed by binocular (Center) or monocular (Right) retinal inactivation. The restoration of balanced DE and NDE neuron size induced by binocular inactivation was the product of recovery rather than generalized shrinkage because (i) compared with monocular inactivation the size of DE neurons after binocular treatment was significantly larger [F(2, 15) = 5.79, P = 0.013; Bonferroni, P < 0.05], and (ii) neuron size after binocular silencing was not different from normal control data (Bonferroni, P > 0.05). Dashed line denotes average value observed from normal kittens. Closed and open symbols indicate measurements from A and A1 LGN layers, respectively, and colors denote normal visual experience (green), the DE (blue), or the NDE (yellow). Center values and error bars, mean ± SD.
Discussion
Our experiments reveal that stable electrophysiological, anatomical, and behavioral consequences of early-life MD can be fully and rapidly reversed in two evolutionarily distant species, kittens and mice, when binocular visual experience is restored following temporary bilateral anesthetic inactivation of the retinas. These findings suggest the possibility of developing a treatment modality for amblyopia with significant advantages over the current standard of care.
Kittens historically have been a preferred animal model to study ocular dominance plasticity, in part because of clear relevance of the findings to human amblyopia. As is the case in humans and nonhuman primates, MD and reverse occlusion are effective in kittens only during a well-defined sensitive period of early postnatal development; the physiological and anatomical changes in LGN and V1 correlate with altered acuity and binocularity assessed behaviorally; and temporary deprivation produces visual deficits that persist even when normal visual experience is restored. However, over the past 20 y, mice have supplanted other species for the investigation of visual cortical plasticity because of their numerous advantages for interventional mechanistic studies. Mice show many of the expected responses to temporary MD, including robust depression of deprived-eye responses. However, one unusual feature of mouse ocular dominance plasticity is that it persists well into adulthood (42), particularly when visual experience is enriched (49, 50). An additional complication is that the ocular dominance shift initiated in adults reverts spontaneously when binocular vision is restored (51, 52) or if both eyelids are closed (53). Thus, for the mouse to serve as a useful model for amblyopia following early-life MD, it was of particular importance to understand the degree to which the juvenile ocular dominance shift persists when binocular experience is restored. Previous studies in juvenile mice have shown that deprived-eye visual deficits persist through adulthood following 13-d MD ending at P32 (10), but not 4- to 5-d MD ending at P30 (51, 54). In our study, we monitored spontaneous recovery following 7-d MD (P26–P33). We took advantage of the fact that the effects of MD could be followed in the same animals through chronically implanted electrodes in V1. These longitudinal recordings revealed that like cats and primates, 7-d early MD in mice depresses responses to stimulation of the deprived eye, and this response depression persists for weeks. Whereas we did observe some evidence of spontaneous recovery during the first week after MD (Fig. S1B), deprived-eye responses in MD animals were still well below sham controls. These findings establish 7 d of early MD in mice as a useful protocol to investigate strategies for reversing ocular dominance plasticity and promoting recovery from amblyopia.
It has been known for many years from work in kittens and monkeys that deprivation-induced synaptic depression can be reversed if normal vision is restored in the weak (amblyopic) eye and the fellow eye is occluded (11, 12). However, gains in the amblyopic eye often come at the expense of vision in the fellow eye, may be temporary, and are rarely accompanied by improvement in binocularity (9, 17–19). Further, as a treatment for human amblyopia, patching is limited by poor compliance, has a variable outcome, and is effective only when initiated in early childhood (16, 19). A better understanding of what allows recovery with patching might suggest improvements over the current standard of care. One view is that recovery is enabled by a reduction in cortical activity that occurs when the strong eye is occluded (27). Support for this idea comes from findings that exposure of rats (28) and kittens (30, 32) to complete darkness for 10–14 d can promote recovery from the effects of MD when visual experience is restored. The fact that darkness, but not bilateral lid closure (31) is effective suggests that a key variable in changing the threshold for cortical plasticity is the level and/or variance of residual retinal activity. Our current findings using TTX to silence retinal activity directly support this hypothesis.
Dark exposure (to lower the plasticity threshold) followed by repetitive (55) or dichoptic (56) visual training exercises (to use binocular cooperativity to strengthen weak synapses in V1) offers a neurobiologically sound alternative to reverse occlusion (patching) as a treatment for amblyopia. However, previous studies in kittens have found that very brief daily light exposure eliminated the therapeutic effects of an otherwise uninterrupted 10-d period of darkness (32). Further, shortening the duration of absolute darkness to 5 d was insufficient to promote recovery of visual acuity or mobilize neuroplasticity mechanisms (30, 32). Therefore, the infrastructure and strict compliance requirements for effective dark exposure therapy could present a serious obstacle for clinical application of this approach, particularly to medically underserved communities (57, 58). In these respects, complete silencing of the retinas could offer significant advantages over dark exposure. Our data show rapid and complete recovery of visual responsiveness and acuity after binocular TTX treatment can be achieved following a single dose. In addition to shortening the treatment duration, pharmacological inactivation of the retinas represents a portable treatment that can circumvent compliance issues associated with enforcing an extended period of darkness. If the risks of the procedure and the duration of blockade can be minimized, while maintaining efficacy, the brevity of the period of visual incapacitation makes it potentially of greater appeal than the longer period of total darkness required to promote equivalent recovery. We believe the current findings are sufficiently compelling to justify additional preclinical studies to establish safety, minimal treatment duration, the maximal age of treatment effectiveness, and the maximal duration of MD that can be corrected.
In light of the translational potential, it is important to consider questions and concerns raised by this approach. First, TTX is a highly potent blocker of sodium-dependent action potentials. Although systemic exposure to TTX can be lethal, this risk is minimized by microinjection into the eyes. TTX was chosen for our experiments because a single treatment produces a long-lasting block of sodium channels, but there are many other approaches to achieve the same goal of silencing retinal output and it will be important for future studies to evaluate these alternatives. Second, although intravitreal injections are a routine ophthalmological procedure, they are not entirely without risk to the retina. The benefits of this or related approaches (e.g., retrobulbar block) will need to be weighed carefully against the risk of complications. Finally, there is an obvious requirement for monitoring patient safety during the period of temporary blindness.
Caveats aside, the remarkable effects of temporary inactivation of the visual pathway demonstrate that V1 synapses can be rejuvenated and at least some forms of amblyopia can be reversed. An urgent goal of future studies will be to assess precisely how retinal inactivation creates conditions in the ascending visual pathway that promote recovery. For example, it has been shown in mice that retinal silencing with TTX can promote bursting in the LGN (41) and burst stimulation of the geniculate can potentiate VEPs (59). Homeostatic adaptations that facilitate experience-dependent synaptic potentiation (24, 60, 61), such as reduced cortical inhibition and alterations in the properties of NMDA receptors (62–64), could also contribute to the therapeutic effects of retinal inactivation. We are encouraged to believe that the approach described here, and/or the knowledge gained by studying it, might ultimately have therapeutic potential for the treatment of amblyopia and other types of neurological rehabilitation.
Materials and Methods
All mouse experiments adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (65) and were approved by the Committee on Animal Care at the Massachusetts Institute of Technology (MIT). All cat experiments conformed to guidelines of the Canadian Council on Animal Care and were conducted according to protocols approved by the University Committee on Laboratory Animals at Dalhousie University. Details on experimental procedures are provided in SI Materials and Methods.
SI Materials and Methods
Mice.
Animals.
Electrophysiological studies were conducted in male wild-type mice on a C57BL/6 background obtained from Charles River Laboratories and maintained at MIT. Animals were housed in groups of three to five with food and water available ad libitum. Mice were maintained on a 12-h light/dark cycle, with all experimental procedures occurring during the light cycle. The final dataset included 34 mice from 11 litters. No explicit strategy for randomization was used, although each treatment group was represented by at least 1 animal per litter. All recordings were conducted blind to the deprivation and treatment conditions. All procedures adhered to the guidelines of the National Institutes of Health and were approved by the Committee on Animal Care at MIT.
Chronic surgical implants.
Analgesics were administered to mice s.c. (buprenorphine, 0.1 mg/kg) before surgery, and for 2 d postoperatively. Mice were anesthetized by inhalation of isoflurane (1–3% in oxygen) and kept on a heated surface (∼37 °C) throughout the surgical procedure. Fur was removed from the scalp, and the exposed skin was cleaned with ethanol [70% (vol/vol)] and povidone-iodine [10% (wt/vol)]. The skin was cut at the midline and connective tissue was removed. A steel post positioned anterior to bregma was fixed to the skull using cyanoacrylate. Small holes were drilled into the skull 3 mm lateral of lambda to expose the cortical surface, and tapered tungsten microelectrodes (300–500 MΩ, FHC) were lowered 450 μm to layer 4 of binocular V1. Silver reference electrodes were placed in prefrontal cortex. All electrodes were secured and the exposed skull was covered using cyanoacrylate and dental cement. Mice recovered in a heated chamber for 1 h before returning to their home cage. Signs of infection and discomfort were monitored by trained veterinary staff for 3 d postoperatively.
Visual stimulus.
Awake, head-fixed mice viewed stimuli on an LCD monitor in an otherwise dark room. Full-field visual stimuli were delivered using custom software written in MATLAB and using the PsychToolbox extension. Stimuli consisted of either a gray screen or sinusoidal gratings. Grating stimuli were presented at 0.05, 0.2, and 0.4 cycles per degree (cpd) and 100% contrast, and phase reversed at 2 Hz. For each spatial frequency, three blocks of 50 phase reversals were presented to each eye in pseudorandom order, with an opaque paddle used to restrict vision to one of the two eyes within a recording session (Fig. 1B). Each block of grating stimuli was separated by a 30-s presentation of gray. Gamma correction was used to ensure a linear gradient and constant total luminance in both gray screen and grating conditions. At each recording time point, a novel orientation of the grating stimuli was presented, and any orientation presented was at least 30° offset from a previously viewed orientation. The order of orientations presented was the same within littermates and varied between cohorts.
V1 electrophysiology.
Local field potentials (LFPs) were recorded continuously at 1 kHz via chronically implanted microelectrodes. Data were amplified and digitized using the Plexon Recorder-64 system, and low-pass filtered (200-Hz cut). Recorded data were extracted and analyzed using custom software written in C++ and MATLAB. VEP waveforms were generated from a phase reversal-triggered average of the LFP for each animal, time point, and viewing eye. VEP magnitude was defined as the peak-to-peak amplitude of this biphasic VEP waveform. All recordings were performed blind to treatment condition.
Eyelid suture.
Mice were anesthetized by inhalation of isoflurane (1–3% in oxygen) and kept on a heated surface (∼37 °C) throughout the procedure. The eye was rinsed with sterile eye drops and coated with ophthalmic ointment containing bacitracin, neomycin, and polymysin. For all animals, mattress stitching of sterile silk 7-0 suture thread was used to close the eye from the temporal to nasal corners. For sham animals, these sutures were removed immediately, and inhalant anesthesia was delivered 45 min before the next recording. For MD animals, eyelids were kept closed for 7 d, and sutures were removed under inhalant anesthesia 45 min before recording. If eyelids came open before the end of 7 d, animals were excluded from the study.
Intravitreal injections.
Mice were anesthetized by inhalation of isoflurane (1–3% in oxygen) and kept on a heated surface (∼37 °C) throughout the procedure. A sterile silk 7–0 suture thread was gently pulled through the conjunctiva, and this suture was secured anteriorly to expose the temporal portion of the eyeball. A sterile 30-gauge needle was used to puncture the globe at the limbus to gain access to the vitreous chamber. A glass micropipette containing TTX (1 mM in citrate buffer) or saline was introduced into the vitreous chamber, and a nanoliter injector was used to deliver 1 µL of solution. After removing the micropipette, the eye was rinsed with sterile eye drops and coated with ophthalmic ointment containing bacitracin, neomycin, and polymysin. For TTX-injected animals, retinal inactivation was verified by the absence of direct and consensual pupillary light reflexes.
Postmortem analyses.
At the conclusion of the study, mice were deeply anesthetized by inhalation of isoflurane and subsequently decapitated. Brains and eyeballs of all subjects were harvested for further analysis. For brains, tissue was fixed in a solution containing 4% (wt/vol) paraformaldehyde for 72 h at room temperature and then sectioned into 50-µm coronal slices using a vibratome. Slices were mounted and allowed to dry for 24 h, followed by staining of the Nissl bodies using cresyl violet. Images of binocular V1 were acquired using a confocal microscope, and these images were analyzed for the presence and location of electrode tracks. Animals that did not display an electrode track localized to layer 4 of binocular V1 were excluded from the study. For eyeballs, corneas were examined and retinas were rapidly dissected out of each eye. Animals displaying any signs of corneal or retinal damage were excluded from the study. All exclusion criteria were determined before conducting the study. All analyses were performed blind to treatment.
Statistics.
Statistical analyses were performed using Prism (GraphPad). VEP magnitudes were analyzed using a two-way repeated measures analysis of variance to examine changes over time and across treatment conditions. To compare changes from baseline values over time while correcting for multiple comparisons, we used Dunnett’s post hoc test (Fig. 1D). To compare differences across different treatment conditions at each time point while correcting for multiple comparisons, we used Tukey’s post hoc test (Fig. 1E and Fig. S1B). All post hoc tests were two sided. Reported sample sizes denote individual animals (biological replicates). At the outset of the study, our target sample sizes were 10–12 mice per treatment condition, although no explicit statistical tests were performed a priori as effect sizes were unknown. Variance was similar between mice in the three treatment groups.
Kittens.
Animals.
Behavioral and anatomical studies were conducted on, respectively, 5 kittens (two males and three females) from two litters and 13 kittens (five males and eight females) from six litters that were all born and raised in a closed breeding colony at Dalhousie University. No explicit strategy for randomization was used. No animals were excluded from the datasets. Rearing and experimental procedures were conducted according to protocols approved by the University Committee on Laboratory Animals at Dalhousie University and conformed to guidelines of the Canadian Council on Animal Care.
Rearing.
Kittens used for anatomy were either normally reared (n = 3), were monocularly deprived for 7 d at the peak of the critical period (66) (P30; n = 4), or were monocularly deprived for 7 d at P30 and then subjected to binocular retinal silencing for 4 d (n = 2; two TTX injections), 6 d (n = 1; three TTX injections), or 10 d (n = 3; five TTX injections). Five kittens used in behavioral experiments were monocularly deprived at P30 for 7 d after which the deprived eye was opened so that acuity measurements for both eyes could be made and the depth of amblyopia in the deprived eye documented. Four of the kittens received two intravitreal injections of TTX spaced 2 d apart at either P94 and P96 (C363, C364, and C365), or at P99 and P101 (C349). The fifth kitten (C363) also received two intravitreal TTX injections but separated by 6 wk at P94 and P135.
Surgical procedures.
Monocular deprivation was performed under general gaseous anesthesia (3–4% isoflurane in oxygen) and involved closure of the upper and lower palpebral conjunctivae of the left eye with vicryl suture material, followed by closure of the eyelids with silk suture. Anesthetized animals received a s.c. injection of Anafen for postprocedure analgesia, local anesthesia was produced with Alcaine sterile ophthalmic solution (1% proparacaine hydrochloride), and a broad-spectrum topical antibiotic (1% chloromycetin) was administered to mitigate infection after surgery. Following the period of MD, sutures were removed under general gaseous anesthesia to open the eye. For anatomical studies, general anesthesia was maintained immediately after the eyelids of the deprived eye had been opened to allow intravitreal injections of TTX (ab120055, Abcam) made after first making a small puncture through the sclera just posterior to the ora serrata on the temporal side of the eye using a sterile 30-gauge needle. Using a surgical microscope, injections of TTX (3 mM dissolved in citrate buffer; 0.5 µL/100 gm body weight) were made using a sterilized Hamilton syringe with a 30-gauge needle (point style 4) positioned about 5–10 mm into the vitreous chamber through the original scleral puncture. The full volume of TTX was slowly dispensed into the vitreous chamber and after waiting about 1 min the needle was slowly retracted. Topical antibiotic (1% chloromycetin) and Alcaine solution were administered to the eye after injection. Subsequent TTX injections were made every 48 h using the original injection site to avoid having to make additional punctures. The same procedure was followed for the intravitreal injections of TTX for the kittens of the behavioral studies with the exception that they occurred later at P92–P94. Basic assessment of visual behavior during the period of retinal inactivation were made daily on all animals. In all but one animal (C363) there was an absence of any pupillary light reflex and visuomotor behaviors such as visual placing, visual startle, visual tracking of moving objects or laser pointers, as well as a lack of evidence of vision-mediated behavior when interacting with littermates or staff. For C363, the initial injection did not fully block retinal activity as evidenced by crude visual placing and following responses as well as incomplete pupillary dilation. A second TTX injection was made on this animal 6 wk after the first and all observations after the second injection indicated complete retinal inactivation.
Histology.
In preparation for histology, animals were euthanized with a lethal dose of sodium pentobarbital (pentobarbital sodium; 150 mg/kg) and shortly thereafter exsanguinated by transcardial perfusion with ∼150 mL of PBS followed by an equal volume of PBS containing 4% (wt/vol) dissolved paraformaldehyde. Brain tissue was immediately extracted and the thalamus was dissected from the remainder of the brain to prepare the LGN for sectioning and histological processing. Tissue containing the LGN was cryoprotected and then cut coronally into 25-µm thick sections by use of a sliding microtome. A subset of sections was mounted onto glass slides and stained for Nissl substance using 0.1% cresyl violet acetate dye dissolved in distilled water. The cross-sectional area of neuron somata within A and A1 layers of the left and right LGN was measured from Nissl-stained sections using the nucleator probe from a computerized stereology system (newCAST, VisioPharm). All area measurements were performed using a BX-51 compound microscope with a 60× oil-immersion objective (Olympus Canada, Inc.). Neurons were distinguished from glial cells using established selection criteria (67–69) that included measurement of cells with dark cytoplasmic and nucleolar staining and with light nuclear staining. Adherence to these criteria permitted avoidance of cell caps and inclusion only of neurons cut through the somal midline. Approximately 1,500–2,000 neurons were measured from each animal. For each animal, the effect of MD was determined using a deprivation metric that calculated the percentage difference between deprived and nondeprived layers (69). Soma sizes were assessed using a one-way analysis of variance, followed by two-tailed post hoc t tests with Bonferroni correction for multiple comparisons. Reported sample sizes denote individual animals (biological replicates).
Behavioral testing.
Measurements of the visual acuity for square-wave gratings were made by use of a jumping stand and procedures (17, 32, 44) that have been refined over four decades. Kittens were required to make a two-alternative forced-choice discrimination between a vertical and an adjacent horizontal grating of the same spatial frequency on a jumping stand (Fig. 2A). The gratings were 19 cm square surrounded on all sides by a gray border 3 cm wide, had a luminance of 80 cd/m2, and a Michelson contrast close to 1.0. They were printed by a dot matrix printer on heavy paper (56 lb), mounted on cardboard, and spray painted with a clear protective coating. A correct jump to the vertical grating was rewarded with wet kitten food and petting but after an error they were denied the rewards and required immediately to repeat the trial until correct. After an error it was necessary for kittens to make five consecutively correct responses or else seven correct out of a maximum of 10 trials presented at each spatial frequency. The side of the vertical grating was changed in a pseudorandom manner according to a Gellerman sequence (70). The spatial frequency of the gratings was increased gradually in small and equal logarithmic steps that were larger at the start of a testing session but increased to as many as 12 steps/octave within 1–2 octaves of threshold. At the start of a testing session, the spatial frequency of the grating was increased after each correct response but the minimum number of trials was increased thereafter to at least 3–5 trials within an octave of the presumed threshold. As a possible consequence of small incremental steps of spatial frequency, the performance of kittens usually was flawless until it dropped to chance over only one or two steps. Effects of treatment were examined on an individual animal basis, so explicit statistical tests were performed.
Acknowledgments
We thank Yasemin Atiyas, Julia Deere, and Filia Van Dessel for assistance with the collection of mouse data; Paige Northrup for assistance with the kitten studies; and Jeffrey Gavornik and Arnold Heynen for performing key pilot experiments. This research was supported by National Eye Institute Grant 5R01EYO23037-03 (to M.F.B.), Canadian Institutes of Health Research Grant 102653 (to K.R.D. and D.E.M.), Natural Sciences and Engineering Research Council Discovery Grants 298167 (to K.R.D.) and 7660 (to D.E.M.), the Picower Institute Innovation Fund, and the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: The authors disclose a patent filing on the use of retinal inactivation to treat human amblyopia.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1613279113/-/DCSupplemental.
References
- 1.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 2.Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16(10):3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191(1):1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 5.Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44(6):917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Khibnik LA, Cho KK, Bear MF. Relative contribution of feedforward excitatory connections to expression of ocular dominance plasticity in layer 4 of visual cortex. Neuron. 2010;66(4):493–500. doi: 10.1016/j.neuron.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman JE, et al. Rapid structural remodeling of thalamocortical synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. J Neurosci. 2010;30(29):9670–9682. doi: 10.1523/JNEUROSCI.1248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harwerth RS, Smith EL, 3rd, Boltz RL, Crawford ML, von Noorden GK. Behavioral studies on the effect of abnormal early visual experience in monkeys: Spatial modulation sensitivity. Vision Res. 1983;23(12):1501–1510. doi: 10.1016/0042-6989(83)90162-1. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell DE. The extent of visual recovery from early monocular or binocular visual deprivation in kittens. J Physiol. 1988;395:639–660. doi: 10.1113/jphysiol.1988.sp016939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci. 2003;17(1):167–173. doi: 10.1046/j.1460-9568.2003.02420.x. [DOI] [PubMed] [Google Scholar]
- 11.Blakemore C, Van Sluyters RC. Reversal of the physiological effects of monocular deprivation in kittens: Further evidence for a sensitive period. J Physiol. 1974;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blakemore C, Garey LJ, Vital-Durand F. The physiological effects of monocular deprivation and their reversal in the monkey’s visual cortex. J Physiol. 1978;283:223–262. doi: 10.1113/jphysiol.1978.sp012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond GT, Scott WE, Keech RV. Management of monocular congenital cataracts. Arch Ophthalmol. 1989;107(1):45–51. doi: 10.1001/archopht.1989.01070010047025. [DOI] [PubMed] [Google Scholar]
- 14.Robb RM, Mayer DL, Moore BD. Results of early treatment of unilateral congenital cataracts. J Pediatr Ophthalmol Strabismus. 1987;24(4):178–181. doi: 10.3928/0191-3913-19870701-07. [DOI] [PubMed] [Google Scholar]
- 15.Birch EE, Swanson WH, Stager DR, Woody M, Everett M. Outcome after very early treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1993;34(13):3687–3699. [PubMed] [Google Scholar]
- 16.Lewis TL, Maurer D. Multiple sensitive periods in human visual development: Evidence from visually deprived children. Dev Psychobiol. 2005;46(3):163–183. doi: 10.1002/dev.20055. [DOI] [PubMed] [Google Scholar]
- 17.Murphy KM, Mitchell DE. Reduced visual acuity in both eyes of monocularly deprived kittens following a short or long period of reverse occlusion. J Neurosci. 1987;7(5):1526–1536. doi: 10.1523/JNEUROSCI.07-05-01526.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell DE. The long-term effectiveness of different regimens of occlusion on recovery from early monocular deprivation in kittens. Philos Trans R Soc Lond B Biol Sci. 1991;333(1266):51–79. doi: 10.1098/rstb.1991.0060. [DOI] [PubMed] [Google Scholar]
- 19.Birch EE. Amblyopia and binocular vision. Prog Retin Eye Res. 2013;33:67–84. doi: 10.1016/j.preteyeres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birch EE, Stager DR. The critical period for surgical treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1996;37(8):1532–1538. [PubMed] [Google Scholar]
- 21.Ellemberg D, Lewis TL, Maurer D, Brent HP. Influence of monocular deprivation during infancy on the later development of spatial and temporal vision. Vision Res. 2000;40(23):3283–3295. doi: 10.1016/s0042-6989(00)00165-6. [DOI] [PubMed] [Google Scholar]
- 22.Mioche L, Singer W. Chronic recordings from single sites of kitten striate cortex during experience-dependent modifications of receptive-field properties. J Neurophysiol. 1989;62(1):185–197. doi: 10.1152/jn.1989.62.1.185. [DOI] [PubMed] [Google Scholar]
- 23.Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19(4):126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381(6582):526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- 25.Philpot BD, Cho KK, Bear MF. Obligatory role of NR2A for metaplasticity in visual cortex. Neuron. 2007;53(4):495–502. doi: 10.1016/j.neuron.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo MC, Dringenberg HC. Short-term (2 to 5 h) dark exposure lowers long-term potentiation (LTP) induction threshold in rat primary visual cortex. Brain Res. 2009;1276:58–66. doi: 10.1016/j.brainres.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 27.Cooper LN, Bear MF. The BCM theory of synapse modification at 30: Interaction of theory with experiment. Nat Rev Neurosci. 2012;13(11):798–810. doi: 10.1038/nrn3353. [DOI] [PubMed] [Google Scholar]
- 28.He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat Neurosci. 2007;10(9):1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- 29.Montey KL, Quinlan EM. Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nat Commun. 2011;2:317. doi: 10.1038/ncomms1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffy KR, Mitchell DE. Darkness alters maturation of visual cortex and promotes fast recovery from monocular deprivation. Curr Biol. 2013;23(5):382–386. doi: 10.1016/j.cub.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Duffy KR, Bukhamseen DH, Smithen MJ, Mitchell DE. Binocular eyelid closure promotes anatomical but not behavioral recovery from monocular deprivation. Vision Res. 2015;114:151–160. doi: 10.1016/j.visres.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell DE, MacNeill K, Crowder NA, Holman K, Duffy KR. Recovery of visual functions in amblyopic animals following brief exposure to total darkness. J Physiol. 2016;594(1):149–167. doi: 10.1113/JP270981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell DE, Crowder NA, Holman K, Smithen M, Duffy KR. Ten days of darkness causes temporary blindness during an early critical period in felines. Proc Biol Sci. 2015;282(1803):20142756. doi: 10.1098/rspb.2014.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho KK, Bear MF. Promoting neurological recovery of function via metaplasticity. Future Neurol. 2010;5(1):21–26. doi: 10.2217/fnl.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397(6717):347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- 36.Heynen AJ, et al. Molecular mechanism for loss of visual cortical responsiveness following brief monocular deprivation. Nat Neurosci. 2003;6(8):854–862. doi: 10.1038/nn1100. [DOI] [PubMed] [Google Scholar]
- 37.Rittenhouse CD, et al. Stimulus for rapid ocular dominance plasticity in visual cortex. J Neurophysiol. 2006;95(5):2947–2950. doi: 10.1152/jn.01328.2005. [DOI] [PubMed] [Google Scholar]
- 38.Dräger UC. Observations on monocular deprivation in mice. J Neurophysiol. 1978;41(1):28–42. doi: 10.1152/jn.1978.41.1.28. [DOI] [PubMed] [Google Scholar]
- 39.Smith GB, Heynen AJ, Bear MF. Bidirectional synaptic mechanisms of ocular dominance plasticity in visual cortex. Philos Trans R Soc Lond B Biol Sci. 2009;364(1515):357–367. doi: 10.1098/rstb.2008.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooke SF, Bear MF. How the mechanisms of long-term synaptic potentiation and depression serve experience-dependent plasticity in primary visual cortex. Philos Trans R Soc Lond B Biol Sci. 2013;369(1633):20130284. doi: 10.1098/rstb.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linden ML, Heynen AJ, Haslinger RH, Bear MF. Thalamic activity that drives visual cortical plasticity. Nat Neurosci. 2009;12(4):390–392. doi: 10.1038/nn.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawtell NB, et al. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38(6):977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 43.Murphy WJ, et al. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science. 2001;294(5550):2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell DE, Giffin F, Timney B. A behavioural technique for the rapid assessment of the visual capabilities of kittens. Perception. 1977;6(2):181–193. doi: 10.1068/p060181. [DOI] [PubMed] [Google Scholar]
- 45.Guillery RW. Binocular competition in the control of geniculate cell growth. J Comp Neurol. 1972;144(1):117–129. doi: 10.1002/cne.901440106. [DOI] [PubMed] [Google Scholar]
- 46.Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- 47.Bear MF, Colman H. Binocular competition in the control of geniculate cell size depends upon visual cortical N-methyl-D-aspartate receptor activation. Proc Natl Acad Sci USA. 1990;87(23):9246–9249. doi: 10.1073/pnas.87.23.9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duffy KR, Slusar JE. Monocular deprivation provokes alteration of the neuronal cytoskeleton in developing cat lateral geniculate nucleus. Vis Neurosci. 2009;26(3):319–328. doi: 10.1017/S0952523809090130. [DOI] [PubMed] [Google Scholar]
- 49.Matthies U, Balog J, Lehmann K. Temporally coherent visual stimuli boost ocular dominance plasticity. J Neurosci. 2013;33(29):11774–11778. doi: 10.1523/JNEUROSCI.4262-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greifzu F, et al. Environmental enrichment extends ocular dominance plasticity into adulthood and protects from stroke-induced impairments of plasticity. Proc Natl Acad Sci USA. 2014;111(3):1150–1155. doi: 10.1073/pnas.1313385111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Prior experience enhances plasticity in adult visual cortex. Nat Neurosci. 2006;9(1):127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- 52.Rose T, Jaepel J, Hübener M, Bonhoeffer T. Cell-specific restoration of stimulus preference after monocular deprivation in the visual cortex. Science. 2016;352(6291):1319–1322. doi: 10.1126/science.aad3358. [DOI] [PubMed] [Google Scholar]
- 53.Pham TA, et al. A semi-persistent adult ocular dominance plasticity in visual cortex is stabilized by activated CREB. Learn Mem. 2004;11(6):738–747. doi: 10.1101/lm.75304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaneko M, Hanover JL, England PM, Stryker MP. TrkB kinase is required for recovery, but not loss, of cortical responses following monocular deprivation. Nat Neurosci. 2008;11(4):497–504. doi: 10.1038/nn2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montey KL, Eaton NC, Quinlan EM. Repetitive visual stimulation enhances recovery from severe amblyopia. Learn Mem. 2013;20(6):311–317. doi: 10.1101/lm.030361.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, et al. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol. 2013;23(8):R308–R309. doi: 10.1016/j.cub.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 57.Simons K. Amblyopia characterization, treatment, and prophylaxis. Surv Ophthalmol. 2005;50(2):123–166. doi: 10.1016/j.survophthal.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Courtright P, Hutchinson AK, Lewallen S. Visual impairment in children in middle- and lower-income countries. Arch Dis Child. 2011;96(12):1129–1134. doi: 10.1136/archdischild-2011-300093. [DOI] [PubMed] [Google Scholar]
- 59.Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30(48):16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arendt KL, Sarti F, Chen L. Chronic inactivation of a neural circuit enhances LTP by inducing silent synapse formation. J Neurosci. 2013;33(5):2087–2096. doi: 10.1523/JNEUROSCI.3880-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Félix-Oliveira A, Dias RB, Colino-Oliveira M, Rombo DM, Sebastião AM. Homeostatic plasticity induced by brief activity deprivation enhances long-term potentiation in the mature rat hippocampus. J Neurophysiol. 2014;112(11):3012–3022. doi: 10.1152/jn.00058.2014. [DOI] [PubMed] [Google Scholar]
- 62.Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci USA. 1999;96(22):12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Philpot BD, Sekhar AK, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29(1):157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 64.Whitt JL, Petrus E, Lee HK. Experience-dependent homeostatic synaptic plasticity in neocortex. Neuropharmacology. 2014;78:45–54. doi: 10.1016/j.neuropharm.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed Natl Acad Press; Washington, DC: 1985. [Google Scholar]
- 66.Olson CR, Freeman RD. Profile of the sensitive period for monocular deprivation in kittens. Exp Brain Res. 1980;39(1):17–21. doi: 10.1007/BF00237065. [DOI] [PubMed] [Google Scholar]
- 67.Wiesel TN, Hubel DH. Effects of visual deprivation on morphology and physiology of cells in the cats lateral geniculate body. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- 68.Guillery RW, Stelzner DJ. The differential effects of unilateral lid closure upon the monocular and binocular segments of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1970;139(4):413–421. doi: 10.1002/cne.901390403. [DOI] [PubMed] [Google Scholar]
- 69.Duffy KR, Holman KD, Mitchell DE. Shrinkage of X cells in the lateral geniculate nucleus after monocular deprivation revealed by FoxP2 labeling. Vis Neurosci. 2014;31(3):253–261. doi: 10.1017/S0952523813000643. [DOI] [PubMed] [Google Scholar]
- 70.Gellerman LW. Chance orders of alternating stimuli in visual discrimination. J Genet Psychol. 1933;42:206–208. [Google Scholar]