Abstract
REST/NRSF is a transcriptional repressor that acts at the terminal stage of the neuronal differentiation pathway and blocks the transcription of several differentiation genes. REST/NRSF is generally downregulated during induction of neuronal differentiation. The recombinant transcription factor REST-VP16 binds to the same DNA binding site as does REST/NRSF but functions as an activator instead of a repressor and can directly activate the transcription of REST/NRSF target genes. However, it is not known whether REST-VP16 expression is sufficient to cause formation of functional neurons from neural stem cells (NSCs). Here we show that regulated expression of REST-VP16 in a physiologically relevant NSC line growing under cycling conditions converted the cells rapidly to the mature neuronal phenotype. Furthermore, when grown in the presence of retinoic acid, REST-VP16-expressing NSCs activated their target, as well as other differentiation genes that are not their direct target, converting them to the mature neuronal phenotype and enabling them to survive in the presence of mitotic inhibitors, which is a characteristic of mature neurons. In addition, these neuronal cells were physiologically active. These results showed that direct activation of REST/NRSF target genes in NSCs with a single transgene, REST-VP16, is sufficient to cause neuronal differentiation, and the findings suggested that direct activation of genes involved in the terminal stage of differentiation may cause neuronal differentiation of NSCs.
Differentiation of neural stem cells (NSCs) into neurons is generally believed to occur in four steps characterized by the expression and action of specific gene products (7, 11). Thus, NSCs, which can multiply and produce their own kind under one set of conditions and differentiate under other conditions, express p75 and nestin. The neuronal determination step is characterized by the action of basic helix-loop-helix proteins such as MASH, MATH, and NeuroD3/neurogenin. Next is the commitment step, in which genes such as those that encode neuroD1/2, Myt1, and neurofilament 150 are expressed. The terminal differentiation step is characterized by the expression of genes such as those that encode SCG10, sodium channel type II, synapsin, glutamate receptor, and acetylcholine receptor. These terminal differentiation genes are the main direct targets of the repressor element 1 (RE1)-silencing transcription factor (REST)/neuron-restrictive silencer factor (NRSF) (5, 21). REST/NRSF contains a DNA binding domain and two repressor domains, one at the N terminal and the other at the C terminal (5, 21). REST/NRSF blocks transcription of its target genes by binding to a specific consensus RE1 binding site/neuron-restrictive silencer element (RE1/NRSE) that is present in the target genes' regulatory regions (5, 21). REST/NRSF-dependent promoter repression requires interaction with several cellular cofactors, including Co-REST, N-CoR, mSin3A, and histone deacetylase complex, and activity of histone deacetylase (1, 2, 6, 8). Although REST/NRSF is expressed mainly in nonneural cells (5, 21), some studies have shown that REST/NRSF is expressed in certain mature neurons in adults (6, 9), suggesting that it has a complex role that depends on its cellular and physiological environment. There are several differentially spliced isoforms of REST/NRSF (16). One such isoform, REST4, functions as a dominant-negative regulator by competing with REST/NRSF for DNA binding in neurons (23, 26). In addition, both REST/NRSF and REST4 interact with RILP, a LIM domain protein, for nuclear translocation (22). Thus, several mechanisms have the potential to regulate the activity of REST/NRSF in cells. REST/NRSF is downregulated for the induction and maintenance of the neuronal phenotype (1); overexpression of REST/NRSF in differentiating neurons disrupts neuronal gene expression and causes axon guidance errors (17).
To activate the target genes of REST/NRSF, we previously constructed a recombinant transcription factor, REST-VP16, by replacing the repressor domains of REST/NRSF with the activation domain of herpes simplex virus protein VP16. Because REST-VP16 still contains the DNA binding domain of REST/NRSF, we found that REST-VP16, when transiently expressed in mammalian neuronal cells, operates through the RE1/NRSE, competes with endogenous REST/NRSF for DNA binding, and activates REST/NRSF target genes (7, 11). In the present study, we investigated whether direct activation of REST/NRSF target genes through a single transgene, REST-VP16, would convert NSCs to the mature neuronal phenotype.
MATERIALS AND METHODS
Plasmids.
The NheI/XhoI fragment of pcDNA3.1-REST-VP16 (7) was subcloned into NheI/XhoI-digested plasmid pBig2r (25). The clone obtained was confirmed by sequencing the junction region. Construction of pNaCh, pNaChΔRE1, pT.luc, pRE.T.luc, pREST/NRSF, pGal4-VP16, and pREST-VP16 has been described elsewhere (7, 11, 28). pCMV-βGAL was purchased from Stratagene.
Cell culture and differentiation.
Mouse multipotent C17.2 NSCs were originally described by Snyder et al. (24) and were used throughout this study. The NSCs were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (GIBCO) and 5% horse serum (GIBCO) (growth medium) and never grown to confluence. For REST-VP16-mediated differentiation, cells were grown on poly-l-lysine (PLL; Sigma)-coated CC2 (Lab-Tek) chamber slides for 2, 8, or 16 days in the medium described above. For retinoic acid (RA) and mitotic inhibitor treatments, cells were grown in growth medium for 24 h on Corning dishes coated with PLL at 10 μg/ml for 24 h; the medium was then changed to growth medium with 10 μM RA (Sigma) for 4 weeks to induce neuronal differentiation. Cells were split 1:4 by trypsinization and subcultured on PLL- and laminin (Sigma)-coated Corning dishes for 2 days. Cells were then treated with DMEM containing 5% fetal calf serum and the mitotic inhibitors 10 μM 5-fluoro-2′-deoxyuridine, 10 μM uridine, and 1 μM cytosine β-d-arabinofuranoside for 5 days. All media were changed every 2 days.
PLL and laminin coating.
Coating of Corning culture dishes or CC2 chamber slides with PLL or PLL plus laminin was done with 10 μg of PLL per ml in distilled water. This solution was applied for 15 min, aspirated, washed five times for 10 min each time, aspirated, and air dried. For laminin coating of PLL-coated Corning dishes or CC2 slides, 15 μg of laminin per ml in phosphate-buffered saline (PBS) containing 1 mM CaCl2 and 1 mM MgCl2 at room temperature was applied, and the dishes or slides were incubated overnight. Excess laminin solution was aspirated from the dishes or slides, and the cells were rinsed twice with culture medium, air dried, and used immediately.
Stable transfection.
C17.2 cells were transfected with plasmid pBig2r or pBig2r-REST-VP16 with Fugene 6 (Roche) in accordance with the manufacturer's instructions. Two days after transfection, 750 μg of hygromycin B (Roche) per ml was added for clone selection. The culture medium was refreshed every 2 days with 4 μg of doxycycline (Sigma) per ml. Doxycycline-inducible clones, pBig2r-REST-VP16, and vector pBig2r, were selected by induction of REST-VP16 and tTA after removal of doxycycline for 2 days and identified by Western blot analysis. After selection, clones were maintained in medium containing 500 μg of hygromycin B per ml. During the assays, the concentration of hygromycin B was reduced to 250 μg/ml to avoid removal of the transgene.
Calcium imaging.
Living-cell microscopy to detect depolarization-dependent calcium influx was performed as previously described (18, 20, 28). A confocal laser system (Olympus Fluoview FV500) and the fluorescent calcium indicator Fluo-3 (Molecular Probes) were used to measure the intracellular free calcium concentration. Cells were loaded with Fluo-3 and depolarized with high-KCl (100 mM) Ringer solution as previously described (3). Fluorescence was measured every 0.25 s for a total of 12 s. For the glutamate experiment, 10 μM glutamate was included in Ringer solution.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Cells were cultured on six-well Corning plates for 2 days or on PLL-coated six-well Corning plates for 8 days without doxycycline. Cells were then rinsed and lysed directly in Laemmli sample buffer (Bio-Rad). Lysates were vortexed vigorously, boiled for 5 min, and electrophoresed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis-ready gels (Bio-Rad). Gels were transferred to Hybond-P polyvinylidene difluoride membranes (Amersham Biosciences, Buckinghamshire, United Kingdom), and blots were detected with ECL-plus reagents (Amersham).
Antibodies.
The following antibodies were used: anti-VP16 (1:100; Clontech), anti-unique β-tubulin (Western blotting, 1:500; immunofluorescence assay, 1:1,000; Tuj1; Covance Research Products), anti-synaptotagmin I (1:500; AB5600; Chemicon), anti-microtubule-associated protein 2 (MAP2) (1:1,000; HM-2; Sigma), anti-α-tubulin (1:1,000; B-5-1-2; Sigma), antiactin (1:2,000; I-19; Santa Cruz), horseradish peroxidase-conjugated anti-mouse, anti-rabbit immunoglobulin G (heavy and light chains; 1:20,000; Amersham), and Cy3-labeled anti-mouse or anti-rabbit immunoglobulin G (heavy and light chains; 1:1,000; Amersham).
Reporter gene assays.
C17.2 cells and c17.2-pBig2r and C17.2-REST-VP16 clones were plated in six-well Corning plates for 3 days in complete DMEM without doxycycline. pT-Luc or pRE-T-Luc (2 μg) was transfected by Fugene 6 (Roche) in accordance with the manufacturer's instructions. Cells were collected after 48 h and assayed for protein concentration with the bicinchoninic acid protein assay kit (Pierce) and for luciferase activity as previously described (7).
Immunofluorescence assay.
Cells were fixed on PLL-coated or PLL-laminin-coated CC2 chamber slides (see description of cell culture and differentiation) for 20 to 30 min at room temperature with either 4% paraformaldehyde in PBS or 10% buffered formalin, rinsed in PBS, permeabilized, and blocked in blocking buffer (5% nonfat dry milk [Nestle], 0.3% Triton X-100 σ, PBS) for 30 min at room temperature. Primary antibodies were diluted in the blocking buffer and incubated overnight at 4°C under humidified conditions. Secondary antibodies were diluted in PBS and incubated for 1 h at room temperature. For nuclear staining, 0.1 μg of Hoechst 33328 (Molecular Probes) per ml in water was used. Photographs were taken with a Hamamatsu 5880 color charge-coupled device camera equipped with a Leica DMR microscope. Double-label images were assembled in Adobe Photoshop.
RESULTS
Construction of NSC clones stably expressing doxycycline-regulated REST-VP16.
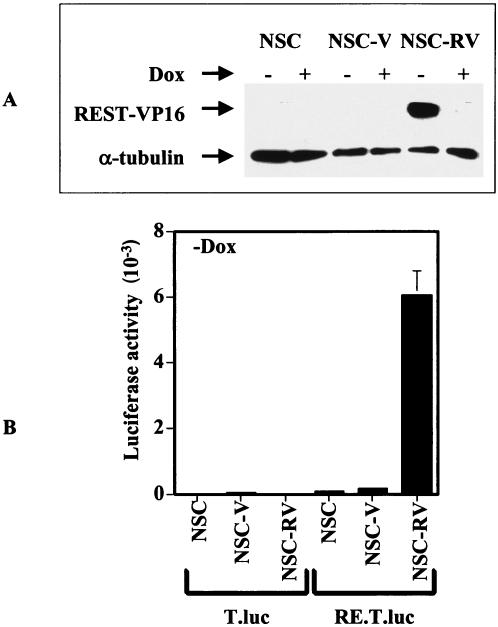
To determine the effect of REST-VP16 expression in NSCs, we generated stable C17.2 mouse clonal NSCs by using a bidirectional doxycycline-regulated vector, pBig2r (25). These NSC lines were generated by transduction of v-myc into the external granule layer cells of normal mouse cerebellum (14). These cells can differentiate into normal neuronal and glial cells in vitro and in vivo, do not form tumors in the brain, and can be integrated into the normal brain structures, indicating that they have the functional endogenous machinery to perform normal biological functions of NSCs (14). The clonal cell line NSC-RVs stably expressed the REST-VP16 protein when grown without doxycycline (−Dox) for 2 days, as shown by Western blotting with anti-VP16 antibodies (Fig. 1A). In contrast, C17.2 cells (NSCs) or C17.2 cells expressing the vector alone (NSC-Vs) and grown with or without doxycycline (+Dox) did not produce detectable levels of the REST-VP16 protein. Similarly, NSC-RVs grown with doxycycline produced a low level of REST-VP16, probably as a result of the leakiness of the doxycycline-regulated system (25, 28). In these experiments, anti-α-tubulin was used as an internal control. Taken together, these experiments showed that NSC-RVs efficiently expressed REST-VP16 in the absence of doxycycline. The behavior of another REST-VP16 clone, NSC-RV′, was similar to that of NSC-RVs in all of our experiments described below (data not shown), indicating that the effect of the REST-VP16 clone was independent of the genomic integration site.
FIG. 1.
Expression of functional REST-VP16 in stable NSC clones. (A) C17.2 cells (NSC) and stable C17.2 clones encoding the vector (NSC-V), or REST-VP16 (NSC-RV) were grown in growth medium (as described in Materials and Methods) with and without doxycycline (Dox) for 2 days, and the cell extracts (20 μg) were analyzed by Western blotting with anti-VP16 and anti-α-tubulin antibodies. (B) NSC-RV (−Dox) cells producing the REST-VP16 protein can activate the basal TATA box promoter through the RE1 binding sites. To determine whether the REST-VP16 protein was transcriptionally active, we constructed synthetic promoters containing a luciferase reporter gene under the control of a TATA box alone (pT.luc) or a TATA box plus two copies of the wild-type RE1/NRSE sequence (pRE.T.luc). The cells were grown without doxycycline and transfected with the reporter plasmid pT.luc or pRE.T.luc along with an internal control plasmid (pβ-gal) and analyzed as described in the text and elsewhere (7). Luciferase expression is shown for pT.luc and pRE.T.luc.
To determine whether the REST-VP16 protein was transcriptionally active, we devised a reporter gene assay system to be used before using a reporter plasmid system (7, 11). We constructed synthetic promoters containing a luciferase reporter gene under the control of a TATA box alone (pT.luc) or a TATA box plus two copies of the wild-type RE1/NRSE sequence (pRE.T.luc). A transfection assay was performed with NSCs, NSC-Vs, and NSC-RVs and these plasmids. The plasmid pβ-gal was used as an internal control in these experiments, and the luciferase activity obtained from each experiment was divided by the corresponding β-galactosidase activity. As shown in Fig. 1B, a high level of luciferase activity was observed only from pRE.T.luc and only in NSC-RVs grown without doxycycline, indicating that NSC-RVs produced large amounts of transcriptionally active REST-VP16 protein. When cells were grown with doxycycline, little luciferase activity was observed (data not shown).
REST-VP16 converts NSCs to the mature neuronal phenotype.
To determine the ability of stably expressed REST-VP16 protein to induce neuronal differentiation of NSCs, NSC-Vs and NSC-RVs were allowed to grow under cycling conditions in the presence of full growth medium as described in Materials and Methods with and without doxycycline. As shown in Fig. 2A, by day 8 NSC-RVs grown without doxycycline expressed neuronal β-tubulin, a neuronal differentiation marker, as assayed by the anti-TuJ-1 antibody, but control cells did not. This indicated that the REST-VP16 protein produced in these cells could activate its cellular target genes. By day 16, REST-VP16-expressing NSCs were highly differentiated and expressed markedly higher levels of neuronal β-tubulin than did the control cells. A low level of neuronal β-tubulin expression was detected in NSC-RVs grown with doxycycline, presumably because of the leakiness of the doxycycline-regulated system, similar to what was observed previously (25, 28). The magnified panel in Fig. 2 shows characteristic neurite-like structures in these cells. To confirm the differentiation of NSC-RVs, we performed a Western blot analysis of 16-day-differentiated NSC-V and NSC-RV cell extracts with antibodies against another neuronal differentiation marker, synaptotagmin I, a major synaptic vesicle protein; the expression of synaptotagmin I is also directly regulated by REST/NRSF (9, 10). In this experiment, anti-actin antibody was used as the internal control and mouse brain extract was used as a positive control for synaptotagmin I expression. As shown in Fig. 2B, NSC-RVs produced the REST-VP16 and synaptotagmin I proteins but NSC-Vs did not.
FIG. 2.
REST-VP16 causes neuronal differentiation of NSCs in vitro. (A) NSC-RVs show neuronal β-tubulin (β-tub) expression. NSCs, NSC-Vs, or NSC-RVs were plated on PLL-laminin-coated dishes and grown in growth medium as described in Materials and Methods with and without doxycycline. After the indicated times in culture, the cellular nuclei were stained with Hoechst dye and the cells were then analyzed by immunofluorescence microscopy (original magnification, ×60) with anti-Tuj1 antibodies to measure expression of neuronal β-tubulin. The magnified views of the 16-day images (−Dox) show the cellular morphology. (B) NSC-RVs show synaptotagmin I expression. Cell extracts (20 μg) from NSC-Vs and NSC-RVs cultured for 16 days as described in panel A were subjected to Western blot analysis with anti-VP16 (VP16), anti-synaptotagmin I (syt), and antiactin (actin) antibodies. Mouse brain extract was used as a positive control for synaptotagmin I expression.
REST-VP16 causes sensitization to RA and expression of REST target and REST nontarget neuronal differentiation genes in NSCs and converts them into the neuronal phenotype.
The vitamin A derivative RA is known to be responsible for embryonic patterning and has been found to induce general neuronal differentiation, as well as the formation of different types of neurons, in vivo and in vitro (10, 12, 13, 15). We previously found that our untreated NSCs were not sensitive to RA-dependent neuronal differentiation (see below). We sought to determine whether the expression of REST-VP16 in NSCs would produce RA sensitivity and cause them to differentiate and survive in the presence of mitotic inhibitors, a characteristic of differentiated neurons. We grew NSCs, NSC-Vs, and NSC-RVs with RA and without and with doxycycline and then treated the cells with the mitotic inhibitors 5-fluoro-2′-deoxyuridine, uridine, and cytosine-β-d-arabinofuranoside. The cellular nuclei were labeled with Hoechst dye, stained with antibodies for the neuronal differentiation markers neuronal β-tubulin (Tuj1) and MAP2, and then examined by immunofluorescence microscopy. Most of the NSC-RVs grown without doxycycline, unlike the NSCs or NSC-Vs grown with or without doxycycline, survived the mitotic inhibitors well, produced neuronal β-tubulin (Fig. 3) and MAP2 (Fig. 4) proteins, and formed neurite-like structures. Under these conditions, only a few control cells were still present, indicating that REST-VP16-expressing NSCs differentiated into neurons and that these cells survived mitotic inhibitors more efficiently than did control cells. When these experiments were repeated with antibodies against synapsin, another direct target of REST-VP16, the results were similar to those obtained for neuronal β-tubulin and MAP2 (data not shown). Interestingly, NSC-RVs produced MAP2, a neuronal differentiation marker not known to be a direct target of REST, indicating that expression of REST-regulated neuronal differentiation genes in NSCs through REST-VP16 also triggers the activation of other neuronal differentiation genes that are not direct targets of REST/NRSF. Taken together, the results of these experiments showed that REST-VP16 could produce neuronal differentiation of NSCs.
FIG. 3.
REST-VP16 caused expression of neuronal β-tubulin (β-tub) in NSCs. NSCs, NSC-Vs, or NSC-RVs were grown with RA and mitotic inhibitors as described in Materials and Methods, in the presence (+Dox) or absence (−Dox) of doxycycline, and the cellular nuclei were stained with Hoechst dye. The cells were then analyzed by immunofluorescence microscopy (original magnification, ×60) for neuronal β-tubulin (Tuj1).
FIG. 4.
REST-VP16 caused expression of MAP2 in NSCs. NSCs, NSC-Vs, or NSC-RVs were grown with RA and mitotic inhibitors as described in Materials and Methods, in the presence (+Dox) or absence (−Dox) of doxycycline, and the cellular nuclei were stained with Hoechst dye. The cells were then analyzed by immunofluorescence microscopy (original magnification, ×60) for MAP2.
REST-VP16 expression in NSCs produces physiological activity of neurons.
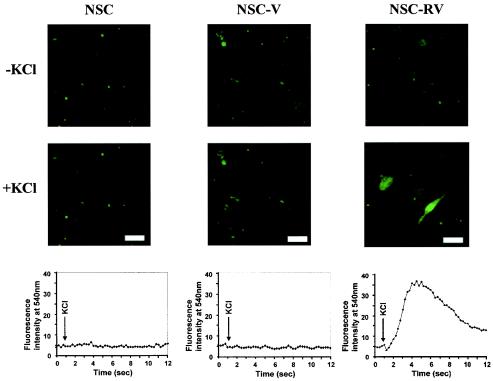
Depolarization-dependent calcium influx is an intrinsic property of synaptic vesicles, occurring in both mature and developing neuronal processes, and is independent of the presence of synaptic contacts (3, 14, 18, 20, 27). To determine whether NSC-RVs grown without doxycycline, which survived mitotic inhibitors, also undergo depolarization-dependent calcium influx, we measured intracellular Ca2+ levels by loading live NSCs, NSC-Vs, and NSC-RVs with the fluorescent calcium indicator Fluo-3 in the absence of doxycycline and examined them with a confocal laser system. Fluo-3 is practically nonfluorescent unless bound to Ca2+ (18, 20). These cells were then depolarized with a high K+ concentration, and the fluorescence was measured every 0.25 s for a total of 12 s. As shown in Fig. 5, a rapid, reversible calcium influx occurred specifically in response to K+ in NSC-RVs but not in NSCs or NSC-Vs. The K+-specific signal returned to the baseline upon washing (data not shown). Thus, NSC-RVs that survived mitotic inhibitors were physiologically active.
FIG. 5.
NSC-RVs show depolarization-dependent calcium influx. NSCs, NSC-Vs, or NSC-RVs (−Dox) from the cells shown in Fig. 4 were loaded with 25 μM Fluo-3 AM for 60 min at room temperature. The Fluo-3 AM solution was replaced with high-KCl Ringer solution, and an image was taken every 0.25 s for a total of 12 s after depolarization. Fluorescence intensity is shown at the bottom. Bars, 60 μm.
Glutamate-induced calcium influx is another intrinsic property of neurons. To determine whether NSC-RVs have this property, we measured the cells' intracellular Ca2+ levels with and without glutamate. As shown in Fig. 6, NSC-RVs but not NSCs or NSC-Vs underwent a rapid increase in intracellular Ca2+ in the presence of glutamate. The signal returned to the baseline upon washing (data not shown). These experiments showed that NSC-RVs had the physiological properties of neurons.
FIG. 6.
NSC-RVs show glutamate-induced calcium influx. NSCs, NSC-Vs, or NSC-RVs (−Dox) were loaded with 25 μM Fluo-3 AM for 60 min at room temperature, and the cells were treated as described in the legend to Fig. 5, except that 10 μM glutamate (18, 28) was added instead of KCl, and an image was taken every 0.25 s for a total of 12 s. Fluorescence intensity is shown at the bottom. Bars, 60 μm.
DISCUSSION
We showed that expression of a single transgene, that which encodes REST-VP16, causes rapid conversion of NSCs to the mature, physiologically active neuronal phenotype. These results suggest that direct activation of the REST/NRSF target is sufficient to produce neuronal differentiation of NSCs. However, whether REST-VP16-mediated neuronal differentiation occurs without transiently activating regulators that control the preceding stages of differentiation is unknown. We are currently examining these possibilities.
Direct activation of REST/NRSF target genes through REST-VP16 in NSCs also activates MAP2, which is not known to be a direct target of REST/NRSF. We recently observed such activation of MAP2 by REST-VP16 in myoblasts (28). Whether activation of MAP2 is accomplished directly by REST-VP16 or indirectly by the gene products activated by REST-VP16 remains unclear.
Our preliminary observations indicate that although NSCs express REST/NRSF activity, transcription of the REST/NRSF gene is blocked as NSCs differentiate along the neuronal pathway (data not shown). Whether REST-VP16-mediated neuronal differentiation also causes such a blockage of endogenous REST/NRSF gene transcription is unknown. However, the findings that REST-VP16 can successfully compete with endogenous REST/NRSF in neuronal cells and activate REST/NRSF's target genes (7, 11) suggest that the REST-VP16-mediated neuronal differentiation of NSCs would occur even in the presence of endogenous REST/NRSF.
Future studies will determine whether REST-VP16-mediated production of the neuronal phenotype, resulting in a rapid fate change, also accompanies global chromatin modification, as was recently found for normal NSC development (29). As described elsewhere, REST/NRSF regulates transcription through chromatin modification (1, 2, 6, 8). REST-VP16 binds to the same DNA binding site as does REST/NRSF but functions as an activator instead of a repressor (7, 11) and therefore would be expected to block REST/NRSF-mediated chromatin modification. We are currently examining the chromatin modification generated by REST/NRSF and REST-VP16 in our mammalian chromatin reconstitution system (19). Interestingly, a previous study showed that neurons dying of ischemic insults re-expressed REST/NRSF (4); experiments should be performed to determine whether expression of REST-VP16 can reverse such neuronal death. Recently, the wild-type huntingtin protein was found to bind to REST/NRSF and thereby sequester REST/NRSF in the cytoplasm (30). It was postulated that in the pathology of Huntington's disease, the REST/NRSF-huntingtin protein interaction is lost, causing REST/NRSF to enter the nucleus and repress its target genes (19). Examining whether REST-VP16 can be used to block this role of REST/NRSF in Huntington's disease is an exciting prospect.
Acknowledgments
We are very grateful to Gail Mandel for the generous gift of pREST-Express, p73, pBS.REST, and pSDK7; to Craig Strathdee for plasmid pBig2r; and to Evan Snyder for C17.2 cells. We are also grateful to the anonymous reviewers, whose comments made the paper better.
This work was supported by grants from the National Cancer Institute (CA 81255 and CA97124). DNA sequencing was supported by NIH Cancer Center Support (Core) grant CA16672.
REFERENCES
- 1.Ballas, N., E. Battaglioli, F. Atouf, M. E. Andres, J. Chenoweth, M. E. Anderson, C. Burger, M. Moniwa, J. R. Davie, W. J. Bowers, H. J. Federoff, D. W. Rose, M. G. Rosenfeld, P. Brehm, and G. Mandel. 2001. Regulation of neuronal traits by a novel transcriptional complex. Neuron 31:353-365. [DOI] [PubMed]
- 2.Battaglioli, E., M. E. Andres, D. W. Rose, J. G. Chenoweth, M. G. Rosenfeld, M. E. Anderson, and G. Mandel. 2002. REST repression of neuronal genes requires components of the hSWI.SNF complex. J. Biol. Chem. 277:41038-41045. [DOI] [PubMed] [Google Scholar]
- 3.Bischofberger, J., and D. Schild. 1995. Different spatial patterns of [Ca2+] increase caused by N- and L-type Ca2+ channel activation in frog olfactory bulb neurones. J. Physiol. 487(Pt. 2):305-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calderone, A., T. Jover, K. M. Noh, H. Tanaka, H. Yokota, Y. Lin, S. Y. Grooms, R. Regis, M. V. Bennett, and R. S. Zukin. 2003. Ischemic insults derepress the gene silencer REST in neurons destined to die. J. Neurosci. 23:2112-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong, J. A., J. Tapia-Ramirez, S. Kim, J. J. Toledo-Aral, Y. Zheng, M. C. Boutros, Y. M. Altshuller, M. A. Frohman, S. D. Kraner, and G. Mandel. 1995. REST: a mammalian silencer protein that restricts sodium channel gene expression to neurons. Cell 80:949-957. [DOI] [PubMed] [Google Scholar]
- 6.Griffith, E. C., C. W. Cowan, and M. E. Greenberg. 2001. REST acts through multiple deacetylase complexes. Neuron 31:339-340. [DOI] [PubMed] [Google Scholar]
- 7.Immaneni, A., P. Lawinger, Z. Zhao, W. Lu, L. Rastelli, J. H. Morris, and S. Majumder. 2000. REST-VP16 activates multiple neuronal differentiation genes in human NT2 cells. Nucleic Acids Res. 28:3403-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jepsen, K., O. Hermanson, T. M. Onami, A. S. Gleiberman, V. Lunyak, R. J. McEvilly, R. Kurokawa, V. Kumar, F. Liu, E. Seto, S. M. Hedrick, G. Mandel, C. K. Glass, D. W. Rose, and M. G. Rosenfeld. 2000. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102:753-763. [DOI] [PubMed] [Google Scholar]
- 9.Koenigsberger, C., J. J. Chicca II, M. C. Amoureux, G. M. Edelman, and F. S. Jones. 2000. Differential regulation by multiple promoters of the gene encoding the neuron-restrictive silencer factor. Proc. Natl. Acad. Sci. USA 97:2291-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawinger, P., L. Rastelli, Z. Zhao, and S. Majumder. 1999. Lack of enhancer function in mammals is unique to oocytes and fertilized eggs. J. Biol. Chem. 274:8002-8011. [DOI] [PubMed] [Google Scholar]
- 11.Lawinger, P., R. Venugopal, Z. S. Guo, A. Immaneni, D. Sengupta, W. Lu, L. Rastelli, A. Marin Dias Carneiro, V. Levin, G. N. Fuller, Y. Echelard, and S. Majumder. 2000. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nat. Med. 6:826-831. [DOI] [PubMed] [Google Scholar]
- 12.Linville, A., E. Gumusaneli, R. A. Chandraratna, and T. F. Schilling. 2004. Independent roles for retinoic acid in segmentation and neuronal differentiation in the zebrafish hindbrain. Dev. Biol. 270:186-199. [DOI] [PubMed] [Google Scholar]
- 13.Maden, M. 2002. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 3:843-853. [DOI] [PubMed] [Google Scholar]
- 14.Matteoli, M., C. Verderiom, K. Krawzeski, O. Mundigl, S. Coco, G. Fumagalli, and P. Camilli. 1995. Mechanisms of synaptogenesis in hippocampal neurons in primary culture. J. Physiol. Paris 89:51-55. [DOI] [PubMed] [Google Scholar]
- 15.Novitch, B. G., H. Wichterle, T. M. Jessell, and S. Sockanathan. 2003. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron 40:81-95. [DOI] [PubMed] [Google Scholar]
- 16.Palm, K., N. Belluardo, M. Metsis, and T. Timmusk. 1998. Neuronal expression of zinc finger transcription factor REST/NRSF/XBR gene. J. Neurosci. 18:1280-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paquette, A. J., S. E. Perez, and D. J. Anderson. 2000. Constitutive expression of the neuron-restrictive silencer factor (NRSF)/REST in differentiating neurons disrupts neuronal gene expression and causes axon pathfinding errors in vivo. Proc. Natl. Acad. Sci. USA 97:12318-12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pincus, D. W., H. M. Keyoung, C. Harrison-Restelli, R. R. Goodman, R. A. Fraser, M. Edgar, S. Sakakibara, H. Okano, M. Nedergaard, and S. A. Goldman. 1998. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann. Neurol. 43:576-585. [DOI] [PubMed] [Google Scholar]
- 19.Rastelli, L., K. Robinson, Y. Xu, and S. Majumder. 2001. Reconstitution of enhancer function in paternal pronuclei of one-cell mouse embryos. Mol. Cell. Biol. 21:5531-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy, N. S., S. Wang, L. Jiang, J. Kang, A. Benraiss, C. Harrison-Restelli, R. A. Fraser, W. T. Couldwell, A. Kawaguchi, H. Okano, M. Nedergaard, and S. A. Goldman. 2000. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat. Med. 6:271-277. [DOI] [PubMed] [Google Scholar]
- 21.Schoenherr, C. J., and D. J. Anderson. 1995. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science 267:1360-1363. [DOI] [PubMed] [Google Scholar]
- 22.Shimojo, M., and L. B. Hersh. 2003. REST/NRSF-interacting LIM domain protein, a putative nuclear translocation receptor. Mol. Cell. Biol. 23:9025-9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimojo, M. A., A. J. Paquette, D. J. Anderson, and L. B. Hersh. 1999. Protein kinase A regulated cholinergic gene expression on PC12 cells: REST4 silences the silencing activity of neuron-restrictive silencer factor/REST. Mol. Cell. Biol. 19:6788-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder, E. Y., D. L. Deitcher, C. Walsh, S. Arnold-Aldea, E. A. Hartwieg, and C. L. Cepko. 1992. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell 68:33-51. [DOI] [PubMed] [Google Scholar]
- 25.Strathdee, C. A., M. R. McLeod, and J. R. Hall. 1999. Efficient control of tetracycline-responsive gene expression from an autoregulated bi-directional expression vector. Gene 229:21-29. [DOI] [PubMed] [Google Scholar]
- 26.Tabuchi, A., T. Yamada, S. Sasagawa, Y. Naruse, N. Mori, and M. Tsuda. 2002. REST4-mediated modulation of REST/NRSF-silencing function during BDNF gene promoter activation. Biochem. Biophys. Res. Commun. 290:415-420. [DOI] [PubMed] [Google Scholar]
- 27.Tooze, S. A., G. J. Martens, and W. B. Huttner. 2001. Secretory granule biogenesis: rafting to the SNARE. Trends Cell Biol. 11:116-122. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe, Y., S. Kameoka, V. Gopalakrishnan, K. D. Aldape, Z. Z. Pan, F. F. Lang, and S. Majumder. 2004. Conversion of myoblasts to physiologically active neuronal phenotype. Genes Dev. 18:889-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao, X., T. Ueba, B. R. Christie, B. Barkho, M. J. McConnell, K. Nakashima, E. S. Lein, B. D. Eadie, A. R. Willhoite, A. R. Muotri, R. G. Summers, J. Chun, K. F. Lee, and F. H. Gage. 2003. Mice lacking methyl-CpG binding protein 1 have deficits in adult neurogenesis and hippocampal function. Proc. Natl. Acad. Sci. USA 100:6777-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuccato, C., M. Tartari, A. Crotti, D. Goffredo, M. Valenza, L. Conti, T. Cataudella, B. R. Leavitt, M. R. Hayden, T. Timmusk, D. Rigamonti, and E. Cattaneo. 2003. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat. Genet. 35:76-83. [DOI] [PubMed] [Google Scholar]