Significance
We report fossil evidence of feather structural protein (beta-keratin) from a 130-My-old basal bird (Eoconfuciusornis) from the famous Early Cretaceous Jehol Biota, which has produced many feathered dinosaurs, early birds, and mammals. Multiple independent molecular analyses of both microbodies and associated matrix recovered from the fossil feathers confirm that these microbodies are indeed melanosomes. We use transmission electron microscopy and immunogold to show localized binding of antibodies raised against feather protein to matrix filaments within these ancient feathers. Our work sheds new light on molecular constituents of tissues preserved in fossils.
Keywords: keratinous protein, immunogold, ChemiSTEM, melanosome, Early Cretaceous
Abstract
Microbodies associated with feathers of both nonavian dinosaurs and early birds were first identified as bacteria but have been reinterpreted as melanosomes. Whereas melanosomes in modern feathers are always surrounded by and embedded in keratin, melanosomes embedded in keratin in fossils has not been demonstrated. Here we provide multiple independent molecular analyses of both microbodies and the associated matrix recovered from feathers of a new specimen of the basal bird Eoconfuciusornis from the Early Cretaceous Jehol Biota of China. Our work represents the oldest ultrastructural and immunological recognition of avian beta-keratin from an Early Cretaceous (∼130-Ma) bird. We apply immunogold to identify protein epitopes at high resolution, by localizing antibody–antigen complexes to specific fossil ultrastructures. Retention of original keratinous proteins in the matrix surrounding electron-opaque microbodies supports their assignment as melanosomes and adds to the criteria employable to distinguish melanosomes from microbial bodies. Our work sheds new light on molecular preservation within normally labile tissues preserved in fossils.
Feathers and feather-like epidermal structures are now well-documented in several groups of nonavian dinosaurs and basal birds, with the most abundant evidence coming from the Middle Jurassic-to-Early Cretaceous deposits in northeastern China (1–3). Round-to-elongated microbodies associated with these feathers and feather-like structures were first interpreted as microbes (4, 5), leading to the hypothesis that microbial activity played a key role in the preservation of these normally labile remains (5). This hypothesis was based on and supported by experiments and observations of bacterial decomposition of feather keratins in modern birds (6, 7).
More recently, these bodies were reinterpreted as remnant melanosomes (pigment-containing intracellular organelles) (8–11) and, subsequently, hypotheses of dinosaurian color (8–13), behavior (10), habitat (14), and physiology (15) were proposed based upon this reinterpretation. However, melanosomes and microbes overlap completely in size and shape (16–18), and thus these hypotheses are equally plausible. Furthermore, the majority of the data presented to support a melanosome origin are based not on the morphology of the bodies themselves but rather on their impressions within a structural matrix (9–15, 19) that is presumed, but not demonstrated, to be keratin.
The most rigorous test for the origin of these microbodies is the chemical characterization of both the microbodies and the matrix in which they were embedded. However, where chemical data were presented previously, with few exceptions (20, 21) the data were not sufficiently specific to support or eliminate either hypothesis (22–26). Whether the melanin-related chemical signals originated from the microbodies or from the surrounding matrix (22–26) could not be determined, with the exception of one study by Lindgren et al. (21). No detailed morphological or chemical studies have been conducted on the matrix in which the reported microbodies were embedded. Whether keratinous proteins were preserved in these feathers, and the potential extent of this preservation, has not been explored.
Indeed, if both microbodies and matrix are preserved, tests can be conducted to chemically characterize the composition of each. If these bodies are melanosomes, they should be contained within a keratinous matrix; if they are microbial in origin, this matrix should consist of exopolymeric substances secreted by the microbes and subsequently mineralized. To distinguish between these alternative hypotheses, we applied multiple methods, well-established for molecular and chemical characterization of modern materials, to chicken (Gallus gallus) feathers and to preserved feathers of a new specimen of the bird Eoconfuciusornis [Shandong Tianyu Museum of Nature (STM) 7-144] (Pygostylia: Confuciusornithiformes) (Fig. 1A) from the 130-Ma Protopteryx horizon of the Huajiying Formation in Fengning, northern Hebei, China. Our results are consistent with the retention of original organic components derived from both keratin and melanin, thus supporting a melanosome origin for these ancient microstructures.
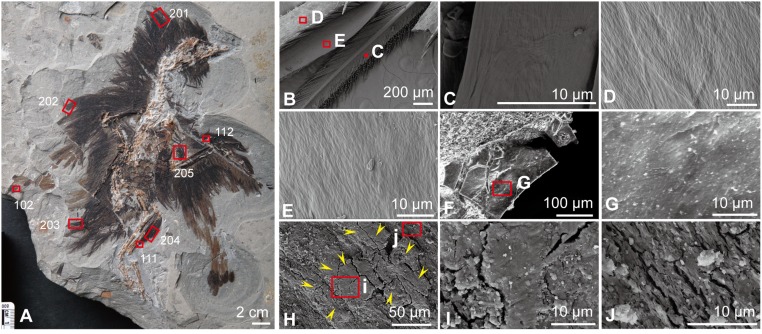
Fig. 1.
New specimen of Eoconfuciusornis (STM 7-144) collected from the Early Cretaceous lake deposits in Hebei, northern China, and SEM images of associated feather material, compared with SEM images of a black feather from the extant chicken G. gallus. (A) Photograph of the new fossil specimen, indicating sample locations (boxes; not to scale). (B–E) SEM images of a black feather from G. gallus. (B) Low-magnification image. (C–E) High-magnification images of the boxed areas in B. (F–J) SEM images of the feather samples 111 (crural feathers) and 202 (interpreted as the tertials of the left wing) from Eoconfuciusornis. (F) Low-magnification image. (G) High-magnification image of the boxed area in F. (H) Melanosomes are shown located immediately below the structured thin layer, which is indicated with yellow arrowheads. (I and J) High-magnification images of the boxed areas in H. These bodies are embedded within the feathers and not observed on the surface of the feathers. (Scales are as indicated.)
Results
Fossil Feather Histology.
Scanning electron microscopy (SEM) was used to demonstrate that samples 201 to 205 were preserved as three-dimensional, thick carbon sheets. SEM images of the fossil feathers (Fig. 1 F–J) were compared with those of G. gallus remiges (Fig. 1 B–E). At higher magnifications, much of the fossil is composed of 3D microbodies, with some regions retaining a very thin carbon layer (Fig. 1 F–I) that covers the microbodies (Fig. 1J). This thin layer displays fibrous bundles in arrays that form regular angles (Fig. 1 G and I), a feature previously reported in the epicortex of G. gallus feathers (27) and also observed in Fig. 1 C and D.
Transmission electron microscopy (TEM) confirms microstructural similarity between Eoconfuciusornis and extant G. gallus feathers (Fig. 2). The microbodies preserved in Eoconfuciusornis STM 7-144 are distinct, relatively uniform, elongated, electron-dense, and embedded in a less electron-dense filamentous matrix (Fig. 2 A and B), patterns also observed in extant feathers (Fig. 2 C and D). Both filaments and microbodies are more densely packed in the fossils, suggesting some diagenetic alteration perhaps involving compression.
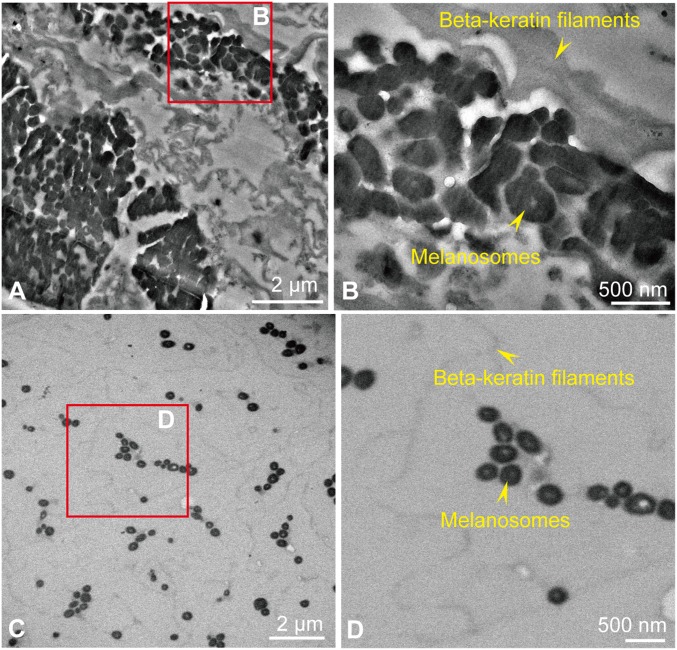
Fig. 2.
TEM images of the crural feathers sampled in Eoconfuciusornis STM 7-144 (A and B, sample 111) compared with that of a black feather from G. gallus (C and D). (A) Low-magnification image. (B) High-magnification image of the boxed area in A. (C) Low-magnification image. (D) High-magnification image of the boxed area in C. Both melanosomes and beta-keratin filaments are more densely distributed in the fossil sample. (Scales are as indicated.)
Immunohistochemistry.
A polyclonal antiserum raised against extracts of mature modern white chicken feathers (28) was used to test the hypothesis that the exceptional microstructural preservation in these fossil feathers extended to the molecular level. Both immunofluorescence and immunogold labeling support the presence of epitopes in fossil tissues consistent with beta-keratin, a family of proteins not produced by either mammals or microbes but that comprise ∼90% of proteins in extant feathers (28) and are coexpressed with alpha-keratin in the skin, beak, and claw tissues of reptiles and birds (29). These are also referred to as corneous proteins (30).
Specific immunoreactivity to components within the fossil feathers of Eoconfuciusornis (Fig. 3) was revealed by tissue-specific, although weaker, localization of fluorescence signal (Fig. 3 C and D) in a pattern comparable to extant black chicken feathers used as a positive control (Fig. 3 G and H). No fluorescence was present in any controls, where the primary antiserum was omitted, but all other steps were kept identical (Fig. 3 A, B, E, and F). Furthermore, antibodies to hemoglobin (a protein found in vertebrate red blood cells, used as an irrelevant control) and peptidoglycan (a glycosaminoglycan produced exclusively by bacteria) did not react with the fossil feather sample (Fig. S1), supporting both the persistence of keratinous components and the specificity of the anti-feather antibody.
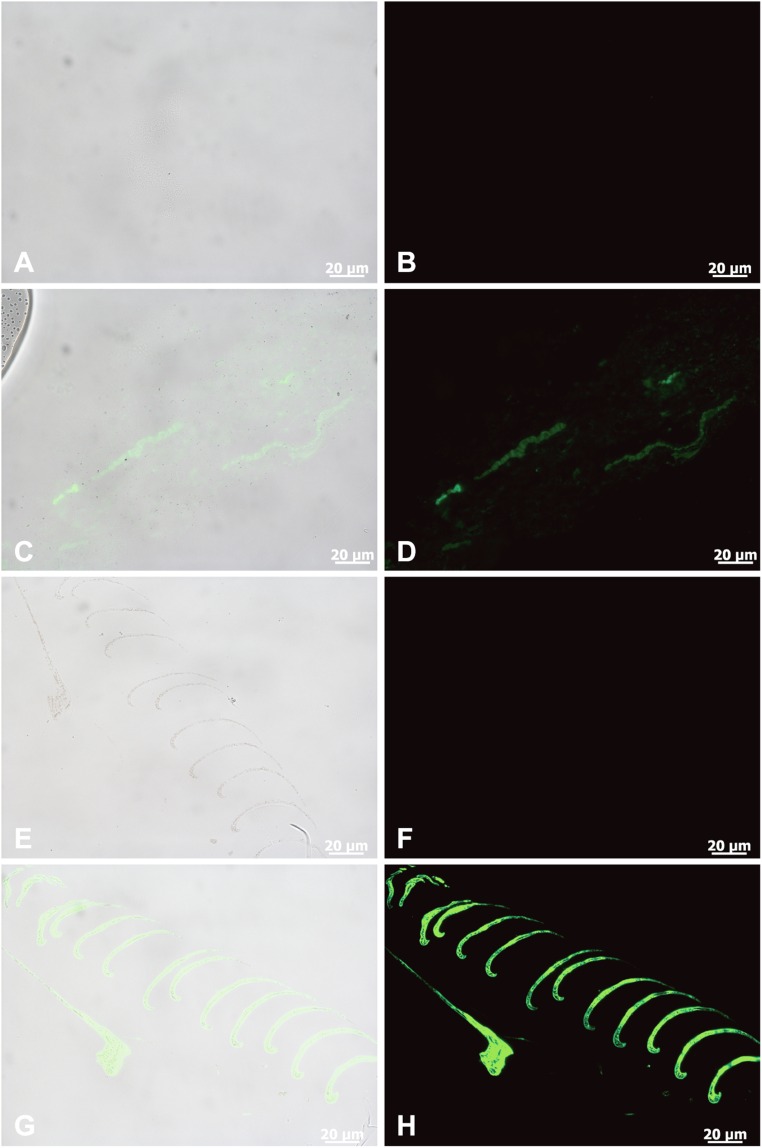
Fig. 3.
Immunofluorescence results of fossil feathers (sample 205, propatagium, which is covered dorsally and ventrally by feathers) compared with feathers of G. gallus. (A, C, E, and G) Overlay images showing where the antibodies bind to tissues (green) superimposed on transmitted light images. (B, D, F, and H) Fluorescence images showing only antibody binding, as represented by green fluorescence of the FITC label. (A and B) Negative control corresponding to C and D, where the primary antibody is omitted. (C and D) Eoconfuciusornis feathers exposed to anti-feather antibody demonstrates specific and localized antibody binding, as reflected by green fluorescence signal. (E and F) Negative control, with the primary antibody omitted. (G and H) In situ fluorescence signal corresponding to bound antibodies on the feather tissue of G. gallus. Binding patterns are similar between samples, although the ancient feathers show reduced binding as indicated by less intense fluorescence signal. All data were collected under identical conditions and all images were processed identically. (Scales are as indicated.)
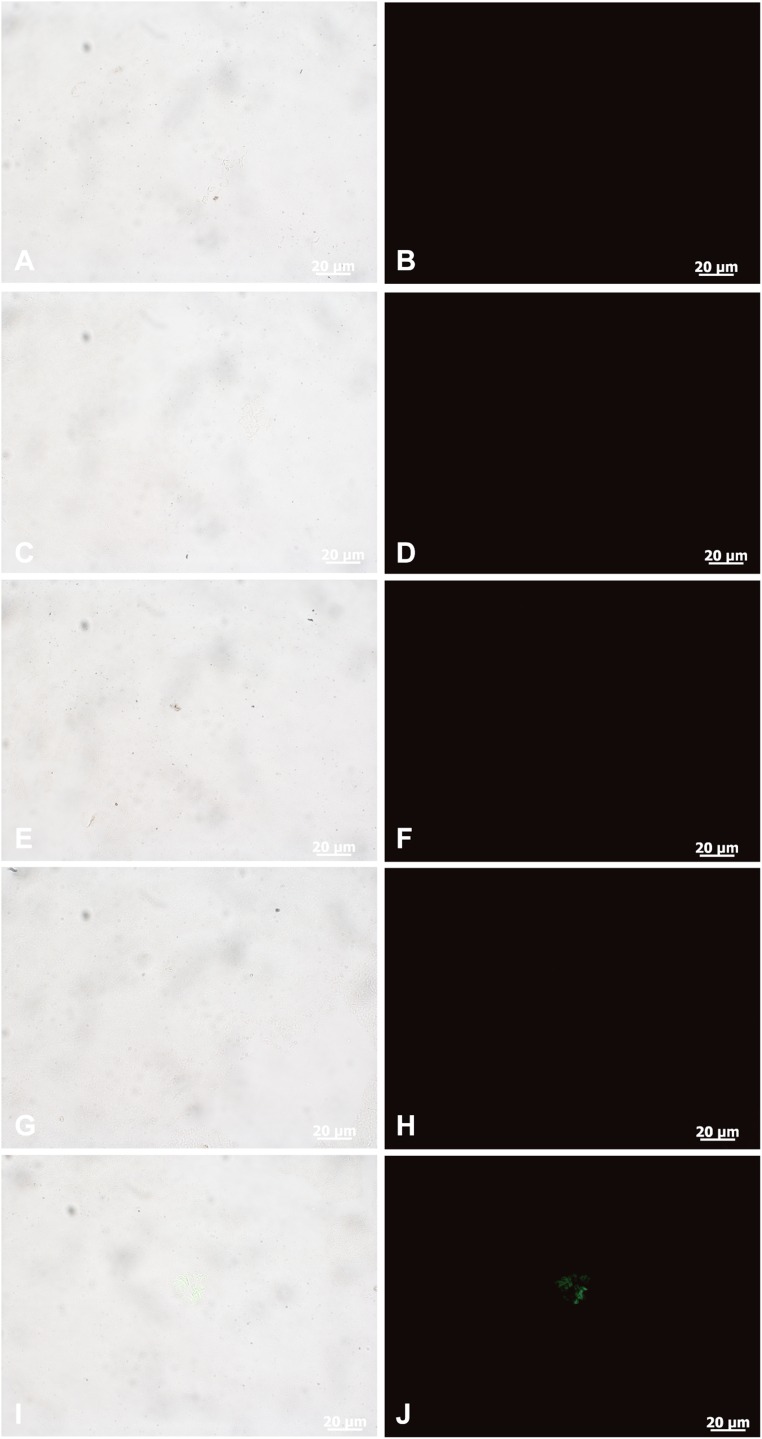
Fig. S1.
Immunofluorescence results of fossil feathers (sample 203). (A, C, E, G, and I) Overlying images showing where the antibodies bind to tissues. (B, D, F, H, and J) Fluorescence images showing only antibody binding, as represented by the green fluorescence of the FITC label. (A and B) Negative control of E, F, I, and J, when the primary antibody is omitted. (E and F) In situ fluorescence immunohistochemistry of Eoconfuciusornis feathers exposed to anti-hemoglobin antibody, showing no immunoreactivity. (I and J) In situ fluorescence immunohistochemistry of Eoconfuciusornis feathers exposed to anti-feather antibody, showing clear fluorescence. (C and D) Negative control of G and H, when the primary antiserum is omitted. (G and H) In situ fluorescence immunohistochemistry of Eoconfuciusornis feathers exposed to anti-peptidoglycan antibody, showing no immunoreactivity. (Scales are as indicated.)
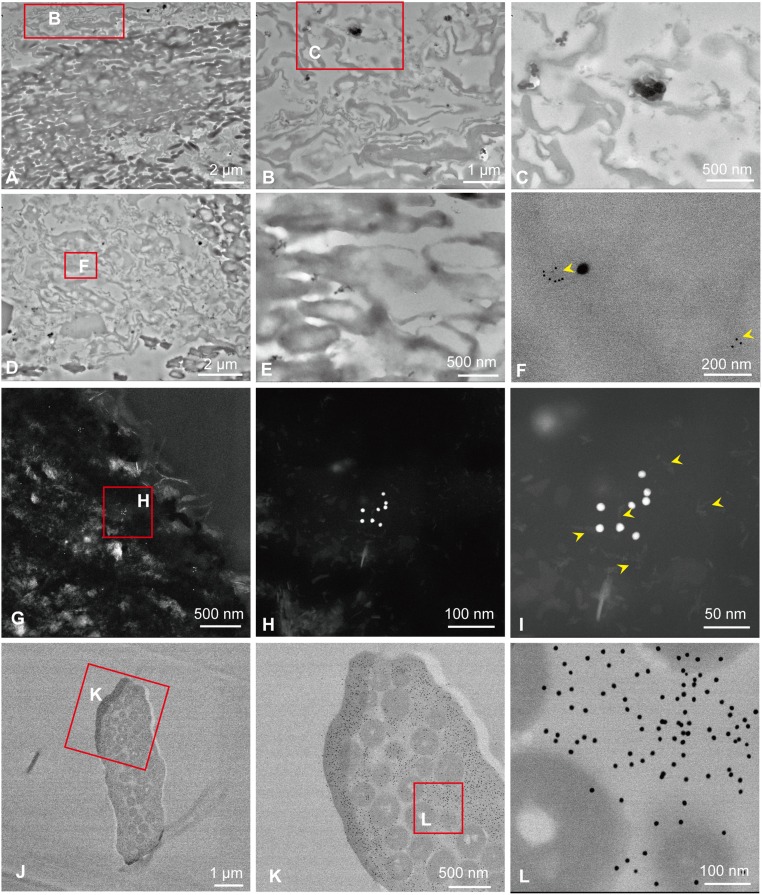
We applied the same anti-feather antiserum (28) to ultrathin sections (∼90-nm) of fossil and modern black feathers. Binding was detected using a secondary antibody conjugated to 12-nm gold particles. The gold particles were seen associated with fibril bundles measuring ∼3 nm, consistent with filaments of beta-keratin matrix (Fig. 4 G–I), but were sparsely distributed relative to binding in modern controls (Fig. 4 D and F–L). However, other structures, including the electron-dense microbodies and needle-shaped structures probably of diagenetic origin, were completely unlabeled (Figs. 4E and 5A). No gold particles were visible in the negative controls (omitting the primary antiserum) (Fig. 4 A–C), similar to results from immunofluorescence. The higher-resolution immunoelectron localization of gold particles generally confirms the binding of the anti-feather antiserum visualized in fluorescence microscopy, and verifies that these fossil feathers partially preserved the molecular structure of the feather–keratin epitopes, which remains immunoreactive.
Fig. 4.
Immunogold labeling of fossil feathers (sample 201) compared with feathers of G. gallus. (A–C) Negative control of D–F, when the primary antiserum is omitted. (B) High-magnification image of the boxed area in A. (C) High-magnification image of the boxed area in B. (D–I) In situ nanogold immunohistochemistry of Eoconfuciusornis feathers exposed to anti-feather antibody. (E) Melanosomes from the same section of D and F; no nanogold particles were seen to specifically bind. (F) High-magnification image of the boxed area in D; arrowheads show binding of the nanogold beads. (G–I) High-angle annular dark-field (HADDF) images show binding of the nanogold beads from the same section of D–F. (H) High-magnification image of the boxed area in G. (I) High-magnification image of H; arrowheads show the filaments at the bead-binding regions. (J–L) In situ nanogold immunohistochemistry of G. gallus feathers exposed to anti-feather antibody. Nanogold particles specifically bind the keratinous tissues but do not bind the melanosomes, visualized in cross-section. (K) High-magnification image of the boxed area in J. (L) High-magnification image of the boxed area in K. (Scales are as indicated.)
Fig. 5.
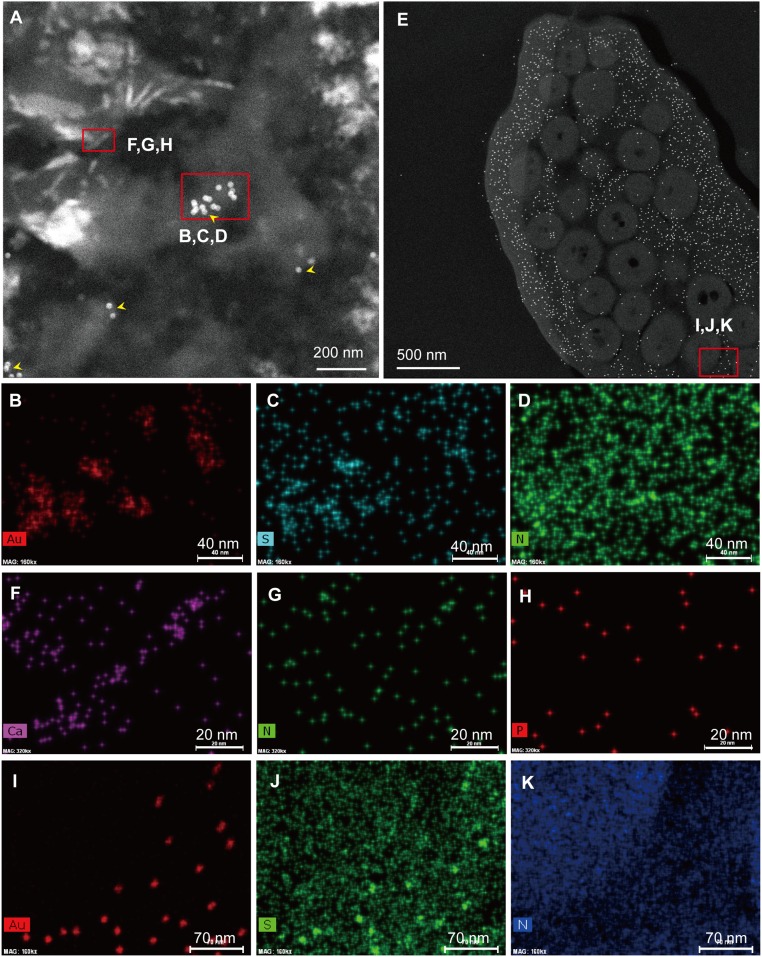
ChemiSTEM data derived from the region immunoreactively binding nanogold particles and needle-shaped structures of the Eoconfuciusornis feathers (sample 205), and compared with feathers of G. gallus. (A) HADDF image of an immunogold-labeled section of the Eoconfuciusornis feather, indicating the region selected for elemental mapping. (B–D) Various element distributions for the immunogold-labeled region (arrowhead). (B) Gold (Au) distribution map indicating the localization of nanoparticles. (C) Sulfur map showing the distribution of this element of overlap with antibody-bound Au beads. (D) Nitrogen map suggesting the entire tissue is dominated by N, consistent with a proteinaceous source. (F–H) Element distributions for the needle-shaped structures observed in some regions of the fossil feather. (F) Calcium distribution outlines the shape of the structure, indicating it contains a relatively high Ca level. (G and H) Nitrogen and phosphorus distribution maps, respectively, which are not specifically localized within the needle-shaped structures. (E) HADDF image of an immunogold-labeled section of G. gallus feather, indicating the region selected for elemental mapping. (I–K) Various element distributions for the immunogold-labeled region. (I) Au distribution map. (J) S map. (K) N map. (Scales are as indicated.)
ChemiSTEM.
We obtained high-resolution elemental maps of ultrathin sections of fossil feathers using ChemiSTEM technology [FEI Titan G2 series of Cs-corrected STEM (scanning/transmission electron microscopy)]. This technology reveals the elemental distribution of nanogold particles bound to antibodies (Fig. 5 B–D and I–K) and identifies, at high spatial resolution, other elements (e.g., N) that would be expected to be present in organic remains. This technology also identifies and localizes potential diagenetic compounds (e.g., calcium mineral morphs). Antibodies tagged with gold localize to and overlap with relatively high concentrations of sulfur in both ancient (Fig. 5 A–C) and modern (Fig. 5 E, I, and J) feathers. Keratin molecules incorporate the S-bearing amino acid residue cysteine in relatively high concentrations (31, 32), supporting the hypothesis that these antibodies are most likely binding keratin.
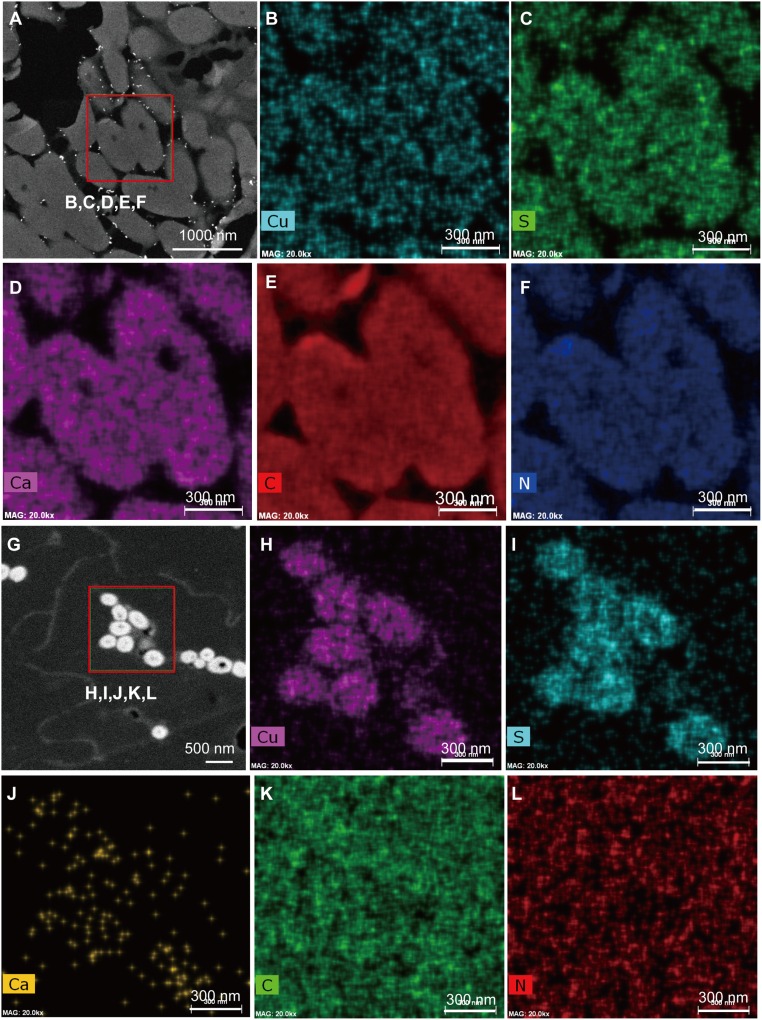
We applied the same technology to modern feathers to show that extant melanosomes contain higher concentrations of copper, sulfur, and calcium (Fig. 6 G–J) than are seen in the keratin matrix. Similarly, the elements Cu, S, and Ca localize to electron-dense microbodies visualized in the fossil feathers (Fig. 6 A–D). We also note that the keratinous matrix surrounding melanosomes in modern feathers (Fig. 6 K and L) contains greater concentrations of carbon and nitrogen than are seen in fossil feathers (Fig. 6 E and F), indicating a possible diagenetic effect.
Fig. 6.
ChemiSTEM data derived from the microbodies of feather samples from Eoconfuciusornis (A–F), compared with melanosomes of a black feather from G. gallus (G–L). (A) HADDF image showing the selected region for elemental mapping. (B–F) Various element distributions for the selected region. (B) Cu map. (C) S map. (D) Ca map. (E) C map. (F) N map. (G) HADDF image showing the region on the G. gallus feather selected for elemental mapping. (H–L) Various element distributions for the selected region. (H) Cu map. (I) S map. (J) Ca map. (K) C map. (L) N map. (Scales are as indicated.)
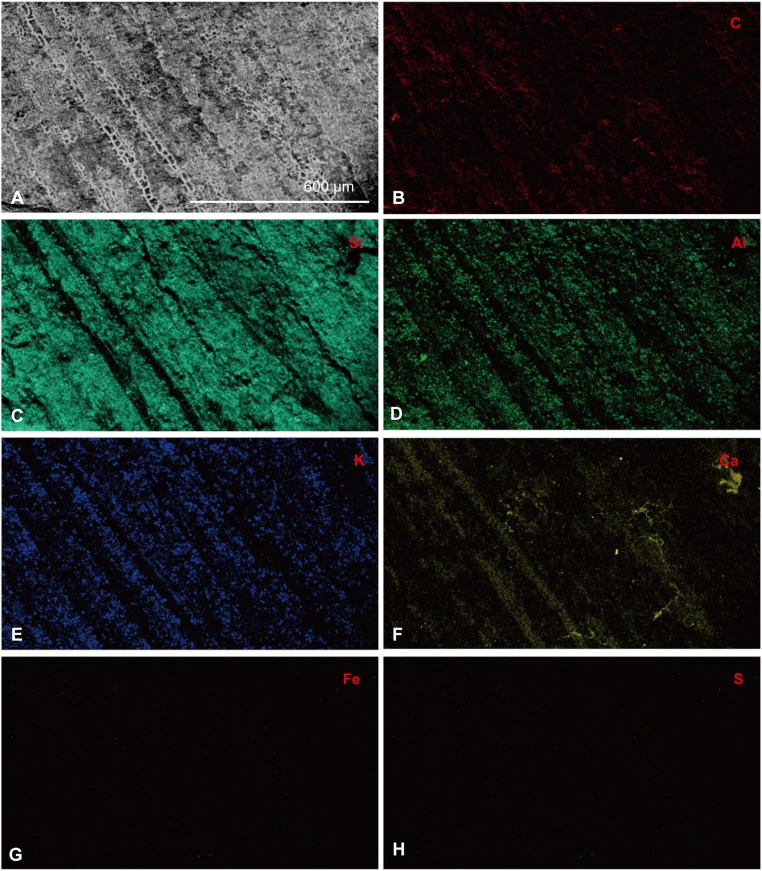
The fossil feathers also show needle-shaped structures (Fig. 5A) that are high in Ca (Fig. 5F). We propose that these structures are most likely of diagenetic origin (Fig. 5F) and possibly microbially mediated, because they are not found in modern feathers and their shape is consistent with early diagenetic morphs of calcium-containing minerals. However, phosphorus is barely measurable, and does not map to the Ca signal, so a CaPO4 mineral morph can be excluded. The elemental maps obtained from lower-magnification images using environmental scanning electron microscope with energy dispersive spectroscopy (ESEM-EDS) (Fig. S2) indicate a higher concentration of Ca in the fossil feather material, which maps to both the microbodies and the needle-like mineral structures.
Fig. S2.
SEM-EDS data derived from feather sample 102 of Eoconfuciusornis. (A) SEM image of the selected area for elemental mapping. Altogether, seven elements are presented. (B) C. (C) Si. (D) Al. (E) K. (F) Ca. (G) Fe. (H) S. (The scale is as indicated.)
Discussion
The smooth and dense outer cortex of the feathers of Eoconfuciusornis was imaged using SEM and compared with that of extant chicken feathers at the same magnification. In both samples, immediately below this dense cortex, 3D microbodies were present. Melanosomes are suspended in a beta-keratin matrix (33), a pattern and distribution similar to what is seen in Eoconfuciusornis. SEM analyses of modern feathers rarely showed melanosomes, as these are obscured by the dense outer feather cortex, and melanosomes herein were not visualized unless this cortex was removed (27). However, this dense cortex is rarely observed in fossil feathers, exposing microbodies for easier observation. It is likely that the cortex disintegrated early in diagenesis, as seen in taphonomic experiments of feather degradation.
TEM of 90-nm sections taken from Eoconfuciusornis feathers was comparable to images of modern feathers in that the matrix of both samples contained filaments consistent with fibrillar keratin and electron-dense bodies consistent with melanosomes in size, orientation, and location.
However, although suggestive, imaging alone is not conclusive. Therefore, we hypothesized that the exceptional ultrastructural preservation may extend to the molecular level. Capitalizing on the specificity and sensitivity of the vertebrate immune response, which has been used successfully to characterize other originally keratinous fossil material (34, 35), we show that antibodies raised against feather proteins (28) react specifically with ancient feathers in in situ experiments. Binding, as visualized by green fluorescence signal, is reduced in intensity but comparable in pattern and distribution to that of extant feathers. The decreased intensity of antibody binding in the fossil feather may be due to several factors: (i) greater degradation resulting in a reduced number of recognizable epitopes; (ii) some epitopes present in modern feathers (against which the antibody was raised) may not have been present at this early stage in avian evolution (29, 36); or (iii) epitopes may be blocked or masked by infiltration with minerals or other factors resulting from diagenetic processes.
To confirm the results we observed by immunofluorescence labeling, we applied nanogold (12-nm)-labeled antibodies to 90-nm sections of fossil feathers and observed localized binding under TEM, which allows much higher resolution. We show that the anti-feather antibodies localize to filaments within the matrix, supporting the hypothesis that the matrix is composed of material consistent with filamentous beta-keratin proteins. No gold labeling was detected in or associated with the microbodies embedded in this matrix in either fossil or extant feathers (Fig. 4), supporting the specificity of antibody binding to keratinous matrix but not other components of the feathers. These experiments produced complementary data that confirm the presence of molecules consistent with feather keratins in the fossil tissues.
High-resolution elemental mapping (ChemiSTEM) adds additional information. This technique showed that regions binding antibodies complexed to gold also demonstrate a relatively high concentration of both sulfur and nitrogen, which would be expected in keratinous material. The keratin protein family incorporates high concentrations of amino acids rich in sulfur (31, 32), whereas carbon and nitrogen are common to all amino acids and proteins. However, the possibility that the sulfur signals might be enlarged by the antibodies cannot be fully excluded based on the present experiments.
Recently, it has been demonstrated that certain trace elements, such as copper, sulfur, and calcium, are present in both fossil and modern melanosome-bearing tissues, and it has been suggested that these elements may be used as biomarkers for melanin-derived compounds in fossils (23–25). This is problematic, however, in that all Cu, S, and Ca are also derived from bacterially induced decay as well as many diagenetic processes (37, 38). One of the ways to differentiate between melanosomes and morphologically similar bacteria is to directly correlate, at high resolution, these trace elements to the microstructures but not the surrounding matrix. However, the resolution of either ESEM-EDS or synchrotron rapid scanning X-ray fluorescence (SRS-XRF) maps is not sufficient to attribute the elements specifically to any organelles (25). The high-resolution elemental maps achieved by ChemiSTEM technology display high levels of copper and sulfur in both the modern melanosomes and ancient microbodies. It thus confirms that both Cu and S colocalize to proposed melanin-bearing organelles. Although S is also observed in both the fossil feather matrix and the microbodies, Cu is found only within the melanosomes, indicating little diagenetic movement and supporting the original distribution of this element. Hence, we conclude that the microbodies examined herein are indeed the fossilized remains of melanin-bearing organelles and that they contain high concentrations of the original trace elements of the living organisms preserved through geological time.
Another notable trace metal is calcium, which was once thought to be chelated by and affect the chemical properties of melanin (39). Because of this association, it was proposed that Ca distribution could be used as a proxy for melanin distribution in fossil feathers (23). In previous studies, the SRS-XRF maps of Ca in the neck region of a specimen of Confuciusornis sanctus showed a strong correlation with Cu (23). Our ESEM-EDS maps (Fig. S2) also suggest that the distribution of Ca strongly correlates with diagenetically altered feather structures. However, the high-resolution elemental maps show that Ca signal is much weaker in melanosomes of extant feathers compared with that in the microbodies of fossil feathers and, although Cu is localized within proposed melanosomes in the fossil, Ca maps to both microbodies and the needle-shaped structures within the feather–keratin matrix. These Ca-concentrated needle-shaped structures are absent in extant feathers. Thus, we hypothesize that precipitation of calcium, possibly mediated by microbes during the fossilization process, facilitated ultrastructural and molecular preservation of the feathers by stabilizing them at the molecular level before they could completely degrade (5, 40). It has long been known that the association with mineral substrates greatly enhances the preservation potential of biomolecules (35). Furthermore, it has been proposed that calcium may incorporate into molecular fragments, conferring stability, and that this process may be microbially mediated (35). Because the surrounding sediments of this Eoconfuciusornis specimen consist mainly of Al silicates, with little or no calcium detected, the source of the calcium observed in these feathers remains unknown.
Molecular dating techniques suggest that feather beta-keratin diverged from “feather-like” beta-keratins about 143 Ma (29). If these molecular dates are correct, feathers of Archaeopteryx and Anchiornis were constitutively distinct from those of extant bird feathers, and thus feather structure preceded modern feather proteins. Molecular data show that only in the Early Cretaceous did feather beta-keratins begin to diversify to their modern forms. These molecular changes conferred greater elasticity to feathers than is seen in materials composed of other types of beta-keratin (29), thus optimizing the material properties of feathers for flight (41). The diversification of feather beta-keratin genes coincided with and perhaps facilitated the radiation of modern birds.
Another factor that may contribute to the unique biomaterial properties of feathers is the epidermal differentiation cysteine-rich protein (42). This molecule has the same gene structure as beta-keratins, and is coexpressed with them at certain stages of development in most keratinized tissues of living birds. However, it is only in feathers that this protein continues to be detected throughout life (42). The greatly elevated cysteine levels in this protein facilitate intramolecular cross-linking, and contribute to the stability and resistance of feathers to degradation. Further molecular recovery of ancient feather materials may allow direct testing of these hypotheses.
Conclusion
The multiple independent analyses used in this study provide strong evidence for the retention of original and phylogenetically significant protein components in Eoconfuciusornis STM 7-144, and are consistent with the identification of melanosomes as well as keratin filaments in these ancient (∼130-Ma) tissues. This is the oldest report of ultrastructure consistent with beta-keratin from an Early Cretaceous bird feather. Identifying keratin is key to ruling out a microbial source for microbodies identified in fossils. Both the depositional context and mode of preservation of this bird are similar to those of numerous other Early Cretaceous Jehol birds (and dinosaurs) and Jurassic Yanliao dinosaurs in China (1–3, 10, 11, 13, 15). This similarity suggests that other fossils preserved at these localities could provide additional sources of molecular information. More importantly, our study incorporating specific antibody reactivity in this Early Cretaceous specimen demonstrates the benefit of testing other fossil tissue samples with antisera generated against specific protein epitopes. Such tests have the potential not only to distinguish between keratinous feathers and skin-derived collagen fibers (43, 44) but also to characterize the molecular composition of early feathers and elucidate steps leading to the evolution, at the molecular level, of modern bird feathers.
Materials and Methods
Eight samples (S102, S111, S112, S201, S202, S203, S204, and S205) removed from STM 7-144 were used in this study. Samples S102, S111, and S112 were directly observed by SEM without coating. Samples S201, S202, S203, S204, and S205, which were selected for molecular analysis, were washed successively in 75% (vol/vol) ethanol and E-pure water and ultrasonically cleaned to avoid potential contaminants. The samples were then dried under a biological hood, and the surface structure was documented using SEM. Subsequently, the samples were put into 50% HF for 3 to 5 d to dissolve the silicon minerals of the matrix and washed with E-pure water three times. Approximately 1- to 2-mm3 samples were selected to be dehydrated in two changes of 70% ethanol for 30 min each, followed by one change of a mixture of LR White (medium-grade) and 70% ethanol (2:1) for 1 h, followed by another two changes of 100% LR White for 1 h each. The process was carried out at room temperature (RT). Then the samples were moved to gelatin capsules, filled with 100% LR White, covered to exclude oxygen, and allowed to polymerize for 24 h at 60 °C. A Leica EM UC6 ultramicrotome with a DiATOME 45° knife was used to cut 200-nm sections for immunohistochemistry analysis and 90-nm sections for TEM observations, immunogold tests, and ChemiSTEM analysis. Extant feathers were treated in the same way but sectioned with a different diamond knife. Neither fossils nor extant feathers were fixed, and only the extant feathers were stained with uranyl acetate (5 min) and Reynold’s lead citrate (8 min) before the TEM morphological observation. All experiments were repeated multiple times to validate results.
SEM.
The dried samples were mounted on stubs with double-sided carbon tape (S111 and S112), and one sample from an extant chicken feather was coated with ∼10 nm of gold/palladium for 30 s. The SEM-EDS data were collected from uncoated S102, S201, S203, S204, and S205. Samples were imaged with a Leo 1530 VP analytical scanning electron microscope controlled by JEOL InTouchScope version 1.05A software. Two detectors were used for taking SEM images: (i) a normal secondary electron detector, used at high vacuum and 1 to 5 keV, and (ii) a variable-pressure secondary electron detector, used at low vacuum and 10 to 20 keV.
TEM.
The 90-nm sections were mounted on carbon-coated copper grids. Samples were stained with 5% methanolic uranyl acetate (5 min), washed in E-pure water, stained with Reynold’s lead citrate (8 min), and washed in E-pure water. Sections were imaged using a JEM-2100 transmission electron microscope (AATC) from Nanjing Forestry University and a JEM-2100Plus transmission electron microscope from the State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, at 80 to 100 keV.
Immunohistochemistry.
For the production of anti-feather antibody, see Moyer et al. (28).
The autofluorescence immune-labeling protocol was modified from Schweitzer et al. (45). The 200-nm sections were mounted on six-well Teflon-coated slides, with about 10 sections in each well, and dried overnight at 42 °C. Sections were etched with 25 μg/mL proteinase K in 1× PBS at 37 °C for 15 min, followed by 0.5 M EDTA (pH 8.0) (30 min) and last with NaBH4 (twice for 10 min each). Incubations were interrupted by two 5-min washes in PBS. Following these steps to accomplish epitope retrieval and quenching of autofluorescence, 4% normal goat serum in PBS was applied to occupy nonspecific binding sites and prevent spurious binding. Sections were incubated in primary antibody (polyclonal rabbit anti-feather, 1:200; polyclonal rabbit anti-hemoglobin, 1:200; monoclonal mouse anti-peptidoglycan, 1:200) in primary dilution buffer overnight at 4 °C. All sections were then incubated with secondary antibody [biotinylated goat anti-rabbit IgG (H+L) (antibody recognizes both heavy and light chains)] (Vector Laboratories; BA-1000) diluted 1:500 for rabbit primary antibodies, and biotinylated goat anti-mouse IgG (H+L) (Vector Laboratories; BA-9200) diluted 1:500 for monoclonal mouse anti-peptidoglycan, for 2 h at RT. Sections were then incubated with fluorescein avidin D [fluorescein isothiocyanate (FITC); Vector Laboratories; A-2001] for 1 h at RT. All incubations were interrupted by sequential washes (twice for 10 min each) in PBS/Tween 20 followed by two 10-min rinses in PBS. Finally, sections were mounted with Vectashield mounting media (Vector Laboratories; H-1000) and coverslips were applied. Sections were examined with a Zeiss Axioskop 2 Plus biological microscope and captured using an AxioCam MRc 5 (Zeiss) with 10× ocular magnification on the Axioskop 2 Plus in the AxioVision software package (version 4.7.0.0).
The postembedding TEM immunogold-labeling protocol was modified from the methods shared in the protocol database from IHCWORLD Life Science Products & Services (www.ihcworld.com/_protocols/em/post_immunoem_embed812.htm). The 90-nm sections were collected on carbon-coated nickel grids (EMS; CFT200-NI). The grids were incubated in PBS/Tween 20 for 10 min. Four percent normal donkey serum in PBS was applied to occupy nonspecific binding sites and prevent spurious binding for 1 h at RT. The grids were incubated in primary antibody (polyclonal rabbit anti-feather, 1:20) in primary dilution buffer for 3 h at RT and then washed with PBS/Tween 20, 10 times for 2 min each. All grids were then incubated with secondary antibody [12-nm Colloidal Gold AffiniPure Donkey Anti-Rabbit IgG (H+L), 1:20; Jackson ImmunoResearch; 711-205-152] for 1 h. The grids were rinsed with PBS/Tween 20, 10 times for 2 min each and in E-pure water three times for 30 s each, and dried with filter paper. The sections were observed using the FEI Titan G2 80-300 electron microscope at the Analytical Instrumentation Facility (AIF) of North Carolina State University.
ChemiSTEM.
Sections (90-nm) were collected on carbon-coated nickel grids (EMS; CFT200-NI) and analyzed with an FEI Titan G2 80-300 electron microscope at the AIF of North Carolina State University.
The FEI Titan G2 series of Cs-corrected scanning/transmission electron microscopes with the revolutionary ChemiSTEM technology is applied in this study. This new technology greatly enhances EDX (energy dispersive X-ray spectroscopy) detection sensitivity due to a number of innovations in the system architecture, including the X-FEG (a high-brightness Schottky field emission gun source), Super-X EDX detector system (four windowless silicon drift detectors with shutters integrated deeply into the objective lens), and high-speed electronics capable of 100,000 spectra readouts. This new system architecture provides many performance benefits, such as improved light element detection, better sample tilt response, faster mapping, and atomic chemical mapping capabilities of crystalline structures.
Acknowledgments
We thank technician Ms. Chunzhao Wang of the State Key Laboratory of Palaeobiology and Stratigraphy (LPS) of Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Research Scholars Chuanzhen Zhou and Yang Liu of the Analytical Instrumentation Facility (AIF) of North Carolina State University, Dr. Lulu Cong of the Analytical Center of Nanjing Institute of Geography & Limnology, Chinese Academy of Sciences, and Dr. Lingfei Ding and Dr. Yang Jing of the Advanced Analysis Test Center of Nanjing Forestry University for technical assistance. We also benefited from discussions with Dr. Johan Lindgren and Prof. Franz Fürsich. This work was performed in part at the AIF at North Carolina State University, which is supported by the State of North Carolina and the National Science Foundation (Award ECCS-1542015). The AIF is a member of the North Carolina Research Triangle Nanotechnology Network, a site in the National Nanotechnology Coordinated Infrastructure. The research was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB18030501), National Natural Science Foundation of China (41472009 and 91514302), Open-Lab Grants of the LPS and National Science Foundation USA (INSPIRE Program EAR-1344198 to M.H.S., W.Z., and E.R.S., and DGE01252376 to A.E.M.), as well as funds from the David and Lucile Packard Foundation (to M.H.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1617168113/-/DCSupplemental.
References
- 1.Xu X, Zheng X, You H. A new feather type in a nonavian theropod and the early evolution of feathers. Proc Natl Acad Sci USA. 2009;106(3):832–834. doi: 10.1073/pnas.0810055106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, You H, Du K, Han F. An Archaeopteryx-like theropod from China and the origin of Avialae. Nature. 2011;475(7357):465–470. doi: 10.1038/nature10288. [DOI] [PubMed] [Google Scholar]
- 3.Godefroit P, et al. Reduced plumage and flight ability of a new Jurassic paravian theropod from China. Nat Commun. 2013;4:1394. doi: 10.1038/ncomms2389. [DOI] [PubMed] [Google Scholar]
- 4.Wuttke M. Weichteil-Erhaltung durch lithifizierte Mikroorganismen bei mittel-Eozänen Vertebraten aus den Ölschiefern der Grube Messel bei Darmstadt. Senckenb Biol. 1983;64(5–6):509–527. [Google Scholar]
- 5.Davis PG, Briggs DEG. Fossilization of feathers. Geology. 1995;23(9):783–786. [Google Scholar]
- 6.Burtt EH, Ichida JM. Occurrence of feather-degrading bacilli in the plumage of birds. Auk. 1999;116(2):364–372. [Google Scholar]
- 7.Clayton DH. Feather-busting bacteria. Auk. 1999;116(2):302–304. [Google Scholar]
- 8.Vinther J, Briggs DEG, Prum RO, Saranathan V. The colour of fossil feathers. Biol Lett. 2008;4(5):522–525. doi: 10.1098/rsbl.2008.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vinther J, Briggs DEG, Clarke J, Mayr G, Prum RO. Structural coloration in a fossil feather. Biol Lett. 2010;6(1):128–131. doi: 10.1098/rsbl.2009.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, et al. Plumage color patterns of an extinct dinosaur. Science. 2010;327(5971):1369–1372. doi: 10.1126/science.1186290. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F, et al. Fossilized melanosomes and the colour of Cretaceous dinosaurs and birds. Nature. 2010;463(7284):1075–1078. doi: 10.1038/nature08740. [DOI] [PubMed] [Google Scholar]
- 12.Carney RM, Vinther J, Shawkey MD, D’Alba L, Ackermann J. New evidence on the colour and nature of the isolated Archaeopteryx feather. Nat Commun. 2012;3:637. doi: 10.1038/ncomms1642. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, et al. Reconstruction of Microraptor and the evolution of iridescent plumage. Science. 2012;335(6073):1215–1219. doi: 10.1126/science.1213780. [DOI] [PubMed] [Google Scholar]
- 14.Vinther J, et al. 3D camouflage in an ornithischian dinosaur. Curr Biol. 2016;26(18):2456–2462. doi: 10.1016/j.cub.2016.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Q, et al. Melanosome evolution indicates a key physiological shift within feathered dinosaurs. Nature. 2014;507(7492):350–353. doi: 10.1038/nature12973. [DOI] [PubMed] [Google Scholar]
- 16.Moyer AE, et al. Melanosomes or microbes: Testing an alternative hypothesis for the origin of microbodies in fossil feathers. Sci Rep. 2014;4:4233. doi: 10.1038/srep04233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindgren J, et al. Interpreting melanin-based coloration through deep time: A critical review. Proc Biol Sci. 2015;282(1813):20150614. doi: 10.1098/rspb.2015.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweitzer MH, Lindgren J, Moyer AE. Melanosomes and ancient coloration re-examined: A response to Vinther 2015 (DOI 10.1002/bies.201500018) BioEssays. 2015;37(11):1174–1183. doi: 10.1002/bies.201500061. [DOI] [PubMed] [Google Scholar]
- 19.Clarke JA, et al. Fossil evidence for evolution of the shape and color of penguin feathers. Science. 2010;330(6006):954–957. doi: 10.1126/science.1193604. [DOI] [PubMed] [Google Scholar]
- 20.Lindgren J, et al. Molecular preservation of the pigment melanin in fossil melanosomes. Nat Commun. 2012;3:824. doi: 10.1038/ncomms1819. [DOI] [PubMed] [Google Scholar]
- 21.Lindgren J, et al. Molecular composition and ultrastructure of Jurassic paravian feathers. Sci Rep. 2015;5:13520. doi: 10.1038/srep13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barden HE, et al. Morphological and geochemical evidence of eumelanin preservation in the feathers of the Early Cretaceous bird, Gansus yumenensis. PLoS One. 2011;6(10):e25494. doi: 10.1371/journal.pone.0025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wogelius RA, et al. Trace metals as biomarkers for eumelanin pigment in the fossil record. Science. 2011;333(6049):1622–1626. doi: 10.1126/science.1205748. [DOI] [PubMed] [Google Scholar]
- 24.Manning PL, et al. Synchrotron-based chemical imaging reveals plumage patterns in a 150 million year old early bird. J Anal At Spectrom. 2013;28(7):1024–1030. [Google Scholar]
- 25.Egerton VM, et al. The mapping and differentiation of biological and environmental elemental signatures in the fossil remains of a 50 million year old bird. J Anal At Spectrom. 2015;30(3):627–634. [Google Scholar]
- 26.Glass K, et al. Direct chemical evidence for eumelanin pigment from the Jurassic Period. Proc Natl Acad Sci USA. 2012;109(26):10218–10223. doi: 10.1073/pnas.1118448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingham-Soliar T, Murugan N. A new helical crossed-fibre structure of β-keratin in flight feathers and its biomechanical implications. PLoS One. 2013;8(6):e65849. doi: 10.1371/journal.pone.0065849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer AE, Zheng W, Schweitzer MH. Keratin durability has implications for the fossil record: Results from a 10 year feather degradation experiment. PLoS One. 2016;11(7):e0157699. doi: 10.1371/journal.pone.0157699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenwold MJ, Sawyer RH. Linking the molecular evolution of avian beta (β) keratins to the evolution of feathers. J Exp Zool B Mol Dev Evol. 2011;316B(8):609–616. doi: 10.1002/jez.b.21436. [DOI] [PubMed] [Google Scholar]
- 30.Alibardi L, Dalla Valle L, Toffolo V, Toni M. Scale keratin in lizard epidermis reveals amino acid regions homologous with avian and mammalian epidermal proteins. Anat Rec A Discov Mol Cell Evol Biol. 2006;288(7):734–752. doi: 10.1002/ar.a.20342. [DOI] [PubMed] [Google Scholar]
- 31.Murphy ME, King JR, Taruscio TG, Geupel GR. Amino acid composition of feather barbs and rachises in three species of pygoscelid penguins: Nutritional implication. Condor. 1990;92(4):913–921. [Google Scholar]
- 32.Hallahan DL, et al. Analysis of gene expression in gecko digital adhesive pads indicates significant production of cysteine- and glycine-rich beta-keratins. J Exp Zool B Mol Dev Evol. 2009;312B(1):58–73. doi: 10.1002/jez.b.21242. [DOI] [PubMed] [Google Scholar]
- 33.Durrer H. Colouration. In: Bereiter-Hahn J, Matoltsy AG, Richards KS, editors. Biology of the Integument, Vertebrates. Vol 2. Springer; Heidelberg: 1986. pp. 239–247. [Google Scholar]
- 34.Schweitzer MH, et al. Beta-keratin specific immunological reactivity in feather-like structures of the cretaceous alvarezsaurid, Shuvuuia deserti. J Exp Zool. 1999;285(2):146–157. [PubMed] [Google Scholar]
- 35.Schweitzer MH, et al. Keratin immunoreactivity in the Late Cretaceous bird Rahonavis ostromi. J Vertebr Paleontol. 1999;19(4):712–722. [Google Scholar]
- 36.Greenwold MJ, et al. Dynamic evolution of the alpha (α) and beta (β) keratins has accompanied integument diversification and the adaptation of birds into novel lifestyles. BMC Evol Biol. 2014;14:249. doi: 10.1186/s12862-014-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta R, Ramnani P. Microbial keratinases and their prospective applications: An overview. Appl Microbiol Biotechnol. 2006;70(1):21–33. doi: 10.1007/s00253-005-0239-8. [DOI] [PubMed] [Google Scholar]
- 38.Brandelli A, Daroit DJ, Riffel A. Biochemical features of microbial keratinases and their production and applications. Appl Microbiol Biotechnol. 2010;85(6):1735–1750. doi: 10.1007/s00253-009-2398-5. [DOI] [PubMed] [Google Scholar]
- 39.Niecke M, Heid M, Krüger A. Correlations between melanin pigmentation and element concentration in feathers of white-tailed eagles (Haliaeetus albicilla) J Ornithol. 1999;140(3):355–362. [Google Scholar]
- 40.Briggs DEG. The role of decay and mineralization in the preservation of soft-bodied fossils. Annu Rev Earth Planet Sci. 2003;31:275–301. [Google Scholar]
- 41.Weiss IM, Kirchner HO. Plasticity of two structural proteins: Alpha-collagen and beta-keratin. J Mech Behav Biomed Mater. 2011;4(5):733–743. doi: 10.1016/j.jmbbm.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Strasser B, Mlitz V, Hermann M, Tschachler E, Eckhart L. Convergent evolution of cysteine-rich proteins in feathers and hair. BMC Evol Biol. 2015;15:82. doi: 10.1186/s12862-015-0360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lingham-Soliar T, Feduccia A, Wang X. A new Chinese specimen indicates that ‘protofeathers’ in the Early Cretaceous theropod dinosaur Sinosauropteryx are degraded collagen fibres. Proc Biol Sci. 2007;274(1620):1823–1829. doi: 10.1098/rspb.2007.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lingham-Soliar T. A unique cross section through the skin of the dinosaur Psittacosaurus from China showing a complex fibre architecture. Proc Biol Sci. 2008;275(1636):775–780. doi: 10.1098/rspb.2007.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schweitzer MH, Moyer AE, Zheng W. Testing the hypothesis of biofilm as a source for soft tissue and cell-like structures preserved in dinosaur bone. PLoS One. 2016;11(2):e0150238. doi: 10.1371/journal.pone.0150238. [DOI] [PMC free article] [PubMed] [Google Scholar]