Significance
Ammonia-oxidizing archaea (AOA), key players in global biogeochemical cycles, represent a heterogeneous group with a broad environmental distribution. Understanding their activity and physiology is of great importance due to the impact of the overuse of agricultural fertilizers on the N cycle and the production of the greenhouse gas N2O during nitrification. Despite their prominent ecological role, little is known about the fundamental metabolic processes of AOA. Here, we show that AOA of marine and terrestrial environments share unique and well-conserved pathways of carbon and nitrogen metabolism, and we raise hypotheses about missing steps in these pathways. Our approach also highlights the extensive environmental adaptations of the soil clade, including the capacity for cell surface modifications, carbohydrate conversions, detoxification, and biofilm formation.
Keywords: ammonia oxidation, proteomics, archaea, comparative genomics, biofilm
Abstract
Ammonia-oxidizing archaea (AOA) are among the most abundant microorganisms and key players in the global nitrogen and carbon cycles. They share a common energy metabolism but represent a heterogeneous group with respect to their environmental distribution and adaptions, growth requirements, and genome contents. We report here the genome and proteome of Nitrososphaera viennensis EN76, the type species of the archaeal class Nitrososphaeria of the phylum Thaumarchaeota encompassing all known AOA. N. viennensis is a soil organism with a 2.52-Mb genome and 3,123 predicted protein-coding genes. Proteomic analysis revealed that nearly 50% of the predicted genes were translated under standard laboratory growth conditions. Comparison with genomes of closely related species of the predominantly terrestrial Nitrososphaerales as well as the more streamlined marine Nitrosopumilales [Candidatus (Ca.) order] and the acidophile “Ca. Nitrosotalea devanaterra” revealed a core genome of AOA comprising 860 genes, which allowed for the reconstruction of central metabolic pathways common to all known AOA and expressed in the N. viennensis and “Ca. Nitrosopelagicus brevis” proteomes. Concomitantly, we were able to identify candidate proteins for as yet unidentified crucial steps in central metabolisms. In addition to unraveling aspects of core AOA metabolism, we identified specific metabolic innovations associated with the Nitrososphaerales mediating growth and survival in the soil milieu, including the capacity for biofilm formation, cell surface modifications and cell adhesion, and carbohydrate conversions as well as detoxification of aromatic compounds and drugs.
Ten years of extensive research on ammonia-oxidizing archaea (AOA) have provided important insights into their environmental distribution, ecophysiology, and general genome content. AOA belong to the archaeal phylum Thaumarchaeota (1, 2) and are a major component of many microbial communities, with abundances up to 107 cells per 1 mL in sea waters (3, 4) and even 100-fold more per gram of soil (5, 6). They perform a key role in global nitrogen cycling but also participate in carbon cycling and production of metabolites essential to many members of the microbial and eukaryotic community (7–9). Since their discovery in 2005 (10, 11), about a dozen strains have been enriched under aerobic and autotrophic conditions, and some were obtained in pure laboratory cultures. About an equal number of full or nearly complete genome sequences are now available, allowing the first comparative analyses of their core and variable gene repertoires (12, 13). Despite their vast distribution and large numbers in the environment, AOA are delicate organisms with which to work. Their small cell sizes, slow growth rates, and autotrophic (or mixotrophic) growth mode on low concentrations of ammonia as energy source (compared with their bacterial counterparts) have limited the ecophysiological and metabolic characterization of these oligotrophic organisms (12, 14, 15). Nitrososphaera viennensis, isolated from soil, represents the first deposited type strain of the class Nitrososphaeria within the phylum Thaumarchaeota that encompasses all known AOA (16, 17) and is one of the best studied AOA from soil. The growth characteristics of N. viennensis on ammonia and urea as energy sources have been described (16), as well as its dependence on small organic acids for growth (16), resistance to common nitrification inhibitors (18, 19), production of NO and contribution to greenhouse gas emissions (20, 21), thaumarchaeal ether–lipid components (22), and typical archaeal S-layer structure with p3 symmetry (17).
Despite this accumulated knowledge on N. viennensis and other AOA, many fundamental questions about their core and variable metabolism and niche differentiation remain unanswered. Although it is generally assumed that the archaeal version of ammonia monooxygenase encoded in all studied AOA is responsible for the first step of ammonia oxidation, the second step to nitrite production and the contributing cofactors and electron carriers have still not been identified. Moreover, although a unique and highly energy efficient aerobic pathway of carbon assimilation conserved in all AOA has been biochemically investigated in the marine strain “Candidatus (Ca.) Nitrosopumilus maritimus” (23), regulation mechanisms that maintain the balance over the key metabolic processes of nitrogen and carbon assimilation have not been elucidated. In addition, adaptive features of different AOA to their respective ecological niches have not been investigated, which are needed to understand their metabolic flexibility and their reaction to changing environmental conditions. A better understanding of the ecosphysiology of terrestrial AOA compared with their ammonia-oxidizing bacteria (AOB) counterparts that coexist in the same habitats would help to elucidate the relative contribution of both AOA and AOB to nitrogen (and carbon) turnover in soils. This knowledge of AOA (eco)physiology is not only of fundamental ecological interest but also might eventually be of importance for mitigation strategies aimed at reducing N2O emissions in agriculture, since AOB but not AOA can produce significant amounts of N2O as a side reaction of ammonia oxidation or via the nitrifier denitrification pathway (21, 24, 25).
To gain deeper insights into the central metabolism of AOA and specific adaptations of the terrestrial group, we have performed a manual annotation of the genome of N. viennensis, the type strain of the phylum Thaumarchaeota, and determined its proteome under standard growth conditions. In a comparative genomic analysis with seven closed genomes of Thaumarchaeota (26–31), we have dissected the conserved gene repertoire of AOA and compared this with the expressed proteome of N. viennensis and “Ca. Nitrosopelagicus brevis” (30). This comparative analysis allows us to narrow down the minimal active gene set of AOA, reconstruct central metabolic pathways, including possible regulatory mechanisms, and identify candidates for potential missing steps in the core energy metabolism of AOA. In addition, we identify and discuss in the context of their ecophysiology the gene complement specific to N. viennensis and the terrestrial strains.
Results and Discussion
With 2.52 Mb and 3,123 predicted protein-coding genes, the N. viennensis genome is similar in size to the other genomes of the Nitrososphaerales [formerly group I.1b (17, 28, 29)] and larger than genomes of the other major order “Ca. Nitrosopumilales” (formerly group I.1a) that are mostly marine strains and range in size from 1.23 to 1.85 Mb (26, 27, 30, 32–38) [except marine symbiont “Ca. Cenarchaeum symbiosum”, with 2.05 Mb (39)].
The proteome of late exponential-phase cultures of N. viennensis was analyzed on an LC-Orbitrap Mass Spectrometer. Total cellular lysates were fractionated in membrane-enriched and cytoplasmic fractions by ultracentrifugation to enrich and enable identification of otherwise poorly represented membrane-associated proteins. From the combined datasets, we recovered a total of 1,503 proteins, representing 48% of all predicted proteins encoded in the N. viennensis genome (Dataset S1). The absolute number of proteins identified in the proteome of N. viennensis was considerably higher than 1,012 proteins reported recently for (early stationary phase) cultures of the marine AOA Ca. N. brevis (30). The expressed proteome of Ca. N. brevis, however, represented a greater fraction (70%) of all proteins predicted from its genome (1,445 coding sequences) as expected from its more streamlined genome that results in a smaller size of its nonconserved (shell) gene complement. Assuming that the larger gene repertoire of N. viennensis is a consequence of its adaptations to a diverse range of environmental changes and stresses in soil, we do not expect expression of a higher protein complement of the genome under stable laboratory culture conditions.
The Core Gene Repertoire of AOA.
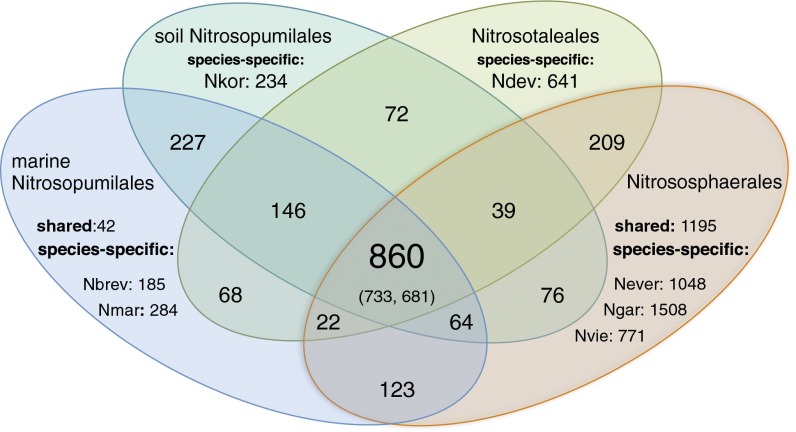
Clusters of orthologous groups (COGs) of proteins were defined for seven closed genomes of free-living Thaumarchaeota representing the order Nitrososphaerales and the two candidate orders Ca. Nitrosopumilales and “Ca. Nitrosotaleales” (formerly clades I.1b, I.1a, and I.1a-associated, respectively) (features of the genomes are summarized in Table S1). This dataset included, in addition to N. viennensis, “Ca. Nitrososphaera gargensis” (28) and “Ca. Nitrososphaera evergladensis” (29) of the order Nitrososphaerales; Ca. N. maritimus (26), “Ca. Nitrosoarchaeum koreensis” (27), and Ca. N. brevis, a marine strain for which a proteome dataset is also available (30), from the order Ca. Nitrosopumilales; and “Ca. Nitrosotalea devanaterra”, an acidophilic soil strain that, according to its divergence from other strains, probably represents a separate order, Ca. Nitrosotaleales, associated with Ca. Nitrosopumilales (31) (the Candidatus designation of the strains was omitted below to improve readability). This comparison enabled us to identify a conserved core set of 860 protein families (Fig. 1 and Dataset S2) (i.e., families shared by all analyzed genomes and likely representing the “core” gene repertoire of AOA; from here on, they are referred to as core COG families). Among these core COG families, we encountered all of the highly conserved information processing genes (involved in replication, transcription, translation, etc.) that are signatures of the phylum Thaumarchaeota (as outlined in ref. 2). A summary of the functional categories of the core COG families is depicted in Fig. S1.
Table S1.
Selected features of the AOA strains and genomes used in this study
| Strains | N. gargensis Ga9.2 | N. viennensis EN76 | N. evergladensis SR1 | N. maritimus SCM1 | N. koreensis MY1 | N. brevis CN25 | N. devanaterra Nd1 |
| Order | Nitrososphaerales | Nitrososphaerales | Nitrososphaerales | Nitrosopumilales | Nitrosopumilales | Nitrosopumilales | Nitrosotaleales |
| Family | Nitrososphaeraceae | Nitrososphaeraceae | Nitrososphaeraceae | Nitrosopumilaceae | Nitrosopumilaceae | Nitrosopelagicaceae | Nitrosotaleaceae |
| Culture | Yes (pure) | Yes (pure) | Yes (enrichment) | Yes (pure) | Yes (enrichment) | Yes (enrichment) | Yes (pure) |
| Source | Microbial mat | Garden soil | Agricultural soil | Marine aquarium gravel | Rhizosphere soil | Subtropical surface ocean | Acidic agricultural soil |
| Genome size (Mb) | 2.83 | 2.53 | 2.95 | 1.65 | 1.61 | 1.23 | 1.81 |
| CDS NCBI (protein coding) | 3,565 | 3,123 | 3,499 | 1,796 | 1,810 | 1,445 | 2,103 |
| GC, % | 48.4 | 52.7 | 50 | 34.2 | 32.7 | 33 | 37.7 |
| Reference culture | Hatzenpichler et al. (104) | Stieglmeier et al. (17) | Zhalnina et al. (29) | Könneke et al. (10) | Jung et al. (35) | Santoro et al. (105) | Lehtovirta-Morley (106) |
| Reference genome | Spang et al. (28) | This study | Zhalnina et al. (29) | Walker et al. (26) | Kim et al. (27) | Santoro et al. (30) | Lehtovirta-Morley et al. (31) |
| Genome accession number | CP002408 | CP007536 | CP007174 | CP000866 | AFPU00000000 | CP007026 | ERS884509 |
Fig. 1.
Simplified Venn diagram illustrating the numbers of COGs shared between marine Ca. Nitrosopumilales (COGs shared between N. maritimus and N. brevis and between either of them and the other categories), soil Ca. Nitsosopumilales (COGs shared between N. koreensis and the other categories), Ca. Nitrosotaleales (COGs shared between N. devanaterra and the other categories), and Nitrososphaerales [COGs shared between N. viennensis, N. gargensis, and N. evergladensis (either among all three or pairwise) and between either of them and the other categories]. Proteins that were not grouped into COGs are represented as specific proteins for each organism. Not all combinations of shared COGs are represented in this Venn diagram. Numbers in parentheses indicate the numbers of clusters detected in the experimentally determined proteomes of N. viennensis and N. brevis, respectively. Genome abbreviations are Nbrev, N. brevis; Ndev, N. devanaterra; Never, N. evergladensis; Ngar, N. gargensis; Nkor, N. koreensis; Nmar, N. maritimus; and Nvie, N. viennensis.
Fig. S1.
Distribution of the arCOG functional categories assigned to the defined core COGs.
Members of the core COG families represent a large fraction of the expressed proteome in both available proteomic datasets, composing 48.8% of the identified proteins in N. viennensis (733 in a total of 1,503) (Fig. 1) and 67.3% in N. brevis (681 in 1,012 identified proteins), whereas 611 were expressed in both (Dataset S1). Both proteomic datasets were generated from cultures grown in defined media [as defined for each organism (17, 30)] under ammonia-oxidizing conditions. Comparative analysis of the genomic and proteomic datasets highlights and confirms the common processes supporting the aerobic, autotrophic ammonia-oxidizing lifestyle in these representatives, although they stem from two distinct orders associated with radically different ecological habitats (12, 13, 40). This conservation among the core protein families enabled us to raise hypotheses about missing steps in shared metabolisms and dissect highly specialized distinct functions in the variable (accessory) genomes (see below).
Conserved Central Carbon Metabolism in AOA.
All pathways of the central carbon metabolism including the genes (canonical and putative) participating in the hydroxypropionate/hydroxybutyrate CO2 fixation pathway (23, 41), tricarboxylic acid (TCA) cycle, gluconeogenesis, and nonoxidative pentose phosphate pathways are present in all analyzed AOA genomes and both proteomes, verifying the conserved metabolic features of the phylum (Datasets S1 and S2). A full reconstruction of the central carbon metabolism based on our comparative analysis is given in Fig. 2, distinguishing the core AOA gene repertoire (genes labeled in gray) from genes with a more limited distribution (orange and blue). Notably, Nitrososphaerales seem to be particularly versatile with respect to interconversions of the key metabolite pyruvate (28, 29), as indicated by the presence of a pyruvate/phosphate dikinase, converting pyruvate to phosphoenolpyruvate (PEP), a putative pyruvate dehydrogenase/oxidase converting it to acetate, and an alanine dehydrogenase (Dataset S2).
Fig. 2.
Reconstruction of the predicted central carbon metabolism modules in AOA emphasizing the conservation of the core pathway enzymes and carriers and features of the Nitrososphaerales. Canonical enzymes belonging to the core COGs are depicted in light gray boxes, whereas candidate enzymes according to the work by Spang et al. (28) are in dark gray boxes. COGs conserved among Nitrososphaerales are depicted in orange boxes, and COGs present in some of the analyzed genomes are in blue (refer to the color key in Fig. 3). Proteins catalyzing all displayed reactions were detected in the proteome of N. viennensis by proteotypic peptides. Gene accession numbers and enzyme abbreviations are listed in Dataset S2.
All Thaumarchaeota harbor candidate enzymes for all steps of an oxidative TCA cycle, the functionality of which in vivo is, however, debated. In particular, the substrate specificity of the 2-oxoacid:ferredoxin oxidoreductase (NVIE_029480–NVIE_029490), the candidate enzyme for conversion of both pyruvate and 2-oxoglutarate to acetyl-CoA and succinyl-CoA, respectively, remains unknown (42), and its functioning in both steps of the cycle might be unlikely. Moreover, classical TCA bypass routes seem to be incomplete or noncanonical, as only one candidate enzyme (isocitrate lyase) catalyzing the first step of the glyoxylate shunt could be identified in the genome and is also detected in the proteome (Datasets S1 and S2).
As shown for Metallosphaera sedula (43) and proposed by Könneke et al. (23) for the AOA N. maritimus, succinyl-CoA is probably the primary carbon fixation product in the hydroxypropionate/hydroxybutyrate pathway. Notably, N. viennensis and other Nitrososphaerales contain a putative malic enzyme apart from the PEP carboxykinase, which could connect the C4/C3 compound pool in the absence of a full oxidative TCA cycle under autotrophic growth. For a better understanding of AOA central carbon metabolism (and other metabolisms), it will be crucial to perform biochemical analyses to verify and/or determine substrate specificities of the predicted components.
Although this analysis and other genomic and physiological investigations support the assumption that AOA are, in principle, autotrophic ammonia oxidizers, the possibility of alternative metabolisms was indicated by studies showing growth of AOA without apparent ammonia oxidation activity (44, 45) and the finding of transporters for organics (13) as well as incorporation studies of amino acids (46). However, despite the large amount of transporters encoded in the genome of N. viennensis (13), we have so far not been able to identify alternate energy or carbon sources in growth experiments. In addition, Kim et al. (47) have convincingly shown that the dependency of N. viennensis and other strains on small amounts of organic acids (16, 17, 48) can be overcome by reducing reactive oxygen species in the medium. Consequently, based on growth experiments and genomic predictions, all AOA currently in pure culture can grow strictly autotrophically.
The AOA Core Genomic Repertoire Involved in Ammonia Oxidation.
Based on the genomic and proteomic data, we have reconstructed the possible pathways of energy metabolism (via ammonia oxidation) and ammonia assimilation as they are found in all investigated thaumarchaeal genomes and translated in N. viennensis (Fig. 3 and Dataset S2, gene accession numbers of N. viennensis). Among the core protein families involved in energy metabolism are the ammonia monooxygenase (AMO) subunits A, B, and C, which are homologs of the bacterial counterparts. A gene for a fourth potential membrane-associated subunit that we earlier referred to as ORF38 (11) or amoX (49) is linked to amoA in all sequenced genomes. The AmoC protein family includes a single homolog in the Ca. Nitrosopumilales genomes but is enlarged in the soil-dwelling Nitrososphaerales and AOB (28, 29, 50), with N. viennensis encoding six homologs (72–96% amino acid identity). The core AmoC COG includes two copies in Nitrososphaerales (AmoC3 and C6 in N. viennensis). The additional homologs either belong to COGs shared between the Nitrososphaerales genomes (AmoC4,C5) or cluster with the organism-specific COGs (AmoC1,C2), reflecting possible regulatory or stress response functions. Transcription studies in the AOB Nitrosomonas europaea implicate a role of additional amoC copies in the recovery from ammonia starvation or other types of stress by ensuring the stability of the AMO holoenzyme (51, 52). The A, B, and X subunits were detected in both proteomic datasets, whereas the core C subunit was only detected in the N. brevis proteome. AmoB was the second most abundant protein in the N. viennensis proteome (Dataset S1), supporting observations on the environmental abundance (or ease of detection) of this subunit from metaproteomic studies (53).
Fig. 3.
Reconstruction of putative ammonia oxidation and primary nitrogen assimilation in AOA emphasizing the conservation of the core pathway enzymes and carriers and features of the Nitrososphaerales. The ammonia oxidation module was adapted from ref. 21. Dark gray indicates candidate enzyme [in this case, the unidentified HURM (21)]. Dashed lines indicate binding/regulation. Dotted lines indicate absent reaction, which is included here for clarity. All depicted proteins except the urea transporters were detected in the proteome of N. viennensis. Gene accession numbers are in Dataset S2. The presence and function of urea transporters (UT and SSS) and urease (Ure) as well as the two Amt ammonium transporters have been presented earlier (refs. 16 and 13, respectively) and are not discussed here.
Although the identity of the enzyme(s) for the second step in ammonia oxidation remains unknown, hydroxylamine has been verified as an intermediate compound in N. maritimus (54). Currently, models for ammonia oxidation in AOA and AOB assume that the active site of the AMO is located on the periplasmic side of the membrane (21, 24, 26, 55). Consequently, it is suggested that the following oxidation of the intermediate hydroxylamine should also take place in the (pseudo)periplasmic space of AOA (i.e., in the space between the cytoplasmic membrane and the S-layer). An extracellular location of the oxidation of hydroxylamine into nitrite is bioenergetically favorable, because it contributes to the establishment of the proton gradient across the cell membrane via the release of scalar protons in the periplasmic space (24, 55). The intracellular oxidation of hydroxylamine would instead require the transport of the highly reactive and toxic hydroxylamine into the cytoplasm and the secretion of nitrite outside the cell, which seems, at least theoretically, unfavorable. The immediate implication of those considerations is that the enzyme(s) participating in the oxidation of hydroxylamine would be secreted as shown for the hydroxylamine oxidoreductase (HAO) in AOB.
We assume that all core and accessory enzymes in the energy metabolism would be among the core COGs in our analysis, and therefore, we searched for enzymes capable of performing oxidoreductase reactions among the core protein families. Naturally, this search is limited to the fraction of core COGs for which a function could be proposed, but we did not limit our search to putative candidates predicted to be membrane associated or secreted.
Five protein candidates were identified, which include two putative F420-dependent luciferase-like monooxygenase proteins (NVIE_004930 and NVIE_015060), with a domain found in flavin monooxygenases catalyzing mostly the oxidation of aldehydes in various pathways. Both proteins are present in high amounts in the proteomes of N. viennensis and N. brevis, suggesting an important role in the metabolism of those organisms. A putative NADPH-dependent F420 reductase (NVIE_027500) and two putative FAD- and FAD/NAD(P)-dependent oxidoreductases (NVIE_016850 and NVIE_007970) were also expressed in both proteome datasets. The predicted localization of these five enzymes is cytoplasmic (Materials and Methods), making them incompatible with the extracellular ammonia oxidation hypothesis. However, secreted proteins do not always possess a signal peptide, and studies of archaeal signal peptides are scarce; we cannot rule out the possibility that some of these enzymes are secreted via nonclassical protein secretion pathways (56).
The production of significant amounts of F420 by AOA was earlier verified by Spang et al. (28). The genes for the biosynthesis of this cofactor are among the core COGs, supporting the proposed important role of this cofactor in AOA metabolism (28). As noted by Spang et al. (28), reduced F420 generated by an F420 glucose-6-phosphate dehydrogenase in mycobacteria has a protective role against reactive nitrogen species (57). Thus, a similar F420-dependent mechanism might operate in AOA. Recently, a membrane-attached small cupredoxin from N. maritimus with a type 1 copper center (T1Cu) was shown to have reversible NO oxidation/NO2− reduction activity in vitro (58), indicating a need for detoxification of extracellularly produced reactive nitrogen species. Biochemical analyses will be needed to clarify whether F420 is involved in detoxification, is part of the archaeal hydroxylamine:ubiquinone redox module (HURM), catalyzing the oxidation of hydroxylamine (see below), or participates in both functions.
Conservation of Multicopper Proteins in AOA.
It has been repeatedly proposed that a multicopper oxidase (MCO) could be the archaeal counterpart to the bacterial HAO or participate in the archaeal HURM (Fig. 3), as multiple MCOs that are predicted to be membrane associated or secreted were discovered in all AOA genomes (12, 21, 26, 55). However, none of the MCOs were found among the core COGs in this analysis. To examine the distribution of this protein family further, we conducted a thorough phylogenetic analysis on all available AOA genomes and representative genomes of other archaeal phyla and AOB/nitrite-oxidizing bacteria (details are in SI Results and Discussion and Table S2). The phylogenetic analysis reveals four well-supported major lineages of two-domain MCOs with type 1 (T1Cu) and trinuclear type 2/type 3 copper centers (labeled MCO1–4 in Fig. 4). MCO1 comprises only sequences from thaumarchaeal genomes, whereas MCO4 (with an additional blue copper domain) is present only in Nitrososphaerales and the genera Nitrosopumilus and Nitrosoarchaeum. MCO2 and MCO3 comprise nonthaumarchaeal sequences (with one exception) (Fig. S2 shows an uncollapsed tree). MCO1 orthologs are present in all described AOA, with the sole exception of N. devanaterra. All AOA (except N. devanaterra) encode a transporter of the Zinc-Iron Permease (ZIP) family immediately adjacent to MCO1 and on the same strand (except for Nitrosotenuis chungbukensis MY2 and Nitrosotenuis uzonensis N4, where the gene is unlinked). The co-occurrence pattern indicates a functional link and coregulation between these two proteins, which are also reflected in a shared evolutionary history (Fig. S3 shows the permease tree). Given that transporters of the ZIP family are involved in the transport of a range of divalent cations, including copper (Cu2+), this pair of genes (MCO plus the ZIP family permease) could be involved in copper sequestration provided that the MCO is secreted. The absence of both genes in N. devanaterra further supports a functional link of the two and could be explained by the particularly low pH conditions that this archaeon encounters in the environment, which likely influences the bioavailability of copper in these soils (59) and might impose different copper uptake strategies. Alternatively, this MCO could still represent the missing module in ammonia oxidation (HURM in Fig. 3), whereas in the acidophile N. devanaterra, a substitution by a different enzyme might have occurred due to pH constraints. Such cases of nonorthologous gene replacements in acidophiles have already been reported. For example, a completely novel type of tetrathionate hydrolase essential for the dissimilatory oxidation of inorganic sulfur compounds has been exclusively found in acidophilic Bacteria and Archaea (60).
Table S2.
Genomes screened for the presence of MCOs
| No. code | Genus and species | Strain | Taxon identification |
| BEOBS1 | Thaumarchaeota archaeon | BS1 | IMG_2502957026 |
| BEOBS4 | Thaumarchaeota archaeon | BS4 | IMG_2519899514 |
| NFN1 | Thaumarchaeota group 1.1c | (bin Fn1) | |
| CAERO | Calditenuis aerorheumensis | ?? | IMG_2545555825_21516 |
| CSUB | Caldiarchaeum subterraneum | ?? | BA000048 |
| DRAGT | Thaumarchaeota archaeon | DS1 | IMG_2263082000 |
| CENSY | C. symbiosum | A | NC_014820.1 |
| CSP1 | Thaumarchaeota archaeon | CSP1 | LDXL00000000.1 |
| NADR | Nitrosopumilus adriaticus | NF5 | CP011070.1 |
| NBREV | N. brevis | CN25 | CP007026.1 |
| NCHUN | Nitrosotenuis chungbukensis | MY2 | AVSQ00000000 |
| NDEVA | N. devanaterra | Nd1 | LN890280.1 |
| NEVER | N. evergladensis | SR1 | CP007174.1 |
| NEXA | Nitrosocosmicus exaquare | G61 | (MAGE) |
| NGAR | N. gargensis | Ga9.2 | CP002408.1 |
| NIKO | N. koreensis | MY1 | NZ_AFPU00000000 |
| NISA | Nitrosopumilus salaria | BD31 | NZ_AEXL00000000 |
| NKOR | Nitrosopumilus koreensis | AR1 | CP003842 |
| NLIBG20 | Nitrosoarchaeum limnia | BG20 | GCF_000241145.1 |
| NLIM | Nitrosoarchaeum limnia | SFB1 | CM001158.1 |
| NMAR | N. maritimus | SCM1 | CP000866.1 |
| NOLEO | Nitrosocosmicus oleophilus | MY3 | CP012850.1 |
| NPIR | Nitrosopumilus piranensis | D3C | CP010868.1 |
| NSED | Nitrosopumilus sediminis | AR2 | CP003843.1 |
| NUZO | Nitrosotenuis uzonensis | N4 | GCF_000723185.1 |
| NVIE | N. viennensis | EN76 | CP007536.1 |
| SU86 | Nitrosotenuis cloacae | SAT1 | CP011097.2 |
| xob-BRI | Nitrosospira briensis | C-128 | NZ_CP012371.1 |
| xob-DEF | Nitrospira defluvii | ?? | NC_014355.1 |
| xob-EUR | Nitrosomonas europaea | ATCC19718 | NC_004757 |
| xob-EUT | Nitrosomonas eutropha | C91 | NC_008344.1 |
| xob-HAM | Nitrobacter hamburgensis | X14 | NC_007964.1 |
| xob-INO | Nitrospira inopinata | ENR4 | NZ_LN885086.1 |
| xob-MOS | Nitrospira moscoviensis | NSP M-1 | NZ_CP011801.1 |
| xob-MUL | Nitrosospira multiformis | ATCC 25196 | NC_007614.1 |
| xob-OCE | Nitrosococcus oceanii | ?? | NC_007484.1 |
| xob-WIN | Nitrobacter winograskyi | ?? | NC_007406.1 |
| xob-WIN | Sulfolobus solfataricus | P2 | AE006641 |
| xob-WIN | Pyrobaculum aerophilum | IM2 | AE009441 |
| xob-WIN | Hyperthermus butylicus | DSM 5456 | CP000493 |
| xob-WIN | Thermofilum pendens | Hrk5 | CP000505 |
| xob-WIN | Acidilobus saccharovorans | 345–15 | CP001742 |
| xob-WIN | Desulfurococcus fermentans | DSM 16532 | CP003321 |
| xob-WIN | Caldisphaera lagunensis | DSM 15908 | CP003378 |
| xob-WIN | Fervidicoccus fontis | Kam940 | CP003423 |
Fig. 4.
Maximum likelihood tree (after automatic model selection with IQ-Tree, version 1.4.2) of multicopper proteins found in all available AOA genomes (25 in total) and Aigarchaeota genomes and selected genomes from AOB and nitrite-oxidizing bacteria. NirK is as identified in ref. 61. The two uncollapsed clades contain exclusively genes from AOA and are represented in almost all 25 species (MCO1 is not in N. devanaterra, and NirK is not in C. symbiosum and N. yellowstonii). More details and strain abbreviations are in the text and SI Materials and Methods. Values at nodes represent ultrafast bootstraps («UF-boot »). BC, additional blue copper domain.
Fig. S2.
Maximum likelihood tree (after automatic model selection with IQ-Tree, version 1.4.2) of multicopper proteins found in all available AOA genomes (25 in total) and Aigarchaeota genomes and selected genomes from AOB and nitrite-oxidizing bacteria. NirK was as identified in the work by Bartossek et al. (49). This tree is an uncollapsed version of Fig. 4. Values at nodes represent ultrafast bootstraps («UF-boot »). BC, additional blue copper domain.
Fig. S3.
Maximum likelihood tree (after automatic model selection with IQ-Tree, version 1.4.2) of the core ZIP family permease associated with MCO1. More details and strain abbreviations are in the text and SI Materials and Methods. Values at nodes represent ultrafast bootstraps («UF-boot »).
The second major lineage represents the putative NO-forming nitrite reductase (NirK) family enzymes in both AOA and AOB (61), containing sequences with only two cupredoxin domains and T1, T2 Cu centers. NirK is broadly distributed in AOA with the exceptions of the symbiont C. symbiosum (39), for which evidence for capability of chemolithoautotrophic growth is still missing, and Nitrosocaldus yellowstonii (12), for which the full genome is not published. Nevertheless, NirK (or a noncanonical NirK) has been suggested to assist in the process of ammonia oxidation (14, 21, 26), because NO (the product of the NirK reaction) is essential for ammonia oxidation in AOA (18, 21, 62). In support of this hypothesis, NirK is found highly expressed on the mRNA level in cultivated AOA and environmental samples (63–65) and was identified in the N. viennensis proteome (NVIE_000530).
The AOA Core Proteome Involved in Ammonia Assimilation.
Components of the ammonia assimilation machinery are mostly conserved among the analyzed genomes, but some genomic features differ between the three orders of AOA being compared (Fig. 3). All sequenced AOA encode two copies of glutamine synthetase (GS), but only one is among the core COGs. Glutamate dehydrogenase (GDH), the other key enzyme involved in the regulation of the glutamine/glutamate pool in the cell, also clusters within the core COGs, and both proteins (GDH and GS) are found in the proteomics datasets from N. viennensis and N. brevis. A homolog of glutamate synthase (GOGAT) was not identified in any of the analyzed genomes. The GS/GOGAT pathway usually exhibits higher affinity for ammonia, which might reflect an adjustment of AOA to higher intracellular substrate concentrations (discussion is in SI Results and Discussion).
Only genomes of Nitrososphaerales encode alanine dehydrogenases (Fig. 3) (28, 29). The amination of pyruvate could be a possible mechanism of ammonia storage or alleviation of ammonia toxicity or could also represent another primary nitrogen assimilation process as in AOB and other bacteria (66, 67).
Members of the PII superfamily of signal transduction proteins, key players in nitrogen regulation, are overrepresented in all analyzed AOA genomes with five to six homologs. These proteins have been shown to integrate information regarding the carbon/nitrogen ratio and energy status of the cell and regulate the ammonia uptake and use in response to changes in the extracellular nitrogen availability (68). This function is achieved by binding the key molecules 2-oxoglutarate and ATP/ADP/AMP and subsequently regulating a broad collection of target proteins (e.g., Amt family transporters, GS, and others) (68). The overrepresentation of these regulatory proteins in thaumarchaeal genomes, their relatively conserved genetic linkage to amt in a number of AOA, and the high relative abundance in the proteome of N. viennensis (five homologs detected of six encoded in the genome and three among the top 50 most abundant proteins) (Dataset S1) reinforce the putative central role of these molecules in maintaining a tight regulation and balance over the key metabolic processes of nitrogen and carbon assimilation.
Environmental Adaptations of the Terrestrial Organisms.
A closer look at 1,195 COGs shared between the Nitrososphaerales genomes (present in at least two of three) (Fig. 1) enabled us to identify distinguishing characteristics of this mostly terrestrial lineage. The consistently larger genome sizes compared to the marine organisms, together with an apparently conserved core metabolism suggest a variety of environmental adaptations (see below). A total of 789 of the shared COGs are of unknown function, and proteins assigned to 411 of them are identified in the proteome, making up one-third of the dataset.
An additional 324 COGs are shared between members of the Nitrososphaerales and the soil-dwelling organisms of the Ca. Nitrosopumilales (i.e., N. koreensis) and Ca. Nitrosotaleales (N. devanaterra) (sum of 209, 39, and 76 shared COGs, respectively, in Fig. 1). Among these COGs, we encounter protein families related to detoxification, motility, chemotaxis, and cell surface modifications, the importance of which as soil adaptations is elaborated in the subsequent paragraphs.
Osmotic Regulation, CO2 Uptake, and Detoxification in Terrestrial AOA.
The presence of a putative mannosyl-3-phosphoglycerate synthase in the Nitrososphaerales-specific protein families indicates their capability to synthesize the compatible solute mannosyl-glycerate from mannose-6-phosphate (Fig. 2). This enzyme was detected in the N. viennensis proteome, suggesting the production of this osmotic regulator, which also functions as a thermoprotectant in hyperthermophiles (69).
N. viennensis, N. evergladensis, and N. devanaterra encode a γ-class carbonic anhydrase (CA) homolog (NVIE_017960) (Fig. 2), which contains an N-terminal signal peptide indicating its extracellular localization. This enzyme is responsible for the reversible hydration of CO2 to bicarbonate and widely distributed in both autotrophic and heterotrophic organisms. It mainly fulfills the role of CO2/bicarbonate transport between tissues/compartments and maintains the CO2 partial pressure in a range favorable for the activity of carboxylating enzymes (70). The presence and localization of this enzyme in N. viennensis suggest a role in facilitating carbon transfer into the cell by extracellularly converting bicarbonate to CO2, which can subsequently diffuse through the cell membrane. At a stable intracellular pH of 7, equilibrium favors rehydration to bicarbonate, which can then be used by carbon fixation enzymes, such as the acetyl-CoA/propionyl-CoA carboxylase. An analogous role has been proposed for CA homologs (of mostly the α and β classes) in AOBs, where under carbon-limiting conditions the activity of CA correlated with the rate of CO2 fixation (71). In the case of terrestrial AOA, CA might constitute an adaptation to the discontinuous soil environment, where local and frequent changes in CO2 pressure and pH can lead to low CO2 availability. In contrast, marine AOA encode a sodium-bicarbonate family transporter (13) and would not require a CA homolog, since bicarbonate is the predominant form of inorganic carbon in marine environments. As this gene does not occur in N. gargensis (isolated from a biofilm), it might constitute a bona fide adaptation to the terrestrial lifestyle of soil AOA, which remains to be validated upon availability of more genomes.
Genomes of soil-associated Nitrososphaerales encode a higher number of genes belonging to the glyoxalase/bleomycin resistance/dioxygenase superfamily (SSF54593; 9 in N. viennensis and 14 in N. evergladensis as opposed to 3–6 in the Ca. Nitrosopumilales genomes and 6 in both N. gargensis and Nitrosotalea), an observation that stands true for some soil AOB genomes as well (e.g., 11 in Nitrosospira multiformis). This superfamily contains proteins involved in antibiotic resistance and degradation of aromatic compounds, an essential trait for soil organisms (72) and a likely function of at least some of the thaumarchaeal homologs. Six homologs are present in the proteome. An additional member of this superfamily is glyoxalase I, an enzyme involved in the glyoxal pathway for detoxification of methylglyoxal, which converts this toxic product of central carbon metabolism [also shown to be produced in archaea by dephosphorylation of glyceraldehyde-3-phosphate catalyzed by triose-P-isomerase (73)] to lactate, a precursor of F420 biosynthesis (74). Interestingly, glyoxalase II (the second enzyme of the glyoxal pathway) is a metallo-beta-lactamase fold-containing protein (PF00753), a number of which are also encoded in the genomes of Thaumarchaeota, suggesting the possible existence of this pathway.
Evidence for Exopolysaccharide Production and Cell Surface Modification Pathways in Nitrososphaerales.
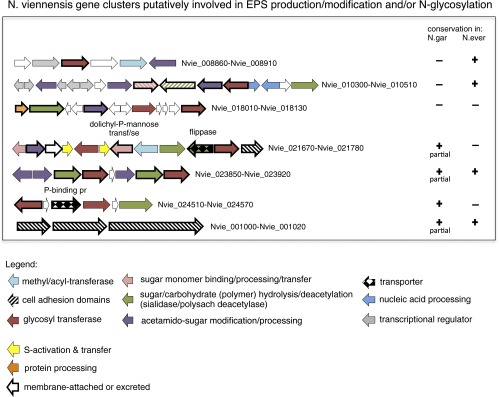
Nitrososphaerales encode an extensive gene repertoire for exopolysaccharide production and cell surface modification pathways, which is a prerequisite of biofilm formation (Fig. 5 and Dataset S2). Compared with the Ca. Nitrosopumilales, they have expanded gene families of glycosyl transferases (GTs) represented by the CAZy families GT1 (PF04101), GT2 (PF00535), and GT4 (PF00534). GTs are involved in the modification of lipids and proteins by the synthesis and covalent attachment of polysaccharides and the biosynthesis of high-molecular weight heteropolymeric exopolysaccharides (75, 76). A number of the GTs in N. viennensis are related to the putative Vectorial Glycosyl Polymerization transporters (4.D.1), a family of transporters that couples polysaccharide biosynthesis with membrane translocation. The carbohydrate esterase family 4 (CE4; 13–15 in N. viennensis and N. evergladensis compared with 1–4 in all others) (Fig. 5 and Dataset S2) includes enzymes responsible for the hydrolysis of N- or O-linked acetyl groups from N-acetylglucosamine or acetylxylose residues, respectively, which comprise the subunits of polymeric compounds such as chitin, peptidoglycan, and xylan (77). The majority of the thaumarchaeal homologs encode signal peptides and are predicted to be extracellular or membrane attached (Dataset S2). Additionally, all sequenced AOA possess an E-type oligosaccharyl-transferase AglB (NVIE_025270), indicating the ability to perform N-linked glycosylation (75, 76), and a Multidrug/Oligosaccharidyl-lipid/Polysaccharide (MOP) flippase. The above-mentioned protein families (GT, CE4, MOP, and AglB) feature prominently in the cell surface modification and extracellular polymeric substances (EPS) production pathways of biofilm-forming bacteria and archaea (75, 76, 78–80). Genes involved in these pathways are often organized in operons (76, 78–80), and in N. viennensis we can identify at least six gene clusters encoding genes with related functions (Fig. S4), a number of which are detected in the proteome (Fig. 5).
Fig. 5.
Distribution of polysaccharide biosynthesis and adhesion-related domains in the analyzed AOA genomes. Shading reflects the expansion of the respective gene family/domain: darker shades of red represent an increasing number of homologs, and white represents absence of the respective category in the genome. The number of homologs detected in the proteome of N. viennensis, out of the total number of homologs in the N. viennensis genome, are listed in the last column. An extended version of this table, including the numbers and the protein family accession numbers, can be found in Dataset S2. Nbrev, N. brevis; Ndev, N. devanaterra; Never, N. evergladensis; Ngar, N. gargensis; Nkor, N. koreensis; Nmar, N. maritimus; and Nvie, N. viennensis.
Fig. S4.
Gene clusters of N. viennensis putatively involved in EPS production/modification and/or N-glycosylation.
Although the molecular mechanisms of biofilm formation among the archaea remain largely unknown (81), production and modification of various types of EPS are essential processes in biofilm development and the establishment of cell–cell and cell–surface interactions (82). These complex phenotypes entail the existence of pathways for acetamidosugar biosynthesis (UDP-Glu, UDP-Gal, GDP-Man, UDP-GlcNAc, and UDP-ManNAc), oligosaccharide assembly on an appropriate carrier (usually dolichol), membrane translocation, polymerization, and modification (Fig. 5 and Dataset S2). Distribution patterns of key enzymes for the pathways involved reveal distinct capacities for the different thaumarchaeal orders [e.g., a deoxythymidine diphosphate (dTDP)-rhamnose biosynthesis pathway in Ca. Nitrosopumilales] (Fig. 5).
Targets and Role of Cell Surface Modifications in Terrestrial AOA.
Little is known regarding the targets for cell surface modifications in Thaumarchaeota. The S-layer proteins and archaella components (archaellins) have been shown to be heavily glycosylated in archaea, with the degree and type of glycosylation varying greatly among species (83). In N. viennensis and N. evergladensis, the two putative S-layer subunits present in all Thaumarchaeota and identified in high abundance in the proteomics dataset (NVIE_016730 and NVIE_016740) are encoded adjacent to a series of genes involved in polysaccharide production and modification. Both S-layer proteins contain four and five canonical N-glycosylation sequons (N-X-S/T motifs) respectively, suggesting that they might be heavily modified.
Interestingly, the modification of S-layer proteins seems to be of a highly dynamic nature in Archaea and can be modified reversibly in response to altering environmental conditions (84). Glycosylation seems to have an adaptive role, enabling the alteration of cell surface properties (e.g., charge distribution, hydration layer, or interaction between cell surface proteins) to provide protection, promote survival against rapidly changing environmental conditions, and establish inter- or intraspecies interactions (82, 85). Moreover, the large S-layer subunit conserved in all Thaumarchaeota clusters in two different COGs representing the two orders Ca. Nitrosopumilales and Nitrososphaerales, suggesting adaptations to the different environmental habitats at a single-protein level (Fig. 5 and Dataset S2).
Additional evidence for cell surface modification mechanisms comes from the fact that all sequenced AOA encode an archaeosortase/exosortase family transpeptidase (also detected in the proteome). This family is found in Bacteria and Euryarchaea, where it correlates with biofilm-forming strains and has been implicated in biofilm formation (86, 87). Distinct subfamilies recognize specific C-terminal sorting signals in target proteins (which include the S-layer proteins) and mediate their membrane translocation, proteolysis, and covalent attachment to the membrane, usually accompanied by extensive posttranslational modifications (86, 87).
Motility and Cell Adhesion Mechanisms in Terrestrial AOA.
Motility-associated genes are encoded in all investigated genomes of the genus Nitrososphaera as well as in N. devanaterra (17, 28, 29, 31) (Fig. 5). These organisms possess gene clusters dedicated to chemotaxis, biosynthesis, and assembly of an archaellum and a type IV-related pili cluster. Cell appendages have been implicated in biofilm formation and surface adhesion (reviewed in ref. 88), and it is interesting to speculate an equivalent function in AOA given the genomic repertoire in support of the capacity for biofilm formation. We observed both types of cell appendages during morphological characterization of the strain using transmission EM and SEM (17).
All AOA isolated from soil encode a large number of predicted membrane proteins with diverse cell adhesion-related domains, a number of which are detected in the proteome of N. viennensis (Fig. 5). The presence of these protein families suggests an increased capacity for recognition of and adhesion to an array of different surfaces.
Biofilm Production in Nitrososphaerales.
Our analysis suggests that N. viennensis, all analyzed Nitrososphaerales, and to a much lesser extent Ca. Nitrosopumilales, seem to encode the genomic inventory for both EPS production and cell surface modification, with an apparent versatility in the types of polysaccharides produced. Moreover, the strains have the potential for surface adhesion and to switch between a sessile and motile lifestyle. These observations indicate an increased ability to adapt to their extracellular environment in order to achieve the optimal conditions for cell–surface and interspecies interactions, and to form synergistic microconsortia in the context of a biofilm community (89).
Indeed, we describe here extensive biofilm formation in N. viennensis continuous batch and fed batch cultures provided with appropriate attachment surfaces. In particular, aggregates and EPS-like structures were observed during microscopic investigations of stationary cultures. Fig. 6 shows an example of biofilm formation in a fully grown culture stained with the lectin wheat germ agglutinin (WGA), indicating the presence of N-acetylglucosamine and/or N-acetylneuraminic acid residues composing the EPS.
Fig. 6.
N. viennensis biofilm grown on a glass coverslip: (A) stained with DAPI (blue) and FITC-conjugated WGA (green), the latter binding to N-acetylglucosamine and/or N-acetylneuraminic acid residues; (B) phase-contrast image of the biofilm. (Scale bar: 5 μm.)
SI Results and Discussion
This material presents additional interesting gene families present in the core genomic repertoire of the analyzed AOA that could not be discussed in the text because of space considerations.
Moreover, specific features of the publicly available but not yet discussed genome of Nitrososphaera viennensis are included here.
Additional Features of the Core Genomic Repertoire of AOA.
Abundance of regulatory PII proteins.
As discussed in the text, regulation of the carbon and nitrogen metabolism is likely to involve PII superfamily proteins, multiple homologs of which are encoded by all AOA (five to six homologs on average except Cenarchaeum symbiosum, with one homolog). Although very small (∼115 amino acids) and conserved, the PII proteins cluster in distinct COGs mostly following the two orders. These N-regulatory proteins have been shown in other organisms to integrate information regarding the carbon/nitrogen ratio and energy status of the cell by binding the key molecules 2-oxoglutarate and ATP/ADP/AMP and subsequently regulating a broad collection of target proteins [e.g., Amt family transporters, GS, and others (68)]. Other central molecules, such as pyruvate, have also been implicated in PII-mediated regulation (68). Sequence analysis indicates that thaumarchaeal homologs belong to the glnK/B subfamily of PII proteins, with members that maintain a relatively conserved linkage with their targets amtB and GS, regulate the ammonia uptake, and use in response to changes in the extracellular nitrogen availability (68). The genetic linkage with the canonical target amtB is conserved mostly in Ca. Nitrosopumilales (13). The genomic neighborhood of PII genes in Nitrososphaerales genomes is very diverse, although conserved associations include certain transporter families [Vacuolar Iron Transporter and Iron Lead Transporter (ILT)], an iron-dependent DtxR family repressor, and a remote linkage to the urease cluster. Given that members of another PII subfamily, the PII-NG, are located adjacent to heavy metal efflux pumps and proposed to be implicated in heavy metal sensing (68), we speculate that those latter PII orthologs play an equivalent role [e.g., in iron (or other heavy metals) sensing and uptake regulation].
Reactive oxygen species (ROS) detoxification.
Multiple families dedicated to oxygen species detoxification also feature within the core thaumarchaeal COGs, such as the bacterial-type superoxide dismutase and three alkyl hydroperoxide reductases. Interestingly, the superoxide dismutase is among the most abundant proteins in our dataset. In addition, we also observe stress response genes and chaperones, such as the bacterial type DNA repair system uvrABC, dnaK, dnaJ, and grpE, with acquisition (probably by horizontal gene transfer) in combination with the above-mentioned oxygen detoxification genes that can be considered responsible for the ability of Thaumarchaeota to colonize aerobic environments. A key role in the organism homeostasis is supported by the relatively high presence of these proteins in both available proteomes (Dataset S1).
N. viennensis and other recently isolated soil and marine strains (48) need the supply of small amounts of organic acids (∼0.1 mM) in their medium to grow in pure cultures, in particular pyruvate, oxaloacetate, 2-oxoglutarate, and glyoxylate (16, 17). It was recently shown that these α-keto acids participate in a nonenzymatic decarboxylation reaction that simultaneously consumes endogenously produced H2O2, thereby protecting the organisms from oxidative stress (47). This elegant study thus offered an explanation to a long-standing question on the possible capability for mixotrophic growth of certain AOA.
DNA repair.
An interesting observation regarding DNA repair mechanisms is that the presence of the helicase:nuclease pair involved in eukaryotic and archaeal nucleotide excision repair (NER) pathway XPB-Bax1 (95) seems confined to genomes of the Nitrososphaerales, whereas other factors (its helicase partner XPD and the XPF nuclease) are encoded by all AOA. The functionality of NER in archaea has not yet been elucidated, but the presence of both the bacterial-type UvrABC system and an extended set of the eukaryotic-type genes in archaea has been proposed to signify the existence of two independent NER pathways or different role(s) for the eukaryotic-type genes (95). From a functional perspective, the enrichment of DNA repair enzymes in Nitrososphaerales (including DNA polymerases IV and family X, both present in the proteome) could indicate an increased exposure to conditions that induce DNA damage.
Transport systems.
The shared thaumarchaeal- and Nitrososphaerales-specific transport capabilities are discussed in detail in ref. 13. In this analysis, we observe that 4% of the shared COG families among AOA are dedicated to transport processes. Represented systems include the Amt, MZT, CopD, and CbtAB family transporters (of which the first three are present in the proteomes), indicating the importance of inorganic ions and metals uptake and homeostasis systems. Core systems implicated in the uptake of various types of organic compounds are the PepT, TauT, MFS, MIP, and TSUP family transporters, of which the first two families are present in both proteomes. Also part of the core COGs and detected in the proteomes are the MacB, Drug Exporter-1 family (DrugE1), and DedA family transporters, with mostly unspecified substrates so far. In addition, Nitrososphaerales share transporters belonging to the Nucleobase:Cation symporter (NCS) family (NCS1), mediating the transport of nucleosides.
Specific Features of N. viennensis, an AOA from Soil.
Energy metabolism and ammonia assimilation.
The reconstructed possible pathways of energy metabolism (via ammonia oxidation) and ammonia assimilation for N. viennensis are presented in Fig. 3. All of the proteins discussed in this section were detected in the proteome (Dataset S1), except where indicated. N. viennensis contains single copies of the amoA, amoB, and amoX genes and six copies of amoC (72–96% amino acid identity). It encodes four periplasmic MCOs in addition to the NirK (Dataset S2), three of which are detected in the proteome (Dataset S1).
An alternative ammonia source for N. viennensis stems from the breakdown of urea by the urease holoenzyme, the main subunits of which are found in two copies in the genome (Dataset S2) (16). The genes encoding the urease subunits and urea transporters belonging to the UT and SSS families are found in the Nitrososphaerales-specific gene complement, although they do not represent a unique feature of this order, because urease is also present in the marine strains Ca. C. symbiosum and Ca. Nitrosopumilus sediminis AR2 (32, 39). N. viennensis possesses two copies of the main subunits of the urease holoenzyme that are present in the proteome data, revealing expression even in the absence of urea as an external energy substrate. The urea transporters, however, were not detected in the proteome, indicating either the intracellular production of urea as a product of arginine catabolism, as proposed recently by Palatinszky et al. (96), or the presence of the urease holoenzyme in an inactive state, suggesting nevertheless the readiness of the organism to take advantage of all possible energy sources on availability.
Transport systems in N. viennensis.
Seventy-nine transport systems are present in the genome of N. viennensis (13) encoded by 109 genes and distributed in 44 families (Dataset S2). In comparison with other Thaumarchaeota, primary active transporter systems seem to be overrepresented in the genome of N. viennensis, composing 24.4% of the total number of transport systems (13).
N. viennensis has the capacity to transport ammonia and urea, the substrates for ammonia oxidation, via three Amt family homologs and UT and SSS homologs, respectively. The putative functional differences of the two types of Amt transporters encoded in the genome are discussed extensively in the work by Offre et al. (13). Curiously and in contrast to other Thaumarchaeota, both N. viennensis and Nitrososphaera evergladensis lack a homolog of the H+, Na+-translocating pyrophosphatase, raising questions regarding the efficient generation and maintenance of a proton motive force solely from the activity of the electron transport chain. Twenty-eight transport systems were detected in the proteome (Dataset S1), including both types of Amt as well as MscS, MIT, MFS, APC, CDF, ZIP, ILT, P-ATPase, ArsAB, RDD, CopD, PepT, MZT, TauT, DrugE1, and MacB families.
Unique genes in N. viennensis.
In total, 771 genes are specific to N. viennensis (Fig. 1), and although no known function could be assigned to 80% of the genes, interesting features are contained within the remaining interpretable fraction. Of the strain-specific genes, 147 are present in the proteome of N. viennensis, and 73 are present in the proteome of Nitrosopelagicus brevis, indicating the need of both organisms to make use of their specific adaptations. For example, in the proteome of N. viennensis, we detect proteins responsible for attachment, cell surface modification, and EPS production as well as two AmoC subunits specific to N. viennensis, reflecting possible regulatory or stress response functions fine-tuned to its particular niche. Unique in N. viennensis is a Carbohydrate Uptake-2 family transporter that is also detected in the proteome and exhibits similarity to general nucleoside uptake porters. In combination with the NCS1 family, this observation suggests an expanded ability to mediate the transport of nucleosides, the role of which (either in catabolic reactions or to reduce the energy cost of de novo nucleoside synthesis) is unclear. Moreover, N. viennensis encodes the highest number of the DrugE1 transport systems (13), suggesting an enhanced capability for detoxification of drugs and toxic compounds excreted by other soil inhabitants.
Conclusions and Outlook
With the increasing number of genomes of Thaumarchaeota becoming available, it is now possible to obtain a better picture of conserved and specific adaptations of AOA. Therefore, we discuss in this study the genome and proteome of N. viennensis in the context of six other fully closed genomes of free-living AOA to identify commonalities and predict specific adaptive features of the terrestrial organisms. From this study, it becomes clear that AOA share an impressive number of conserved genes and that their central energy and carbon metabolism is highly conserved and evolved independently from their bacterial counterparts. AOA contain an ammonia monooxygenase that is only remotely related to that of AOB and contains four rather than three subunits. Most probably, it catalyzes the first step in ammonia oxidation, whereas the enzymes for the subsequent reactions remain enigmatic, in particular since earlier suggested candidate proteins (MCOs) do not belong to the conserved gene repertoire. Nevertheless, we suggest that multicopper proteins play an important role for AOA metabolism and we also suggest candidates for the oxidation of hydroxylamine that should be considered in future biochemical analyses, including F420-dependent enzymes.
The enlarged genomes of the Nitrososphaerales reveal the extensive environmental adaptation strategies of these soil organisms, including detoxification, biofilm formation, adhesion, and cell–cell recognition, which indicate the potential for extensive interactions with other microorganisms in soil. Studying these features in an environmental context will be important to identify microbial interaction partners of AOA (for example, specific associations with nitrite oxidizers or other groups that could participate in the process of nitrogen species interconversions connected through the substrate–product chain) (90). It will be equally interesting to elucidate those adaptations that are the basis for the enormous ecological success of AOA in terrestrial environments.
Materials and Methods
Genome Annotation and Determination of COGs.
Preparation of genomic DNA and sequencing have been described (16). Genome was assembled using single-end and mate-pair 454 pyrosequencing reads and remaining gaps were closed using Sanger sequencing of PCR-amplified gap regions. Prediction and annotation of ORFs were performed on the Microscope platform (91) with subsequent manual curation as in ref. 28. The annotated genome sequence has been deposited into GenBank under accession number CP007536. Predicted protein coding sequences from six thaumarchaeal genomes were retrieved from the National Center for Biotechnology Information RefSeq database (March of 2016). Bidirectional best hits (BBHs) between all genomes were calculated and clustered into COGs using the COGtriangles algorithm in the COGsoft package (92) with cutoff e values of 1e−10 and 70% minimal coverage for both proteins in each protein pair. Because of the small number of involved genomes, BBHs not part of any triangle were represented as specific proteins for each organism.
Phylogenetic Analysis.
MCO and ZIP permease sequences were retrieved by using hidden Markov model (HMM) profiles based on manually curated alignments. From the filtered alignments, phylogenetic trees were built by maximum likelihood after automatic model selection with IQ-Tree, version 1.4.2 (93).
Cultivation and Biofilm Analysis.
The strain was cultivated as described (17) in 20 mL with glass coverslips to allow adherence. The glass coverslips were dried, fixed, and stained with DAPI and FITC-labeled WGA (Sigma).
Proteome Analysis.
Late exponential-phase cells were lysed by homogenization and sonication, and the lysate was fractionated into membrane and cytoplasmic fractions by ultracentrifugation in 60% (wt/vol) sucrose gradient. Proteins were extracted and processed as in ref. 94, and generated peptides were analyzed on an LC-LTQ-Orbitrap Mass Spectrometer (Thermo Scientific Inc.) coupled to ultrahigh-pressure nanoLC according to ref. 94. Data are available via ProteomeXchange with identifier PXD005297.
Detailed information on the bioinformatic tools and experimental procedures can be found in SI Materials and Methods.
SI Materials and Methods
Sequencing, Annotation, Metabolic Reconstruction, and Bioinformatic Tools.
Genomic DNA used for the sequencing of the N. viennensis genome was extracted from N. viennensis batch cultures (200 mL each) in early stationary phase. A genomic DNA sample was sheared to an average fragment size of 3.4 kb for the preparation of a mate-pair library, which was sequenced on a 454/FLX-Titanium Sequencer (Roche). The sequencing run generated 690,476 mate-pair reads (average read length of 337 bp), which were assembled de novo as described in ref. 16. The assembly resulted in a single circular scaffold of 2,527,938 bp containing 18 short (<1 kb) gaps. Six gaps were closed by merging the scaffold with unordered contigs previously assembled from single-end 454-pyrosequencing reads (16). Remaining gaps were closed using Sanger sequencing of PCR amplicons covering the gaps. Ambiguous and miscalled bases as well as homopolymer length errors were called and corrected by analyzing the alignment of 66,966,722 paired-end GAII Illumina reads (library average insert size: 370 bp; average read length: 108 bp) to the closed genome sequence using Nesoni (https://github.com/Victorian-Bioinformatics-Consortium/nesoni).
Prediction and annotation of ORFs were performed on the comparative genome analysis and manual functional annotation platform Microscope (91). The annotation of all genes discussed in the paper was manually curated following the annotation guideline described in the work by Spang et al. (28). KEGG pathway mapping and homology searches using HMMER profile-based (97) approaches were used to assist pathway curation. Transport systems were classified as previously reported in the work by Offre et al. (13).
Signal peptide, transmembrane domains, and putative subcellular localization were predicted using web-based tools, such as Pred-Signal (98), TatP (99), Phobius (100), and SecretomeP (56), in addition to tools already implemented in the Microscope platform. The combined output of all tools was taken into account to infer the putative subcellular localization of predicted protein coding sequences (CDS).
The annotated genome sequence has been deposited to GenBank under accession number CP007536.
Determination of COGs.
Predicted CDS from six thaumarchaeal genomes, namely Nitrososphaera gargensis, N. evergladensis, Nitrosopumilus maritimus, N. brevis, Nitrosoarchaeum koreensis, and Nitrosotalea devanaterra, were retrieved from the March 2016 release of the National Center for Biotechnology Information (NCBI) RefSeq database. All of the available complete and published genomes of Nitrososphaerales (genus Nitrososphaera), three representative complete genomes of Ca. Nitrosopumilales, and the only representative of Ca. Nitrosotaleales were used for the determination of COGs. The genome of the symbiont C. symbiosum was not included in this study. BBHs between all genomes (including N. viennensis) were calculated and clustered into COGs using the COGtriangles algorithm in the COGsoft package (92) using cutoff e values of 1e−10 and 70% minimal coverage for both proteins in each protein pair. Because of the small number of involved genomes, BBHs not part of any triangle were represented as specific proteins for each organism. The total number of shared COGs exceeds the number of CDS per genome because of the fact that a gene can belong to multiple COGs.
Phylogenetic Analysis.
An HMM protein profile was built with HMMER (97) based on a manually curated alignment [MAFFT, version v7.215, “auto,” default parameters (101)] of core thaumarchaeal families (MCO and ZIP permease) plus representative MCO sequences (putative NirK from archaeal genomic fragment 54d9: GenBank accession no. AJ627422 and NirK from Nitrosomonas europaea: GenBank accession no. KXK47907). A sequence similarity search was performed with this profile using HMMER on all Thaumarchaeota genomes available in the databases (RefSeq of NCBI and IMG of JGI; last accessed June 6, 2016), Aigarchaeota genomes, genomes representative of each class of Crenarchaeota, and a selection of AOB and nitrite-oxidizing bacteria bacterial genomes (Table S2). Sequences matched with an i-e value ≤ 10–6 and at least 80% of profile coverage were selected for additional analysis and aligned with MAFFT (same parameters as above). For the MCO, the manual inspection of the conserved features of the alignment and a first tree (see below) allowed us to discard dissimilar sequences (i.e., having zero or one of three copper centers present in other members of the MCOs and long branches) and sequences having terminal branches above one substitution per site, because they are likely to be mutationally saturated and induce artifacts in the trees. The positions of the alignments were filtered using the BMGE program, version 1.12. (BLOSUM30 similarity matrix, gap rate cutoff = 0.20, sliding window size = 3, entropy score cutoff = 0.5). From the filtered alignments, phylogenetic trees were built by maximum likelihood after automatic model selection with IQ-Tree, version 1.4.2 (93) (1,000 ultrafast bootstraps “UF-boot,” “-m TESTNEW -madd LG4M,LG4X,EX2,EX3,C20”).
Biomass Production for Proteomics and Biofilm Growth Conditions.
The strain was cultivated as described in the work by Stieglmeier et al. (17) with the supplementation of 3 mM NH4Cl and 0.1 mM sodium pyruvate. Cells from replicate cultures grown in 2.5 L medium in 5-L flasks were harvested in late exponential phase by three centrifugation steps. In the first step, cells were concentrated at 40,000 × g using a CEPA Laboratory Centrifuge Series LE, and ∼250 mL cell concentrate were centrifuged at 24,471 × g for 40 min at 4 °C in 500-mL centrifugation cups; finally, the pellet was resuspended, aliquoted in 2-mL reaction tubes, and centrifuged at 16,100 × g for 20 min at 4 °C. Harvested cells were stored at −80 °C until further use.
For biofilm formation, 20-mL cultures supplemented with 0.5 mM sodium pyruvate were inoculated in cultivation flasks in which glass coverslips were inserted to allow adherence.
Biofilm Staining and Microscopy.
Overgrown glass coverslips were dried overnight at room temperature and fixed with 70% (vol/vol) ethanol for 30 min. FITC-labeled WGA (selectively binding to N-acetylglucosamine and N-acetylneuraminic acid; Sigma) was used to visualize glycoconjugates and EPS, and DAPI (Sigma) was used for cell visualization. Briefly, 100 µL 25 µg/mL WGA solution was added to the coverslip and incubated for 20 min in the dark at room temperature; 200 µL 13.3 ng/mL DAPI solution was then applied, and the coverslip was incubated at room temperature overnight in the dark. The coverslip was washed with PBS to remove excess dye before mounting with 5 µL PBS on a microscope slide, and observation was with a Nikon Eclipse 50i Microscope equipped with a MFCool Camera (Jenoptik).
Sample Preparation for Proteomics.
Frozen cell pellets (200–500 mg) from three biological replicate culture batches were resuspended in 20 mM NaHPO4, pH 7.4, 500 mM NaCl, 1 mM EDTA, 10% (vol/vol) Glycerol, and Protease Inhibitor (Pefabloc SC; Roche) and lysed by homogenization and sonication. The total cellular lysate was fractionated in membrane and cytoplasmic fractions by ultracentrifugation in 60% (wt/vol) sucrose gradient at 133,000 × g for 2 h at 4 °C. Proteins were extracted from both fractions using methanol/chloroform with an additional phenol cleaning step as described in the work by Valledor and Weckwerth (94). Tryptic digestion of proteins from the enriched membrane fractions was enhanced by the addition of 0.2% cleavable detergent RapiGest SF Surfactant (Waters). Purified peptides were resuspended in 0.1% formic acid and 2% (vol/vol) acetonitrile before analysis.
Proteome Analysis.
MS analysis was performed on an LC-LTQ-Orbitrap Mass Spectrometer (Thermo Scientific Inc.) coupled to ultrahigh-pressure nanoliquid chromatography (nano-LC) according to the work by Valledor and Weckwerth (94). Peptides were loaded onto a 1D Nanoflow LC-MS/MS System (Eksigent) equipped with an inline premicrofilter (Sciex). Peptides were eluted using a monolithic C18 column Chromolith RP-18r (Merck) of 15 cm in length and 0.1 mm i.d. during a 90-min gradient from 5 to 40% B with a controlled flow rate of 400 nL/min. LC was coupled to MS using an ESI (electrospray ionization) source: mobile phase A: 0.1% formic acid; mobile phase B: 90% (vol/vol) acetonitrile and 0.1% formic acid. Specific tune settings for the MS were set as follows: spray voltage was set to 1.8 kV using a 30-µm i.d. needle (PicoTip Emitter; NewObjective), and temperature of the heated transfer capillary was set to 180 °C. FTMS (Fourier transform mass spectrometry) was operated as follows: full-scan mode, centroid, resolution of 30,000, covering the range 300–1,800 m/z, and Cyclomethicone used as lock mass. Each full MS scan was followed by 10 dependent MS/MS scans, in which the 10 most abundant peptide molecular ions were dynamically selected, with a dynamic exclusion window set to 90 s and an exclusion list set to 500. Dependent fragmentations were performed in CID (collision-induced dissociation) mode, with a normalized collision energy of 35, iso width of 1.0, activation Q of 0.250, and activation time of 30 ms. Ions with unassigned charge or +1 were excluded for fragmentation. The minimum signal threshold was set to 750.
Mass spectral libraries were searched using the SEQUEST algorithm within the Thermo Proteome Discoverer software against a six-frame translation of the genome and the translated sequences of all ORFs predicted on the Microscope platform (91). Precursor and fragment ion mass tolerances were set to 10 ppm and 0.8 Da, respectively. Minimum precursor ion size was set to 350 Da. The following dynamic modifications because of sample preparation were taken into account: N-terminal acetylation, methionine oxidation, and carbamidomethylation. For protein identifications, high-confidence peptides [false discovery rate (FDR), 0.01] were considered, and the following score vs. charge state parameters were applied (charge state:minimal score): 1:1.5; 2:2.3; 3:3; 4:4; 6:6; 7:7; and >7:7. Protein identifications based on a single peptide match with score (Xcorr) below 2.3 were checked manually. Relative quantification of detected proteins relied on a spectral counting method [i.e., the calculation of normalized spectral abundance factors (102)]. The sum of peptide-spectrum matches for each identified protein from each fraction/replicate was used for relative quantification across all samples, resulting in a final combined dataset. In total, 1,503 proteins were identified based on the detection of 9,274 peptides derived from the automated analysis of 214,786 spectra. The generated proteomics data have been deposited to the ProteomeXchange Consortium (www.proteomexchange.org) via the PRIDE partner repository with the dataset identifier PXD005297, and are also accessible in PROMEX (promex.pph.univie.ac.at/promex/).
Only a single previously unpredicted ORF of unknown function was identified with this proteogenomics approach (NVIE_019901; 62-aa residues) using a six-frame translation of the genome as a search database for peptide identification according to the work by May et al. (103).
Supplementary Material
Acknowledgments
We thank all current and former members of the Archaea Biology and Ecogenomics Division for continuous discussions on the N. viennensis genome, in particular Anja Spang, Michaela Stieglmeier, Simon Rittmann, and Filipa Sousa. We also thank Thomas Weinmaier, Romana Bittner, and Tobias Reinelt for technical assistance and the reviewers for their valuable comments on the manuscript. This work was financed through Austrian Science Fund Grants P27017 and W1257 (to C.S.) and P26342 (to W.W.); European Research Council Advanced Grant ERC-ADG 695192 (to C.S.); and Marie Skłodowska-Curie Grants FP7-PEOPLE-2009-IEF-255109 (to L.V.) and H2020-MSCA-IF-2017:701981 (to S.S.A.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. CP007536), and the mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium, www.proteomexchange.org (identifier PXD005297).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601212113/-/DCSupplemental.
References
- 1.Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic Crenarchaeota: Proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol. 2008;6(3):245–252. doi: 10.1038/nrmicro1852. [DOI] [PubMed] [Google Scholar]
- 2.Spang A, et al. Distinct gene set in two different lineages of ammonia-oxidizing archaea supports the phylum Thaumarchaeota. Trends Microbiol. 2010;18(8):331–340. doi: 10.1016/j.tim.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Karner MB, DeLong EF, Karl DM. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature. 2001;409(6819):507–510. doi: 10.1038/35054051. [DOI] [PubMed] [Google Scholar]
- 4.Lin X, et al. Comparison of vertical distributions of prokaryotic assemblages in the anoxic Cariaco Basin and Black Sea by use of fluorescence in situ hybridization. Appl Environ Microbiol. 2006;72(4):2679–2690. doi: 10.1128/AEM.72.4.2679-2690.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leininger S, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442(7104):806–809. doi: 10.1038/nature04983. [DOI] [PubMed] [Google Scholar]
- 6.Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W. Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev. 2009;33(5):855–869. doi: 10.1111/j.1574-6976.2009.00179.x. [DOI] [PubMed] [Google Scholar]
- 7.Metcalf WW, et al. Synthesis of methylphosphonic acid by marine microbes: A source for methane in the aerobic ocean. Science. 2012;337(6098):1104–1107. doi: 10.1126/science.1219875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Offre P, Spang A, Schleper C. Archaea in biogeochemical cycles. Annu Rev Microbiol. 2013;67(1):437–457. doi: 10.1146/annurev-micro-092412-155614. [DOI] [PubMed] [Google Scholar]
- 9.Doxey AC, Kurtz DA, Lynch MD, Sauder LA, Neufeld JD. Aquatic metagenomes implicate Thaumarchaeota in global cobalamin production. ISME J. 2015;9(2):461–471. doi: 10.1038/ismej.2014.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Könneke M, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 2005;437(7058):543–546. doi: 10.1038/nature03911. [DOI] [PubMed] [Google Scholar]
- 11.Treusch AH, et al. Novel genes for nitrite reductase and Amo-related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ Microbiol. 2005;7(12):1985–1995. doi: 10.1111/j.1462-2920.2005.00906.x. [DOI] [PubMed] [Google Scholar]
- 12.Stahl DA, de la Torre JR. Physiology and diversity of ammonia-oxidizing archaea. Annu Rev Microbiol. 2012;66:83–101. doi: 10.1146/annurev-micro-092611-150128. [DOI] [PubMed] [Google Scholar]
- 13.Offre P, Kerou M, Spang A, Schleper C. Variability of the transporter gene complement in ammonia-oxidizing archaea. Trends Microbiol. 2014;22(12):665–675. doi: 10.1016/j.tim.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Schleper C, Nicol GW. Ammonia-oxidising archaea--physiology, ecology and evolution. Adv Microb Physiol. 2010;57:1–41. doi: 10.1016/B978-0-12-381045-8.00001-1. [DOI] [PubMed] [Google Scholar]
- 15.Stieglmeier M, Alves R, Schleper C. Thaumarchaeota. In: Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F, editors. The Prokaryotes—Other Major Lineages of Bacteria and the Archaea. 4th Ed. Springer; Berlin: 2014. pp. 1–40. [Google Scholar]
- 16.Tourna M, et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci USA. 2011;108(20):8420–8425. doi: 10.1073/pnas.1013488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stieglmeier M, et al. Nitrososphaera viennensis gen. nov., sp. nov., an aerobic and mesophilic, ammonia-oxidizing archaeon from soil and a member of the archaeal phylum Thaumarchaeota. Int J Syst Evol Microbiol. 2014;64:2738–2752. doi: 10.1099/ijs.0.063172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen T, Stieglmeier M, Dai J, Urich T, Schleper C. Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett. 2013;344(2):121–129. doi: 10.1111/1574-6968.12164. [DOI] [PubMed] [Google Scholar]
- 19.Taylor AE, et al. Inhibitory effects of C2 to C10 1-alkynes on ammonia oxidation in two Nitrososphaera species. Appl Environ Microbiol. 2015;81(6):1942–1948. doi: 10.1128/AEM.03688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stieglmeier M, et al. Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J. 2014;8(5):1135–1146. doi: 10.1038/ismej.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J. 2016;10(8):1836–1845. doi: 10.1038/ismej.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinninghe Damsté JS, et al. Intact polar and core glycerol dibiphytanyl glycerol tetraether lipids of group I.1a and I.1b thaumarchaeota in soil. Appl Environ Microbiol. 2012;78(19):6866–6874. doi: 10.1128/AEM.01681-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Könneke M, et al. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc Natl Acad Sci USA. 2014;111(22):8239–8244. doi: 10.1073/pnas.1402028111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arp DJ, Stein LY. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit Rev Biochem Mol Biol. 2003;38(6):471–495. doi: 10.1080/10409230390267446. [DOI] [PubMed] [Google Scholar]
- 25.Stein LY. Surveying N2O-producing pathways in bacteria. In: Klotz MG, Stein LY, editors. Methods in Enzymology: Research on Nitrification and Related Processes Part A. Vol. 486. Elsevier; Amsterdam: 2011. pp. 131–152. [DOI] [PubMed] [Google Scholar]
- 26.Walker CB, et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA. 2010;107(19):8818–8823. doi: 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BK, et al. Genome sequence of an ammonia-oxidizing soil archaeon, “Candidatus Nitrosoarchaeum koreensis” MY1. J Bacteriol. 2011;193(19):5539–5540. doi: 10.1128/JB.05717-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spang A, et al. The genome of the ammonia-oxidizing Candidatus Nitrososphaera gargensis: Insights into metabolic versatility and environmental adaptations. Environ Microbiol. 2012;14(12):3122–3145. doi: 10.1111/j.1462-2920.2012.02893.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhalnina KV, et al. Genome sequence of Candidatus Nitrososphaera evergladensis from group I.1b enriched from Everglades soil reveals novel genomic features of the ammonia-oxidizing archaea. PLoS One. 2014;9(7):e101648. doi: 10.1371/journal.pone.0101648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santoro AE, et al. Genomic and proteomic characterization of “Candidatus Nitrosopelagicus brevis”: An ammonia-oxidizing archaeon from the open ocean. Proc Natl Acad Sci USA. 2015;112(4):1173–1178. doi: 10.1073/pnas.1416223112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehtovirta-Morley LE, et al. Identifying potential mechanisms enabling acidophily in the ammonia-oxidising archaeon “ Candidatus Nitrosotalea devanaterra.”. Appl Environ Microbiol. 2016;82(9):2608–2619. doi: 10.1128/AEM.04031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blainey PC, Mosier AC, Potanina A, Francis CA, Quake SR. Genome of a low-salinity ammonia-oxidizing archaeon determined by single-cell and metagenomic analysis. PLoS One. 2011;6(2):e16626. doi: 10.1371/journal.pone.0016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosier AC, Allen EE, Kim M, Ferriera S, Francis CA. Genome sequence of “Candidatus Nitrosopumilus salaria” BD31, an ammonia-oxidizing archaeon from the San Francisco Bay estuary. J Bacteriol. 2012;194(8):2121–2122. doi: 10.1128/JB.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebedeva EV, et al. Enrichment and genome sequence of the group I.1a ammonia-oxidizing Archaeon “Ca. Nitrosotenuis uzonensis” representing a clade globally distributed in thermal habitats. PLoS One. 2013;8(11):e80835. doi: 10.1371/journal.pone.0080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung M-Y, et al. A mesophilic, autotrophic, ammonia-oxidizing archaeon of thaumarchaeal group I.1a cultivated from a deep oligotrophic soil horizon. Appl Environ Microbiol. 2014;80(12):3645–3655. doi: 10.1128/AEM.03730-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park S-J, et al. Genomes of two new ammonia-oxidizing archaea enriched from deep marine sediments. PLoS One. 2014;9(5):e96449. doi: 10.1371/journal.pone.0096449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayer B, et al. Physiological and genomic characterization of two novel marine thaumarchaeal strains indicates niche differentiation. ISME J. 2016;10(5):1051–1063. doi: 10.1038/ismej.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, et al. A novel ammonia-oxidizing archaeon from wastewater treatment plant: Its enrichment, physiological and genomic characteristics. Sci Rep. 2016;6:23747. doi: 10.1038/srep23747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallam SJ, et al. Genomic analysis of the uncultivated marine crenarchaeote Cenarchaeum symbiosum. Proc Natl Acad Sci USA. 2006;103(48):18296–18301. doi: 10.1073/pnas.0608549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prosser JI, Nicol GW. Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends Microbiol. 2012;20(11):523–531. doi: 10.1016/j.tim.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs G. Alternative pathways of carbon dioxide fixation: Insights into the early evolution of life? Annu Rev Microbiol. 2011;65:631–658. doi: 10.1146/annurev-micro-090110-102801. [DOI] [PubMed] [Google Scholar]
- 42.Park YJ, Yoo CB, Choi SY, Lee HB. Purifications and characterizations of a ferredoxin and its related 2-oxoacid:ferredoxin oxidoreductase from the hyperthermophilic archaeon, Sulfolobus solfataricus P1. J Biochem Mol Biol. 2006;39(1):46–54. doi: 10.5483/bmbrep.2006.39.1.046. [DOI] [PubMed] [Google Scholar]
- 43.Estelmann S, et al. Labeling and enzyme studies of the central carbon metabolism in Metallosphaera sedula. J Bacteriol. 2011;193(5):1191–1200. doi: 10.1128/JB.01155-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alves RJE, et al. Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J. 2013;7(8):1620–1631. doi: 10.1038/ismej.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mussmann M, et al. Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proc Natl Acad Sci USA. 2011;108(40):16771–16776. doi: 10.1073/pnas.1106427108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouverney CC, Fuhrman JA. Marine planktonic archaea take up amino acids. Appl Environ Microbiol. 2000;66(11):4829–4833. doi: 10.1128/aem.66.11.4829-4833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim J-G, et al. Hydrogen peroxide detoxification is a key mechanism for growth of ammonia-oxidizing archaea. Proc Natl Acad Sci USA. 2016;113(28):7888–7893. doi: 10.1073/pnas.1605501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin W, et al. Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation. Proc Natl Acad Sci USA. 2014;111(34):12504–12509. doi: 10.1073/pnas.1324115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bartossek R, Spang A, Weidler G, Lanzen A, Schleper C. Metagenomic analysis of ammonia-oxidizing archaea affiliated with the soil group. Front Microbiol. 2012;3:208–222. doi: 10.3389/fmicb.2012.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norton JM, Alzerreca JJ, Suwa Y, Klotz MG. Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch Microbiol. 2002;177(2):139–149. doi: 10.1007/s00203-001-0369-z. [DOI] [PubMed] [Google Scholar]
- 51.Berube PM, Samudrala R, Stahl DA. Transcription of all amoC copies is associated with recovery of Nitrosomonas europaea from ammonia starvation. J Bacteriol. 2007;189(11):3935–3944. doi: 10.1128/JB.01861-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berube PM, Stahl DA. The divergent AmoC3 subunit of ammonia monooxygenase functions as part of a stress response system in Nitrosomonas europaea. J Bacteriol. 2012;194(13):3448–3456. doi: 10.1128/JB.00133-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris RM, et al. Comparative metaproteomics reveals ocean-scale shifts in microbial nutrient utilization and energy transduction. ISME J. 2010;4(5):673–685. doi: 10.1038/ismej.2010.4. [DOI] [PubMed] [Google Scholar]
- 54.Vajrala N, et al. Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci USA. 2013;110(3):1006–1011. doi: 10.1073/pnas.1214272110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon J, Klotz MG. Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations. Biochim Biophys Acta. 2013;1827(2):114–135. doi: 10.1016/j.bbabio.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Bendtsen JD, Kiemer L, Fausbøll A, Brunak S. Non-classical protein secretion in bacteria. BMC Microbiol. 2005;5:58. doi: 10.1186/1471-2180-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Purwantini E, Mukhopadhyay B. Conversion of NO2 to NO by reduced coenzyme F420 protects mycobacteria from nitrosative damage. Proc Natl Acad Sci USA. 2009;106(15):6333–6338. doi: 10.1073/pnas.0812883106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosseinzadeh P, et al. A purple cupredoxin from Nitrosopumilus maritimus containing a mononuclear type 1 copper center with an open binding site. J Am Chem Soc. 2016;138(20):6324–6327. doi: 10.1021/jacs.5b13128. [DOI] [PubMed] [Google Scholar]
- 59.Reddy KJ, Wang L, Gloss SP. Solubility and mobility of copper, zinc and lead in acidic environments. Plant Soil. 1995;171(1):53–58. [Google Scholar]
- 60.Protze J, et al. An extracellular tetrathionate hydrolase from the thermoacidophilic archaeon Acidianus ambivalens with an activity optimum at pH 1. Front Microbiol. 2011;2:68. doi: 10.3389/fmicb.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartossek R, Nicol GW, Lanzen A, Klenk HP, Schleper C. Homologues of nitrite reductases in ammonia-oxidizing archaea: Diversity and genomic context. Environ Microbiol. 2010;12(4):1075–1088. doi: 10.1111/j.1462-2920.2010.02153.x. [DOI] [PubMed] [Google Scholar]
- 62.Sauder LA, Ross AA, Neufeld JD. Nitric oxide scavengers differentially inhibit ammonia oxidation in ammonia-oxidizing archaea and bacteria. FEMS Microbiol Lett. 2016;363(7):1–8. doi: 10.1093/femsle/fnw052. [DOI] [PubMed] [Google Scholar]
- 63.Shi Y, Tyson GW, Eppley JM, DeLong EF. Integrated metatranscriptomic and metagenomic analyses of stratified microbial assemblages in the open ocean. ISME J. 2011;5(6):999–1013. doi: 10.1038/ismej.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hollibaugh JT, Gifford S, Sharma S, Bano N, Moran MA. Metatranscriptomic analysis of ammonia-oxidizing organisms in an estuarine bacterioplankton assemblage. ISME J. 2011;5(5):866–878. doi: 10.1038/ismej.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lund MB, Smith JM, Francis CA. Diversity, abundance and expression of nitrite reductase (nirK)-like genes in marine thaumarchaea. ISME J. 2012;6(10):1966–1977. doi: 10.1038/ismej.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caballero FJ, Cárdenas J, Castillo F. Purification and properties of L-alanine dehydrogenase of the phototrophic bacterium Rhodobacter capsulatus E1F1. J Bacteriol. 1989;171(6):3205–3210. doi: 10.1128/jb.171.6.3205-3210.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stein LY, et al. Whole-genome analysis of the ammonia-oxidizing bacterium, Nitrosomonas eutropha C91: Implications for niche adaptation. Environ Microbiol. 2007;9(12):2993–3007. doi: 10.1111/j.1462-2920.2007.01409.x. [DOI] [PubMed] [Google Scholar]
- 68.Huergo LF, Chandra G, Merrick M. P(II) signal transduction proteins: Nitrogen regulation and beyond. FEMS Microbiol Rev. 2013;37(2):251–283. doi: 10.1111/j.1574-6976.2012.00351.x. [DOI] [PubMed] [Google Scholar]
- 69.Empadinhas N, da Costa MS. Diversity and biosynthesis of compatible solutes in hyper/thermophiles. Int Microbiol. 2006;9(3):199–206. [PubMed] [Google Scholar]
- 70.Ferry JG. The γ class of carbonic anhydrases. Biochim Biophys Acta. 2010;1804(2):374–381. doi: 10.1016/j.bbapap.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jahnke L, Lyman C, Hooper AB. Carbonic anhydrase, carbondioxide levels and growth of Nitrosomonas. Arch Microbiol. 1984;140(2-3):291–293. [Google Scholar]
- 72.Tropel D, van der Meer JR. Bacterial transcriptional regulators for degradation pathways of aromatic compounds. Microbiol Mol Biol Rev. 2004;68(3):474–500. doi: 10.1128/MMBR.68.3.474-500.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White RH, Xu H. Methylglyoxal is an intermediate in the biosynthesis of 6-deoxy-5-ketofructose-1-phosphate: A precursor for aromatic amino acid biosynthesis in Methanocaldococcus jannaschii. Biochemistry. 2006;45(40):12366–12379. doi: 10.1021/bi061018a. [DOI] [PubMed] [Google Scholar]
- 74.Grochowski LL, Xu H, White RH. Identification of lactaldehyde dehydrogenase in Methanocaldococcus jannaschii and its involvement in production of lactate for F420 biosynthesis. J Bacteriol. 2006;188(8):2836–2844. doi: 10.1128/JB.188.8.2836-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magidovich H, Eichler J. Glycosyltransferases and oligosaccharyltransferases in Archaea: Putative components of the N-glycosylation pathway in the third domain of life. FEMS Microbiol Lett. 2009;300(1):122–130. doi: 10.1111/j.1574-6968.2009.01775.x. [DOI] [PubMed] [Google Scholar]
- 76.Jarrell KF, et al. N-linked glycosylation in archaea: A structural, functional, and genetic analysis. Microbiol Mol Biol Rev. 2014;78:304–341. doi: 10.1128/MMBR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caufrier F, Martinou A, Dupont C, Bouriotis V. Carbohydrate esterase family 4 enzymes: Substrate specificity. Carbohydr Res. 2003;338(7):687–692. doi: 10.1016/s0008-6215(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 78.Péant B, et al. Comparative analysis of the exopolysaccharide biosynthesis gene clusters from four strains of Lactobacillus rhamnosus. Microbiology. 2005;151:1839–1851. doi: 10.1099/mic.0.27852-0. [DOI] [PubMed] [Google Scholar]
- 79.Zhao D, et al. Improving polyhydroxyalkanoate production by knocking out the genes involved in exopolysaccharide biosynthesis in Haloferax mediterranei. Appl Microbiol Biotechnol. 2013;97(7):3027–3036. doi: 10.1007/s00253-012-4415-3. [DOI] [PubMed] [Google Scholar]
- 80.Chimileski S, Franklin MJ, Papke RT. Biofilms formed by the archaeon Haloferax volcanii exhibit cellular differentiation and social motility, and facilitate horizontal gene transfer. BMC Biol. 2014;12:65. doi: 10.1186/s12915-014-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orell A, Fröls S, Albers S. Archaeal biofilms: The great unexplored. Annu Rev Microbiol. 2013;67(1):337–354. doi: 10.1146/annurev-micro-092412-155616. [DOI] [PubMed] [Google Scholar]
- 82.Flemming H, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 83.Jarrell KF, Jones GM, Kandiba L, Nair DB, Eichler J. S-layer glycoproteins and flagellins: Reporters of archaeal posttranslational modifications. Archaea. 2010;2010:612948. doi: 10.1155/2010/612948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guan Z, Naparstek S, Calo D, Eichler J. Protein glycosylation as an adaptive response in Archaea: Growth at different salt concentrations leads to alterations in Haloferax volcanii S-layer glycoprotein N-glycosylation. Environ Microbiol. 2012;14(3):743–753. doi: 10.1111/j.1462-2920.2011.02625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaminski L, et al. Add salt, add sugar: N-glycosylation in Haloferax volcanii. Biochem Soc Trans. 2013;41(1):432–435. doi: 10.1042/BST20120142. [DOI] [PubMed] [Google Scholar]
- 86.Haft DH, Payne SH, Selengut JD. Archaeosortases and exosortases are widely distributed systems linking membrane transit with posttranslational modification. J Bacteriol. 2012;194(1):36–48. doi: 10.1128/JB.06026-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abdul Halim MF, et al. Haloferax volcanii archaeosortase is required for motility, mating, and C-terminal processing of the S-layer glycoprotein. Mol Microbiol. 2013;88(6):1164–1175. doi: 10.1111/mmi.12248. [DOI] [PubMed] [Google Scholar]
- 88.Lassak K, Ghosh A, Albers SV. Diversity, assembly and regulation of archaeal type IV pili-like and non-type-IV pili-like surface structures. Res Microbiol. 2012;163(9-10):630–644. doi: 10.1016/j.resmic.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 89.Comolli LR, Banfield JF. Inter-species interconnections in acid mine drainage microbial communities. Front Microbiol. 2014;5:367. doi: 10.3389/fmicb.2014.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Daebeler A, et al. Interactions between Thaumarchaea, Nitrospira and methanotrophs modulate autotrophic nitrification in volcanic grassland soil. ISME J. 2014;8(12):2397–2410. doi: 10.1038/ismej.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vallenet D, et al. MicroScope: A platform for microbial genome annotation and comparative genomics. Database (Oxford) 2009;2009:bap021. doi: 10.1093/database/bap021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kristensen DM, et al. A low-polynomial algorithm for assembling clusters of orthologous groups from intergenomic symmetric best matches. Bioinformatics. 2010;26(12):1481–1487. doi: 10.1093/bioinformatics/btq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valledor L, Weckwerth W. An improved detergent-compatible gel-fractionation LC-LTQ-Orbitrap-MS workflow for plant and microbial proteomics. In: Walker JM, editor. Methods in Molecular Biology. Humana; Clifton, NJ: 2014. pp. 347–358. [DOI] [PubMed] [Google Scholar]
- 95.Rouillon C, White MF. The evolution and mechanisms of nucleotide excision repair proteins. Res Microbiol. 2011;162(1):19–26. doi: 10.1016/j.resmic.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Palatinszky M, et al. Cyanate as an energy source for nitrifiers. Nature. 2015;524(7563):105–108. doi: 10.1038/nature14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eddy SR. Accelerated profile HMM searches. PLOS Comput Biol. 2011;7(10):e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bagos PG, Tsirigos KD, Plessas SK, Liakopoulos TD, Hamodrakas SJ. Prediction of signal peptides in archaea. Protein Eng Des Sel. 2009;22(1):27–35. doi: 10.1093/protein/gzn064. [DOI] [PubMed] [Google Scholar]
- 99.Bendtsen JD, Nielsen H, Widdick D, Palmer T, Brunak S. Prediction of twin-arginine signal peptides. BMC Bioinformatics. 2005;6:167. doi: 10.1186/1471-2105-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Käll L, Krogh A, Sonnhammer EL. Advantages of combined transmembrane topology and signal peptide prediction–the Phobius web server. Nucleic Acids Res. 2007;35:W429–W432. doi: 10.1093/nar/gkm256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zybailov B, et al. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J Proteome Res. 2006;5(9):2339–2347. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
- 103.May P, et al. Metabolomics- and proteomics-assisted genome annotation and analysis of the draft metabolic network of Chlamydomonas reinhardtii. Genetics. 2008;179(1):157–166. doi: 10.1534/genetics.108.088336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hatzenpichler R, et al. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proc Natl Acad Sci USA. 2008;105(6):2134–2139. doi: 10.1073/pnas.0708857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Santoro AE, Casciotti KL. Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: Phylogeny, physiology and stable isotope fractionation. ISME J. 2011;5(11):1796–1808. doi: 10.1038/ismej.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA. 2011;108(38):15892–15897. doi: 10.1073/pnas.1107196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.