Significance
This work provides a complete experimental answer to the longstanding question of which local factors determine the success (i.e., establishment, growth, and flowering) of common nonnative plant invaders at their coldest physiological limits. Using a multifactorial seed-addition experiment along repeated elevational gradients in two sub(ant)arctic mountain regions, we pinpoint the relative contribution of all main candidate determinants: temperature, disturbance, nutrient addition, and propagule pressure. We warn that climate change and direct human disturbances will together result in increased plant invasion in cold-climate ecosystems in the near future.
Keywords: alien plant species, biological invasions, climate change, disturbance, mountains
Abstract
Until now, nonnative plant species were rarely found at high elevations and latitudes. However, partly because of climate warming, biological invasions are now on the rise in these extremely cold environments. These plant invasions make it timely to undertake a thorough experimental assessment of what has previously been holding them back. This knowledge is key to developing efficient management of the increasing risks of cold-climate invasions. Here, we integrate human interventions (i.e., disturbance, nutrient addition, and propagule input) and climatic factors (i.e., temperature) into one seed-addition experiment across two continents: the subantarctic Andes and subarctic Scandinavian mountains (Scandes), to disentangle their roles in limiting or favoring plant invasions. Disturbance was found as the main determinant of plant invader success (i.e., establishment, growth, and flowering) along the entire cold-climate gradient, explaining 40–60% of the total variance in our models, with no indication of any facilitative effect from the native vegetation. Higher nutrient levels additionally stimulated biomass production and flowering. Establishment and flowering displayed a hump-shaped response with increasing elevation, suggesting that competition is the main limit on invader success at low elevations, as opposed to low-growing-season temperatures at high elevations. Our experiment showed, however, that nonnative plants can establish, grow, and flower well above their current elevational limits in high-latitude mountains. We thus argue that cold-climate ecosystems are likely to see rapid increases in plant invasions in the near future as a result of a synergistic interaction between increasing human-mediated disturbances and climate warming.
Although plant invasions in cold environments have increased significantly in recent decades, and are expected to advance even more under future scenarios of global change (1–5), high-elevation and high-latitude areas are still mostly invader-free (6–10). Whether the current barriers are mostly abiotic, biotic, or anthropogenic, however, is hard to deduce from observations alone, because propagule pressure, anthropogenic disturbance, biotic interactions, and bioavailable nutrients all covary along a temperature gradient (6, 11) and have all been observed to correlate with the spread of nonnative species in cold regions (3, 7, 12, 13). Experiments have also revealed declining invasiveness and/or invader performance (14–16) with increasing elevation and lower temperatures in several mountain regions (17). However, we still lack the experimental data to disentangle the roles of all potential determinants (i.e., temperature, disturbance, soil nutrients, and propagule pressure) underlying plant invasion in cold-climate regions. Such knowledge is key for developing strategies to limit the spread of nonnative species toward colder areas.
In mountains, both large-scale (e.g., roads) and small-scale (e.g., gaps of a few centimeters) disturbances have been demonstrated to promote invader establishment and to facilitate range shifts (1, 3, 17–20). More precisely, in lowland conditions, the physical removal of neighboring plant aboveground and belowground biomass (compare reduced competition) and its associated soil perturbation has been shown many times to promote plant invasion (21, 22). However, the stress-gradient hypothesis predicts that, in extreme environments, this type of disturbance would disrupt facilitative interactions between plants, thereby potentially hindering invader establishment (23, 24). Indeed, disturbance has been shown to suppress nonnative seedling emergence at high elevations (25, 26), and facilitation by alpine cushion plants promotes the invasion of nonnative species in dry mountain regions (27). Apart from the expected shift from competition to facilitation with increasing abiotic stress (28) (compare cold stress here), the biotic resistance from resident communities also declines toward colder climates, making invader success more likely in the undisturbed vegetation (1). However, the precise role played by disturbance in cold climates currently is unresolved, although evidence points toward a shift from a positive to a negative effect with decreasing temperatures (24).
Low nutrient levels also constrain plant growth in alpine and arctic ecosystems (29, 30). A recent metaanalysis has shown consistently less invasion in nutrient-poor environments (31), and nutrient addition is known to promote nonnative species within and outside mountain regions (22, 32, 33). However, atmospheric nitrogen deposition, agriculture, and stimulated mineralization under climate change are rapidly increasing the availability of nutrients in colder regions (29). Nonetheless, experimental proof of their effects on nonnative species is limited.
Most observations of mountain plant invasions can be linked to the introduction of propagules with human vectors (34). The proximity of such vectors is indeed often a prerequisite for invasion (35–38). For example, the link between roads or trails and plant invasion is widely recognized (2, 3, 6, 18, 39), although it remains unclear whether this association arises from their role as corridors or as providers of suitable microenvironments (31). Experiments have shown that a low propagule pressure, together with poor soils, controlled the invasion of Taraxacum officinale in dry-climate mountains (40), thus overruling disturbance. However, experiments comparing the performance of plant invaders in their native and invasive range in mountain regions showed no limiting effect of propagule pressure (16, 41).
High-latitude mountain gradients provide an ideal study system to disentangle the roles of the main determinants of plant invasion in cold climates. Nonnative plant species reach their current cold climate limits along the steep climatic gradients in sub(ant)arctic mountains, with high levels of invasion in lowlands, yet currently still invader-free environments at higher elevations (1, 9). The presence of invaders in the lowlands, the predicted climate warming, and increasing human intervention is soon likely to lead to increased levels of invasion at high elevations.
Here, we report a multifactorial experiment (Fig. 1) with 10 mountain plant invader species added as seeds along elevational gradients in two high-latitude mountain regions, the subantarctic Andes (hereafter called Andes) and the subarctic northern Scandes (hereafter called Scandes). The experiment disentangles the effects of all the above-mentioned determinants and their interactions on invader success (establishment, growth, and flowering) along gradients of elevation, temperature, and native community productivity. We answer the question: Which combination of temperature, disturbance, soil nutrient levels, and propagule pressure promotes plant invasion across all studied species in cold environments? By disturbance, we here (and throughout the work) refer to neighbor plant removal and its associated soil perturbation. We hypothesize that, as elevation increases, abiotic resistance will increase until it eventually becomes too cold to allow invader establishment. Disturbance is expected to promote establishment at lower elevations (i.e., due to competition release), but to have a negative effect (i.e., due to facilitation suppression) higher up in the mountains. Nutrient addition and increased propagule pressure could play a synergistic role to overcome limitations in the most stressful circumstances.
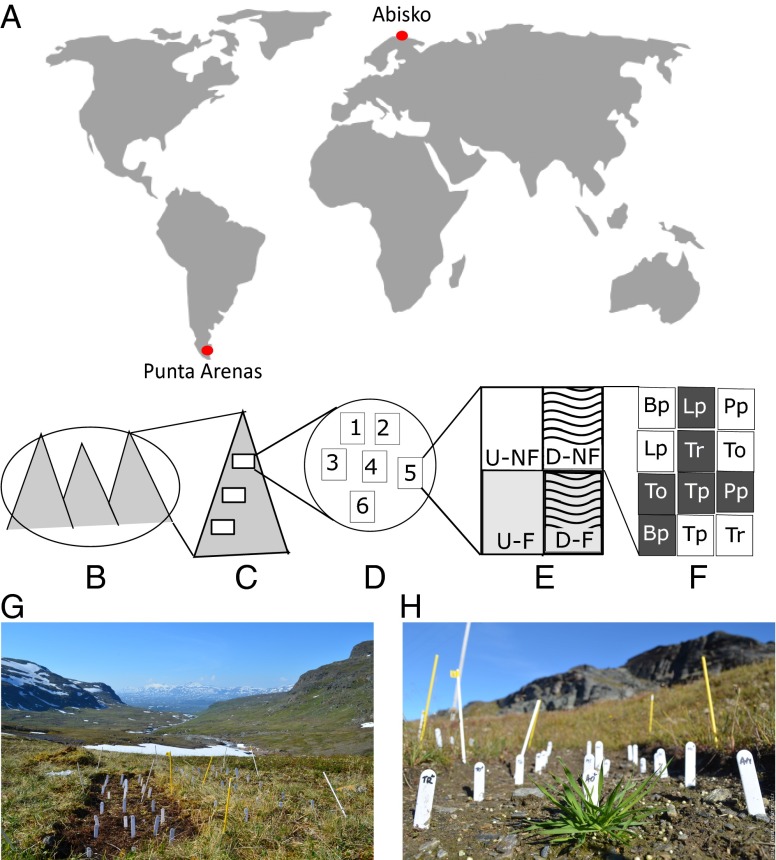
Fig. 1.
Experimental design. (A) The two study regions (Punta Arenas in the subantarctic Andes, Chile, and Abisko in the subarctic Scandes, Sweden) are marked with a red dot on the map. (B and C) In each region, we selected three elevation gradients across three different mountains (B) and chose three sites per elevational gradient (C). (D and E) At each site, we installed six plots (D) with the right half of each plot disturbed (waved lines, E) and the bottom half with added nutrients (gray, E), resulting in four subplots (D-F, disturbed, fertilized; D-NF, disturbed, not fertilized; U-F, undisturbed, fertilized; U-NF, undisturbed, not fertilized). (F) Within each subplot, six species were sown randomly at low (5 seeds; white small squares) and high (30 seeds; gray small squares) propagule pressure, resulting in 4 times 12 microplots per plot. Species used in the Scandes were as follows: Am, Achillea millefolium L.; Ac, Agrostis capillaris L.; Ao, Anthoxanthum odoratum L.; Ml, Medicago lupulina L.; To, Taraxacum officinale F. H. Wigg.; and Tr, Trifolium repens L. Species used in the Andes were as follows: Bp, Bellis perennis L.; Lp, Lolium perenne L.; Pp, Poa pratensis L.; To, T. officinale; Tp, Trifolium pratense L.; and Tr, T. repens. (G) The picture gives an overview of a plot in the Scandes, H shows a detail of a microplot in a disturbed and fertilized subplot.
Results
We found that disturbance was the primary determinant (from 40% to 56% of variance explained) of plant invasion in cold environments in both the Andes and Scandes, irrespective of the chosen gradient [elevation or temperature, expressed as growing degree days (GDDs)] and irrespective of the invasion stage (establishment, biomass, or flowering after 2 y) (Table 1 and Fig. 2). This effect was especially pronounced toward the warmest end of the gradient (Figs. 2 and 3), where disturbance and temperature acted in synergy on biomass production (Fig. 3 B and E). Surprisingly, even at the coldest end of the gradient and at the highest elevations, the effect of disturbance on invader success was still positive (Figs. 2 and 3 and SI Appendix, Tables S1–S4). Its effect was stronger on later stages of invasion (biomass and flowering) than on establishment.
Table 1.
Total percentage of variance explained by each factor in the best models for survival and biomass production
| Factor | Survival, % explained | Biomass, % explained |
| Elevation | 8.48 | 20.61 |
| Disturbance | 43.31 | 56.35 |
| Nutrients | 1.32 | 15.12 |
| Propagules | 5.77 | 6.98 |
| Region | 38.03 | 1.81 |
| GDD | 20.20 | 8.34 |
| Disturbance | 39.81 | 52.36 |
| Nutrients | 0.15 | 15.01 |
| Propagules | 7.35 | 6.87 |
| Region | 21.87 | 2.29 |
Models with either elevation (m a.s.l., top) and GDD (°C days, bottom) as continuous variables. Values are based on a variance partitioning procedure. For detailed calculations, see Methods and SI Appendix, Tables S7 and S8.
Fig. 2.
Graphical representation of the best models (SI Appendix, Table S1) for the different invader success measurements over all 10 species against elevation. Shown are models for invader establishment (A and D), biomass (B and E), and probability of flower production (C and F) in the Andes (Upper) and the Scandes (Lower). Different lines represent different treatments (A, Inset): disturbed (D; red) vs. undisturbed (U; black), fertilized (F; full line) vs. not fertilized (NF; dashed line), and high propagule pressure (high; thick line) vs. low propagule pressure (low; thin line). Black dots on graphs A and D indicate elevation of study plots. When treatment levels were not significantly different, only the line type for the high treatment level is shown. Establishment and biomass production were expressed per microplot.
Fig. 3.
Graphical representation of the best models (SI Appendix, Table S3) for the different invader success measurements over all 10 species against GDDs. Shown are models for invader establishment (A and D), biomass (B and E), and probability of flower production (C and F) in the Andes (Upper) and the Scandes (Lower). Different lines represent different treatments (A, Inset): disturbed (D; red) vs. undisturbed (U; black), fertilized (F; full line) vs. not fertilized (NF; dashed line), and high propagule pressure (high; thick line) vs. low propagule pressure (low; thin line). When treatment levels were not significantly different, only the line type for the high treatment level is shown.
Within disturbed and fertilized plots, higher temperatures were the most important determinant increasing the total biomass produced by nonnative plants and their probability of flower production (Fig. 3 B, C, E, and F and SI Appendix, Tables S3 and S4). The probability of nonnative plant establishment in the Scandes also increased with temperature (Fig. 3D). For establishment in the Andes, a hump-shaped relationship with temperature was observed (Fig. 3A). Despite the overall positive correlation of survival, biomass production, and probability of flower production with temperature, most measures of success were related quadratically with elevation (i.e., they peaked midway the elevation gradient; Fig. 2 and SI Appendix, Tables S1 and S2). The influence of native productivity on nonnative biomass, conversely, was negative (Fig. 4 and SI Appendix, Tables S5 and S6). Patterns thus differed among gradients of elevation, temperature, and nonnative productivity, despite the negative correlation of the latter two with elevation (SI Appendix, Fig. S1).
Fig. 4.
Graphical representation of the best model (SI Appendix, Table S5) for invader biomass production against plot productivity. Predictions for the disturbed plots in the Andes (A) and the Scandes (B). Different lines represent different treatments: fertilized (F; full line) vs. not fertilized (NF; dashed line) and high propagule pressure (high; thick line) vs. low propagule pressure (low; thin line).
Propagule pressure predominantly influenced establishment (Table 1), whereas nutrient addition only determined biomass and flower production. In the Scandes, added nutrients were essential to flowering (Fig. 3F and SI Appendix, Table S3). The effects of propagule pressure on establishment and of nutrient addition on biomass production interacted synergistically with disturbance (SI Appendix, Tables S1 and S3).
Although there were some regional differences, with lower invader establishment, yet higher biomass and flowering, and more pronounced relationships with temperature and elevation in the Andes, overall patterns were consistent across both regions (Figs. 2 and 3).
Discussion
Despite the fact that a switch would be expected from competitive release at low elevations to facilitation suppression at high elevations, disturbance was found as the main positive determinant of invader success along the entire elevation gradient, and its importance increased even more toward later stages of development. This finding proves that the overriding positive effect of disturbance holds true from lowland forests up to the alpine zone, in two different cold-climate regions and for different measures of invader success. Part of the positive effect of disturbance can be attributed to warmer soils during the growing season (SI Appendix, Fig. S1; ref. 42), yet the main underlying mechanism is likely to be found in the combined reduction of competition and increase in resource release (43), given the high resistance against invasion observed in the undisturbed vegetation (17, 18, 23). Unexpectedly, invader success at the coldest extreme of the gradient was never higher in plots with neighbors than in those without them, indicating that disturbance, and not facilitation, matters for plant invasion in cold environments. This prevalence of disturbance regardless of elevation could be caused by reduced competition for space, light, and nutrients with the dense, yet slow-growing, vegetation at high elevations (22, 44). The primary influence of disturbance, and thus neighbor removal, in our study system is consistent with the often observed positive correlation between anthropogenic disturbances like roadsides and nonnative species richness in cold environments (3, 19), suggesting that the roadside environment is a competitor-free and disturbed environment and as such beneficial for nonnative species success, and that roads are more than just corridors (20).
Unlike our original hypothesis, invader success did not generally correlate negatively with elevation. Instead, we found a consistent hump-shaped relationship for both regions and for establishment as well as flowering (Fig. 2). This hump-shaped pattern indicates that different processes limit invaders at both ends of the studied elevation gradients. At high elevations, climate is the most likely barrier (4), supported here by the dwindling invader success with decreasing temperature in both regions, even though disturbance partially alleviated this constraint (Fig. 3). At low elevations in our study systems, temperature is unlikely to be limiting (29). A more probable explanation of the curtailed invader success there is competition (6), given the negative correlation between native productivity and invader biomass (Fig. 4), especially in the (more productive) fertilized subplots. Native productivity itself was greatest in the lowlands and amplified by nutrient addition (SI Appendix, Fig. S1). At midelevations, competition with the regrowing vegetation in the disturbed plots was most likely sufficiently low to allow successful invasion, while the microclimate was not as extreme as at high elevations and thus not preventing alien plant establishment. The hump-shaped response of establishment and flowering success with elevation deviates from the widely reported negative response of invader species richness to elevation (1–3, 45). In observational studies, a high propagule pressure at low elevations is indeed usually associated with dispersal routes and human movement along roads and trails (3), where competitive pressure is likely to be alleviated, and therefore extreme differences in propagule pressure tend to override competition at the community level. These observations contrast with the high levels of competition in the lowlands and the constant propagule pressure along the gradient in our experiment.
As expected, nutrient addition contributed significantly to the production of biomass and flowers of the nonnative species in both regions in our study (29–33). However, the positive effects of small-scale fertilization on the later stages of development were only visible in combination with disturbance and higher temperatures: Nutrient addition and disturbance or temperature acted synergistically to generate the highest invader success (Fig. 3 B, C, E, and F and SI Appendix, Table S3). These results emphasize the importance of anthropogenic disturbances like roadsides as sources of invasion into cold environments, not only because of reductions in competition through neighbor removal, but also because these disturbances locally enhance nutrient availability (46).
Propagule pressure played a less important role as a determinant of invasion than expected based on previous experiments and the observed correlation of invasion with vectors and propagule sources in mountains (1, 13, 40, 45, 47–49). It did promote establishment and biomass production, yet never explained >10% of the total variance in the models (Table 1). In optimal combinations of the other determinants, even a low propagule pressure sufficed to start invasion, whereas in all other combinations, even a high propagule pressure failed. This contrasting result supports the idea that disturbance is the main driver of plant invasion as soon as any propagules are present.
Despite the large ecological and biogeographical differences between the Andes and the Scandes, we found most patterns to be consistent in both study regions. This convergence across subpolar regions is striking and may indicate the generality of our results. The Andes has only recently encountered Eurasian species (since Europeans settled in the late 19th century) and has a relatively low number of plant species due to strong glaciation and reduced landmass (50). The Scandes has a more continental climate with harsher conditions for plant growth across the entire gradient and a strong dominance of the Eurasian flora because of its connections to the rest of the continent. The more productive ecosystem in the Andes, likely influenced by the maritime climate, has a higher average growing-season temperature (SI Appendix, Fig. S1). This warmer climate did reduce invader survival and thus invasibility more than in the Scandes, presumably through more intense competition. Invader survival also declined at the highest growing season temperatures in the Andes (Fig. 3A), which might result from the extreme values of plot productivity (and thus competition) in the warmer plots there. This decline is explained by the domination of European grasses and herbs in the heavily grazed lowland areas of the subantarctic Andes, which are extremely strong competitors (51, 52). A theoretical native community would probably also profit from the mild climate and high productivity there, yet we speculate that the competitive effect would have been smaller and the observed decline in invader success at high temperatures (Fig. 3A) less pronounced. Nevertheless, although the high-latitude Scandes and Andes are very different, our experiments show solid similarities, which may hold true for other cold ecosystems.
Based on our results, we argue that invasion by nonnative plant species with a lowland origin (currently the majority of mountain invaders; refs. 53 and 54) has not yet reached its upper limit in cold-climate mountain ecosystems (3). Indeed, the peak of invasion success in our experiment occurred at the current elevational limits of the selected nonnative species in an observational study in the northern Scandes (1). This finding implies that invasion is likely to increase at a faster rate at the current range edge of the studied species as soon as propagules are introduced—which is most likely just a matter of time (6, 7, 54). Invasion would eventually slow down again at high elevations, however, because these areas are currently protected through their cold climate. Additionally, we have shown that invader establishment correlates with disturbance from plant removal, independent of elevation or temperature, and that high nutrient levels or higher temperatures promote biomass production and flowering. The interplay between these three determinants thus regulates successful invader establishment. Unfortunately, those conditions are on the rise in cold climates (6, 55). However, as disturbance from plant removal is revealed as a predisposing factor for cold-climate invasions, neither temperature increase, nutrient addition, nor increased propagule pressure are likely to trigger plant invasion in the absence of such disturbance. These invasions may thus remain limited to disturbed sites, even if propagules arrive.
The results of our cross-continental experiment have important implications for cold-climate ecosystem management across the globe. Because air temperatures and human interventions will likely increase further in the near future, plant invasion will become a greater threat (6), and nonnative species will probably exploit anthropogenic disturbances to expand their ranges to higher elevations and colder environments than they currently occupy. It will thus be important to keep disturbance in these systems to a minimum and to protect remote areas against uncontrolled expansion of anthropogenic activities. Although it may not seem feasible to prevent invasion along anthropogenic disturbances, such as mountain trails, roads, and railroads, our data suggest that invasions could be contained within such disturbed environments. Caution should be taken, however, where disturbances penetrate the natural vegetation either naturally (e.g., landslides or animal trampling) or anthropogenically (e.g., hikers wandering off trail or ski slopes). Handling mountain invasions thus requires a comprehensive preventive scheme for biological invasions in cold environments, integrating the multiple drivers of global change—not just climate warming.
Methods
Study Regions and Site Characteristics.
We performed a 2-y multifactorial split plot experiment in two high-latitude mountain regions (Fig. 1): the subarctic part of the northern Scandes around Abisko, Sweden (N 68° 21′ E 18° 49′) and the subantarctic Andes around Punta Arenas, Chile (S 53° 09′ W 70° 53′). Abisko has a subarctic climate, with a mean annual air temperature and precipitation of −0.6 °C and 310 mm, respectively [Abisko Scientific Research Station, 400 m above sea level (a.s.l.); www.eu-interact.org/]. Punta Arenas is characterized by a subantarctic cold temperate climate, with a mean annual air temperature of 5.9 °C and 375 mm mean annual precipitation at sea level (56, 57).
In each study region, three elevation gradients and three study sites per elevation gradient were selected (Fig. 1 and SI Appendix, Table S10), spanning a range of ∼500 m from the lowlands (situated at 400 m a.s.l. in the subarctic Scandes and 6 m a.s.l. in the subantarctic Andes) and covering a range from lowland forests up to the alpine zone. Such an elevation range approximates an average temperature increase of 3 °C, based on an adiabatic lapse rate of 0.6 °C per 100 m (29), a range shown in local and global analyses to have a 50% up to 80% drop in nonnative species diversity, especially when extending above the tree line (1, 3). At each elevation level, representative sites with similar characteristics were chosen: productive understory vegetation at the lowest elevation, productive tree line meadows at intermediate elevation, and poor alpine meadows at the highest elevation in the Scandes; and productive open grasslands, productive meadows in forest clearings, and poor alpine meadows, respectively, in the Andes. We focused on grass and forb vegetation, because in the subarctic Scandes, this vegetation type is known to be highly invasive relative to other high-latitude habitats (17).
Experimental Setup.
We installed six 160 × 120-cm plots at random locations in each of the three sites on every gradient (Fig. 1). Half of each plot (160 × 60 cm) was experimentally disturbed by completely removing the vegetation (plant canopies and roots) and top 3-cm soil layer, to disrupt biotic interactions with neighbor plants as happens in anthropogenic or natural disturbances like construction works, roadsides and trailsides, avalanches, trampling, or digging by animals. The downhill half of the plot (80 × 120 cm) was fertilized with 50 g of Substral Osmocote slow release fertilizer NPKMg (19-9-11-2, equaling 35 kg of N per ha/y), evenly spread on the soil surface to release plants from nutrient limitation in generally nutrient-limited arctic and alpine ecosystems. These contrasting nutrient levels are representative of the range of variation that can be expected through deposition of nitrogen and faster nutrient release through an increased N decomposition under a warming climate (58, 59). Each plot was thus divided in four subplots (Fig. 1). Within every subplot, six species of nonnative forbs and grasses were sown in microplots (3 cm diameter), either at a low (5 seeds) or high (30 seeds) propagule pressure (the latter to release any seed limitation), resulting in 12 evenly distributed microplots per subplot, each of them 20 cm apart. Each combination of species and propagule pressure was randomly assigned to one of the 12 microplots.
In both regions, we chose two species from each of three distinct taxa characterized by a large number of globally invasive species (60) (i.e., from Asteraceae, Fabaceae, and Poaceae). For species, see the Fig. 1 legend. These species covered a broad range of types of invasive plant species, allowing us to be more general in our conclusions. All chosen species (except Bellis perennis) belonged to the 50 most widely spread global mountain invaders (2) and were present as nonnative species in the study regions (1, 61, 62). Seeds were available from local merchants (except for T. officinale in the Andes, for which seeds where collected manually in the field). All species showed germination rates >60% in a 2-wk germination test on moist filter paper in Petri dishes in a greenhouse, except for Agrostis capillaris in Sweden and B. perennis and T. officinale in the Andes, showing germination rates of 54%, 44%, and 20%, respectively. Species was analyzed as a random factor in all models to search for patterns larger than the species-specific differences, because the sample size would be too small to test patterns between species or species groups.
The experiment was installed in spring: early July 2013 in the Scandes and November 2014 in the Andes. At the end of the second growing season (beginning of September 2014 in the Scandes; beginning of April 2015 in the Andes), we recorded invader success and probability of flower production as presence/absence per microplot. Aboveground biomass of the sown species was harvested per microplot and dried and weighed, and belowground biomass was removed. Plot productivity, the regrowth of the surrounding vegetation in the disturbed subplots, was estimated by harvesting the aboveground biomass separately in both disturbed subplots within a randomly located 400-cm2 square, drying this biomass, weighing it, and extrapolating it to productivity on a 1-m2 scale.
Soil Temperature.
Soil temperature was logged every 2 h (iButtons: DS1921G with 0.5 °C accuracy in the Andes; DS1922L with 0.0625 °C accuracy in the Scandes; Maxim Integrated). Thermometers were placed 3 cm deep in two randomly chosen plots per site, on the central axis of the plot, with one in the disturbed and one in the undisturbed plot half. The resulting temperature time series were used to calculate GDDs (being the sum of all positive daily averages within the growing season) for the second growing season for every plot half. Temperatures of the second growing season were used, because we then had temperature loggers in both disturbed and undisturbed plots, allowing more detailed analysis. For plots without loggers, we calculated the average GDD per plot half and per site from the available data within that site.
Statistical Analysis.
Each invader success measure (invader survival, biomass production, and probability of flower production; all per microplot) was modeled separately against elevation, GDD, and plot productivity, with the beyond-optimal models for all of them containing a quadratic and linear term of the continuous variable, and additionally disturbance (yes/no), nutrient addition (yes/no), propagule pressure (high/low), region (Scandes/Andes), and all two-way interactions as fixed effects.
Invader survival was modeled with generalized linear mixed models (GLMMs) with a binomial family and a logit-link [function glmer in R; package lme4 (63]]. Probability of flower production was also modeled with GLMMs with a binomial family and a logit-link, yet only values for those microplots (n = 1,230 of 5,184) with successful survival after the second growing season were incorporated in the model. Nonnative species biomass was analyzed with linear mixed models [LMMs; function lme, from the package lme4 (63)], similarly after removing those microplots without successful survival and after log-transforming the data to achieve normal distribution. A different variance structure (nutrients*disturbed) was included by means of the varIdent function [package nlme (64)] to take into account differences in variance between the different subplots (65).
In each model, we corrected for random effects, with species nested within plot, within site, and within gradient as a random intercept. Elevation, GDD, and plot productivity were all scaled to a normal distribution with mean 0 and SD 1 (function scale; ref. 66). The models against plot productivity were limited to the disturbed plot halves, because plot productivity was only measured in these subplots. The optimal model was always selected based on the lowest Akaike information criterion (AIC) value and the significance levels of the fixed effects, by step-by-step removal of the fixed effect with the highest P value starting from the beyond-optimal model (SI Appendix, Tables S2, S4, and S6), until the lowest AIC was reached (65) (SI Appendix, Tables S1, S3, and S5).
Additionally, we modeled GDD against elevation, disturbance, and their two-way interactions with a LMM. Normal distribution of the data was achieved by adjusting the variance structure to the region (function varIdent). The same model was used for plot productivity against elevation, but disturbance was replaced by nutrient addition, and plot productivity was log-transformed to obtain a normal distribution.
The function r.squaredGLMM from the MuMIn-package (67) was used to calculate the marginal (fixed effects) and conditional (full model) R2 for all optimal and beyond-optimal models. We then followed a variation partitioning procedure to determine the relative proportion of variation for each response variable in the models with elevation, GDD, and plot productivity. To perform our variation partitioning, we constructed a series of models with (i) only one focal variable, (ii) all variables except that focal variable, or (iii) the full model with all explanatory variables. The proportion of variation explained by each fixed factor was represented by calculating the difference between the marginal R2 of the full model and of the model without the focal variable and dividing it by the marginal R2 of the full model (68). For all factors in all models, the variation explained by the model without the focal variable (R2 of full model minus R2 of model with only the focal variable, divided by full model) and the shared variance (R2 of model of focal variable plus R2 of model without focal variable minus R2 of full model, divided by R2 of full model) were calculated as well (SI Appendix, Tables S7–S9). This variance partitioning could not be performed for the models of probability of flower production, because this probability was zero in the undisturbed subplots in the Scandes.
All data analyses were performed in R (Version 3.0.1) (69).
Supplementary Material
Acknowledgments
We thank three anonymous reviewers for their constructive comments and John Gash for manuscript editing. This work was supported by Swedish Research Council Grant VR 2012-6252 (to A.M., A.P., and M.A.N.) and a grant from the Research Foundation–Flanders (to J.J.L.). A.P. is supported by the Institute of Ecology and Biodiversity (IEB), the Chilean Ministry of Economy and Tourism Grant Iniciativa Científica Milenio ICM P05-002 and Comisión Nacional de Ciencia y Tecnología CONICYT Grant PFB-23.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1608980113/-/DCSupplemental.
References
- 1.Lembrechts JJ, Milbau A, Nijs I. Alien roadside species more easily invade alpine than lowland plant communities in a subarctic mountain ecosystem. PLoS One. 2014;9(2):e89664. doi: 10.1371/journal.pone.0089664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seipel T, et al. Processes at multiple scales affect richness and similarity of non-native plant species in mountains around the world. Glob Ecol Biogeogr. 2012;21(2):236–246. [Google Scholar]
- 3.Alexander JM, et al. MIREN Consortium Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc Natl Acad Sci USA. 2011;108(2):656–661. doi: 10.1073/pnas.1013136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marini L, et al. Beta-diversity patterns elucidate mechanisms of alien plant invasion in mountains. Glob Ecol Biogeogr. 2013;22(4):450–460. [Google Scholar]
- 5.Becker T, Dietz H, Billeter R, Buschmann H, Edwards PJ. Altitudinal distribution of alien plant species in the Swiss Alps. Perspect Plant Ecol. 2005;7(3):173–183. [Google Scholar]
- 6.Pauchard A, et al. Ain’t no mountain high enough: Plant invasions reaching new elevations. Front Ecol Environ. 2009;7(9):479–486. [Google Scholar]
- 7.Pyšek P, Jarošík V, Pergl J, Wild J. Colonization of high altitudes by alien plants over the last two centuries. Proc Natl Acad Sci USA. 2011;108(2):439–440. doi: 10.1073/pnas.1017682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDougall KL, et al. Plant invasions in mountains: Global lessons for better management. Mt Res Dev. 2011;31(4):380–387. [Google Scholar]
- 9.Pauchard A, et al. Non-native and native organisms moving into high elevation and high latitude ecosystems in an era of climate change: New challenges for ecology and conservation. Biol Invasions. 2016;18(2):345–353. [Google Scholar]
- 10.Alsos IG, Ware C, Elven R. Past Arctic aliens have passed away, current ones may stay. Biol Invasions. 2015;17(11):3113–3123. [Google Scholar]
- 11.Körner C. Why are there global gradients in species richness? Mountains might hold the answer. Trends Ecol Evol. 2000;15(12):513–514. [Google Scholar]
- 12.Marini L, Gaston KJ, Prosser F, Hulme PE. Contrasting response of native and alien plant species richness to environmental energy and human impact along alpine elevation gradients. Glob Ecol Biogeogr. 2009;18(6):652–661. [Google Scholar]
- 13.Kalwij JM, Robertson MP, van Rensburg BJ. Annual monitoring reveals rapid upward movement of exotic plants in a montane ecosystem. Biol Invasions. 2015;17(12):3517–3529. [Google Scholar]
- 14.Haider S, Alexander JM, Kueffer C. Elevational distribution limits of non-native species: Combining observational and experimental evidence. Plant Ecol Divers. 2011;4(4):363–371. [Google Scholar]
- 15.Haider S, Kueffer C, Edwards PJ, Alexander JM. Genetically based differentiation in growth of multiple non-native plant species along a steep environmental gradient. Oecologia. 2012;170(1):89–99. doi: 10.1007/s00442-012-2291-2. [DOI] [PubMed] [Google Scholar]
- 16.Ross LC, Lambdon PW, Hulme PE. Disentangling the roles of climate, propagule pressure and land use on the current and potential elevational distribution of the invasive weed Oxalis pes-caprae L. on Crete. Perspect Plant Ecol. 2008;10(4):251–258. [Google Scholar]
- 17.Milbau A, Shevtsova A, Osler N, Mooshammer M, Graae BJ. Plant community type and small-scale disturbances, but not altitude, influence the invasibility in subarctic ecosystems. New Phytol. 2013;197(3):1002–1011. doi: 10.1111/nph.12054. [DOI] [PubMed] [Google Scholar]
- 18.Pollnac F, Seipel T, Repath C, Rew LJ. Plant invasion at landscape and local scales along roadways in the mountainous region of the Greater Yellowstone Ecosystem. Biol Invasions. 2012;14(8):1753–1763. [Google Scholar]
- 19.Arévalo JR, et al. Distribution of alien vs. native plant species in roadside communities along an altitudinal gradient in Tenerife and Gran Canaria (Canary Islands) Perspect Plant Ecol. 2005;7(3):185–202. [Google Scholar]
- 20.Lembrechts JJ, et al. Mountain roads shift native and non-native plant species’ ranges. Ecography. 2016 doi: 10.1111/ecog.02200. [DOI] [Google Scholar]
- 21.Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: A general theory of invasibility. J Ecol. 2000;88(3):528–534. [Google Scholar]
- 22.Hobbs RJ, Huenneke LF. Disturbance, diversity, and invasion - implications for conservations. Conserv Biol. 1992;6(3):324–337. [Google Scholar]
- 23.Ansari S, Daehler CC. Life history variation in a temperate plant invader, Verbascum thapsus along a tropical elevational gradient in Hawaii. Biol Invasions. 2010;12(12):4033–4047. [Google Scholar]
- 24.Lembrechts JJ, Milbau A, Nijs I. Trade-off between competition and facilitation defines gap colonization in mountains. AoB Plants. 2015;7:plv128. doi: 10.1093/aobpla/plv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paiaro V, Mangeaud A, Pucheta E. Alien seedling recruitment as a response to altitude and soil disturbance in the mountain grasslands of central Argentina. Plant Ecol. 2007;193(2):279–291. [Google Scholar]
- 26.Poll M, Naylor BJ, Alexander JM, Edwards PJ, Dietz H. Seedling establishment of Asteraceae forbs along altitudinal gradients: A comparison of transplant experiments in the native and introduced ranges. Divers Distrib. 2009;15(2):254–265. [Google Scholar]
- 27.Cavieres LA, Quiroz CL, Molina-Montenegro MA, Munoz AA, Pauchard A. Nurse effect of the native cushion plant Azorella monantha on the invasive non-native Taraxacum officinale in the high-Andes of central Chile. Perspect Plant Ecol. 2005;7(3):217–226. [Google Scholar]
- 28.Callaway RM, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417(6891):844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- 29.Körner C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems. Springer; New York: 2003. [Google Scholar]
- 30.Concilio AL, Loik ME, Belnap J. Global change effects on Bromus tectorum L. (Poaceae) at its high-elevation range margin. Glob Change Biol. 2013;19(1):161–172. doi: 10.1111/gcb.12032. [DOI] [PubMed] [Google Scholar]
- 31.Zefferman E, et al. Plant communities in harsh sites are less invaded: A summary of observations and proposed explanations. AoB Plants. 2015;7:plv056. doi: 10.1093/aobpla/plv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leishman MR, Thomson VP. Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia. J Ecol. 2005;93(1):38–49. [Google Scholar]
- 33.He WM, Yu GL, Sun ZK. Nitrogen deposition enhances Bromus tectorum invasion: Biogeographic differences in growth and competitive ability between China and North America. Ecography. 2011;34(6):1059–1066. [Google Scholar]
- 34.Wasowicz P. Non-native species in the vascular flora of highlands and mountains of Iceland. PeerJ. 2016;4:e1559. doi: 10.7717/peerj.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansong M, Pickering C. Are weeds hitchhiking a ride on your car? A systematic review of seed dispersal on cars. PLoS One. 2013;8(11):e80275. doi: 10.1371/journal.pone.0080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ansong M, Pickering C. Weed seeds on clothing: A global review. J Environ Manage. 2014;144:203–211. doi: 10.1016/j.jenvman.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 37.Whinam J, Chilcott N, Bergstrom DM. Subantarctic hitchhikers: expeditioners as vectors for the introduction of alien organisms. Biol Conserv. 2005;121(2):207–219. [Google Scholar]
- 38.Giorgis MA, et al. Factors associated with woody alien species distribution in a newly invaded mountain system of central Argentina. Biol Invasions. 2011;13(6):1423–1434. [Google Scholar]
- 39.Barros A, Pickering CM. Non-native plant invasion in relation to tourism use of Aconcagua Park, Argentina, the highest protected area in the Southern Hemisphere. Mt Res Dev. 2014;34(1):13–26. [Google Scholar]
- 40.Quiroz CL, Cavieres LA, Pauchard A. Assessing the importance of disturbance, site conditions, and the biotic barrier for dandelion invasion in an Alpine habitat. Biol Invasions. 2011;13(12):2889–2899. [Google Scholar]
- 41.Alexander JM, Poll M, Dietz H, Edwards PJ. Contrasting patterns of genetic variation and structure in plant invasions of mountains. Divers Distrib. 2009;15(3):502–512. [Google Scholar]
- 42.Ford KR, Ettinger AK, Lundquist JD, Raleigh MS, Hille Ris Lambers J. Spatial heterogeneity in ecologically important climate variables at coarse and fine scales in a high-snow mountain landscape. PLoS One. 2013;8(6):e65008. doi: 10.1371/journal.pone.0065008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Compagnoni A, Adler PB. Warming, competition, and Bromus tectorum population growth across an elevation gradient. Ecosphere. 2014;5(9):1–34. [Google Scholar]
- 44.Kaarlejarvi E, Olofsson J. Concurrent biotic interactions influence plant performance at their altitudinal distribution margins. Oikos. 2014;123(8):943–952. [Google Scholar]
- 45.Pauchard A, Alaback PB. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of south-central Chile. Conserv Biol. 2004;18(1):238–248. [Google Scholar]
- 46.Müllerová J, Vítková M, Vítek O. The impacts of road and walking trails upon adjacent vegetation: Effects of road building materials on species composition in a nutrient poor environment. Sci Total Environ. 2011;409(19):3839–3849. doi: 10.1016/j.scitotenv.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 47.Dullinger S, Dirnbock T, Grabherr G. Patterns of shrub invasion into high mountain grasslands of the Northern Calcareous Alps, Austria. Arct Antarct Alp Res. 2003;35(4):434–441. [Google Scholar]
- 48.Von Holle B, Simberloff D. Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology. 2005;86(12):3212–3218. [Google Scholar]
- 49.Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends Ecol Evol. 2005;20(5):223–228. doi: 10.1016/j.tree.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 50.Kilian R, Lamy F. A review of Glacial and Holocene paleoclimate records from southernmost Patagonia (49-55 degrees S) Quat Sci Rev. 2012;53:1–23. [Google Scholar]
- 51.Domínguez E, Elvebakk A, Marticorena C, Pauchard A. Plantas introducidas en el Parque Nacional Torres del Paine, Chile. Gayana Bot. 2006;63:131–141. [Google Scholar]
- 52.Sottile GD, Meretta PE, Tonello MS, Bianchi MM, Mancini MV. Disturbance induced changes in species and functional diversity in southern Patagonian forest-steppe ecotone. For Ecol Manage. 2015;353:77–86. [Google Scholar]
- 53.McDougall KL, et al. Alien flora of mountains: global comparisons for the development of local preventive measures against plant invasions. Divers Distrib. 2011;17(1):103–111. [Google Scholar]
- 54.Haider S, et al. The role of bioclimatic origin, residence time and habitat context in shaping non-native plant distributions along an altitudinal gradient. Biol Invasions. 2010;12(12):4003–4018. [Google Scholar]
- 55.Walther GR, et al. Ecological responses to recent climate change. Nature. 2002;416(6879):389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- 56.Butorovic N. Resumen meterológica año 2014 Estación Jorge Shythe (53°08‘S; 70°53’W; 6 m snm) Anales del Instituto de la Patagonia (Chile) 2015;43(1):175–184. [Google Scholar]
- 57.Santana A, Butorovic N, Olave C. Variación de la temperatura en Punta Arenas en los últimos 120 años. Anales del Instituto de la Patagonia (Chile) 2009;37(1):85–96. [Google Scholar]
- 58.Portillo-Estrada M, et al. Climatic controls on leaf litter decomposition across European forests and grasslands revealed by reciprocal litter transplantation experiments. Biogeosciences. 2016;13(5):1621–1633. [Google Scholar]
- 59.Flechard CR, et al. Dry deposition of reactive nitrogen to European ecosystems: A comparison of inferential models across the NitroEurope network. Atmos Chem Phys. 2011;11(6):2703–2728. [Google Scholar]
- 60.Daehler CC. The taxonomic distribution of invasive angiosperm plants: ecological insights and comparison to agricultural weeds. Biol Conserv. 1998;84(2):167–180. [Google Scholar]
- 61.Weidema IR. Introduced Species in the Nordic Countries. Nordic Council of Ministers; Copenhagen: 2000. [Google Scholar]
- 62.Fuentes N, Pauchard A, Sanchez P, Esquivel J, Marticorena A. A new comprehensive database of alien plant species in Chile based on herbarium records. Biol Invasions. 2013;15(4):847–858. [Google Scholar]
- 63.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 64.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2016. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-128. Available at https://cran.r-project.org/web/packages/nlme/index.html. Accessed July 2016.
- 65.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. Springer; New York: 2009. [Google Scholar]
- 66.Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol. 2010;1(2):103–113. [Google Scholar]
- 67.Barton K. 2016. MuMIn: Multi-Model Inference. R Package Version 1.15.6. Available at https://cran.r-project.org/web/packages/MuMIn/index.html. Accessed July 2016.
- 68.Legendre P, Legendre J. Numerical Ecology. Elsevier; Amsterdam: 1998. [Google Scholar]
- 69.R Core Team 2013. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.