Abstract
Cholesterol uptake and efflux are key metabolic processes associated with macrophage physiology and atherosclerosis. Peroxisome proliferator-activated receptor gamma (PPARγ) and liver X receptor alpha (LXRα) have been linked to the regulation of these processes. It remains to be identified how activation of these receptors is connected and regulated by endogenous lipid molecules. We identified CYP27, a p450 enzyme, as a link between retinoid, PPARγ, and LXR signaling. We show that the human CYP27 gene is under coupled regulation by retinoids and ligands of PPARs via a PPAR-retinoic acid receptor response element in its promoter. Induction of the enzyme's expression results in an increased level of 27-hydroxycholesterol and upregulation of LXR-mediated processes. Upregulated CYP27 activity also leads to LXR-independent elimination of CYP27 metabolites as an alternative means of cholesterol efflux. Moreover, human macrophage-rich atherosclerotic lesions have an increased level of retinoid-, PPARγ-, and LXR-regulated gene expression and also enhanced CYP27 levels. Our findings suggest that nuclear receptor-regulated CYP27 expression is likely to be a key integrator of retinoic acid receptor-PPARγ-LXR signaling, relying on natural ligands and contributing to lipid metabolism in macrophages.
Handling of lipids by macrophages is an important metabolic process in the context of hypercholesterolemia and the development of atherosclerotic lesions (20, 32, 44). For this reason it is critical to understand the regulatory processes associated with cholesterol and fatty acid uptake and release (efflux) in this cell type. A regulatory network has been associated with macrophage lipid metabolism in recent years. First, it has been shown that peroxisome proliferator-activated receptor gamma (PPARγ), a member of the nuclear receptor superfamily, can be linked to macrophage maturation and uptake of modified (oxidized) low-density lipoprotein (LDL) (35, 45). Later, the oxysterol receptor liver X receptor (LXR) was linked to macrophage lipid metabolism by showing that LXRα is a direct transcriptional target of PPARγ and could induce lipid transporters such as ABCA1 (9, 40) and ABCG1 (26). A coordinated lipid transport is likely to be regulated by these receptors. Linking of the two receptor systems (PPARγ and LXRα) provides an attractive but not well understood pathway to explain lipid and cholesterol uptake and efflux from macrophages. The issue of how the activation of the two receptors may be coupled has not been addressed yet. It was assumed that the lipid contents of lipoproteins may act as activators or ligands for both PPARγ (35) and LXR (30). The fact that LXR signaling is activated in macrophages exposed to acetylated LDL (30), which does not contain oxidized cholesterol, suggests that there must be other ways to activate or produce ligand for this receptor. Furthermore, LXR-RXR heterodimers were originally identified as mediators of an alternative retinoid signaling pathway (47, 48) showing that the LXR-RXR heterodimer is highly permissive and can be activated from either the RXR side by retinoids or the LXR side by oxysterols (40). These observations and the fact that RXR agonists have atheroprotective effects in ApoE−/− animals (11) suggest that retinoids may also participate in the regulation of lipid homeostasis. A number of oxysterols were identified as potential endogenous ligands for LXR (24, 25, 31, 36). One of these compounds, 27-hydroxycholesterol, is produced by a p450 enzyme, CYP27. CYP27 is a mitochondrial enzyme representing an alternative bile acid synthesis pathway (1, 8, 37, 42, 43) and was reported to be expressed, besides in the liver, in the lung and also in macrophages (2, 5). It has been also associated with atherosclerotic lesions (13, 23). A mutation in this enzyme leads to the human disease cerebrotendinous xanthomatosis (CTX), a rare sterol storage disease characterized by xanthomas in tendons and also in the central nervous system leading to ataxia, spinal cord paresis, neurological dysfunctions, normolipidemic xanthomatosis, and accelerated atherosclerosis (6, 7, 33). The enzyme's product, 27-hydroxycholesterol, has been shown to activate LXR (17, 25). By carrying out global gene expression profiling in a search for common elements of monocyte-macrophage transition and PPARγ-RXR-mediated transcriptional events, we identified CYP27 as an element of monocyte-macrophage transition (22) and also as part of the realm of PPARγ-RXR-regulated genes in myeloid cells. Here we show that human CYP27 is transcriptionally regulated by both retinoids and PPAR ligands, based on interactions on a response element in the promoter region of the gene. Furthermore, retinoids induce LXR-regulated lipid transporters mediating cholesterol efflux. Finally, all elements of this pathway can be found in human macrophage-rich atherosclerotic lesions.
MATERIALS AND METHODS
Materials.
Cells were treated with the following ligands: AM580 (Biomol); LG268 (a gift from R. Heyman) (Ligand Pharmaceuticals); Wy14643, Rosiglitazone, and T0901317 (Alexis Biochemicals); GW501516 and GW9662 (gifts from T. M. Willson) (GlaxoSmithKline); 27-hydroxycholesterol, 25-hydroxycholesterol, 24S,25-epoxycholesterol, and 22R-hydroxycholesterol (Steraloids Inc.); 9-hydroxyoctadecadienoic acid (Cayman Chemicals); LDL and oxidized LDL (Intracel); and AGN193109 (a gift from Roshantha A. S. Chandraratna) (Allergan Inc.). All other reagents were obtained from Sigma or as indicated.
Plasmids.
Mammalian expression vectors expressing human retinoic acid receptor alpha (RARα), human retinoid X receptor alpha (RXRα), mouse PPARγ (mPPARγ), β-galactosidase, and thymidine kinase (TK)-Luc were described previously (10). The hCYP27-853-pCAT, hCYP27-649-pCAT, and hCYP27-217-pCAT plasmids were constructed earlier (18). pcDNA3.1-hCYP27 was a gift from E. G. Lund (Merck Research Laboratories, Rahway, N.J.).
Cell culture.
MonoMac-6 cells were kind gift of E. Duda (Biological Research Center, Szeged, Hungary). The cells were grown in RPMI 1640 (Sigma) supplemented with 10% fetal bovine serum (FBS) (Invitrogen), 10% charcoal-stripped serum (Invitrogen), or 10% lipoprotein-deficient serum (Intracel), along with 2 mM glutamine, penicillin, and streptomycin (Sigma), or without serum in the presence of insulin-transferrin-sodium selenite medium supplement (Sigma), or in AIMV (Invitrogen). Primary human fibroblasts were isolated from healthy adults and patients with CTX as described earlier (19) and were cultured in DMEM (Sigma) supplemented with 10% FBS, 2 mM glutamine, penicillin, and streptomycin.
Microarray analysis.
Total RNA was isolated by using Trizol reagent (Invitrogen) and further purified by using the RNeasy kit (Qiagen). cRNA was generated from 5 μg of total RNA by using the SuperScript Choice kit (Invitrogen) and the high-yield RNA transcription labeling kit (Enzo Diagnostics). Fragmented cRNA was hybridized to Affymetrix (Santa Clara, Calif.) arrays (HU95A) according to standard Affymetrix protocols. Preliminary data analysis was performed by the Microarray Core Facility of the Howard Hughes Medical Institute at Stanford University. Further analysis was performed with GeneSpring 6.0 (Silicon Genetics, Redwood City, Calif.). These analyses provided a signal for each specific transcript that was subsequently normalized by comparison to the median signal (arbitrary value of 1.0) obtained from the whole array.
Isolation and culture of human primary monocytes.
Human monocytes were isolated from healthy volunteer buffy coat obtained from the regional blood bank. Monocyte separation was carried out according to the manufacturer's instructions with CD14 MicroBeads (Miltenyi Biotec). Monocytes were differentiated for 2 to 4 days. Primary human cells were cultured in RPMI 1640 supplemented with 10% FBS, 2 mM glutamine, penicillin, and streptomycin or as indicated.
RNA extraction and real-time quantitative PCR.
Total RNA was isolated from cells by using Trizol reagent (Invitrogen) according to the instructions of the manufacturer. Atherosclerotic lesions were dissected off from underlying tissues and homogenized. Control samples were obtained from the neighboring nonatherosclerotic part of the vessel wall. For the human samples, lesion areas (abnormal, fatty, nonulcering lesions of the femoral artery and the common and internal carotid arteries) were dissected off. After homogenization, total RNA was extracted with Trizol reagent. Transcript quantitation was performed by quantitative real-time reverse transcriptase PCR (RT-PCR) with TaqMan probes. Transcript levels were normalized to the level of cyclophilin D and 36B4. The sequences of primers and TaqMan probes used in transcript quantitation are available at http://biochem.dote.hu/nagylab.
Northern blot analysis.
Total RNA (10 to 15 μg) was separated by formaldehyde-1% agarose gel electrophoresis and transferred to a nylon membrane that was hybridized with radioactive [α-32P]dATP-labeled DNA probes by using a random hexamer DNA labeling kit (Fermentas) and QuickHyb hybridization solution (Stratagene). Transcript levels were determined after overnight exposure to Kodak X-OMAT films and were normalized for 36B4 expression levels.
Western blot analysis.
Cells were treated for 2 days as indicated, washed in phosphate-buffered saline, and then lysed in buffer A (Tris-HCl [pH 7.5], 1 mM EDTA, 15 mM β-mercaptoethanol, 0.1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride. Twenty-five micrograms of total protein was separated by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane (Bio-Rad Laboratories). After blocking with 5% dry milk, the membrane was probed with anti-CYP27 antibody, a kind gift from D. W. Russell (University of Texas Southwestern Medical Center, Dallas) and subsequently with peroxidase-conjugated secondary antibody. An enhanced chemiluminescence detection kit (Pierce) was used for signal detection.
Chromatin immunoprecipitation.
Chromatin immunoprecipitation was carried out as described previously (27) with modifications. Briefly, cells were fixed with 1% formaldehyde for 10 min at room temperature. Fixation was stopped by adding chilled glycine to a final concentration of 150 mM. The cells were scraped and washed twice with ice-cold phosphate-buffered saline containing proteinase inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin per μl, and 1 μg of pepstatin A per μl). Nuclei were prepared by incubation for 10 min on ice in a buffer containing 5 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] (pH 8), 85 mM KCl, 0.5% NP-40, and proteinase inhibitors. After centrifugation at 3,000 × g for 10 min at 4°C, nuclei were resuspended in sonication buffer (1% SDS, 0.1 M NaHCO3, and proteinase inhibitors), lysed on ice for 10 min, and sonicated on ice to an average fragment size of 300 bp. Cell debris was pelleted twice by centrifugation at 10,000 × g for 30 min at 4°C in a bench top centrifuge. Soluble chromatin was aliquoted, frozen in liquid nitrogen, and stored at −70°C. For immunoprecipitation, chromatin was diluted 10-fold in immunoprecipitation buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris [pH 8.1], 16.7 mM NaCl, and proteinase inhibitors). One milliliter of diluted chromatin was precleared twice with 40 μl of blocked protein A/G-Sepharose beads. Immunoprecipitation was carried out with specific antibodies purchased from Upstate Biotechnology (anti-acetyl H4 2) and Santa Cruz (anti-PPARγ and anti-RXRα). Anti-RARα was a kind gift of L. Tora (CNRS/INSERM/ULP, Strasburg, France). Immunoprecipitated nucleosomes were eluted twice from beads with elution buffer (1% SDS, 0.1 M NaHCO3), and eluates were combined. Cross-links were reversed by incubation for 6 h at 65°C after addition of 20 μl of 5 M NaCl. The eluate was combined with 10 μl of 0.5 M EDTA, 20 μl 1 M Tris (pH 6.5), and 2 μg of proteinase K and incubated for 1 h at 45°C. DNA was recovered after phenol-chloroform extraction and ethanol precipitation with 20 μg of glycogen as a carrier. The DNA was resuspended in 50 μl of yeast tRNA (50 ng/μl) (Invitrogen). Two microliters of this solution was used for real-time Q-PCR in a 25-μl reaction mixture. All measurements were done in triplicate. All chromatin results were verified from independent chromatin preparations.
Transient transfections and reporter gene assays.
Human CYP27-chloramphenicol acetyltransferase (CAT) promoter plasmids were constructed as described earlier (18). The following deletion constructs were used and cotransfected with the indicated full-length receptors: hCYP27-853-pCAT, hCYP27-649-pCAT, hCYP27-217-pCAT (numbers indicate positions relative to the translational start site, +1). The response elements were cloned into TK-Luc to obtain Enhancer trap vectors that were transfected along with the indicated receptors. All transfection experiments were performed with CV1 cells (green monkey kidney fibroblasts) as described earlier (45).
Electrophoretic mobility shift assays.
Electrophoretic mobility shift assays were performed as described earlier (4). Full-length hRARα, hRXRα, and mPPARγ receptors were produced by using the T7 Quick TNT in vitro transcription-translation kit (Promega). For supershift experiments, the receptors were preincubated with the indicated antibody prior to binding reaction: anti-RAR (Santa Cruz), anti-RXR (a kind gift from L. Tora, CNRS/INSERM/ULP, Strasburg, France), or anti-PPARγ (Biomol). The oligonucleotides that were used are available at http://biochem.dote.hu/nagylab.
Determination of 27-hydroxycholesterol and 3β-hydroxy-5-cholestenoic acid.
Levels of 27-oxygenated sterols were determined as described previously (2). Briefly, 106 cells were treated as indicated in the figures, and 27-hydroxycholesterol and 3β-hydroxy-5-cholestenoic acid contents were measured by quantitative mass spectrometry with use of deuterium-labeled 27-hydroxycholesterol and 3β-hydroxy-5-norcholestenoic acid, respectively, as internal standards.
Determination of retinoid levels.
Retinoid levels were determined by high-performance liquid chromatography (HPLC) as described previously (41).
Statistical analysis.
All data are presented as means ± standard deviations (SD). In real-time quantitative PCR and transient-transfection experiments, the mean and SD were calculated for both the normalized and the normalizer values. To incorporate the random errors of the measurements, we used the propagation of errors to determine the SD of the normalized values. For all experiments we made at least four biological replicates, and on the fold changes we performed an F test followed by an unpaired (two-tailed) t test; results were considered significant with a P value of <0.01.
RESULTS
Human CYP27 is acutely regulated by retinoids and PPARγ agonists.
In order to identify the common elements of the transcriptional changes during the monocyte-macrophage transition and that of PPAR-RXR heterodimer activation, we have carried out DNA microarray analysis on monocytic leukemia cells (MonoMac-6) treated with PPARγ (Rosiglitazone) and the RXR-selective retinoid LG268 and also on primary human monocytes induced to differentiate to macrophages as described in Materials and Methods. As part of these global gene expression profiling efforts, we found that transcript levels of the p450 enzyme CYP27 increased in monocyte-derived macrophages and also in PPARγ and RXR ligand-treated myeloid cells, along with several other genes associated with lipid metabolism (data not shown), suggesting that these genes may be under PPARγ and/or retinoid regulation. In order to provide a baseline for further studies, we determined the transcript levels of various nuclear receptors (PPARs, retinoid receptors, and LXR) during the monocyte-macrophage transition. Comparing the relative abundances of nuclear receptor transcript levels by quantitative PCR, we found increased PPARγ and LXRα levels (Fig. 1A and B). Strikingly, this was paralleled by an increase in transcript levels of CYP27 (Fig. 1C), the enzyme with the potential to produce a soluble cholesterol metabolite and a ligand for LXRα.
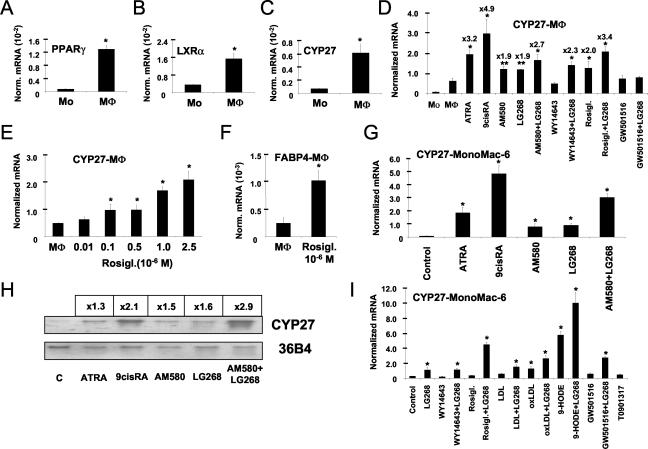
FIG. 1.
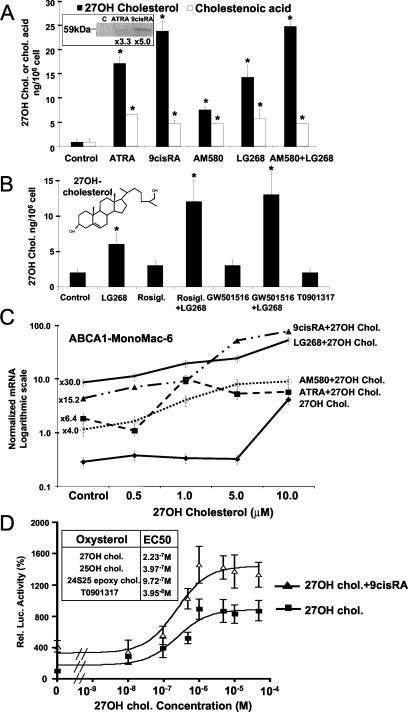
Regulated expression of CYP27 by retinoids and PPARγ activators. Total RNAs isolated from primary human monocytes (Mo) and macrophages (MΦ) or MonoMac-6 cells after 2 days in culture were used for real-time quantitative RT-PCR analysis as described in Materials and Methods. Absolute molecule numbers of PPARγ (A), LXRα (B), and CYP27 (C) were determined and normalized to cyclophilin levels. Every transcript was measured in triplicates. Primary human monocytes were treated with Rosiglitazone (D, E, and F) or MonoMac-6 cells (G, H, and I) and cultured in the presence of the indicated ligands or vehicle (ATRA. 1 μM; 9-cis-RA, 1 μM; AM580 [RARα agonist], 100 nM; LG268 [RXR agonist], 100 nM; WY14643 [PPARα agonist], 10 μM; Rosiglitazone [PPARγ agonist], 1 μM; GW501516 [PPARδ agonist], 1 μM; T0901317 [LXRα agonist], 1 μM). (D, E, F, G, and I) After 2 days, total RNA was isolated and real-time quantitative RT-PCR analysis was performed. The means of at least three independent measurements ± SD are presented. (H) Northern analysis was performed on total RNA isolated from MonoMac-6 cells as described in Materials and Methods. Membranes were probed with labeled CYP27 and 36B4 cDNA probe. *, P < 0.01; **, P < 0.05 (compared to the respective control value).
It was intriguing to speculate that there was a link between the well-established PPARγ-LXR signaling and induction of CYP27 in macrophages. Therefore, we decided to determine whether ligand activation of nuclear receptor pathways could activate CYP27 gene transcription. Using monocyte-derived macrophages and the monocytic leukemia cell line (MonoMac-6), we tested the effect of RAR-, RXR-, PPAR-, and LXR-specific ligands on the expression of CYP27. The results of Northern blot and quantitative RT-PCR analyses are presented in Fig. 1D to I. We found that retinoids and activators of PPARγ could induce the CYP27 transcript to high levels in human macrophages (Fig. 1D and E). To further substantiate the Rosiglitazone induction, a dose-response experiment was carried out (Fig. 1E) and showed that CYP27 is induced by Rosiglitazone in a dose-dependent manner similarly to the bone fide target gene for fatty acid binding protein (FABP4/aP2) (21) (Fig. 1F). In MonoMac-6 cells this represents the induction of a single transcript (∼2 kb) (Fig. 1H and 2A). Compounds with RAR selectivity (such as all-trans-retinoic acid [ATRA] and AM580) and also one with RXR selectivity (LG268) proved to be effective inducers, and we could detect an increased induction upon treatment with the PPARγ-specific Rosiglitazone in combination with the RXR-specific LG268 (Fig. 1G, H, and I). In MonoMac-6 cells the retinoid induction profile of CYP27 was very similar to that of macrophages (Fig. 1G). Among all of the PPARγ activators tested, interestingly, only oxidized LDL and its lipid component 9-hydroxyoctadecadienoic acid proved to be active on their own in MonoMac-6 cells (Fig. 1I). Rosiglitazone induced CYP27 mRNA expression only in combination with the RXR-selective LG268. This profile is very similar to the one reported by us on the regulation of CD36 in another myeloid leukemia cell line THP-1 (45). Significantly, neither PPARδ- nor LXR-selective ligands induced CYP27 in this cell type. PPARδ agonist showed an at least additive or synergistic response when combined with LG268 (Fig. 1I).
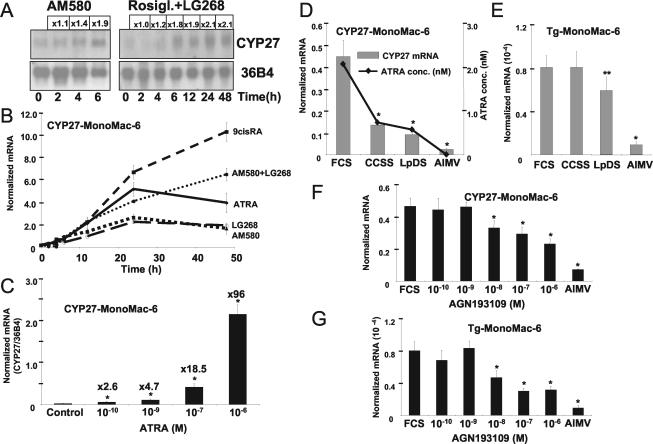
FIG. 2.
Regulation of CYP27 is dose and time dependent. (A) Northern analysis was performed on RNA isolated from retinoid and PPARγ agonist-treated MonoMac-6 cells. (B) Real-time quantitative PCR analysis, showing a detailed time course of CYP27 induction, on RNA isolated from MonoMac-6 cells treated as indicated. Cyclophilin D was used as a normalizer during quantitative PCR, unless indicated otherwise. (C) ATRA was used to show the dose dependence of CYP27 induction in MonoMac-6 cells. (D and E) MonoMac-6 cells were cultured in the indicated serum-containing medium for 2 days, and CYP27 (D) and transglutaminase (Tg) (E) mRNA levels (bars) were determined by quantitative RT-PCR. The ATRA concentrations (D) in the various sera were measured by HPLC as described in Materials and Methods. CCSS, charcoal-stripped medium; LpDS, lipoprotein-deficient medium. (F and G) MonoMac-6 cells cultured in fetal calf serum (FCS) were treated with the RAR inverse agonist AGN193109, and CYP27 (F) and transglutaminase (G) mRNA levels were determined by quantitative PCR and compared to those in AIMV. The means of at least three determinations ± SD are shown. *, P < 0.01; **, P < 0.05 (compared to the respective control value).
In order to gain insight into the mechanism of induction, we next wanted to test if this induction with PPARγ agonists and retinoids was acute or required prolonged exposure to the compounds. As shown in Fig. 2A, Northern blot analysis revealed that both retinoids (AM580 in this case) and the combination of PPARγ and RXR agonist induced an acute (after 2 to 6 h) induction of CYP27 transcription. These experiments strongly suggested that RAR-RXR and PPARγ-RXR heterodimers can transcriptionally regulate the human CYP27 gene. These observations were further substantiated by carrying out time course experiments with RAR- and RXR-selective compounds. As shown in Fig. 2B, both the RARα-selective AM580 and the RXR-selective LG268 induced CYP27 gene expression, while the combination of the two or the pan-agonist 9-cis-retinoic acid (9-cis-RA) proved to be more effective inducers of gene expression. The efficacy of ATRA-induced expression was between those of the receptor-selective compounds and the pan-agonists, indicating that perhaps some of ATRA is converted into pan- or RXR-agonist compounds during the course of the experiment.
In order to establish that the induction is dose dependent and induced with doses biologically relevant for receptor activation, dose responses experiments were carried out. As shown in Fig. 2C, ATRA induced CYP27 expression in a dose-dependent manner in the range of 0.1 nM to 1 μM. These experiments established that CYP27 is acutely regulated by retinoids in a dose- and time-dependent manner and that the doses required for activation were in the same range as the Kds of the compounds for the receptors. This raised the possibility that endogenous retinoids might regulate CYP27 expression also. We compared the expression levels of human CYP27 and a known retinoid receptor target gene, that for tissue transglutaminase (14, 33), in MonoMac-6 cells cultured in four different media (containing FBS, charcoal stripped, lipoprotein deficient, or serum free), in which we determined endogenous ATRA concentrations. We then plotted the mRNA levels of CYP27 along with ATRA levels (Fig. 2D), which showed a remarkable correlation. The more ATRA detectable in the serum, the more transcripts were detectable. A similar profile was obtained for tissue transglutaminase expression (Fig. 2E). RAR levels did not change under the conditions used (data not shown). Obviously, other differences between the different sera may exist; therefore, in order to obtain further evidence on the role of endogenous retinoids in the induction of CYP27, we used a well-characterized RAR antagonist-inverse agonist (AGN193109) to block activation through endogenous RARs. Increasing amount of antagonist decreased the expression level of CYP27 in serum (FBS)-containing medium by 50 to 60% (Fig. 2F). Similarly, tissue transglutaminase expression was also reduced (Fig. 2G). These two pieces of evidence did not prove but strongly suggested that normal levels of endogenous retinoids in serum are likely to contribute to the regulation of CYP27 in myeloid cells and established human CYP27 as a gene regulated by endogenous levels of retinoids.
RAR-RXR and PPARγ-RXR heterodimers bind to and activate the human CYP27 promoter.
Our findings prompted us to look at the previously defined promoter region of human CYP27 (18). Using an 853-bp fragment, we carried out transient-transfection experiments examining whether receptor heterodimers can activate this promoter. Cotransfection of RAR and RXR and addition of 9-cis-RA induced a more than a fivefold increase in promoter activity (Fig. 3A). Similarly, cotransfection of PPARγ and RXR in the presence of both PPARγ- and RXR-specific ligands increased transcriptional activity (Fig. 3A and data not shown). These results indicated that the 853-bp fragment contained all of the necessary information to mediate both retinoid- and PPARγ-dependent regulation. To define the response elements, we looked at various deletion mutants of the 853-bp fragment (a 649-bp and a 217-bp fragment) and found that the detected induction was approximately half of that for the 853-bp promoter fragment (Fig. 3B) when a shorter fragment (649 bp) was used. An additional deletion (217-bp fragment) led to retained core promoter activity and to the complete loss of inducibility. These experiments suggested that transcriptional regulation takes place on the human CYP27 promoter by RAR-RXR and PPARγ-RXR heterodimers and raised the possibility that more than one response element may be localized between bp −853 and −217. In silico analysis of the promoter identified two regions with potential binding sites for RAR-RXR and PPARγ-RXR heterodimers. We termed these PPAR retinoid response elements (PRREs) A and B. The A element contained an unusual arrangement of binding sites, with three binding sites arranged in an overlapping DR1-DR5 configuration (i.e., two direct repeats sharing a half site) (28, 34, 38, 46). The B element looked simpler; it was a direct repeat separated by one nucleotide (DR1).
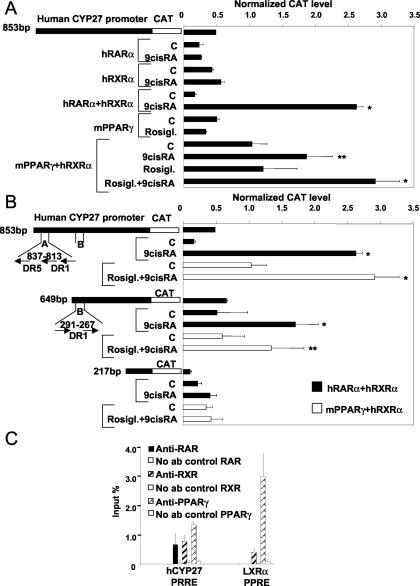
FIG. 3.
The promoter of the human CYP27 gene is a direct target for complex regulation by RAR-RXR and PPARγ-RXR heterodimers. (A and B) CV-1 cells were cotransfected with hCYP27-853-pCAT, hCYP27-649-pCAT, hCYP27-217-pCAT, the indicated receptors, and β-galactosidase and treated with the receptor agonists 9-cis-RA (1 μM), Rosiglitazone (1 μM), both, or vehicle for 48 h. Cells were lysed, and the CAT level and β-galactosidase activity were assayed as described in Materials and Methods. CAT levels were normalized to β-galactosidase activity. All experiments were done in triplicates. The means of at least three determinations ± SD are shown. (C) Chromatin immunoprecipitation was performed with anti-RAR, anti-RXR, and anti-PPARγ antibodies (ab), and the DNA content was determined with two quantitative PCR assays specific for PRRE-B. DNA precipitated with anti-RXR and anti-PPARγ antibodies was analyzed by a human LXRα PPAR response element (PPRE)-specific assay. The results are shown as percentages of input DNA. All measurements were done in triplicate. All chromatin results were verified from independent chromatin preparations. *, P < 0.01; **, P < 0.05 (compared to the respective control [C] value).
Before embarking on a detailed promoter analysis, we wanted to see whether the promoter was transcriptionally active in myeloid cells and whether endogenous levels of receptors were bound to the identified elements. We first carried out chromatin immunoprecipitation experiments to assess the acetylation status of histone 4 lysines. This would provide direct evidence for promoter activation. Both 9-cis-RA and the combination of LG268 and Rosiglitazone induced significant acetylation of the promoter (data not shown). Next, we used receptor-specific antibodies to immunoprecipitate chromatin with RARα, RXRα, and PPARγ from ligand-treated MonoMac6 cells. TaqMan probes were designed for PRRE-A and PRRE-B, and the LXRα PPAR response element as a positive control, to quantitate reliably immunoprecipitated genomic DNA. Both elements could be precipitated with the PPARγ antibody, while the RARα and RXR antibody readily immunoprecipitated the B element and to a much lesser degree immunoprecipitated the A element. The LXR-PRRE was precipitated with the anti-RXR and anti-PPARγ antibodies. Collectively, the transfection and immunoprecipitation data established that the identified region of the promoter was transcriptionally active and that the B element could be important in the regulation of the gene, while the A element might have an accessory role. Therefore, we continued with the characterization of the B element.
Characterization of a response element mediating retinoid and PPARγ signaling.
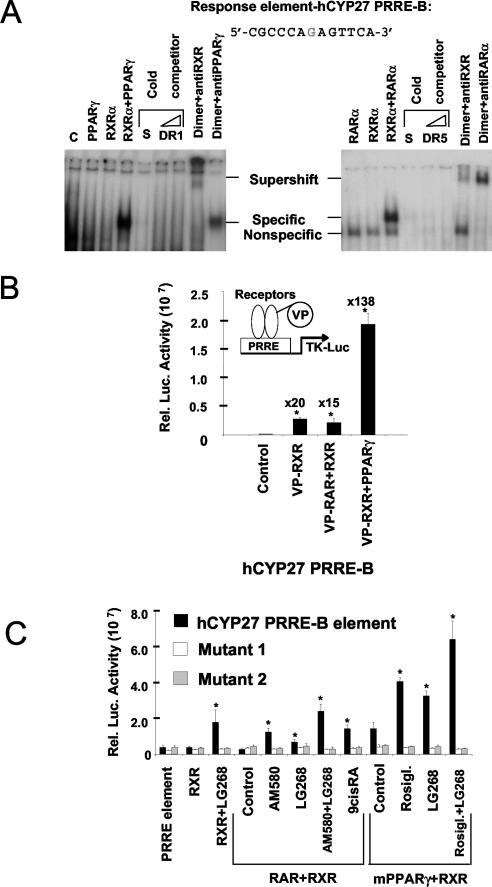
In order to test whether the identified element could indeed bind receptor heterodimers in vitro, we carried out electrophoretic mobility shift assays. Figure 4A shows that the major element (hCYP27-PRRE-B) has a DR1 arrangement. Electrophoretic mobility shift assay analysis revealed that this DR1 is able to mediate both PPARγ-RXR and RAR-RXR binding (Fig. 4A). Neither PPARγ nor RXR alone was able to bind to the labeled oligonucleotide; the two receptors together show strong specific binding, which can be effectively competed by either unlabeled self or a canonical DR1 oligonucleotide (Fig. 4A, left panel). The specificity of binding was demonstrated by the use of antibodies against RXR and PPARγ, inducing supershifts or reduced binding, respectively (Fig. 4A). A similar set of experiments was carried out with RAR-RXR heterodimers. As shown in Fig. 4A (right panel) RXR-RAR heterodimers can bind to hCYP27-PRRE and this binding can be competed by self and canonical DR5 elements. RAR-RXR and PPARγ-RXR can bind to the same element utilizing a DR1. We have established a transfection-based assay for the determination of receptor binding independent of transactivation in CV-1 cells, in which the transfection experiments were carried out. By using VP16 fusion receptors (ligand mimic receptors) and transient transfection, we could show a strong and robust binding of PRRE-B with VP-RXR and with VP-RXR and PPARγ (Fig. 4B). To prove that the identified response element is a functional enhancer, enhancer trap vectors were constructed by fusing two copies of the element to a minimal TK promoter and a luciferase reporter gene. Cotransfection analysis revealed that the identified element conferred both RAR-RXR and PPARγ-RXR heterodimer responsiveness (Fig. 4C). Similar experiments carried out with mutations in either of the two half-sites resulted in a complete loss of induced transcription.
FIG. 4.
Identification of RXR-RAR and RXR-PPARγ binding sites in the human CYP27 promoter. (A) Electrophoretic mobility shift analysis for hCYP27-PRRE-B was performed by using in vitro-translated receptors of mPPARγ and hRXRα (left panel) and hRARα and hRXRα (right panel) and α32P-labeled oligonucleotides in the absence or presence of the indicated ligands (Rosiglitazone [1 μM] or 9-cis-RA [1 μM]) as described in Materials and Methods. For competition experiments, cold hCYP27-PRRE-B (self [S]), consensus DR1, or DR5 was used at ×10 and ×20 concentrations. For supershift experiments, the receptors were preincubated with the indicated antibodies prior to the binding reaction. (B) The response element was analyzed in a cell-based binding assay. The indicated VP fusion nuclear receptors were cotransfected with the response element containing a luciferase reporter plasmid, and the normalized reporter activity ± SD are shown. (C) Two copies of hCYP27-PRRE-B and half-site mutants were cloned upstream from the TK-Luc reporter. They were cotransfected into CV-1 cells with the indicated receptors and β-galactosidase and treated with the indicated ligands: AM580 (100 nM), Rosiglitazone (1 μM), LG268 (100 nM), or vehicle. Luciferase activity was normalized to β-galactosidase activity. *, P < 0.01 compared to the respective control value.
We have also analyzed the other in silico-found response element (hCYP27-PRRE-A). There appears to be a discrepancy between the in vitro binding and the apparent enhancer activity of this element, because it shows strong in vitro binding but only weak enhancer activity (data not shown). Therefore, the role of the A element may be cell type specific or, more likely, part of a more complex enhancer interaction in the context of the full promoter. Based on these data, the B element (hCYP27-PRRE-B) appeared to be critical in mediating transcriptional activation, with a potentially minor contribution of a weak, accessory element (hCYP27-PRRE-A). Identification and analysis of this element provided evidence for direct regulation and a potentially complex interplay between retinoid and PPARγ signaling on the human CYP27 promoter.
Retinoid- and PPARγ-induced CYP27 expression results in 27-hydroxycholesterol formation and efflux.
Having established that the human CYP27 gene is transcriptionally regulated by two nuclear receptor heterodimers (RAR-RXR and PPARγ-RXR), we next wanted to see what the biological consequences of this regulation were. We carried out Western blot analysis with MonoMac-6 cells treated with natural retinoids (ATRA or 9-cis-RA). Protein expression was increased, as demonstrated by the appearance of the expected single 59-kDa band (Fig. 5A, inset). In order to establish that the protein is functional, we carried out mass spectrometric determination of the metabolic products of CYP27 from cell pellets and medium supernatants. The determined compounds were 27-hydroxycholesterol and 3β-hydroxy-5-cholestenoic acid. MonoMac-6 cells were treated with the indicated nuclear receptor ligand and simultaneously loaded with cholesterol. After 2 days, 27-hydroxycholesterol and 3β-hydroxy-5-cholestenoic acid levels in the supernatant and cell pellet were determined. As shown in Fig. 5A and B, treatment with the naturally occurring retinoids ATRA and 9-cis-RA as well as a synthetic ligand for RARα (AM580) or RXR (LG268) induced 27-hydroxycholesterol formation and release to the medium (data not shown). Combinations of RAR and RXR ligands, i.e., PPARγ and RXR or PPARδ and RXR ligands, also induced this effect. Neither Rosiglitazone nor a synthetic PPARδ ligand (GW501516) was able to induce 27-hydroxycholesterol formation on its own. Ligands for LXR alone or in combination with RXR ligands failed to induce it also. In molar terms, the supernatant contained 20 to 50 nM 27-hydroxycholesterol, while the highest intracellular levels deduced from the pellets were approximately 12 to 20 μM. It is unsettled whether high levels of 27-hydroxycholesterol are able to activate LXR-RXR heterodimers to sufficiently high levels.
FIG. 5.
Induction of CYP27 is accompanied by increased enzyme activity that leads to production of 27-hydroxycholesterol. (A) MonoMac-6 cells were treated with water-soluble cholesterol (100 μg/ml) and the indicated ligands for 2 days in RPMI supplemented with insulin-transferrin-sodium selenite medium supplement. 27-Hydroxyxholesterol and 3β-hydroxy-5-cholestenoic acid contents in the cell pellet were determined and normalized to cell number as described in Materials and Methods. The inset shows Western blot analysis of CYP27 protein levels in MonoMac-6 cells treated with ATRA (1 μM), 9-cis-RA (1 μM), or vehicle (for details, see Materials and Methods). (B) As for panel A, MonoMac-6 cells were cholesterol loaded and treated with ligands or vehicle as indicated, and 27-hydroxycholesterol was determined. (C) MonoMac-6 cells were treated with increasing amounts of 27-hydroxycholesterol, and a dose-response curve was determined. To analyze synergy, cells were treated with ATRA (1 μM), 9-cis-RA (1 μM), AM580 (100 nM), or LG268 (100 nM) and 27-hydroxycholesterol simultaneously. Transcript levels of ABCA1 were measured by real-time quantitative RT PCR and normalized to cyclophilin levels. The results are shown on a logarithmic scale. (D) CV-1 cells were cotransfected with Gal-hLXRα-LBD, hRXRα-LDB, pMH100-TK-Luc, and β-galactosidase plasmids and treated with LXR activators 25-hydroxycholesterol, 27-hydroxycholesterol, and 24(S)25-epoxycholesterol at the indicated concentrations in the absence or presence of 9-cis-RA (1 μM). Luciferase activity was normalized to β-galactosidase activity, and EC50s values were calculated from the dose-response curves (inset). *, P < 0.01 compared to the respective control value.
The fact that retinoids were able to induce the enzyme's expression and also to synergize with the enzyme's product offered a testable hypothesis. It is possible that under some conditions both retinoids and 27-hydroxycholesterol are required for full activation of LXR-RXR heterodimers. We tested this hypothesis by looking at LXR-dependent gene expression induced by retinoids and 27-hydroxycholesterol. As shown in Fig. 5C, LG268 and 9-cis-RA synergize with 27-hydroxycholesterol to induce gene expression to a very significant degree. Note that 27-hydroxycholesterol at 10 μM was able to induce a 14-fold induction, while if it was combined with 9-cis-RA, the induction was further induced by an additional 19-fold, resulting in a nearly 300-fold induction altogether. These data suggest that retinoids via the induction of a partial agonist of LXR gain competence to induce robust LXR-dependent transcription. This notion was further substantiated by determining the 50% effective concentrations (EC50s) for 27-hydroxycholesterol alone and in combination with 9-cis-RA in better-defined transient-transfection experiments. Figure 5D shows that 27-hydroxycholesterol is able to induce LXR-dependent gene expression with an EC50 of 2 × 10−7 M and that addition of 9-cis-RA increased the efficacy of the response. EC50s of other oxysterols were also determined (Fig. 5D, inset) for reference in the same assay. These fall in the same range as that of 27-hydroxycholesterol.
Retinoids induce LXR-mediated gene expression, which involves activation of CYP27.
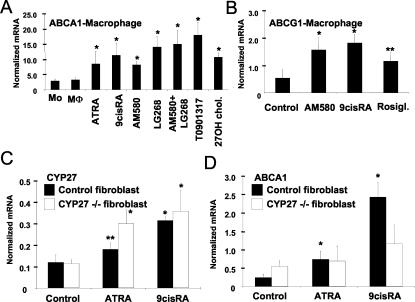
After having established that retinoids and PPARγ ligands induce CYP27 expression and that this leads to the formation of 27-hydroxycholesterol in biologically relevant quantities, we sought to understand the biological consequence of this induction. One of the key biological consequences of the pathway described, one may predict, is the induction of LXR responses. To address this issue, we treated human monocyte-derived macrophages with various RAR, RXR, and PPARγ ligands and determined the expression levels of key LXR target genes, ABCA1 and ABCG1. As shown in Fig. 6A and B, retinoids and LXR ligand induced ABCA1 and ABCG1 to various degrees. The induction of ABCA1 and ABCG1 by RXR or pan-retinoid agonist ligands is not unexpected, since they are believed to regulate LXR-RXR heterodimers from the RXR side, but the RAR-selective ligand AM580 could also induce ABCA1 and ABCG1 (Fig. 6A and B). Combinations of RAR and RXR ligands were slightly better than the RXR ligand alone. Our findings therefore suggest that retinoid induction of LXR target genes could involve activation of CYP27 and production of 27-hydroxycholesterol. To obtain genetic evidence for the role of CYP27 in the described regulatory network and its biological relevance, we used primary cell lines derived from patients with CYP27 deficiency (CTX). We could demonstrate that retinoids induced CYP27 expression not only in myeloid cells but also in fibroblasts (Fig. 6C) and that retinoid-induced ABCA1 expression is strongly attenuated in the absence of CYP27 (in CTX fibroblasts) (Fig. 6D).
FIG. 6.
Retinoid-induced CYP27 promotes an LXR response. Primary human monocytes were isolated and treated with the indicated retinoids. Total RNA was isolated, and ABCA1 (A) and ABCG1 (B) mRNA levels were determined by real-time quantitative RT-PCR. (C and D) Primary human fibroblasts isolated from healthy adults and patients with CTX (CYP27−/−) were treated with the indicated ligands for 2 days. Total RNA was isolated, and CYP27 (C) and ABCA1 (D) mRNA levels were determined by real-time quantitative RT-PCR. *, P < 0.01; **, P < 0.05 (compared to the respective control value).
Evidence for retinoid- and PPARγ-regulated gene expression in human atherosclerotic lesions.
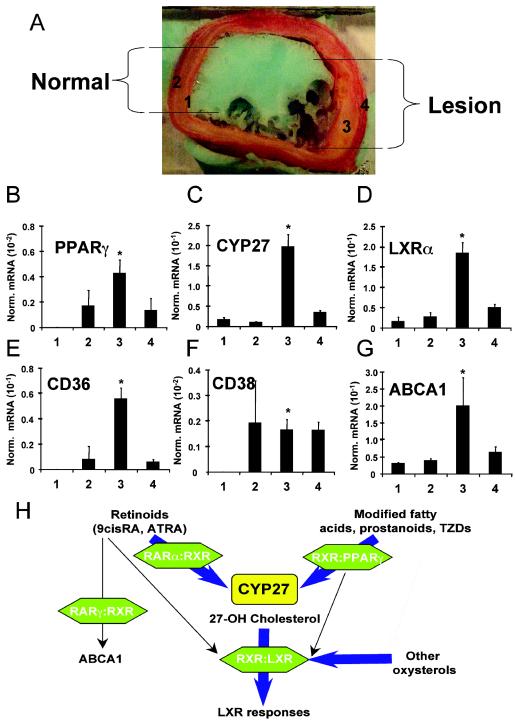
In order to obtain evidence for the physiological relevance and disease relatedness of the described pathway in atherosclerosis, a disease linked to macrophage cholesterol metabolism, we analyzed the gene expression profile of human macrophage-rich atherosclerotic lesions. mRNA levels of LXRα, PPARγ, and their relevant target genes ABCA1, CD36, and CYP27 were determined from lesions and lesion-free intima and vessel wall tissue, along with transcript levels of enzymes responsible for the production of ATRA retinaldehyde dehydrogenase (RALDH2) and a marker gene for retinoid action, CD38. Lesions were defined as abnormal fatty tissue on the luminar side of the internal elastic membrane of arteries. As shown in Fig. 7, in human lesions remarkable increases of PPARγ (Fig. 7B), LXRα (Fig. 7D), and their target genes ABCA1 (Fig. 7G), CD36 (Fig. 7E), and CYP27 (Fig. 7C) were detected, compared to those in normal vessel wall, along with high expression of RALDH2 and CD38 (Fig. 7F and data not shown). These data suggest that lesion tissue (mostly macrophages) has an expression profile similar to that of in vitro differentiated macrophages showing retinoid-, PPARγ-, and LXR-regulated transcription, including increased CYP27 expression. These data collectively provide a very strong link and correlation between retinoid, PPARγ, and LXR signaling in the context of human macrophage-rich atherosclerotic lesions and also establish CYP27 as a potentially key mediator of this interrelated signaling network.
FIG. 7.
Elements of the retinoid-PPARγ-CYP27-LXRα pathway are present in human macrophage-rich atherosclerotic lesions. (A) A human femoral artery containing a macrophage-rich fatty lesion was dissected, and total RNA was extracted from the indicated parts. 1, normal intima; 2, normal media and adventitia; 3, intima of atherosclerotic lesion; 4, media and adventitia of atherosclerotic lesion. (B to G) Transcript levels of the indicated genes were measured by real-time PCR and normalized to cyclophilin levels. Three similar lesions from various individuals were analyzed with essentially the same results. Results from a representative experiment are presented. *, P < 0.01 compared to the respective control value. (H) Integration of retinoid, PPAR, and LXR signaling via CYP27, leading to enhanced LXR-dependent and -independent cholesterol efflux. TZD, thiazolidinedione.
DISCUSSION
We have identified the gene for a p450 enzyme, CYP27, as a gene commonly induced during monocyte-macrophage transition and as a PPARγ-RXR- and RAR-RXR-regulated gene in myeloid cells. Promoter analysis revealed complex regulation by retinoid receptors and PPARs via a response element on the promoter of human CYP27, further underscoring the interrelatedness of these pathways. These findings tie retinoid, PPAR, and LXR signaling into one regulatory network requiring natural ligands: retinoids and modified fatty acids or prostanoids to activate an entire metabolic pathway and leading to coordinate regulation of lipid and cholesterol uptake, metabolism, and efflux (Fig. 7H). Furthermore, we provided evidence that all components of the described pathways exist in human atherosclerotic lesions.
Applying synthetic ligands to biological systems allows the identification of pathways attributable to the receptors. This proved to be a very fruitful approach to identify biological processes activated by the receptors, but it also overrides the need for the identification of natural ligands and, more importantly, sources and regulation of natural ligand production. This approach may also represent superphysiological and ectopic activation of receptors. This is a particular concern in cases of the metabolite receptors (PPARs, LXRs, farnesoid X receptor, and pregnane X receptor), for which only low-affinity natural ligands have been identified so far, because activation with low-affinity (partial agonist) ligands may lead to a biological outcome different from that for activation with a synthetic full agonist. Therefore, the processes and enzymes leading to endogenous ligand production and their regulation should be also considered when assigning biological functions to receptors. This line of argument led us to search for mechanisms linking PPARγ-RXR and LXR-RXR signaling pathways. In the case of LXR, several low-affinity oxysterols [22(R)-cholesterol, 20(S)-cholesterol, and 24-, 25- and 27-hydoxycholesterol] have been identified as endogenous ligands (17, 24, 25, 31, 36), but none of them has an affinity higher than 10 μM. It is important to note that among these oxysterols 27-hydroxycholesterol is present at the highest concentration in the circulation (15), suggesting an in vivo relevance in activation of LXR. We identified CYP27 as a nuclear receptor-regulated enzyme that is capable of producing endogenous ligands for LXR. Moreover, pan-agonists or RXR-activating retinoids can contribute to receptor activation, resulting in a robust synergistic response between retinoids and oxysterols. The linkage of regulated enzyme expression and heterodimer activation suggests that partial agonists such as 27-hydroxycholesterol can contribute to full activation in the presence of the appropriate retinoid. This adds an additional layer of control to the receptor's activity by the regulation of the production of two endogenous lipid molecules.
CYP27 is an attractive target for regulated transcription because its product is an alternative bile acid synthesis precursor in the liver. Identification of a complex PPAR-RAR regulation and the fact that retinoids present in the serum regulate the basal expression level of the enzyme in myeloid cells are significant novel aspects of the enzyme's regulation. The product generated, 27-hydroxycholesterol, is a polar compound capable of transversing membranes and therefore provides an alternative cholesterol efflux mechanism (3, 5, 16). The fact that 27-hydroxycholesterol is an endogenous ligand of LXR has been noted previously (17, 25). It was even suggested that 27-hydroxycholesterol is a partial agonist of LXR-RXR heterodimers (17). Our results are in agreement with this assessment, but they go further and show that ligand production can be regulated and that, in combination with retinoids, 27-hydroxycholesterol becomes a full agonist on LXR-regulated target genes. The fact that retinoids (RAR- and RXR-selective compounds) and combinations of PPAR- and RXR-selective compounds were able to induce CYP27 expression suggested that there is a cross talk between retinoid and PPAR signaling. This became apparent when the promoter analysis revealed the two enhancers mediating the effects. Further studies identified PRRE-B as the major element and regulator of human CYP27 gene transcription, while PRRE-A might serve as an accessory site required for full activation. Further studies are needed to define the relationship between the two elements and the two heterodimers (RAR-RXR and PPARγ-RXR) binding to them. The chromatin immunoprecipitation assays suggest two possibilities: one is that both heterodimers could bind in one complex, and the other is that there is heterogeneity between the cells and that some bind one heterodimer and some bind the other heterodimer. It may also be possible that the two elements and two heterodimers are part of a larger complex (i.e., an enhancesome). Traditional promoter-enhancer analysis is not sufficient to dissect such complex relationships. Another interesting aspect of the cross talk between retinoid and PPAR signaling is the fact that PPAR ligands are active in regulating CYP27 expression only if rexinoids are present in some of the cell lines used. We termed this the retinoid-enabled PPAR response. This phenomenon can be observed in the monocytic leukemia cell line MonoMac-6, and a similar observation was also made during the transient-transfection-based analysis of the promoter. This is in line with our initial observations on oxidized LDL uptake regulated by PPARγ-RXR heterodimers (35). At this point it is not clear whether the identified elements are solely responsible for this effect or whether other factors contribute to it.
Regulated expression of CYP27 is also of interest because CYP27 has a key metabolic function, converting cholesterol into a more polar compound, 27-hydroxycholesterol. Consequently, it has two major effects on cholesterol efflux. It induces ABC transporter expression and subsequent high-density lipoprotein-dependent efflux via the activation of LXR-RXR. 27-Hydroxycholesterol also represents an alternative cholesterol efflux pathway from macrophages that is independent of known transporters, including ABCs and high-density lipoprotein, and allows converted cholesterol to leave the cells and be cleared by the liver as bile acids. It is estimated that under steady-state conditions it may represent as much as 10 to 20% of total cholesterol efflux, as was shown by Babiker et al. (2). Our data suggest that this efflux in macrophages may be regulated by retinoid- and PPARγ-mediated induction of CYP27. It is also apparent that besides cholesterol efflux, LXR-RXR heterodimers are capable of inducing multiple other pathways involved in lipid metabolism, such as SREBP1c induction (39, 49) or the induction of phospholipid transport protein (29), and that they have a more global effect on macrophage lipid homeostasis. As far as the physiological relevance of the pathway is concerned, due to the species specificity of this regulation (it exists in human but not in mouse) and the lack of suitable mouse models, we had to rely on approaches involving gathering of data from CTX (CYP27−/−) human fibroblasts and atherosclerotic lesions of humans. The evidence from these approaches clearly demonstrates that CYP27 is required, at least in part, for retinoid and PPARγ ligand-induced LXR-mediated gene expression and potentially for cholesterol efflux. The striking similarity between gene expression patterns, including retinoid-, PPARγ-, and LXR-regulated gene expression as well as high levels of CYP27, in in vitro-differentiated macrophages and in tissue samples of macrophage-rich atherosclerotic lesions also underscores that this regulatory network is likely to have physiological and disease relevance. A recent study by Costet et al. (12) suggested that ABCA1 and cholesterol efflux can be regulated directly by retinoids via an RARγ-mediated pathway. Our data presented here show that RARα activation has a broader effect on human macrophage cholesterol metabolism, as those authors suggested. We suggest that retinoid-regulated CYP27 is likely to act as a modulator of a robust LXR response. In our view, the identified pathway also represents a potential new target for the regulation of macrophage cholesterol efflux and for the management of diseases with increased foam cell formation and cholesterol overload. It also suggests that retinoids may have a more profound effect on lipid metabolism than previously suspected. This notion is further underscored by the observation that RXR agonists have a atheroprotective effects in ApoE−/− mice (11). Clearly, more work needs to be done before regulation of CYP27 can be considered a valid target for pharmacological intervention in these conditions.
Acknowledgments
We thank F. J. Schweigert for the use of HPLC equipment for retinoid analysis at the Institute of Nutritional Science, University of Potsdam, Potsdam, Germany. We acknowledge the excellent technical assistance of Marta Beladi.
This work was supported by grants from the HFSP, an RTN from the EU FP5 (to L.N.), a Research Award from the Boehringer Ingelheim Fund (to L.N.), a grant from the Swedish Heart Lung Foundation (to U.D.), and a grant from the Hungarian Scientific Research Fund (T034434) (to L.N.). L.N. is an International Scholar of HHMI and an EMBO Young Investigator.
REFERENCES
- 1.Andersson, S., D. L. Davis, H. Dahlback, H. Jornvall, and D. W. Russell. 1989. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 264:8222-8229. [PubMed] [Google Scholar]
- 2.Babiker, A., O. Andersson, D. Lindblom, J. van der Linden, B. Wiklund, D. Lutjohann, U. Diczfalusy, and I. Bjorkhem. 1999. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J. Lipid Res. 40:1417-1425. [PubMed] [Google Scholar]
- 3.Babiker, A., O. Andersson, E. Lund, R. J. Xiu, S. Deeb, A. Reshef, E. Leitersdorf, U. Diczfalusy, and I. Bjorkhem. 1997. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J. Biol. Chem. 272:26253-26261. [DOI] [PubMed] [Google Scholar]
- 4.Benko, S., J. D. Love, M. Beladi, J. W. Schwabe, and L. Nagy. 2003. Molecular determinants of the balance between co-repressor and co-activator recruitment to the retinoic acid receptor. J. Biol. Chem. 278:43797-43806. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkhem, I., O. Andersson, U. Diczfalusy, B. Sevastik, R. J. Xiu, C. Duan, and E. Lund. 1994. Atherosclerosis and sterol 27-hydroxylase: evidence for a role of this enzyme in elimination of cholesterol from human macrophages. Proc. Natl. Acad. Sci. USA 91:8592-8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bjorkhem, I., and E. Leitersdorf. 2000. Sterol 27-hydroxylase deficiency: a rare cause of xanthomas in normocholesterolemic humans. Trends Endocrinol. Metab. 11:180-183. [DOI] [PubMed] [Google Scholar]
- 7.Cali, J. J., C. L. Hsieh, U. Francke, and D. W. Russell. 1991. Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis. J. Biol. Chem. 266:7779-7783. [PMC free article] [PubMed] [Google Scholar]
- 8.Cali, J. J., and D. W. Russell. 1991. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J. Biol. Chem. 266:7774-7778. [PubMed] [Google Scholar]
- 9.Chawla, A., W. A. Boisvert, C. H. Lee, B. A. Laffitte, Y. Barak, S. B. Joseph, D. Liao, L. Nagy, P. A. Edwards, L. K. Curtiss, R. M. Evans, and P. Tontonoz. 2001. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 7:161-171. [DOI] [PubMed] [Google Scholar]
- 10.Chen, J. D., and R. M. Evans. 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454-457. [DOI] [PubMed] [Google Scholar]
- 11.Claudel, T., M. D. Leibowitz, C. Fievet, A. Tailleux, B. Wagner, J. J. Repa, G. Torpier, J. M. Lobaccaro, J. R. Paterniti, D. J. Mangelsdorf, R. A. Heyman, and J. Auwerx. 2001. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc. Natl. Acad. Sci. USA 98:2610-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costet, P., F. Lalanne, M. C. Gerbod-Giannone, J. R. Molina, X. Fu, E. G. Lund, L. J. Gudas, and A. R. Tall. 2003. Retinoic acid receptor-mediated induction of ABCA1 in macrophages. Mol. Cell. Biol. 23:7756-7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crisby, M., J. Nilsson, V. Kostulas, I. Bjorkhem, and U. Diczfalusy. 1997. Localization of sterol 27-hydroxylase immuno-reactivity in human atherosclerotic plaques. Biochim. Biophys. Acta 1344:278-285. [DOI] [PubMed] [Google Scholar]
- 14.Davies, P. J., M. P. Murtaugh, W. T. Moore, Jr., G. S. Johnson, and D. Lucas. 1985. Retinoic acid-induced expression of tissue transglutaminase in human promyelocytic leukemia (HL-60) cells. J. Biol. Chem. 260:5166-5174. [PubMed] [Google Scholar]
- 15.Dzeletovic, S., O. Breuer, E. Lund, and U. Diczfalusy. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225:73-80. [DOI] [PubMed] [Google Scholar]
- 16.Escher, G., Z. Krozowski, K. D. Croft, and D. Sviridov. 2003. Expression of sterol 27-hydroxylase (CYP27A1) enhances cholesterol efflux. J. Biol. Chem. 278:11015-11019. [DOI] [PubMed] [Google Scholar]
- 17.Fu, X., J. G. Menke, Y. Chen, G. Zhou, K. L. MacNaul, S. D. Wright, C. P. Sparrow, and E. G. Lund. 2001. 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 276:38378-38387. [DOI] [PubMed] [Google Scholar]
- 18.Garuti, R., M. A. Croce, L. Piccinini, R. Tiozzo, S. Bertolini, and S. Calandra. 2002. Functional analysis of the promoter of human sterol 27-hydroxylase gene in HepG2 cells. Gene 283:133-143. [DOI] [PubMed] [Google Scholar]
- 19.Garuti, R., N. Lelli, M. Barozzini, R. Tiozzo, M. T. Dotti, A. Federico, A. M. Ottomano, A. Croce, S. Bertolini, and S. Calandra. 1996. Cerebrotendinous xanthomatosis caused by two new mutations of the sterol-27-hydroxylase gene that disrupt mRNA splicing. J. Lipid Res. 37:1459-1467. [PubMed] [Google Scholar]
- 20.Glass, C. K., and J. L. Witztum. 2001. Atherosclerosis: the road ahead. Cell 104:503-516. [DOI] [PubMed] [Google Scholar]
- 21.Graves, R. A., P. Tontonoz, and B. M. Spiegelman. 1992. Analysis of a tissue-specific enhancer: ARF6 regulates adipogenic gene expression. Mol. Cell. Biol. 12:1202-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansson, M., E. Ellis, M. C. Hunt, G. Schmitz, and A. Babiker. 2003. Marked induction of sterol 27-hydroxylase activity and mRNA levels during differentiation of human cultured monocytes into macrophages. Biochim. Biophys. Acta 1593:283-289. [DOI] [PubMed] [Google Scholar]
- 23.Hulten, L. M., H. Lindmark, U. Diczfalusy, I. Bjorkhem, M. Ottosson, Y. Liu, G. Bondjers, and O. Wiklund. 1996. Oxysterols present in atherosclerotic tissue decrease the expression of lipoprotein lipase messenger RNA in human monocyte-derived macrophages. J. Clin. Investig. 97:461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janowski, B. A., M. J. Grogan, S. A. Jones, G. B. Wisely, S. A. Kliewer, E. J. Corey, and D. J. Mangelsdorf. 1999. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA 96:266-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janowski, B. A., P. J. Willy, T. R. Devi, J. R. Falck, and D. J. Mangelsdorf. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383:728-731. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy, M. A., A. Venkateswaran, P. T. Tarr, I. Xenarios, J. Kudoh, N. Shimizu, and P. A. Edwards. 2001. Characterization of the human ABCG1 gene: liver X receptor activates an internal promoter that produces a novel transcript encoding an alternative form of the protein. J. Biol. Chem. 276:39438-39447. [DOI] [PubMed] [Google Scholar]
- 27.Kuo, M. H., and C. D. Allis. 1999. In vivo cross-linking and immunoprecipitation for studying dynamic protein:DNA associations in a chromatin environment. Methods 19:425-433. [DOI] [PubMed] [Google Scholar]
- 28.Kurokawa, R., J. DiRenzo, M. Boehm, J. Sugarman, B. Gloss, M. G. Rosenfeld, R. A. Heyman, and C. K. Glass. 1994. Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature 371:528-531. [DOI] [PubMed] [Google Scholar]
- 29.Laffitte, B. A., S. B. Joseph, M. Chen, A. Castrillo, J. Repa, D. Wilpitz, D. Mangelsdorf, and P. Tontonoz. 2003. The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions. Mol. Cell. Biol. 23:2182-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laffitte, B. A., S. B. Joseph, R. Walczak, L. Pei, D. C. Wilpitz, J. L. Collins, and P. Tontonoz. 2001. Autoregulation of the human liver X receptor alpha promoter. Mol. Cell. Biol. 21:7558-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann, J. M., S. A. Kliewer, L. B. Moore, T. A. Smith-Oliver, B. B. Oliver, J. L. Su, S. S. Sundseth, D. A. Winegar, D. E. Blanchard, T. A. Spencer, and T. M. Willson. 1997. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272:3137-3140. [DOI] [PubMed] [Google Scholar]
- 32.Lusis, A. J. 2000. Atherosclerosis. Nature 407:233-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghadasian, M. H., G. Salen, J. J. Frohlich, and C. H. Scudamore. 2002. Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch. Neurol. 59:527-529. [DOI] [PubMed] [Google Scholar]
- 34.Nagy, L., M. Saydak, N. Shipley, S. Lu, J. P. Basilion, Z. H. Yan, P. Syka, R. A. Chandraratna, J. P. Stein, R. A. Heyman, and P. J. Davies. 1996. Identification and characterization of a versatile retinoid response element (retinoic acid receptor response element-retinoid X receptor response element) in the mouse tissue transglutaminase gene promoter. J. Biol. Chem. 271:4355-4365. [DOI] [PubMed] [Google Scholar]
- 35.Nagy, L., P. Tontonoz, J. G. Alvarez, H. Chen, and R. M. Evans. 1998. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93:229-240. [DOI] [PubMed] [Google Scholar]
- 36.Peet, D. J., B. A. Janowski, and D. J. Mangelsdorf. 1998. The LXRs: a new class of oxysterol receptors. Curr. Opin. Genet. Dev. 8:571-575. [DOI] [PubMed] [Google Scholar]
- 37.Pikuleva, I. A., A. Babiker, M. R. Waterman, and I. Bjorkhem. 1998. Activities of recombinant human cytochrome P450c27 (CYP27) which produce intermediates of alternative bile acid biosynthetic pathways. J. Biol. Chem. 273:18153-18160. [DOI] [PubMed] [Google Scholar]
- 38.Rastinejad, F., T. Wagner, Q. Zhao, and S. Khorasanizadeh. 2000. Structure of the RXR-RAR DNA-binding complex on the retinoic acid response element DR1. EMBO J. 19:1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Repa, J. J., G. Liang, J. Ou, Y. Bashmakov, J. M. Lobaccaro, I. Shimomura, B. Shan, M. S. Brown, J. L. Goldstein, and D. J. Mangelsdorf. 2000. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 14:2819-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Repa, J. J., S. D. Turley, J. A. Lobaccaro, J. Medina, L. Li, K. Lustig, B. Shan, R. A. Heyman, J. M. Dietschy, and D. J. Mangelsdorf. 2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289:1524-1529. [DOI] [PubMed] [Google Scholar]
- 41.Ruhl, R., and F. J. Schweigert. 2003. Automated solid-phase extraction and liquid chromatographic method for retinoid determination in biological samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 798:309-316. [DOI] [PubMed]
- 42.Russell, D. W. 1999. Nuclear orphan receptors control cholesterol catabolism. Cell 97:539-542. [DOI] [PubMed] [Google Scholar]
- 43.Russell, D. W. 2000. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta 1529:126-135. [DOI] [PubMed] [Google Scholar]
- 44.Skalen, K., M. Gustafsson, E. K. Rydberg, L. M. Hulten, O. Wiklund, T. L. Innerarity, and J. Boren. 2002. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417:750-754. [DOI] [PubMed] [Google Scholar]
- 45.Tontonoz, P., L. Nagy, J. G. Alvarez, V. A. Thomazy, and R. M. Evans. 1998. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93:241-252. [DOI] [PubMed] [Google Scholar]
- 46.Vivanco Ruiz, M. M., T. H. Bugge, P. Hirschmann, and H. G. Stunnenberg. 1991. Functional characterization of a natural retinoic acid responsive element. EMBO J. 10:3829-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willy, P. J., and D. J. Mangelsdorf. 1997. Unique requirements for retinoid-dependent transcriptional activation by the orphan receptor LXR. Genes Dev. 11:289-298. [DOI] [PubMed] [Google Scholar]
- 48.Willy, P. J., K. Umesono, E. S. Ong, R. M. Evans, R. A. Heyman, and D. J. Mangelsdorf. 1995. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 9:1033-1045. [DOI] [PubMed] [Google Scholar]
- 49.Yoshikawa, T., H. Shimano, M. Amemiya-Kudo, N. Yahagi, A. H. Hasty, T. Matsuzaka, H. Okazaki, Y. Tamura, Y. Iizuka, K. Ohashi, J. Osuga, K. Harada, T. Gotoda, S. Kimura, S. Ishibashi, and N. Yamada. 2001. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol. Cell. Biol. 21:2991-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]