Abstract
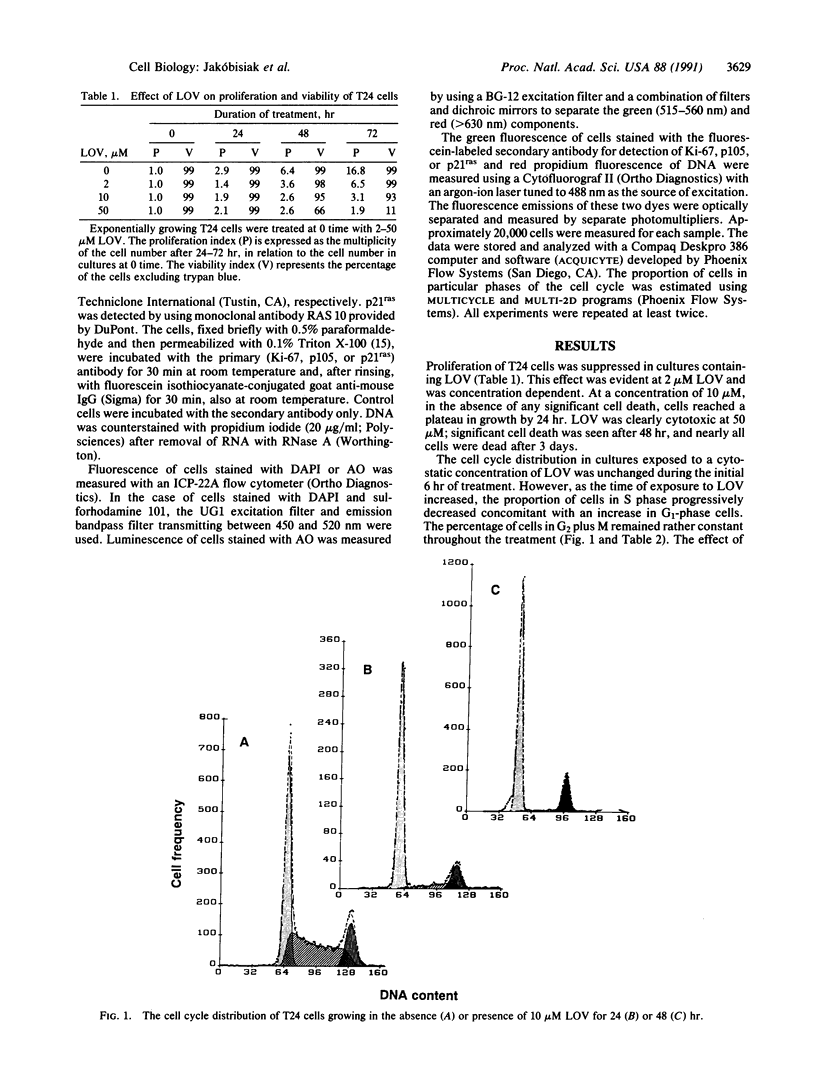
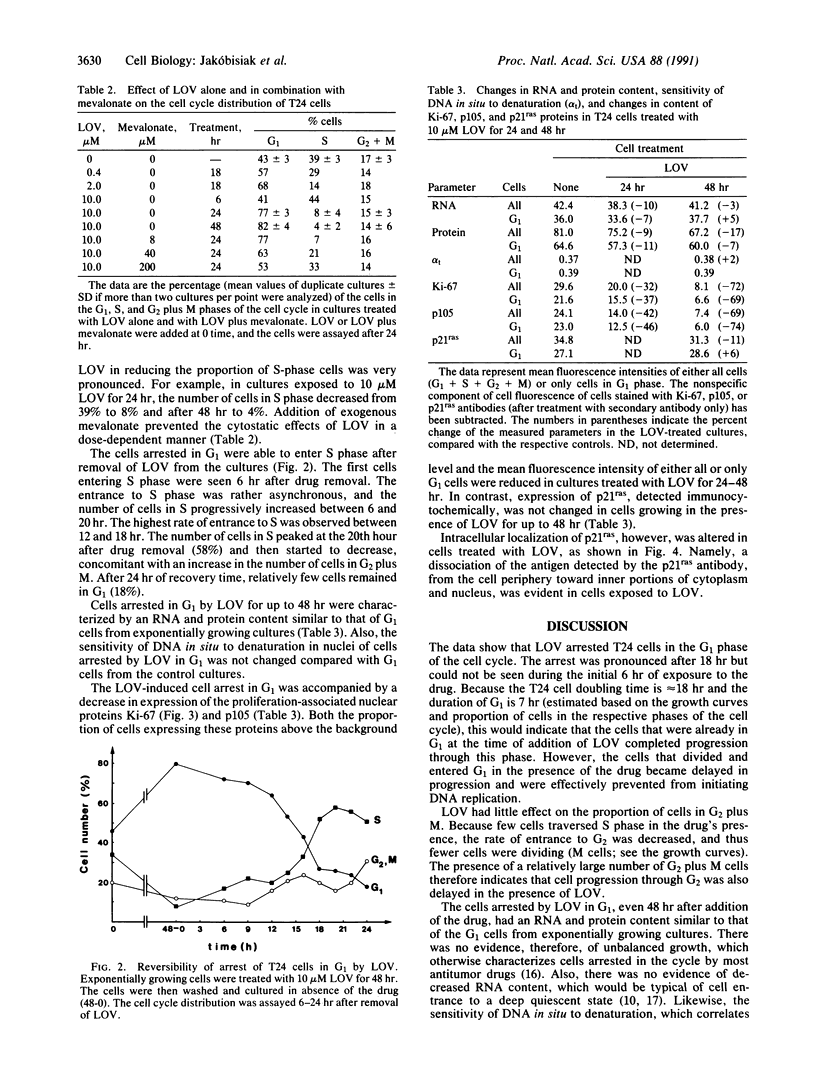
Lovastatin (LOV), the drug recently introduced to treat hypercholesteremia, inhibits the synthesis of mevalonic acid. The effects of LOV on the cell cycle progression of the human bladder carcinoma T24 cell line expressing activated p21ras were investigated. At a concentration of 2-10 microM, LOV arrested cells in G1 and also prolonged--or arrested a minor fraction of cells in--the G2 phase of the cell cycle; at a concentration of 50 microM, LOV was cytotoxic. The cytostatic effects were reversed by addition of exogenous mevalonate. Cells arrested in the cycle by LOV were viable for up to 72 hr and did not show any changes in RNA or protein content or chromatin condensation, which would be typical of either unbalanced growth or deep quiescence. The expression of the proliferation-associated nuclear proteins Ki-67 and p105 in these cells was reduced by up to 72% and 74%, respectively, compared with exponentially growing control cells. After removal of LOV, the cells resumed progression through the cycle; they entered S phase asynchronously after a lag of approximately 6 hr. Because mevalonate is essential for the posttranslational modification (isoprenylation) of p21ras, which in turn allows this protein to become attached to the cell membrane, the data suggest that the LOV-induced G1 arrest may be a consequence of the loss of the signal transduction capacity of p21ras. Indeed, while exposure of cells to LOV had no effect on the cellular content of p21ras (detected immunocytochemically), it altered the intracellular location of this protein, causing its dissociation from the cell membrane and translocation toward the cytoplasm and nucleus. However, it is also possible that inhibition of isoprenylation of proteins other than p21ras (e.g., nuclear lamins) by LOV may be responsible for the observed suppression of growth of T24 cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer K. D. Analysis of proliferation-associated antigens. Methods Cell Biol. 1990;33:235–247. [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger C. V., Bauer K. D., Epstein A. L. A method for simultaneous nuclear immunofluorescence and DNA content quantitation using monoclonal antibodies and flow cytometry. Cytometry. 1985 May;6(3):208–214. doi: 10.1002/cyto.990060306. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Melamed M. R. New cell cycle compartments identified by multiparameter flow cytometry. Cytometry. 1980 Sep;1(2):98–108. doi: 10.1002/cyto.990010203. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T. K., Melamed M. R. Cell cycle-related changes in nuclear chromatin of stimulated lymphocytes as measured by flow cytometry. Cancer Res. 1977 Dec;37(12):4635–4640. [PubMed] [Google Scholar]
- Darzynkiewicz Z., Traganos F., Sharpless T., Melamed M. R. Lymphocyte stimulation: a rapid multiparameter analysis. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2881–2884. doi: 10.1073/pnas.73.8.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Williamson B., Carswell E. A., Old L. J. Cell cycle-specific effects of tumor necrosis factor. Cancer Res. 1984 Jan;44(1):83–90. [PubMed] [Google Scholar]
- DeClue J. E., Vass W. C., Papageorge A. G., Lowy D. R., Willumsen B. M. Inhibition of cell growth by lovastatin is independent of ras function. Cancer Res. 1991 Jan 15;51(2):712–717. [PubMed] [Google Scholar]
- Doyle J. W., Kandutsch A. A. Requirement for mevalonate in cycling cells: quantitative and temporal aspects. J Cell Physiol. 1988 Oct;137(1):133–140. doi: 10.1002/jcp.1041370116. [DOI] [PubMed] [Google Scholar]
- Fabricant M., Broitman S. A. Evidence for deficiency of low density lipoprotein receptor on human colonic carcinoma cell lines. Cancer Res. 1990 Feb 1;50(3):632–636. [PubMed] [Google Scholar]
- Fairbanks K. P., Barbu V. D., Witte L. D., Weinstein I. B., Goodman D. S. Effects of mevinolin and mevalonate on cell growth in several transformed cell lines. J Cell Physiol. 1986 May;127(2):216–222. doi: 10.1002/jcp.1041270205. [DOI] [PubMed] [Google Scholar]
- Farnsworth C. C., Gelb M. H., Glomset J. A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science. 1990 Jan 19;247(4940):320–322. doi: 10.1126/science.2296721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. C., Wolda S. L., Gelb M. H., Glomset J. A. Human lamin B contains a farnesylated cysteine residue. J Biol Chem. 1989 Dec 5;264(34):20422–20429. [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Glomset J. A., Gelb M. H., Farnsworth C. C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990 Apr;15(4):139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Grogan T. M., Lippman S. M., Spier C. M., Slymen D. J., Rybski J. A., Rangel C. S., Richter L. C., Miller T. P. Independent prognostic significance of a nuclear proliferation antigen in diffuse large cell lymphomas as determined by the monoclonal antibody Ki-67. Blood. 1988 Apr;71(4):1157–1160. [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., Ross R. Relation of cholesterol and mevalonic acid to the cell cycle in smooth muscle and swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1980 Jun 10;255(11):5134–5140. [PubMed] [Google Scholar]
- Hall A. The cellular functions of small GTP-binding proteins. Science. 1990 Aug 10;249(4969):635–640. doi: 10.1126/science.2116664. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Landberg G., Tan E. M., Roos G. Flow cytometric multiparameter analysis of proliferating cell nuclear antigen/cyclin and Ki-67 antigen: a new view of the cell cycle. Exp Cell Res. 1990 Mar;187(1):111–118. doi: 10.1016/0014-4827(90)90124-s. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Defendini R., Green R. A., Sheridan K. M., Donley D. K. Suppression of murine neuroblastoma growth in vivo by mevinolin, a competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Clin Invest. 1985 Nov;76(5):1748–1754. doi: 10.1172/JCI112165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesney-Huneeus V., Wiley M. H., Siperstein M. D. Essential role for mevalonate synthesis in DNA replication. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5056–5060. doi: 10.1073/pnas.76.10.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Reynolds R. K., Santos E., Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982 Nov 11;300(5888):149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- Repko E. M., Maltese W. A. Post-translational isoprenylation of cellular proteins is altered in response to mevalonate availability. J Biol Chem. 1989 Jun 15;264(17):9945–9952. [PubMed] [Google Scholar]
- Sasaki K., Murakami T., Kawasaki M., Takahashi M. The cell cycle associated change of the Ki-67 reactive nuclear antigen expression. J Cell Physiol. 1987 Dec;133(3):579–584. doi: 10.1002/jcp.1041330321. [DOI] [PubMed] [Google Scholar]
- Schmidt R. A., Schneider C. J., Glomset J. A. Evidence for post-translational incorporation of a product of mevalonic acid into Swiss 3T3 cell proteins. J Biol Chem. 1984 Aug 25;259(16):10175–10180. [PubMed] [Google Scholar]
- Sinensky M., Logel J. Defective macromolecule biosynthesis and cell-cycle progression in a mammalian cell starved for mevalonate. Proc Natl Acad Sci U S A. 1985 May;82(10):3257–3261. doi: 10.1073/pnas.82.10.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr M., Vogt-Schaden M., Knobloch M., Vogel R., Futterman G. Evaluation of eight fluorochrome combinations for simultaneous DNA-protein flow analyses. Stain Technol. 1978 Jul;53(4):205–215. doi: 10.3109/10520297809111467. [DOI] [PubMed] [Google Scholar]
- Traganos F., Darzynkiewicz Z., Melamed M. R. The ratio of RNA to total nucleic acid content as a quantitative measure of unbalanced cell growth. Cytometry. 1982 Jan;2(4):212–218. doi: 10.1002/cyto.990020403. [DOI] [PubMed] [Google Scholar]
- Verheijen R., Kuijpers H. J., Schlingemann R. O., Boehmer A. L., van Driel R., Brakenhoff G. J., Ramaekers F. C. Ki-67 detects a nuclear matrix-associated proliferation-related antigen. I. Intracellular localization during interphase. J Cell Sci. 1989 Jan;92(Pt 1):123–130. doi: 10.1242/jcs.92.1.123. [DOI] [PubMed] [Google Scholar]