Abstract
Protein kinase Cδ (PKCδ) is an important regulator of apoptosis in epidermal keratinocytes. However, little information is available regarding the downstream kinases that mediate PKCδ-dependent keratinocyte death. This study implicates p38δ mitogen-activated protein kinase (MAPK) as a downstream carrier of the PKCδ-dependent death signal. We show that coexpression of PKCδ with p38δ produces profound apoptosis-like morphological changes. These morphological changes are associated with increased sub-G1 cell population, cytochrome c release, loss of mitochondrial membrane potential, caspase activation, and PARP cleavage. This death response is specific for the combination of PKCδ and p38δ and is not produced by replacing PKCδ with PKCα or p38δ with p38α. A constitutively active form of MEK6, an upstream activator of p38δ, can also produce cell death when coupled with p38δ. In addition, concurrent p38δ activation and extracellular signal-regulated kinase 1/2 (ERK1/2) inactivation are required for apoptosis. Regarding this inverse regulation, we describe a p38δ-ERK1/2 complex that may coordinate these changes in activity. We further show that this p38δ-ERK1/2 complex relocates into the nucleus in response to PKCδ expression. This regulation appears to be physiological, since H2O2, a known inducer of keratinocyte apoptosis, promotes identical PKCδ and p38δ-ERK1/2 activity changes, leading to similar morphological changes.
Identifying the intracellular signal transduction pathways that regulate keratinocyte death and differentiation is an important goal. The protein kinase C (PKC) family of lipid-activated serine/threonine kinases appears to play a key role in this process (2, 19). The PKC family includes three subtypes: classical, novel, and atypical (49-52). The classical isoforms (PKCα, -βI, -βII, and -γ) are calcium, diacylglycerol, and phospholipid dependent, the novel isoforms (PKCδ, -ɛ, -η, -θ, and -μ) are calcium independent, and the atypical isoforms (PKCζ and -λ) are calcium and diacylglycerol independent (49-52). Each PKC isozyme displays a unique tissue distribution, subcellular localization, and substrate specificity. Epidermal keratinocytes express PKCα, -δ, -ɛ, -η, and -ζ (18, 27, 30, 44, 55), and PKCδ is implicated as a regulator of epidermal apoptosis (8, 15, 16, 43). For example, Denning et al. showed that PKCδ is proteolytically cleaved in UVB-treated keratinocytes, and the released catalytic fragment then acts to destabilize the mitochondria and cause apoptosis (15). However, other studies, with pharmacologic agents, suggest that PKCδ-dependent activation of downstream signaling cascades is required for death, although the identity of relevant signaling cascade(s) has not been reported (43).
Mitogen-activated protein kinases (MAPKs) are important intracellular regulators of keratinocyte differentiation that are activated by PKC-dependent pathways (17, 23, 25). Several MAPK subtypes have been identified, including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 MAPK (7, 9, 78). The p38 MAPK family includes p38α, -β, -γ, and -δ (54). Only p38α, -β, and -δ are expressed in keratinocytes (14, 20). Among these isoforms, p38δ has been implicated as a regulator of keratinocyte function and is required for cell response to a variety of differentiating agents, including phorbol ester, calcium, okadaic acid, and green tea polyphenol (1, 22, 23). In addition, p38δ MAPK is a known downstream target of PKC via a cascade that includes novel PKC, Ras, MEKK1, and MEK3/6 (23). Therefore, it is possible that p38δ may mediate PKCδ-associated keratinocyte cell death. The present experiments were designed to assess this possibility. Our studies show that PKCδ acts to regulate the function of a novel p38δ-ERK1/2 complex that is constitutively present in the cell. PKCδ stimulation results in relocation of p38δ and ERK1/2 to the nucleus, along with a coordinate increase in p38δ activity and decrease in ERK1/2 activity. We hypothesize that the complex mediates the coordinate inverse regulation of p38δ and ERK1/2. Both the increase in p38δ activity and the decrease in ERK1/2 activity are required for subsequent procaspase and PARP activation leading to cell death. We show here that hydrogen peroxide (H2O2), a known inducer of keratinocyte apoptosis (6, 12, 29, 57), produces similar changes in keratinocyte morphology and similar enzyme activities, suggesting that the results are meaningful in normal keratinocyte physiology.
MATERIALS AND METHODS
Antibodies and reagents.
Keratinocyte serum-free medium, gentamicin, trypsin, and Hanks balanced salt solution were obtained from Life Technologies, Inc. Dispase was obtained from Boehringer Mannheim. Z-VAD-FMK was purchased from BD Pharmingen. Polybrene (hexadimethrine bromide) was obtained from Sigma. Anti-PKCδ (sc-937), anti-PKCα (sc-208), anti-p38δ/SAPK4 (sc-7585), anti-MEK6 (sc-6073), and anti-phospho-ERK1/2 (sc-7383) antibodies were obtained from Santa Cruz Biotechnology. Anti-caspase 3 (AHZ0052) and anti-phospho-Tyr311-PKCδ (44-950) antibodies were from Biosource International. Anti-PARP antibody 556494 was from BD Pharmingen. Anti-ERK1/2 (M5670), anti-FLAG (F3165), and anti-β-actin antibodies were obtained from Sigma. Anti-phospho-ATF2 antibody 9221 was from Cell Signaling Technology. Hydrogen peroxide (H2O2) was obtained from Calbiochem.
Cell culture and infection with recombinant adenovirus vectors.
The methods for culturing normal human foreskin keratinocytes were described previously (13). Adenoviruses encoding wild-type PKCα and PKCδ and dominant-negative PKCδ were provided by T. Kuroki (53). Adenovirus encoding constitutively active PKCɛ was provided by A. Samarel (69). Adenoviruses encoding constitutively active Raf-1 (Raf-BXB), constitutively active MEK6 (caMEK6), and wild-type FLAG-tagged p38 MAPK isoforms α and δ were obtained from Y. Wang (76). An empty control adenovirus was generated by recombining pCA3 plasmid with the pJM17 adenovirus backbone in 293 cells. Recombinant adenoviruses were propagated in 293 cells and purified by cesium chloride centrifugation. The optimal multiplicity of adenoviral infection was determined by using the green fluorescent protein-encoding adenovirus (13). The adenoviruses were administered at the indicated MOIs in the presence of 2.5 μg of Polybrene/ml.
Immunoblot analysis.
Total cell extracts were prepared from cultured human epidermal keratinocytes. Equivalent amounts of protein were electrophoresed on reducing and denaturing polyacrylamide gels and transferred to nitrocellulose. The membranes were blocked, incubated with an indicated primary antibody, washed, and exposed to an appropriate horseradish peroxidase-conjugated secondary antibody. Secondary antibody binding was visualized by using chemiluminescence detection methods.
MAPK assays.
The adenovirus-delivered FLAG-tagged p38 MAPK isoforms were precipitated with anti-FLAG monoclonal antibody. Expression of individual FLAG-p38 isoforms was confirmed by immunoblotting with anti-FLAG antibody. The activities of precipitated FLAG-tagged kinases were measured by using a nonisotopic p38 MAPK assay method (Cell Signaling Tech). For p38 MAPK assays, FLAG-tagged p38 α and δ isoforms are delivered to keratinocytes by using adenovirus. After 48 h, individual FLAG-tagged isoforms are precipitated with anti-FLAG monoclonal antibody. The activity of the precipitated FLAG-tagged kinase was measured as follows. Briefly, keratinocyte total cell lysates are prepared and equal amounts of total protein (100 μg) are precipitated for kinase assay. Precipitated kinases were then allowed to phosphorylate the p38 substrate, ATF2, in the presence of ATP. Phosphorylation of ATF2 was then analyzed by immunoblotting with an antibody specific for phospho-ATF2. ERK1/2 activity was assessed by immunoblotting with anti-phospho-ERK1/2 (Cell Signaling Tech). ERK1/2 activity was also measured by kinase assay (data not shown).
Mitochondrial membrane potential and cytochrome c release.
To monitor changes in mitochondrial membrane potential, keratinocytes growing on coverslips were stained with the MitoSensor Reagent (BD Biosciences) for 20 min at 37°C and then examined by fluorescence microscopy by using a band-pass filter that detects fluorescein and rhodamine. To assay for cytochrome c release, mitochondrial and cytosolic fractions were separated by using an ApoAlert cell fractionation kit (BD Biosciences/Clontech) in accordance with the manufacturer's instructions, and the fractions were analyzed for the presence of cytochrome c by immunoblotting with anti-cytochrome c antibody. The purity of each cytosol and mitochondrial fraction was controlled by monitoring the level of cytochrome c oxidase subunit IV (COX4, a mitochondrial marker) and β-actin (cytosolic marker).
Flow cytometry analysis of DNA content.
To measure DNA content, keratinocytes were treated with trypsin, fixed in methanol, washed with phosphate-buffered saline (PBS), and treated with DNase-free RNase (40 μg/ml final concentration) at 37°C for 30 min. The cells were then stained with propidium iodide (50 μg/ml final concentration) and DNA content was analyzed by flow cytometry.
Confocal microscopy.
Human keratinocytes were plated onto 22-by-22-mm coverslips. After 24 h, cells were infected with the appropriate virus. After 24 to 48 h, the cells were fixed with 2% paraformaldehyde for 1 h, permeabilized with methanol for 10 min, and incubated with a primary antibody cocktail containing rabbit anti-ERK1/2 (M5670, 1:1,000; Sigma-Aldrich) and mouse anti-FLAG M2 (F3165, 1:1,000; Sigma-Aldrich). The secondary antibody cocktail contained the appropriate combination of Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin G (IgG; A11034, 1:1,000; Molecular Probes) and Cy3-conjugated sheep anti-mouse IgG (C2181, 1:1,000; Sigma-Aldrich). The coverslips were then sealed onto microscope slides by using DABCO antifade reagent (Molecular Probes) and examined by laser scanning confocal microscopy (LSM510; Zeiss, Thornwood, N.Y.) with a ×63 N.A. 1.4 oil immersion plan-Apochromat objective. Green fluorescent ERK1/2 images were collected by using a 488-nm excitation light from an argon/krypton laser, a 488-nm dichroic mirror and a 500- to 550-nm band-pass barrier filter. Images of red FLAG-p38δ fluorescence were collected by using a 543-nm excitation light from the HeNeI laser, a 543-nm dichroic mirror, and a 560-nm pass-filter. The images were analyzed, combined, and processed by using Adobe Photoshop (version 7.0) and are representative of at least three separate experiments in which five fields were examined.
RESULTS
PKCδ, MEK6, and p38δ regulate cell morphology.
To determine whether PKCδ and p38δ cooperate to promote keratinocyte cell death, we expressed PKCδ in the presence or absence of p38δ and monitored the effects on cell survival. As shown in Fig. 1A, expression of PKCδ or p38δ alone produces minimal change, and the phenotypes are similar to those observed after infection with the empty expression vector (EV). In contrast, expression of PKCδ in combination with p38δ results in nuclear shrinkage, plasma membrane blebbing, cell rounding, detachment, and formation of spherical structures (Fig. 1A). We have previously shown that a PKCδ/Ras/MEKK1/MEK6 cascade regulates p38 function in keratinocytes (13, 54). Consistent with it being a member of this signaling cascade, constitutively active MEK6 (caMEK6) also cooperates with p38δ to produce a remarkably similar morphological change. Figure 1B confirms that the adenovirus-delivered kinases are expressed. These morphological changes are reminiscent of those associated with programmed cell death, and so we next examined whether the cells were undergoing apoptosis. Figure 2 shows that treatment with a combination of PKCδ and p38δ (PKCδ+p38δ) or caMEK6+p38δ results in a 10-fold increase in the number of sub-G1 cells present in the population compared to untreated cultures. Individual expression of PKCδ, p38δ, or caMEK6 produces no change (not shown). It is important to note that the fraction of subG1 cells in the PKCδ+p38δ- and caMEK6+p38δ-treated groups represents a minimal estimate of the extent of cell damage, since a substantial fraction of dead cells is released from the dish or disintegrates during harvest. These results suggest that caMEK6+p38δ- and PKCδ+p38δ-expressing cells undergo cell death. However, the cell cycle evidence alone is not adequate to support a definitive diagnosis of apoptosis, and so we endeavored to measure additional death indicators.
FIG. 1.
PKCδ and MEK6 cooperate with p38δ to regulate cell morphology. (A) Normal human keratinocytes were infected with empty control adenovirus vector (EV) at a multiplicity of infection (MOI) of 30 or coinfected with PKCδ- or caMEK6-encoding adenovirus at an MOI of 15 in the presence of empty adenovirus or p38δ-adenovirus (MOI = 15). After 48 h the cells were photographed by phase-contrast microscopy. The arrows in the enlarged images point to spherical blebs. (B) Expression of adenovirus-delivered proteins was confirmed by immunoblotting. PKCδ was detected by using a PKCδ-selective antibody, whereas FLAG-p38δ and hemagglutinin (HA)-caMEK6 were detected by using FLAG- and MEK6-specific antibodies, respectively. The β-actin level was assayed as a control to normalize protein loading.
FIG. 2.
PKCδ and MEK6 cooperate with p38δ to increase sub-G1 DNA content. Keratinocytes were infected with of empty adenovirus (EV) (MOI = 30) or PKCδ- or caMEK6-encoding virus (MOI = 15) with of p38δ virus (MOI = 15). DNA content was assayed by flow cytometry of propidium iodide-stained cells. The percentage of sub-G1 cells is indicated. PKCδ, caMEK6, or p38δ expression alone did not increase sub-G1 DNA content (not shown).
PKCδ+p38δ treatment stimulates cytochrome c release, mitochondrial membrane depolarization and killer caspase activation.
In many cell types, including keratinocytes (15, 16, 43), apoptosis involves the loss of the mitochondrial membrane potential with accompanied release of apoptogenic factors, including cytochrome c, and activation of cysteine-dependent aspartate-directed proteases known as caspases (65, 77, 83). We therefore measured the extent of cytochrome c release and mitochondrial membrane status. As shown in Fig. 3A, the altered morphology observed in Fig. 1A is associated with enhanced release of cytochrome c into the cytosol in PKCδ+p38δ- or caMEK6+p38δ-treated cells. Expression of PKCδ, caMEK6, or p38δ alone produced no cytochrome c release (results not shown). We also measured the COX4 level. COX4 is a mitochondrial protein that is not released from damaged mitochondria and serves as a marker of the mitochondrial fraction. The β-actin level was measured as a cytosolic marker. The distribution of these markers confirms that the compartments were successfully separated. We next assessed the effects of PKCδ+p38δ or caMEK6+p38δ on mitochondrial membrane potential. Mitochondria of healthy cells accumulate MitoSensor dye, forming intramitochondrial aggregates that fluoresce red. In apoptotic cells, dye monomers reside in the cytoplasm and fluoresce green. As anticipated, red fluorescence, which is indicative of intact mitochondria, was observed in empty vector-infected cells. In addition, cells expressing only PKCδ, caMEK6 or p38δ also fluoresced red (Fig. 3B). In contrast, cells treated with PKCδ+p38δ or caMEK6+p38δ, fluoresce green, indicating a loss of mitochondrial membrane potential.
FIG. 3.
p38δ coexpression with PKCδ or MEK6 alters mitochondrial membrane potential, and potentiates cytochrome c release. A Keratinocytes were infected with empty adenovirus (EV) (MOI = 30) or PKCδ-, p38δ-, or caMEK6-encoding adenovirus vectors (MOI = 15) in the presence of empty vector (MOI = 15). Parallel groups were treated with PKCδ- or caMEK6-encoding vector (MOI = 15) in thepresence of p38δ-encoding virus (MOI = 15). The cells were harvested at 30 h postinfection. Cytosolic and mitochondrial fractions were prepared, and the cytochrome c level was monitored by immunoblotting. Successful separation of the mitochondrial and cytosolic fractions was confirmed by detection of COX4 (a mitochondrial marker) and β-actin (a cytosol marker). (B) Keratinocytes, grown on coverslips, were infected as described above. At 48 h postinfection, the cells were stained with MitoSensor reagent and examined by fluorescence microscopy.
The release of cytochrome c into the cytoplasm ultimately results in caspase cleavage and activation (28, 60, 83). Figure 4A shows that the expression of PKCδ+p38δ or caMEK6+p38δ results in increased cleavage of procaspase 3, a caspase that is activated via mitochondrion-dependent mechanisms (28, 60, 83). Figure 4B shows that the expression of PKCδ+p38δ or caMEK6+p38δ also results in enhanced cleavage of PARP, a caspase 3 target. These findings are consistent with a model wherein mitochondrion destruction permits the release of cytochrome c that, in turn, activates the caspase-dependent cell death cascade. Activation of each apoptotic marker requires the simultaneous presence of p38δ plus the upstream activator kinase (i.e., MEK6 and PKCδ).
FIG. 4.
PKCδ, MEK6, and p38δ activation of caspases. Keratinocytes were infected with the indicated adenoviruses. (A and B) At 48 h, total cell extracts were prepared and electrophoresed, and the procaspase 3 and PARP levels were monitored by immunoblotting. β-Actin was included as a loading normalization control. (C) Keratinocytes were incubated without (− Z VAD) or with (+ Z VAD) 20 μM Z-VAD-FMK for 30 min prior to infection with empty vector (MOI = 30) or PKCδ or caMEK6 vector (MOI = 15) with p38δ-encoding adenovirus (MOI = 15). At 48 h postinfection, the cells were photographed by phase-contrast microscopy. The arrows indicate apoptosis-associated spherical blebs.
If the process is really caspase dependent, it should be possible to attenuate the response by inhibiting caspase activity. We therefore treated cells with empty vector, PKCδ+p38δ, or caMEK6+p38δ in the presence or absence of Z-VAD-FMK, a pan-caspase inhibitor. Indeed, as shown in Fig. 4C, treatment with this agent nearly completely inhibited the appearance of the apoptotic morphology. In addition, Z-VAD-FMK blocks procaspase 3 and PARP degradation induced by the coexpression of p38δ+PKCδ or p38δ+caMEK6 (not shown).
PKCδ, MEK6, and regulation of p38δ activity.
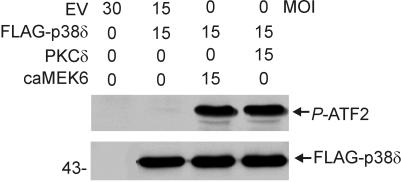
The findings presented above suggested that PKCδ- or MEK6-dependent activation of p38δ may be required for keratinocyte cell death. To test this hypothesis, PKCδ or caMEK6 was expressed with p38δ and, after 48 h, FLAG-p38δ was precipitated with a FLAG-specific antibody. The activity of the precipitated enzyme was measured based on its ability to phosphorylate the p38 substrate, ATF2 (23). When expressed alone, FLAG-p38δ is active at a low level that is only detected when the blot in Fig. 5 is overexposed (not shown). In contrast, expression of PKCδ or caMEK6 with p38δ results in marked p38δ activation (Fig. 5).
FIG. 5.
p38δ activity is increased by PKCδ and MEK6. Keratinocytes were infected with the indicated doses of empty or PKCδ-, caMEK6-, or p38δ-encoding virus. At 48 h, total cell extracts were prepared, and FLAG-p38δ was precipitated (100 μg of protein/sample) by using anti-FLAG (5 μg/precipitation). The activity of precipitated FLAG-p38δ was measured by in vitro kinase assay that measures the formation of P-ATF2. Phosphorylated ATF2 was detected by immunoblotting with anti-phospho-ATF2 antibody. A parallel blot was treated with the anti-FLAG to assure that equivalent amounts of precipitated FLAG-p38δ were present in each reaction.
Required reduction in ERK1/2 activity.
It has been suggested, in a variety of cell models, that the balance between activity of JNK and p38 versus ERK1/2 activity regulates cell fate (22, 46, 79). We therefore considered whether ERK1/2 activity is altered in cells displaying increased p38δ activity and undergoing apoptosis. Cells were infected with PKCδ, caMEK6, or p38δ encoding adenovirus, and the endogenous ERK1/2 activity was monitored after 48 h. Figure 6 shows that when p38δ activity is increased (i.e., cells expressing PKCδ or caMEK6 with p38δ), the P-ERK1/2 level is markedly reduced. This represents an authentic reduction in activity, since the absolute level of ERK1/2 protein is not altered (ERK1/2).
FIG. 6.
PKCδ, MEK6, and p38δ regulation of ERK1/2 activity. Keratinocytes were incubated for 48 h with individual viruses as in Fig. 5. The total cell extract was then utilized to measure the endogenous ERK1/2 level and activity. Phosphorylated ERK1/2 level were monitored by using anti-phospho-ERK1/2, whereas the total ERK1/2 level was determined by using anti-ERK1/2. The β-actin level was assayed by immunoblotting to ensure equal protein loading.
To determine whether reduced ERK1/2 activity is necessary for apoptosis, we forced maintenance of ERK1/2 activity by expressing constitutively active Raf-1 (caRaf-1), a specific upstream activator of ERK1/2, in the presence of various combinations of PKCδ, caMEK6 and p38δ. Figure 7A confirms that PKCδ+p38δ expression suppresses ERK1/2 activity and that this suppression is partially reversed when caRaf-1 is present. We were also concerned to demonstrate that shifting the p38δ/ERK1/2 activity balance in favor of ERK1/2 inhibits the apoptotic response. We therefore determined whether forced ERK1/2 activation inhibits the PKCδ+p38δ-dependent activation of PARP cleavage. As shown in Fig. 7B, expressing PKCδ+p38δ in keratinocytes results in PARP cleavage. Figure 7B also shows that expression of caRaf-1 inhibits the PKCδ+p38δ-dependent PARP cleavage.
FIG. 7.
Expression of constitutively activated Raf-1 alleviates PKCδ+p38δ-dependent inhibition of ERK1/2 activity and activation of PARP degradation. Keratinocytes were infected with caRaf-1. After 24 h, the cells were secondarily infected with p38δ or PKCδ. Total cell extracts were prepared after an additional 48 h. (A) Phospho-ERK1/2 level was measured by immunoblotting with anti-P-ERK1/2, and the total ERK1/2 level was monitored by using anti-ERK1/2. (B) The total extracts were immunoblotted to measure the PARP level by using anti-PARP antibody. The β-actin level was monitored to normalize protein loading.
Evidence for PKCδ-dependent intranuclear relocation of p38δ-ERK1/2.
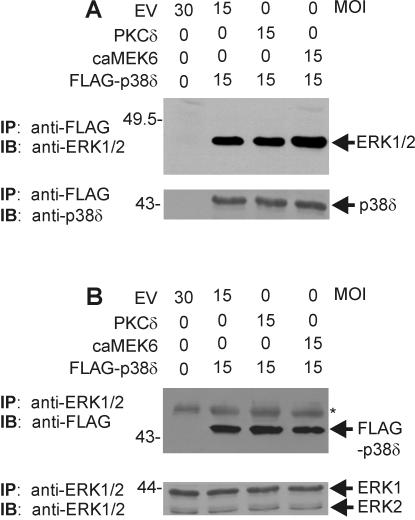
Our results (Fig. 5 and 6) clearly show that p38δ activity and ERK1/2 activity are coordinately and inversely regulated by PKCδ and MEK6. One possible explanation for the p38δ-dependent reduction in ERK1/2 activity is an interaction of p38δ with ERK1/2. A p38α-ERK1/2 complex has been identified in HeLa cells (80), and we have previously shown that an endogenous p38δ-ERK1/2 complex is constitutively present in keratinocytes (22). Regulatory stimuli, including treatment with 12-O-tetradecanoylphorbol-13-acetate (TPA) and okadaic acid, alter activity in this complex to increase p38δ and decrease ERK1/2 activity (22). We therefore assessed whether such a p38δ-ERK1/2 complex, which could function to integrate the reciprocal regulation, is observed during PKCδ/MEK6/p38δ-dependent keratinocyte apoptosis. Cells were infected for 48 h with FLAG-p38δ-encoding virus in the presence or absence of PKCδ- or caMEK6-encoding virus. Extracts were prepared and FLAG-p38δ was immunoprecipitated. The p38δ immunoblot in Fig. 8A (lower blot) confirms that FLAG-p38δ is precipitated. The ERK1/2 immunoblot (Fig. 8A, upper panel) demonstrates that ERK1 is precipitated with p38δ. We also performed the inverse experiment: precipitation with anti-ERK1/2. The anti-ERK1/2 immunoblot shown in Fig. 8B (lower panel) confirms that ERK1 and ERK2 are precipitated. The anti-FLAG blot (Fig. 8B, upper panel) shows that FLAG-p38δ coprecipitates with ERK1/2.
FIG. 8.
p38δ and ERK1/2 exist as a complex in keratinocytes. Keratinocytes were infected with empty adenovirus (EV) (MOI = 30) or PKCδ- or caMEK6-encoding adenovirus (MOI = 15) in the presence of empty virus or FLAG-p38δ-encoding virus (MOI = 15) and then harvested 48 h postinfection. (A) Total cell extracts were prepared and immunoprecipitated with anti-FLAG antibody, and the precipitate was then monitored for the presence of ERK1/2 or p38δ by immunoblotting with anti-ERK1/2 or anti-p38δ. (B) Total cell extract was precipitated with anti-ERK1/2, and the precipitated proteins were immunoblotted with anti-FLAG or anti-ERK1/2. Primary antibody binding was visualized by using an appropriate secondary detection reagent. The asterisk identifies a nonspecific band.
These findings confirm that a FLAG-p38δ-ERK1/2 complex exists in keratinocytes (Fig. 8) and that PKCδ or caMEK6 cause p38δ activation with coordinate ERK1/2 inactivation (Fig. 5 and 6). A recent study indicates that mitogen stimulation of cells results in rapid ERK1/2 accumulation in the nucleus and that prolonged stimulation results in intranuclear ERK1/2 inactivation (58, 75). We therefore explored the possibility that some p38δ and ERK1/2 may relocate to the nucleus after PKCδ stimulation. As shown in Fig. 9A, p38δ and ERK1/2 colocalize in the cytoplasm of resting keratinocytes (empty vector). PKCδ expression, in contrast, results in the appearance of some p38δ and ERK1/2 in the nucleus. To provide additional evidence of FLAG-p38δ and ERK1/2 movement into the nucleus and to assess the activation state of these kinases, we isolated nuclear extracts from keratinocytes expressing PKCδ in the presence or absence of p38δ. Figure 9B shows that a substantial quantity of FLAG-p38δ is nucleus associated in the absence of PKCδ and that this amount does not change in the presence of PKCδ. However, only when PKCδ is present does activated, P-FLAG-p38δ, accumulate inside the nucleus. A similar distribution is observed for endogenous p38δ (not shown). The lower panels in Fig. 9B show that intranuclear ERK1/2 levels increase in the presence of PKCδ; however, this ERK1/2 is dephosphorylated (inactive). The association of FLAG-p38δ with the nuclear fraction in Fig. 9B, in the absence of PKCδ, is surprising considering that no nuclear FLAG-p38δ is present as measured by immunohistology (Fig. 9A). However, additional images (not shown) suggest that some FLAG-38δ is constitutively associated with the outer nuclear surface in the absence of PKCδ and only moves into the nucleus in the presence of PKCδ.
FIG. 9.
PKCδ-dependent movement of p38δ and ERK1/2 into the nucleus. (A) Keratinocytes were infected with the indicated virus (MOI = 15). At 48 h postinfection, the cells were fixed and costained with mouse anti-FLAG+Cy3-conjugated sheep anti-mouse IgG to detect FLAG-p38δ and with rabbit anti-ERK1/2+Alexa Fluor 488-conjugated goat anti-rabbit IgG to detect ERK1/2. Red and green fluorescent and combination images were obtained by confocal microscopy. The yellow color in the combined image indicates colocalization. (B) Keratinocytes were incubated for 48 h with the indicated MOI of each virus. At 48 h, the cells were harvested, and nuclear extracts were prepared as described in Materials and Methods. Equivalent quantities of nuclear extract, based on protein concentration, were electrophoresed and transferred to nitrocellulose. Separate gels were probed with anti-FLAG (to detect total p38δ), anti-P-p38 to detect P-FLAG-p38δ, anti-ERK1/2 to detect ERK1/2, and anti-P-ERK1/2, which detects phosphorylated ERK1/2.
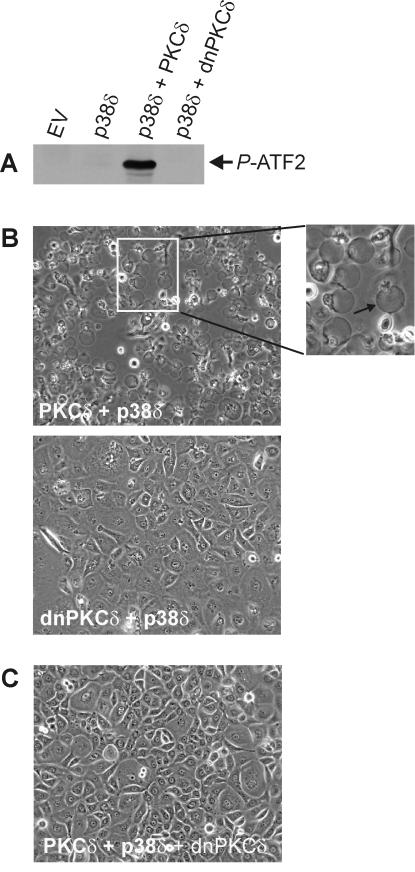
Active PKCδ is required for regulation.
The above results suggest a key role for PKCδ in driving apoptosis. However, it was not clear whether PKCδ catalytic activity is required for a response. We therefore monitored changes in cell morphology and p38δ activity after expression of intact or kinase-inactive (dominant-negative) PKCδ. As shown in Fig. 10A, expression of intact PKCδ increases p38δ activity (Fig. 10A) and stimulates morphological change (Fig. 10B), but inactive, dominant-negative PKCδ (dnPKCδ) does not activate p38δ or cause altered morphology. These finding indicate that PKCδ kinase activity is required to promote apoptosis. We also anticipated that dominant-negative PKCδ should inhibit the ability of native PKCδ to induce apoptosis. Indeed, Fig. 10C shows that expression of dnPKCδ inhibits the PKCδ+p38δ-associated apoptosis.
FIG. 10.
PKCδ kinase activity is required for p38δ activation and appearance of apoptotic morphology. (A) Keratinocytes were infected with empty virus (EV) (MOI = 30), PKCδ or dnPKCδ-encoding virus (MOI = 15) plus FLAG-p38δ virus (MOI = 15), or EV (MOI = 15) with p38δ virus (MOI = 15) for 48 h. Total cell extracts were prepared, FLAG-p38δ was immunoprecipitated by using anti-FLAG, and the activity of the immunoprecipitated p38δ was measured by using in vitro kinase with ATF2 as a substrate. The P-ATF2 level was measured by immunoblotting with anti-phospho-ATF2. The total p38δ level was found to be equal in each infection as measured by immunoblotting with anti-FLAG (not shown). An immunoblot of extracts prepared from PKCδ- and dnPKCδ-expressing cells reveals comparable levels of expression at approximately four times endogenous levels (not shown). (B) Keratinocytes were infected with PKCδ+p38δ or dnPKCδ+p38δ as described above and photographed after 48 h. The arrow in the enlarged panel indicates the apoptotic spheres. (C) Keratinocytes were triply infected with of PKCδ, p38δ, and dnPKCδ (MOI = 15 for each) and photographed after 48 h.
A second important issue is whether activation of apoptosis is a property shared by all PKC and p38 isoforms. We first sought to determine whether the classical PKC isoform, PKCα, can promote cell death when coupled with p38δ. As shown in Fig. 11A, this combination does not cause altered morphology. We also show that the combination of PKCα+p38α produces no response. Thus, PKCα does not promote cell death in keratinocytes when coupled to α or δ isoforms of p38 MAPK. We next determined whether p38α plays a role as a downstream mediator of PKCδ-induced death signal. As shown in Fig. 11A, coupling of PKCδ with p38α does not stimulate morphological change. In addition, coupling of caMEK6 with p38α does not produce an apoptotic response (not shown). Moreover, p38α activity can be inhibited without altering p38δ-dependent cell death. Treatment of cells with SB203580, an inhibitor of p38α and -β, but not p38δ (11, 40), does not suppress PKCδ+p38δ (Fig. 11B)- or caMEK6+p38δ (not shown)-associated cell death, further supporting the notion that p38α and p38β do not have a role in this process.
FIG. 11.
Do PKCα, PKCɛ, and p38α stimulate keratinocyte apoptosis? (A) Keratinocytes were infected with EV (MOI = 30) or PKCα or PKCδ (MOI = 15), with p38α or p38δ (MOI = 15). The cells were photographed at 48 h postinfection. (B) p38α and p38β activity is not required for PKCδ+p38δ-dependent apoptosis. Keratinocytes were infected with empty vector (MOI = 30) or PKCδ- and p38δ-encoding viruses (MOI = 15) and then incubated for 48 h in the presence of 2 μM SB203580. At 48 h, the cells were photographed. (C) Keratinocytes were infected with PKCδ or PKCɛ (MOI = 15) with p38δ (MOI = 15) and then photographed at 48 h postinfection. (D) Expression of PKCα, FLAG-p38α, and PKCɛ was confirmed by immunoblotting with anti-PKCα, anti-FLAG, and anti-PKCɛ, respectively.
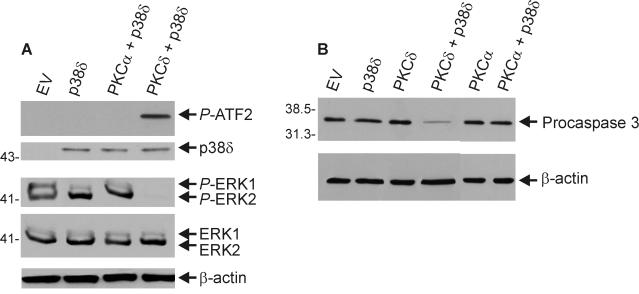
PKCδ is a member of the novel PKC subfamily (49, 50). Thus, it is possible that novel PKC isoforms may share an ability to function with p38δ to promote keratinocyte apoptosis, since multiple novel PKC isoforms can promote keratinocyte apoptosis (8, 15, 16). To address this possibility, we tested the combination of PKCɛ+p38δ. As shown in Fig. 11C, PKCɛ+p38δ expression produces a morphological change that is very similar to that produced by PKCδ+p38δ. Moreover, PKCɛ induces increased p38δ activity and decreased ERK1/2 activity similar to that observed when PKCδ or caMEK6 are expressed with p38δ (not shown). In addition, the combination of PKCɛ+p38δ promotes increased procaspase 3 cleavage (not shown). The immunoblot in Fig. 11D confirms that the viral expression vectors actually delivered the p38α, PKCα, and PKCɛ proteins.
These results suggest that the PKCα+p38δ combination does not influence morphology. We would anticipate that this combination should also not regulate the biochemical responses associated with the morphological change. Figure 12A tests the ability of PKCα to increase p38δ and reduce ERK1/2 activity. These results confirm that PKCα cannot substitute for PKCδ as a regulator of p38δ or ERK1/2 activity. As a measure of a downstream biochemical response, we measured PKCα+p38δ-dependent regulation of procaspase 3 cleavage. The results shown in Fig. 12B confirm that PKCα cannot substitute for PKCδ and function, in conjunction with p38δ, to promote procaspase 3 cleavage.
FIG. 12.
PKCα does not activate p38δ, suppress ERK1/2 activity, or stimulate procaspase 3 cleavage. Keratinocytes were infected with empty vector (MOI = 30) or FLAG-p38δ (MOI = 15) plus empty vector, PKCα, or PKCδ-encoding virus (MOI = 15). At 48 h, FLAG-p38δ was immunoprecipitated, and the kinase activity was measured by determining the in vitro formation of P-ATF2. ATF2 phosphorylation was analyzed by immunoblotting with anti-phospho-ATF2. The ERK1/2 level and the P-ERK1/2 level were monitored by using total extract prepared from the same cells and total ERK1/2- and P-ERK1/2-specific antibodies. (B) PKCα does not stimulate procaspase 3 cleavage. Keratinocytes were infected for 48 h with each kinase-encoding adenovirus (MOI = 15). The total viral load was adjusted to an MOI of 30 in each group by the addition of empty vector. Procaspase 3 levels were measured by immunoblotting, and equivalent gel loading was confirmed by assay of β-actin. As shown in Fig. 11B, the level of expressed PKCα is robust in these cells. Thus, the lack of ability of PKCα+p38δ to drive cell death is not due to a low level of PKCα expression.
PKCδ tyrosine phosphorylation.
Covalent modification can alter PKCδ stability and activity (3, 37, 50, 68). Phosphorylation of PKCδ at Y311 is thought to increase activity (29, 39, 63). We therefore examined whether PKCδ Y311 phosphorylation is altered in cells undergoing PKCδ+p38δ-dependent apoptosis. Keratinocytes were treated with empty vector, PKCδ, or PKCδ+p38δ. Cell extracts were then assayed by immunoblotting for the presence of PKCδ and PKCδ-P-Y311. Figure 13A shows that vector-delivered PKCδ is expressed, and Fig. 13B shows that it is phosphorylated at Y311. In contrast, endogenous PKCδ is not phosphorylated at Y311 in unstimulated cells. This finding will be considered further in the context of H2O2-dependent regulation of endogenous PKCδ phosphorylation (below).
FIG. 13.
PKCδ phosphorylation in keratinocytes. Keratinocytes were infected with PKCδ (MOI = 15)- and/or p38δ (MOI = 15)-encoding virus as indicted. Total viral load was adjusted to an MOI of 30 by the addition of empty vector. Total extract was prepared at 48 h postinfection and electrophoresed on parallel gels. (A) The total PKCδ level was assayed by immunoblotting with anti-PKCδ. (B) The level of PKCδ phosphorylation at Y311 was analyzed by immunoblotting with antibodies against phosphorylated Y311. As a control to normalize sample level, comparable levels of β-actin were observed for each loading (results not shown).
H2O2 promotes keratinocyte apoptosis via a PKCδ/p38δ-dependent signaling pathway.
We next determined whether known inducers of keratinocyte apoptosis operate via a PKCδ- and p38δ-dependent mechanism. H2O2 is a known keratinocyte stress agent, produced in response to UV light treatment, that promotes keratinocyte apoptosis (6, 12, 29, 57). We determined whether H2O2-dependent cell death is mediated via the PKCδ and p38δ-ERK1/2 pathway. As shown in Fig. 14A and B, H 2O2 treatment of keratinocytes produces marked morphological changes, including shrinkage of the nuclei, cell rounding, formation of balloon-like blebs (spheres) (Fig. 14B, arrow), and cell detachment. The phenotype is virtually indistinguishable from that observed following overexpression of PKCδ+p38δ (Fig. 1). The H2O2-induced apoptotic morphology is completely blocked by pretreatment of the cells with rottlerin, a PKCδ-selective inhibitor (31). A recent report indicates that rottlerin also uncouples respiration and thus is not an ideal PKCδ inhibitor (67). However, we believe it can still have value, when used in conjunction with other evidence, since it inhibits the PKCδ-dependent keratinocyte morphological change.
FIG. 14.
H2O2 treatment causes apoptosis-associated morphological changes. (A) Keratinocytes were pretreated for 1 h with 10 μM rottlerin and then treated for 2 h with 10 mM H2O2 in the presence or absence of 10 μM rottlerin. The cells were then photographed. (B) Photographic enlargement of the H2O2-treated morphology. The arrow indicates an apoptotic-associated sphere. (C) H2O2 treatment enhances loss of mitochondrial membrane potential. Human keratinocytes, grown on coverslips, were treated in the absence or presence of H2O2 for 4 h and then stained with MitoSensor reagent. Fluorescence was monitored by epifluorescence microscopy. The arrows show the location of a representative apoptotic sphere.
H2O2 enhances keratinocyte cell death.
We next determined whether treatment with H2O2 causes keratinocyte apoptosis as measured by loss of mitochondrial membrane potential. Keratinocytes were treated in the absence or presence of H2O2 for 4 h and stained with MitoSensor dye prior to the detection of fluorescence. The dye fluoresces red in untreated cells, a finding indicative of intact mitochondria, but green in H2O2-treated cells, a result indicative of a loss of mitochondrial membrane potential (Fig. 14C).
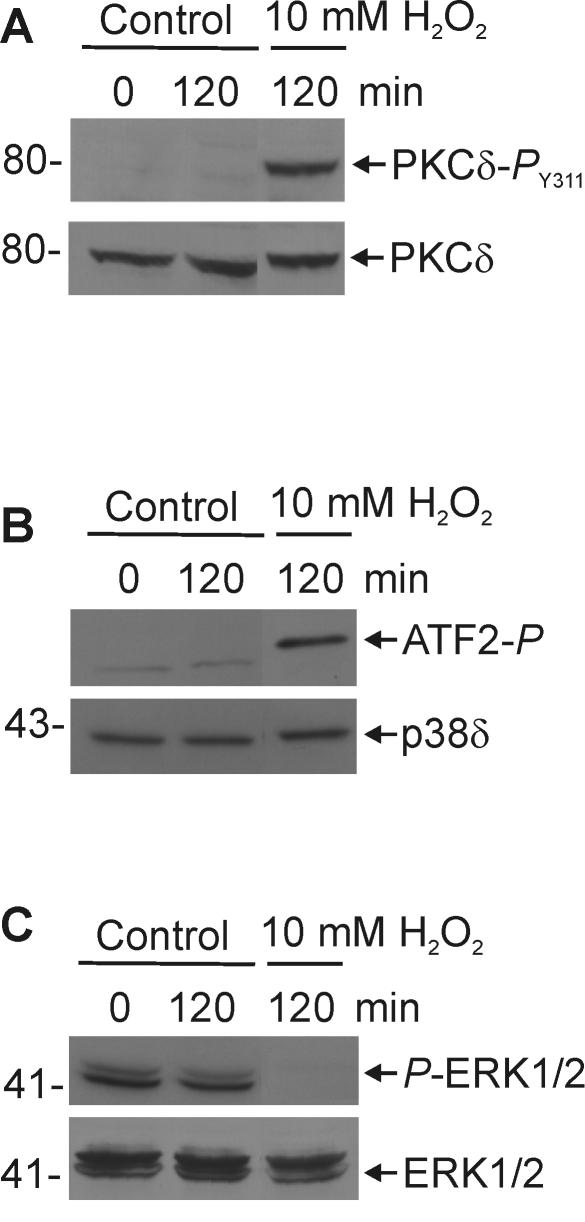
H2O2 causes PKCδ phosphorylation and inversely regulates p38δ-ERK1/2 activity.
We next examined the role of PKCδ and p38δ-ERK1/2 signaling in mediating the response to H2O2. We monitored the effects of H2O2 treatment on PKCδ phosphorylation and on ERK1/2 and p38δ activity. As shown in Fig. 15A, H2O2 treatment increases phosphorylation of PKCδ at Y311, a modification that is known to be associated with PKCδ activation (29, 39, 63). This response is similar to that observed after expression of PKCδ in keratinocytes (compare with Fig. 13B). We would anticipate that if H2O2 works to promote apoptosis via a mechanism similar to that observed after PKCδ+p38δ expression, then treatment would increase p38δ and decrease ERK1/2 activity. Indeed, as shown in Fig. 15B and C, H2O2 treatment increased p38δ and inhibited ERK1/2 activity. It should be noted that, although keratinocytes are relatively tolerant of H2O2 (74, 81), all of the H2O2-dependent responses described in Fig. 14 and 15 were observed at concentrations ranging from 1 to 10 mM. The only difference was the rate of response: treatment with 1 mM H2O2 for 24 h matched the response after 10 mM H2O2 treatment for 2 h.
FIG. 15.
H2O2 promotes PKCδ phosphorylation, p38δ activation and ERK1/2 inhibition. Keratinocytes were treated for 120 min with 10 mM H2O2 or vehicle. (A) Total cell extracts were prepared for immunoblotting with anti-PKCδ-PY311 and anti-PKCδ antibodies. (B) FLAG-p38δ was precipitated with anti-FLAG antibody, and the activity of the precipitated p38δ kinase was assayed based on the ability to phosphorylate ATF2. (C) Total cell extracts were prepared and assayed for total ERK1/2 level and P-ERK1/2.
DISCUSSION
The PKC family includes 11 serine/threonine kinases classified into three groups. Conventional/classical PKCs (cPKCα, -βI, -βII, and -γ) are calcium, phospholipid-, and diacylglycerol-dependent, novel PKCs (nPKCδ, -ɛ, -η, -θ, and -μ) are calcium independent, and atypical PKCs (aPKCζ and -λ) are calcium- and diacylglycerol independent (49, 50, 52). Differences in subcellular localization, cofactor requirements, substrate specificity, and tissue distribution suggest distinct biological functions for each isozyme (47, 52). Each of the five PKC isozymes (PKCα, -δ, -ɛ, -η, and -ζ), expressed in keratinocytes (18, 27, 30, 44, 55), differentially regulate keratinocyte proliferation and differentiation. For example, PKCδ and -η regulate keratinocyte cell cycle protein function (34-36, 53), and expression of differentiation-dependent genes (41) such as type I transglutaminase (73), involucrin (22-25), and transcriptional regulators (23, 25). Taken together, these findings suggest that novel PKCs are prodifferentiation and antisurvival (21). In contrast, classical PKC isoforms (e.g., PKCα) can oppose these responses (17, 21).
PKC isoforms have also been implicated in cell death responses. In keratinocytes, cell death is a distinct, but related process, to cell differentiation. Apoptotic keratinocyte cell death involves killer caspase activation and is frequently associated with exposure to a toxic stimuli (e.g., UVB irradiation, etc.) (15, 16, 46, 66, 71). UVB-dependent apoptosis is associated with release of the PKCδ catalytic subunit (15). In addition, apoptotic stimuli may induce translocation of PKCδ from the cytoplasm to the plasma membrane, mitochondria, Golgi, endoplasmic reticulum, or nucleus (3). Translocation of PKCδ to the cell membrane in response to UVB irradiation has been reported in mouse epidermal cell lines (8). Yuspa and coworkers have shown that treatment of PKCδ overexpressing keratinocytes with phorbol ester results in activation of PKCδ and its subsequent movement to mitochondria, followed by loss of mitochondrial membrane potential and apoptosis (43).
PKCδ and p38δ act to stimulate mitochondrion-dependent apoptosis.
Given the central role of PKC isoforms in regulating keratinocyte death and differentiation, understanding the signaling pathways that mediate PKC action is an important goal. Our previous studies have implicated a signaling cascade that includes novel PKCs, Ras, MEKK1, and MEK6 that targets p38δ and ERK1/2 to regulate keratinocyte differentiation (25). In the present study, we identify this pathway as having a central role in regulating keratinocyte cell death. Our studies show that PKCδ, when coupled with p38δ, causes keratinocyte apoptosis. This response requires functional PKCδ kinase activity and is associated with potent and sustained PKCδ-stimulated activation of p38δ. This pathway produces a classical apoptotic response that includes accumulation of cells in sub-G1 growth phase, mitochondrial cytochrome c release, loss of mitochondrial membrane potential, caspase activation, and PARP degradation. The absence of procaspase 8 cleavage (not shown) suggests that apoptosis does not involve nonmitochondrial, death receptor-dependent pathways (28, 72). This is interesting, since receptor-dependent caspase 8 activation and apoptosis is observed in keratinocytes treated with UVB (38), undergoing anoikis (59) or treated with DNA-damaging agents (61). Thus, although UVB, for example, can trigger both receptor-dependent and mitochondrion-associated caspase activity, PKCδ/MEK6/p38δ appears to specifically activate only mitochondrion-associated apoptosis.
Which PKC isoforms activate apoptosis?
Our results indicate that PKCδ, when coupled with p38δ, promotes apoptosis. Substitution of PKCɛ for PKCδ results in a similar pattern of regulation; however, if PKCα, a classical isoform, replaces PKCδ, no apoptosis is observed. These observations are consistent with previous reports suggesting that classical PKC isoforms (e.g., PKCα) promote cell survival and inhibit differentiation (21, 32). Moreover, the observation that two novel PKC isoforms (PKCɛ and PKCδ) promote a similar apoptotic process, suggests that the novel PKC isoforms are major mediators of this response. These findings are consistent with other studies of human keratinocytes (8, 15, 21, 34-36, 53) and of other cell types, suggesting that the novel PKCs are prodifferentiation/antisurvival kinases. However, it is clear that the regulation is not always straightforward (32). In our cell culture model, coupling PKCη with p38δ fails to cause apoptosis (T. Efimova and R. L. Eckert, unpublished data). This is despite the fact that PKCη is a potent p38δ activator (23) and is consistent with the observed opposite roles of PKCδ and PKCη in UVB-induced apoptosis. In this context, PKCη is a negative regulator of the apoptotic response (45). In addition, both classical PKCα and novel PKCδ mediate apoptosis in prostate cancer cells (70), and PKCɛ is prosurvival in cardiac myocytes (33). Moreover, in PKCα-overexpressing transgenic mouse skin, treatment with TPA causes apoptosis (5). Thus, it is clear that additional studies will be required to understand the subtle differences that account for this diverse regulation.
Phosphorylation of expressed PKCδ is associated with PKCδ/MEK6/p38δ-dependent keratinocyte apoptosis.
PKCδ can be modified by enzymatic cleavage and by phosphorylation (3, 37). We find that expressed PKCδ is phosphorylated constitutively at Y311. Phosphorylation at Y311 renders PKCδ resistant to caspase cleavage and appears to promote the ability of the intact PKCδ to mediate death (37). It is possible that the tyrosine phosphorylation activates PKCδ - thereby facilitating its apoptotic role (37). As noted below, PKCδ Y311 phosphorylation is also observed when keratinocytes are treated with physiological stress agents.
Moreover, PKCδ proteolysis during apoptosis is a caspase 3-dependent event that results in release of the active PKCδ catalytic domain (3, 26). Catalytic fragment release is observed after keratinocyte treatment with UV radiation (15). In contrast, in the present study, we detected no increase in PKCδ catalytic fragment production during PKCδ+p38δ-dependent apoptosis. This finding agrees with previous reports indicating that caspase-dependent PKCδ cleavage is not necessarily required for apoptosis (3, 37).
Role for p38δ.
The p38 MAPK cascade includes four isozymes (i.e., p38α, -β, -δ, and -γ), and each can produce distinct cellular responses (54). Activation of p38 isoforms can be either pro- or antiapoptotic (48, 54). p38α isoform is by far the most thoroughly studied member of the p38 family (48, 54). Numerous reports document proapoptotic and prosurvival effects of p38α (10, 76, 82). Keratinocytes express p38α, -β, and -δ, but not p38γ (14). Several lines of evidence presented here suggest that p38δ activity is required for keratinocyte apoptosis. First, PKCδ and MEK6 can couple with p38δ to drive apoptosis. Second, p38α cannot couple with PKCδ or MEK6 to induce apoptosis. Third, treatment with an inhibitor, SB203580, that inhibits p38α and -β, but not p38δ (11, 40), does not inhibit apoptosis. It is interesting that although a role for p38δ has been confirmed in only a limited number of systems, a clear role for p38δ as a keratinocyte prodifferentiation regulator has been confirmed in several contexts and in response to several stimuli (1, 20, 22, 23). The present study is the first to document a role for p38δ in regulating keratinocyte apoptosis.
Physiological role of PKCδ- and p38δ-ERK1/2-associated cell death.
An important issue is whether similar changes in morphology and signaling kinase activity occur in the presence of physiologic regulators of keratinocyte apoptosis. In this regard, okadaic acid, a known inducer of keratinocyte apoptosis, promotes keratinocyte death via a mitochondrial/caspase/PARP pathway, and this response requires increased endogenous p38δ activity and decreased ERK1/2 activity (Efimova and Eckert, unpublished). PKCδ expression has been reported to sensitize keratinocytes to phorbol ester-dependent apoptosis (43). If p38δ acts downstream of PKCδ to promote apoptosis, we would expect that p38δ expression would also facilitate cell death in response to phorbol ester treatment. Indeed, we observed that p38δ expression renders keratinocytes susceptible to phorbol ester-induced apoptosis with changes identical to that observed in PKCδ/p38δ-expressing cells (results not shown).
Moreover, in the present study, we show that hydrogen peroxide, an important mediator of oxidative stress-induced cell death and a known activator of keratinocyte apoptosis, promotes PKCδ Y311 phosphorylation, increases p38δ activity, and decreases ERK1/2 activity. Hydrogen peroxide is produced in response to keratinocyte exposure to UV light (6, 12). These changes are coupled with the same morphological alterations (nuclear shrinkage, cell death, membrane blebbing, and sphere formation) that accompany PKCδ+p38δ overexpression-dependent apoptosis. Taken together, these findings suggest that the PKCδ- and p38δ-ERK1/2-dependent pathway mediates cell death in response to keratinocyte apoptotic agents. These findings are in agreement with data from PKCδ knockout mice which show that PKCδ-deficient smooth muscle cells are resistant to apoptosis induced by various stimuli, including H2O2 (42). These cells exhibit diminished cytochrome c release, caspase activation, and PARP cleavage in response to H2O2 treatment, suggesting that PKCδ function is required for this response. Moreover, stress-induced p38 activity is significantly reduced in these cells, a finding consistent with our suggestion that PKCδ is an upstream activator of p38.
p38δ activation and ERK1/2 suppression: coordination mediated via a p38δ-ERK1/2 complex?
As noted above, our studies suggest that p38δ activity is required for PKC-induced keratinocyte apoptosis. However, a remarkable feature of this regulation is the coupling of p38δ activation with suppression of ERK1/2 activity. This finding is consistent with the suggestion that cell fate is decided by a balance between prosurvival ERK1/2 signaling and prodeath stress-activated protein kinase signaling (46, 79). Our studies reveal two significant aspects of this regulation. First, as first described in our recent report (22) and as confirmed here, p38δ and ERK1/2 are part of a complex that is constitutively present in stimulated and nonstimulated keratinocytes. The presence of this complex suggests that p38δ activation is, either directly or indirectly, coupled to suppression of ERK1/2 activity. Second, the present study indicates that PKCδ stimulation of keratinocytes results in coaccumulation of p38δ and ERK1/2 into the cell nucleus. Comovement of the p38δ/ERK partners to the nucleus in response to PKCδ is an important finding. In resting fibroblasts, ERK1/2 is anchored to the upstream ERK1/2 activator, MEK1, in the cytoplasm (58). In response to MAPK stimulation, ERK1/2 is released from MEK1 and ERK1/2 accumulates in the nucleus (4, 56). Nuclear accumulation of ERK1/2 is ultimately associated with activation of phosphatases that inactivate ERK1/2 (75). Our studies suggest that p38δ and ERK1/2 are distributed in the cytoplasm and on the outer nuclear surface in resting keratinocytes. In the presence of PKCδ, some p38δ and ERK1/2 kinases are distributed to the nucleus interior. Both kinases remain nuclear even 48 h after initiation of treatment, and this localization is associated with maintenance of p38δ activity and inactivation of ERK1/2. Although ERK1/2 dephosphorylation may also occur in the cytoplasm, our findings are consistent with the idea that that the nucleus is a site of ERK1/2 inactivation by resident phosphatases (75). Interestingly, a recent study describes a direct association of a p38α splice isoform, Mxi2, with ERK1/2 that has a profound effect on the function of ERK1/2 in the nucleus (64). Specifically, Mxi2 binding to ERK1/2 appears to hinder ERK1/2 dephosphorylation, which results in prolonged ERK1/2 activation in the nucleus (64). Zhang et al. (80) also describe an interaction between p38α and ERK1/2. However, this interaction results in ERK1/2 inhibition, possibly by blocking MEK1-dependent ERK1/2 phosphorylation. Our previous study on p38δ-ERK1/2 interaction (22), together with the present study, firmly establishes the existence of p38δ-ERK1/2 complexes in keratinocytes and suggests that they have an important functional role.
These findings may also have important implications for the regulation of p38δ and ERK1/2 activity by upstream kinases. ERK1/2 activation is generally thought to be MEK1 dependent (7, 9). However, we show here that the regulation may be more complicated in keratinocytes. It is clear that expression of caRaf1 can enhance ERK1/2 activity, presumably via a mechanism that involves MEK1. However, as we demonstrate here, expression of MEK6, a MEK isoform that is traditionally associated with p38 isoform activation, suppresses ERK1/2 activity. This suggests that ERK1/2 may be part of a MEK6-dependent regulatory pathway.
Mechanism whereby p38δ/ERK1/2 regulates apoptosis.
Based on these observations, we propose the signaling cascade outlined in Fig. 16. In this model, p38δ and ERK1/2 in resting cells are present as a cytoplasmic-perinuclear complex in which ERK1/2 is active and p38δ is relatively inactive. It is likely that this complex also includes other proteins, such as one or more MEKs (not shown). Stimulus-dependent activation of PKCδ ultimately leads, possibly via a previously described pathway that involves Ras and MEKK1 (25), to MEK6 activation. MEK6, in turn, activates p38δ. The p38δ-ERK1/2 complex then moves into the nucleus where intranuclear ERK1/2-specific phosphatases inactivate ERK1/2. The idea that ERK1/2 may be inactivated in the nucleus is based on previous reports (75), and data are presented here showing that nuclear ERK1/2 is not phosphorylated. In this manner, ERK1/2 functions as an indirect target of the MEK6/p38δ pathway. The ultimate result of these modifications is a shift in the balance between ERK1/2 activity and p38δ activity in favor of p38δ. It should be noted that partial relocation of p38δ to the nucleus may occur in the absence of PKCδ stimulation; however, nuclear p38δ is strongly activated by PKCδ. This shift in activity results in activation of nuclear proapoptotic events, including phosphorylation of c-Jun (Efimova and Eckert, unpublished), as well as, conveyance of a nuclear signal to drive mitochondrion-dependent apoptosis. Ultimately, breakdown of mitochondrial integrity leads to the release of cytochrome c, the activation of caspases, PARP cleavage, and cell death. The dotted line in Fig. 16 accounts for the possibility that the p38δ-ERK1/2 complex may act to alter mitochondrial function via a direct, nonnuclear mechanism. This is at least possible, since another p38 isoform, p38α, causes apoptosis in a lymphoblastoid B-cell line by translocating directly to the mitochondria (62). Nuclear localization of activate p38δ suggests that this kinase may regulate gene expression or activity of apoptosis-related proteins. The identify of these proteins is currently under investigation.
FIG. 16.
A PKCδ, MEK6, p38δ/ERK1/2 pathway regulates keratinocyte apoptosis. Based on previous evidence, we hypothesize that stimulus-dependent activation of PKCδ (or PKCɛ) leads to RAS and MEKK1 activation and that this ultimately leads to MEK6 activation. The role of RAS and MEKK1 in this pathway is inferred based on previous reports, suggesting that these kinases provide a link between PKCδ and MEK6 (25). In resting keratinocytes, the balance of ERK1/2 to p38δ activity favors ERK1/2 (+ERK-p38δ−). MEK6 activation of p38δ increases p38δ activity (+ERK-p38δ+) and promotes movement of the p38δ/ERK1/2 complex into the nucleus. We propose that ERK1/2 is dephosphorylated in the nucleus by MAPK phosphatase (MKP), thus terminating ERK1/2 activity (−ERK-p38δ+). The change in the ratio of p38δ to ERK1/2 activity promotes activation of nuclear apoptotic responses, such as the phosphorylation of c-jun, and also signals to the mitochondria to begin membrane breakdown and release of proapoptotic effects, including cytochome c. Release of these reagents ultimately leads to caspase activation and cell death. The dotted line indicates the possibility that the p38δ/ERK1/2 complex may also have direct effects on the mitochondria.
Acknowledgments
This study utilized the facilities of the Skin Diseases Research Center of Northeast Ohio (NIH AR39750) and the Case Western Reserve University Comprehensive Cancer Center (P30 CA43703) and was supported by a grant from the National Institutes of Health (to R.L.E.). T.E. is the recipient of a Career Development Award from the Dermatology Foundation.
REFERENCES
- 1.Balasubramanian, S., T. Efimova, and R. L. Eckert. 2002. Green tea polyphenol stimulates a Ras, MEKK1, MEK3, and p38 cascade to increase activator protein 1 factor-dependent involucrin gene expression in normal human keratinocytes. J. Biol. Chem. 277:1828-1836. [DOI] [PubMed] [Google Scholar]
- 2.Bollinger, B. W., and R. J. Bollag. 2001. 1,25-Dihydroxyvitamin D3, phospholipase D and protein kinase C in keratinocyte differentiation. Mol. Cell Endocrinol. 177:173-182. [DOI] [PubMed] [Google Scholar]
- 3.Brodie, C., and P. M. Blumberg. 2003. Regulation of cell apoptosis by protein kinase Cδ. Apoptosis 8:19-27. [DOI] [PubMed] [Google Scholar]
- 4.Brunet, A., D. Roux, P. Lenormand, S. Dowd, S. Keyse, and J. Pouyssegur. 1999. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cataisson, C., E. Joseloff, R. Murillas, A. Wang, C. Atwell, S. Torgerson, M. Gerdes, J. Subleski, J. L. Gao, P. M. Murphy, R. H. Wiltrout, C. Vinson, and S. H. Yuspa. 2003. Activation of cutaneous protein kinase Cα induces keratinocyte apoptosis and intraepidermal inflammation by independent signaling pathways. J. Immunol. 171:2703-2713. [DOI] [PubMed] [Google Scholar]
- 6.Cerutti, P. A. 1985. Prooxidant states and tumor promotion. Science 227:375-381. [DOI] [PubMed] [Google Scholar]
- 7.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 8.Chen, N., W. Ma, C. Huang, and Z. Dong. 1999. Translocation of protein kinase Cɛand protein kinase Cδ to membrane is required for ultraviolet B-induced activation of mitogen-activated protein kinases and apoptosis. J. Biol. Chem. 274:15389-15394. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Z., T. B. Gibson, F. Robinson, L. Silvestro, G. Pearson, B. E. Xu, A. Wright, C. Vanderbilt, and M. H. Cobb. 2001. MAP kinases. Chem. Rev. 101:2449-2476. [DOI] [PubMed] [Google Scholar]
- 10.Chouinard, N., K. Valerie, M. Rouabhia, and J. Huot. 2002. UVB-mediated activation of p38 mitogen-activated protein kinase enhances resistance of normal human keratinocytes to apoptosis by stabilizing cytoplasmic p53. Biochem. J. 365:133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuenda, A., J. Rouse, Y. N. Doza, R. Meier, P. Cohen, T. F. Gallagher, P. R. Young, and J. C. Lee. 1995. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 12.Darr, D., and I. Fridovich. 1994. Free radicals in cutaneous biology. J. Investig. Dermatol. 102:671-675. [DOI] [PubMed] [Google Scholar]
- 13.Dashti, S. R., T. Efimova, and R. L. Eckert. 2001. MEK6 regulates human involucrin gene expression via a p38α- and p38δ-dependent mechanism. J. Biol. Chem. 276:27214-27220. [DOI] [PubMed] [Google Scholar]
- 14.Dashti, S. R., T. Efimova, and R. L. Eckert. 2001. MEK7-dependent activation of p38 MAP kinase in keratinocytes. J. Biol. Chem. 276:8059-8063. [DOI] [PubMed] [Google Scholar]
- 15.Denning, M. F., Y. Wang, B. J. Nickoloff, and T. Wrone-Smith. 1998. Protein kinase Cδ is activated by caspase-dependent proteolysis during ultraviolet radiation-induced apoptosis of human keratinocytes. J. Biol. Chem. 273:29995-30002. [DOI] [PubMed] [Google Scholar]
- 16.Denning, M. F., Y. Wang, S. Tibudan, S. Alkan, B. J. Nickoloff, and J. Z. Qin. 2002. Caspase activation and disruption of mitochondrial membrane potential during UV radiation-induced apoptosis of human keratinocytes requires activation of protein kinase C. Cell Death. Differ. 9:40-52. [DOI] [PubMed] [Google Scholar]
- 17.Deucher, A., T. Efimova, and R. L. Eckert. 2002. Calcium-dependent involucrin expression is inversely regulated by protein kinase C (PKC)α and PKCδ. J. Biol. Chem. 277:17032-17040. [DOI] [PubMed] [Google Scholar]
- 18.Dlugosz, A. A., H. Mischak, J. F. Mushinski, and S. H. Yuspa. 1992. Transcripts encoding protein kinase C-alpha, -delta, -epsilon, -zeta, and -eta are expressed in basal and differentiating mouse keratinocytes in vitro and exhibit quantitative changes in neoplastic cells. Mol. Carcinog. 5:286-292. [DOI] [PubMed] [Google Scholar]
- 19.Dotto, G. P. 1999. Signal transduction pathways controlling the switch between keratinocyte growth and differentiation. Crit. Rev. Oral Biol. Med. 10:442-457. [DOI] [PubMed] [Google Scholar]
- 20.Eckert, R. L., T. Efimova, S. Balasubramanian, J. F. Crish, F. Bone, and S. Dashti. 2003. p38 mitogen-activated protein kinases on the body surface: a function for p38δ. J. Investig. Dermatol. 120:823-828. [DOI] [PubMed] [Google Scholar]
- 21.Eckert, R. L., T. Efimova, S. R. Dashti, S. Balasubramanian, A. Deucher, J. F. Crish, M. Sturniolo, and F. Bone. 2002. Keratinocyte survival, differentiation, and death: many roads lead to mitogen-activated protein kinase. J. Investig. Dermatol. Symp. Proc. 7:36-40. [DOI] [PubMed] [Google Scholar]
- 22.Efimova, T., A. M. Broome, and R. L. Eckert. 2003. A regulatory role for p38δ MAPK in keratinocyte differentiation: evidence for p38δa-ERK1/2 complex formation. J. Biol. Chem. 278:34277-34285. [DOI] [PubMed] [Google Scholar]
- 23.Efimova, T., A. Deucher, T. Kuroki, M. Ohba, and R. L. Eckert. 2002. Novel protein kinase C isoforms regulate human keratinocyte differentiation by activating a p38δ mitogen-activated protein kinase cascade that targets CCAAT/enhancer-binding protein alpha. J. Biol. Chem. 277:31753-31760. [DOI] [PubMed] [Google Scholar]
- 24.Efimova, T., and R. L. Eckert. 2000. Regulation of human involucrin promoter activity by novel protein kinase C isoforms. J. Biol. Chem. 275:1601-1607. [DOI] [PubMed] [Google Scholar]
- 25.Efimova, T., P. LaCelle, J. F. Welter, and R. L. Eckert. 1998. Regulation of human involucrin promoter activity by a protein kinase C, Ras, MEKK1, MEK3, p38/RK, AP1 signal transduction pathway. J. Biol. Chem. 273:24387-24395. [DOI] [PubMed] [Google Scholar]
- 26.Emoto, Y., H. Kisaki, Y. Manome, S. Kharbanda, and D. Kufe. 1996. Activation of protein kinase Cdelta in human myeloid leukemia cells treated with 1-beta-d-arabinofuranosylcytosine. Blood 87:1990-1996. [PubMed] [Google Scholar]
- 27.Fisher, G. J., A. Tavakkol, K. Leach, D. Burns, P. Basta, C. Loomis, C. E. Griffiths, K. D. Cooper, N. J. Reynolds, and J. T. Elder. 1993. Differential expression of protein kinase C isoenzymes in normal and psoriatic adult human skin: reduced expression of protein kinase C-beta II in psoriasis. J. Investig. Dermatol. 101:553-559. [DOI] [PubMed] [Google Scholar]
- 28.Fraser, A., and G. Evan. 1996. A license to kill. Cell 85:781-784. [DOI] [PubMed] [Google Scholar]
- 29.Fukunaga, M., M. Oka, M. Ichihashi, T. Yamamoto, H. Matsuzaki, and U. Kikkawa. 2001. UV-induced tyrosine phosphorylation of PKC delta and promotion of apoptosis in the HaCaT cell line. Biochem. Biophys. Res. Commun. 289:573-579. [DOI] [PubMed] [Google Scholar]
- 30.Gherzi, R., B. Sparatore, M. Patrone, A. Sciutto, and P. Briata. 1992. Protein kinase C mRNA levels and activity in reconstituted normal human epidermis: relationships to cell differentiation. Biochem. Biophys. Res. Commun. 184:283-291. [DOI] [PubMed] [Google Scholar]
- 31.Gschwendt, M., H. J. Muller, K. Kielbassa, R. Zang, W. Kittstein, G. Rincke, and F. Marks. 1994. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 199:93-98. [DOI] [PubMed] [Google Scholar]
- 32.Gutcher, I., P. R. Webb, and N. G. Anderson. 2003. The isoform-specific regulation of apoptosis by protein kinase C. Cell Mol. Life Sci. 60:1061-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heidkamp, M. C., A. L. Bayer, J. L. Martin, and A. M. Samarel. 2001. Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase C epsilon and delta in neonatal rat ventricular myocytes. Circ. Res. 89:882-890. [DOI] [PubMed] [Google Scholar]
- 34.Ishino, K., M. Ohba, M. Kashiwagi, S. Kawabe, K. Chida, and T. Kuroki. 1998. Phorbol ester-induced G1 arrest in BALB/MK-2 mouse keratinocytes is mediated by delta and eta isoforms of protein kinase C. Jpn. J. Cancer Res. 89:1126-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kashiwagi, M., M. Ohba, K. Chida, and T. Kuroki. 2002. Protein kinase Cη (PKCη): its involvement in keratinocyte differentiation. J. Biochem. 132:853-857. [DOI] [PubMed] [Google Scholar]
- 36.Kashiwagi, M., M. Ohba, H. Watanabe, K. Ishino, K. Kasahara, Y. Sanai, Y. Taya, and T. Kuroki. 2000. PKCη associates with cyclin E/cdk2/p21 complex, phosphorylates p21, and inhibits cdk2 kinase in keratinocytes. Oncogene 19:6334-6341. [DOI] [PubMed] [Google Scholar]
- 37.Kikkawa, U., H. Matsuzaki, and T. Yamamoto. 2002. Protein kinase Cδ (PKCδ): activation mechanisms and functions. J. Biochem. 132:831-839. [DOI] [PubMed] [Google Scholar]
- 38.Kim, P. K., R. Weller, Y. Hua, and T. R. Billiar. 2003. Ultraviolet irradiation increases FADD protein in apoptotic human keratinocytes. Biochem. Biophys. Res. Commun. 302:290-295. [DOI] [PubMed] [Google Scholar]
- 39.Konishi, H., E. Yamauchi, H. Taniguchi, T. Yamamoto, H. Matsuzaki, Y. Takemura, K. Ohmae, U. Kikkawa, and Y. Nishizuka. 2001. Phosphorylation sites of protein kinase Cδ in H2O2-treated cells and its activation by tyrosine kinase in vitro. Proc. Natl. Acad. Sci. USA 98:6587-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar, S., P. C. McDonnell, R. J. Gum, A. T. Hand, J. C. Lee, and P. R. Young. 1997. Novel homologues of CSBP/p38 MAP kinase: activation, substrate specificity, and sensitivity to inhibition by pyridinyl imidazoles. Biochem. Biophys. Res. Commun. 235:533-538. [DOI] [PubMed] [Google Scholar]
- 41.Kuroki, T., T. Ikuta, M. Kashiwagi, S. Kawabe, M. Ohba, N. Huh, K. Mizuno, S. Ohno, E. Yamada, and K. Chida. 2000. Cholesterol sulfate, an activator of protein kinase C mediating squamous cell differentiation: a review. Mutat. Res. 462:189-195. [DOI] [PubMed] [Google Scholar]
- 42.Leitges, M., M. Mayr, U. Braun, U. Mayr, C. Li, G. Pfister, N. Ghaffari-Tabrizi, G. Baier, Y. Hu, and Q. Xu. 2001. Exacerbated vein graft arteriosclerosis in protein kinase Cδ-null mice. J. Clin. Investig. 108:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, L., P. S. Lorenzo, K. Bogi, P. M. Blumberg, and S. H. Yuspa. 1999. Protein kinase Cδ targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol. Cell. Biol. 19:8547-8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsui, M. S., S. L. Chew, and V. A. DeLeo. 1992. Protein kinase C in normal human epidermal keratinocytes during proliferation and calcium-induced differentiation. J. Investig. Dermatol. 99:565-571. [DOI] [PubMed] [Google Scholar]
- 45.Matsumura, M., N. Tanaka, T. Kuroki, M. Ichihashi, and M. Ohba. 2003. The eta isoform of protein kinase C inhibits UV-induced activation of caspase-3 in normal human keratinocytes. Biochem. Biophys. Res. Commun. 303:350-356. [DOI] [PubMed] [Google Scholar]
- 46.Matsuzawa, A., and H. Ichijo. 2001. Molecular mechanisms of the decision between life and death: regulation of apoptosis by apoptosis signal-regulating kinase 1. J. Biochem. 130:1-8. [DOI] [PubMed] [Google Scholar]
- 47.Mellor, H., and P. J. Parker. 1998. The extended protein kinase C superfamily. Biochem. J. 332(Pt. 2):281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 49.Newton, A. C. 2001. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 101:2353-2364. [DOI] [PubMed] [Google Scholar]
- 50.Newton, A. C. 2003. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem. J. 370:361-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishizuka, Y. 1992. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258:607-614. [DOI] [PubMed] [Google Scholar]
- 52.Nishizuka, Y. 1995. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 9:484-496. [PubMed] [Google Scholar]
- 53.Ohba, M., K. Ishino, M. Kashiwagi, S. Kawabe, K. Chida, N. H. Huh, and T. Kuroki. 1998. Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol. Cell. Biol. 18:5199-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ono, K., and J. Han. 2000. The p38 signal transduction pathway: activation and function. Cell Signal. 12:1-13. [DOI] [PubMed] [Google Scholar]
- 55.Osada, S., K. Mizuno, T. C. Saido, Y. Akita, K. Suzuki, T. Kuroki, and S. Ohno. 1990. A phorbol ester receptor/protein kinase, nPKC eta, a new member of the protein kinase C family predominantly expressed in lung and skin. J. Biol. Chem. 265:22434-22440. [PubMed] [Google Scholar]
- 56.Pages, G., P. Lenormand, G. L'Allemain, J. C. Chambard, S. Meloche, and J. Pouyssegur. 1993. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA 90:8319-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peus, D., R. A. Vasa, A. Beyerle, A. Meves, C. Krautmacher, and M. R. Pittelkow. 1999. UVB activates ERK1/2 and p38 signaling pathways via reactive oxygen species in cultured keratinocytes. J. Investig. Dermatol. 112:751-756. [DOI] [PubMed] [Google Scholar]
- 58.Pouyssegur, J., V. Volmat, and P. Lenormand. 2002. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem. Pharmacol. 64:755-763. [DOI] [PubMed] [Google Scholar]
- 59.Puviani, M., A. Marconi, E. Cozzani, and C. Pincelli. 2003. Fas ligand in pemphigus sera induces keratinocyte apoptosis through the activation of caspase-8. J. Investig. Dermatol. 120:164-167. [DOI] [PubMed] [Google Scholar]
- 60.Ravagnan, L., T. Roumier, and G. Kroemer. 2002. Mitochondria, the killer organelles and their weapons. J. Cell Physiol. 192:131-137. [DOI] [PubMed] [Google Scholar]
- 61.Rosenthal, D. S., A. Velena, F. P. Chou, R. Schlegel, R. Ray, B. Benton, D. Anderson, W. J. Smith, and C. M. Simbulan-Rosenthal. 2003. Expression of dominant-negative Fas-associated death domain blocks human keratinocyte apoptosis and vesication induced by sulfur mustard. J. Biol. Chem. 278:8531-8540. [DOI] [PubMed] [Google Scholar]
- 62.Rosini, P., G. De Chiara, M. Lucibello, E. Garaci, F. Cozzolino, and M. Torcia. 2000. NGF withdrawal induces apoptosis in CESS B-cell line through p38 MAPK activation and Bcl-2 phosphorylation. Biochem. Biophys. Res. Commun. 278:753-759. [DOI] [PubMed] [Google Scholar]
- 63.Rybin, V. O., J. Guo, A. Sabri, H. Elouardighi, E. Schaefer, and S. F. Steinberg. 2004. Stimulus-specific differences in PKCδ localization and activation mechanisms in cardiomyocytes. J. Biol. Chem. 279:19350-19361. [DOI] [PubMed]
- 64.Sanz-Moreno, V., B. Casar, and P. Crespo. 2003. p38alpha isoform Mxi2 binds to extracellular signal-regulated kinase 1 and 2 mitogen-activated protein kinase and regulates its nuclear activity by sustaining its phosphorylation levels. Mol. Cell. Biol. 23:3079-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scorrano, L., and S. J. Korsmeyer. 2003. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 304:437-444. [DOI] [PubMed] [Google Scholar]
- 66.Sitailo, L. A., S. S. Tibudan, and M. F. Denning. 2002. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J. Biol. Chem. 277:19346-19352. [DOI] [PubMed] [Google Scholar]
- 67.Soltoff, S. P. 2001. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks PKCδ tyrosine phosphorylation. J. Biol. Chem. 276:37986-37992. [DOI] [PubMed]
- 68.Srivastava, J., K. J. Procyk, X. Iturrioz, and P. J. Parker. 2002. Phosphorylation is required for PMA- and cell cycle-induced degradation of protein kinase Cδ. Biochem. J. 368:349-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strait, J. B., III, J. L. Martin, A. Bayer, R. Mestril, D. M. Eble, and A. M. Samarel. 2001. Role of protein kinase C-epsilon in hypertrophy of cultured neonatal rat ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 280:H756-H766. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka, Y., M. V. Gavrielides, Y. Mitsuuchi, T. Fujii, and M. G. Kazanietz. 2003. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through the activation of p38 MAPK and inhibition of the Akt survival pathway. J. Biol. Chem. 278:33753-33762. [DOI] [PubMed]
- 71.Teraki, Y., and T. Shiohara. 1999. Apoptosis and the skin. Eur. J. Dermatol. 9:413-425. [PubMed] [Google Scholar]
- 72.Tibbetts, M. D., L. Zheng, and M. J. Lenardo. 2003. The death effector domain protein family: regulators of cellular homeostasis. Nat. Immunol. 4:404-409. [DOI] [PubMed] [Google Scholar]
- 73.Ueda, E., S. Ohno, T. Kuroki, E. Livneh, K. Yamada, K. Yamanishi, and H. Yasuno. 1996. The eta isoform of protein kinase C mediates transcriptional activation of the human transglutaminase 1 gene. J. Biol. Chem. 271:9790-9794. [DOI] [PubMed] [Google Scholar]
- 74.Vessey, D. A., K. H. Lee, and K. L. Blacker. 1992. Characterization of the oxidative stress initiated in cultured human keratinocytes by treatment with peroxides. J. Investig. Dermatol. 99:859-863. [DOI] [PubMed] [Google Scholar]
- 75.Volmat, V., M. Camps, S. Arkinstall, J. Pouyssegur, and P. Lenormand. 2001. The nucleus, a site for signal termination by sequestration and inactivation of p42/p44 MAP kinases. J. Cell Sci. 114:3433-3443. [DOI] [PubMed] [Google Scholar]
- 76.Wang, Y., S. Huang, V. P. Sah, J. Ross, Jr., J. H. Brown, J. Han, and K. R. Chien. 1998. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273:2161-2168. [DOI] [PubMed] [Google Scholar]
- 77.Waterhouse, N. J., J. E. Ricci, and D. R. Green. 2002. And all of a sudden it's over: mitochondrial outer-membrane permeabilization in apoptosis. Biochimie 84:113-121. [DOI] [PubMed] [Google Scholar]
- 78.Whitmarsh, A. J., and R. J. Davis. 1999. Signal transduction by MAP kinases: regulation by phosphorylation-dependent switches. Sci. STKE. 1999:E1. [DOI] [PubMed] [Google Scholar]
- 79.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 80.Zhang, H., X. Shi, M. Hampong, L. Blanis, and S. Pelech. 2001. Stress-induced inhibition of ERK1 and ERK2 by direct interaction with p38 MAP kinase. J. Biol. Chem. 276:6905-6908. [DOI] [PubMed] [Google Scholar]
- 81.Zhang, T., T. L. Woods, and J. T. Elder. 2002. Differential responses of S100A2 to oxidative stress and increased intracellular calcium in normal, immortalized, and malignant human keratinocytes. J. Investig. Dermatol. 119:1196-1201. [DOI] [PubMed] [Google Scholar]
- 82.Zhang, X., P. Shan, J. Alam, R. J. Davis, R. A. Flavell, and P. J. Lee. 2003. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J. Biol. Chem. 278:22061-22070. [DOI] [PubMed] [Google Scholar]
- 83.Zimmermann, K. C., C. Bonzon, and D. R. Green. 2001. The machinery of programmed cell death. Pharmacol. Ther. 92:57-70. [DOI] [PubMed] [Google Scholar]