Abstract
The F-box protein βTrcp1 controls the stability of several crucial regulators of proliferation and apoptosis, including certain inhibitors of the NF-κB family of transcription factors. Here we show that mammary glands of βTrcp1−/− female mice display a hypoplastic phenotype, whereas no effects on cell proliferation are observed in other somatic cells. To investigate further the role of βTrcp1 in mammary gland development, we generated transgenic mice expressing human βTrcp1 targeted to epithelial cells under the control of the mouse mammary tumor virus (MMTV) long terminal repeat promoter. Compared to controls, MMTV βTrcp1 mammary glands display an increase in lateral ductal branching and extensive arrays of alveolus-like protuberances. The mammary epithelia of MMTV βTrcp1 mice proliferate more and show increased NF-κB DNA binding activity and higher levels of nuclear NF-κB p65/RelA. In addition, 38% of transgenic mice develop tumors, including mammary, ovarian, and uterine carcinomas. The targeting of βTrcp1 to lymphoid organs produces no effects on these tissues. In summary, our results support the notion that βTrcp1 positively controls the proliferation of breast epithelium and indicate that alteration of βTrcp1 function and expression may contribute to malignant behavior of breast tumors, at least in part through NF-κB transactivation.
F-box proteins (FBPs) are defined by the presence of an approximately 40-amino-acid domain named the F box after the protein, cyclin F, in which it was originally identified (2). Studies in different species have shown that FBPs play a crucial role in the ubiquitin-mediated degradation of cellular regulatory proteins (e.g., cyclins, cyclin-dependent kinase inhibitors, β-catenin, IκB, etc.) (reviewed in references 11, 29, and 31). Indeed, FBPs are subunits of ubiquitin ligases named SCFs because they comprise Skp1, Cul1, and one of many FBPs.
βTrcp1 (β-transducin repeat-containing protein), the mammalian ortholog of Xenopus βTrCP (53), was identified by using either Skp1 or the pseudosubstrate Vpu as bait in two-hybrid screens (13, 41). Mammalian βTrcp1 and the paralogous protein βTrcp2 have been reported to be involved in the degradation of IκB (inhibitor of NF-κB [nuclear factor κB]) family members in response to NF-κB-activating stimuli (17, 21, 24, 25, 32, 45, 49, 52, 59-61). Several studies have reported that βTrcp1 and βTrcp2 also control β-catenin stability in mammalian cultured cells (23, 24, 30, 37, 43, 59). Furthermore, additional βTrcp substrates have been proposed: Atf4/Creb2 (35), Smad3 (18), Smad4 (57), the alpha interferon receptor (33), the prolactin receptor (38), and the disks large tumor suppressor (40). More recently, βTrcp1 and βTrcp2 have been implicated in cell cycle control. During the S and G2 phases, these two FBPs keep Cdk1 inactive by inducing the degradation of its activating phosphatase Cdc25a (9, 28). At the G2/M transition, βTrcp1 and βTrcp2 change their specificity by targeting the Cdk1-inactivating kinase Wee1 for degradation (58), resulting in Cdc25a accumulation and Cdk1 activation. Finally, in mitosis βTrcp1 and βTrcp2 promote the degradation of Emi1 (22, 42, 47), an inhibitor of the anaphase-promoting complex/cyclosome, thereby attenuating Cdk1 activity via the anaphase-promoting complex/cyclosome-mediated degradation of two activating cyclin subunits (cyclin A and cyclin B). Thus, βTrcp1 and βTrcp2 contribute to “turning off” Cdk1 in the S and G2 phases, turning it on at the G2/M transition, and turning it off again in late mitosis.
Previously, we have shown that βTrcp1 loss of function in mice does not affect viability but induces an impairment of spermatogenesis and reduced male fertility (22). In the present study, we show that mammary glands of βTrcp1−/− mice display a hypoplastic phenotype. To investigate further the role of βTrcp1 in mammary gland development, we generated and characterized a transgenic mouse model in which we targeted βTrcp1 expression to epithelial cells using the mouse mammary tumor virus (MMTV) promoter. In addition, the epithelial-tissue-specific phenotype observed in both the loss-of-function and gain-of-function mouse models was confirmed by generating a transgenic mouse mutant in which βTrcp1 expression was targeted to the lymphoid organs using the CD4 promoter and in which no phenotype was observed. The results of these studies are herein presented.
MATERIALS AND METHODS
Generation of MMTV βTrcp1 and CD4 βTrcp1 transgenic mice.
The MMTV βTrcp1 transgene construct was generated by cloning the human βTrcp1 cDNA into the pMSG expression vector that contains an MMTV long terminal repeat promoter and a simian virus 40 polyadenylation signal (15, 56). The CD4 βTrcp1 transgene construct was generated by cloning the human βTrcp1 cDNA in a plasmid containing the minimal CD4 enhancer, the minimal murine CD4 promoter, the transcriptional initiation site, and 70 bp of the untranslated first exon and part of the first intron of the murine CD4 gene (36). Constructs were injected into the pronuclei of fertilized eggs from B6D2F1 donors, which were subsequently transferred into pseudopregnant mice. The strain was maintained on a BALB/c genetic background. Screening of founder animals and their offspring was performed by genomic PCR and confirmed by Southern hybridization on genomic DNA from tail biopsy samples. The following primers were used for genomic PCR: 5′-CTGGCTATCATCACAAGAGCGGAACGGA-3′ and 5′-CCCAAGCTTCTTCCACAGCATGCCGTCA-3′.
RNA extraction and reverse transcription-PCR (RT-PCR) analysis.
Total RNA was extracted using RNeasy (QIAGEN) according to the manufacturer's instructions. RNA was quantified, and its purity was determined by standard spectrophotometric methods. cDNA was synthesized from 1 μg of the total RNA by using the Omniscript RT kit (QIAGEN). The following primers specific to human βTrcp1 were used: F, 5′-AATTCCTCAGAGAGAGAAGACT-3′, and R, 5′-TCTGGCAAAACACTAAATATAT-3′. To amplify mouse GAPDH, the following primers were used: F, 5′-GGGTGGAGCCAAACGGGTC-3′, and R, 5′-GGAGTTGCTGTTGAAGTCGCA-3′. cDNA was amplified using 1 U of Taq DNA polymerase (Fermentas Inc., Hanover, Md.), and amplification was performed in a PTC-100 thermal cycler (MJ Research Inc.) for 35 cycles (denaturation at 94°C for 1 min, annealing for 1 min, and extension at 72°C for 2 min). The annealing temperatures were as follows: for human βTrcp1, 55°C; for mouse GAPDH, 58°C. The amplification reaction products were resolved on 2.0% agarose-Tris-acetate-EDTA gels, electrophoresed at 100 V, and visualized by ethidium bromide staining.
Histology and whole-mount preparation.
The animals were monitored daily. Necropsies were performed on all animals that died spontaneously during the observation period. For histological analysis, mammary glands and other tissues were fixed overnight or longer in 10% phosphate-buffered formalin, embedded in paraffin, and sectioned. They were stained with hematoxylin and eosin for histological analysis. For whole-mount tissue preparations, mammary glands were processed as described previously (27).
Immunohistochemistry and bromodeoxyuridine (BrdU) incorporation.
For immunohistochemical staining, deparaffinized sections were immersed in methanol containing 0.03% hydrogen peroxide for 30 min to block endogenous peroxidase activity. The slides were subjected to microwaving in 10 mM citrate buffer three times for 5 min and then incubated with Protein Block Serum-Free (Dako, Tucson, Ariz.) for 30 min to block the nonspecific antibody binding sites. The slides were treated with an anti-p65/RelA polyclonal antibody (1:100; Santa Cruz) at 4°C overnight and then with EnVision+ (Dako) for 30 min. Peroxidase activity was developed using 3,3′-diaminobenzidine tetrahydrochloride in 50 mM Tris-HCl (pH 7.5) containing 0.001% hydrogen peroxidase. The sections were slightly counterstained with Mayer's hematoxylin. For the negative control, the primary antibodies were replaced with normal mouse serum.
For BrdU incorporation, a sterile solution containing 10 mg of BrdU (Sigma)/ml and 0.6 mg of fluorodeoxyuridine (Sigma)/ml in phosphate-buffered saline was injected intraperitoneally (100 μl/10 g of body weight). After 2 h, the mice were sacrificed, mammary glands were collected and fixed, and 5-μm sections were prepared. BrdU incorporation was visualized by immunohistochemistry using a BrdU detection kit (Zymed Laboratories), and the nuclei were counterstained with hematoxylin. For quantification, 10 random fields per section at a magnification of 40× were documented by photomicroscopy, and the percentage of BrdU-positive epithelial-cell nuclei relative to the total number of epithelial-cell nuclei was calculated.
Electrophoretic mobility shift assay.
The electrophoretic mobility shift assay was performed as described previously (4, 46). Briefly, 3 μl (approximately 3 μg) of cell extract was incubated for 20 min at room temperature in 20 μl of buffer (20 mM Tris [ph 7.4], 5% glycerol, 0.1% Tween 20, 0.5 mM MgCl2, 1 mM dithiothreitol, 1 mM EDTA, 50 mM KCl) containing 2 μg of poly(dI-dC) and a κB probe (100,000 cpm) labeled using Klenow fill-in. The probe was the palindromic κB probe previously described (6). The mixture was then separated on a native polyacrylamide gel that was dried and exposed for autoradiography.
Immunoblotting and Northern blotting.
Immunoblotting and Northern blotting were performed as previously described (12, 22). Antibodies to cyclin A (3), Emi1, β-catenin, and IκBα (22) were previously described. Antibodies to IκBβ, NF-κB1/p105, and keratin 18 were from Santa Cruz Biotechnology, antibody to c-Myc was from Sigma, and antibody to cyclin D1 was from Zymed.
RESULTS
Hypoplastic phenotype of βTrcp1−/− mammary gland.
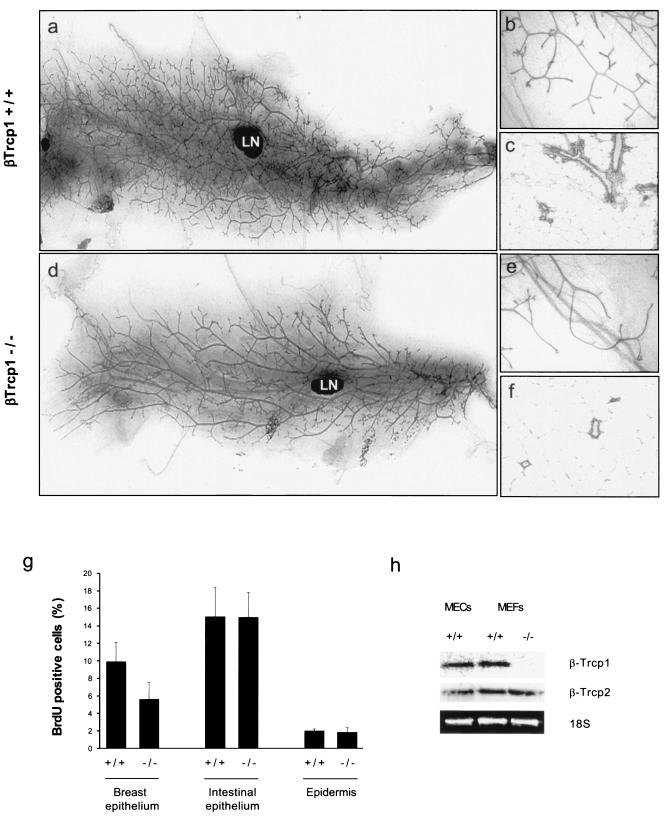
To study the function of βTrcp1 in the development of the mammary gland, we analyzed whole-mount preparations of mammary glands from wild-type and βTrcp1-deficient female virgin mice. In comparison with those of wild-type mice, mammary glands from βTrcp1−/− mice showed a hypobranching phenotype (Fig. 1a, b, d, and e). Histological examination confirmed the hypoplasia of βTrcp1−/− mammary glands (Fig. 1c and f). In virgin adults (approximately 6 months of age), this phenotype was highly penetrant (found in 9 of 10 mice). In contrast, no significant differences between the two phenotypes were observed during pubertal ductal elongation, pregnancy, and lactation, the times of maximal hormonal stimulation.
FIG. 1.
Mammary gland hypoplasia in βTrcp1−/− mice. (a to f) Whole-mount preparations (a, b, d, and e) and hematoxylin and eosin-stained sections (c and f) of inguinal mammary glands from 6-month-old virgin βTrcp1−/− mice (d to f) and wild-type littermates (a to c). LN, lymph node. (g) BrdU incorporation in the indicated organs of virgin βTrcp1−/− mice and wild-type littermates. The percentages of BrdU-positive nuclei were calculated. Error bars represent standard errors of the means. (h) Levels of βTrcp1 and βTrcp2 mRNAs in mammary epithelial cells (MECs) and mouse embryonic fibroblasts (MEFs).
To determine whether the observed defects in mammary gland development were the result of reduced cell proliferation, DNA synthesis was measured in an in vivo BrdU incorporation assay. Six-month-old virgin mice were administered BrdU, whose incorporation into DNA was then detected by immunohistochemistry (Fig. 1g). In βTrcp1−/− mammary glands, the proliferation index, calculated as the percentage of BrdU-positive cells out of the total epithelial cells, was approximately 50% of that measured in estrus-matched wild-type mice (5.6% ± 1.9% versus 9.9% ± 2.2%; P = 0.001). In contrast, no differences in BrdU incorporation were detected in other organs, such as intestine and skin (Fig. 1g). Apoptosis, measured as the percentage of cells positive by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL), at different ages was not different between the two genotypes (data not shown). The presence of a hyperplastic phenotype in the mammary glands of βTrcp1−/− mice was not the result of a lack of βTrcp2 expression in mammary epithelial cells, as shown by Northern blot analysis (Fig. 1h).
Thus, we conclude that βTrcp1 is necessary for the proper development of the mouse mammary gland under conditions of low hormonal stimuli.
Targeted expression of βTrcp1 induces hyperplasia and transformation in epithelial organs.
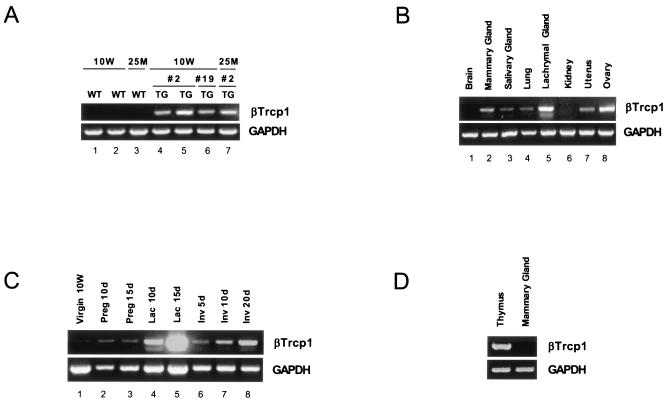
To further analyze the role of βTrcp1 in the development of the mammary gland epithelium, we generated βTrcp1 transgenic mice by placing the human βTrcp1 cDNA under the control of the MMTV long terminal repeat promoter (Fig. 2A to C). Using this construct, three independent βTrcp1 transgenic lines of mice were obtained, but all the experiments described herein were performed with two MMTV βTrcp1 transgenic lines (lines 2 and 19) that expressed similar βTrcp1 levels in the breast epithelium (Fig. 2A).
FIG. 2.
Characterization of MMTV βTrcp1 and CD4 βTrcp1 transgene expressions. (A) RT-PCR analysis of the βTrcp1 transgene expression in mammary glands. RT-PCR was performed with primers specific to human βTrcp1 or to mouse GAPDH; 1 μg of total RNA prepared from mammary glands of the indicated lines of 10-week-old (10W) and 25-month-old (25M) transgenic (TG) and wild-type (WT) virgin mice. (B) RT-PCR analysis of MMTV βTrcp1 transgene expression in the indicated organs. (C) RT-PCR analysis of transgene expression in mammary glands of 10W virgin mice or during pregnancy (Preg), lactation (Lac), or involution (Inv) at different days (d). (D) RT-PCR analysis of the CD4-βTrcp1 transgene expression in thymus and mammary gland.
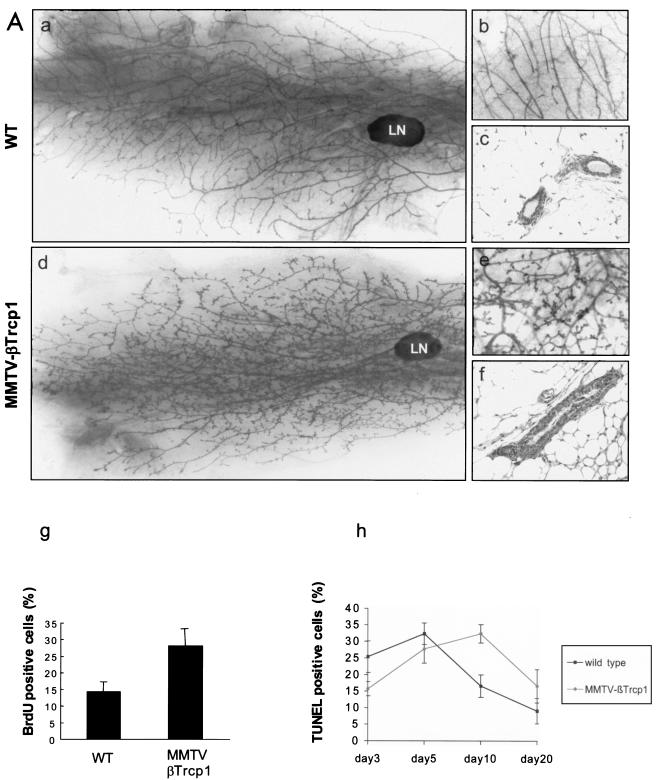
MMTV βTrcp1 mice appeared normal at birth, and their growth was indistinguishable from that of their wild-type littermates. Compared to those of wild-type virgin female mice, the mammary glands of MMTV βTrcp1 mice (six mice, approximately 10 weeks of age, for each genotype) were found by whole-mount analysis to display an increase in lateral ductal branching and extensive arrays of alveolus-like hyperplasia (Fig. 3A, panels a and d). At a higher magnification, the ducts were shorter, wider, and covered with small alveolus-like protuberances (Fig. 3A, panels b and e). In histological sections the number of ducts was increased in comparison to wild-type glands. In addition, the ductal epithelial-cell layers of MMTV βTrcp1 glands were thicker than those from wild-type mice (Fig. 3A, panels c and f), confirming the pervasive ductal hyperplasia. The observed hyperplasia of the mammary gland correlated with a twofold increase in cell proliferation, as measured by an in vivo BrdU incorporation assay (28.1% ± 5.2% versus 14.4% ± 2.9%; P = 0.016) (Fig. 3A, panel g). In contrast, apoptosis, measured as the percentage of TUNEL-positive cells, was not significantly different in either virgin (data not shown) or breeder transgenic mice during mammary involution (Fig. 3A, panel h), although it appeared somehow delayed (values at day 10 for transgenic mice were similar to values at day 5 for wild-type animals, and vice versa).
FIG. 3.
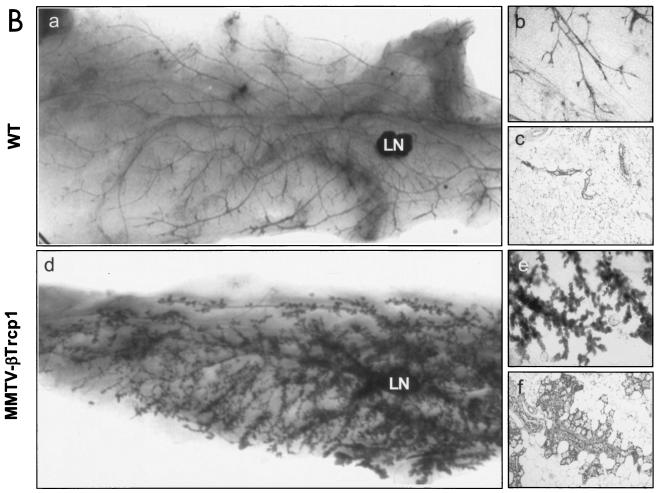
Mammary gland hyperplasia in MMTV βTrcp1 transgenic mice. (A) Whole mounts (a, b, d, and e) and hematoxylin and eosin-stained sections (c and f) of inguinal mammary glands from 10-week-old virgin MMTV βTrcp1 transgenic mice (d to f) and wild-type (WT) littermates (a to c). LN, lymph node. (g) BrdU incorporation in breast epithelium of 10-week-old virgin WT and estrus-matched MMTV βTrcp1 transgenic littermates. The percentages of BrdU-positive nuclei were calculated. (h) Percentages of TUNEL-positive nuclei at different days after removal of pups. Error bars represent standard errors of the means. (B) Whole mounts (a, b, d, and e) and hematoxylin and eosin-stained (c and f) sections of inguinal mammary glands from 22-month-old WT mice (a to c) and MMTV βTrcp1 transgenic littermates (d to f).
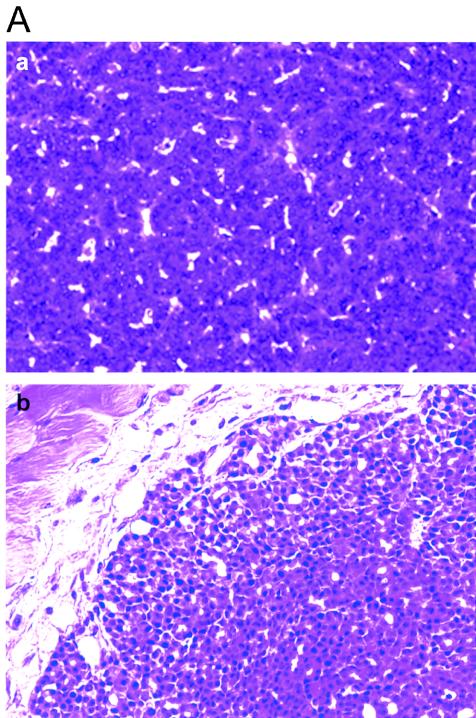
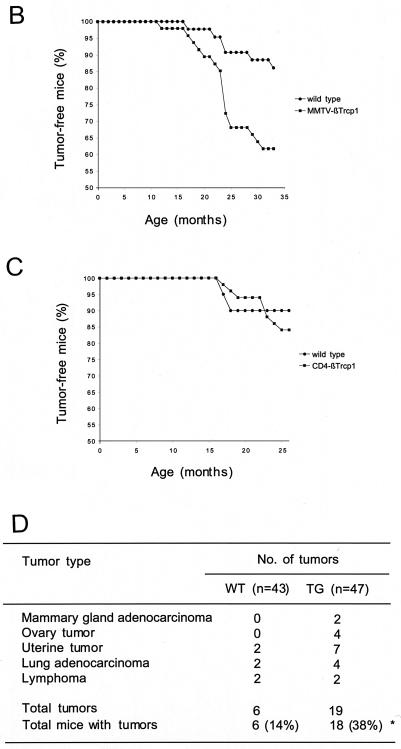
The aberrant hyperplasia of mammary glands of βTrcp1 transgenic mice was maintained throughout life. In older animals (14 to 22 months of age), the difference with the controls was dramatic (Fig. 3B, panels a to f). This mammary-gland hyperplasia was observed in 8 out of 10 virgin aged βTrcp1 transgenic mice examined, but not in wild-type virgin aged mice (0 out of 8). In addition, malignant transformation of the mammary gland was observed in two βTrcp1 transgenic animals (n = 47) but was never observed in the control mice (n = 43). Histopathological analysis indicated that these two mammary tumors were adenocarcinomas, one showing both a tubular pattern (well-formed duct-like structures) and a papillary pattern and the other displaying a solid tubular pattern (Fig. 4A). Overall, 38% of MMTV βTrcp1 females developed epithelial tumors, including mammary, ovarian, and uterine tumors, at an incidence significantly higher than that in wild-type mice (Fig. 4B and D). The presence of tumors in other epithelial tissues is not surprising since the MMTV promoter targets βTrcp1 expression not only to the mammary gland but also to uterine, ovarian, and other epithelial cells, such as those of the salivary glands, lachrymal glands, and lung (Fig. 2B).
FIG. 4.
βTrcp1 expression induces tumorigenesis in epithelial organs. (A) Hematoxylin and eosin-stained section of two mammary adenocarcinomas found in MMTV βTrcp1 mice, one with a papillo-tubular pattern (a) and the other displaying a solid tubular pattern (b). (B) Graph of tumor incidence in MMTV βTrcp1 transgenic mice and control wild-type littermates. The percentage of animals in each cohort remaining free of palpable tumors was plotted as a function of age for βTrcp1 transgenic mice (n = 47) and wild-type mice (n = 43). (C) Graph of tumor incidence in CD4 βTrcp1 transgenic mice and control wild-type littermates. The percentage of animals in each cohort remaining free of palpable tumors was plotted as a function of age for βTrcp1 transgenic mice (n = 49) and wild-type mice (n = 20). (D) Table showing tumors arising in MMTV βTrcp1 mice (TG) and control littermates (WT). There was no significant difference in the development of tumors between virgin and breeder MMTV βTrcp1 females. The difference between total number of tumors and total number of mice in the TG column (indicated by an asterisk) is due to the presence of two tumors found in the same mouse.
These results suggest that targeted expression of βTrcp1 induces hyperplasia and transformation in epithelial organs, particularly those devoted to reproduction and lactation. To confirm this specificity, we generated transgenic mice expressing βTrcp1 targeted to the T-lymphoid lineage by using the murine CD4 promoter to drive the expression in thymic T cells (Fig. 2D). Despite a βTrcp1 expression similar to that achieved in breast cells, no difference in cell proliferation (data not shown) and tumor incidence (Fig. 4C) was observed between CD4 transgenic mice and controls.
βTrcp1 activates NF-κB in breast epithelium.
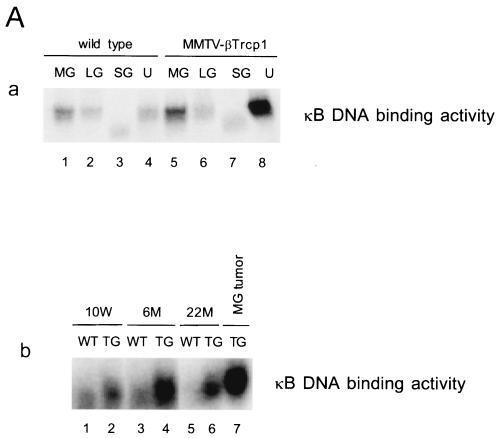
βTrcp1 mediates the ubiquitination of several cellular substrates including IκB family members (i.e., IκBα, IκBβ, and IκBɛ) resulting in their proteasome-dependent degradation and consequent activation of the NF-κB transcription activity. To determine whether βTrcp1 expression in mammary glands was associated with an increased activation of NF-κB, we examined the DNA binding activity of NF-κB in the mammary, lachrymal, and salivary glands, in the uterus, and in the ovary. Mammary gland, ovary, and uterus of 10-week-old virgin transgenic females showed higher NF-κB activity in comparison with that of wild-type females (Fig. 5A and data not shown). This increase was also evident in mammary glands of older mice (6 and 22 months old) and in breast tumors in MMTV βTrcp1 transgenic mice (Fig. 5A, panel b).
FIG. 5.
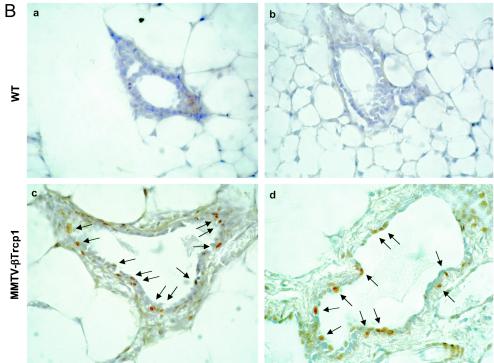
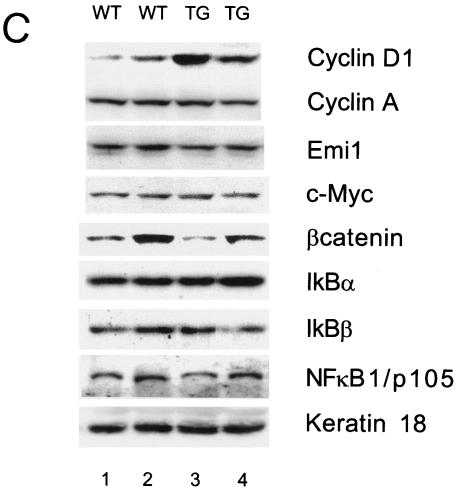
NF-κB activity is increased in MMTV βTrcp1 transgenic mice. (A) DNA binding activity of NF-κB in mammary gland (MG), lachrymal gland (LG), salivary gland (SG), and uterine horn (U) (a). NF-κB activity during the development of mammary gland (virgin mice at 10 weeks and 6 and 22 months of age) and in a breast tumor (MG tumor) in an MMTV βTrcp1 transgenic mouse (b). (B) Nuclear expression of p65/RelA in mammary glands of 10-week-old wild-type and MMTV βTrcp1 transgenic mice. Arrows show representative immunopositive cells with nuclear staining. (C) Extracts from mammary glands of two representative 10-week-old wild-type (WT) and two βTrcp1 transgenic (TG) mice were subjected to immunoblotting with the indicated antibodies (keratin 18 is shown as a control for the epithelial content of the sample).
As an independent way to test the status of the NF-κB pathway (7), we examined by immunohistochemistry the nuclear localization of p65/RelA, which increases in response to NF-κB activation. The nuclear localization of p65/RelA in mammary epithelial cells of 10-week-old MMTV βTrcp1 transgenic mice was much higher than that in wild-type animals (Fig. 5B).
Lastly, we analyzed by immunoblotting the expression of cell cycle regulatory proteins and members of the NF-κB and β-catenin pathways in mammary glands from 10-week-old virgin wild-type and βTrcp1 transgenic mice (Fig. 5C). In 75% of the cases (eight mice for each genotype), we observed a significant increase in the levels of cyclin D1 in mammary glands from βTrcp1 transgenic mice compared with those in wild-type glands. In contrast, the levels of IκBα, IκBβ, NF-κB1/p105, NF-κB2/p100, β-catenin, cyclin A, Emi1, Wee1, and c-Myc were identical in wild-type and βTrcp1 transgenic mammary glands (Fig. 5C and data not shown).
DISCUSSION
A βTrcp1-deficient mouse mutant was previously generated, and what at that time was an unsuspected role for βTrcp1 in controlling both meiosis and mitosis was found (22). In the present study, we have found that in the absence of elevated hormonal stimuli, loss of βTrcp1 induces a hypoplastic phenotype in mammary glands (Fig. 1). In addition, cell proliferation is decreased in breast epithelium but not in other organs, such as skin and intestine (Fig. 1). Similarly, we were unable to find alterations in the lymphoid compartments of βTrcp1−/− mice. We investigated the development of T and B cells and extensively tested for the presence of alterations in the immune response and defects in the apoptotic response without finding any significant difference between mutant and wild-type mice (data not shown). Accordingly, we did not observe any aberrant response of thymocytes and macrophages of βTrcp1−/− mice to a variety of stimuli or stresses (22).
To confirm a role for βTrcp1 in the development of the mammary gland, we generated an MMTV βTrcp1 transgenic mouse mutant (Fig. 2A). We found that targeting expression of βTrcp1 to the mammary gland enhances proliferation and induces hyperbranching and hyperplastic nodules (Fig. 3A), a phenotype exactly opposite to that observed in the loss-of-function model. The phenotype observed in MMTV βTrcp1 mice is most evident in aged animals, in which mammary hyperplasia is observed in 80% of cases (Fig. 3B). Moreover, 38% of MMTV βTrcp1 mice developed tumors (Fig. 4D) localized to the breast, ovary, uterus, and lung, all organs in which the MMTV promoter induces βTrcp1 expression (Fig. 2B). In contrast, targeting βTrcp1 expression to lymphoid organs using the T-cell-specific promoter CD4 did not produce any effect on either cell proliferation (data not shown) or tumor development (Fig. 4C).
NF-κB is the generic term for a family of transcription factors that regulates key genes involved in cell proliferation, apoptosis, immune responses, and inflammation (19). NF-κB is activated by a wide variety of different stimuli, including proinflammatory cytokines such as tumor necrosis factor alpha and interleukin-1, bacteria, and bacterial lipopolysaccharide, viruses, viral proteins, double-stranded RNA, and physical and chemical stresses. After stimulation, the IκB kinase, IKK, mediates phosphorylation of IκB on two critical serine residues, leading to the immediate recognition by βTrcp and consequent polyubiquitinylation of IκB via the SCFβTrcp complex (21, 24, 25, 32, 45, 49, 52, 59-61). The consequent rapid degradation of IκB by the 26S proteasome exposes the nuclear localization sequence of NF-κB family members (e.g., p50 and p65/RelA), resulting in their translocation to the nucleus, where they induce the transcription of target genes. NF-κB1/p105 functions both as a precursor of NF-κB/p50 and as a cytoplasmic inhibitor of NF-κB, and its degradation is also mediated by βTrcp (26, 34).
We found that the increase in cell proliferation observed in MMTV βTrcp1 mice associates with an activation of the NF-κB pathway determined by measuring both κB DNA binding activity and the abundance of nuclear p65/RelA (Fig. 5A and B). The positive regulation of NF-κB activity by βTrcp1 is likely to represent one of the reasons for the changes in cell proliferation observed in the mammary glands of our mouse models. Consistent with this hypothesis, an increase or a decrease in BrdU incorporation was observed in breast epithelium lacking IκBα (7) or expressing an inactive IKKα (10), respectively. These two former studies also agree with ours on another issue: in all three cases, no significant differences in apoptosis rates were observed in mammary glands upon NF-κB activation, despite the well-established role of this transcription factor in inhibiting apoptosis. Since NF-κB is particularly activated during pregnancy, peaking around days 15 to 16 post coitum and during involution, it is possible that a further increase in activity is not achievable because of a plateau. Finally, the work by Cao and colleagues shows that cyclin D1 is a critical effector of the network that controls mammary epithelial proliferation in response to NF-κB activation (10). Similarly, we found elevated levels of cyclin D1 in mammary glands of βTrcp1 transgenic mice (Fig. 5C), which display high NF-κB activity.
NF-κB activation not only plays an important role in the development of the mouse mammary gland (7, 8, 10), but it is also known as a key player in oncogenesis, promoting proliferation and inhibiting apoptosis (reviewed in reference 39). Elevated and/or constitutive NF-κB activation is found in a variety of tumors (20), including breast cancers (14, 44, 50, 51). In addition, transgenic mice overexpressing the NF-κB family member c-Rel in breast epithelium develop late-onset mammary carcinomas (48). Our data confirm and extend this notion by showing a role for βTrcp1, an activator of NF-κB, in controlling proliferation of breast epithelial cells and in contributing to their transformation. Given the well-established role of cyclin D1 in the growth control and transformation of breast epithelium, it is possible that the effect of βTrcp1 on breast tumorigenesis is, at least in part, mediated by the increase in cyclin D1 levels (Fig. 5C). In agreement with our studies of breast tumors, βTrcp1 has been found to be overexpressed in human colorectal carcinomas, and Wnt signaling induces the expression of βTrcp1 in colon cancer cell lines with a consequent upregulation of NF-κB transactivation (54). Furthermore, βTrcp2 is upregulated in tumor cell lines (including breast cancer lines) (55) and in chemically induced mouse papillomas and squamous-cell carcinomas, and this overexpression is associated with constitutive activation of NF-κB (5).
How does βTrcp1 activate NFκB in the MMTV βTrcp1 mice? Are other substrates affected by the lack or overexpression of βTrcp1 in breast and other epithelial cells? The SCFβTrcp complex targets several substrates for degradation, including IκB family members, NF-κB1/p105, β-catenin, Emi1, Cdc25a, Wee1, Atf4/Creb2, Smad3/4, the alpha interferon receptor, the prolactin receptor, and the disks large tumor suppressor. In addition, βTrcp1 activates NF-κB by inducing the processing of NF-κB2/p100 (1, 16). We could not observe in MMTV βTrcp1 or βTrcp1−/− mice any significant changes in the levels of βTrcp1 substrates by either immunoblotting (for IκBα, IκBβ, NF-κB1/p105, NF-κB2/p100, β-catenin, Emi1, and Wee1) or immunohistochemistry (β-catenin) (Fig. 5C and data not shown). However, we could observe a clear effect on NF-κB activation. A possible explanation for the lack of changes in βTrcp1-deficient mice is the redundancy with βTrcp2, which is well expressed in breast epithelial cells (Fig. 1h). In transgenic animals the explanation is more difficult; however, it needs to be reiterated that while on one hand βTrcp induces IκBα degradation, on the other, by activating NF-κB, it induces the synthesis of IκBα, obscuring IκBα proteolysis. In addition, it is possible that levels of endogenous βTrcp1 are limiting and that the forced expression of βTrcp1 induces small perturbations in the degradation of IκBα, IκBβ, IκBɛ, and NF-κB1/p105 and in the processing of NF-κB2/p100, the sum of which produces, as a final result, a prolonged and enhanced activation of NF-κB.
So far, of more than 70 human FBPs, only 4 have been well characterized and matched to downstream substrates: βTrcp1, βTrcp2, Skp2, and Fbw7. Of these, Skp2 is the product of a protooncogene, while Fbw7 is a tumor suppressor protein. The results herein demonstrate a role for βTrcp1 in the development of the mammary gland. Furthermore, our results indicate that βTrcp1 might contribute to the malignant behavior of breast and other epithelial cells by providing a gain of function, at least in part via the overactivation of NF-κB.
Acknowledgments
We thank A. Beg, P. Cowin, T. Cardozo, G. Franzoso, and S. Fuchs for helpful discussions and critical reading of the paper.
This work was supported by a fellowship from the Japanese Ministry of Culture (2001) and an IARC fellowship (2002) to Y.K., an American-Italian Cancer Foundation fellowship (1999 to 2000) and a Susan Komen Breast Cancer Foundation fellowship (2001 to 2004) to D.G., a Bernard B. Levine Foundation award to R.K.-N., an Irma T. Hirschl scholarship and grants from the NIH (R01-CA76584 and R01-GM57587) to M.P., and a Pew scholarship and an NIH grant (R01-CA79646) to L.Y.
REFERENCES
- 1.Amir, R. E., H. Haecker, M. Karin, and A. Ciechanover. 2004. Mechanism of processing of the NF-kappaB2 p100 precursor: identification of the specific polyubiquitin chain-anchoring lysine residue and analysis of the role of NEDD8-modification on the SCF(β-TrCP) ubiquitin ligase. Oncogene 23:2540-2547. [DOI] [PubMed] [Google Scholar]
- 2.Bai, C., P. Sen, K. Hofman, L. Ma, M. Goebel, W. Harper, and S. Elledge. 1996. Skp1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86:263-274. [DOI] [PubMed] [Google Scholar]
- 3.Bashir, T., N. V. Dorrello, V. Amador, D. Guardavaccaro, and M. Pagano. 2004. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428:190-193. [DOI] [PubMed] [Google Scholar]
- 4.Beg, A. A., T. S. Finco, P. V. Nantermet, and A. S. Baldwin, Jr. 1993. Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of IκBα: a mechanism for NF-κB activation. Mol. Cell. Biol. 13:3301-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia, N., J. R. Herter, T. J. Slaga, S. Y. Fuchs, and V. S. Spiegelman. 2002. Mouse homologue of HOS (mHOS) is overexpressed in skin tumors and implicated in constitutive activation of NF-kappaB. Oncogene 21:1501-1509. [DOI] [PubMed] [Google Scholar]
- 6.Bours, V., P. R. Burd, K. Brown, J. Villalobos, S. Park, R.-P. Ryseck, R. Bravo, K. Kelly, and U. Siebenlist. 1992. A novel mitogen-inducible gene product related to p50/p105-NF-κB participates in transactivation through a κB site. Mol. Cell. Biol. 12:685-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brantley, D. M., C.-L. Chen, R. S. Muraoka, P. B. Bushdid, J. L. Bradberry, F. Kittrell, D. Medina, L. M. Matrisian, L. D. Kerr, and F. E. Yull. 2001. Nuclear factor-κB (NF-κB) regulates proliferation and branching in mouse mammary epithelium. Mol. Biol. Cell 12:1445-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brantley, D. M., F. E. Yull, R. S. Muraoka, D. J. Hicks, C. M. Cook, and L. D. Kerr. 2000. Dynamic expression and activity of NF-kappaB during post-natal mammary gland morphogenesis. Mech. Dev. 97:149-155. [DOI] [PubMed] [Google Scholar]
- 9.Busino, L., M. Donzelli, M. Chiesa, D. Guardavaccaro, D. Ganoth, N. V. Dorrello, A. Hershko, M. Pagano, and G. F. Draetta. 2003. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature 426:87-91. [DOI] [PubMed] [Google Scholar]
- 10.Cao, Y., G. Bonizzi, T. N. Seagroves, F. R. Greten, R. Johnson, E. V. Schmidt, and M. Karin. 2001. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107:763-775. [DOI] [PubMed] [Google Scholar]
- 11.Cardozo, T., and M. Pagano. The SCF ubiquitin ligase: insights into a molecular machine. Nat. Rev. Mol. Cell Biol., in press. [DOI] [PubMed]
- 12.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for the ubiquitin-mediated degradation of the Cdk inhibitor p27. Nat. Cell Biol. 1:193-199. [DOI] [PubMed] [Google Scholar]
- 13.Cenciarelli, C., D. S. Chiaur, D. Guardavaccaro, W. Parks, M. Vidal, and M. Pagano. 1999. Identification of a human family of F-box proteins. Curr. Biol. 9:1177-1179. [DOI] [PubMed] [Google Scholar]
- 14.Cogswell, P. C., D. C. Guttridge, W. K. Funkhouser, and A. S. Baldwin, Jr. 2000. Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene 19:1123-1131. [DOI] [PubMed] [Google Scholar]
- 15.Daphna-Iken, D., D. B. Shankar, A. Lawshe, D. M. Ornitz, G. M. Shackleford, and C. A. MacArthur. 1998. MMTV-Fgf8 transgenic mice develop mammary and salivary gland neoplasia and ovarian stromal hyperplasia. Oncogene 17:2711-2717. [DOI] [PubMed] [Google Scholar]
- 16.Fong, A., and S. C. Sun. 2002. Genetic evidence for the essential role of beta-transducin repeat-containing protein in the inducible processing of NF-kappa B2/p100. J. Biol. Chem. 277:22111-22114. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs, S. Y., A. Chen, Y. Xiong, Z. Q. Pan, and Z. Ronai. 1999. HOS, a human homolog of Slimb, forms an SCF complex with Skp1 and Cullin1 and targets the phosphorylation-dependent degradation of IkappaB and beta-catenin. Oncogene 18:2039-2046. [DOI] [PubMed] [Google Scholar]
- 18.Fukuchi, M., T. Imamura, T. Chiba, T. Ebisawa, M. Kawabata, K. Tanaka, and K. Miyazono. 2001. Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol. Biol. Cell 12:1431-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore, T. D. 1999. The Rel/NF-kappaB signal transduction pathway: introduction. Oncogene 18:6842-6844. [DOI] [PubMed] [Google Scholar]
- 21.Gonen, H., B. Bercovich, A. Orian, A. Carrano, C. Takizawa, K. Yamanaka, M. Pagano, K. Iwai, and A. Ciechanover. 1999. Identification of the ubiquitin carrier proteins, E2s, involved in signal-induced conjugation and subsequent degradation of IkappaBalpha. J. Biol. Chem. 274:14823-14830. [DOI] [PubMed] [Google Scholar]
- 22.Guardavaccaro, D., Y. Kudo, J. Boulaire, M. Barchi, L. Busino, M. Donzelli, F. Margottin-Goguet, P. K. Jackson, L. Yamasaki, and M. Pagano. 2003. Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Dev. Cell 4:799-812. [DOI] [PubMed] [Google Scholar]
- 23.Hart, M., J. P. Concordet, I. Lassot, I. Albert, R. del los Santos, H. Durand, C. Perret, B. Rubinfeld, F. Margottin, R. Benarous, and P. Polakis. 1999. The F-box protein β-TrCP associates with phosphorylated beta-catenin and regulates its activity in the cell. Curr. Biol. 9:207-210. [DOI] [PubMed] [Google Scholar]
- 24.Hatakeyama, S., M. Kitagawa, K. Nakayama, M. Shirane, M. Matsumoto, K. Hattori, H. Higashi, H. Nakano, K. Okumura, K. Onoe, R. A. Good, and K. I. Nakayama. 1999. Ubiquitin-dependent degradation of IKBα is mediated by a ubiquitin ligase Skp1/Cul1/F-box protein Fwd1. Proc. Natl. Acad. Sci. USA 96:3859-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattori, K., S. Hatakeyama, M. Shirane, M. Matsumoto, and K. Nakayama. 1999. Molecular dissection of the interactions among IkappaBalpha, FWD1, and Skp1 required for ubiquitin-mediated proteolysis of IkappaBalpha. J. Biol. Chem. 274:29641-29647. [DOI] [PubMed] [Google Scholar]
- 26.Heissmeyer, V., D. Krappmann, E. N. Hatada, and C. Scheidereit. 2001. Shared pathways of IκB kinase-induced SCFβTrCP-mediated ubiquitination and degradation for the NF-κB precursor p105 and IκBα. Mol. Cell. Biol. 21:1024-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imbert, A., R. Eelkema, S. Jordan, H. Feiner, and P. Cowin. 2001. ΔN89β-Catenin induces precocious development, differentiation, and neoplasia in mammary gland. J. Cell Biol. 153:555-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin, J., T. Shirogane, L. Xu, G. Nalepa, J. Qin, S. J. Elledge, and J. W. Harper. 2003. SCFbeta-TRCP links Chk1 signaling to degradation of the Cdc25A protein phosphatase. Genes Dev. 17:3062-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kipreos, E., and M. Pagano. 2000. The F-box protein family. Genome Biol. 1:3002.1-3002.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitagawa, M., S. Hatakeyama, M. Shirane, M. Matsumoto, N. Ishida, K. Hattori, I. Nakamichi, A. Kikuchi, K. I. Nakayama, and K. Nakayama. 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of beta-catenin. EMBO J. 18:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koepp, D., J. W. Harper, and S. J. Elledge. 1999. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell 97:431-433. [DOI] [PubMed] [Google Scholar]
- 32.Kroll, M., F. Margottin, A. Kohl, P. Renard, H. Durand, J. P. Concordet, F. Bachelerie, F. Arenzana-Seisdedos, and R. Benarous. 1999. Inducible degradation of IkappaBalpha by the proteasome requires interaction with the F-box protein h-betaTrCP. J. Biol. Chem. 274:7941-7945. [DOI] [PubMed] [Google Scholar]
- 33.Kumar, K. G., W. Tang, A. K. Ravindranath, W. A. Clark, E. Croze, and S. Y. Fuchs. 2003. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 22:5480-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang, V., J. Janzen, G. Z. Fischer, Y. Soneji, S. Beinke, A. Salmeron, H. Allen, R. T. Hay, Y. Ben-Neriah, and S. C. Ley. 2003. βTrCP-mediated proteolysis of NF-κB1 p105 requires phosphorylation of p105 serines 927 and 932. Mol. Cell. Biol. 23:402-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassot, I., E. Ségéral, C. Berlioz-Torrent, H. Durand, L. Groussin, T. Hai, R. Benarous, and F. Margottin-Gogue. 2001. ATF4 degradation relies on a phosphorylation-dependent interaction with the SCFβTrCP ubiquitin ligase. Mol. Cell. Biol. 21:2192-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latres, E., R. Chiarle, B. Schulman, A. Pellicer, G. Inghirani, and M. Pagano. 2001. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl. Acad. Sci. USA 98:2515-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latres, E., D. S. Chiaur, and M. Pagano. 1999. The human F-box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18:849-855. [DOI] [PubMed] [Google Scholar]
- 38.Li, Y., K. G. S. Kumar, W. Tang, V. S. Spiegelman, and S. Y. Fuchs. 2004. Negative regulation of prolactin receptor stability and signaling mediated by SCFβ-TrCP E3 ubiquitin ligase. Mol. Cell. Biol. 24:4038-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin, A., and M. Karin. 2003. NF-kappaB in cancer: a marked target. Semin. Cancer Biol. 13:107-114. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani, F., and L. Banks. 2003. Regulation of the discs large tumor suppressor by a phosphorylation-dependent interaction with the beta-TrCP ubiquitin ligase receptor. J. Biol. Chem. 278:42477-42486. [DOI] [PubMed] [Google Scholar]
- 41.Margottin, F., S. P. Bour, H. Durand, L. Selig, S. Benichou, V. Richard, D. Thomas, K. Strebel, and R. Benarous. 1998. A novel human WD protein, h-βTrCP, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1:565-574. [DOI] [PubMed] [Google Scholar]
- 42.Margottin-Goguet, F., J. Y. Hsu, A. Loktev, H. M. Hsieh, J. D. Reimann, and P. K. Jackson. 2003. Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4:813-826. [DOI] [PubMed] [Google Scholar]
- 43.Nakayama, K., S. Hatakeyama, S. Maruyama, A. Kikuchi, K. Onoe, R. A. Good, and K. I. Nakayama. 2003. Impaired degradation of inhibitory subunit of NF-kappa B (I kappa B) and beta-catenin as a result of targeted disruption of the beta-TrCP1 gene. Proc. Natl. Acad. Sci. USA 100:8752-8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakshatri, H., P. Bhat-Nakshatri, D. A. Martin, R. J. Goulet, Jr., and G. W. Sledge, Jr. 1997. Constitutive activation of NF-κB during progression of breast cancer to hormone-independent growth. Mol. Cell. Biol. 17:3629-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohta, T., J. J. Michel, A. J. Schottelius, and Y. Xiong. 1999. ROC1, a homolog of APC11, represents a family of cullin partners with an associated ubiquitin ligase activity. Mol. Cell 3:535-541. [DOI] [PubMed] [Google Scholar]
- 46.Pagano, M., G. Draetta, and P. Jansen-Dürr. 1992. Association of cdk2 kinase with the transcription factor E2F during S phase. Science 255:1144-1147. [DOI] [PubMed] [Google Scholar]
- 47.Peters, J. M. 2003. Emi1 proteolysis: how SCF(beta-Trcp1) helps to activate the anaphase-promoting complex. Mol. Cell 11:1420-1421. [DOI] [PubMed] [Google Scholar]
- 48.Romieu-Mourez, R., D. W. Kim, S. M. Shin, E. G. Demicco, E. Landesman-Bollag, D. C. Seldin, R. D. Cardiff, and G. E. Sonenshein. 2003. Mouse mammary tumor virus c-rel transgenic mice develop mammary tumors. Mol. Cell. Biol. 23:5738-5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shirane, M., S. Hatakeyama, K. Hattori, and K. Nakayama. 1999. Common pathway for the ubiquitination of IkappaBalpha, IkappaBbeta, and IkappaBepsilon mediated by the F-box protein FWD1. J. Biol. Chem. 274:28169-28174. [DOI] [PubMed] [Google Scholar]
- 50.Sovak, M. A., M. Arsura, G. Zanieski, K. T. Kavanagh, and G. E. Sonenshein. 1999. The inhibitory effects of transforming growth factor beta1 on breast cancer cell proliferation are mediated through regulation of aberrant nuclear factor-kappaB/Rel expression. Cell Growth Differ. 10:537-544. [PubMed] [Google Scholar]
- 51.Sovak, M. A., R. E. Bellas, D. W. Kim, G. J. Zanieski, A. E. Rogers, A. M. Traish, and G. E. Sonenshein. 1997. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J. Clin. Investig. 100:2952-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer, E., J. Jiang, and Z. J. Chen. 1999. Signal-induced ubiquitination of IKB by the F-box protein Slimb/β-TrCP. Genes Dev. 13:284-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spevak, W., B. D. Keiper, C. Stratowa, and M. J. Castañón. 1993. Saccharomyces cerevisiae cdc15 mutants arrested at a late stage in anaphase are rescued by Xenopus cDNAs encoding N-ras or a protein with β-transducin repeats. Mol. Cell. Biol. 13:4953-4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiegelman, V., T. Slaga, M. Pagano, T. Minamoto, Z. Ronai, and S. Fuchs. 2000. Wnt/β-catenin signaling induces the expression and activity of β-Trcp ubiquitin ligase receptor. Mol. Cell 5:877-882. [DOI] [PubMed] [Google Scholar]
- 55.Spiegelman, V. S., W. Tang, A. M. Chan, M. Igarashi, S. A. Aaronson, D. A. Sassoon, M. Katoh, T. J. Slaga, and S. Y. Fuchs. 2002. Induction of homologue of Slimb ubiquitin ligase receptor by mitogen signaling. J. Biol. Chem. 277:36624-36630. [DOI] [PubMed] [Google Scholar]
- 56.Wagner, K. U., K. McAllister, T. Ward, B. Davis, R. Wiseman, and L. Hennighausen. 2001. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 10:545-553. [DOI] [PubMed] [Google Scholar]
- 57.Wan, M., Y. Tang, E. M. Tytler, C. Lu, B. Jin, S. M. Vickers, L. Yang, X. Shi, and X. Cao. 2004. Smad4 protein stability is regulated by ubiquitin ligase SCF beta-TrCP1. J. Biol. Chem. 279:14484-14487. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe, N., H. Arai, Y. Nishihara, M. Taniguchi, N. Watanabe, T. Hunter, and H. U. Osada. Ubiquitination of somatic Wee1 by SCFβTrcp is required for mitosis. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 59.Winston, J. T., P. Strack, P. Beer-Romero, C. Y. Chu, S. J. Elledge, and J. W. Harper. 1999. The SCFβ-TRCP ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13:270-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, C., and S. Ghosh. 1999. beta-TrCP mediates the signal-induced ubiquitination of IkappaBbeta. J. Biol. Chem. 274:29591-29594. [DOI] [PubMed] [Google Scholar]
- 61.Yaron, A., A. Hatzubai, M. Davis, I. Lavon, S. Amit, A. M. Manning, J. S. Andersen, M. Mann, F. Mercurio, and N. Y. Ben. 1998. Identification of the receptor component of the IkappaBalpha-ubiquitin ligase. Nature 396:590-594. [DOI] [PubMed] [Google Scholar]