Abstract
The persistence of latently HIV-infected cellular reservoirs represents the major obstacle to virus eradication in patients under antiretroviral therapy (ART). Cure strategies to eliminate these reservoirs are thus needed to reactivate proviral gene expression in latently infected cells. In this study, we tested optimal concentrations of PKC agonist candidates (PEP005/Ingenol-3-angelate, prostratin, bryostatin-1, and JQ1) to reactivate HIV latency in vitro, and examined their effects on cell survival, activation and epigenetic histone methylation after treatment alone or in combination in cell line and isolated CD4 T cells from SIV-infected macaques. The results showed that PKC agonists increased cell activation with different degrees of latency reactivation, concomitant with reduced levels of histone methylation. With increasing concentrations, prostratin and byrostain-1 treatment rapidly reduced cell survival and cell activation. The PKC agonist combinations, or in combination with JQ1, led to modest levels of synergistic reactivation of HIV. Remarkably, PEP005 treatment alone caused marked reactivation of HIV latency, similar to PMA stimulation. These findings suggested that PEP005 alone, as indicated its lower cytotoxicity and lower effective dose inducing maximal reactivation, might be a candidate for effectively reactivating HIV latency as part of a therapeutic strategy for HIV infection.
Antiretroviral therapy (ART), using a combination of three or more antiretroviral drugs, has dramatically reduced HIV-1 replication and viremia below the clinical limit of detection and prevents the rate of progression to AIDS. However, residual low-level replication-competent HIV-1 still persists in a latent state in the form of integrated and transcriptionally silent proviruses. Long-lived viral reservoirs are unaffected by long-term ART or host immune responses, resulting in lifelong infection and viral rebound to pre-treatment levels in the vast majority of HIV-1-infected individuals when ART is discontinued1,2,3,4,5,6,7. Lymphoid tissues such as tonsil and lymph node have been described as the major sites for HIV persistence, replication and latency8,9. The persistence of latent HIV-infected cellular reservoirs represents the major hurdle to virus eradication in patients treated with ART. The stability of HIV reservoirs is consistent with long-term survival of naïve, resting memory CD4+ cells (over 20 years). Since the transcription of HIV genes depends on the activation state of cells, the integrated HIV DNA is transcriptionally silent in these cells, and therefore unaffected by ART10. Thus, the “shock and kill” strategy has been proposed to antagonize HIV-1 latency in viral reservoirs by several therapeutic agents in combination with ART. Recently, doctors and scientists in the UK report that new pioneering anti-retroviral treatment in HIV+ patients, in combination with boosted immune responses and a latent reversing agent (LRA)-vorinostat called the “kick and kill” strategy, may have worked in an HIV patient, as indicated undetectable viral load in blood, albeit it is uncertain whether this patient has truly been cured (http://www.iflscience.com/health-and-medicine/a-british-man-may-have-been-cured-of-hiv/). Cells harboring latent HIV provirus are activated by cytokines (e.g., IL-2)11,12, lipopolysaccharides13, bacterial superantigens14, anti-T cell antibodies (OKT3)15, and small-molecule LRAs including histone deacetylase inhibitors and Protein Kinase C (PKC) agonists16. Upon reactivated by LRAs, the virus latently infected cells could be eliminated though viral cytopathic effects or host cytolytic T lymphocyte (CTL) responses17,18,19. Histone modification contributes to regulation of active gene expression and latency reactivation. For examples, histone acetylation is associated with increased transcription while deacetylation induces gene repression. Histone deacetylase inhibitors (HDACi), including suberoylanilide hydroxamic acid (SAHA; Vorinostat), romidepsin, and panobinostat, are thus used to activate viral latency or cancer cells through suppression of histone deacetylases that enzymatically remove the acetyl group from histones20,21,22,23,24. In contrast to HDACi, natural or semisynthetic protein kinase C (PKC) activators, could strongly reactivate HIV in cell line models and primary CD4+ T cells without inducing tumor formation17,25,26,27. The PKC pathway plays an important role in cellular latency reactivation via a NF-κB signaling as well as by a positive transcription elongation factor b (P-TEFb)-dependent manner. Various small-molecule PKC activators, including PEP005, prostratin and bryostatin-1, are helpful to reactivate HIV latency. PEP005 (ingenol-3-angelate), an activator of protein kinase C (PKC), induces nuclear translocation of PKCδ, exhibiting activity of clinical anti-tumor and actinic keratosis28,29,30,31, and potential for HIV latency reactivation32,33,34. Prostratin is another PKC activator that extracted from the tropical plant, Homalanthus nutans with potent anti-tumor and cell activation properties. Prostratin not only induces HIV expression from latently infected cells through phosphorylation and degradation of IκBα, leading to the rapid nuclear translocation of NF-κB35, but also inhibits HIV entry by interacting with a cellular target necessary for viral entry, displaying potent antiviral activity against different strains of HIV-1 and SIV36,37,38,39. Likewise, PKC agonist bryostatin-1, isolated from the marine invertebrate Bugula neritina40,41, also shows excellent potential as a therapeutic agent that acts through modulation of PKC signal transduction, as indicated its potency to revert HIV-1 latency via the adenosine monophospate (AMP)-activated protein kinase (AMPK) pathway25,42,43,44,45,46. However, recent study of bryostatin-1 treatment shows inhibitory effects on the HIV-specific CD8+ T cells47. To fully enhance reactivation of HIV latency, these LRAs, their combination or in combination with others, are examined in cell line models in vitro48. JQ1 is another small molecule inhibitor of the bromodomain and extra-terminal (BET) family of bromodomain proteins with high affinity for BRD4 that associates with acetylated chromatin for active transcription. JQ1 competitively binds to BRD4, thereby preventing BRD4 from binding to positive transcription elongation factor b (P-TEFb)49. Since JQ1 displaces P-TEFb from BRD4, resulting in formation of P-TEFb/HIV Tat complexes and subsequent recruitment to the HIV LTR, JQ1 is thus believed to specifically initiate HIV gene expression in HIV-infected cells50,51. Therefore, it is valuable to seek compounds suitable for HIV reactivation from reservoirs with low or minimal cytotoxicity, especially for assessing their potential for therapeutic applications in vivo.
In this study, we tested various concentrations of Protein kinase C (PKC) agonists (PEP005/Ingenol-3-angelate, prostratin and bryostatin-1) for their reactivation potential in HIV latency cell models in vitro, and compared their effects on cell survival, activation and histone methylation after treatment alone, in combination, or in combination with JQ1. Effects of optimized PKC agonist treatment alone or combination on the reactivation of primary CD4 T cell isolated from SIV-infected macaques were also evaluated.
Results
Optimal dosing for maximal effects of small-molecule compounds, and their combination on reactivation of HIV latency in vitro
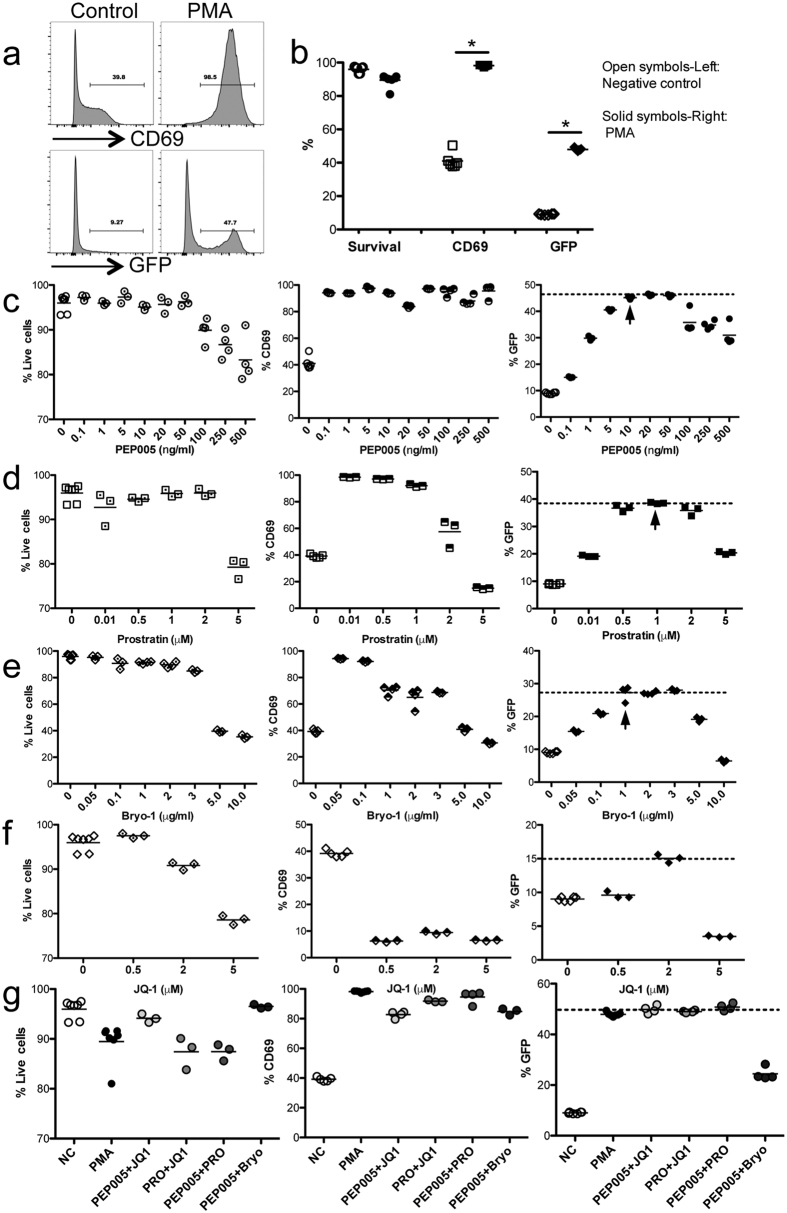
The J-Lat-Tat-GFP cell line is derived from Jurkat T lymphocytes, which bear the integrated HIV LTR-Tat and GFP gene52,53,54. Resting cells are GFP negative, but cell activation leads to HIV-1 LTR-driven GFP expression. As indicated in Fig. 1a and b, after PMA (final 5 ng/ml) stimulation for 24 h, higher percentage of cells (~45%) expressed GFP, compared with unstimulated controls (~5%). In addition to GFP expression, cells stimulated by PMA also significantly increased early cell activation (CD69). These results indicate the J-Lat-Tat-GFP cell line represents an appropriate cell model to evaluate reactivation of HIV latency in vitro. To determine the optimal concentration of each PKC agonist and JQ1 in inducing HIV latency reversal, cells were cultured in the presence of a varying concentrations of each compound for 24 h, then harvested to detect cell survival, GFP and CD69 expression. The data show PKC agonists displayed different concentration-dependent effects on cell activation and GFP expression. Optimal concentrations accompanied by >90% cell survival were as follows: PEP005 (10 ng/ml; GFP, ~45%; CD69, ~94%); prostratin (1 μM or 390 μg/ml; GFP, ~38.5%; CD69, ~92%); and bryostatin-1 (1 μg/ml; GFP, ~28%; CD69, ~70%). At these concentrations, PEP005 treatment showed the maximal potential HIV latency reactivation as indicated by GFP expression (~45%), followed by prostratin (~38.5%), and the lowest was bryostatin-1 (~28%). With increasing concentrations, GFP expression was rapidly reduced in prostratin treated (from 1 μM to 5 μM) or byrostatin-1 treated (from 1 μg/ml to 10 μg/ml) cells, concomitant with reduced cell survival at the highest concentrations tested (<80% or 40%, respectively), compared with a broad range of tolerance to PEP005 treatment (from 10 ng/ml to 500 ng/ml, with cell survival >80% even at the highest concentration) (Fig. 1c–e).
Figure 1. Small-molecule compounds reactivate HIV latency in a dose-dependent manner in J-Lat-Tat-GFP cells in vitro.
(a) Representative histogram of CD69 and GFP expression in cells in the presence or absence of PMA stimulation for 24 hrs; (b) Levels of cell survival, CD69 and GFP expression in control or PMA stimulated cells. *P < 0.05. The small-molecule compounds include PEP005 (c), prostratin (d) and bryostatin-1 (e), and JQ1 (f). Cells (5 × 105/well) were cultured in the presence of different concentration of each compound for 24 hrs. Cell survival, the early cell activation marker CD69, and GFP expression were analyzed. Note the effects of PEP005 treatment alone on HIV latency reactivation were identical to that of PMA stimulation. (g) The levels of cell survival, CD69 and GFP expression in presence of optimal concentration of PKC activators in combination, with or without JQ1. These data are representative of at least three independent experiments.
We also tested the bromodomain inhibitor JQ1 that has been previously shown to synergize with PEP005 or prostratin to re-activate latently infected cells in vitro32,51. JQ1 is a small molecule inhibitor of the bromodomain and extra-terminal (BET) family of bromodomain proteins with the high affinity for BRD4. BRD4 is associates with acetylated chromatin for active transcription. JQ1 competitively binds to BRD4, thereby preventing BRD4 from binding to positive transcription elongation factor b (P-TEFb)49. Here we tested the effects of JQ1 treatment alone for its capacity to induce HIV latency reactivation, as indicated in Fig. 1f. However, note only ~15% cells expressed GFP after JQ1 treatment. PEP005 treatment alone induced identical cell reactivation levels to those of PMA, and significantly higher levels of cell reactivation than JQ1, prostratin, bryostatin-1 or SAHA (data not shown) treatments alone. Combined, these findings indicated that of the drugs tested alone, PEP005 was the best candidate to efficiently reactivate HIV latency by inducing the highest levels of cell activation and latency reversal but with the lowest cytotoxicity.
Recent reports indicate that PKC agonists, in combination with JQ1, exhibit synergism in reactivation of latent HIV32,45. In this study, PKC agonists in combination, and with JQ1 were evaluated for their potential to reactivate HIV latency. Using the combination of optimal concentration of each compound, our data showed that combinations of PEP005+JQ1, prostratin+JQ1, or PEP005+prostratin, synergistically induced HIV latency reactivation to some extent, but the PEP005+bryostatin-1 combination did not (Fig. 1g). Consistent with results in J-Lat cell lines, treatment with PKC agonist alone or in combination also showed activation of primary CD4 T cells isolated from rhesus macaques, as indicated by significant increases of CD69 and HLA-DR expression. However, PEP005, in combination with prostratin, bryotatin-1 or JQ1, did not increase cell activation, compared with PEP005 alone (Fig. 2a and b). These results suggested that PEP005 alone may be enough to efficiently reactivate HIV latency as combinations provided minimal to no benefits.
Figure 2. Effects of compound treatment alone or/and combination on HIV latency reactivation in primary CD4 T cells from SIV-infected rhesus macaques in vitro.
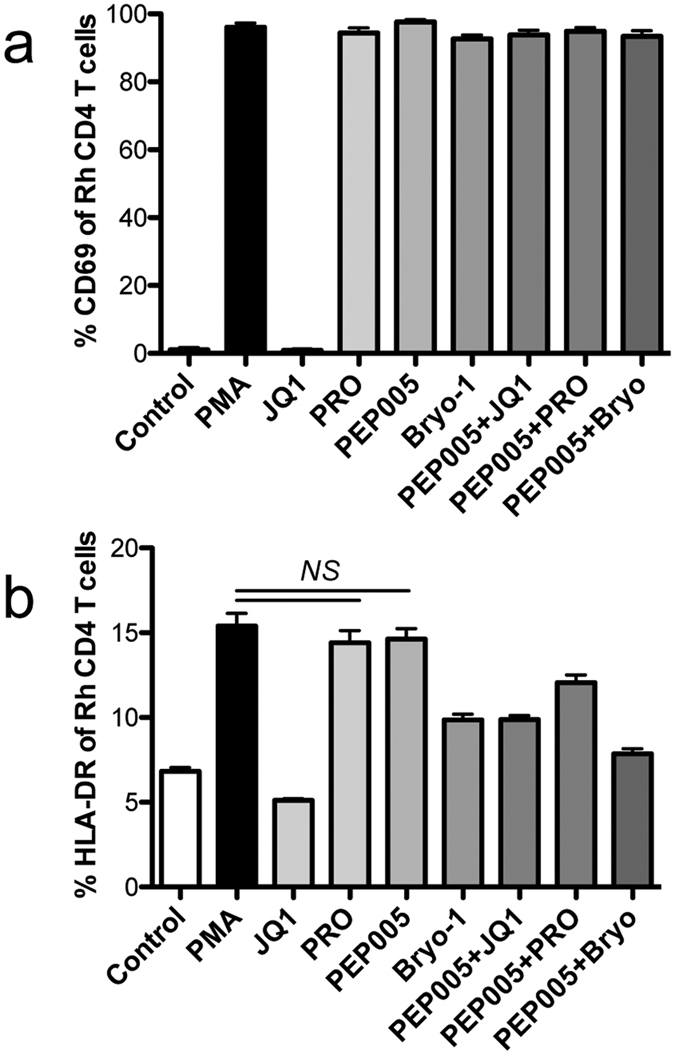
CD4 T cell activation (CD69, (a) or HLA-DR, (b) were analyzed before and after stimulation for 24 h. CD4 T cells were negatively isolateed by microbeads. These data are representative of at least three independent experiments.
Effects of PKC agonist, in combination with PEP005 or JQ1, on levels of H3K27me3 in latently infected cells
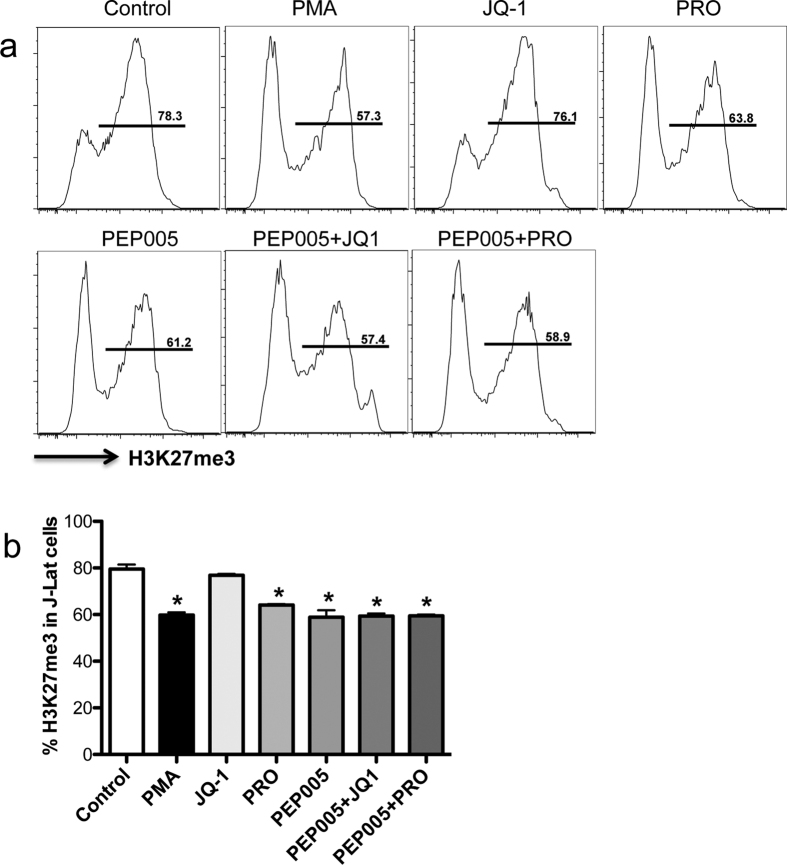
Epigenetic histone modification at histone post-translational levels, such as histone 3 lysine 27 (H3K27) trimethylation (H3K27me3), is affiliated with chromosomal condensation and gene repression55,56. We thus examined the changes of H3K27me3 in compound-treated J-Lat cells. The data showed that PKC agonist treatment alone or in combination could significantly reduce levels of H3K27me3, compared with JQ1 treatment alone or unstimulated controls (Fig. 3a and b). These results indicated that levels of H3K27me3 reflected activation states of latently infected cells, which suggest the PKC agonists promote histone demethylation, cell reactivation, and downstream active transcription.
Figure 3. Changes in histone 3 lysine 27 (H3K27) trimethylation in Jat-Lat-Tat-GFP cells treated with compounds alone, and/or in combination in vitro.
(a) Representative histogram of H3K27me3 expression in cell lines treated with compounds alone or combination; (b) Statistical analysis of H3K27me3 expression in cell lines treated with compounds alone or combination. These data are representative of at least three independent experiments. *P < 0.05.
Discussion
Combined antiretroviral therapy (cART) is effective in suppressing HIV replication, but the persistence of latently HIV-infected cellular reservoirs remains the major obstacle to virus eradication. Given the need for cure strategies to purge residual viral reservoirs in patients on ART, the search for new potential compounds capable of efficiently reactivating viral latency, is a major priority.
To eliminate viral latency, histone deacetylase inhibitors (HDACi), such as SAHA, are used to reactivate virus latently cells, and to promote virion release and lytic gene expression from an extremely small minority of viral reservoir, which is induced by herpes simplex virus (HSV), human cytomegalovirus (HCMV) or HIV20,23,24,57,58. However, recent studies indicate SAHA cannot effectively reverse latency in cells from patients on ART, or reduce the size of the latent reservoir, despite effectively inhibiting histone deacetylases59,60,61. SAHA may successfully increase viral transcription, but fails to effectively enhance viral translation61. Moreover, SAHA treatment may increase susceptibility of uninfected CD4 T cell to HIV, and impair virus-specific CTLs62,63. In comparison, synthetic analogues of protein kinase C (PKC) agonists represent HIV latency reversing agents (LRAs) of interest. We thus tested and comprehensively evaluated these PKC agonists for maximal efficiency in inducing cell reactivation, without inducing cytotoxicity in vitro.
The effects of these three PKC agonists on cell activation, HIV LTR-driven GFP expression, and cytotoxicity were tested over a broad range of doses and combinations in vitro. As indicated in Fig. 1c–e, these three PKC agonists demonstrated different extents of latency reactivation in our assays while maintaining >90% cell survival. Notably, PEP005 induced maximal GFP expression (~45%), compared with prostratin (~38.5%), bryostatin-1 (~28%) and PMA positive controls (~47%). Importantly, the optimal concentration of PEP005 was far lower in inducing maximal HIV latency reactivation (10 ng/ml), compared with prostratin (1 μM or 390 μg/ml) and bryostatin-1 1 μg/ml). With increasing concentrations, the levels of GFP expression were rapidly reduced to 20% in prostratin (5 times of optimal concentration) or byrostatin-1 treatment (5 times of optimal concentration), accompanied with lower cell survival (prostratin, <80%; bryostatin-1, 40%), whereas PEP005 treatment still maintained high levels of GFP expression (>30%) as well as cell survival (>80%) even at 50 times the optimal concentration. These findings indicated that PEP005 possesses high efficiency to reactivate HIV latency with minimal cytotoxicity.
Given JQ-1 could specifically reactivate HIV-infected cells50,51, JQ1 treatment combined with other PKC agonists may increase specificity of target cells. We tested the ability of JQ1 treatment alone and in combination with PKC agonists here. However, HIV Tat expression is limited in latently HIV-infected cells, and JQ1 treatment alone cannot fully initiate HIV gene expression, as indicated in our results that JQ1 treatment induces minimal GFP expression from cells (~15%, compared to ~10% in controls) (Fig. 1f). In contrast, PKC agonists treatment in combination with JQ1, modestly increased levels of cell activation and reactivation of HIV latency, and reduced histone methylation (Figs 1g and 3), consistent with recent reports showing combined treatment causes release of HIV viruses to levels similar to positive control stimulation in ex vivo cultures45. However, Jiang et al. reports that PEP005, in combination with JQ1, increases reactivation (~30% GFP+ cells) and increased levels of HIV RNA transcripts in HIV latently infected cells (~260) in vitro, compared with PEP005 treatment alone in reactivation (~15%) and relative levels of HIV RNA (~40) by LTR region measurement32. This discrepancy may be attributed to differences in data analysis, as it is known that HIV transcripts in infected cells largely correlate with cell activation64. For examples, raltegravir treatment reduces T-cell activation, which is higher at baseline in subjects with detectable 2-LTR circles65,66,67. In our experiments, cell reactivation and GFP expression were examined in live cells, as dead cells were excluded by Aqua Live/Dead cell staining. JQ1 plays role in reactivation of HIV latency via HIV Tat expression in HIV-infected cells, relying on state of cell activation. In fact JQ1 treatment alone did not increase, but reduced T cell activation, as indicated by lower levels of CD69/HLA-DR in J-Lat cell or rhesus primary CD4 T cells (Fig. 2). Importantly, the in vivo half-life of JQ1 in plasma is only ~1 h (intravenous injection) or 1.4 hrs (oral administration)68,69, thus limiting its potential for clinical applications. Due to this limitation of JQ1 in HIV latency reactivation, we thus evaluated whether other PKC agonists or combinations could increase reactivation of virus-infected cells. Our data showed that PEP005 could significantly increase GFP expression, cell activation and histone demethylation, similar to PMA stimulation. However, the combination of PEP005 with others did not significantly produce reactivation of HIV latency. These findings suggested that PEP005 alone, showing higher efficiency and lower cytotoxicity, may be the better candidate for HIV latency reactivation and for potential cure strategies in HIV therapy.
Methods
Ethics statement
All animals in this study were housed at the Tulane National Primate Research Center in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International standards. All studies were reviewed and approved by the Tulane University Institutional Animal Care and Use Committee. Animal housing and studies were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH, AAALAC #000594) and with the recommendations of the Weather all report; “The use of non-human primates in research”. All clinical procedures were carried out under the direction of a laboratory animal veterinarian. All procedures were performed under anesthesia using ketamine, and all efforts were made to minimize stress, improve housing conditions, and to provide enrichment opportunities (e.g., objects to manipulate in cage, varied food supplements, foraging and task-oriented feeding methods, interaction with caregivers and research staff).
Cell culture and treatments in vitro
Jurkat-Lat Tat-GFP (A1) T cell line, which is infected with retroviruses containing LTR-Tat-IRES-GFP, and expresses green fluorescence protein (GFP) upon reactivation53,54, was used to study HIV latency and reactivation in this study. This cell line was obtained from NIH AIDS Reagent Program. For primary CD4 T cells, mononuclear cells from peripheral blood were isolated from chronically SIV-infected rhesus macaques. CD4 T cells were isolated by MACS Pan T-Cell Isolation Kit, biotinylated anti-CD8 (BioLegend) and anti-Biotin microbeads (Miltenyi). The cells were cultured in an RPMI 1640 medium with 10% fetal bovine serum (FBS), 1% of Pen/Strep, and 1% L-Glutamine. The cells were maintained in 37 °C with 5% CO2. To test cell reactivation, cells (5 × 105) were treated with a series of concentrations of PEP005 (R&D), Prostratin (R&D), Bryostatin-1 (SIGMA), SAHA (SIGMA), or JQ1 (R&D) in 96-well U-bottom plates for 24 h. The phorbol-12-myristate-13-acetate (PMA, SIGMA) was used as positive control (5 ng/ml). In combination treatments, all compounds were tested using the optimized concentrations to reactivated HIV latency. After stimulation, cells were harvested and analyzed by flow cytometry using Live/Dead cell discrimination/activation markers, and for GFP expression as below.
Phenotyping
Cells were stained with: CD3 (SP34), CD8 (SK1), CD4 (L200), CD69 (FN50), and HLA-DR (L243). All antibodies and reagents were purchased from BD Biosciences Pharmingen (San Diego, CA) unless otherwise noted. For J-Lat Tat-GFP cell line, cells were stained with surface CD69 and intracellular H3K27me3 (Cell Signaling). Cell viability was analyzed by Aqua Live/Dead cell staining kit (Invitrogen). Stained samples were resuspended in BD Stabilizing Fixative (BD Biosciences) and acquired on a BD FACS Verse flow cytometer (Becton Dickinson). Data was analyzed with Flow Jo software (Tree star, Ashland, OR).
Statistics
Graphical presentation and statistical analysis of the data were performed using GraphPad Prism 4.0 (GraphPad Software, SanDiego, CA). Comparisons between groups were analyzed by a one-way ANOVA and Mann-Whitney T-test. P values < 0.05 were considered statistically significant.
Additional Information
How to cite this article: Brogdon, J. et al. In vitro effects of the small-molecule protein kinase C agonists on HIV latency reactivation. Sci. Rep. 6, 39032; doi: 10.1038/srep39032 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We thank Meagan Watkins and Eunice C. Vincent for technical support. This work was supported by NIH grants R01 DE025432, R01 AI084793, and R01 AI099795. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author Contributions J.B. and W.Z. performed most of the experiments; X.W. assisted with manuscript preparation; H.X. wrote the manuscript and designed the experiments. R.S.V. edited the manuscript.
References
- Barouch D. H. & Deeks S. G. Immunologic strategies for HIV-1 remission and eradication. Science 345, 169–174, doi: 10.1126/science.1255512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997). [DOI] [PubMed] [Google Scholar]
- Siliciano J. D. et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature medicine 9, 727–728, doi: 10.1038/nm880 (2003). [DOI] [PubMed] [Google Scholar]
- Strain M. C. et al. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci USA 100, 4819–4824, doi: 10.1073/pnas.0736332100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun T. W., Davey R. T. Jr., Engel D., Lane H. C. & Fauci A. S. Re-emergence of HIV after stopping therapy. Nature 401, 874–875, doi: 10.1038/44755 (1999). [DOI] [PubMed] [Google Scholar]
- Dinoso J. B. et al. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. Journal of virology 83, 9247–9257, doi: 10.1128/JVI.00840-09 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeraert L., Kraus G. & Pomerantz R. J. Hide-and-seek: the challenge of viral persistence in HIV-1 infection. Annu Rev Med 59, 487–501, doi: 10.1146/annurev.med.59.062806.123001 (2008). [DOI] [PubMed] [Google Scholar]
- Xu H. et al. Persistent Simian Immunodeficiency Virus Infection Drives Differentiation, Aberrant Accumulation, and Latent Infection of Germinal Center Follicular T Helper Cells. Journal of virology 90, 1578–1587, doi: 10.1128/JVI.02471-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connick E. et al. Compartmentalization of Simian Immunodeficiency Virus Replication within Secondary Lymphoid Tissues of Rhesus Macaques Is Linked to Disease Stage and Inversely Related to Localization of Virus-Specific CTL. J Immunol 193, 5613–5625, doi: 10.4049/jimmunol.1401161 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankson J. N., Persaud D. & Siliciano R. F. The challenge of viral reservoirs in HIV-1 infection. Annu Rev Med 53, 557–593, doi: 10.1146/annurev.med.53.082901.104024 (2002). [DOI] [PubMed] [Google Scholar]
- Chun T. W., Engel D., Mizell S. B., Ehler L. A. & Fauci A. S. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med 188, 83–91 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag R. M. et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J Clin Immunol 21, 218–226 (2001). [DOI] [PubMed] [Google Scholar]
- Moriuchi H., Moriuchi M., Mizell S. B., Ehler L. A. & Fauci A. S. In vitro reactivation of human immunodeficiency virus 1 from latently infected, resting CD4+ T cells after bacterial stimulation. J Infect Dis 181, 2041–2044, doi: 10.1086/315496 (2000). [DOI] [PubMed] [Google Scholar]
- Shen Y. et al. Clinical latency and reactivation of AIDS-related mycobacterial infections. J Virol 78, 14023–14032, doi: 10.1128/JVI.78.24.14023-14032.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu G. K. et al. Anti-HIV designer T cells progressively eradicate a latently infected cell line by sequentially inducing HIV reactivation then killing the newly gp120-positive cells. Virology 446, 268–275, doi: 10.1016/j.virol.2013.08.002 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T. A., Tolstrup M. & Sogaard O. S. Reversal of Latency as Part of a Cure for HIV-1. Trends Microbiol 24, 90–97, doi: 10.1016/j.tim.2015.11.003 (2016). [DOI] [PubMed] [Google Scholar]
- Bullen C. K., Laird G. M., Durand C. M., Siliciano J. D. & Siliciano R. F. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 20, 425–429, doi: 10.1038/nm.3489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks S. G. HIV: Shock and kill. Nature 487, 439–440, doi: 10.1038/487439a (2012). [DOI] [PubMed] [Google Scholar]
- Archin N. M. & Margolis D. M. Emerging strategies to deplete the HIV reservoir. Current opinion in infectious diseases 27, 29–35, doi: 10.1097/QCO.0000000000000026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin N. M. et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485, doi: 10.1038/nature11286 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin N. M. et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci USA 109, 9523–9528, doi: 10.1073/pnas.1120248109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. Q. et al. Effect of suberoylanilide hydroxamic acid (SAHA) administration on the residual virus pool in a model of combination antiretroviral therapy-mediated suppression in SIVmac239-infected indian rhesus macaques. Antimicrob Agents Chemother 58, 6790–6806, doi: 10.1128/AAC.03746-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaher R. J. et al. Histone deacetylase inhibitors induce reactivation of herpes simplex virus type 1 in a latency-associated transcript-independent manner in neuronal cells. J Neurovirol 11, 306–317, doi: 10.1080/13550280590952817 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna B. A. et al. Transient activation of human cytomegalovirus lytic gene expression during latency allows cytotoxic T cell killing of latently infected cells. Sci Rep 6, 24674, doi: 10.1038/srep24674 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChristopher B. A. et al. Designed, synthetically accessible bryostatin analogues potently induce activation of latent HIV reservoirs in vitro. Nat Chem 4, 705–710, doi: 10.1038/nchem.1395 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary D. C. & Peterlin B. M. Targeting the latent reservoir to achieve functional HIV cure. F1000Res 5, doi: 10.12688/f1000research.8109.1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beans E. J. et al. Highly potent, synthetically accessible prostratin analogs induce latent HIV expression in vitro and ex vivo. Proc Natl Acad Sci USA 110, 11698–11703, doi: 10.1073/pnas.1302634110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson P. et al. PEP005, a selective small-molecule activator of protein kinase C, has potent antileukemic activity mediated via the delta isoform of PKC. Blood 106, 1362–1368, doi: 10.1182/blood-2004-10-4117 (2005). [DOI] [PubMed] [Google Scholar]
- Fidler B. & Goldberg T. Ingenol mebutate gel (picato): a novel agent for the treatment of actinic keratoses. P T 39, 40–46 (2014). [PMC free article] [PubMed] [Google Scholar]
- Li L. et al. The skin cancer chemotherapeutic agent ingenol-3-angelate (PEP005) is a substrate for the epidermal multidrug transporter (ABCB1) and targets tumor vasculature. Cancer Res 70, 4509–4519, doi: 10.1158/0008-5472.CAN-09-4303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S. et al. A randomized prospective trial of the postoperative quality of life between laparoscopic uterine artery ligation and laparoscopy-assisted vaginal hysterectomy for the treatment of symptomatic uterine fibroids: clinical trial design. Trials 10, 8, doi: 10.1186/1745-6215-10-8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G. et al. Synergistic Reactivation of Latent HIV Expression by Ingenol-3-Angelate, PEP005, Targeted NF-kB Signaling in Combination with JQ1 Induced p-TEFb Activation. PLoS Pathog 11, e1005066, doi: 10.1371/journal.ppat.1005066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrilow D., Gardner J., Darnell G. A., Suhrbier A. & Harrich D. HIV type 1 inhibition by protein kinase C modulatory compounds. AIDS Res Hum Retroviruses 22, 854–864, doi: 10.1089/aid.2006.22.854 (2006). [DOI] [PubMed] [Google Scholar]
- Spivak A. M. et al. Ex Vivo Bioactivity and HIV-1 Latency Reversal by Ingenol Dibenzoate and Panobinostat in Resting CD4(+) T Cells from Aviremic Patients. Antimicrob Agents Chemother 59, 5984–5991, doi: 10.1128/AAC.01077-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. A. et al. Prostratin antagonizes HIV latency by activating NF-kappaB. J Biol Chem 279, 42008–42017, doi: 10.1074/jbc.M402124200 (2004). [DOI] [PubMed] [Google Scholar]
- Biancotto A. et al. Dual role of prostratin in inhibition of infection and reactivation of human immunodeficiency virus from latency in primary blood lymphocytes and lymphoid tissue. J Virol 78, 10507–10515, doi: 10.1128/JVI.78.19.10507-10515.2004 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witvrouw M. et al. Potent and selective inhibition of HIV and SIV by prostratin interacting with viral entry. Antivir Chem Chemother 14, 321–328 (2003). [DOI] [PubMed] [Google Scholar]
- Sanchez-Duffhues G. et al. Activation of latent HIV-1 expression by protein kinase C agonists. A novel therapeutic approach to eradicate HIV-1 reservoirs. Curr Drug Targets 12, 348–356 (2011). [DOI] [PubMed] [Google Scholar]
- Hezareh M. Prostratin as a new therapeutic agent targeting HIV viral reservoirs. Drug News Perspect 18, 496–500, doi: 10.1358/dnp.2005.18.8.944543 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Preclinical pharmacology of the natural product anticancer agent bryostatin 1, an activator of protein kinase C. Cancer Res 56, 802–808 (1996). [PubMed] [Google Scholar]
- Amador M. L., Jimeno J., Paz-Ares L., Cortes-Funes H. & Hidalgo M. Progress in the development and acquisition of anticancer agents from marine sources. Ann Oncol 14, 1607–1615 (2003). [DOI] [PubMed] [Google Scholar]
- Mehla R. et al. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS One 5, e11160, doi: 10.1371/journal.pone.0011160 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C. et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 30, 1385–1392, doi: 10.1097/QAD.0000000000001064 (2016). [DOI] [PubMed] [Google Scholar]
- Martinez-Bonet M. et al. Synergistic Activation of Latent HIV-1 Expression by Novel Histone Deacetylase Inhibitors and Bryostatin-1. Sci Rep 5, 16445, doi: 10.1038/srep16445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcis G. et al. An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression. PLoS Pathog 11, e1005063, doi: 10.1371/journal.ppat.1005063 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz L. et al. Bryostatin activates HIV-1 latent expression in human astrocytes through a PKC and NF-kB-dependent mechanism. Sci Rep 5, 12442, doi: 10.1038/srep12442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker-Sperling V. E., Pohlmeyer C. W., Tarwater P. M. & Blankson J. N. The Effect of Latency Reversal Agents on Primary CD8+ T Cells: Implications for Shock and Kill Strategies for Human Immunodeficiency Virus Eradication. EBioMedicine 8, 217–229, doi: 10.1016/j.ebiom.2016.04.019 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird G. M. et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest 125, 1901–1912, doi: 10.1172/JCI80142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loven J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334, doi: 10.1016/j.cell.2013.03.036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D. et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell Cycle 12, 452–462, doi: 10.4161/cc.23309 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Guo J., Wu Y. & Zhou Q. The BET bromodomain inhibitor JQ1 activates HIV latency through antagonizing Brd4 inhibition of Tat-transactivation. Nucleic Acids Res 41, 277–287, doi: 10.1093/nar/gks976 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula S. V. et al. HIV-1 induced nuclear factor I-B (NF-IB) expression negatively regulates HIV-1 replication through interaction with the long terminal repeat region. Viruses 7, 543–558, doi: 10.3390/v7020543 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A., Defechereux P. & Verdin E. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J 20, 1726–1738, doi: 10.1093/emboj/20.7.1726 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A., Bisgrove D. & Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J 22, 1868–1877, doi: 10.1093/emboj/cdg188 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barski A. et al. High-resolution profiling of histone methylations in the human genome. Cell 129, 823–837, doi: 10.1016/j.cell.2007.05.009 (2007). [DOI] [PubMed] [Google Scholar]
- Ferrari K. J. et al. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol Cell 53, 49–62, doi: 10.1016/j.molcel.2013.10.030 (2014). [DOI] [PubMed] [Google Scholar]
- Contreras X. et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem 284, 6782–6789, doi: 10.1074/jbc.M807898200 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei D. G. et al. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog 10, e1004071, doi: 10.1371/journal.ppat.1004071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cillo A. R. et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci USA 111, 7078–7083, doi: 10.1073/pnas.1402873111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. Q. et al. Effect of SAHA administration on the residual virus pool in a model of combination antiretroviral therapy-mediated suppression in SIVmac239-infected Indian rhesus macaques. Antimicrob Agents Chemother, doi: 10.1128/AAC.03746-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi P. et al. Dynamics of HIV latency and reactivation in a primary CD4+ T cell model. PLoS Pathog 10, e1004156, doi: 10.1371/journal.ppat.1004156 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucera M. B. et al. The histone deacetylase inhibitor vorinostat (SAHA) increases the susceptibility of uninfected CD4+ T cells to HIV by increasing the kinetics and efficiency of postentry viral events. Journal of virology 88, 10803–10812, doi: 10.1128/JVI.00320-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B. et al. Histone deacetylase inhibitors impair the elimination of HIV-infected cells by cytotoxic T-lymphocytes. PLoS Pathog 10, e1004287, doi: 10.1371/journal.ppat.1004287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun T. W. et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest 115, 3250–3255, doi: 10.1172/JCI26197 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon M. J. et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med 16, 460–465, doi: 10.1038/nm.2111 (2010). [DOI] [PubMed] [Google Scholar]
- Llibre J. M. et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antivir Ther 17, 355–364, doi: 10.3851/IMP1917 (2012). [DOI] [PubMed] [Google Scholar]
- Wu Y. HIV-1 gene expression: lessons from provirus and non-integrated DNA. Retrovirology 1, 13, doi: 10.1186/1742-4690-1-13 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabucco S. E. et al. Inhibition of bromodomain proteins for the treatment of human diffuse large B-cell lymphoma. Clin Cancer Res 21, 113–122, doi: 10.1158/1078-0432.CCR-13-3346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073, doi: 10.1038/nature09504 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]