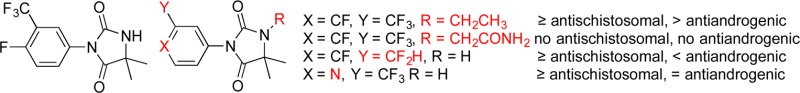Abstract
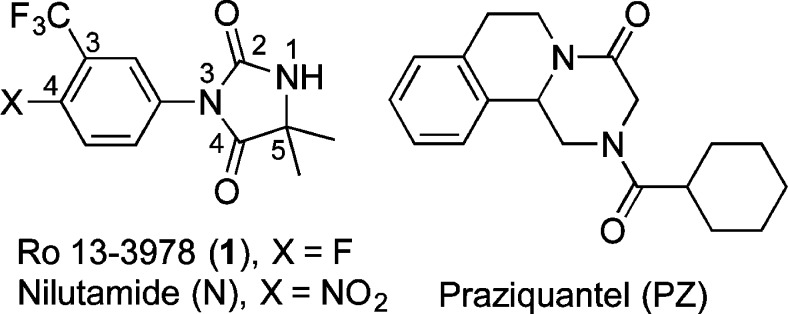
The aryl hydantoin 1 (Ro 13-3978) was identified in the early 1980s as a promising antischistosomal lead compound. However, this series of aryl hydantoins produced antiandrogenic side effects in the host, a not unexpected outcome given their close structural similarity to the antiandrogenic drug nilutamide. Building on the known SAR of this compound series, we now describe a number of analogs of 1 designed to maximize structural diversity guided by incorporation of substructures and functional groups known to diminish ligand–androgen receptor interactions. These analogs had calculated polar surface area (PSA), measured LogD7.4, aqueous kinetic solubility, and estimated plasma protein binding values in ranges predictive of good ADME profiles. The principal SAR insight was that the hydantoin core of 1 is required for high antischistosomal activity. We identified several compounds with high antischistosomal efficacy that were less antiandrogenic than 1. These data provide direction for the ongoing optimization of antischistosomal hydantoins.
Schistosomiasis is a tropical parasitic disease caused by infections with flukes of the genus Schistosoma.1 Of these, Schistosoma mansoni, S. hematobium, and S. japonicum cause the largest public health burden.2,3 Praziquantel (PZ) is the only drug available for treatment of this disease.4−6 The high drug pressure from the widespread administration of PZ could lead to problematic drug resistance.7,8 Even so, the discovery of a new drug for schistosomiasis continues to elude us, although several antischistosomal lead compounds and repurposed drugs have been identified in recent years.9−14
The introduction of PZ in 1982 likely led to decisions to abandon the development of a number of promising antischistosomal agents that were discovered during the same time period. One of these was 1 (Ro 13-3978) (Figure 1), the lead compound from a series of aryl hydantoins that were investigated in some detail at Hoffmann La-Roche.15−18 As reported by Link and Stohler,181 has high oral efficacy against all three major schistosome species—S. mansoni, S. hematobium, and S. japonicum—in a range of animal models. Confirming these data, we found that 1 had single oral dose ED50 values of 15 and 140 mg/kg against adult and juvenile S. mansoni in a mouse model.19 In this same schistosome mouse model, PZ is considerably less effective against adult S. mansoni, with reported ED50 values ranging from 172 to 202 mg/kg,18,20 and it has no significant activity against juvenile stages of the parasite. Despite the high in vivo antischistosomal efficacy of 1, we found that this aryl hydantoin at concentrations up to 170 μM had almost no effect on adult S. mansoni in vitro.19 Data generated so far indicate that active metabolites do not account for the striking difference between the in vitro and in vivo antischistosomal activity of 1.19
Figure 1.
However, this series of aryl hydantoins produced antiandrogenic side effects in the host,15 a not unexpected outcome given their close structural similarity to the antiandrogenic drug nilutamide (N). We recently demonstrated that N, but not the three structurally diverse androgen receptor (AR) antagonists flutamide, bicalutamide, and cyproterone acetate, has weak, but measurable, antischistosomal activity in S. mansoni-infected mice.21 As phylogenetic evidence indicates that schistosome species do not appear to have AR’s,22 these data led us to hypothesize that, for aryl hydantoins and related heterocycles, the structural requirements for antischistosomal efficacy and AR binding interactions are divergent. In this respect, 1 had no measurable interaction with the AR in a ligand competition assay, but it did block DHT-induced cell proliferation in an androgen-dependent cell line.23 Despite its antiandrogenic liability, Link and Stohler18 observed no apparent toxicity following administration of a single 1250 mg/kg dose of 1 to mice.
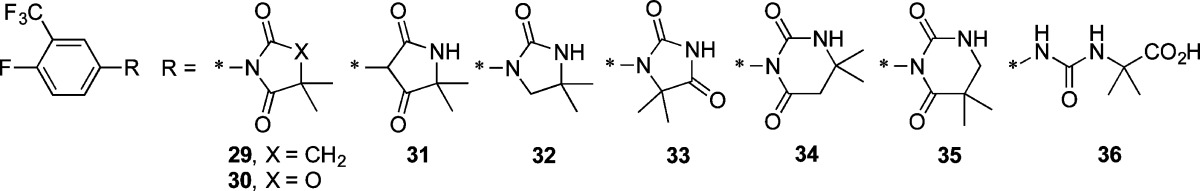
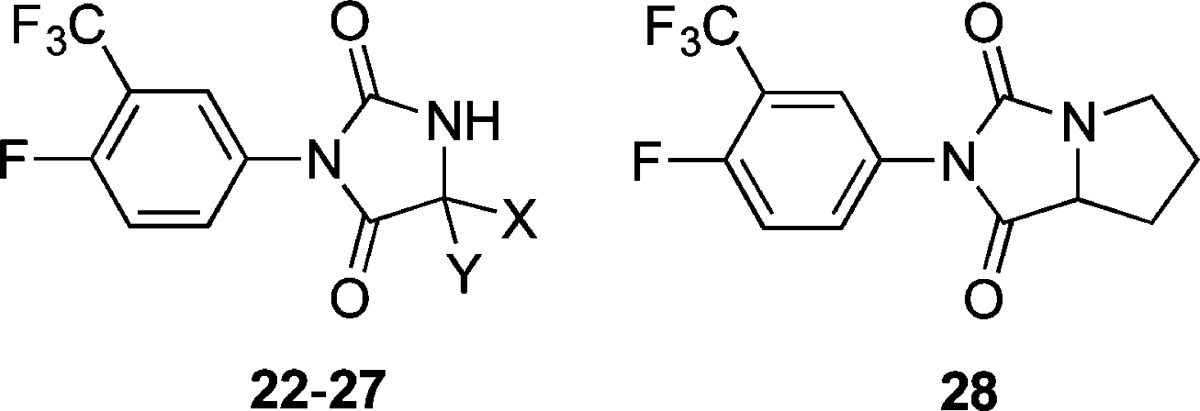
Highlights from the SAR of this aryl hydantoin compound series conducted by Hoffmann La-Roche18 are (1) a combination of halogens (F, Cl) and/or CF3 groups at positions 3 and 4 of the phenyl ring was optimal; (2) electron-donating groups such as methoxy and dimethylamino at these same positions diminished activity; (3) 4-imino derivatives were active; and (4) some N1-substituted analogs were active. Building on this foundation, we now describe a number of analogs of 1 (2–36, Tables 1–4) designed to maximize structural diversity guided by incorporation of substructures and functional groups known to diminish ligand–AR interactions. For example, several target compounds maintain the 5,5-dimethylhydantoin core of 1 and incorporate phenethyl (6), sulfonamide (7, 8), aromatic halogens (10), and C=N bonds (15–20), functional groups and structural elements demonstrated to abolish or diminish AR ligand affinity.24−28 The final set of target compounds maintains the 4-fluoro-3-trifluoromethylphenyl substructure of 1 in heterocycle variants of the 5,5-dimethylhydantoin substructure: succinimide 29, oxazolidinedione 30, oxolactam 31, urea 32, hydantoin transpositional isomer 33, and the ring-expanded dihydropyrimidinediones 34 and 35. Hydantoins analogous to 33 had relatively weak AR binding affinity.24 We now report physicochemical profiling, in vitro ADME, antiandrogenic assessment, plasma exposure, and in vivo antischistosomal activities of these compounds.
Table 1. Physicochemical, in Vitro ADME, Antiandrogenic, and Antischistosomal Data for N1-Substituted 4-Fluoro-3-trifluoromethylphenyl Hydantoins.

| Compd | R | LogD7.4a | PSA (Å2)b | cPPB (%)c | Sol2.0/Sol6.5 (μg/mL)d | h/m CLint (μL/min/mg protein)e | LAPC4 IC50 (μM)f | S. mansoni WBR (%) 1 × 100 mg/kg pog |
|---|---|---|---|---|---|---|---|---|
| 1 | H | 2.7 | 49.4 | 52.1 | >100/>100 | 8/<7 | 4.4 | 95h |
| 2 | CH3 | 3.2 | 40.6 | 40.9 | 50–100/50–100 | 16/65 | 1.3 | 93* |
| 3 | CH2CH3 | 3.5 | 40.6 | 49.3 | 50–100/25–50 | 30/32 | 0.14 | 98* |
| 4 | CH2CONH2 | 2.2 | 83.7 | 20.3 | 50–100/50–100 | <7/<7 | agonist | 25 |
| 5 | CH2CN | 3.4 | 64.4 | 48.2 | 25–50/25–50 | NDj | 0.29 | 91* |
| 6 | (CH2)2C6H5 | 4.6 | 40.6 | 96.2 | 12.5–25/25–50 | >870/740 | 2.4 | 54 |
| 7 | SO2CH3 | 3.8 | 74.8 | 66.0 | 1.6–3.1/1.6–3.1 | <7/<7 | 6.3 | 19 |
| 8 | SO2C6H5 | 4.6 | 74.8 | 96.1 | <1.6/<1.6 | 210/160 | 4.3 | 44 |
| PZ | 3.0 | 40.6 | ND | >100/>100 | 52/790 | ND | 18i |
LogD values were estimated by correlation of their chromatographic retention properties using gradient HPLC.46
Calculated using ChemAxon JChem for Excel.
Protein binding values were estimated by correlation of their chromatographic retention properties on a human albumin column.47
Compounds in DMSO were spiked into either pH 6.5 phosphate buffer or 0.01 M HCl (approximately pH 2.0) and analyzed by nephelometry to determine a concentration range.
In vitro intrinsic clearance measured in human and mouse liver microsomes.
Cells were then exposed to 10 nM DHT for 24 h in the presence of varying concentrations of test compounds.
Groups of five S. mansoni-infected NMRI mice were treated on day 49 postinfection with compounds dissolved or suspended in 7% v/v Tween 80, 3% v/v ethanol. At 28 d post-treatment, animals were sacrificed and dissected to assess total worm burden reduction (WBR). *p < 0.05 from the Kruskal–Wallis test comparing the medians of the responses between the treatment and control groups.
Data from Keiser et al.19
Data from Keiser et al.21
ND = not determined.
Table 4. Physicochemical, in Vitro ADME, Antiandrogenic, and Antischistosomal Data for 4-Fluoro-3-trifluoromethylphenyl Hydantoin Heterocyle Variants.
| Compd | LogD7.4 | PSA (Å2) | cPPB (%) | Sol2.0/Sol6.5 (μg/mL) | h/m CLint (μL/min/mg protein) | LAPC4 IC50 (μM) | S. mansoni WBR (%) 1 × 100 mg/kg po |
|---|---|---|---|---|---|---|---|
| 29 | 3.4 | 37.4 | 42.5 | 12.5–25/12.5–25 | 11/10 | 0.91 | 42 |
| 30 | 3.7 | 49.6 | 58.1 | 3.1–6.3/3.1–6.3 | 12/12a | 2.1 | 11 |
| 31 | 0.5 | 46.2 | 93.3 | 25–50/>100 | <7/<7 | >10 | 0 |
| 32 | 3.2 | 32.3 | 78.3 | 12.5–25/12.5–25 | <7/30 | 8.1 | 59 |
| 33 | 2.5 | 49.4 | 67.1 | 25–50/25–50 | <7/<7 | 2.8 | 0 |
| 34 | 2.4 | 49.4 | 15.0 | 25–50/25–50 | <7/<7 | >10 | 51b |
| 35 | 2.5 | 49.4 | 21.5 | 12.5–25/12.5–25 | 9/15 | 6.9 | 19 |
| 36 | 1.1 | 81.3 | 94.3 | 25–50/>100 | <7/<7 | 3.1 | 67 |
non-NADPH-mediated degradation observed.
2/4 mice died.
Chemistry
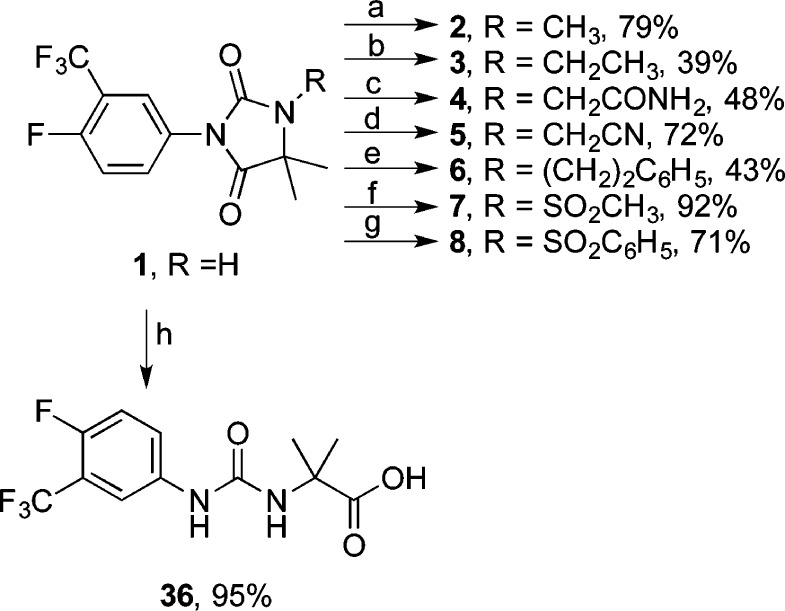
N1-Alkyl and aralkyl hydantoins 2–6 and sulfonamides 7 and 8 were obtained by N-alkylation and sulfonylation of 1 according to the methods of Van Dort and Jung28 and Jung et al.24 (Scheme 1). Urea carboxylic acid 36 was obtained in high yield by hydrolysis of 1 with aqueous NaOH followed by acidification with dilute HCl.
Scheme 1.
Reagents and conditions: (a) NaH, DMF, rt, 0.5 h, then MeI, rt, 2 h; (b) NaH, THF, 0 °C to rt, 0.5 h, then EtI, rt, 3 d; (c) NaH, THF, 0 °C to rt, 0.5 h, then 2-bromoacetamide, rt, 3 d; (d) NaH, DMF, rt, 0.5 h, then 2-bromoacetonitrile, rt, 2 h; (e) NaH, DMF, rt, 0.5 h, then 2-bromoethylbenzene, rt, 2 h; (f) NaH, THF, 0 °C to rt, 0.5 h, then MeSO2Cl, rt, 3 d; (g) NaH, DMF, rt, 0.5 h, then benzenesulfonyl chloride, rt, 2 h; (h) 2 M NaOH, rt, 4 h, then 2 M HCl.
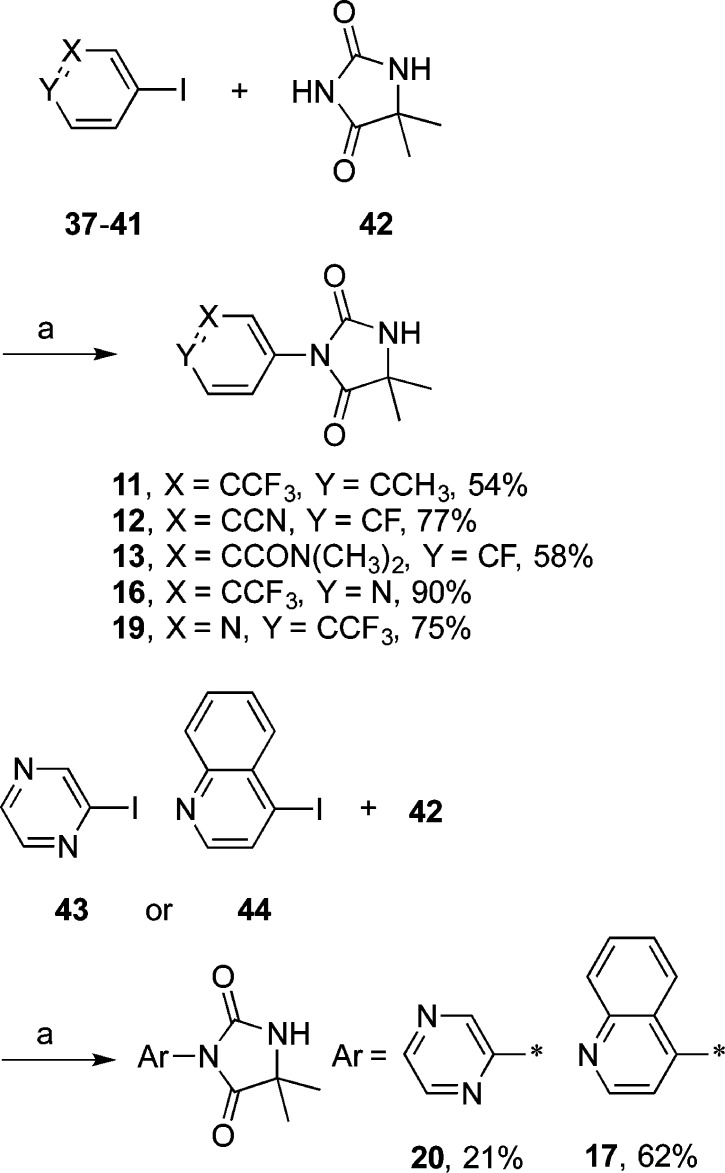
Target compounds 11–13, 16, 17, 19, and 20 were obtained in variable yields by high-temperature reactions of the corresponding aryl iodides (37–41, 43, and 44) and hyantoin 42 with cuprous oxide29,30 in dimethylacetamide (DMA) (Scheme 2).
Scheme 2.
Reagents and conditions: (a) Cu2O, DMA, 140–160 °C; 12–72 h.
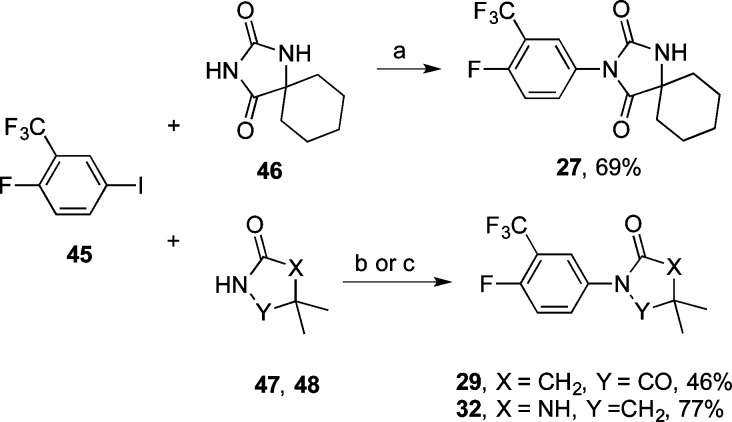
Reactions between aryl iodide 45 and spiro hydantoin 46 or succinimide 47 under these same conditions afforded 27 and 29, respectively (Scheme 3). Similarly, 14 was obtained by copper-catalyzed coupling of aryl bromide 49 and 42, whereas 21 was obtained by alkylation of benzyl bromide 50 with 42 (Scheme 4). Compound 32 was obtained in a palladium-catalyzed N-arylation reaction31 between aryl iodide 45 and imidazolidin-2-one 48(32) (Scheme 3).
Scheme 3.
Reagents and conditions: (a) Cu2O, DMA, 160 °C; 24 h; (b) Cu2O, DMF, 160 °C; 48 h (29); (c) Pd2(dba)3, Xanthphos, Cs2CO3, toluene, 90 °C; 12 h (32).
Scheme 4.
Reagents and conditions: (a) Cu2O, DMA, 160 °C, 24 h; (b) K2CO3, DMA, 85 °C; 24 h.
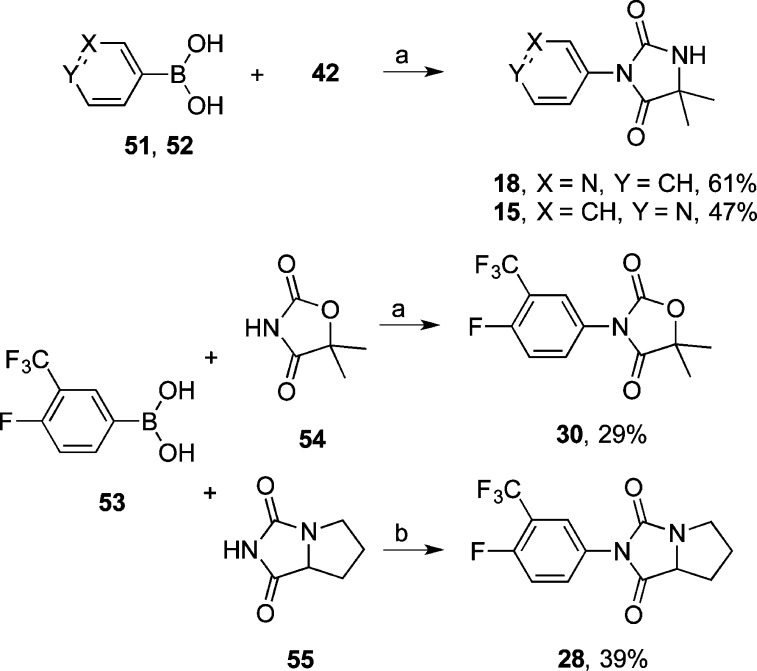
N-Arylation via copper(II) acetate promoted cross-coupling33−35 of boronic acids 51 and 52 with 42 afforded 18 and 15; the same cross-coupling reaction between boronic acid 53 and 1,3-oxazolidine-2,4-dione 54 or bicyclic hydantoin 55 afforded 30 and 28, respectively (Scheme 5).
Scheme 5.
Reagents and conditions: (a) Cu(OAc)2, MeOH, O2, 70 °C; 12 h; (b) Cu(OAc)2, pyridine, CH2Cl2, rt, 7 d.
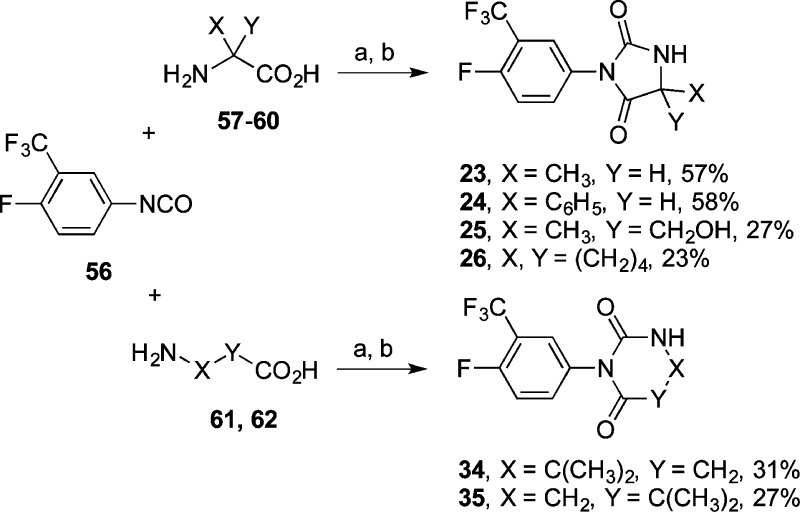
Compounds 23–26 were obtained in a two-step sequence18,27,36 by reactions between aryl isocyanate 56 and α-amino acids 57–60 in aqueous NaOH to form the corresponding urea carboxylic acids that then cyclized to the hydantoins when exposed to 2–4 M HCl at 110 °C (Scheme 6). Compounds 34 and 35 were obtained by parallel two-step reactions between 56 and β-amino acids 61 and 62. In some instances, the acyclic urea carboxylic acid reaction intermediates precipitated from the initial reactions after acidification, but these were not usually purified, and were converted directly to the desired hydantoin reaction products. For almost all of these reactions, small amounts of the insoluble symmetrical N,N-diarylurea derived from isocyanate 56 were formed.
Scheme 6.
Reagents and conditions: (a) 1–2 M NaOH, 0 °C to rt, 3–12 h; (b) 2–4 M HCl, 110 °C, 2–12 h.
Oxolactam 31 was obtained by cyclization of the anion of benzyl amide ester 66 in a Dieckmann-type condensation;3766 was obtained from the corresponding acid chloride 65 (Scheme 7). The key intermediate in the synthesis of 33 was gem-dimethyl α-amino amide 64, which was obtained in a one-pot free radical multicomponent reaction38 from aniline 63. Exposure of 64 to 2,6-diisopropylphenyl isocyanate at high temperature39 effected carbonylative ring closure to hydantoin 33. Compounds 1, 9, 10, and 22 were synthesized following procedures described by Link et al.18
Scheme 7.
Reagents and conditions: (a) 1 M TiCl4 in CH2Cl2, formamide, acetone, CH2Cl2, 0 °C, 0.5 h, then Zn, 50% H2O2 in formamide, 0 °C, 3 h; (b) 2,6-diisopropylphenyl isocyanate, toluene, 250 °C, 5 bar, 10 min, MW; (c) methyl 2-amino-2-methylpropanoate HCl, Et3N, THF, rt, 12 h; (d) NaH, THF, rt, 12 h, then aq AcOH.
Physicochemical and in Vitro ADME
It is instructive to first consider the physicochemical and in vitro ADME properties of these analogs of 1 (Tables 1–4). The calculated polar surface area (PSA) values of between 32 and 84 Å2 indicate that the polarity of these compounds is unlikely to be a rate-limiting factor for membrane permeability and oral bioavailability.40 The measured LogD7.4 values for all but two of the compounds ranged from 0 to 4, suggesting that high lipophilicity and the resulting poor aqueous solubility are unlikely to be limiting factors for oral absorption; this was largely borne out by the fairly high aqueous kinetic solubilities of many of these compounds. Compounds with low solubilities were sulfonamides 7 and 8, 5-phenylhydantoin 24, 5,5-spirocyclopentylhydantoin 27, and 1,3-oxazolidine-2,4-dione 30.
The only compounds with estimated plasma protein binding values ≥90% were aryl sulfonamide 8, N1-phenethyl 6, 5-phenyl 24, oxolactam imide 31, and urea carboxylic acid 36. Notably, these compounds featured either additional aryl substructures (6, 8, 24) or weak acid functional groups (31 and 36). In this respect, we note that 1, with its pKa value of 12.1,17 is largely un-ionized at physiological pH. The metabolic stabilities of the N1-alkyl and N1-sulfonamide hydantoin derivatives (Table 1) varied from highly stable (7), to intermediate (2, 3), to unstable (6, 8). For N1-alkyl hydantoins 2 and 3 we observed N-dealkylation metabolic reactions to form 1. Notably, 6 and 8, with their additional aryl groups, were the most lipophilic members of the series and also had the lowest metabolic stabilities. However, as seen for carboxamide 4, addition of polar functional groups in the N1-alkyl substructure can increase metabolic stability. The N3-aryl hydantoins (Table 2) and hydantoin heterocycle variants (Table 4) had high metabolic stabilities. Hydantoins substituted at the 5 position (Table 3) with combinations of methyl, hydrogen, and hydroxymethyl (1, 22, 23, 25) had high metabolic stabilities. However, incorporation of aryl (24) or spirocycloalkyl (26, 27) substructures, or linking the 5 and N1 positions by way of a pyrrolidine heterocycle (28), decreased the metabolic stabilities.
Table 2. Physicochemical, in Vitro ADME, Antiandrogenic, and Antischistosomal Data for N3-Substituted Aryl Hydantoins.
| Compd | LogD7.4 | PSA (Å2) | cPPB (%) | Sol2.0/Sol6.5 (μg/mL) | h/m CLint (μL/min/mg protein) | LAPC4 IC50 (μM) | S. mansoni WBR (%) 1 × 100 mg/kg po |
|---|---|---|---|---|---|---|---|
| N | 3.0 | 92.6 | 70.3 | 50–100/50–100 | <7/<7 | 0.60/0.45 | 31a |
| 9 | 2.5 | 49.4 | 49.3 | >100/>100 | <7/<7 | 6.0 | 80* |
| 10 | 3.0 | 49.4 | 79.6 | >100/>100 | <7/<7 | 1.3 | 75*b |
| 11 | 2.8 | 49.4 | 64.8 | >100/>100 | <7/16 | >10 | 4.8 |
| 12 | 1.7 | 73.2 | 14.6 | >100/>100 | <7/<7 | 3.7 | 10 |
| 13 | 0.9 | 69.7 | 5.9 | >100/>100 | <7/<7 | 9.1 | 30 |
| 14 | 2.2 | 49.4 | 20.4 | >100/>100 | <7/<7 | ≥10 | 94* |
| 15 | 0.5 | 62.3 | 6.7 | 50–100/>100 | <7/11 | 3.8 | 1.8 |
| 16 | 1.9 | 62.3 | 22.7 | >100/>100 | <7/<7 | 4.5 | 100* |
| 17 | 1.2 | 62.3 | 16.9 | >100/>100 | <7/<7 | 1.3 | 17 |
| 18 | 0.2 | 62.3 | 6.6 | >100/>100 | <7/<7 | 9.6 | 38 |
| 19 | 1.8 | 62.3 | 18.3 | >100/>100 | <7/<7 | >10 | 0 |
| 20 | 0.3 | 75.2 | 5.8 | >100/>100 | <7/<7 | >10 | 42 |
| 21 | 2.9 | 49.4 | 82.1 | 50–100/>100 | 17/11 | 4.2 | 56* |
Data from Keiser et al.21
1/4 mice died.
Table 3. Physicochemical, in Vitro ADME, Antiandrogenic, and Antischistosomal Data for 5-Substituted 4-Fluoro-3-trifluoromethylphenyl Hydantoins.
| Compd | X, Y | LogD7.4 | PSA (Å2) | cPPB (%) | Sol2.0/Sol6.5 (μg/mL) | h/m CLint (μL/min/mg protein) | LAPC4 IC50 (μM) | S. mansoni WBR (%) 1 × 100 mg/kg po |
|---|---|---|---|---|---|---|---|---|
| 22 | H, H | 2.0 | 49.4 | 43.6 | >100/>100 | <7/13 | 0.26 | 0 |
| 23 | CH3, H | 2.4 | 49.4 | 38.6 | >100/>100 | 17/34 | >10 | 66* |
| 24 | C6H5, H | 3.5 | 49.4 | 90.0 | 6.3–12.5/6.3–12.5 | 22/181 | 5.3 | 41 |
| 25 | CH3, CH2OH | 1.9 | 69.6 | 34.3 | 25–50/25–50 | <7/<7 | 4.8 | 0 |
| 26 | (CH2)4 | 3.2 | 49.4 | 70.4 | 50–100/50–100 | 33/93 | 0.99 | 61 |
| 27 | (CH2)5 | 3.5 | 49.4 | 74.9 | 3.1–6.3/3.1–6.3 | 25/122 | 4.2 | 42 |
| 28 | 3.0 | 40.6 | 46.0 | 25–50/25–50 | 30/144 | 3.5 | 0 |
Antiandrogenic and Antischistosomal Activities
We now consider the in vitro antiandrogenic and in vivo antischistosomal properties of these analogs of 1. The former was assessed by inhibition of dihydrotestosterone (DHT)-induced androgen luciferase reporter activity in the LAPC4 cell line, a cell line with a wild-type androgen receptor (AR), and the latter by measuring worm burden reduction (WBR) in S. mansoni-infected mice. As we had previously observed for 1, none of the compounds at concentrations up to 100 μM had activity against schistosomula or adult S. mansoni in vitro. Similarly, none of the compounds was cytotoxic at concentrations up to 30 μM against the rat skeletal myoblast L6 cell line. Contrary to our expectation based on the previous SAR for this compound class, we did not observe decreased antiandrogenic potencies for N1-substituted sulfonamides 7 and 8 or N1-phenethyl 6 (Table 1). N1-Substitution with small alkyl (2, 3) groups increased antiandrogenic potencies, most strongly for the latter. Incorporation of nitrile (5) and carboxamide (4) functional groups into the N1-alkyl substructure had very different effects; the former was a potent antiandrogen whereas the latter had no antiandrogenic properties and was instead a weak AR agonist. It is known41 that hydantoins with N1-cyanomethyl substructures are potent antiandrogens; thus, 5 served as a “negative control compound” for this SAR study. Similar to that of 1, N1-substituted hydantoins 2, 3, and 5 had high antischistosomal activities; of the remaining compounds in this series, 6 had weak antischistosomal activity.
The antiandrogenic and antischistosomal properties of the N3-substituted aryl hydantoins exhibit several interesting trends (Table 2). Replacing the 4-F in 1 with a NO2 (N) or Cl (10) increased antiandrogenic potency, whereas a H (9) or Me (11) at this same position decreased antiandrogenic potency. Of these, hydantoins 9 and 10 had high antischistosomal activities, similar to previously reported data.18 Replacing the 3-trifluoro in 1 with a nitrile (12) had no effect on antiandrogenic potency and abolished antischistosomal activity. However, replacing the 3-trifluoro in 1 with a tertiary carboxamide (13) or difluoromethyl (14) decreased antiandrogenic potency, and the latter had high antischistosomal activity. Hydantoin 21, the benzyl derivative of 1, had similar antiandrogenic potency but substantially reduced antischistosomal activity compared to the latter. As we had anticipated based on the known SAR for antiandrogenic hydantoins, we observed decreased antiandrogenic potencies for some of the derivatives with aromatic C=N bonds; these included pyridines 18 and 19 and pyrazine 20. However, the only one of the C=N containing hydantoins to have high activity against S. mansoni in vivo was 16, the 4-pyridyl derivative with a trifluoromethyl group, which at 100 mg/kg resulted in cure of all of the infected mice.
As the data in Table 3 illustrate, our initial foray into the SAR of the 5-position of 1 did not bear much fruit. The principle insight gained was to note that removing one, but not both, of the methyl groups (23) decreases antiandrogenic activity and retains significant antischistosomal activity. 5,5-Spirocycloalkyl derivatives 26 and 27 had measurable but insignificant worm burden reduction (WBR) values and were no less antiandrogenic than 1. Bicyclic hydantoin 28 reveals that connecting the 5- and N1-positions with a pyrrolidine substructure completely abolished antischistosomal activity and offered no improvement in antiandrogenic activity. Finally, the data in Table 4 shows that the hydantoin core of 1 is required for high antischistosomal efficacy. Of these, only cyclic urea 32 and urea carboxylic acid 36 had moderate antischistosomal activities. The latter is the hydrolysis product of 1 and is formed in small quantities when 1 is administered at high doses (vide infra, Figure 2a). Interestingly, as reported by Link and Stohler,18 the methyl ester of 36, with a single-dose ED50 of 62 mg/kg, has significant antischistosomal activity.
Figure 2.
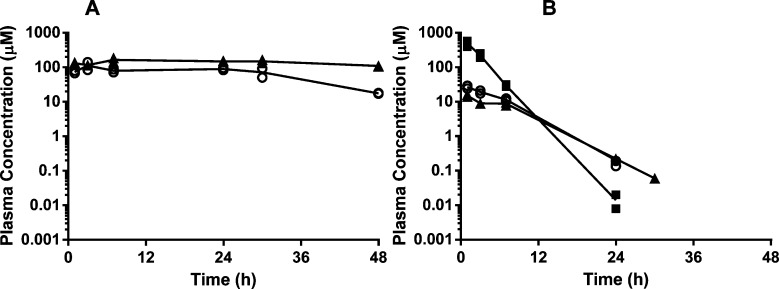
Plasma concentration versus time profiles of (A) 1 (filled triangles) and the metabolites 36 (filled circles) and 25 (open diamonds); (B) 2 (open circles) and the metabolite 1 (filled triangles); and (C) 3 (open circles) and the metabolite 1 (filled triangles) following oral administration of 100 mg/kg to male Swiss outbred mice. Symbols represent individual data points, and the lines represent the mean profiles.
Mouse Exposure
With this compound series, we have the unusual situation where lead compounds have no in vitro activity but generally exhibit in vivo activity. Therefore, to see if there was a correlation between antischistosomal efficacy and plasma exposure and to begin to assess the effect of aryl hydantoin structure on pharmacokinetics, the most active aryl hydantoins (1–3, 14, and 16) and three less active hydantoins (13, 23, 26) were administered to noninfected mice at single oral doses of 100 mg/kg. From a practical standpoint, it was necessary to assume that exposure profiles generated in noninfected mice provided a reasonable estimation of exposure in S. mansoni-infected mice.
After oral administration of 1, plasma concentrations increased until 2 h postdose, after which concentrations remained relatively constant up to 24 h, most likely due to saturated clearance processes at the very high concentrations (Figure 2A, Table 5). We also observed low but measurable concentrations of urea carboxylic acid 36, the hydantoin hydrolysis product, and 25, the hydroxymethyl metabolite. Based on values for AUC0–24h, the exposures of 36 and 25 were <1% and approximately 3%, respectively, relative to 1. Following oral administration of 2 or 3 (Figure 2B and 2C), concentrations of the parent compounds rapidly declined with subsequent formation of 1 at maximum concentrations comparable to that seen following dosing with 1 (Figure 2A).
Table 5. Exposure Parameters for Selected Aryl Hydantoins in Male Swiss Outbred Mice Following Oral Administration of 100 mg/kga.
| Compd | Cmax (μM) | Tmax (h) | AUC0-last (μM·h) |
|---|---|---|---|
| 1 | 124 | c.n.d. | 2740 |
| 2 | c.n.d. | 1 | c.n.d. |
| 3 | 11.1 | 1 | 103 |
| 13 | 488 | 1 | 1360 |
| 14 | 112 | 3 | 3190 |
| 16 | 155 | 7 | 6480 |
| 23 | 26.6 | 1 | 163 |
| 26 | 15.0 | 1 | 106 |
c.n.d. = could not determine.
Hydantoins 13, 14, 16, 23, and 26 were all rapidly absorbed; however, they differed substantially in their plasma exposure profiles. The highest and most prolonged exposures were observed for 14 and 16, where plasma concentrations were comparable to those for 1 (Figure 3A and Table 5). Both the maximum concentrations and the duration of exposures of 23 and 26 were substantially lower, likely due in part to their higher intrinsic clearance, as determined in mouse microsomes (Figure 3B and Table 5). While 13 reached a higher maximum plasma concentration than either 14 or 16, concentrations declined at a much faster rate, resulting in a considerably reduced duration of exposure (Figure 3B and Table 5). As 13 had a low intrinsic clearance in mouse microsomes, its more rapid rate of elimination is likely due to its lower log D value (0.9), resulting in a lower volume of distribution and, possibly, significant renal excretion.
Figure 3.
Plasma concentration versus time profiles of (A) 16 (filled triangles) and 14 (open circles) and (B) 13 (filled squares), 23 (open circles), and 26 (filled triangles), following oral administration of 100 mg/kg to male Swiss outbred mice. Symbols represent individual data points, and the lines represent the mean profiles.
Summary
Hydantoin 1 and its analogs 2–36 had calculated polar surface area (PSA), measured LogD7.4, aqueous kinetic solubility, and estimated plasma protein binding values in ranges predictive of good ADME profiles. For N1-alkyl and N1-sulfonamide derivatives of 1, incorporation of polar functional groups increased metabolic stability, whereas incorporation of phenyl substructures decreased metabolic stability. However, none of these possessed both decreased antiandrogenic potency and increased antischistosomal activity. N3-Aryl hydantoins, hydantoin heterocycle variants, and hydantoins substituted at the 5 position with combinations of methyl, hydrogen, and hydroxymethyl had high metabolic stabilities. The SAR of the N3-substituted aryl hydantoins was complex, but three of these had high antischistosomal efficacy and were less antiandrogenic than 1. The SAR of the 5-substituted aryl hydantoins reveals that replacing one of the methyl groups with a hydrogen atom decreases antiandrogenic activity and retains significant antischistosomal activity. Investigation of heterocycle variants showed that the hydantoin core of 1 is required for high antischistosomal activity. In this respect, recent investigations of a series of structurally distinct 4-thiohydantoins reveal that they have activity against S. mansoni in vitro;42 the best of these effected a 71% worm burden reduction (WBR) in S. mansoni-infected mice when it was administered as five daily 100 mg/kg oral doses.43
Exposure data for selected compounds reveals the following. First, N1-alkyl hydantoins 2 and 3 had high antischistosomal activities, due most likely to their extensive conversion to 1 by metabolic N-dealkylation reactions. Second, N3-aryl hydantoins 14 and 16, two of the most active compounds, had, like 1, high plasma exposures; conversely, N3-aryl hydantoin 13, 5-methyl hydantoin 23, and 5,5-dispirocyclopentyl hydantoin 26, three less effective compounds, had relatively low plasma exposures. These data are suggestive that antischistosomal efficacy and plasma exposure may correlate. This study provides several insights for the ongoing identification of more effective antischistosomal hydantoins.
Experimental Section
General
All reagents were purchased from Sigma-Aldrich, Fisher, or Acros Organics and used without further purification, unless otherwise stated. Melting points were determined with a Stanford Research Systems melting point apparatus and are uncorrected. 1D 1H and 13C NMR spectra were recorded on a Bruker 500 MHz spectrometer using CDCl3 or DMSO-d6 as solvents. All chemical shifts are reported in parts per million (ppm) and are relative to internal (CH3)4Si (0 ppm) for 1H and CDCl3 (77.2 ppm) or DMSO-d6 (39.5 ppm) for 13C NMR. EI GC-MS data were obtained using an Agilent quadrapole mass spectrometer with 30 m DB-XLB type columns and a He flow rate of 1 mL/min. Silica gel (sg) particle size 32–63 μm was used for all flash column chromatography. Reported reaction temperatures are those of the oil bath. A Biotage microwave reactor was used for selected reactions. Combustion analysis confirmed that all target compounds have a purity of at least 95%.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-1,5,5-trimethylimidazolidine-2,4-dione (2)
To a solution of 1 (700 mg, 2.4 mmol) in DMF was added NaH (87 mg, 3.6 mmol) under Ar. The mixture was stirred at rt for 30 min, and then iodomethane (514 mg, 3.6 mmol) was added dropwise. After stirring for 2 h, the mixture was evaporated in vacuo to give a residue which was extracted with brine (30 mL) and EtOAc (3 × 30 mL). The combined organic phase was washed with brine (2 × 30 mL) and dried over MgSO4. After removal of the solvents, the residue was purified by chromatography (sg, hexane:EtOAc, 4:1) to afford 2 as a white solid (580 mg, 79%). mp 110–112 °C; 1H NMR (CDCl3) δ 1.51 (s, 6H), 2.98 (s, 3H), 7.29 (t, J = 9.3 Hz, 1H), 7.66–7.69 (m, 1H), 7.77 (d, J = 6.3 Hz, 1H); 13C NMR (CDCl3) δ 22.28, 24.68, 61.22, 117.48 (m), 118.96 (qd, J = 33.6, 13.9 Hz), 122.03 (q, J = 272.5 Hz), 124.80 (m), 128.07 (d, J = 3.6 Hz), 131.23 (d, J = 8.6 Hz), 153.44, 158.48 (q, J = 258.2 Hz), 175.14. Anal. Calcd for C13H12F4N2O2: C, 51.32; H, 3.98; N, 9.21; Found: C, 51.20; H, 3.92; N, 9.39.
1-Ethyl-3-[4-fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethylimidazolidine-2,4-dione (3)
To a solution of 1 (700 mg, 2.4 mmol) in THF (7.5 mL) was added NaH (87 mg, 3.6 mmol) in THF (7.5 mL) at 0 °C under Ar. The reaction mixture was then stirred at rt for 3 h before dropwise addition of iodoethane (561 mg, 3.6 mmol). The reaction mixture was stirred at rt for 72 h before quenching with acetic acid (600 mg, 10 mmol) and H2O (1 mL). Solvent removal in vacuo gave a residue which was crystallized from H2O. The residue was further purified by chromatography (sg, EtOAc:hexane, 1:4) to afford 3 as a white solid (301 mg, 39%). mp 90–91 °C; 1H NMR (CDCl3) δ 1.32 (t, J = 7.2 Hz, 3H), 1.53 (s, 6H), 3.43 (q, J = 7.2 Hz, 2H), 7.29 (t, J = 9.4 Hz, 1H), 7.65–7.74 (m, 1H), 7.78 (dd, J = 6.3, 2.6 Hz, 1H). 13C NMR (CDCl3) δ 15.14, 23.58, 35.08, 61.97, 117.60 (d, J = 22.1 Hz), 119.11 (qd, J = 33.6, 13.6 Hz), 122.21 (q, J = 272.5 Hz), 124.95 (q, J = 4.5 Hz), 128.25 (d, J = 3.6 Hz), 131.34 (d, J = 8.9 Hz), 153.46, 158.60 (d, J = 258.1 Hz), 175.28. Anal. Calcd for C14H14F4N2O2: C, 52.83; H, 4.43; N, 8.80. Found: C, 52.80, H, 4.60, N, 8.71.
2-[3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-2,4-dioxoimidazolidin-1-yl]acetamide (4)
To a solution of 1 (1.04 g, 3.6 mmol) in THF (15 mL) was added NaH (163 mg, 6.8 mmol) in THF (15 mL) at 0 °C under Ar. The mixture was then stirred at rt for 3 h before dropwise addition of 2-bromoacetamide (937 mg, 6.8 mmol). The reaction mixture was stirred at rt for 72 h before quenching with acetic acid (600 mg, 10 mmol). Solvent removal in vacuo gave a residue which was crystallized from CH2Cl2 to afford 4 as a white solid (600 mg, 48%). mp 186–187 °C; 1H NMR (DMSO-d6) δ 1.43 (s, 6H), 3.90 (s, 2H), 7.25 (s, 1H), 7.50 (s, 1H), 7.69 (t, J = 9.7 Hz, 1H), 7.80–7.86 (m, 1H), 7.90 (dd, J = 6.6, 2.5 Hz, 1H); 13C NMR (DMSO-d6) δ 22.04, 41.90, 61.62, 116.72 (qd, J = 32.9, 13.6 Hz), 117.84 (d, J = 21.8 Hz), 122.21 (q, J = 272.4 Hz), 125.36 (q, J = 5.0 Hz), 128.74 (d, J = 3.3 Hz), 133.37 (d, J = 9.3 Hz), 153.50, 157.56 (dq, J = 254.9, 1.9 Hz), 169.63, 175.02. Anal. Calcd for C14H13F4N3O3: C, 48.42; H, 3.77; N, 12.10. Found: C, 48.70, H, 4.01, N, 11.89.
2-[3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-2,4-dioxoimidazolidin-1-yl]acetonitrile (5)
To a solution of 1 (700 mg, 2.4 mmol) in DMF was added NaH (116 mg, 4.8 mmol) under Ar. The mixture was stirred at rt for 30 min, and then 2-bromoacetonitrile (578 mg, 4.8 mmol) was added dropwise. After stirring for 2 h, the mixture was evaporated in vacuo to give a residue which was extracted with brine (30 mL) and EtOAc (3 × 30 mL). The combined organic phase was washed with brine (2 × 30 mL) and dried over MgSO4. After removal of the solvents, the residue was purified by chromatography (sg, hexane:EtOAc, 2:1) to afford 5 as a white solid (573 mg, 72%). mp 147–149 °C; 1H NMR (CDCl3) δ 1.65 (s, 6H), 4.37 (s, 2H), 7.32 (t, J = 9.3 Hz, 1H), 7.66–7.69 (m, 1H), 7.75 (dd, J = 2.4, 5.9 Hz, 1H); 13C NMR (CDCl3) δ 22.75, 27.14, 62.11, 114.73, 117.81 (d, J = 22.1 Hz), 119.28 (qd, J = 33.6, 13.9 Hz), 121.90 (q, J = 273.0 Hz), 124.89 (q, J = 1.9 Hz), 127.28(d, J = 4.4 Hz), 131.27 (d, J = 9.6 Hz), 153.13, 158.83(q, J = 257.2 Hz), 173.71. Anal. Calcd for C14H11F4N3O2: C, 51.07; H, 3.37; N, 12.76; Found: C, 51.29; H, 3.60; N, 12.52.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-1-phenethylimidazolidine-2,4-dione (6)
To a solution of 1 (700 mg, 2.4 mmol) in DMF (15 mL) was added NaH (87 mg, 3.6 mmol) under Ar. The mixture was stirred at rt for 30 min, and then 2-bromoethylbenzene (670 mg, 3.6 mmol) was added dropwise. After stirring for 2 h, the mixture was evaporated in vacuo to give a residue which was extracted with brine (30 mL) and EtOAc (3 × 30 mL). The combined organic phase was washed with brine (2 × 30 mL) and dried over MgSO4. After removal of the solvents, the residue was purified by chromatography (sg, hexane:EtOAc, 4:1) to afford 6 as a white solid (410 mg, 43%). mp 98–100 °C; 1H NMR (CDCl3) δ 1.39 (s, 6H), 3.05 (t, J = 7.8 Hz, 2H), 3.53 (t, J = 8.3 Hz, 2H), 7.24–7.34 (m, 6H), 7.69–7.72 (m, 1H), 7.78 (dd, J = 2.4, 6.9 Hz, 1H); 13C NMR (CDCl3) δ 23.12, 35.34, 42.23, 61.86, 117.46 (d, J = 22.2 Hz), 118.94 (qd, J = 33.1, 13.9 Hz), 122.04 (q, J = 272.0 Hz), 124.72 (m), 126.81, 128.69, 128.81, 131.14 (d, J = 8.5 Hz), 138.23, 153.62, 158.49 (d, J = 258.2 Hz), 174.94. Anal. Calcd for C20H18F4N2O2: C, 60.91; H, 4.60; N, 7.10; Found: C, 60.70; H, 4.89; N, 7.26.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-1-(methylsulfonyl)imidazolidine-2,4-dione (7)
To a solution of 1 (700 mg, 2.4 mmol) in THF (7.5 mL) was added NaH (100 mg, 4.1 mmol) in THF (7.5 mL) at 0 °C under Ar. The reaction mixture was then stirred at rt for 30 min before dropwise addition of methanesulfonyl chloride (410 mg, 3.6 mmol). After further stirring at rt for 72 h, the reaction was quenched with acetic acid (4 mL). Removal of solvents in vacuo gave a residue to which was added H2O (30 mL). The resulting precipitate was filtered and crystallized from 1:5 EtOAc:hexane to afford 7 as a white solid (812 mg, 92%). mp 207–208 °C; 1H NMR (CDCl3) δ 1.86 (s, 6H), 3.44 (s, 3H), 7.35 (t, J = 9.2 Hz, 1H), 7.67 (dt, J = 7.4, 3.5 Hz, 1H), 7.75 (dd, J = 6.1, 2.7 Hz, 1H). 13C NMR (CDCl3) δ 24.19, 43.53, 66.73, 118.19 (d, J = 22.3 Hz), 119.70 (qd, J = 33.9, 13.9 Hz), 121.95 (q, J = 272.6 Hz), 125.36 (qd, J = 6.9, 4.5 Hz), 126.56, 131.70 (d, J = 9.4 Hz), 151.70, 159.31 (d, J = 259.9 Hz), 173.02. Anal. Calcd for C13H12F4N2O4S: C, 42.39; H, 3.28; N, 7.61. Found: C, 42.40; H, 3.46; N, 7.49.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyl-1-(phenylsulfonyl)imidazolidine-2,4-dione (8)
To a solution of 1 (700 mg, 2.4 mmol) in DMF (15 mL) was added NaH (87 mg, 3.6 mmol) under Ar. The mixture was stirred at rt for 30 min, and then benzenesulfonyl chloride (639 mg, 3.6 mmol) was added dropwise. After stirring for 2 h, the mixture was evaporated in vacuo to give a residue which was extracted with brine (30 mL) and EtOAc (3 × 30 mL). The combined organic phase was washed with brine (2 × 30 mL) and dried over MgSO4. After removal of the solvents, the residue was purified by chromatography (sg, hexane:EtOAc, 4:1) to afford 8 as a white solid (739 mg, 71%). mp 153–155 °C; 1H NMR (CDCl3) δ 1.92 (s, 6H), 7.27 (t, J = 9.4 Hz, 1H), 7.57–7.71 (m, 5H), 8.13 (d, J = 8.3 Hz, 2H); 13C NMR (CDCl3) δ 24.26, 66.75, 117.80 (d, J = 22.2 Hz), 119.29 (qd, J = 34.1, 13.9 Hz), 121.77(q, J = 272.5 Hz), 125.14 (m), 126.46 (d, J = 3.8 Hz), 128.73, 129.16, 131.54 (d, J = 9.1 Hz), 134.59, 138.32, 150.57, 158.99 (d, J = 259.6 Hz), 172.81. Anal. Calcd for C18H14F4N2O4S 0.67 H2O: C, 48.87; H, 3.49; N, 6.33; Found: C, 48.71; H, 3.09; N, 6.12.
5,5-Dimethyl-3-[4-methyl-3-(trifluoromethyl)phenyl]imidazolidine-2,4-dione (11)
To a solution of 5,5-dimethylimidazolidine-2,4-dione (42) (568 mg, 4.44 mmol) and Cu2O (240 mg, 1.68 mmol) in DMA (15 mL) was added 4-iodo-1-methyl-2-(trifluoromethyl)benzene (37) (1.00 g, 3.5 mmol). After stirring at 160 °C for 12 h, the solvent was evaporated in vacuo to give a crude product which was further purified by sg chromatography using successive elution with hexane, EtOAc, and EtOH to afford 11 as a white solid (536 mg, 54%). mp 134–135 °C; 1H NMR (CDCl3) δ 1.57 (s, 6H), 2.51 (s, 3H), 5.52 (s, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.50 (d, J = 7.9 Hz, 1H), 7.70 (s, 1H); 13C NMR (DMSO-d6) δ 18.43, 24.65, 57.85, 123.92 (q, J = 5.2 Hz), 124.09 (q, J = 273.6 Hz), 127.54 (q, J = 29.6 Hz), 130.37, 130.41, 132.59, 135.53, 153.85, 176.21. Anal. Calcd for C13H13F3N2O2: C, 54.55; H, 4.58; N, 9.79; Found: C, 54.45; H, 4.73; N, 9.62.
5-(4,4-Dimethyl-2,5-dioxoimidazolidin-1-yl)-2-fluorobenzonitrile (12)
To a solution of 42 (0.674, 5.3 mmol) and Cu2O (290 mg, 2 mmol) in DMA (4 mL) was added 2-fluoro-5-iodobenzonitrile (38) (1.0 g, 4.1 mmol). After it was heated to 160 °C for 48 h, the solvent was evaporated in vacuo. After addition of EtOAc (100 mL), the resulting precipitate was filtered and the filtrate was concentrated and purified by chromatography (sg, EtOAc) followed by recrystallization from 1:20 ethanol:H2O to afford 12 as a white solid (768 mg, 77%). mp 145–147 °C; 1H NMR (CDCl3) δ 1.56 (s, 6H), 6.70 (s, 1H), 7.33 (t, J = 8.8 Hz, 1H), 7.78–7.81 (m, 1H), 7.84 (dd, J = 2.4, 5.4 Hz, 1H); 13C NMR (DMSO-d6) δ 25.15, 58.83, 102.08(d, J = 17.3 Hz), 112.99, 117.08 (d, J = 21.1 Hz), 128.52 (d, J = 3.4 Hz), 130.48, 132.28 (d, J = 8.6 Hz), 154.41, 161.74 (d, J = 261.0 Hz),175.47. Anal. Calcd for C12H10FN3O2: C, 58.30; H, 4.08; N, 17.00; Found: C, 58.08; H, 4.26; N, 17.28.
5-(4,4-Dimethyl-2,5-dioxoimidazolidin-1-yl)-2-fluoro-N,N-dimethylbenzamide (13)
To a solution of 42 (640 mg, 5.0 mmol) and Cu2O (259 mg, 1.8 mmol) in DMA (10 mL) was added 2-fluoro-5-iodo-N,N-dimethylbenzamide (39) (496 mg, 1.7 mmol). The mixture was then heated to 150 °C for 48 h. Solvent removal in vacuo gave a crude product which was further purified by sg chromatography using successive elution with hexane, EtOAc, and ethanol followed by crystallization from a 1:10 EtOAc:hexane mixture to afford 13 as a white solid (296 mg, 58%). mp 166–168 °C; 1H NMR (DMSO-d6) δ 1.40 (s, 6H), 2.87 (s, 3H), 3.01 (s, 3H), 7.41 (t, J = 9.1 Hz, 1H), 7.44 (dd, J = 6.0, 2.5 Hz, 1H), 7.51 (ddd, J = 8.9, 4.8, 2.6 Hz, 1H), 8.59 (s, 1H). 13C NMR (DMSO-d6) δ 24.68, 34.33, 37.85, 57.85, 116.21 (d, J = 23.3 Hz), 124.76 (d, J = 20.0 Hz), 127.11 (d, J = 4.7 Hz), 128.69 (d, J = 3.0 Hz), 129.62 (d, J = 8.7 Hz), 153.93, 156.32 (d, J = 246.5 Hz), 164.45, 176.27. Anal. Calcd for C14H16FN3O3: C, 57.33; H, 5.50; N, 14.33. Found: C, 57.51; H, 5.25; N, 14.22.
3-[3-(Difluoromethyl)-4-fluorophenyl]-5,5-dimethylimidazolidine-2,4-dione (14)
To a solution of 42 (2.54 g, 19.8 mmol) and Cu2O (1.09 g, 7.6 mmol) in DMA (30 mL) was added 4-bromo-2-(difluoromethyl)-1-fluorobenzene (49) (3.50 g, 15.5 mmol). After the reaction mixture was heated at 160 °C for 24 h, the solvent was removed in vacuo. The residue was purified by chromatography (sg, EtOAc) and then crystallized successively from hexane (15 mL) and H2O (20 mL) to afford 14 as a white solid (3.43 g, 81%). mp 158–159 °C; 1H NMR (DMSO-d6) δ 1.41 (s, 6H), 7.26 (t, J = 54.1 Hz, 1H), 7.51 (t, J = 9.5 Hz, 1H), 7.59–7.68 (m, 1H), 7.72 (dd, J = 6.4, 2.5 Hz, 1H), 8.62 (s, 1H). 13C NMR (DMSO-d6) δ 24.60, 57.85, 111.52 (td, J = 236.2, 3.5 Hz), 116.72 (d, J = 21.8 Hz), 121.46 (td, J = 23.4, 14.0 Hz), 125.75 (d, J = 3.3 Hz), 128.68 (d, J = 3.3 Hz), 131.64 (d, J = 9.0 Hz), 153.79, 158.38 (dt, J = 251.3, 4.8 Hz), 176.17. Anal. Calcd for C12H11F3N2O2: C, 52.94; H, 4.07; N, 10.29. Found: C, 52.72, H, 4.19, N, 10.12.
5,5-Dimethyl-3-(pyridin-4-yl)imidazolidine-2,4-dione (15)
To a solution of 42 (5.38 g, 42 mmol) and Cu(OAc)2 (543 mg, 3 mmol) in methanol (150 mL) was added pyridin-4-ylboronic acid (53) (3.6 g, 29 mmol) under O2. The mixture was heated at 70 °C overnight. The solvent was then filtered through silica gel to remove copper, and the resulting cake was washed with methanol (100 mL). Evaporation of the filtrate gave a crude product which was further purified by crystallization from a saturated Na2CO3 solution (20 mL) to afford 15 as a white solid (2.8 g, 47%). mp 201–203 °C; 1H NMR (DMSO-d6) δ 1.42 (s, 6H), 7.57 (d, J = 5.6 Hz, 2H), 8.67 (d, J = 6.1 Hz, 2H), 8.74 (brs, 1H). 13C NMR (DMSO-d6) δ 24.70, 57.75, 119.62, 139.85, 150.26, 153.08, 175.88. Anal. Calcd for C10H11N3O2: C, 58.53; H, 5.40; N, 20.48. Found: C, 58.14; H, 5.64; N, 20.57.
5,5-Dimethyl-3-[2-(trifluoromethyl)pyridin-4-yl]imidazolidine-2,4-dione (16)
To a solution of 42 (776 mg, 6.1 mmol) and Cu2O (288 mg, 2.0 mmol) in DMA (30 mL) was added 4-iodo-2-(trifluoromethyl)pyridine (39) (750 mg, 2.8 mmol). After stirring at 140 °C for 24 h, the solvent was evaporated in vacuo to give a crude product which was further purified by sg chromatography using successive elution with hexane and EtOAc to afford 16 as a white solid (674 mg, 90%). mp 195–197 °C; 1H NMR (DMSO-d6) δ 1.43 (s, 6H), 7.95 (dd, J = 5.4, 1.9 Hz, 1H), 8.14 (d, J = 1.8, 1H), 8.87 (d, J = 5.4 Hz, 1H), 8.89 (br s, 1H); 13C NMR (DMSO-d6) δ 24.61, 57.82, 116.17, 121.39 (q, J = 274.3 Hz), 122.29, 141.72, 146.94 (q, J = 34.1 Hz), 151.15, 152.68, 175.71. Anal. Calcd for C11H10F3N3O2: C, 48.36; H, 3.69; N, 15.38. Found: C, 47.97; H, 4.00; N, 15.64.
5,5-Dimethyl-3-(quinolin-4-yl)imidazolidine-2,4-dione (17)
To a solution of 42 (384 mg, 3 mmol) and Cu2O (142 mg, 1 mmol) in DMA (5 mL) was added 4-iodoquinoline (44) (163 mg, 0.64 mmol). After the reaction mixture was heated at 150 °C for 72 h, the solvent was removed in vacuo. The residue was purified by sg chromatography using successive elution with hexane, EtOAc, and ethanol to afford 17 as a pale yellow solid (101 mg, 62%). mp 194–196 °C; 1H NMR (DMSO-d6) δ 1.50 (s, 3H), 1.57 (s, 3H), 7.61 (d, J = 4.6 Hz, 1H), 7.65–7.76 (m, 2H), 7.86 (t, J = 7.2 Hz, 1H), 8.15 (d, J = 8.4 Hz, 1H), 8.83 (s, 1H), 9.05 (d, J = 4.6 Hz, 1H); 13C NMR (DMSO-d6) δ 24.50, 25.26, 58.77, 121.60, 122.83, 124.72, 127.58, 129.42, 130.15, 137.08, 148.77, 150.73, 153.60, 176.45. Anal. Calcd for C14H13N3O2 H2O: C, 61.53; H, 5.53; N, 15.38. Found: C, 61.82; H, 5.08; N, 15.30.
5,5-Dimethyl-3-(pyridin-3-yl)imidazolidine-2,4-dione (18)
To a solution of 42 (1.792 g, 14 mmol) and Cu(OAc)2 (181 mg, 1 mmol) in methanol (50 mL) was added pyridin-3-ylboronic acid (52) (1.23 g, 10 mmol) under O2. The mixture was heated at 70 °C overnight. The solvent was then filtered through silica gel to remove copper, and the resulting cake was washed with methanol (100 mL). Evaporation of the filtrate gave a crude product which was further purified by successive crystallizations from diethyl ether (10 mL) and saturated Na2CO3 (10 mL) to afford 18 as a white solid (1.258 g, 61%). mp 160–162 °C; 1H NMR (CDCl3) δ 1.56 (s, 6H), 6.85 (s, 1H), 7.43 (dd, J = 4.4, 7.8 Hz, 1H), 7.84 (d, J = 8.3 Hz, 1H), 8.62 (d, J = 4.4 Hz, 1H), 8.78 (s, 1H); 13C NMR (CDCl3) δ 25.22, 58.87, 123.57, 128.78, 133.15, 146.79, 148.65, 154.72, 175.79. Anal. Calcd for C10H11N3O2: C, 58.53; H, 5.40; N, 20.48; Found: C, 58.70; H, 5.36; N, 20.19.
5,5-Dimethyl-3-[6-(trifluoromethyl)pyridin-3-yl]imidazolidine-2,4-dione (19)
To a solution of 42 (384 mg, 3.00 mmol) and Cu2O (143 mg, 1.00 mmol) in DMA (10 mL) was added 5-iodo-2-(trifluoromethyl)pyridine (41) (476 mg, 1.74 mmol), and the mixture was heated to 140 °C for 48 h. After solvent removal in vacuo, the crude product was purified by sg chromatography using successive elution with hexane and EtOAc. After removal of the solvents in vacuo, the residue was crystallized from 1:20 EtOAc:hexane. The resulting precipitate was collected by filtration and rinsed by H2O (5 mL) to give 19 as a white solid (357 mg, 75%). mp 173–174 °C; 1H NMR (DMSO-d6) δ 1.44 (s, 6 H), 8.08 (d, J = 8.3 Hz, 1 H), 8.20 (d, J = 8.3 Hz, 1 H), 8.80 (s, 1 H), 8.88 (s, 1 H); 13C NMR (DMSO-d6) δ 24.80, 58.36, 121.16, 121.61(q, J = 273.9 Hz), 132.27, 135.50, 144.63 (q, J = 34.4 Hz), 147.43, 153.32,176.10. Anal. Calcd for C11H10F3N3O2: C, 48.36; H, 3.69; N, 15.38. Found C, 48.48; H, 3.81; N, 15.16.
5,5-Dimethyl-3-(pyrazin-2-yl)imidazolidine-2,4-dione (20)
To a solution of 42 (1.28 g, 10.0 mmol) and Cu2O (357.5 mg, 2.5 mmol) in DMA (20 mL) was added 2-iodopyrazine (43) (1.00 g, 4.9 mmol). The reaction mixture was then heated to 140 °C for 24 h. After solvent removal in vacuo, the crude product was purified by sg chromatography using successive elutions with hexane, EtOAc, and ethanol. After removal of the solvents in vacuo, the residue was crystallized from a mixture of ether (0.5 mL) and hexane (10 mL). The resulting precipitate was collected by filtration to give 20 as a pale yellow solid (210 mg, 21%). mp 149–151 °C; 1H NMR (DMSO-d6) δ 1.44 (s, 6H), 8.66–8.90 (m, 4H); 13C NMR (DMSO-d6) δ 24.65, 58.38, 142.76, 143.72, 143.91, 144.38, 153.05, 175.80. Anal. Calcd for C9H10N4O2; C, 52.42; H, 4.89; N, 27.17. Found: C, 52.34; H, 5.00; N, 27.21.
3-[4-Fluoro-3-(trifluoromethyl)benzyl]-5,5-dimethylimidazolidine-2,4-dione (21)
A mixture of 4-(bromomethyl)-1-fluoro-2-(trifluoromethyl)benzene (50) (1.552 g, 6.0 mmol), 42 (647 mg, 5.1 mmol), and K2CO3 (2.004 g, 14.5 mmol) in DMA (12.5 mL) was stirred at 85 °C for 24 h. The mixture was extracted with EtOAc (3 × 50 mL) and H2O (50 mL). The organic phase was combined, dried, and evaporated in vacuo to give a residue which was further purified by chromatography (sg, EtOAc:hexane, 1:3) and crystallized from H2O to afford 21 as a white solid (1.16 g, 75%). mp 86–87 °C; 1H NMR (DMSO-d6) δ 1.32 (s, 6H), 4.63 (s, 2H), 7.50 (dd, J = 10.7, 8.6 Hz, 1H), 7.56–7.63 (m, 1H), 7.66 (d, J = 7.0 Hz, 1H), 8.46 (s, 1H); 13C NMR (DMSO-d6) δ 24.53, 39.91, 58.03, 116.53 (qd, J = 32.4, 12.6 Hz), 117.55 (d, J = 20.7 Hz), 122.47 (q, J = 272.0 Hz), 126.07 (q, J = 4.7 Hz), 133.99, 134.04 (d, J = 5.3 Hz), 154.91, 158.10 (dq, J = 253.2, 2.3 Hz), 177.22. Anal. Calcd for C13H12F4N2O2: C, 51.32; H, 3.98; N, 9.21. Found: C, 51.49; H, 4.12; N, 9.34.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5-methylimidazolidine-2,4-dione (23)
Step 1. To a solution of 2-aminopropanoic acid (57) (2.225 g, 25 mmol) in 1 M NaOH (40 mL) was added 1-fluoro-4-isocyanato-2-(trifluoromethyl)benzene (56) (6.15 g, 30 mmol) in CH3CN (15 mL) at 0 °C dropwise. The reaction mixture was stirred at 0 °C for 3 h and then warmed to rt overnight. Step 2. The reaction mixture was adjusted to pH 3.0 by 32% HCl and concentrated in vacuo. The resulting precipitate was crystallized from 1:40 EtOAc:hexane to give 2-[3-[4-fluoro-3-(trifluoromethyl)phenyl]ureido]propanoic acid as a white solid (5.157 g, 70%), 1.1 g (3.74 mmol) of which was suspended in 4 M HCl (50 mL) and then heated at 110 °C overnight. After the reaction mixture was cooled to rt, the resulting precipitate was filtered to afford 23 as a white solid (845 mg, 82%). mp 153–154 °C; 1H NMR (DMSO-d6) δ 1.38 (d, J = 6.9 Hz, 3H), 4.27 (q, J = 6.9 Hz, 1H), 7.65 (t, J = 9.7 Hz, 1H), 7.76–7.83 (m, 1H), 7.87 (dd, J = 6.7, 2.5 Hz, 1H), 8.59 (s, 1H); 13C NMR (DMSO-d6) δ 16.94, 52.23, 116.73 (qd, J = 32.8, 13.4 Hz), 117.76 (d, J = 21.8 Hz), 122.23 (q, J = 272.1 Hz), 125.47 (q, J = 5.1 Hz), 128.95 (d, J = 3.5 Hz), 133.43 (d, J = 9.3 Hz), 154.84, 157.50 (dq, J = 254.3, 2.1 Hz), 173.81. Anal. Calcd for C11H8F4N2O2·0.5H2O: C, 46.33; H, 3.18; N, 9.82; Found: C, 46.61; H, 3.19; N, 9.81.
(R)-3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5-phenylimidazolidine-2,4-dione (24)
Step 1. To a solution of (R)-2-amino-2-phenylacetic acid (58) (1.057 g, 7.0 mmol) in 1 M NaOH (10 mL) was added 56 (2.05 g, 10 mmol) in CH3CN (5 mL) at 0 °C dropwise. The mixture was stirred at 0 °C for 3 h, and then 1,4-dioxane (10 mL) was added according to the method of Cooper et al.44 After stirring at rt for 12 h, the reaction mixture was adjusted to pH 2 by 32% HCl and concentrated in vacuo. Step 2. The resulting precipitate was successively crystallized from 1:10 EtOH:H2O and 1:10 EtOAc:hexane to give (R)-2-[3-[4-fluoro-3-(trifluoromethyl)phenyl]ureido]-2-phenylacetic acid as a white solid (1.661 g, 67%), 818 mg of which was then suspended in 4 M HCl (50 mL) and heated at 110 °C overnight. After the reaction mixture was cooled to 0 °C, the resulting precipitate was filtered to give 24 as a white solid (678 mg, 87%). mp 166–168 °C; 1H NMR (DMSO-d6) δ 5.37 (s, 1H), 7.33–7.54 (m, 5H), 7.67 (dd, J = 10.0, 9.7 Hz, 1H), 7.78–7.88 (m, 1H), 7.92 (dd, J = 6.7, 2.5 Hz, 1H), 9.10 (s, 1H). 13C NMR (DMSO-d6) δ 60.36, 117.01 (qd, J = 32.8, 13.5 Hz), 118.05 (d, J = 21.8 Hz), 122.40 (q, J = 272.7 Hz), 125.89, 127.55, 128.76, 128.89, 128.97 (d, J = 3.4 Hz), 133.80 (d, J = 9.3 Hz), 135.55, 155.33, 157.85 (d, J = 258.6 Hz), 171.60. Anal. Calcd for C16H10F4N2O2: C, 56.81; H, 2.98; N, 8.28. Found: C, 56.66; H, 3.12; N, 8.18.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5-(hydroxymethyl)-5-methylimidazolidine-2,4-dione (25)
Step 1. To a solution of 2-amino-3-hydroxy-2-methylpropanoic acid (59) (537 mg, 4.5 mmol) in dioxane (23 mL) was added 56 (8.10 g, 39.5 mmol) under Ar. After the reaction mixture was stirred at 80 °C for 6 h, it was cooled to rt, diluted with CH2Cl2 (25 mL), and extracted with 2 M NaOH (3 × 25 mL). After the combined aqueous layers were separated and filtered to remove the insoluble N,N-diarylurea side product, the filtrate was acidified with 2 M HCl (100 mL) and concentrated. Step 2. The residue was suspended in 2 M HCl (50 mL) and then heated at 110 °C for 4 h. After the reaction mixture was cooled to rt, the resulting precipitate was collected by filtration to give 25 (370 mg, 27%) as a white solid. mp 166–168 °C; 1H NMR (DMSO-d6) δ 1.30 (s, 3H), 3.43 (d, J = 10.7 Hz, 1H), 3.67 (d, J = 11.2 Hz, 1H), 7.66 (t, J = 10.3 Hz, 1H), 7.76–7.77 (m, 1H), 7.81 (d, J = 6.3, 1H), 8.52 (s, 1H); 13C NMR (DMSO-d6) 18.97, 63.52, 65.38, 117.00 (q, J = 32.5 Hz), 118.07 (d, J = 22.0 Hz), 122.40 (q, J = 273.0 Hz), 125.20, 129.15, 133.29 (d, J = 9.2 Hz), 154.78, 157.61 (d, J = 254.2 Hz), 175.04. Anal. Calcd for C12H10F4N2O3: C, 47.07; H, 3.29; N, 9.15. Found: C, 46.91, H, 3.46, N, 9.32.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-1,3-diazaspiro[4.4]nonane-2,4-dione (26)
Step 1. To a solution of 1-aminocyclopentane-1-carboxylic acid (60) (1.161 g, 9.0 mmol) in 1 M NaOH (15 mL) was added a solution of 56 (1.83 g, 8.9 mmol) in CH3CN (10 mL) at 0 °C dropwise. The mixture was stirred at 0 °C for 3 h and then warmed to rt overnight. The reaction mixture was adjusted to pH 1.0 by 32% HCl and concentrated in vacuo. Step 2. The resulting precipitate was collected by filtration, and the solid was then suspended in 4 M HCl (50 mL) and heated at 110 °C overnight. After the reaction mixture was cooled to rt, the resulting precipitate was filtered and crystallized from diethyl ether to give 26 as a white solid (660 mg, 23%). mp 151–152 °C; 1H NMR (DMSO-d6) δ 1.67–1.92 (m, 6H), 2.01–2.16 (m, 2H), 7.65 (dd, J = 10.5, 9.0 Hz, 1H), 7.78–7.84 (m, 1H), 7.90 (dd, J = 6.6, 2.6 Hz, 1H), 8.86 (s, 1H); 13C NMR (DMSO-d6) δ 24.82, 37.58, 67.49, 116.86 (qd, J = 33.2, 13.4 Hz), 117.89 (d, J = 21.9 Hz), 122.43 (q, J = 272.4 Hz), 125.72, 129.14, 133.61 (d, J = 9.0 Hz), 154.16, 157.65 (d, J = 253.1 Hz), 176.55. Anal. Calcd for C14H12F4N2O2: C, 53.17; H, 3.82; N, 8.86. Found: C, 52.89; H, 4.00; N, 8.89.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-1,3-diazaspiro[4.5]decane-2,4-dione (27)
To a solution of 1,3-diazaspiro[4.5]decane-2,4-dione (46) (840 mg, 5.0 mmol) and Cu2O (290 mg, 2.0 mmol) in DMA (20 mL) was added 1-fluoro-4-iodo-2-(trifluoromethyl)benzene (45) (1170 mg, 4.0 mmol). After it was heated at 160 °C for 24 h, the solvent was evaporated in vacuo. The residue was purified by sg chromatography (EtOAc) to afford 27 as a white solid (917 mg, 69%). mp 213-215 °C; 1H NMR (DMSO-d6) δ 1.25–1.40 (m, 1H) 1.58 (br s, 3H) 1.64–1.83 (m, 6H) 7.65 (t, J = 9.7 Hz, 1H), 7.77–7.84 (m, 1H) 7.89 (d, J = 6.35 Hz, 1H) 9.05 (br s, 1H); 13C NMR (DMSO-d6) δ 20.93, 24.56, 33.33, 61.27, 116.88 (qd, J = 33.0, 13.3), 117.9 (d, J = 22.0), 122.4 (q, J = 272.5), 125.87 (d, J = 4.1), 129.03, 133.76 (d, J = 9.2), 154.27, 157.7 (d, J = 254.7), 175.64. Anal. Calcd for C15H14F4N2O2: C, 54.55; H, 4.27; N, 8.48. Found: C, 54.38; H, 4.40; N, 8.49.
2-[4-Fluoro-3-(trifluoromethyl)phenyl]tetrahydro-1H-pyrrolo[1,2-c]imidazole-1,3(2H)-dione (28)
To a solution of tetrahydro-1H-pyrrolo[1,2-c]imidazole-1,3(2H)-dione (55) (538 mg, 3.8 mmol), Cu(OAc)2 (1.370 g, 7.6 mmol), and pyridine (900 mg, 11.4 mmol) in CH2Cl2 (38 mL) was added [4-fluoro-3-(trifluoromethyl)phenyl]boronic acid (53) (2.359 g, 11.3 mmol). After stirring at rt for 7 days, the solvent was removed in vacuo. The residue was purified by chromatography (sg, EtOAc) and then crystallized from hexane to afford 28 as a white solid (443 mg, 39%). mp 146–148 °C; 1H NMR (CDCl3) δ 1.78–1.93 (m, 1H), 2.06–2.30 (m, 2H), 2.32–2.45 (m, 1H), 3.37 (ddd, J = 11.6, 8.5, 4.6 Hz, 1H), 3.80 (dt, J = 11.5, 7.8 Hz, 1H), 4.27 (dd, J = 9.4, 7.5 Hz, 1H), 7.29 (t, J = 9.3 Hz, 1H), 7.60–7.68 (m, 1H), 7.74 (dd, J = 6.2, 2.6 Hz, 1H); 13C NMR (DMSO-d6) δ 26.38, 26.50, 45.30, 62.94, 116.77 (qd, J = 33.1, 13.5 Hz), 117.87 (d, J = 21.8 Hz), 122.20 (q, J = 272.0 Hz), 125.68, 128.89 (d, J = 3.4 Hz), 133.59 (d, J = 9.4 Hz), 157.66 (d, J = 255.0 Hz), 158.26, 172.45. Anal. Calcd for C13H10F4N2O2: C, 51.66; H, 3.34; N, 9.27. Found: C, 51.70; H, 3.40; N, 9.55.
1-[4-Fluoro-3-(trifluoromethyl)phenyl]-3,3-dimethylpyrrolidine-2,5-dione (29)
To a solution of 3,3-dimethylpyrrolidine-2,5-dione (47) (1.71 g, 13.5 mmol) and Cu2O (0.74 g, 5.2 mmol) in DMF (15 mL) was added 1-fluoro-4-iodo-2-(trifluoromethyl)benzene (45) (3.0 g, 10.3 mmol), and the mixture was heated to reflux for 48 h. After cooling to rt, the solvent was evaporated in vacuo. After addition of concentrated NH4OH (20 mL) and H2O (15 mL), the resulting precipitate was filtered and purified by chromatography (sg, hexane:EtOAc, 5:1) to afford 29 as a white solid (1.39 g, 46%). mp 134–136 °C; 1H NMR (CDCl3) δ 1.44 (s, 6H), 2.74 (s, 2H), 7.33 (t, J = 9.3 Hz, 1H), 7.53–7.56 (m, 1H), 7.62 (d, J = 5.9 Hz, 1H);13C NMR (CDCl3) δ 25.60, 40.23, 43.62, 117.67 (d, J = 22.1 Hz), 119.15 (qd, J = 34.6, 13.4 Hz), 121.95(q, J = 272.5 Hz), 125.33 (m), 128.0 (d, J = 3.8 Hz), 131.9(d, J = 8.6 Hz), 158.89 (d, J = 258.6 Hz), 174.20, 181.67. Anal. Calcd for C13H11F4NO2: C, 53.99; H, 3.83; N, 4.84; Found: C, 54.00; H, 3.82; N, 4.89.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyloxazolidine-2,4-dione (30)
To a solution of 5,5-dimethyloxazolidine-2,4-dione (54) (903 mg, 7.0 mmol) and Cu(OAc)2 (90 mg, 0.5 mmol) in methanol (25 mL) was added (4-fluoro-3-(trifluoromethyl)phenyl)boronic acid (53) (1.04 g, 5.0 mmol) under O2. The mixture was heated at 70 °C overnight. After cooling to rt, the solvent was evaporated in vacuo. The residue was purified by chromatography with successive elution with hexane and EtOAc followed by crystallization from 1:10 ethanol:H2O to afford 30 as a white solid (421 mg, 29%). mp 174–176 °C; 1H NMR (CDCl3) δ 1.70 (s, 6H), 7.34 (t, J = 8.8 Hz, 1H), 7.69–7.72 (m, 1H), 7.79 (dd, J = 2.4, 5.9 Hz, 1H); 13C NMR (CDCl3) δ 23.74, 83.80, 117.99 (d, J = 22.1 Hz), 119.48 (qd, J = 34.1, 20.2 Hz), 121.81 (q, J = 272.5 Hz), 124.53 (m), 127.12 (d, J = 3.4 Hz), 130.89 (d, J = 9.1 Hz), 152.54, 159.01 (dq, J = 259.6, 1.9 Hz), 174.32. Anal. Calcd for C12H9F4NO3: C, 49.49; H, 3.12; N, 4.81; Found: C, 49.21; H, 3.06; N, 4.80.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethylpyrrolidine-2,4-dione (31)
Step 1. A mixture of 2-[4-fluoro-3-(trifluoromethyl)phenyl]acetic acid (2.22 g, 10 mmol) and SOCl2 (4 mL) was stirred at 80 °C overnight. The reaction mixture was concentrated in vacuo to afford 2-[4-fluoro-3-(trifluoromethyl)phenyl]acetyl chloride (65), which was used for the next step without further purification. Step 2. To a solution of methyl 2-amino-2-methylpropanoate HCl salt (1.53 g, 10 mmol) and Et3N (5.0 g, 50 mmol) in THF (47.5 mL) was added 65 in THF (2.5 mL), and the reaction mixture was stirred at rt overnight. After filtration to remove insoluble TEA HCl salt, the filtrate was concentrated in vacuo. The residue was purified by chromatography (sg, EtOAc:hexane, 1:1) to afford methyl 2-[2-[4-fluoro-3-(trifluoromethyl)phenyl]acetamido]-2-methylpropanoate (66) as a white solid (1.13 g, 35%). 1H NMR (DMSO-d6) δ 1.35 (s, 6H), 3.50 (s, 3H), 3.52 (s, 2H), 7.45 (dd, J = 10.9, 8.6 Hz, 1H), 7.52–7.61 (m, 1H), 7.63 (d, J = 7.7 Hz, 1H), 8.54 (s, 1H). Step 3. To a solution of 66 (860 mg, 2.68 mmol) in THF (7 mL) was added a suspension of NaH (300 mg, 12.5 mmol) in THF (6.4 mL) dropwise under Ar. The reaction mixture was stirred at rt for 12 before quenching with a mixture of acetic acid (1.2 g, 20 mmol) and H2O (1 mL). Solvent removal in vacuo gave a crude product which was crystallized from hexane to afford 31 as a white solid (406 mg, 52%). mp 217–219 °C; 1H NMR (DMSO-d6) δ 1.36 (s, 6H), 7.46 (dd, J = 10.8, 8.9 Hz, 1H), 7.83 (s, 1H), 8.30–8.40 (m, 1H), 8.47 (dd, J = 7.4, 2.2 Hz, 1H), 11.68 (s, 1H). 13C NMR (DMSO-d6) δ 24.54, 56.49, 98.64, 115.88 (qd, J = 31.8, 12.2 Hz), 116.56 (d, J = 20.1 Hz), 122.87 (q, J = 271.4 Hz), 124.55 (q, J = 4.8 Hz), 129.75 (d, J = 3.7 Hz), 132.87 (d, J = 8.0 Hz), 156.37 (d, J = 250.9 Hz), 170.39, 176.13. Anal. Calcd for C13H11F4NO2: C, 53.99; H, 3.83; N, 4.84. Found: C, 54.09; H, 3.92; N, 4.60.
1-[4-Fluoro-3-(trifluoromethyl)phenyl]-4,4-dimethylimidazolidin-2-one (32)
To a solution of 4,4-dimethyl-2-imidazolidinone (48) (500 mg, 4.4 mmol), Cs2CO3 (2.460 g, 7.5 mmol), Xantphos (108 mg, 0.19 mmol), and Pd2(dba)3 (84 mg, 0.09 mmol) in toluene (40 mL) was added 1-fluoro-4-iodo-2-(trifluoromethyl)benzene (45) (1.270 g, 4.4 mmol). The reaction mixture was stirred at 90 °C for 12 h under Ar. Evaporation gave a crude product which was purified by washing with water (25 mL), chromatography (sg, EtOAc), and recrystallizion from hexane to afford 32 as a white solid (939 mg, 77%). mp 147–148 °C; 1H NMR (DMSO-d6) δ 1.28 (s, 6H), 3.64 (s, 2H), 7.39 (s, 1H), 7.45 (t, J = 9.8 Hz, 1H), 7.72 (dt, J = 9.2, 3.6 Hz, 1H), 8.05 (dd, J = 6.4, 2.9 Hz, 1H); 13C NMR (DMSO-d6) δ 28.20, 50.57, 57.07, 114.69 (q, J = 5.2 Hz), 116.22 (qd, J = 32.1, 12.9 Hz), 117.29 (d, J = 21.3 Hz), 122.30 (d, J = 7.7 Hz), 122.66 (q, J = 272.3 Hz), 137.58, 153.26 (d, J = 247.5 Hz), 157.02. Anal. Calcd for C12H12F4N2O: C, 52.18; H, 4.38; N, 10.14. Found: C, 52.32; H, 4.57; N, 10.26.
1-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethylimidazolidine-2,4-dione (33)
Step 1. To a mixture of 4-fluoro-3-(trifluoromethyl)aniline (63) (1.791 g, 10 mmol), formamide (50 mL), and acetone (25 mL) was added 1 M TiCl4 in CH2Cl2 (12.5 mL) at 0 °C under Ar. After 1 h, Zn powder (1.5 g, 23 mmol) was added followed by dropwise addition of 50% H2O2 (1.75 mL) in formamide (23.25 mL) over 3 h. The reaction was quenched with H2O (25 mL), and the pH adjusted to 8 with concentrated ammonium hydroxide followed by extraction with EtOAc (3 × 50 mL). The combined organic layers were concentrated in vacuo, and the residue was dissolved in CHCl3 (100 mL). The solution was then washed with H2O (3 × 25 mL). Evaporation gave a crude product which was further purified by successive recrystallizations from a mixture of CH2Cl2 (5 mL) and hexane (1 mL) and a mixture of acetic acid (150 mg) and H2O (1 mL) to afford 2-[[4-fluoro-3-(trifluoromethyl)phenyl]amino]-2-methylpropanamide (66) as a white solid (418 mg, 16%); 1H NMR (DMSO-d6) δ 1.35 (s, 6H), 6.18 (s, 1H), 6.71 (dt, J = 9.0, 3.6 Hz, 1H), 6.81 (dd, J = 6.0, 3.0 Hz, 1H), 7.05 (s, 1H), 7.22 (t, J = 9.8 Hz, 1H), 7.34 (s, 1H). 13C NMR (DMSO-d6) δ 25.22, 56.63, 111.09 (q, J = 4.7 Hz), 116.20 (qd, J = 31.5, 13.0 Hz), 117.16 (d, J = 21.2 Hz), 118.79 (d, J = 7.4 Hz), 122.92 (q, J = 272.0 Hz), 143.20 (d, J = 1.9 Hz), 150.44 (dq, J = 241.0, 2.4 Hz), 177.13. Step 2. To a solution of 66 (160 mg, 0.6 mmol) in toluene (5 mL) was added 2-isocyanato-1,3-diisopropylbenzene (143 mg, 0.7 mmol). After the mixture was heated to 250 °C at 5 bar for 10 min under microwave irradiation in a sealed tube, the solvent was evaporated in vacuo. The residue was purified by sg chromatography eluting successively with CH2Cl2 and 1:3 acetone:hexane to afford 33 as a white solid (108 mg, 62%). mp 193–195 °C; 1H NMR (DMSO-d6) δ 1.34 (s, 6H), 7.63 (t, J = 9.7 Hz, 1H), 7.71–7.78 (m, 1H), 7.84 (dd, J = 6.6, 2.6 Hz, 1H), 11.26 (s, 1H); 13C NMR (DMSO-d6) δ 23.09, 64.21, 117.25 (qd, J = 32.8, 13.2 Hz), 118.25 (d, J = 21.5 Hz), 122.18 (q, J = 273.5 Hz), 128.19, 131.44 (d, J = 3.4 Hz), 135.86 (d, J = 9.2 Hz), 154.99, 157.87 (d, J = 254.8 Hz), 177.14. Anal. Calcd for C12H10F4N2O2: C, 49.66; H, 3.47; N, 9.65. Found: C, 49.71, H, 3.58, N, 9.80.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-6,6-dimethyldihydropyrimidine-2,4(1H,3H)-dione (34)
Step 1. To a solution of 3-amino-3-methylbutanoic acid (61) (585 mg, 5.0 mmol) in 2 M NaOH (5 mL) was added 56 (1.95 g, 9.5 mmol). After it was stirred at rt for 4 h, the reaction mixture was filtered to remove the insoluble N,N-diarylurea side product, and the filtrate was adjusted to pH 1.0 with 2 M HCl and concentrated. Step 2. The residue was suspended in 2 M HCl (50 mL) and then heated at 110 °C for 2 h. After the reaction mixture was cooled to rt, the resulting precipitate was filtered and washed with H2O (20 mL) to give 34 as a white solid (477 mg, 31%). mp 225–226 °C; 1H NMR (DMSO-d6) δ 1.31 (s, 6H), 2.74 (s, 2H), 7.53–7.64 (m, 2H), 7.71 (dd, J = 6.7, 2.4 Hz, 1H), 8.18 (s, 1H);13C NMR (DMSO-d6) δ 28.11, 44.32, 48.22, 116.88 (qd, J = 32.6, 13.3 Hz), 117.68 (d, J = 21.5 Hz), 122.54 (q, J = 272.3 Hz), 128.60 (d, J = 4.8 Hz), 132.82 (d, J = 3.5 Hz), 136.61 (d, J = 9.1 Hz), 152.51, 158.16 (d, J = 254.0 Hz), 169.81. Anal. Calcd for C13H12F4N2O2 0.5 H2O: C, 49.85; H, 4.18; N, 8.94. Found: C, 49.79; H, 4.51; N, 8.81.
3-[4-Fluoro-3-(trifluoromethyl)phenyl]-5,5-dimethyldihydropyrimidine-2,4(1H,3H)-dione (35)
Step 1. To a solution of 3-amino-2,2-dimethylpropanoic acid hydrochloride (62) (700 mg, 4.6 mmol) in 2 M NaOH (5 mL) was added 56 (1.95 g, 9.5 mmol), and the reaction mixture was stirred at rt for 4 h. After filtration to remove the insoluble N,N-diarylurea side product, the filtrate was adjusted to pH 1.0 with 2 M HCl and concentrated to afford 3-[3-[4-fluoro-3-(trifluoromethyl)phenyl]ureido]-2,2-dimethylpropanoic acid (480 mg, 32%) as a white solid. 1H NMR (DMSO-d6) δ 1.09 (s, 6H), 3.22 (d, J = 6.2 Hz, 2H), 6.25 (t, J = 6.2 Hz, 1H), 7.36 (t, J = 9.8 Hz, 1H), 7.43–7.53 (m, 1H), 7.95 (dd, J = 6.5, 2.8 Hz, 1H), 8.99 (s, 1H), 12.39 (s, 1H). Step 2. 3-[3-[4-Fluoro-3-(trifluoromethyl)phenyl]ureido]-2,2-dimethylpropanoic acid (450 mg, 1.4 mmol) was suspended in 2 M HCl (50 mL) and then heated at 110 °C for 12 h. After the reaction mixture was cooled to rt, the resulting precipitate was filtered and washed with saturated NaHCO3 (30 mL) to give 35 as a white solid (355 mg, 83%). mp 214–215 °C; 1H NMR (DMSO-d6) δ 1.21 (s, 6H), 3.18 (s, 1H), 3.18 (d, J = 2.8 Hz, 1H), 7.52–7.59 (m, 2H), 7.63–7.69 (m, 1H), 8.11 (s, 1H); 13C NMR (DMSO-d6) δ 22.35, 37.39, 46.31, 116.60 (qd, J = 32.9, 13.7 Hz), 117.41 (d, J = 21.5 Hz), 122.34 (q, J = 271.9 Hz), 128.34, 133.07 (d, J = 3.5 Hz), 136.42 (d, J = 9.2 Hz), 153.02, 157.88 (d, J = 253.6 Hz), 175.33. Anal. Calcd for C13H12F4N2O2: C, 51.32; H, 3.98; N, 9.21. Found: C, 51.19; H, 3.99; N, 9.12.
2-[3-[4-Fluoro-3-(trifluoromethyl)phenyl]ureido]-2-methylpropanoic acid (36)
Hydantoin 1 (1.20 g, 4.1 mmol) was added to 2 M NaOH (40 mL). The reaction mixture was stirred at rt for 4 h, and then quenched with 2 M HCl (50 mL). The precipitate was filtered and rinsed with H2O (20 mL) and then dried to afford 36 as a white solid (1.20 g, 95%). mp 196.5–197.5 °C; 1H NMR (DMSO-d6) δ 1.43 (s, 6H), 6.61 (s, 1H), 7.37 (t, J = 9.8 Hz, 1H), 7.47–7.50 (m,1H), 7.96–7.97 (m, 1H), 8.87 (s, 1H), 12.44 (s, 1H); 13C NMR (DMSO-d6) δ 25.42, 55.13, 115.12 (d, J = 5.0 Hz), 116.45 (qd, J = 31.6, 12.8 Hz), 117.65 (d, J = 21.5 Hz), 122.85 (q, J = 272.1 Hz), 123.42 (d, J = 7.8 Hz), 137.34, 153.32 (d, J = 246.9 Hz), 154.37, 176.31. Anal. Calcd for C12H12F4N2O3: C, 46.76; H, 3.92; N, 9.09. Found: C, 46.50; H, 4.04; N, 9.19.
Polar Surface Area (PSA)
PSA values (Å2) were calculated using ChemAxon Instant JChem (ver 16.4).
Kinetic Solubility
Compounds in DMSO (10 mg/mL) were diluted into either pH 6.5 phosphate buffer or 0.01 M HCl (approximately pH 2.0), with the final DMSO concentration being 1%. Samples were then analyzed via nephelometry to determine a solubility range.45
Partition Coefficient
Partition coefficient values (Log D) of the test compounds were estimated by correlation of their chromatographic retention properties against the characteristics of a series of standard compounds with known partition coefficient values using gradient HPLC (modification of a method reported by Lombardo et al.46
Plasma Protein Binding
Plasma protein binding values of the test compounds were estimated by correlation of their chromatographic retention properties on a human albumin column against the characteristics of a series of standard compounds with known protein binding values. The method employed is a gradient HPLC based derivation of the method developed by Valko et al.47
In Vitro Metabolic Stability
Metabolic stability assays were performed by incubating test compounds in liver microsomes at 37 °C and 0.4 mg/mL protein concentration. The metabolic reaction was initiated by the addition of an NADPH-regenerating system and quenched at various time points over a 60 min incubation period by the addition of acetonitrile containing diazepam as internal standard. Control samples (containing no NADPH) were included (and quenched at 2, 30, and 60 min) to monitor for potential degradation in the absence of cofactor. Compound concentrations were determined by LC/MS by comparison to calibration standards prepared in prequenched microsomal matrix.
Mouse Exposure Studies
Pharmacokinetic studies in mice were conducted at Monash University, Parkville, Australia, and were performed in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The study protocol adhered to the principles of reduction, refinement, and replacement and was approved by the Monash Institute of Pharmaceutical Sciences Animal Ethics Committee. The systemic exposure of the aryl hydantoins was studied in nonfasted male Swiss outbred mice weighing 25–33 g (7 mice per compound). Mice had access to food and water ad libitum throughout the pre- and postdose sampling period. Formulations were prepared by dispersing the aryl hydantoins in Tween 80 and then adding ethanol and Milli-Q water (final composition 7% v/v Tween 80, 3% v/v ethanol). Following vortexing and sonication, samples formed either uniform suspensions (1–3, 14, 16, 23, 26) or a colorless solution (13). Compound formulations were mixed by inverting the tubes prior to drawing each dosing volume. All animals were dosed orally by gavage (10 mL/kg dose volume) within 1 h of formulation preparation. Following administration, blood samples were collected from 0.25 to 48 h postdose (n = 2 mice per time point). A maximum of two samples were obtained from each mouse, with samples being taken either via submandibular bleed (approximately 120 μL; conscious sampling) or terminal cardiac puncture (0.6 mL; while mice were under deep anesthesia using inhaled isoflurane). No urine samples were collected, as mice were housed in bedded cages during the study. Blood was collected directly into polypropylene Eppendorf tubes containing heparin as anticoagulant and stabilization cocktail (containing Complete (a protease inhibitor cocktail), potassium fluoride, and EDTA) to minimize the potential for ex vivo degradation of the aryl hydantoins in blood/plasma samples. Once collected, blood samples were centrifuged immediately, and supernatant plasma was removed and stored at −80 °C until analysis by LCMS. Plasma concentrations were determined by comparison to calibration standards prepared in blank plasma and treated the same as the plasma samples. Plasma concentration–time data were analyzed using noncompartmental methods (PKSolver Version 2.0).
S. mansoni in Vivo Studies
The in vivo study was approved by the local veterinary agency, based on Swiss cantonal and national regulations (permission no. 2070). As described by Keiser,48 cercariae of S. mansoni were obtained from infected Biomphalaria glabrata. NMRI mice were infected subcutaneously with approximately 100 S. mansoni cercariae. At 49 d after infection, groups of four mice were treated with single 100 mg/kg oral doses of compounds in a 7% (v/v) Tween 80% and 3% (v/v) ethanol vehicle (10 mL/kg). Untreated mice (n = 8) served as controls. At 21 d post-treatment, animals were killed by the CO2 method and dissected. Worms were removed by picking, then sexed and counted.
Androgen-Dependent Cell-Based Assay
As described by Jones and Diamond,49 LAPC4 cells were cultured in phenol red free RPMI 1640 media supplemented with antibiotics and 10% FBS. For all transfections, pools of cells were transfected using Lipofectamine Plus (Invitrogen) with PSA-luciferase50 and pRL-SV40 (Promega) as a normalization control. The following day, the cells were replated, 0.3 nM DHT and test compounds were added, and 24 h later luciferase production was measured (Dual luciferase assay kit; Promega), normalizing the firefly signal to the renilla signal. Mean-effect plots (log[compound] vs log[fractional effect]) were generated to determine the IC50 values for each test compound or combinations of test compounds at constant ratios.
Acknowledgments
We acknowledge the U.S. National Institutes of Health (AI097802-02 and AI116723-01) and the European Research Council (ERC-2013-CoG 614739-A HERO) for financial support.
Glossary
Abbreviations Used
- AR
androgen receptor
- DMA
dimethylacetamide
- N
nilutamide
- PZ
praziquantel
- WBR
worm burden reduction
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.6b01410.
SMILES data (CSV)
Author Present Address
For Q.Z.: Shanghai Institute of Materia Medica, Chinese Academy of Sciences, No. 501, Haike Rd., Pudong New District, Shanghai 201210, China.
The authors declare no competing financial interest.
Supplementary Material
References
- Hotez P. J.; Brindley P. J.; Bethony J. M.; King C. H.; Pearce E. J.; Jacobson J. Helminth infections: The great neglected tropical diseases. J. Clin. Invest. 2008, 118, 1311–1321. 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryseels B. Schistosomiasis. Infect. Dis. Clin. North Am. 2012, 26, 383–397. 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Colley D. G.; Bustinduy A. L.; Secor W. E.; King C. H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzinger J.; N’goran E. K.; Caffrey C. R.; Keiser J. From innovation to application: Social-ecological context, diagnostics, drugs and integrated control of schistosomiasis. Acta Trop. 2011, 120, S121–S137. 10.1016/j.actatropica.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Olliaro P.; Delgado-Romero P.; Keiser J. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer). J. Antimicrob. Chemother. 2014, 69, 863–870. 10.1093/jac/dkt491. [DOI] [PubMed] [Google Scholar]
- Cioli D.; Pica-Mattoccia L.; Basso A.; Guidi A. Schistosomiasis control: Praziquantel forever?. Mol. Biochem. Parasitol. 2014, 195, 23–29. 10.1016/j.molbiopara.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Melman S. D.; Steinauer M. L.; Cunningham C.; Kubatko L. S.; Mwangi I. N.; Wynn N. B.; Mutuku M. W.; Karanja D. M.; Colley D. G.; Black C. L.; Secor W. E.; Mkoji G. M.; Loker E. S. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Neglected Trop. Dis. 2009, 3, e504. 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinathan R. S.; Greenberg R. M. Pharmacology and potential physiological significance of schistosome multidrug resistance transporters. Exp. Parasitol. 2012, 132, 2–6. 10.1016/j.exppara.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey C. R.; Williams D. L.; Todd M. H.; Nelson D. L.; Utzinger J.. Chemotherapeutic development strategies for schistosomiasis. In Antiparasitic and Antibacterial Drug Discovery; Selzer P. M., Ed.; Wiley–Blackwell: Weinheim, 2009; pp 301–321. [Google Scholar]
- Abdulla M.-H.; Ruelas D. S.; Wolff B.; Snedecor J.; Lim K.-C.; Xu F.; Renslo A. R.; Williams J.; McKerrow J. H.; Caffrey C. R. Drug discovery for schistosomiasis: Hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Neglected Trop. Dis. 2009, 3, e478. 10.1371/journal.pntd.0000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J.; Utzinger J. Antimalarials in the treatment of schistosomiasis. Curr. Pharm. Des. 2012, 18, 3531–3538. 10.2174/138161212801327293. [DOI] [PubMed] [Google Scholar]
- Liu Y.-X.; Wu W.; Liang Y.-J.; Jie Z.-L.; Wang H.; Wang W.; Huang Y.-X. New uses for old drugs: The tale of artemisinin derivatives in the elimination of schistosomiasis japonica in China. Molecules 2014, 19, 15058–15074. 10.3390/molecules190915058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panic G.; Vargas M.; Keiser J.; Scandale I. Activity profile of an FDA-approved compound library against Schistosoma mansoni. PLoS Neglected Trop. Dis. 2015, 9, e0003962. 10.1371/journal.pntd.0003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thétiot-Laurent S. A.-L.; Boissier J.; Robert A.; Meunier B. Schistosomiasis Chemotherapy. Angew. Chem., Int. Ed. 2013, 52, 7936–7956. 10.1002/anie.201208390. [DOI] [PubMed] [Google Scholar]
- Bernauer K.; Link H.; Stohler H.. Antiandrogenic and schistosomicidal imidazolidine derivatives. U. S. Patent 4,234,736. 1980.
- Forget E.; Félix H.; Notteghem M. J.; Léger N. Appréciation de l’activité de dérivés schistosomicides en microscopie électronique. Bull. Soc. Pathol. Ex. 1982, 75, 192–200. [PubMed] [Google Scholar]
- Hansen J. B.; Hafliger O. Determination of the dissociation constant of a weak acid using a dissolution rate method. J. Pharm. Sci. 1983, 72, 429–431. 10.1002/jps.2600720426. [DOI] [PubMed] [Google Scholar]
- Link H.; Stohler H. R. 3-Arylhydantoine, eine substanzklasse mit schistosomizider wirkung. Eur. J. Med. Chem. – Chim. Ther. 1984, 19, 261–265. [Google Scholar]
- Keiser J.; Panic G.; Vargas M.; Wang C.; Dong Y.; Gautam N.; Vennerstrom J. L. Aryl hydantoin Ro 13–3978, a broad spectrum antischistosomal. J. Antimicrob. Chemother. 2015, 70, 1788–1797. 10.1093/jac/dkv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J.; Manneck T.; Vargas M. Interactions of mefloquine with praziquantel in the Schistosoma mansoni mouse model and in vitro. J. Antimicrob. Chemother. 2011, 66, 1791–1797. 10.1093/jac/dkr178. [DOI] [PubMed] [Google Scholar]
- Keiser J.; Vargas M.; Vennerstrom J. L. Activity of antiandrogens against juvenile and adult Schistosoma mansoni in mice. J. Antimicrob. Chemother. 2010, 65, 1991–1995. 10.1093/jac/dkq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendonça R. L.; Escrivá H.; Bouton D.; Laudet V.; Pierce R. J. Hormones and nuclear receptors in schistosome fevelopment. Parasitol. Today 2000, 16, 233–240. 10.1016/S0169-4758(00)01641-0. [DOI] [PubMed] [Google Scholar]
- Wang C.; Zhao Z.; Min J.; Muniyan X.; Vargas M.; Wang X.; Dong Y.; Guy R. K.; Lin M.-F.; Keiser J.; Vennerstrom J. L. Antischistosomal versus antiandrogenic properties of aryl hydantoin Ro 13–3978. Am. J. Trop. Med. Hyg. 2014, 90, 1156–1158. 10.4269/ajtmh.14-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. E.; Ouk S.; Yoo D.; Sawyers C. L.; Chen C.; Tran C.; Wongvipat J. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J. Med. Chem. 2010, 53, 2779–2796. 10.1021/jm901488g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; Su L.; Geng J.; Liu J.; Zhao G. Developments in nonsteroidal antiandrogens targeting the androgen receptor. ChemMedChem 2010, 5, 1651–1661. 10.1002/cmdc.201000259. [DOI] [PubMed] [Google Scholar]
- Vinggaard A. M.; Niemelä J.; Wedebye E. B.; Jensen G. E. Screening of 397 chemicals and development of a quantitative structure-activity relationship model for androgen receptor antagonism. Chem. Res. Toxicol. 2008, 21, 813–823. 10.1021/tx7002382. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Allan G. F.; Sbriscia T.; Linton O.; Lundeen S. G.; Sui Z. Synthesis and SAR of novel hydantoin derivatives as selective androgen receptor modulators. Bioorg. Med. Chem. Lett. 2006, 16, 5763–5766. 10.1016/j.bmcl.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Van Dort M. E.; Jung Y.-W. Synthesis and structure–activity studies of side-chain derivatized arylhydantoins for investigation as androgen receptor radioligands. Bioorg. Med. Chem. Lett. 2001, 11, 1045–1047. 10.1016/S0960-894X(01)00146-9. [DOI] [PubMed] [Google Scholar]
- Li Q.; Wu W.-Z.; Sun J.; Lü W.; Cai J.-C. Synthesis of nilutamide and its analogue. Chin. J. Pharm. 2004, 358, 455–456. [Google Scholar]
- Auvin S.; Lanco C.; Chao Q.; Gu K.. Preparation of novel imidazolidine-2,4-dione derivatives for treating breast or prostate cancers. Int. Pat. Appl. WO 2015100613 A1, July 9 2015.
- Muci A. R.; Buchwald S. L.. Practical palladium catalysts for CN and CO bond formation. In Cross-Coupling Reactions; Miyaura N., Ed.; Springer: MA, 2002; Vol. 219, pp 131–209. [Google Scholar]
- Bergauer M.; Bertani B.; Biagetti M.; Bromidge S. M.; Falchi A.; Leslie C. P.; Merlo G.; Pizzi D. A.; Rinaldi M.; Stasi L. P.; Tibasco J.; Vong A. K. K.; Ward S. E.. Preparation of quinolines and quinazolines as ligands for 5-HT1 receptors and their use in the treatment of CNS disorders, in particular serotonin-related disorders. Int. Pat. Appl. WO 2005014552 A1, February 17, 2005.
- Hügel H. M.; Rix C. J.; Fleck K. Comparison of copper(II) acetate promoted N-arylation of 5,5-dimethylhydantoin and other imides with triarylbismuthanes and arylboronic acids. Synlett 2006, 14, 2290–2292. 10.1055/s-2006-949638. [DOI] [Google Scholar]
- Lan J.; Zhang G.; Yu X.; You J.; Chen L.; Yan M.; Xie R. A simple copper salt catalyzed N-arylation of amines, amides, imides, and sulfonamides with arylboronic acids. Synlett 2004, 35, 1095–1097. 10.1055/s-2004-820059. [DOI] [Google Scholar]
- Chan D. M.; Monaco K. L.; Wang R.-P.; Winters M. P. New N-and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 1998, 39, 2933–2936. 10.1016/S0040-4039(98)00503-6. [DOI] [Google Scholar]
- Hroch L.; Hrusková M.; Schmitz J.; Schnakenburg G.; Gütschow M. 3, 5, 5-Trisubstituted hydantoins from activated (benzyloxycarbonylamino) malonic acids. Synthesis 2012, 44, 1907–1914. 10.1055/s-0031-1290974. [DOI] [Google Scholar]
- Vystrcil A.; Cerny J. Model compounds of physostigmine. Collect. Czech. Chem. Commun. 1959, 24, 804–808. 10.1135/cccc19590804. [DOI] [Google Scholar]
- Pastori N.; Greco C.; Clerici A.; Punta C.; Porta O. Free-radical addition to ketimines generated in situ. New one-pot synthesis of quaternary α-aminoamides promoted by a H2O2/TiCl4– Zn/HCONH2 system. Org. Lett. 2010, 12, 3898–3901. 10.1021/ol1015916. [DOI] [PubMed] [Google Scholar]
- Biadatti T.; Dumais L.; Aubert J.; Lamy L.. Nouveaux derives de N-phenul acatamide, inhibiteurs de l’enzyme soat-1, compositions pharmaceutiques et cosmetiques les contentant. FR2920774 A1, March 13, 2009.
- Palm K.; Stenberg P.; Luthman K.; Artursson P. Polar molecular surface properties predict the intestinal absorption of drugs in humans. Pharm. Res. 1997, 14, 568–571. 10.1023/A:1012188625088. [DOI] [PubMed] [Google Scholar]
- Battmann T.; Branche C.; Bouchoux F.; Cerede E.; Philibert D.; Goubet F.; Teutsch G.; Gaillard-Kelly M. Pharmacological profile of RU 58642, a potent systemic antiandrogen for the treatment of androgen-dependent disorders. J. Steroid Biochem. Mol. Biol. 1998, 64, 103–111. 10.1016/S0960-0760(97)00151-9. [DOI] [PubMed] [Google Scholar]
- Pitta M. G.; Silva A. C.; Neves J. K.; Silva P. G.; Irmão J. I.; Malagueño E.; Santana J. V.; Lima M. C.; Galdino S. L.; Pitta I. R.; Albuquerque M. C. New imidazolidinic bioisosters: Potential candidates for antischistosomal drugs. Mem. Inst. Oswaldo Cruz. 2006, 101, 313–316. 10.1590/S0074-02762006000900049. [DOI] [PubMed] [Google Scholar]
- da Silva A. C.; Neves J. K.; Irmão J. I.; Costa V. M.; Souza V. M.; de Medeiros P. L.; da Silva E. C.; de Lima M. C. A.; Pitta I. R.; Albuquerque M. C.; Galdino S. L. Study of the activity of 3-benzyl-5-(4-chloro-arylazo)-4-thioxo-imidazolidin-2-one against Schistosomiasis mansoni in mice. Sci. World J. 2012, 2012, 520524. 10.1100/2012/520524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. G.; Porter R. A.. Preparation of glycine transporter GlyT1 inhibitors for treating neurological and neuropsychiatric disorders. Int. Pat. Appl. WO 2009034062 A1 (19 March 2009).
- Bevan C. D.; Lloyd R. S. A high-throughput screening method for the determination of aqueous drug solubility using laser nephelometry in microtiter plates. Anal. Chem. 2000, 72, 1781–1787. 10.1021/ac9912247. [DOI] [PubMed] [Google Scholar]
- Lombardo F.; Shalaeva M. Y.; Tupper K. A.; Gao F. ElogDoct:A tool for lipophilicity determination in drug discovery. 2. Basic and neutral compounds. J. Med. Chem. 2001, 44, 2490–2497. 10.1021/jm0100990. [DOI] [PubMed] [Google Scholar]
- Valko K.; Nunhuck S.; Bevan C.; Abraham M. H.; Reynolds D. P. Fast gradient HPLC method to determine compounds binding to human serum albumin. Relationships with octanol/water and immobilized artificial membrane lipophilicity. J. Pharm. Sci. 2003, 92, 2236–2248. 10.1002/jps.10494. [DOI] [PubMed] [Google Scholar]
- Keiser J. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology 2010, 137, 589–603. 10.1017/S0031182009991739. [DOI] [PubMed] [Google Scholar]
- Jones J. O.; Diamond M. I. A cellular conformation-based screen for androgen receptor inhibitors. ACS Chem. Biol. 2008, 3, 412–418. 10.1021/cb800054w. [DOI] [PubMed] [Google Scholar]
- Bolton E. C.; So A. Y.; Chaivorapol C.; Haqq C. M.; Li H.; Yamamoto K. R. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007, 21, 2005–2017. 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.