Abstract
A series of gossypol Schiff bases that were derived from unnatural linear amino acid methyl esters were identified and found to be much more potent than gossypol and ABT-199 in terms of anticancer activity. This is the first example of gossypol Schiff bases with increased activity. The investigation of the Schiff base side chain of gossypol revealed that the unique anticancer effect was achieved by the introduction of hydrophobic ester groups. The optimized products showed low micromolar pan antitumor activities against NCI-60 tumor cell lines, which is promising for further drug development. Studies on the preliminary mechanism of action for their cellular activities was also carried out with antiapoptotic protein (Bcl-2 and Mcl-1) inhibition FP assays. The molecular modeling analysis demonstrated a possible binding mode for these compounds with Bcl-2, which could explain the binding affinity of the novel gossypol Schiff bases with these proteins.
Keywords: Gossypol Schiff bases, linear amino acid esters, antiapoptotic proteins
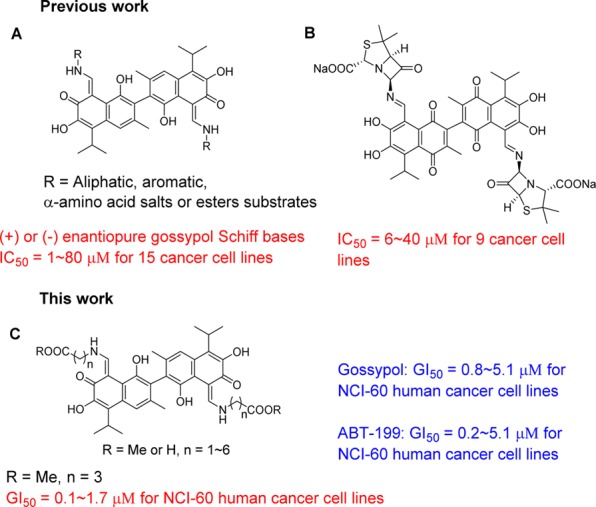
Natural polyphenols have received considerable attention because of their biological activity.1 Gossypol, a polyphenol that is isolated from cotton seeds, has long been investigated for antitumor activity in addition to the reported antiviral (such as anti-HIV2 and anti-H5N13), antifungal,4 and antimalarial5 activity as well as the inhibition of trypanosome T. brucei activity.6 (−)-Gossypol is currently in Phase II clinical trials and displays single-agent antitumor activity in patients with advanced malignancies.7 The removal or mask of aldehyde groups has been shown to significantly reduce the toxicity of gossypol in humans.8 As such, a variety of gossypol derivatives has been developed, including gossypol lactones,9 gossypol nitriles,5 gossypolone,10 modification of the main structure of naphthalene,7,11 etc. Among these, gossypol Schiff bases displayed widespread biological activities. The purpose of the introduction of the imine through the aldehyde was to reduce the toxicity of gossypol without a loss in the activity since the hydroxyl group appears to be essential for the activity in many cases.12 However, from known results, the reported activities of aldehyde derived compounds, including gossypol Schiff bases, decreased dramatically compared with gossypol itself in terms of antitumor activity (Figure 1A).12−15 The antitumor activities of gossypol Schiff bases were improved slightly only when the framework of gossypol was changed into gossypolone (Figure 1B).15 The effects of the introduced substituents of gossypol Schiff bases on the antitumor activity were still ambiguous. As a matter of fact, an investigation of the mechanism of the gossypol Schiff bases was not pursued in these previous studies. On the other hand, studies have shown that gossypol may inhibit tumor cells by interacting with Bcl-2 family proteins, which are the central regulators of apoptosis (programmed cell-death).16 The modulation of the antiapoptotic Bcl-2 family (Bcl-2 and Mcl-1) is necessary for protein–protein interactions (PPIs) during apoptosis. Thus, an ideal inhibitor of antiapoptotic proteins should bind to both Bcl-2 and Mcl-1.17
Figure 1.
Comparison of our newly synthesized Schiff bases with compounds reported to have anticancer activity.
Therefore, to address the role of substituents on gossypol Schiff bases, to explore the SAR, and ultimately to discover agents that increase the broad biological activities with the reduced toxicity of gossypol, we systematically investigated many types of gossypol Schiff bases that targeted both Bcl-2 and Mcl-1 (including a small number of previous synthetic compounds that lacked antitumor activity information, see details in Supporting Information).18 The results demonstrated that gossypol with hydrophobic linear esters increased antitumor activity and reduced cytotoxicity. This finding is different from the conclusion of increased activity that is induced by hydrophilic effects in the literature.14,15
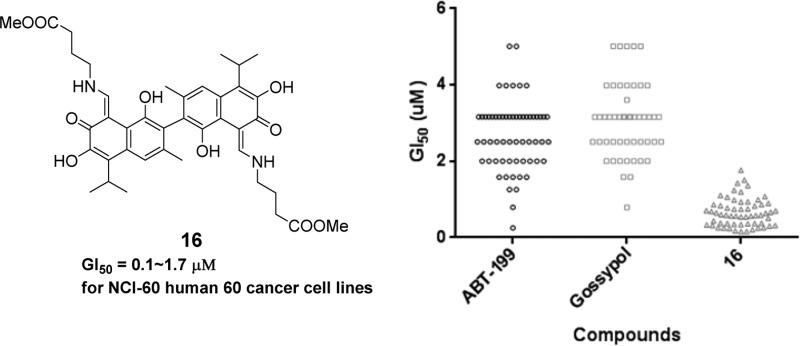
The results indicated that compound 16 with a hydrophobic linear ester was the most active, and the compound showed pan antitumor activity with GI50 values of 0.1 to 1.7 μM in NCI-60 human cancer cell lines (Figure 1C). The therapeutic index of compound 16 was over 30-fold higher than gossypol and showed 10-fold better antitumor activity and 10-fold lower cytotoxicity than gossypol. The competitive FP assays demonstrated that the active compounds had high binding affinity to Bcl-2 and Mcl-1. The results of the molecular modeling study indicated that hydrophobic groups that were introduced by linear amino acid methyl esters were responsible for the extra affinity for the potent compounds.
Cell Viability of Human and Mouse Cancer Cell Lines
The SAR information on the reported substrates of gossypol Schiff bases is limited and includes few discussions about the effect of the substituents. It was reported that the natural product cryptosphaerolide, with an aliphatic chain, exhibited HCT-116 activity through the Mcl-1 pathway.19 Thus, we proposed that the introduction of the hydrophobic moiety into gossypol could be beneficial for the activity of the compound. Therefore, we synthesized a set of different types of amines from gossypol that included aromatic amines, aliphatic amines, amino acid derivatives, etc., described in detail in the Supporting Information. The antiproliferative activity of the target gossypol Schiff bases was initially studied with an MTT cell viability assay for human and mouse cancer cell lines. The results are shown in Table 1.
Table 1. Systematic Evaluation of Gossypol Schiff Bases Using Cell Viability Assays.

IC50 values were the means of three independent experiments.
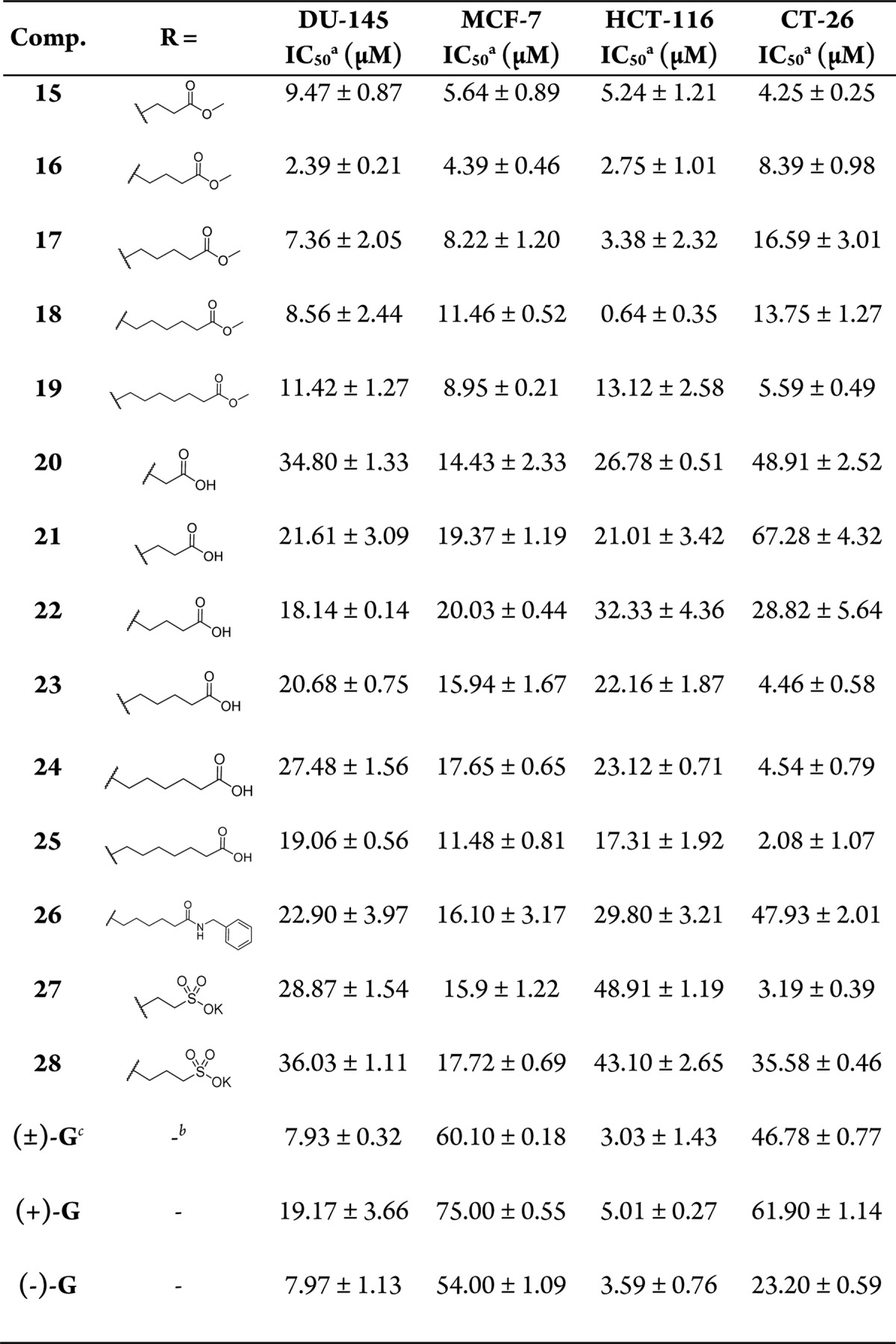
First, compounds 1–6 with different sizes and lengths of aliphatic hydrophobic groups (such as adamantyl and tert-octyl) were attached, but only compounds 1 and 3 with medium size groups (tert-butyl and cyclohexyl) showed moderate inhibitory activity; others did not exhibit activity in cellular assays. We also checked the anticancer activity of Schiff bases with aromatic hydrophobic groups (compounds 7–11), which were more potent than aliphatic groups (compounds 1–6). Compound 7, with a benzoic acid methyl ester, exhibited micromolar inhibitory activity in all of the four cellular assays. We then investigated several natural amino acid methyl ester groups. Gossypol Schiff bases that were derived from natural amino acids have been demonstrated to exhibit good antiviral activity against HIV-1 and H5N1,2,3 but their antitumor activity was not prominent. In this regard, compounds 12, 13, and 14 were prepared from valine, methionine, and glycine methyl esters, respectively. These ester compounds exhibited better activities than aliphatic (compounds 1–6) and aromatic (8–11) ones. Notably, compound 14 is the first modified imine compound with similar inhibitory activity compared to gossypol in all the assays.
With compound 14 in hand, we next investigated gossypol Schiff bases that were derived from unnatural amino acids/esters with different chain lengths (15–26). As shown in Table 2 and Figure 2, in human cancer cell lines MCF-7, DU-145, and HCT-116, gossypol Schiff bases that were conjugated with unnatural amino acid esters (14–19) were more potent than the corresponding amino acids 20–25. For the latter compounds, the chain length had little effect on the cell growth inhibitory activities (Figure 2). For the amino acid methyl esters 14–19, compound 16 (n = 3) exhibited the most potent inhibitory activity (Figure 2). After the replacement of COOH or COOMe groups by a larger group, such as CONHCH2Ph in compound 26, a decreased activity was observed. Compounds 27 and 28, analogues of the gossypol derivative megosin with different chain lengths, were generated to increase water solubility, but displayed modest inhibitory activity in the four cancer cell lines.20 Almost all the compounds exhibited good inhibitory activity in the mouse colon cell line CT-26 (Tables 1 and 2). For example, compounds 2 (IC50 = 5.4 μM) and 9 (IC50 = 12.8 μM) exhibited good results that were absent in the other three cancer cell lines (IC50 > 100 μM).
Table 2. Further Evaluation of the Optimized Gossypol Schiff Bases with Different Linker Lengths Using Cell Viability Assays.
IC50 values were the means of three independent experiments.
“–” means no substitution.
“G′′ means gossypol.
Figure 2.
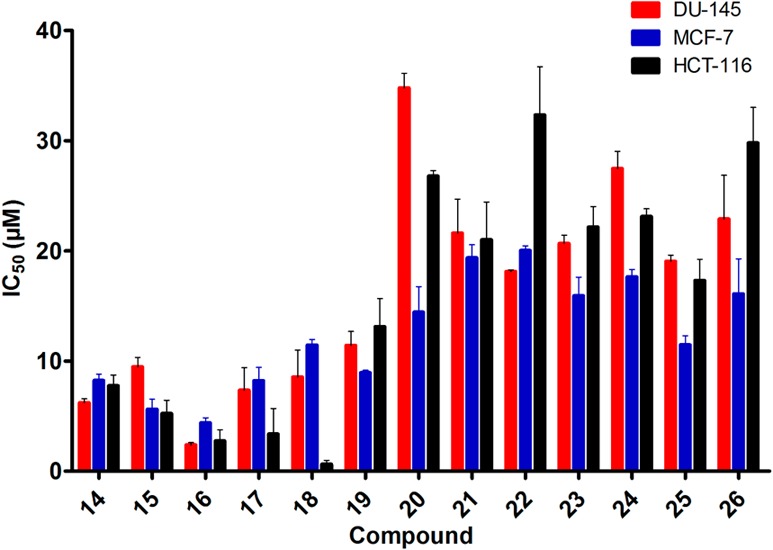
Cell viability assays of the unnatural amino acid methyl esters (14–19), their corresponding acids (20–25), and amide 26 in selected tumor cell lines.
To examine whether these novel gossypol Schiff bases were superior to the parent (±)-gossypols in terms of inhibitory activity, we also prepared (+)-gossypol and (−)-gossypol compounds (see the Supporting Information chemistry section). Antiproliferative data revealed that the linear chain methyl esters of gossypol Schiff bases 14–19 were more active than the parent (±)-gossypols and performed better than the corresponding enantiomers (Table 2). Notably, the newly synthesized compounds significantly affected the growth of MCF-7 and CT-26 cancer cells, in which (±)-gossypol and even (−)-gossypol were ineffective. Compound 16, which was the most potent compound (IC50 = 4.39 μM) that inhibited the growth of MCF-7 cells, was 12-fold more potent than (−)-gossypol (IC50 = 54.0 μM). Similarly, compound 25, with an IC50 value of 2.08 μM, was 11-fold more potent than (−)-gossypol (IC50 = 23.2 μM) in CT-26 cells.
In Vitro NCI-60 Human Cancer Cell Lines Assay
Most of the gossypol Schiff bases in this study (compounds 1-3, 8, 9, 11, 14, 16-20, and 23-28) were also selected for cytotoxicity screening against 60 human tumor cell lines. As anticipated, the data obtained by this cell-based assay correlated well with our previous MTT observations (Tables 1 and 2). Namely, compounds with aliphatic (compounds 1–3) and aromatic substituents (compounds 8, 9, and 11) generally showed poor or modest inhibitory activities at concentrations up to 10 μM in both experimental approaches (see Supplemental Table S1). On the contrary, unnatural amino acids/esters derived gossypol Schiff bases with different chain lengths (compounds 14, 16–20) showed significant cytotoxicity against human cancer cells. It should be noted that the newly synthesized gossypol Schiff bases that were derived from unnatural amino acid esters were more potent than all unnatural amino acids, similar to the results that are presented in Table 2. Notably, the GI50 value for the most potent gossypol Schiff base, compound 16, which was derived from a γ-aminobutyric acid methyl ester, was less than 1.0 μM for 50 tumor cell lines out of the 60 cell lines that were tested, which was approximately 10-fold more potent than the parent (±)-gossypol (average GI50 = 3.1 μM) and the known Bcl-2 inhibitor drug ABT-199 (GI50 = 2.77 μM) (Table 3 and Supplemental Table S2). In addition, in most cases, methyl esters of glycine and 6-aminocaproic acid derived gossypol Schiff bases 14 and 18 showed the same level of cytotoxicity as (±)-gossypol. However, compared to the (±)-gossypol 5-amino valeric acid methyl ester that was derived from gossypol, Schiff base 17 significantly affected the growth of several cancer cells, including a leukemia cell line, the nonsmall cell lung cancer HOP-92 cell line, the CNS cancer SNB-19 and U251 cell lines, the renal cancer RXF 393 cell line (see Supplemental Table S2), etc. Based on the data obtained from NIH and our MTT assays, we proposed that the introduction of a linear amino acid methyl esters side chain could benefit the antitumor activity of gossypol Schiff bases. Cytotoxicity studies that were performed with HEK (human embryonic kidney) 293T and Vero cells showed that most compounds were less toxic than gossypol (see Supplemental Table S5). In addition, in a separate study compound 16 was found to be stable enough at pH 1–10 and to microsomes (Supplemental Tables S3 and S4).
Table 3. Average Cell Growth Inhibition Data for Compounds 14 and 16–18 (GI50, μM) against a Panel of 60 Human Cancer Cells.
| average
cell growth inhibition (GI50, μM)a |
||||||
|---|---|---|---|---|---|---|
| cell type (number of cell lines) | ABT-199 | (±)-gossypol | 14 | 16 | 17 | 18 |
| leukemia (6) | 2.11 | 2.47 | 1.88 | 0.56 | 0.81 | 1.03 |
| nonsmall cell lung cancer (9) | 2.7 | 2.85 | 1.65 | 0.48 | 1.05 | 1.51 |
| colon cancer (7) | 2.85 | 3.19 | 1.63 | 0.46 | 1.00 | 1.44 |
| cns cancer (6) | 3.35 | 2.97 | 1.63 | 0.60 | 1.27 | 1.46 |
| melanoma (9) | 1.75 | 3.22 | 2.84 | 0.99 | 1.42 | 2.35 |
| ovarian cancer (7) | 3.33 | 3.50 | 1.74 | 0.59 | 1.26 | 2.65 |
| renal cancer (8) | 2.6 | 3.16 | 2.19 | 0.82 | 1.14 | 2.08 |
| prostate cancer (2) | 3.51 | NDb | 1.92 | 0.41 | 1.09 | 1.41 |
| breast cancer (6) | 2.77 | ND | 2.44 | 0.74 | 1.08 | 1.72 |
GI50: concentration required for 50% cell growth inhibition.
ND: not determined.
Binding Affinity to Bcl-2 and Mcl-1 Proteins
Gossypol is a potent inhibitor of Bcl-2 and Mcl-1.16,21 It binds to Bcl-2 and Mcl-1 with Ki values of 0.32 and 0.18 μM, respectively, as determined by competitive fluorescence polarization-based (FP-based) binding assays.7 The NIH data and our MTT assay results revealed that our newly synthesized gossypol Schiff bases may have a similar mechanism as the parent compound.
To confirm this point, we evaluated the binding affinity of the selected active compounds for Bcl-2 using FP assays in which Triton X-100 was added as a detergent to prevent the possible aggregation of hydrophobic compounds (Table 4 and Figure 3A). The β-alanine methyl ester derived gossypol Schiff base 15 displayed a high binding affinity for Bcl-2, with a Ki value of 0.090 μM, and the glycine methyl analogue 14 showed an increased binding affinity, with a Ki value of 0.005 μM. The other ester compounds 16–18 and amino acid derivatives 20, 22, and 23 exhibited similar binding affinities with Ki values that ranged from 0.171 to 0.348 μM, and all were better than (±)-gossypol (Ki = 0.511 μM) (Figure 3A). As mentioned above in the MTT assay, unnatural amino acid methyl esters that were derived from the gossypol Schiff bases 14–18 displayed potent efficacy in the inhibition of cell growth in the MCF-7 cell line, which expresses moderate levels of Bcl-2 and Mcl-1 (Table 2).22,23 This observation correlated with the binding data of compounds 14–18 for Bcl-2, which indicated that Bcl-2 was the potential target of these compounds. For the enantiomers of the optimized compound 16 (Supporting Information), both of the binding affinities of (−)-16 (Ki = 0.131 μM) and (+)-16 (Ki = 0.167 μM) were higher than (±)-16 (Ki = 0.341 μM) for Bcl-2. Although gossypol Schiff bases 20, 22, and 23 that were derived from the amino acids displayed strong binding affinity (Ki = 0.224, 0.235, 0.171 μM, respectively) to Bcl-2 in the FP assays, they showed a 2.1-fold lower efficacy in the inhibition of the growth of the MCF-7 cell line, with an average IC50 of 16.8 μM, compared to the corresponding ester analogues 14–19 with an average IC50 value of 7.82 μM. The ester analogues 14–19 are also 7.7 times more potent than gossypol (IC50 = 60.10 μM). This discrepancy is likely due to the high hydrophilicity of these unnatural amino acids, so they always exhibit poor cell membrane permeability compared to the corresponding esters. Therefore, the gossypol unnatural amino acids 20–23 displayed good binding affinity to Mcl-1 and Bcl-2 proteins, and they always showed relatively weaker efficacy in the inhibition of cancer cells. It was also reported the high hydrophilicity of apogossypolone derivatives could result in a low cell permeability and would therefore exhibit moderate antitumor activity, although those compounds displayed good binding affinity to Bcl-2 family proteins.24
Table 4. Cross-Activity of Gossypol Schiff Bases with Selected Linker Length against Bcl-2 and Mcl-1 in Fluorescence Polarization Assays.
| IC50a (μM)
FPA |
Ki (μM) |
|||
|---|---|---|---|---|
| Comp | Bcl-2 | Mcl-1 | Bcl-2 | Mcl-1 |
| 14 | 0.103 ± 0.019 | 1.433 ± 0.33 | 0.005 ± 0.004 | 0.276 ± 0.06 |
| 15 | 0.286 ± 0.039 | 2.959 ± 0.847 | 0.090 ± 0.018 | 0.579 ± 0.166 |
| 16 | 0.825 ± 0.106 | 0.947 ± 0.364 | 0.341 ± 0.05 | 0.180 ± 0.071 |
| 17 | 0.483 ± 0.111 | 0.740 ± 0.154 | 0.182 ± 0.052 | 0.139 ± 0.03 |
| 18 | 0.840 ± 0.075 | 1.021 ± 0.166 | 0.348 ± 0.035 | 0.195 ± 0.032 |
| 20 | 0.573 ± 0.091 | 0.521 ± 0.053 | 0.224 ± 0.042 | 0.121 ± 0.01 |
| 22 | 0.596 ± 0.095 | 0.661 ± 0.071 | 0.235 ± 0.044 | 0.094 ± 0.014 |
| 23 | 0.458 ± 0.053 | 0.478 ± 0.036 | 0.171 ± 0.024 | 0.085 ± 0.007 |
| (+)-16 | 0.452 ± 0.016 | 1.249 ± 0.32 | 0.167 ± 0.008 | 0.240 ± 0.061 |
| (−)-16 | 0.375 ± 0.031 | 0.937 ± 0.175 | 0.131 ± 0.014 | 0.178 ± 0.033 |
| gossypol | 1.191 ± 0.667 | 0.856 ± 0.332 | 0.511 ± 0.312 | 0.162 ± 0.065 |
| ABT-199 | <0.001 | >4 | <0.001 | >0.78 |
IC50 values were the means of three independent experiments.
Figure 3.
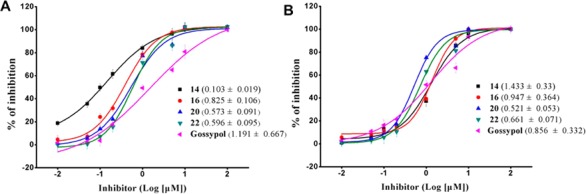
Competitive binding curves of 14, 16, 20, 22, and (±)-gossypol to (A) Bcl-2 and (B) Mcl-1, as determined with the fluorescence-polarization-based binding assay.
Next, we evaluated the binding properties and specificity of selected gossypol Schiff bases against Mcl-1 using FP assays (Table 4); Mcl-1 was expressed at high levels in many cancer cell lines such as the colon cancer HCT-11625 and prostate cancer DU-14526 cell lines. The cell growth inhibition of these compounds in these two cell lines is shown in Tables 1 and 2. All of the tested compounds displayed superior affinity against Mcl-1 in the FP assays, similar to that of gossypol (Ki = 0.162 μM) (Figure 3B). Consistent with binding data, (±)-gossypol exhibited potent efficacy in the inhibition of cell growth in HCT-116 and DU-145 cell lines according to the results of the MTT assay, with IC50 values of 3.03 and 7.93 μM, respectively (Table 2). Other unnatural amino acid methyl esters that were derived as gossypol Schiff bases 14–18 also displayed strong inhibitory properties against the Mcl-1 protein. The most potent compound, compound 17, displaced BH3 binding to Mcl-1, with IC50 values of 7.36 and 3.38 μM to DU-145 and HCT-116, respectively (Table 2). However, the compounds 20, 22, and 23 displayed very high binding affinity to Mcl-1 in the FP assays, with average IC50 values of 0.10 μM; the compounds showed a relatively weak efficacy in inhibiting the growth of DU-145 and HCT-116 cell lines, with average IC50 values of approximately 24.54 and 27.09 μM, respectively. The lower IC50 of cellular data of compounds 20–23 with higher Mcl-1 binding affinity may be due to a permeability issue, as observed in Bcl-2.
To investigate the activities and mechanisms of gossypol imines, ABT-199 was selected as a control compound. ABT-199 is known as a highly Bcl-2 selective antagonist. Our data revealed that compound 16 had lower Bcl-2 binding affinity than ABT-199 but had higher Mcl-1 affinity. According to the NCI cell line data, ABT-199 exhibited the highest activity in the leukemia cancer cell line HL-60, which expresses high levels of Bcl-2. In addition, compound 16 exhibited an equal potency to ABT-199 in the HL-60 cell line (Supplemental Table S2). Furthermore, compound 16 was more potent in most human cancer cell lines than ABT-199 (Supplemental Table S2). Mcl-1, but not Bcl-2, is expressed in most human cancer cell lines.26 Thus, it was possible to infer that the gossypol Schiff bases were superior to ABT-199 via the inhibition of both Bcl-2 and Mcl-1 mechanisms.
With the combination of binding affinities and all the in vitro cell viability data, one can conclude that gossypol Schiff bases that were derived from unnatural linear amino acid methyl esters could enhance the antitumor activity of gossypol. Furthermore, the chain length is also important for Bcl-2 family protein binding; the data revealed that the three-carbon linker was the best in the series. Studies have indicated that small molecular inhibitors of the Bcl-2 family with longer, flexible hydrophobic groups have displayed higher potencies than small, short, and rigid hydrophobic groups.29 Therefore, computational docking studies were carried out to understand the binding model of the selected compounds to Bcl-2 family proteins and the SAR of the different substituents.
Molecular Modeling
The enamine form of the gossypol Schiff base analogue was confirmed by X-ray crystal structure,4 so we used this form in our molecular model study. One advantage of the gossypol enamine tautomer is that it is stabilized by intramolecular hydrogen bonding. Compound (−)-16 was chosen to be studied by molecular docking since the (−)-gossypol conformation exhibits a better binding affinity than the corresponding (+)-conformation.
As observed in Figure 4A,B, the aliphatic chain could occupy the P2 and P3 pockets of the Bcl-2 protein without the flexible loop domain in the model.30 The size and orientation could fit the pocket quite well in the ligand CPK model. We then analyzed the details of the compound (−)-16 and the protein interaction from the molecular modeling point of view.
Figure 4.
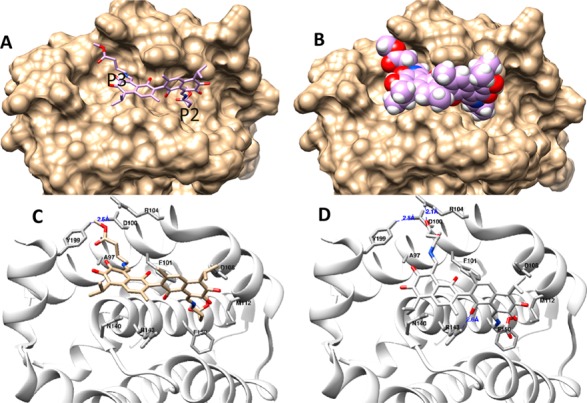
(A,B) The interaction models of compound (−)-16 with Bcl-2. (A) The binding site and orientation of the compound (represented by compound (−)-16) in the hydrophobic groove P2 and P3 of Bcl-2. (B) The protein is rendered in a surface model, while the compound is rendered in a stick model and a van der Waals sphere (CPK) model. (C,D) Two possible poses were assessed in the molecular docking study, (C) with no ligand-protein hydrogen bonding interaction and (D) with a ligand–protein hydrogen bonding interaction; Bcl-2 is rendered as a cartoon, while the compound and the residues around it are rendered as sticks (enlarged pictures, see Supplemental Figure S11).
The major surface of the binding groove is a hydrophobic region that includes A97, F101, M112, N149, and F150 and the polar residue rich region that contains R104, D108, R143 and Y199. The helixes, which contain Y199 and D100, were observed to have strong H bonding according to X-ray structure data and formed the wide hydrophobic pocket P3 for the peptide or ligand interaction. R143 was found to interact with D of the BIM peptide through H bonding. However, in the reported small molecule and Bcl-2 complex, hydrogen bond interactions with the receptor were observed less often, except in one example in which the ABT-199 indole analogue interacted with D100.31 In our cases, two possible binding models are presented in Figure 4C,D; one model included the most observed hydrophobic interaction without the protein–ligand hydrogen bonding interaction, and the other model used the hydrogen bonds between the hydroxyl group of gossypol and the nitrogen of R143 as well as the oxygen of the carbonyl group of gossypol and the nitrogen of R104. We used AmberScore to evaluate the binding energy because AmberScore has been shown to be one of the most precise MD scoring tools for docking studies.33 The first model had a much lower score, −24, than the second model, which had a score of −14 based on the AmberScore. In contrast, the score of gossypol in the form of (−)-16 was −14, as shown in Figure 4D; this score was lower than the form that is presented in Figure 4C. The amino acid ester chains that are shown in Figure 4D did not contribute extra binding energy. The hydrophobic interaction could therefore improve the interaction between the receptor and the ligand, even when the H boning interaction was sacrificed. These results are also consistent with the following findings: from BIM to different types of peptides (stapled or unnatural), the four hydrophobic residues h1 to h4 (ILIF) were widely applied to design novel peptide inhibitors.34 The activity of gossypol imines was improved by the hydrophobic interaction that resulted from the introduction of the aliphatic side chains.
In summary, a series of gossypol Schiff bases with linear unnatural amino acids/methyl esters were demonstrated to be potent inhibitors of antiapoptotic proteins. Compound 16 was found to bind to Bcl-2 and Mcl-1 with IC50 values of 0.341 and 0.180 μM, respectively. The in vitro cellular assay indicated that compound 16 potently inhibited the growth of cancer cells. The cell growth inhibition data from the NIH were consistent with our results, and the GI50 values of compound 16, the most potent compound, ranged from 0.1 to 1.7 μM, which were better than the values that were obtained with gossypol and ABT-199.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (21202125, 31300060, 81573279), the Hubei Province Natural Science Foundation (2014CFB241), the Innovation Seed Fund of Wuhan University School of Medicine, and the Hubei Province Engineering and Technology Research Center for Fluorinated Pharmaceuticals.
Glossary
ABBREVIATIONS
- SARs
structure–activity relationships
- PPIs
protein–protein interactions
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- CNS
central nervous system
- Bcl-2
B-cell lymphoma 2
- Mcl-1
myeloid cell leukemia 1
- FP
fluorescence polarization
- CPK
Corey–Pauling–Koltun
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00302.
Experimental procedures and chemical data for final compounds, molecular modeling, biochemical assays, and stability assays. NCI-60 one dose (10 μM) screening data for compounds 1–3, 8, 9, 11, 14, 16–20, 23–26, and 28. Details of NCI-60 information for compounds ABT-199, 14, 16, 17, and 18 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Tomás-Barberán F. A.; Andrés-Lacueva C. Polyphenols and Health: Current State and Progress. J. Agric. Food Chem. 2012, 60, 8773–8775. 10.1021/jf300671j. [DOI] [PubMed] [Google Scholar]
- An T.; Ouyang W.; Pan W.; Guo D.; Li J.; Li L.; Chen G.; Yang J.; Wu S.; Tien P. Amino acid derivatives of the (−) enantiomer of gossypol are effective fusion inhibitors of human immunodeficiency virus type 1. Antiviral Res. 2012, 94, 276–287. 10.1016/j.antiviral.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Yang J.; Chen G.; Li L. L.; Pan W.; Zhang F.; Yang J.; Wu S.; Tien P. Synthesis and anti-H5N1 activity of chiral gossypol derivatives and its analogs implicated by a viral entry blocking mechanism. Bioorg. Med. Chem. Lett. 2013, 23, 2619–2623. 10.1016/j.bmcl.2013.02.101. [DOI] [PubMed] [Google Scholar]
- Przybylski P.; Pyta K.; Stefanska J.; Ratajczak-Sitarz M.; Katrusiak A.; Huczynski A.; Brzezinski B. Synthesis, crystal structures and antibacterial activity studies of aza-derivatives of phytoalexin from cotton plant--gossypol. Eur. J. Med. Chem. 2009, 44, 4393–4403. 10.1016/j.ejmech.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Royer R. E.; Deck L. M.; Campos N. M.; Hunsaker L. A.; Vander Jagt D. L. Biologically active derivatives of gossypol: synthesis and antimalarial activities of peri-acylated gossylic nitriles. J. Med. Chem. 1986, 29, 1799–1801. 10.1021/jm00159a043. [DOI] [PubMed] [Google Scholar]
- Montamat E.; Burgos C.; Gerez de Burgos N.; Rovai L.; Blanco A.; Segura E. Inhibitory action of gossypol on enzymes and growth of Trypanosoma cruzi. Science 1982, 218, 288–289. 10.1126/science.6750791. [DOI] [PubMed] [Google Scholar]
- Wang G.; Nikolovska-Coleska Z.; Yang C.-Y.; Wang R.; Tang G.; Guo J.; Shangary S.; Qiu S.; Gao W.; Yang D.; Meagher J.; Stuckey J.; Krajewski K.; Jiang S.; Roller P. P.; Abaan H. O.; Tomita Y.; Wang S. Structure-Based Design of Potent Small-Molecule Inhibitors of Anti-Apoptotic Bcl-2 Proteins. J. Med. Chem. 2006, 49, 6139–6142. 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- Shelley M. D.; Hartley L.; Groundwater P. W.; Fish R. G. Structure-activity studies on gossypol in tumor cell lines. Anti-Cancer Drugs 2000, 11, 209–216. 10.1097/00001813-200003000-00009. [DOI] [PubMed] [Google Scholar]
- Deck L. M.; Vander Jagt D. L.; Royer R. E. Gossypol and derivatives: a new class of aldose reductase inhibitors. J. Med. Chem. 1991, 34, 3301–3305. 10.1021/jm00115a021. [DOI] [PubMed] [Google Scholar]
- Shelley M. D.; Hartley L.; Fish R. G.; Groundwater P.; Morgan J. J. G.; Mort D.; Mason M.; Evans A. Stereo-specific cytotoxic effects of gossypol enantiomers and gossypolone in tumour cell lines. Cancer Lett. 1999, 135, 171–180. 10.1016/S0304-3835(98)00302-4. [DOI] [PubMed] [Google Scholar]
- Tang G.; Ding K.; Nikolovska-Coleska Z.; Yang C.-Y.; Qiu S.; Shangary S.; Wang R.; Guo J.; Gao W.; Meagher J.; Stuckey J.; Krajewski K.; Jiang S.; Roller P. P.; Wang S. Structure-Based Design of Flavonoid Compounds As a New Class of Small-Molecule Inhibitors of the Anti-apoptotic Bcl-2 Proteins. J. Med. Chem. 2007, 50, 3163–3166. 10.1021/jm070383c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao V.-T.; Gaspard C.; Mayer M.; Werner G. H.; Nguyen S. N.; Michelot R. J. Synthesis and cytotoxicity of gossypol related compounds. Eur. J. Med. Chem. 2000, 35, 805–813. 10.1016/S0223-5234(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Dao V. T.; Dowd M. K.; Martin M. T.; Gaspard C.; Mayer M.; Michelot R. J. Cytotoxicity of enantiomers of gossypol Schiff’s bases and optical stability of gossypolone. Eur. J. Med. Chem. 2004, 39, 619–624. 10.1016/j.ejmech.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Jiang H.; Cao X.; Zhao H.; Wang F.; Cui Y.; Jiang B. Chiral gossypol derivatives: evaluation of their anticancer activity and molecular modeling. Eur. J. Med. Chem. 2009, 44, 3961–3972. 10.1016/j.ejmech.2009.04.025. [DOI] [PubMed] [Google Scholar]
- Yan F.; Cao X. X.; Jiang H. X.; Zhao X. L.; Wang J. Y.; Lin Y. H.; Liu Q. L.; Zhang C.; Jiang B.; Guo F. A novel water-soluble gossypol derivative increases chemotherapeutic sensitivity and promotes growth inhibition in colon cancer. J. Med. Chem. 2010, 53, 5502–5510. 10.1021/jm1001698. [DOI] [PubMed] [Google Scholar]
- Mohammad R. M.; Wang S.; Aboukameel A.; Chen B.; Wu X.; Chen J.; Al-Katib A. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL [(−)-gossypol] against diffuse large cell lymphoma. Mol. Cancer Ther. 2005, 4, 13–21. [PubMed] [Google Scholar]
- Azmi A. S.; Mohammad R. M. Non-peptidic small molecule inhibitors against Bcl-2 for cancer therapy. J. Cell. Physiol. 2009, 218, 13–21. 10.1002/jcp.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Li Z.; Wang K.; Liu Y.; Li Y.; Wang Q. Synthesis and antiviral, insecticidal, and fungicidal activities of gossypol derivatives containing alkylimine, oxime or hydrazine moiety. Bioorg. Med. Chem. 2016, 24, 474–483. 10.1016/j.bmc.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Oh H.; Jensen P. R.; Murphy B. T.; Fiorilla C.; Sullivan J. F.; Ramsey T.; Fenical W. Cryptosphaerolide, a Cytotoxic Mcl-1 Inhibitor from a Marine-Derived Ascomycete Related to the Genus Cryptosphaeria. J. Nat. Prod. 2010, 73, 998–1001. 10.1021/np1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionov M.; Gordiyenko N.; Olchowik E.; Baram N.; Zijaev K.; Salakhutdinov B.; Bryszewska M.; Zamaraeva M. The immobilization of gossypol derivative on N-polyvinylpyrrolidone increases its water solubility and modifies membrane-active properties. J. Med. Chem. 2009, 52, 4119–4125. 10.1021/jm9002507. [DOI] [PubMed] [Google Scholar]
- Kitada S.; Leone M.; Sareth S.; Zhai D.; Reed J. C.; Pellecchia M. Discovery, Characterization, and Structure–Activity Relationships Studies of Proapoptotic Polyphenols Targeting B-Cell Lymphocyte/Leukemia-2 Proteins. J. Med. Chem. 2003, 46, 4259–4264. 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- Akkoc Y.; Berrak O.; Arisan E. D.; Obakan P.; Coker-Gurkan A.; Palavan-Unsal N. Inhibition of PI3K signaling triggered apoptotic potential of curcumin which is hindered by Bcl-2 through activation of autophagy in MCF-7 cells. Biomed. Pharmacother. 2015, 71, 161–171. 10.1016/j.biopha.2015.02.029. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Dragowska W.; Wallis A.; Duronio V.; Mayer L. Cytotoxicity induced by manipulation of signal transduction pathways is associated with down-regulation of Bcl-2 but not Mcl-1 in MCF-7 human breast cancer. Breast Cancer Res. Treat. 2001, 70, 11–20. 10.1023/A:1012564620853. [DOI] [PubMed] [Google Scholar]
- Wei J.; Kitada S.; Stebbins J. L.; Placzek W.; Zhai D.; Wu B.; Rega M. F.; Zhang Z.; Cellitti J.; Yang L.; Dahl R.; Reed J. C.; Pellecchia M. Synthesis and biological evaluation of Apogossypolone derivatives as pan-active inhibitors of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J. Med. Chem. 2010, 53, 8000–8011. 10.1021/jm100746q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priault M.; Hue E.; Marhuenda F.; Pilet P.; Oliver L.; Vallette F. M. Differential Dependence on Beclin 1 for the Regulation of Pro-Survival Autophagy by Bcl-2 and Bcl-xL in HCT116 Colorectal Cancer Cells. PLoS One 2010, 5, 1–12. 10.1371/journal.pone.0008755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek W. J.; Wei J.; Kitada S.; Zhai D.; Reed J. C.; Pellecchia M. A survey of the anti-apoptotic Bcl-2 subfamily expression in cancer types provides a platform to predict the efficacy of Bcl-2 antagonists in cancer therapy. Cell Death Dis. 2010, 1, e40. 10.1038/cddis.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friberg A.; Vigil D.; Zhao B.; Daniels R. N.; Burke J. P.; Garcia-Barrantes P. M.; Camper D.; Chauder B. A.; Lee T.; Olejniczak E. T.; Fesik S. W. Discovery of potent myeloid cell leukemia 1 (Mcl-1) inhibitors using fragment-based methods and structure-based design. J. Med. Chem. 2013, 56, 15–30. 10.1021/jm301448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacarías-Lara O. J.; Correa-Basurto J.; Bello M. Exploring the conformational and binding properties of unphosphorylated/phosphorylated monomeric and trimeric Bcl-2 through docking and molecular dynamics simulations. Biopolymers 2016, 105, 393–413. 10.1002/bip.22839. [DOI] [PubMed] [Google Scholar]
- Souers A. J.; Leverson J. D.; Boghaert E. R.; Ackler S. L.; Catron N. D.; Chen J.; Dayton B. D.; Ding H.; Enschede S. H.; Fairbrother W. J.; Huang D. C. S.; Hymowitz S. G.; Jin S.; Khaw S. L.; Kovar P. J.; Lam L. T.; Lee J.; Maecker H. L.; Marsh K. C.; Mason K. D.; Mitten M. J.; Nimmer P. M.; Oleksijew A.; Park C. H.; Park C.-M.; Phillips D. C.; Roberts A. W.; Sampath D.; Seymour J. F.; Smith M. L.; Sullivan G. M.; Tahir S. K.; Tse C.; Wendt M. D.; Xiao Y.; Xue J. C.; Zhang H.; Humerickhouse R. A.; Rosenberg S. H.; Elmore S. W. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Lang P. T.; Brozell S. R.; Mukherjee S.; Pettersen E. F.; Meng E. C.; Thomas V.; Rizzo R. C.; Case D. A.; James T. L.; Kuntz I. D. DOCK 6: Combining techniques to model RNA–small molecule complexes. RNA 2009, 15, 1219–1230. 10.1261/rna.1563609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessene G.; Czabotar P. E.; Colman P. M. BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug Discovery 2008, 7, 989–1000. 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.