Abstract
Agrin triggers signaling mechanisms of high temporal and spatial specificity to achieve phosphorylation, clustering, and stabilization of postsynaptic acetylcholine receptors (AChRs). Agrin transiently activates the kinase MuSK; MuSK activation has largely vanished when AChR clusters appear. Thus, a tyrosine kinase cascade acts downstream from MuSK, as illustrated by the agrin-evoked long-lasting activation of Src family kinases (SFKs) and their requirement for AChR cluster stabilization. We have investigated this cascade and report that pharmacological inhibition of SFKs reduces early but not later agrin-induced phosphorylation of MuSK and AChRs, while inhibition of Abl kinases reduces late phosphorylation. Interestingly, SFK inhibition applied selectively during agrin-induced AChR cluster formation caused rapid cluster dispersal later upon agrin withdrawal. We also report that a single 5-min agrin pulse, followed by extensive washing, triggered long-lasting MuSK and AChR phosphorylation and efficient AChR clustering. Following the pulse, MuSK phosphorylation increased and, beyond a certain level, caused maximal clustering. These data reveal novel temporal aspects of tyrosine kinase action in agrin signaling. First, during AChR cluster formation, SFKs initiate early phosphorylation and an AChR stabilization program that acts much later. Second, a kinase mechanism rapidly activated by agrin acts thereafter autonomously in agrin's absence to further increase MuSK phosphorylation and cluster AChRs.
Neuromuscular synapses are cellular contacts of remarkable specialization. The presynaptic terminal is specialized to release neurotransmitters on demand, and the postsynaptic membrane, by accumulating a high density of clustered acetylcholine receptors (AChRs) and associated proteins, is specialized to generate an endplate potential of sufficient magnitude to reliably initiate an action potential in the muscle. To assemble and maintain these structures, it is essential that pre- and postsynaptic cells exchange signals to coordinate their differentiation in time and space.
One such signaling exchange is initiated by agrin, a nerve-derived signal that is concentrated in the synaptic basal lamina (37). Agrin rapidly stimulates MuSK, a receptor tyrosine kinase of skeletal muscle, and agrin-MuSK signaling is essential for the formation of neuromuscular synapses (16, 20). Little is known about the mechanisms by which agrin activates MuSK and how MuSK activation leads to pre- and postsynaptic differentiation. Phosphorylation of tyrosine residues in the MuSK activation loop and the juxtamembrane region, however, is essential for agrin-induced clustering of AChRs (26, 59). Interestingly, agrin-induced activation of MuSK is transient and has largely vanished by the time AChR clusters appear (15). This suggests that a downstream pathway is activated and raises the issue of whether such a pathway operates autonomously in the absence of continuous agrin stimulation.
Pharmacological studies indicate that MuSK stimulation indeed activates a downstream tyrosine kinase cascade important in clustering of AChRs (15). Within this cascade, Src family kinases (SFKs), which are associated with AChRs (14), rapidly become phosphorylated and activated by agrin treatment, and their activation lasts much longer than the activation of MuSK (39). Crucial players in the cascade are Abl kinases, as they are required for AChR clustering, associate with MuSK, and phosphorylate MuSK (10). The downstream cascade also leads to tyrosine phosphorylation of the AChR β and δ subunits (39, 55), and β phosphorylation is required for efficient clustering and cytoskeletal interaction of the AChR (3). It remains unclear which kinase phosphorylates the AChR in response to agrin, although SFKs have been implicated (40, 46), and it is unknown if the downstream kinase cascade requires continuous agrin stimulation to remain active and lead to AChR clustering.
Following their formation in embryogenesis, neuromuscular synapses become structurally and functionally mature during early postnatal life. Although agrin-MuSK signaling is likely to have a role in synaptic maturation, additional signaling mechanisms may regulate synaptic maturation and maintenance without having an essential role in synapse formation (29, 45, 58). Separate pathways for synapse formation and synapse maintenance and maturation are illustrated in mice lacking utrophin and dystrophin or in mice lacking α-dystrobrevin, a component of the dystrophin/utrophin-glycoprotein complex; in these mice, neuromuscular synapses form but fail to mature properly (22, 23). Notably, in the absence of α-dystrobrevin, synaptic AChR clusters are normal at birth but increasingly fragment postnatally, indicating a defect in the mechanisms that stabilize the postsynaptic membrane (23). Similarly, in cultured myotubes lacking α-dystrobrevin, AChR clusters form normally in response to agrin stimulation, but these clusters are unstable and disperse rapidly when agrin is withdrawn from the myotubes (23). α-Dystrobrevin acts at least in part via tyrosine phosphorylation of the α-dystrobrevin-1 isoform, suggesting the involvement of a tyrosine kinase in postsynaptic stabilization (21).
Good candidates for such a kinase are SFKs. We previously analyzed mice that were mutant for Src and Fyn, Src and Yes, or Fyn and Yes and found that neuromuscular synapses appear normal in mice lacking these pairs of SFKs (46). SFKs, however, are important for stabilizing AChR clusters, as AChR clusters, which form normally in cultured myotubes lacking both Src and Fyn, are unstable following the withdrawal of agrin and rapidly fragment into microclusters in these mutant cells (46). Similarly, Src and Fyn stabilize AChR clusters that were induced by laminin, indicating their importance in more than one clustering pathway (36). Together, these observations raise the question of whether the activation of SFKs upon agrin stimulation is linked with the role of SFKs in AChR cluster stabilization.
To elucidate the temporal action of tyrosine kinases in agrin signaling, we have investigated how the downstream kinase cascade acts in clustering and stabilization of AChRs. We find that upon agrin stimulation, SFKs phosphorylate the AChR and MuSK early but not later and that SFKs recruit a pathway for stabilization of AChR clusters during initiation of cluster formation. Furthermore, we find that a single 5-min agrin pulse, followed by efficient washing, is sufficient to trigger maximal AChR clustering, first detectable several hours following the pulse, indicating that agrin rapidly activates an autonomous pathway that subsequently runs in the absence of continual agrin stimulation.
MATERIALS AND METHODS
Expression of agrin and cell culture.
Soluble neurally derived agrin (C-Ag12,4,8) was produced in COS cells as previously described (15). Cell culture reagents were purchased from Life Technologies (Basel, Switzerland). C2C12 (C2), src−/− fyn−/− myoblasts (clones DM11 and DM15), and the corresponding wild-type myoblasts (clones SW5 and SW10) were grown and fused to form myotubes as previously described (15, 46). Most of the experiments were done with clones SW5 and DM11, but the other clones gave identical results.
Antibodies and inhibitors.
Antibodies against phosphotyrosine (PY20, 4G10); the AChR γ and δ subunits (mAb88); the conserved C terminus of Src, Fyn, and Yes (src-CT); MuSK; the AChR β subunit (mAb124); and the AChR α subunit (mAb35) were all used as previously described (35, 39). Sequence-specific phosphorylation of SFKs (at Y215, Y418, or Y529) was detected by rabbit polyclonal phosphopeptide-specific antibodies purified by sequential epitope-specific chromatography as performed by the supplier (BioSource Europe, S.A., Brussels, Belgium). These antibodies were generated against the phosphorylation sites and flanking peptides of Src but are expected to cross-react with Fyn and Yes based on the high sequence conservation. Rabbit polyclonal antibodies raised against the C terminus of agrin that were used in Western blots were detailed previously (48). To analyze the effect of anti-agrin antibody mAb33 on clustering, agrin was preincubated for 1 h at room temperature with the antibody (0.025 to 0.1 mg/ml) and then was applied to myotubes for 6 h. In experiments with brief agrin pulses, agrin was added for 5 min. Agrin was withdrawn and cells were washed with medium, and then they were incubated with media containing mAb33. mAb33 was generated by Werner Hoch (University of Houston) (27) and is commercially available through Stressgen Biotechnologies (Victoria, Canada).
To examine the role of SFKs in agrin-induced AChR phosphorylation and clustering, a novel, potent, and SFK-selective tyrosine kinase inhibitor, CGP77675 (kindly provided by M. Susa, Novartis, Basel, Switzerland) was used (38). Myotubes were preincubated for 90 min in medium containing 10 μM CGP77675, stimulated with agrin in the presence of inhibitor, and subjected to precipitation assays or immunocytochemical stainings as described below. For AChR clustering assays, we occasionally re-added inhibitor (10 μM) several times during agrin incubation to ensure its full activity. We obtained the same results whether or not we re-added CGP77675. CGP77675 had no significant effect on spontaneous AChR clustering or cell morphology. In controls, CGP77675 was omitted and the carrier (dimethyl sulfoxide) was used alone. An established concentration of CGP77675 for use in cell cultures is 10 μM (38, 44), and this is 250-fold greater than the 50% inhibitory concentration for Src autophosphorylation in vitro and 500- to 2,000-fold greater than the 50% inhibitory concentration for phosphorylation of peptide substrates in vitro (38). We also used a range of concentrations of inhibitor, from 3 to 30 μM, for AChR clustering and phosphorylation and obtained results similar to those for 10 μM, although effects were a bit less at 3 μM.
To block Abl kinases, C2 myotubes were treated with 10 μM STI 571 (10), analogous to CGP77675. We also used 20 μM STI 571 and obtained the same results.
Precipitation assays and immunoblot analysis.
To examine the effects of CGP77675 on agrin-induced phosphorylation, myotubes were pretreated for 90 min with the inhibitor, followed by 0.5 nM agrin for 5 or 40 min in the presence of CGP77675. Cell lysates were split into two parts and were analyzed either by MuSK or AChR precipitation followed by phosphotyrosine immunoblotting as previously described (39). To precipitate AChRs, biotin-coupled α-bungarotoxin (α-BT) was used, followed by streptavidin-coupled agarose beads (39). Phosphotyrosine bands were identified based on their molecular weight and by reprobing immunoblots with antibodies reactive with the AChR subunits or with SFKs (src-CT). MuSK was precipitated with polyclonal anti-MuSK antibodies and protein A-Sepharose (15). Quantitation was performed by densitometric scanning as described previously (39). Signals from cells that were mostly not treated with agrin were used as 100% control. For some experiments depicted in the figures, signals of cells treated with agrin for 5 min were used as 100% controls, because in untreated cells signals were sometimes too small (or were even undetectable) to serve as a reference. In all cases tyrosine phosphorylation of AChRs, AChR-associated SFKs, and MuSK were clearly increased by 5 min of agrin treatment compared to tyrosine phosphorylation of untreated cultures. In all quantitations of tyrosine phosphorylation of AChRs or AChR-associated proteins (using α-BT-precipitation), signals were normalized for the amount of precipitated AChRs, as revealed by reprobing blots for the AChR α or γ and δ subunits. Effects of STI 571 on phosphorylation were studied in an analogous way.
Single brief agrin pulse.
Myotubes were stimulated with 0.1 or 0.5 nM agrin for 5 or 40 min, washed twice with fusion medium containing no agrin, and incubated with medium lacking agrin. After the appropriate times, 8 h for clustering, AChR phosphorylation and clustering were analyzed. To analyze the role of SFKs, myotubes were pretreated with CGP77675 and were stimulated with agrin followed by agrin withdrawal, all in the presence of CGP77675.
Quantitation of agrin and MuSK.
To quantify cell-bound agrin per milligram of cellular protein, lysates made from agrin-treated myotubes were analyzed by agrin immunoblotting. Using an agrin standard, we calculated cell-bound agrin (in femtamoles) per milligram of cellular myotube protein in the lysates loaded onto the sodium dodecyl sulfate (SDS) gel. The smallest detectable signal (2.4 fmol/mg), which represents the detection limit of this procedure, indicates the maximal amount of agrin that may remain cell bound after a 5-min pulse and withdrawal (see Fig. 8A). Quantitation of agrin was performed three times, with similar results.
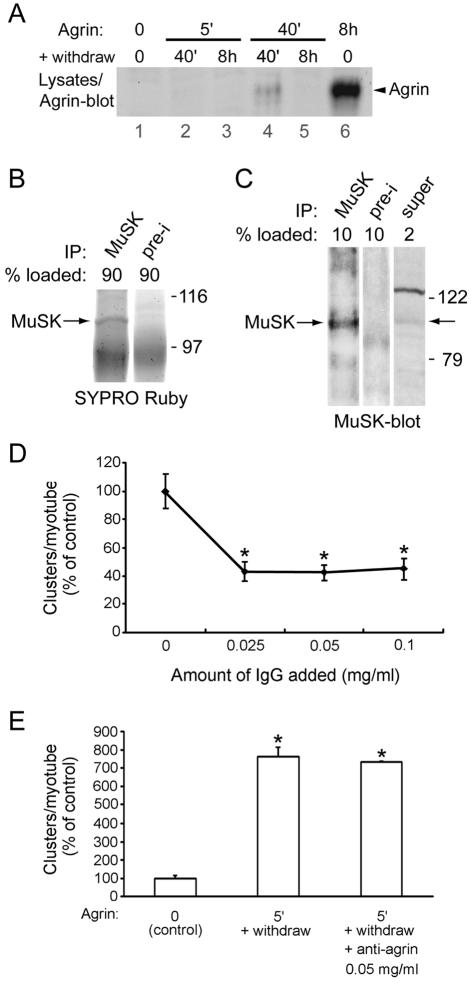
FIG. 8.
Little, if any, agrin remains cell bound after a brief agrin pulse and is not responsible for clustering. (A) C2 myotubes were pulse treated with agrin (0.5 nM for 5 or 40 min), followed by washing and withdrawal for 40 min or 8 h; parallel samples were treated continuously with agrin for 8 h. Cell lysates were analyzed by agrin immunoblotting. No agrin signal of ca. 120 kDa (representing recombinant agrin, C-Ag12,4,8) is detectable in cells treated for 5 or 40 min followed by 8 h of withdrawal (lanes 3 and 5). A small agrin signal is visible in cells treated for 40 min followed by a 40-min withdrawal (lane 4), but this does not correlate with clustering, which is the same after a 5- or 40-min pulse (see Fig. 7). (B) MuSK was immunoprecipitated (IP) from C2 lysates, and 90% of the precipitate was analyzed by SDS-PAGE and SYPRO Ruby protein staining. MuSK was identified based on its molecular weight and its absence in preimmune (pre-i) control precipitation. Using a parallel BSA standard (data not shown), the amount of precipitated MuSK was determined in the gel (100 ng). (C) From the same samples as those used for panel B, 10% of the MuSK precipitate and 2% of the supernatant were analyzed by MuSK immunoblotting. By densitometric scanning, the efficiency of the immunoprecipitation was calculated (60%). (D) In the presence or ab-sence of different concentrations of anti-agrin antibody mAb33, C2 myotubes were treated for 6 h with agrin and AChR clustering was quantified as described for Fig. 5. An asterisk indicates that the result differs significantly from that for the control (no immunoglobulin G [IgG]; P < 0.001; two-tailed paired t test). (E) Myotubes were treated with 0.5 nM agrin for 5 min, followed by withdrawal of agrin, washing, and incubation in agrin-free medium in the presence or absence of 0.05 mg of mAb33/ml. After 8 h, AChRs were stained with rhodamine-α-BT and quantitated as described in the legend to Fig. 5. An asterisk indicates that the result differs significantly from that for the control (P < 0.001) but not from each other (P > 0.09; two-tailed paired t test).
To estimate the amount of MuSK per milligram of cellular protein, MuSK-antibodies known to work properly in precipitation assays (15) were covalently coupled to protein A-Sepharose beads by using dimethyl pimelimidate (25). As control, preimmune serum was coupled. Myotube lysates, typically containing 12 mg of cellular protein, were incubated with MuSK antibodies and the beads were pelleted. Pellets, containing precipitated MuSK, and supernatants, containing soluble MuSK, were boiled in SDS-polyacrylamide gel electrophoresis (SDS-PAGE) buffer. Two steps were performed to estimate MuSK. First, 90% of pellets were subjected to SDS-PAGE and SYPRO Ruby staining, a highly sensitive luminescent protein stain of superior linearity ideal for quantitation of a wide range of proteins (Molecular Probes, Eugene, Oreg.) (2). Precipitated MuSK, identified based on its molecular weight and absence in the preimmune control precipitation, was quantified by blue-light illumination and densitometric scanning using a parallel bovine serum albumin (BSA) standard. Typically, 100 ng of MuSK was found in these precipitates. Second, 10% of pellets and 2% of supernatants were analyzed by MuSK immunoblotting and densitometric scanning, revealing that typically 60% of total MuSK in the original myotube lysate was precipitated while 40% remained in the supernatant. This estimation of MuSK was performed three times and yielded an average amount of 179.1 ± 74.6 (mean ± standard error of the mean [SEM]) fmol of MuSK/mg of cellular protein. Although based on an indirect method of MuSK quantitation, this estimation implies that the amount of agrin remaining myotube associated after withdrawal is only ca. 1% of the level of MuSK.
Immunocytochemical staining, fluorescence microscopy, and quantitation of clusters.
AChRs were visualized by incubating myotubes with 100 nM tetramethylrhodamine-conjugated α-BT (Molecular Probes) in fusion medium for 1 h at 37°C followed by fixation (35). Cells were mounted in glycerol containing p-phenylenediamine (Sigma) to reduce fluorescence fading and were examined with a fluorescence microscope (Axioskop 2; Carl Zeiss, Jena, Germany). To quantitate clusters, 10 to 20 random fields of myotubes taken at ×400 magnification were chosen by phase contrast, and signals were counted as clusters as described previously (35), i.e., if signal intensity was clearly distinguishable from diffuse staining and was well above background levels and if signals had an elongated shape. Within each experiment the number of AChR clusters per myotube was averaged. Data from at least three such experiments were used to calculate means ± SEM.
RESULTS
Agrin increases phosphorylation of the main autophosphorylation site in AChR-associated SFKs.
In our initial experiments, we sought to determine how activation of Src and Fyn, bound to the AChR, is regulated. A hallmark of SFK activation is phosphorylation of a tyrosine in the activation loop, Y418, which represents the main autophosphorylation site (Fig. 1A) (51). In addition, dephosphorylation of a C-terminal phosphotyrosine, Y529, leads to maximal and sustained activity. Dephosphorylation of Y529 displaces the intramolecular pY529-SH2 domain interaction, enhancing kinase reconfiguration and activity (51). Thus, by monitoring Y418 phosphorylation we can assess SFK activation, while the status of Y529 is an indication of the strength and duration of activation.
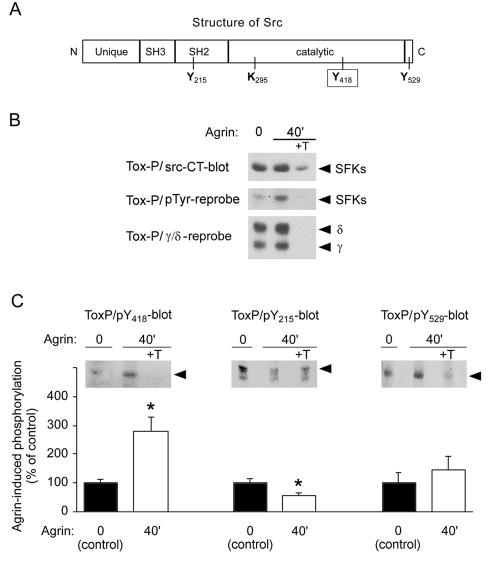
FIG. 1.
Agrin increases phosphorylation of Y418 in AChR-associated SFKs. (A) A cartoon showing the position of tyrosine residues (Y215, Y418, Y529) important in regulation of SFKs. (B) From C2 myotubes treated with 0.5 nM agrin, AChRs were precipitated with biotin-α-BT (Tox-P), followed by immunoblotting. Controls included addition of 10 μM free α-BT (+T). The blot was probed with pan-Src antibodies (src-CT), followed by antiphosphotyrosine and AChR (γ and δ subunit) reprobing. (C) Blots were probed with antibodies against Y418, Y215, and Y529 phosphopeptides, and bands originating from SFKs (arrows) that were identified by src-CT reprobing were quantitated. Background signals from control precipitations (+T) were subtracted from AChR precipitations to yield quantitations (means ± SEM of at least four experiments). An asterisk indicates that the result differs significantly from that for non-agrin-treated control (P < 0.05; two-tailed paired t test).
We monitored phosphorylation of AChR-associated SFKs by treating C2 myotubes with agrin and precipitating AChRs (Tox-P) using α-BT as reported previously (39). We stimulated myotubes with 0.5 nM agrin for 40 min, because phosphorylation of MuSK, SFKs, and AChRs is maximal at this time (15, 39). As shown by immunoblotting, overall tyrosine phosphorylation of AChR-bound SFKs was indeed increased by agrin (Fig. 1B). By probing Western blots with phosphopeptide-specific antibodies to either pY418, pY215, or pY529 in SFKs we found that Y418 phosphorylation increases 2.8-fold following agrin stimulation (Fig. 1C). In contrast, phosphorylation of Y215, residing within the SH2 domain, decreased 1.7-fold, while agrin did not diminish phosphorylation of Y529 (Fig. 1C). These changes in phosphorylation at individual tyrosine residues correspond well with the overall 2.2-fold increase in tyrosine phosphorylation of SFKs induced by agrin (39) (see also Fig. 1B) and suggest that this overall increase largely reflects the increased phosphorylation of Y418. Taken together, agrin stimulates phosphorylation at the activation loop tyrosine Y418 in AChR-associated SFKs, indicating their activation. Because pY529 is not dephosphorylated, this activation appears submaximal and sustained by other mechanisms.
Early but not later agrin-induced phosphorylation is inhibited by CGP77675, a SFK-selective tyrosine kinase inhibitor.
In addition to phosphorylation of AChR-associated SFKs, agrin induces tyrosine phosphorylation of AChR β and δ subunits (39), raising the possibility that the AChR may be a direct substrate for SFKs. To test this idea, we blocked SFK activity by treating myotubes with 10 μM CGP77675, a potent and SFK-specific inhibitor. We stimulated myotubes, pretreated with inhibitor, with agrin for 5 min, because phosphorylation is first detectable at this time (15). CGP77675 was continuously present during the agrin incubation for 40 min, and we analyzed α-BT-AChR precipitates by phosphotyrosine immunoblotting (Fig. 2). We observed that AChR β subunit phosphorylation, induced by a 5-min agrin treatment, is inhibited by CGP77675, whereas β subunit phosphorylation, induced by a 40-min treatment, is not affected (Fig. 2A). Although these experiments indicated that SFKs are ultimately dispensable for stimulating AChR β subunit phosphorylation, we were concerned that the failure of CGP77675 to inhibit this phosphorylation at 40 min may be due to a loss of CGP77675 activity at this time. We therefore determined whether the inhibitor remained active during agrin incubation by measuring the level of total cellular tyrosine phosphorylation in myotube lysates. Figure 2B shows that phosphorylation was equally reduced in CGP77675-treated myotubes incubated with agrin for 5 or 40 min. Thus, CGP77675 is equally efficient at reducing total cellular tyrosine phosphorylation at 5 and 40 min but is effective only in inhibiting AChR β subunit phosphorylation at 5 min following agrin stimulation. Furthermore, we used higher CGP77675 concentrations, up to 30 μM, in these assays and obtained the same results (P. Mittaud and C. Fuhrer, unpublished observations).
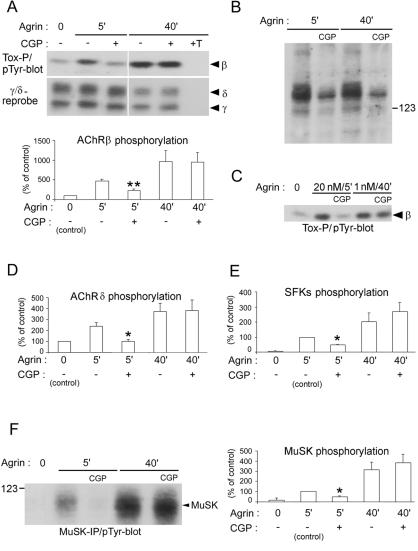
FIG. 2.
CGP77675 inhibits early but not late phosphorylation induced by agrin. C2 myotubes were treated with 1 nM agrin in the presence or absence of 10 μM CGP77675 (CGP) and were processed by α-BT (A, C, D, E) or MuSK (F) precipitation and phosphotyrosine immunoblotting (pTyr-blot). Proteins of interest were identified based on their molecular weight and reprobing results (data not shown). (A) Quantitation of AChR β phosphorylation shows that CGP77675 inhibits early (5 min) but not late (40 min) β phosphorylation. (B) As control for CGP77675 efficiency, total lysates were analyzed by phosphotyrosine immunoblotting. The reduction in phosphorylation of cellular proteins shows that CGP77675 is active and inhibits SFKs. (C) Even when using 20 nM agrin to obtain strong AChR β phosphorylation after 5 min, CGP77675 blocks early but not late (1 nM agrin for 40 min) phosphorylation. (D to F) Densitometric quantitation shows that CGP77675 inhibits early but not late phosphorylation of AChR δ subunits, AChR-associated SFKs, and MuSK. All quantitations represent means ± SEM of at least four experiments. An asterisk indicates that the result differs significantly from that for 5 min of agrin treatment without inhibitor, with a P value of <0.05 (a double asterisk indicates P < 0.005), by two-tailed paired t test. Tox-P, precipitation by biotin-α-BT.
Because AChR β phosphorylation was greater after 40 min of agrin treatment than after 5 min (Fig. 2A), we were concerned that our assay might not detect an inhibitory effect of CGP77675 at 40 min. We therefore stimulated myotubes with 20 nM rather than 1 nM agrin, which induced strong AChR β phosphorylation at 5 min, similar to the β phosphorylation observed at 40 min using 1 nM agrin. We found that CGP77675 nonetheless blocks this strong early β phosphorylation but not the late β phosphorylation (Fig. 2C). Thus, SFK activity is required for early but not later tyrosine phosphorylation of the AChR β subunit.
We next studied the effect of CGP77675 on agrin-induced phosphorylation of AChR δ subunits, AChR-associated SFKs, and MuSK. Quantitation showed that early (5 min), but not late (40 min), phosphorylations of AChR δ, AChR-bound SFKs, and MuSK are inhibited by 10 μM (Fig. 2D to F) or 30 μM CGP77675 (data not shown). To exclude the possibility of nonspecific inhibition of other, non-Src family kinases by CGP77675, we also used this inhibitor at lower concentrations, down to 3 μM, in phosphorylation experiments of AChR β, MuSK, and cellular proteins. We found effects similar to those shown in Fig. 2A, B, and F. Furthermore, we used another SFK inhibitor, PP2 (10 μM) (46), in such assays and again obtained comparable results (P. Mittaud and C. Fuhrer, unpublished).
Together, these data indicate that SFK activity is important for phosphorylation of AChR β and δ subunits of SFKs and of MuSK within the first 5 min of agrin stimulation but not after 40 min of agrin stimulation. Thus, these proteins may be direct substrates for agrin-activated SFKs at this early step, whereas later they are likely phosphorylated by other kinases. Interestingly, this also applies for SFKs themselves, because they are inactivated by CGP77675 at 40 min of agrin treatment as judged by impaired substrate phosphorylation (Fig. 2B), while their own overall phosphorylation is not affected (Fig. 2E). Thus, under these particular conditions of CGP77675 treatment, SFKs, although catalytically inactive, may be phosphorylated by other kinases, and one good candidate for such a kinase is MuSK, because MuSK interacts with SFKs (40).
In the absence of Src and Fyn, AChRs but not MuSK are phosphorylated by SFKs early in agrin signaling.
Cultured myotubes contain three major SFKs: Src, Fyn, and Yes (14). To analyze whether Src and Fyn are the critical SFKs that mediate early agrin-induced phosphorylation, we examined myotubes derived from mice lacking both Src and Fyn (src−/− fyn−/− myotubes) (46). Figure 3A shows that even in these cells, a 5-min agrin treatment increases phosphorylation of AChR β subunits to levels similar to those of wild-type or C2 myotubes (see Fig. 2A for comparison). As the SFK Yes is upregulated and appears to associate with AChRs in src−/− fyn−/− myotubes (46), Yes could be responsible for such phosphorylation. We therefore applied CGP77675, which efficiently inhibits Yes (38), to these cells and found that it indeed inhibited early but not late AChR β subunit phosphorylation (Fig. 3A). Thus, in src−/− fyn−/− myotubes, early AChR β phosphorylation requires an SFK, most likely Yes, whereas later β phosphorylation involves a non-SFK.
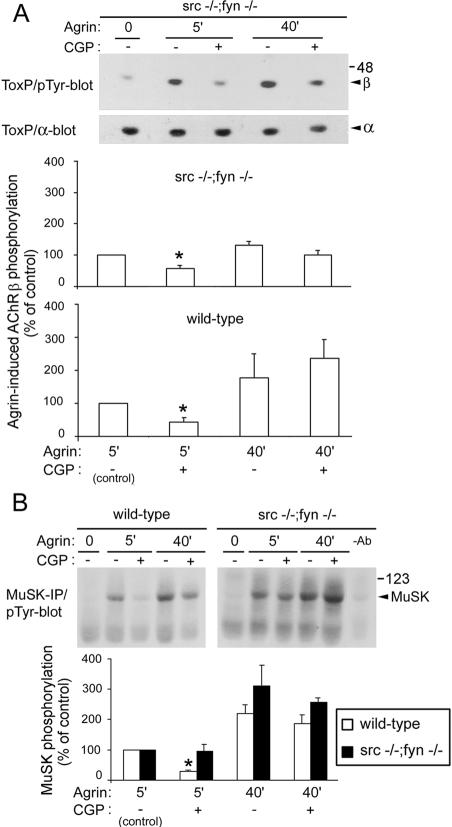
FIG. 3.
In myotubes lacking Src and Fyn, early AChR β subunit phosphorylation induced by agrin is inhibited by CGP77675 (CGP), while MuSK phosphorylation is not affected. (A) src−/− fyn−/− and wild-type myotubes were treated with 1 nM agrin and CGP77675. Phosphorylation of AChR β subunits was analyzed, followed by reprobing for the AChR α subunit. (B) MuSK was precipitated from wild-type and src−/− fyn−/− myotubes and was analyzed by phosphotyrosine immunoblotting (pTyr-blot). As control, the MuSK antibody was omitted (−Ab). For all quantitations, which reflect means ± SEM of at least three experiments, signals of cells treated with agrin for 5 min without inhibitor were set to 100%. An asterisk indicates that the result differs significantly from that for 5 min of agrin treatment without CGP77675 (P < 0.05 in panel A; P < 0.006 in panel B; one-sample student's t test). ToxP, precipitation by biotin-α-BT; IP, immunoprecipitation.
Interestingly, CGP77675 inhibited early MuSK phosphorylation in wild-type but not in src−/− fyn−/− myotubes (Fig. 3B). This shows that in src−/− fyn−/− myotubes Yes cannot replace Src and Fyn in mediating MuSK phosphorylation and that a non-SFK is responsible for early and late MuSK phosphorylation in these mutant cells. Furthermore, overall levels of MuSK phosphorylation appear slightly higher in the mutant (Fig. 3B). This suggests that SFKs may regulate MuSK in both positive and negative ways: if all SFKs are present (wild-type situation), their activity is required for early MuSK phosphorylation; if Src and Fyn are missing (src−/− fyn−/− situation), MuSK phosphorylation occurs more efficiently, is independent of SFK activity, and may occur from MuSK itself or from Abl (10). Thus, Src and Fyn, by interacting with MuSK (40), may normally hinder MuSK and/or Abl from phosphorylating MuSK maximally. In the absence of Src and Fyn, MuSK and/or Abl activity may become upregulated to phosphorylate MuSK to a higher degree. The complex of MuSK, Abl, Src, and Fyn may allow fine tuning of the phosphorylation status of MuSK, which is a central regulator in postsynaptic assembly.
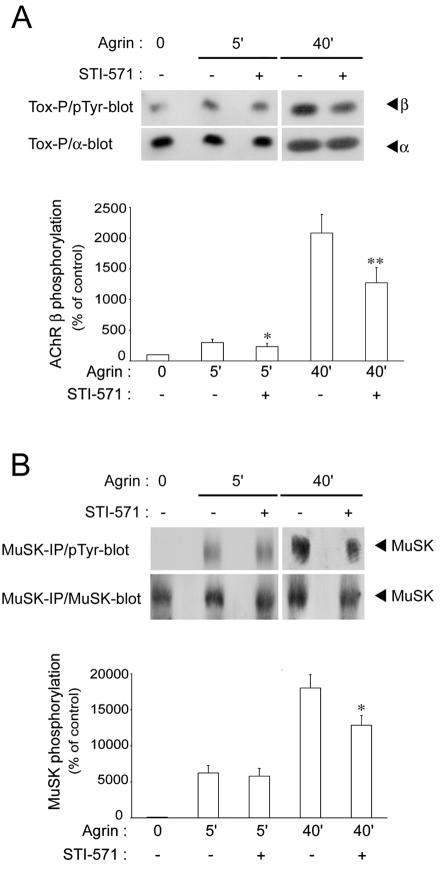
Abl kinases mediate late phosphorylation of AChRs and MuSK.
Because Abl kinases are required for AChR clustering (10), we investigated whether they cause phosphorylation of AChRs and MuSK later in agrin signaling. As before for CGP77675, we pretreated C2 myotubes with 10 μM STI 571, a specific Abl inhibitor (10), and added agrin for 5 or 40 min in the continuous presence of STI 571. Phosphorylation of AChR β subunits and of MuSK was substantially reduced at 40 min of agrin treatment (Fig. 4). At 5 min, AChR β phosphorylation was slightly reduced, while MuSK was not affected (Fig. 4). These data show that Abl kinases preferably act to phosphorylate AChRs and MuSK later (40 min) in agrin signaling, in contrast to SFKs, which cause phosphorylation early (5 min) in agrin signaling.
FIG. 4.
STI 571 inhibits late AChR β and MuSK phosphorylation induced by agrin. C2 myotubes were treated as described in the legend to Fig. 2, but STI 571 was used to inhibit Abl kinases. Phosphotyrosine blots were reprobed with antibodies against AChR α subunits (A) or MuSK (B). Quantitation (means ± SEM from at least eight experiments) revealed a pronounced reduction by STI 571 in phosphorylation of AChR β subunits and MuSK at 40 min of agrin treatment (**, significant difference from corresponding inhibitor-free sample, P < 0.01; *, P < 0.05; two-tailed paired t test). Tox-P, precipitation by biotin-α-BT; pTyr-blot, phosphotyrosine immunoblot; IP, immunoprecipitation.
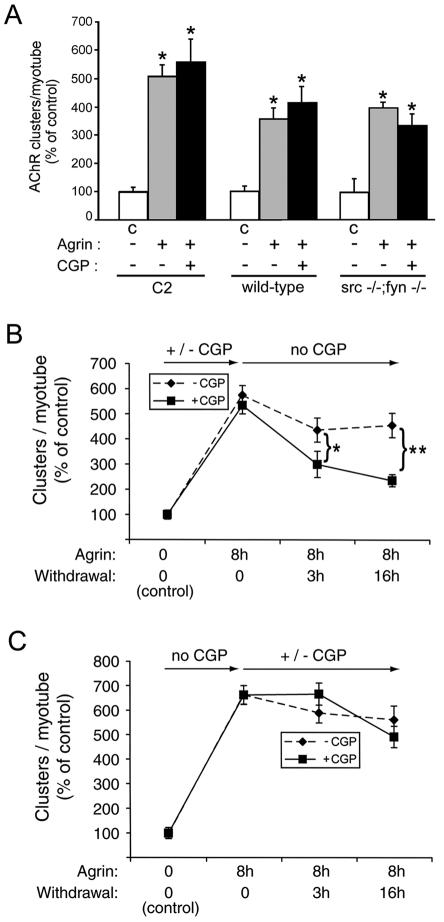
Inhibition of SFKs during the formation of AChR clusters leads to a defect in the stability of AChR clusters.
To investigate how SFK activation relates to AChR clustering, we first examined the effect of CGP77675 on the formation of clusters induced by agrin. As reported previously for other SFK inhibitors, PP1 and PP2 (46), 10 μM CGP77675 had no discernible effect on the overall number, length, shape, or intensity of AChR clusters induced by agrin in C2, wild-type myotubes, and src−/− fyn−/− myotubes (Fig. 5A). We observed the same results when we re-added CGP77675 several times during agrin treatment or when we used higher concentrations (30 μM; data not shown). Thus, SFK activity is not required for agrin-induced AChR cluster formation.
FIG. 5.
SFK activity during cluster formation is required for cluster stabilization later on. (A) Myotubes were stimulated overnight with 0.5 nM agrin in the presence or absence of CGP77675 (CPG), stained with rhodamine-α-BT, and analyzed by fluorescence microscopy. AChR clustering was quantitated and is shown as the percentage of untreated cells (C, control) (means ± SEM of at least three experiments). An asterisk indicates that the result differs significantly from that for the respective untreated cells (P < 0.03) but not from each other (P > 0.09; two-tailed paired t test), showing that CGP77675 does not inhibit agrin-induced formation of AChR clusters. (B) C2 myotubes were treated with 0.5 nM agrin for 8 h in the presence (squares) or absence (diamonds) of CGP77675, followed by withdrawal (for 3 or 16 h) of both CGP77675 and agrin, as indicated by arrows. AChR clustering was quantitated as described for panel A, showing no difference in the number of clusters formed, but the clusters dispersed more rapidly in CGP77675-treated cells (*, P < 0.03; **, P < 0.001; two-tailed paired t test). (C) C2 myotubes were stimulated for 8 h with agrin, followed by withdrawal of agrin and addition (squares) or no addition (diamonds) of CGP77675 into the agrin-free medium. After 3 or 16 h of withdrawal, AChR clustering was quantified as described for panel A, revealing no differences between CGP77675-treated and untreated cells (P > 0.2).
Once induced by agrin, AChR clusters are remarkably stable and disperse only slowly (half-life [t1/2] = 10 to 20 h) following withdrawal of agrin (46). The stability of these AChR clusters is dependent upon SFKs: in myotubes lacking Src and Fyn, agrin-induced AChR clusters disperse rapidly (t1/2 = 80 to 120 min) following agrin withdrawal (46). We therefore asked whether the agrin-stimulated phosphorylation of SFKs, which is maximal after 40 min (Fig. 1) (39), may have a role in stabilizing AChR clusters, which form hours later.
For this purpose, we treated myotubes with CGP77675 and agrin, followed by withdrawal of both CGP77675 and agrin. AChR clusters formed normally but then rapidly dispersed upon withdrawal, with a t1/2 of about 3 h (Fig. 5B). In contrast, when CGP77675 was present during the withdrawal but not the agrin induction period, the stability of AChR clusters was normal (Fig. 5C). These findings demonstrate that SFKs act during the agrin induction period to stabilize AChR clusters hours after agrin withdrawal (Fig. 5B). In addition, these results indicate that the subsequent resumption of SFK activity, during the agrin withdrawal period, is insufficient to stabilize AChR clusters that formed while SFKs were inhibited. We verified this resumption by measuring total tyrosine phosphorylation of cellular proteins by immunoblotting and found it to be normal at the end of the withdrawal period (data not shown).
A single brief agrin pulse is sufficient to trigger long-lasting phosphorylation of MuSK and AChR β.
Agrin (0.5 nM) induces phosphorylation of MuSK and AChRs within 5 min (15) (Fig. 2). In the continuous presence of agrin, these early phosphorylations increase substantially, ca. fourfold, to reach a peak after 40 min (15) (see also Fig. 9). Six to 8 h later, when AChR clusters have formed, MuSK phosphorylation has largely vanished but is still easily detectable, while AChR phosphorylation stays relatively high (15). Thus, agrin triggers a long-lasting downstream tyrosine kinase activity (39) and ultimately leads to clustering of AChRs and many other proteins after several hours. It remains unknown whether all of these aspects are initiated within the first minutes of agrin action, whether agrin's presence is still required thereafter, or whether the downstream cascade, once initiated, is self sustaining and operates autonomously.
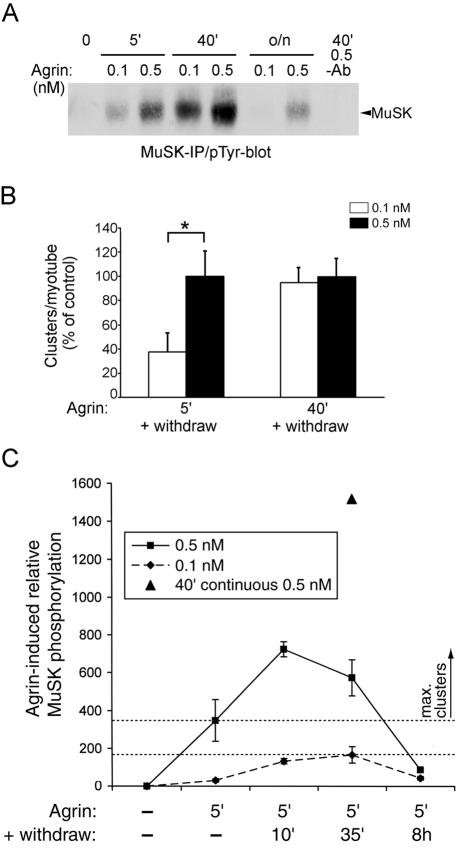
FIG. 9.
The agrin-triggered autonomous pathway is maximally activated if MuSK phosphorylation reaches a certain level. (A) C2 myotubes were continuously treated for 5 min, 40 min, or overnight with 0.1 or 0.5 nM agrin, and cell lysates were analyzed by MuSK immunoprecipitation (IP) and phosphotyrosine immunoblotting (pTyr-blot). As control, MuSK antibody was omitted (−Ab). (B) C2 myotubes were treated for 5 or 40 min with 0.1 or 0.5 nM agrin, followed by withdrawal for 8 h. AChRs were stained with rhodamine-α-BT and were analyzed by fluorescence microscopy. Within each pulse, the numbers of AChR clusters per myotube treated with 0.5 nM agrin, quantitated as described for Fig. 5, were set to 100%. *P < 0.02 by two-tailed paired t test. (C) Myotubes were treated for 5 min with 0.1 nM (diamonds) or 0.5 nM (squares) agrin, followed by 10 min, 35 min, or 8 h of agrin withdrawal, MuSK immunoprecipitation, and phosphotyrosine immunoblotting. Parallel cells were continuously treated for 40 min with 0.5 nM agrin (triangle). After a 5-min agrin pulse, MuSK phosphorylation increased in the withdrawal period. Quantitation shows the means ± SEM of three experiments evaluated by densitometric scanning. The area between the drawn lines indicates a critical level of MuSK phosphorylation. Above this level, maximal clustering occurs even in the subsequent absence of agrin.
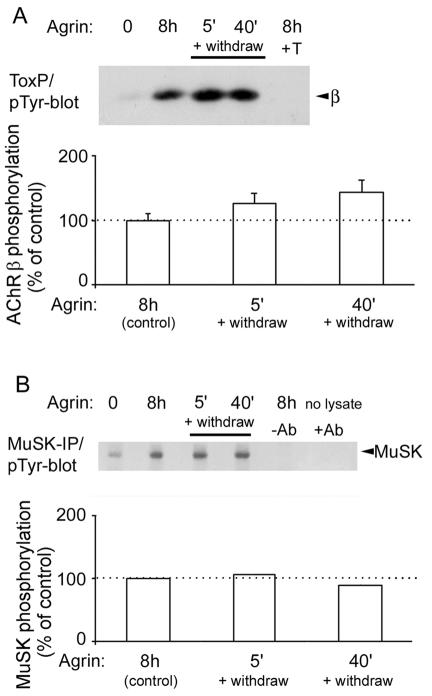
Therefore, we studied the consequences of a brief agrin treatment. C2 myotubes received a single pulse of agrin (0.5 nM) for 5 or 40 min, followed by withdrawal of agrin, extensive washing, and incubation in agrin-free medium for 8 h (Fig. 6A). The level of AChR β subunit phosphorylation following the brief pulse was compared to the level of phosphorylation induced by continuous agrin stimulation for 8 h. Surprisingly, β phosphorylation was the same in all of these treatments, showing that rapid agrin-induced AChR β phosphorylation is maintained long after agrin has been withdrawn (Fig. 6A). Similar results were obtained for MuSK: a single brief agrin pulse (5 min) was sufficient to induce long-lasting MuSK phosphorylation, equivalent to that found in myotubes treated continuously with agrin for 8 h (Fig. 6B). These results demonstrate that agrin acts within 5 min to stimulate a signaling pathway that thereafter is maintained and runs autonomously in the absence of further agrin stimulation.
FIG. 6.
A single 5-min agrin pulse triggers long-lasting MuSK and AChR β phosphorylation. C2 myotubes were treated for 8 h continuously with 0.5 nM agrin. Parallel cultures were incubated for 5 or 40 min with 0.5 nM agrin, followed by agrin withdrawal, washing, and incubation in agrin-free medium for 8 h. All lysates were then split into two parts and were analyzed either by AChR (A) or by MuSK (B) precipitation, followed by phosphotyrosine immunoblotting (pTyr-blot). As controls, we added a 10 μM free α-BT (+T in panel A) or omitted the lysate or the MuSK antibody (−Ab in panel B). (A) Quantitation represents the means ± SEM of three experiments and shows no significant differences between the pulsed or continuous agrin treatments (P > 0.63). (B) Quantitation represents the means of two experiments that showed very small variations between each other. IP, immunoprecipitation.
A single brief agrin pulse leads to efficient AChR clustering.
We examined whether this autonomous pathway, rapidly triggered by agrin, also leads to normal AChR clustering. For this purpose we added agrin for 5 or 40 min, withdrew agrin, washed the cells extensively, incubated them in agrin-free medium, and quantitated AChR clusters 8 h later. We observed no significant differences in the number, size, or density of AChR clusters between the pulsed 5- or 40-min treatment and the continuous 8-h treatment with agrin (Fig. 7). We further investigated whether this AChR clustering is dependent on SFK activity by applying CGP77675. The inhibitor had no significant effect on agrin-induced AChR clustering, even when this clustering was induced by a single brief pulse of agrin as short as 5 min (Fig. 7B). Thus, a brief agrin treatment triggers a mechanism that runs autonomously, in the absence of further agrin stimulation, to cause long-lasting MuSK and AChR phosphorylation and efficient AChR clustering. This clustering, like continuous agrin treatment, is independent of SFK activity.
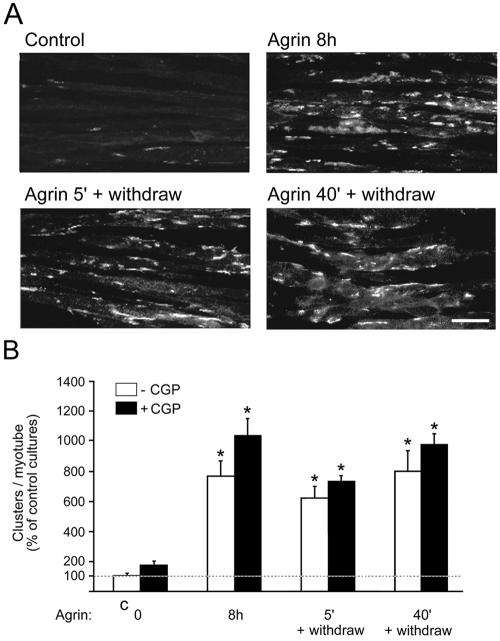
FIG. 7.
A single 5-min agrin pulse leads to efficient AChR clustering that is not affected by CGP77675 (CGP). In the presence or absence of CGP77675, C2 myotubes were continuously treated with 0.5 nM agrin for 8 h or for 5 or 40 min followed by withdrawal of agrin for 8 h. AChRs were stained with rhodamine-α-BT and were analyzed by fluorescence microscopy. Scale bar, 40 μm. (B) The numbers of AChR clusters per myotube are shown as the percentage of untreated cells (C, control) (means ± SEM of at least five experiments). An asterisk indicates that the result differs significantly from that of the control (P < 0.01) but not from each other (P > 0.09; two-tailed paired t test).
It was important to establish that our agrin withdrawal protocol removed the vast majority of cell-bound agrin and that clustering and phosphorylation were not caused by small amounts of agrin potentially remaining after withdrawal. We performed two control experiments to address the issue of whether agrin binds to cells within a 5- or 40 min pulse. First, we stimulated myotubes with 0.5 nM agrin for 5 or 40 min and then transferred the agrin-containing supernatant to a separate myotube culture. Because 0.5 nM soluble agrin is below saturation for AChR clustering and phosphorylation (8), a decrease in the concentration of agrin should reduce AChR clustering and phosphorylation. We found, however, that the transferred supernatant induced AChR clustering and β subunit phosphorylation as efficiently as agrin that had not been exposed to myotubes (P. Mittaud and C. Fuhrer, unpublished). Thus, as no detectable agrin activity was absorbed from agrin-containing media within 5 or 40 min, these data imply that very little agrin binds to myotubes within that time frame. Second, agrin was applied to cells for 5 or 40 min and was withdrawn for 8 h. After the 8 h, the withdrawal medium was transferred to other myotubes, where it failed to induce AChR clustering or β phosphorylation (P. Mittaud and C. Fuhrer, unpublished). Thus, after a brief agrin pulse followed by withdrawal, insufficient agrin is present in the withdrawal medium to stimulate AChR clustering, implying that only low levels of agrin, if any, remain attached to myotubes after the pulse and wash.
We next directly visualized, by immunoblotting, agrin that remained attached to myotubes following a brief treatment and withdrawal. The immunoblot assay was very sensitive: by densitometric scanning and comparison to an agrin standard of known concentration, we calculated that the detection limit of agrin in the immunoblot is 2.4 fmol/mg of cellular protein. While cell-bound agrin was readily detectable (300 fmol/mg) after a continuous 8-h incubation with 0.5 nM agrin (Fig. 8A, lane 6), cell-bound agrin was not detectable after a 5-min pulse followed by withdrawal (Fig. 8A, lanes 2 and 3). Following a 40-min pulse and a 40-min withdrawal period, low levels of agrin were detectable; following a longer withdrawal period (8 h), myotube-associated agrin was no longer detectable (Fig. 8A, lanes 4 and 5). As the extent of AChR clustering is the same in myotubes treated with agrin for 5 min followed by an 8 h withdrawal and in myotubes treated continuously with agrin for 8 h (Fig. 7), the extent of AChR clustering is not correlated with the amount of agrin remaining associated with the myotubes after the pulse (Fig. 8A). Thus, the remaining agrin, if present at all, is not critical for clustering. Rather, the brief stimulation with agrin appears sufficient to trigger a response that occurs several hours later in the absence of agrin.
To rule out that MuSK-bound agrin may remain on MuSK after withdrawal but may represent only a small portion of myotube-associated agrin not detectable by our assay, we used an indirect method to measure the level of MuSK protein. We first precipitated MuSK from myotube lysates by using bead-coupled MuSK antibodies (Fig. 8B). The amount of precipitated MuSK was estimated using a SYPRO Ruby protein stain and a parallel BSA standard. Second, the efficiency of this precipitation was calculated by a parallel MuSK-Western blot, comparing precipitated with remaining soluble MuSK (Fig. 8C). These estimations yielded an amount of 179.1 fmol of MuSK/mg of cellular protein (see Materials and Methods for details). This value is likely an underestimation; due to the background in the SYPRO Ruby stain, we probably underestimate the amount of precipitated MuSK. Nonetheless, our estimation demonstrates that in myotubes the level of MuSK (179.1 fmol/mg) is much higher than the level of agrin remaining cell bound after withdrawal (<2.4 fmol/mg). Because our recombinant agrin (C-terminal half of agrin, or C-Ag12,4,8) (8) not only binds to the MuSK complex but also to heparin and α-dystroglycan (17), the actual level of agrin remaining MuSK bound may be substantially less than 2.4 fmol/mg. Taken together, these data strongly suggest that after withdrawal the vast majority of agrin was released from myotubes and from MuSK on these myotubes.
Finally, we analyzed the effect of an anti-agrin antibody, mAb33 (27), on AChR clustering. When agrin was incubated with the antibody and added to myotubes, clustering of AChRs was severely reduced, as reported previously (27) (Fig. 8D). In contrast, mAb33 did not inhibit AChR clustering when added to myotubes in the withdrawal period immediately after a 5-min pulse of agrin (Fig. 8E). Although we cannot exclude the possibility that myotube-associated agrin is inaccessible to the antibody, these data suggest that agrin, which may be bound to myotubes after the pulse, is no longer required for AChR clustering.
Thus, many lines of evidence indicate that the withdrawal of agrin was effective. First, our agrin, C-Ag12,4,8, lacks the N-terminal domain of agrin, which is required for efficient binding to the extracellular matrix (7). The interaction of C-Ag12,4,8 with myotubes is therefore rather weak, allowing this agrin to be washed off easily. Second, consistent with this notion, no detectable agrin remained associated with myotubes after a 5-min agrin pulse followed by withdrawal and washing. If some agrin remained, it did not appear critical for clustering and was present in much smaller amounts than MuSK. This implies that agrin was efficiently released from MuSK, although we cannot rule out that a very small amount of agrin (ca. 1% of MuSK) may have remained MuSK associated. Third, no agrin activity was detectable in the medium following withdrawal. Fourth, a blocking antibody to agrin, added during the withdrawal phase, failed to inhibit AChR clustering initiated by a 5-min pulse of agrin. Taken together, these experiments show that agrin acts rapidly to trigger a clustering mechanism that subsequently acts autonomously in the absence of further agrin stimulation.
MuSK phosphorylation increases rapidly after the agrin pulse and leads to maximal AChR clustering in the absence of agrin.
Because agrin acts so rapidly, we examined the level of MuSK tyrosine phosphorylation at the end of the agrin pulse and during the withdrawal period. At 0.5 nM agrin, strong MuSK phosphorylation was seen after 5 or 40 min (Fig. 9A). Following an 8-h withdrawal, these levels of MuSK phosphorylation led to a similar number of AChR clusters (Fig. 9B), which were indistinguishable from a continuous 8-h agrin treatment (Fig. 7). As expected, the rate of MuSK phosphorylation was slower when myotubes were stimulated with 0.1 nM agrin. Consequently, the level of MuSK phosphorylation was much less at 5 min in myotubes treated with 0.1 nM agrin than in myotubes treated with 0.5 nM agrin but was similar at 40 min for both agrin concentrations (Fig. 9A). The extent of clustering paralleled these phosphorylations: after a 5-min pulse, clustering was less efficient at 0.1 than at 0.5 nM agrin, while after a 40-min pulse, clustering was comparable at both agrin concentrations (Fig. 9B). This implies that a certain critical level of MuSK activation must be reached within or after the agrin pulse in order to achieve maximal clustering: 5 min of 0.1 nM agrin does not reach this level, while 5 min of 0.5 nM agrin does.
After 5 min of agrin treatment, MuSK phosphorylation increases significantly in the continued presence of agrin, reaching a peak after 40 min (15). We analyzed MuSK phosphorylation after the 5-min agrin pulse, particularly addressing whether this phosphorylation is further increased during the withdrawal period. At both 0.5 and 0.1 nM agrin, MuSK phosphorylation indeed increased substantially after the pulse within 10 to 35 min (Fig. 9C). In comparison, continuous incubation with agrin for 40 min led to a still higher level of MuSK phosphorylation at 40 min (Fig. 9C). After 8 h, the continuous and pulsed agrin treatments resulted in identical MuSK phosphorylation (see Fig. 6). These data demonstrate that MuSK phosphorylation increases rapidly following a brief agrin pulse, even though cell-bound agrin is not detectable during this increase. Thus, agrin triggers a mechanism that increases MuSK phosphorylation in the subsequent absence of agrin. If MuSK phosphorylation reaches a certain critical level, as is the case with a 5-min 0.5 nM agrin pulse, maximal AChR clustering is seen after 8 h of withdrawal. If the MuSK phosphorylation is below this level, as in the case of a 5-min 0.1 nM agrin pulse, maximal clustering does not occur (Fig. 9A), even though MuSK phosphorylation still increases following withdrawal. This defines a critical level of MuSK activation that can be reached within minutes, beyond which the maximal AChR clustering program is initiated even in the absence of further agrin stimulation.
DISCUSSION
Here we reveal novel temporal patterns of tyrosine kinase action in the agrin signaling pathway. First, we showed that SFKs are required for phosphorylation of MuSK and AChRs early (5 min) but not later (40 min) after agrin stimulation, whereas Abl kinases mediate late phosphorylation. Second, we find that SFKs act during the period of AChR cluster formation, and not thereafter, to stabilize AChR clusters following withdrawal of agrin. Thus, during cluster initiation, SFKs recruit a stabilization pathway that acts hours later. Third, we find that a single, brief pulse of agrin is sufficient to induce a pathway that clusters AChRs efficiently in the absence of agrin. This autonomous pathway, once triggered, leads to a rapid increase in MuSK phosphorylation; when a critical level of MuSK phosphorylation is reached, the clustering program is fully activated, leading to AChR clusters hours later.
SFK activity is dispensable for agrin-induced AChR cluster formation.
Our data clarify the role of SFKs in the formation of AChR clusters. Previous studies yielded differing results (40, 46), which may be attributed to inadvertent inhibition of Abl by the inhibitor PP1 (50). As Abl is required for AChR clustering (10), high concentrations (20 μM) of PP1 may have interfered with Abl, leading to defective AChR clustering (40), whereas lower concentrations of PP1 (5 μM) may have been more selective for SFKs, leading to normal clustering (46). Our findings, combining gene elimination with more selective pharmacological inhibition, now demonstrate that the formation of clusters requires very little, if any, SFK activity: in wild-type and even src−/− fyn−/− cells, agrin induces normal AChR clustering in the presence of CGP77675, a potent and specific inhibitor that efficiently blocks SFKs, including Yes (38). Because Yes is the main remaining SFK in src−/− fyn−/− cells (46), these data provide strong evidence that SFK activity is not needed for AChR cluster formation.
Src family and Abl kinases mediate early and late phosphorylation, respectively.
In agrin-induced phosphorylation of AChR β and δ subunits, the role of SFKs has also remained controversial (40, 46). We now find that SFK activity acts only early (5 min) after addition of agrin. These findings may provide an explanation for the reported failure of PP1 to reduce AChR β phosphorylation following 30 to 40 min of agrin stimulation (46) and inhibition of AChR β phosphorylation by PP1 following a 10-min treatment with agrin (40). Thus, our results establish a temporal pattern of SFK action in agrin signaling in which these kinases are required for MuSK and AChR phosphorylation only early (5 min) but not later (40 min and thereafter). In contrast, Abl kinases cause MuSK and AChR β phosphorylation, preferably late (40 min) in agrin signaling. Phosphorylation of MuSK and AChRs is therefore under complex temporal control and involves several nonreceptor tyrosine kinases.
Time-delayed action of SFKs in stabilization of AChR clusters.
We find that SFK activity is required selectively during agrin-induced AChR cluster formation to stabilize these clusters in the hours after agrin withdrawal. These observations functionally link the SFK activation during AChR cluster formation to cluster stabilization later. Because SFK activation is maximal after 40 min of agrin treatment (39) (see also Fig. 1), i.e., hours before AChR clusters are formed, our data imply that SFKs already recruit a stabilization pathway when AChR clustering has just been initiated.
As MuSK and AChR phosphorylation occur independently of SFKs after 40 min of agrin treatment, these data suggest that SFKs phosphorylate proteins in addition to MuSK and AChRs, which are crucial for AChR cluster stabilization. This is in good agreement with our observation that SFKs are not activated by C-terminal dephosphorylation (Fig. 1) but rather, by inference, through protein interactions (51), implying the existence of other SFK-binding proteins and SFK substrates. Agrin-induced SFK activity is thus under constant control rather than being maximal and sustained over prolonged periods (51). Of interest is the reduction in phosphorylation of Y215 within the SH2 domain of SFKs (Fig. 1), a domain known to mediate protein interactions with, for example, phosphorylated MuSK (40). Dephosphorylation of Y215 may well render the SH2 domain capable of binding to other proteins. A good candidate is α-dystrobrevin, whose long isoform (α-dystrobrevin-1) mediates AChR cluster stabilization in part due to its C-terminal tyrosine phosphorylation sites (21), which may be SFK substrates. Other candidates are the Src substrates talin, paxillin, α-fodrin, and cortactin, which are all enriched at neuromuscular junctions (NMJs) and AChR clusters in myotubes (6, 24). All these proteins interact with actin, whose polymerization is a requirement for AChR clustering and may stabilize clusters (6).
The link between cluster formation and their stabilization by SFKs may involve differential phosphorylation of MuSK mediated by SFKs early (5 min) and by Abl kinases late (40 min) in agrin signaling. Although Abl kinases can be phosphorylated by SFKs (49), our observed effects of CGP77675 and STI 571 show that Abl acts independently of SFKs at 40 min and that SFKs act independently of Abl at 5 min to phosphorylate MuSK. Nevertheless, the action of the two kinases is highly intertwined, because early-acting SFKs lead to a delayed pathway that stabilizes AChRs clusters while late-acting Abl kinases lead to a more immediate pathway that forms clusters. This may well occur through phosphorylation of separate tyrosine residues in MuSK by Abl and SFKs. The juxtamembrane domain tyrosine (Y553) is a good candidate for Abl, because both Y553 and Abl are required for AChR cluster formation (10, 26, 59). Tyrosines in the N- and C-terminal region of the MuSK kinase domain (Y576 and Y812, respectively) are candidates for SFKs, because they are not essential in the formation of clusters (26) but may still play a role in their stabilization. Phosphotyrosines may bind to specific adaptor proteins, leading to activation of distinct postsynaptic pathways. These pathways may be initiated and maintained through the increased association of both Abl and SFKs with MuSK, which is rapidly triggered by agrin (10, 40).
Taken together, the emerging picture is that a complex containing MuSK, Abl, and SFKs is rapidly assembled by agrin. Abl and SFKs, possibly through phosphorylation of distinct MuSK residues, activate pathways for cluster formation and stabilization, respectively. In addition to interacting with MuSK (40), SFKs interact with AChRs (14) and agrin stimulates association between MuSK and AChRs (13, 15). Therefore, the stabilization program triggered by SFKs during initiation of clustering is recruited to the right location, nascent clusters at postsynaptic membrane specializations containing MuSK and AChRs. The delay in its manifestation may be important to ensure that the pathway becomes effective only after the initial clusters have been formed to achieve cluster stabilization and maturation. This delay may therefore be related to the complex elaboration of the NMJ, including the formation of postjunctional folds and restriction of AChRs to the crests of these folds, which occur postnatally. Thus, complex interactions between MuSK, Abl, SFKs, and AChRs may ensure that pathways for cluster formation as well as stabilization and maturation converge in an appropriate manner both temporally and spatially at the nascent NMJ.
A single brief agrin pulse activates an autonomous mechanism that leads to AChR clustering.
Our data reveal another temporal aspect of tyrosine kinase action in agrin signaling, because the continuous stimulation by agrin is not required for AChR clustering. Instead, using an agrin pulse and withdrawal protocol, we found that agrin rapidly initiates, within a single 5-min pulse, a clustering pathway that then acts autonomously in agrin's absence.
For other growth or differentiation factors, rapid activation of their tyrosine kinase receptors has been intensively investigated and occurs widely (56). It remains largely unknown, however, to what extent the continual presence of ligand is necessary after the initial trigger to maintain the activity of intracellular signaling. In neuregulin signaling in myogenesis, which requires several days, and in basic fibroblast growth factor-induced calcium influx in fibroblasts, the continuous presence of ligand seems necessary to achieve prolonged effects (11, 42). In contrast, using an approach similar to ours, a single brief pulse of nerve growth factor (NGF), followed by withdrawal and inhibitory antibodies, was exploited to show that NGF acts rapidly to trigger certain long-lasting pathways in PC12 cells (52). The brief application of NGF, epidermal growth factor, or basic fibroblast growth factor induced sodium channel gene expression hours later, leading to global cell excitability (52).
Agrin induces phosphorylation of MuSK within 5 min, but this phosphorylation reaches a peak only after 40 min (15). We showed here that continuous stimulation by agrin is not necessary. In comparison to other receptor tyrosine kinases, MuSK is very unusual and its tasks are far more complex, as previously noted by others (19). MuSK not only triggers intracellular signaling but also has to localize such signaling to the correct site of a huge syncytial cell to achieve new protein synthesis as well as relocalization of dozens of preexisting proteins at the nascent postsynapse. In addition, MuSK is required for presynaptic specialization of the nerve terminal (20). In line with such complex roles of MuSK, experiments with chimeric receptors have shown that, in contrast to growth factor receptors, MuSK's activated intracellular domain alone is not sufficient to reproduce MuSK's biological activity (i.e., AChR clustering)—structural portions of the MuSK extracellular domain are also important (19). Our data propose that even responses as complex as those initiated by MuSK, involving structural roles as well as catalytic tasks, may be triggered by a single brief pulse of ligand. This opens new avenues to better understanding signaling by complex receptor tyrosine kinases in general.
Our data show that within this rapidly triggered, autonomous pathway of agrin, the phosphorylation level of MuSK is of central importance. We find that it increases rapidly even after agrin withdrawal and, when reaching a certain critical level within 40 min of withdrawal, leads to maximal AChR clustering many hours later. This increase in MuSK phosphorylation may originate from MuSK itself through autophosphorylation or from an associated kinase. As mentioned above, MuSK occurs in a multiprotein complex where it associates at least with SFKs (40), Abl kinases (10), some AChRs (13, 15), Dishevelled (34), geranylgeranyltransferase (33), and indirectly with rapsyn (1). MuSK in association with the tyrosine phosphatase SHP-2 in cultured myotubes was recently found (R. Willmann, A. Camilleri, C. Fuhrer, unpublished observations). MAGI-1c is another protein that, at least in a torpedo electric organ, associates with MuSK (47). The plethora of MuSK-associated partners opens the possibility that agrin binding to its receptor complex may rapidly trigger conformational changes in such associated proteins in combination with kinase activation (Abl, SFKs) and/or phosphatase inactivation (possibly SHP-2). Such mechanisms could hold MuSK in an activated state independently of bound agrin and increase its phosphorylation status. In agreement with this, ectopic overexpression in muscle of constitutively active MuSK induces AChR aggregation in the absence of agrin (30), showing that MuSK can cause AChR clustering independently of agrin.
Our mechanism of MuSK activation proposes that when a critical level of MuSK phosphorylation is reached, the maximal clustering program is initiated. This mechanism may explain two prominent aspects of AChR clustering at the NMJ. The first is that myotubes contain several inhibitory mechanisms that counteract clustering. Rapsyn, for example, does not form clusters ectopically in mammalian muscle in vivo (35), and its clustering is marginal in cultured myotubes not treated with agrin, although rapsyn associates with AChRs in such cells (41). In contrast, rapsyn alone efficiently self aggregates in heterologous cells (12, 43). This implies that muscle-specific inhibition prevents rapsyn from clustering unless agrin and a synaptic environment are present. Another inhibitory mechanism is tyrosine phosphatases that dephosphorylate the AChR constitutively, as shown by a strong increase in receptor phosphorylation by pervanadate treatment in the absence of agrin (40, 54). Thus, the tyrosine kinase cascade downstream of MuSK is continuously counteracted by phosphatases which must be overcome by kinase activation through agrin (54). Our data imply that agrin overrides the inhibition associated with rapsyn and phosphatases with a threshold-like mechanism: when a critical level of MuSK phosphorylation is reached after a single agrin pulse, inhibition is overcome, allowing clustering to proceed autonomously and maximally. Besides recruitment of downstream signaling partners to critical phosphotyrosines in MuSK (see above), this may involve MuSK-mediated phosphorylation of downstream effectors that may operate autonomously beyond a certain level of their activation.
The second feature of NMJ development for which our mechanism of MuSK activation may be important is the highly regulated spatial AChR distribution in which high receptor densities lie only microns apart from regions that contain very few AChRs (24, 45). Signaling that underlies clustering must therefore operate with a high spatial resolution. This could be achieved by a mechanism in which agrin stimulation, when producing a critical level of MuSK phosphorylation, triggers maximal clustering autonomously. In regions where stimulation is beyond this level, clustering would occur maximally, while in regions where this level is not reached, the inhibitory mechanisms detailed above would strongly reduce cluster formation. The postsynaptic membrane may thereby be sculpted in vivo, and the effector proteins in such a cascade will be interesting topics of future research.
A rapidly triggered autonomous agrin pathway—a mechanism in the CNS?
In contrast to neurotransmitter receptors at synapses in the central nervous system (CNS), AChR clustering at the NMJ occurs much more slowly. CNS receptors can cluster and synapses can form within 1 to 2 h (5). Increasing evidence suggests that agrin is found concentrated at interneuronal synapses (28, 32) and may also play a role in synapse formation, including receptor clustering, between neurons (4, 9, 18), in addition to its importance at the immunological synapse (31). So how could agrin act in the CNS if it takes so long to cluster muscle AChRs? The features revealed here show that agrin activates its receptor and initiates the AChR clustering pathway extremely rapidly. Thus, it is not the agrin itself that is slow in activating the agrin receptor; rather, the downstream signaling pathway in muscle is slow in ultimately leading to AChR clusters. If coupled to a more rapid downstream pathway in neurons, a CNS agrin receptor, for which there are indications (28), would therefore be in a position to rapidly form a synapse. The rapid action of agrin inferred from our study could thereby rapidly lead to CNS receptor clustering, rapid enough for a CNS synapse to form.
Studies on the NMJ have shown that AChR clustering is under complex control, involving positive as well as negative regulation. Laminin converges with agrin signaling at the level of rapsyn (36), while neurotrophins (BDNF and NT-4) and neuregulin can inhibit AChR clustering (53, 57). Neurons may, in an analogous way, have multiple pathways that affect neurotransmitter receptor clustering. While an autonomous pathway rapidly triggered by agrin may be one mechanism for induction of CNS synapses, CNS synapse formation may be influenced by a more complex network of positive and negative signals that reflects the dynamic nature of synapses in the brain.
Acknowledgments
We are very grateful to M. Susa for generously providing CGP77675, to Roland Schoeb for excellent help with photography and to members of the Fuhrer laboratory for helpful discussions.
This work was supported by the Eric Slack-Gyr Foundation and by grants from the Swiss National Science Foundation, the Swiss Foundation for Research on Muscle Diseases, and the Roche Research Foundation (to C.F.).
REFERENCES
- 1.Apel, E. D., D. J. Glass, L. M. Moscoso, G. D. Yancopoulos, and J. R. Sanes. 1997. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron 18:623-635. [DOI] [PubMed] [Google Scholar]
- 2.Berggren, K., E. Chernokalskaya, T. H. Steinberg, C. Kemper, M. F. Lopez, Z. Diwu, R. P. Haugland, and W. F. Patton. 2000. Background-free, high sensitivity staining of proteins in one- and two-dimensional sodium dodecyl sulfate-polyacrylamide gels using a luminescent ruthenium complex. Electrophoresis 21:2509-2521. [DOI] [PubMed] [Google Scholar]
- 3.Borges, L. S., and M. Ferns. 2001. Agrin-induced phosphorylation of the acetylcholine receptor regulates cytoskeletal anchoring and clustering. J. Cell Biol. 153:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose, C. M., D. Qiu, A. Bergamaschi, B. Gravante, M. Bossi, A. Villa, F. Rupp, and A. Malgaroli. 2000. Agrin controls synaptic differentiation in hippocampal neurons. J. Neurosci. 20:9086-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen-Cory, S. 2002. The developing synapse: construction and modulation of synaptic structures and circuits. Science 298:770-776. [DOI] [PubMed] [Google Scholar]
- 6.Dai, Z., X. Luo, H. Xie, and H. B. Peng. 2000. The actin-driven movement and formation of acetylcholine receptor clusters. J. Cell Biol. 150:1321-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denzer, A. J., M. Gesemann, B. Schumacher, and M. A. Ruegg. 1995. An amino-terminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J. Cell Biol. 131:1547-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferns, M., M. Deiner, and Z. Hall. 1996. Agrin-induced acetylcholine receptor clustering in mammalian muscle requires tyrosine phosphorylation. J. Cell Biol. 132:937-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira, A. 1999. Abnormal synapse formation in agrin-depleted hippocampal neurons. J. Cell Sci. 112:4729-4738. [DOI] [PubMed] [Google Scholar]
- 10.Finn, A. J., G. Feng, and A. M. Pendergast. 2003. Postsynaptic requirement for Abl kinases in assembly of the neuromuscular junction. Nat. Neurosci. 6:717-723. [DOI] [PubMed] [Google Scholar]
- 11.Florini, J. R., D. S. Samuel, D. Z. Ewton, C. Kirk, and R. M. Sklar. 1996. Stimulation of myogenic differentiation by a neuregulin, glial growth factor 2. Are neuregulins the long-sought muscle trophic factors secreted by nerves? J. Biol. Chem. 271:12699-12702. [DOI] [PubMed] [Google Scholar]
- 12.Froehner, S. C., C. W. Luetje, P. B. Scotland, and J. Patrick. 1990. The postsynaptic 43K protein clusters muscle nicotinic acetylcholine receptors in Xenopus oocytes. Neuron 5:403-410. [DOI] [PubMed] [Google Scholar]
- 13.Fuhrer, C., M. Gautam, J. E. Sugiyama, and Z. W. Hall. 1999. Roles of rapsyn and agrin in interaction of postsynaptic proteins with acetylcholine receptors. J. Neurosci. 19:6405-6416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuhrer, C., and Z. W. Hall. 1996. Functional interaction of Src family kinases with the acetylcholine receptor in C2 myotubes. J. Biol. Chem. 271:32474-32481. [DOI] [PubMed] [Google Scholar]
- 15.Fuhrer, C., J. E. Sugiyama, R. G. Taylor, and Z. W. Hall. 1997. Association of muscle-specific kinase MuSK with the acetylcholine receptor in mammalian muscle. EMBO J. 16:4951-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautam, M., P. G. Noakes, L. Moscoso, F. Rupp, R. H. Scheller, J. P. Merlie, and J. R. Sanes. 1996. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 85:525-535. [DOI] [PubMed] [Google Scholar]
- 17.Gesemann, M., V. Cavalli, A. J. Denzer, A. Brancaccio, B. Schumacher, and M. A. Ruegg. 1996. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron 16:755-767. [DOI] [PubMed] [Google Scholar]
- 18.Gingras, J., S. Rassadi, E. Cooper, and M. Ferns. 2002. Agrin plays an organizing role in the formation of sympathetic synapses. J. Cell Biol. 158:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glass, D. J., E. D. Apel, S. Shah, D. C. Bowen, T. M. DeChiara, T. N. Stitt, J. R. Sanes, and G. D. Yancopoulos. 1997. Kinase domain of the muscle-specific receptor tyrosine kinase (MuSK) is sufficient for phosphorylation but not clustering of acetylcholine receptors: required role for the MuSK ectodomain? Proc. Natl. Acad. Sci. USA 94:8848-8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glass, D. J., D. C. Bowen, T. N. Stitt, C. Radziejewski, J. Bruno, T. E. Ryan, D. R. Gies, S. Shah, K. Mattsson, S. J. Burden, P. S. DiStefano, D. M. Valenzuela, T. M. DeChiara, and G. D. Yancopoulos. 1996. Agrin acts via a MuSK receptor complex. Cell 85:513-523. [DOI] [PubMed] [Google Scholar]
- 21.Grady, R. M., M. Akaaboune, A. L. Cohen, M. M. Maimone, J. W. Lichtman, and J. R. Sanes. 2003. Tyrosine-phosphorylated and nonphosphorylated isoforms of alpha-dystrobrevin: roles in skeletal muscle and its neuromuscular and myotendinous junctions. J. Cell Biol. 160:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grady, R. M., H. Teng, M. C. Nichol, J. C. Cunningham, R. S. Wilkinson, and J. R. Sanes. 1997. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell 90:729-738. [DOI] [PubMed] [Google Scholar]
- 23.Grady, R. M., H. Zhou, J. M. Cunningham, M. D. Henry, K. P. Campbell, and J. R. Sanes. 2000. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin-glycoprotein complex. Neuron 25:279-293. [DOI] [PubMed] [Google Scholar]
- 24.Hall, Z. W., and J. R. Sanes. 1993. Synaptic structure and development: the neuromuscular junction. Cell 72(Suppl.):99-121. [DOI] [PubMed] [Google Scholar]
- 25.Harlow, E., and D. Lane. 1999. Using antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Herbst, R., and S. J. Burden. 2000. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 19:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoch, W., J. T. Campanelli, S. Harrison, and R. H. Scheller. 1994. Structural domains of agrin required for clustering of nicotinic acetylcholine receptors. EMBO J. 13:2814-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoover, C. L., L. G. Hilgenberg, and M. A. Smith. 2003. The COOH-terminal domain of agrin signals via a synaptic receptor in central nervous system neurons. J. Cell Biol. 161:923-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huh, K. H., and C. Fuhrer. 2002. Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses. Mol. Neurobiol. 25:79-112. [DOI] [PubMed] [Google Scholar]
- 30.Jones, G., C. Moore, S. Hashemolhosseini, and H. R. Brenner. 1999. Constitutively active MuSK is clustered in the absence of agrin and induces ectopic postsynaptic-like membranes in skeletal muscle fibers. J. Neurosci. 19:3376-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan, A. A., C. Bose, L. S. Yam, M. J. Soloski, and F. Rupp. 2001. Physiological regulation of the immunological synapse by agrin. Science 292:1681-1686. [DOI] [PubMed] [Google Scholar]
- 32.Koulen, P., L. S. Honig, E. L. Fletcher, and S. Kroger. 1999. Expression, distribution and ultrastructural localization of the synapse-organizing molecule agrin in the mature avian retina. Eur. J. Neurosci. 11:4188-4196. [DOI] [PubMed] [Google Scholar]
- 33.Luo, Z. G., H. S. Je, Q. Wang, F. Yang, G. C. Dobbins, Z. H. Yang, W. C. Xiong, B. Lu, and L. Mei. 2003. Implication of geranylgeranyltransferase I in synapse formation. Neuron 40:703-717. [DOI] [PubMed] [Google Scholar]
- 34.Luo, Z. G., Q. Wang, J. Z. Zhou, J. Wang, Z. Luo, M. Liu, X. He, A. Wynshaw-Boris, W. C. Xiong, B. Lu, and L. Mei. 2002. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 35:489-505. [DOI] [PubMed] [Google Scholar]
- 35.Marangi, P. A., J. R. Forsayeth, P. Mittaud, S. Erb-Vogtli, D. J. Blake, M. Moransard, A. Sander, and C. Fuhrer. 2001. Acetylcholine receptors are required for agrin-induced clustering of postsynaptic proteins. EMBO J. 20:7060-7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marangi, P. A., S. T. Wieland, and C. Fuhrer. 2002. Laminin-1 redistributes postsynaptic proteins and requires rapsyn, tyrosine phosphorylation, and Src and Fyn to stably cluster acetylcholine receptors. J. Cell Biol. 157:883-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McMahan, U. J. 1990. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 55:407-418. [DOI] [PubMed] [Google Scholar]
- 38.Missbach, M., M. Jeschke, J. Feyen, K. Muller, M. Glatt, J. Green, and M. Susa. 1999. A novel inhibitor of the tyrosine kinase Src suppresses phosphorylation of its major cellular substrates and reduces bone resorption in vitro and in rodent models in vivo. Bone 24:437-449. [DOI] [PubMed] [Google Scholar]
- 39.Mittaud, P., P. A. Marangi, S. Erb-Vogtli, and C. Fuhrer. 2001. Agrin-induced activation of acetylcholine receptor-bound Src family kinases requires Rapsyn and correlates with acetylcholine receptor clustering. J. Biol. Chem. 276:14505-14513. [DOI] [PubMed] [Google Scholar]
- 40.Mohamed, A. S., K. A. Rivas-Plata, J. R. Kraas, S. M. Saleh, and S. L. Swope. 2001. Src-class kinases act within the agrin/MuSK pathway to regulate acetylcholine receptor phosphorylation, cytoskeletal anchoring, and clustering. J. Neurosci. 21:3806-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moransard, M., L. S. Borges, R. Willmann, P. A. Marangi, H. R. Brenner, M. J. Ferns, and C. Fuhrer. 2003. Agrin regulates rapsyn interaction with surface acetylcholine receptors, and this underlies cytoskeletal anchoring and clustering. J. Biol. Chem. 278:7350-7359. [DOI] [PubMed] [Google Scholar]
- 42.Munaron, L., C. Distasi, V. Carabelli, F. M. Baccino, G. Bonelli, and D. Lovisolo. 1995. Sustained calcium influx activated by basic fibroblast growth factor in Balb-c 3T3 fibroblasts. J. Physiol. 484:557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips, W. D., C. Kopta, P. Blount, P. D. Gardner, J. H. Steinbach, and J. P. Merlie. 1991. ACh receptor-rich membrane domains organized in fibroblasts by recombinant 43-kildalton protein. Science 251:568-570. [DOI] [PubMed] [Google Scholar]
- 44.Recchia, I., N. Rucci, C. Festuccia, M. Bologna, A. R. MacKay, S. Migliaccio, M. Longo, M. Susa, D. Fabbro, and A. Teti. 2003. Pyrrolopyrimidine c-Src inhibitors reduce growth, adhesion, motility and invasion of prostate cancer cells in vitro. Eur. J. Cancer. 39:1927-1935. [DOI] [PubMed] [Google Scholar]
- 45.Sanes, J. R., and J. W. Lichtman. 2001. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2:791-805. [DOI] [PubMed] [Google Scholar]
- 46.Smith, C. L., P. Mittaud, E. D. Prescott, C. Fuhrer, and S. J. Burden. 2001. Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrin-induced clusters of acetylcholine receptors. J. Neurosci. 21:3151-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strochlic, L., A. Cartaud, V. Labas, W. Hoch, J. Rossier, and J. Cartaud. 2001. MAGI-1c: a synaptic MAGUK interacting with muSK at the vertebrate neuromuscular junction. J. Cell Biol. 153:1127-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugiyama, J., D. C. Bowen, and Z. W. Hall. 1994. Dystroglycan binds nerve and muscle agrin. Neuron 13:103-115. [DOI] [PubMed] [Google Scholar]
- 49.Tanis, K. Q., D. Veach, H. S. Duewel, W. G. Bornmann, and A. J. Koleske. 2003. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol. Cell. Biol. 23:3884-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tatton, L., G. M. Morley, R. Chopra, and A. Khwaja. 2003. The Src-selective kinase inhibitor PP1 also inhibits Kit and Bcr-Abl tyrosine kinases. J. Biol. Chem. 278:4847-4853. [DOI] [PubMed] [Google Scholar]
- 51.Thomas, S. M., and J. S. Brugge. 1997. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 13:513-609. [DOI] [PubMed] [Google Scholar]
- 52.Toledo-Aral, J. J., P. Brehm, S. Halegoua, and G. Mandel. 1995. A single pulse of nerve growth factor triggers long-term neuronal excitability through sodium channel gene induction. Neuron 14:607-611. [DOI] [PubMed] [Google Scholar]
- 53.Trinidad, J. C., and J. B. Cohen. 2004. Neuregulin inhibits acetylcholine receptor aggregation in myotubes. J. Biol. Chem. 279:31622-31628. [DOI] [PubMed] [Google Scholar]
- 54.Wallace, B. G. 1995. Regulation of the interaction of nicotinic acetylcholine receptors with the cytoskeleton by agrin-activated protein tyrosine kinase. J. Cell Biol. 128:1121-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallace, B. G., Z. Qu, and R. L. Huganir. 1991. Agrin induces phosphorylation of the nicotinic acetylcholine receptor. Neuron 6:869-878. [DOI] [PubMed] [Google Scholar]
- 56.Weiss, A., and J. Schlessinger. 1998. Switching signals on or off by receptor dimerization. Cell 94:277-280. [DOI] [PubMed] [Google Scholar]
- 57.Wells, D. G., B. A. McKechnie, S. Kelkar, and J. R. Fallon. 1999. Neurotrophins regulate agrin-induced postsynaptic differentiation. Proc. Natl. Acad. Sci. USA 96:1112-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willmann, R., and C. Fuhrer. 2002. Neuromuscular synaptogenesis: clustering of acetylcholine receptors revisited. Cell Mol. Life Sci. 59:1296-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou, H., D. J. Glass, G. D. Yancopoulos, and J. R. Sanes. 1999. Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J. Cell Biol. 146:1133-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]