Abstract

Drug-resistant tuberculosis (TB) is a global threat and innovative approaches such as using adjuvants of anti-TB therapeutics are required to combat it. High-throughput screening yielded two lead scaffolds of inhibitors of Mycobacterium tuberculosis (Mtb) acetyltransferase Eis, whose upregulation causes resistance to the anti-TB drug kanamycin (KAN). Chemical optimization on these scaffolds resulted in potent Eis inhibitors. One compound restored the activity of KAN in a KAN-resistant Mtb strain. Model structures of Eis-inhibitor complexes explain the structure–activity relationship.
Keywords: Aminoglycoside acetyltransferase, resistance, enzyme inactivation, drug combination, structure−activity relationship
Despite extensive efforts to discover new antitubercular agents in recent years, tuberculosis (TB) remains the top bacterial cause of mortality worldwide. The proportion of new multidrug-resistant (MDR)-TB cases has not changed in recent years. MDR strains of Mycobacterium tuberculosis (Mtb) are resistant to the first-line antituberculars isoniazid and rifampicin. Extensively drug-resistant (XDR) strains of Mtb are additionally resistant to fluoroquinolones and at least one of the second-line injectable anti-TB drugs, capreomycin, kanamycin (KAN), or amikacin. XDR-TB has very poor therapeutic outcomes. Thus, the discovery and development of new therapeutics is needed.
KAN is currently used to treat MDR- and XDR-Mtb infections. Resistance to KAN, observed in one-third of KAN-resistant infections, is due to the upregulation of the enhanced intracellular survival (Eis) protein in Mtb.1 We previously established that Eis modifies aminoglycosides (AGs),2 capreomycin,3 and other lysine-containing biological molecules3 by a unique multiacetylating mechanism.5 We also reported crystal structures of Eis-CoA complexes and that of an Eis_Mtb-CoA-tobramycin complex.15,16
New Eis inhibitors that can be used in combination with AGs such as KAN is a potential way to overcome the resistance due to Eis upregulation. We reported some structural scaffolds with inhibitory activity against the purified Eis enzyme in vitro.11 Here, we report two new scaffolds: the methyl 4H-furo[3,2-b]pyrrole-5-carboxylate (scaffold 1) and the 3-(1,3-dioxolano)-2-indolinone (scaffold 2) identified by high-throughput screening (HTS), the synthesis of 27 analogues of these scaffolds, and their biochemical and biological testing.
First, we screened ∼123,000 small molecules for inhibition of KAN acetylation by Eis_Mtb. The HTS yielded two promising scaffolds 1 and 2 (Figure S1A). Sixty compounds containing scaffold 1 (Figure S2) were present in the HTS library. In these 60 compounds, the pyrrole ring was decorated with different groups including alkyl chains, aryl and oxazole rings, and amides. Fifty-nine of these scaffold 1 molecules did not inhibit Eis_Mtb; however, compound 1a (Figure S1B and Scheme 1, also labeled as 4kk in Figure S2) displayed some inhibition in the HTS. Because 1a contained an aryl ketone group, we opted to synthesize 1a along with 12 additional analogues (1b–1m) comprising different aryl groups (Scheme 1). For scaffold 2, ten compounds with different groups attached to the indolinone ring were in the HTS library (Figure S3). A hydrogen (8a), an alkyl (8b), an alkyl ketone (8c), a carboxylic acid (9a), or an amide (9b) substituent resulted in no Eis_Mtb inhibitory activity. Similarly to the scaffold 1 analogues, aryl ketones of scaffold 2 (8f–8h) displayed Eis_Mtb inhibition, with the exception of the trifluoromethyl substituted aryl ketones (8d and 8e). Three compounds synthesized from scaffold 2 (8f–8h, Figure S3) were found to be active. These and 11 of their analogues contain different aryl substituents for further study (Note: 8f–8h are numbered 2g–2i in Scheme 1 and Figure S1 to represent the scaffold 2 series).
Scheme 1. Preparation of Potential Eis Inhibitors: Scaffold 1 and Scaffold 2 Core Structures Generated in This Study.
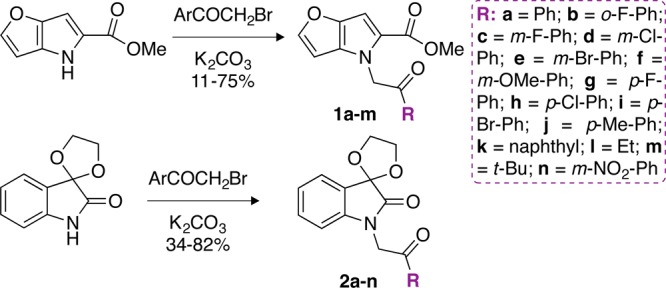
Compound 1a and 12 analogues (1b–1m) as well as 14 analogues for scaffold 2 (2a–2n) with different R substituents were synthesized for structure–activity relationship (SAR) analysis of Eis_Mtb inhibition in vitro and in cellulo (Scheme 1). All new compounds were characterized by 1H, 13C NMR (Figures S5–S56), and mass spectrometry and were established to be ≥95% pure by HPLC prior to further testing.
We evaluated biochemical (inhibition (IC50) of purified Eis_Mtb enzyme) and biological (effect on the KAN MIC values for KAN-sensitive Mtb H37Rv and KAN-resistant Mtb K204 cells) properties of these compounds, in parallel (Table 1; Figure S57). The freshly synthesized 1a, which displayed some inhibition of Eis_Mtb in the HTS campaign, was confirmed to be a good Eis_Mtb inhibitor in vitro (IC50 = 3 ± 1 μM). In the presence of 1a, KAN displayed an MIC of 5–10 μg/mL against Mtb K204. Having confirmed the weak inhibitory activity of 1a, we explored the effect of substitution on the phenyl ring on the aryl ketone part of scaffold 1. Ortho substitution, as in 1b with a o-fluoro substituent, resulted in almost the same Eis_Mtb inhibitory activity (IC50 = 2.9 ± 0.9 μM) as that for the parent 1a. KAN MIC against Mtb K204 was unaffected by 1b (MICKAN = 10 μg/mL). To establish if meta or para substitution would be more favorable than ortho substitution, we generated compounds 1c–1j. For both meta and para substitutions, bulkier substituents led to weaker Eis_Mtb inhibition (IC50 > 200 μM for m-methoxy (1f) and p-bromo (1i) compared to IC50 = 0.16 ± 0.07 and 0.3 ± 0.1 μM for m-fluoro (1c) and p-fluoro (1g), respectively). Most of these derivatives (1c–1i) did not improve KAN activity against Mtb K204. The p-methyl derivative 1j displayed almost the same Eis_Mtb inhibitory activity (IC50 = 5.8 ± 1.8 μM) and MIC value against Mtb K204 as that of 1a. We generated 1k (naphthyl substituted) with the hope of strengthening any possible π–π interactions between the inhibitor and the AG-binding site of the Mtb Eis. This compound was found to be completely inactive (IC50 > 200 μM and MICKAN = 10 μg/mL against Mtb K204). Finally, replacing the phenyl ring with alkyl chains (ethyl (1l) and t-butyl (1m)) did not improve the Eis inhibition or MICKAN for K204 Mtb.
Table 1. IC50 Values against Purified Eis_Mtb and MIC Values against Mtb H37Rv and Mtb K204 with the Compounds at the Concentrations Specified.
| compd | IC50 (μM)a | concentration tested (μM)b | H37Rv MICKAN (μg/mL)c | K204 MICKAN (μg/mL)d |
|---|---|---|---|---|
| ≤1.25 | ≥10, 10 | |||
| 1a | 3 ± 1 | 100 | ≤1.25 | 5, 10 |
| 1b | 2.9 ± 0.9 | 100 | ≤1.25 | 10, 10 |
| 1c | 0.16 ± 0.07 | 15.5 | ≤1.25 | 10, 10 |
| 1d | 0.25 ± 0.08 | 25.1 | ≤1.25 | ≥10, 10 |
| 1e | 0.5 ± 0.2 | 52.6 | ≤1.25 | ≥10, 10 |
| 1f | >200 | 100 | ≤1.25 | ≥10, 10 |
| 1g | 0.3 ± 0.1 | 33.9 | ≤1.25 | 10, 10 |
| 1h | 0.6 ± 0.3 | 64.5 | ≤1.25 | 10, ≥10 |
| 1i | >200 | 100 | ≤1.25 | ≥10, ≥10 |
| 1j | 5.8 ± 1.8 | 100 | ≤1.25 | 5, 10 |
| 1k | >200 | 100 | ≤1.25 | 10, 10 |
| 1l | >200 | 100 | ≤1.25 | ≥10, 10 |
| 1m | >200 | 100 | ≤1.25 | ≥10, 10 |
| 2a | 0.33 ± 0.16 | 32.6 | ≤1.25 | 5, 5 |
| 2b | 23 ± 10 | 100 | ≤1.25 | 10, 10 |
| 2c | 0.30 ± 0.08 | 29.7 | ≤1.25 | 5, 2.5 |
| 2d | 0.015 ± 0.005 | 1.5 | ≤1.25 | ≥10, 10 |
| 2e | 0.54 ± 0.25 | 54.2 | ≤1.25 | 10, 5 |
| 2f | >200 | 100 | ≤1.25 | ≥10, 10 |
| 2g | 0.09 ± 0.03 | 8.9 | ≤1.25 | 5, 5 |
| 2h | 2.2 ± 0.7 | 100 | ≤1.25 | 10, 5 |
| 2i | >200 | 100 | ≤1.25 | ≥10, 10 |
| 2j | 8.7 ± 2.2 | 100 | ≤1.25 | 5, 10 |
| 2k | >200 | 100 | ≤1.25 | 10, 10 |
| 2l | >200 | 100 | ≤1.25 | ≥10, 10 |
| 2m | >200 | 100 | ≤1.25 | ≥10, 10 |
| 2n | 1.2 ± 0.4 | 100 | ≤1.25 | 10, 5 |
IC50 against purified Eis.
Concentrations of Eis inhibitor in the MIC assays. At these concentrations, these compounds did not inhibit the growth of Mtb H37Rv or that of Mtb K204 when tested in the absence of KAN. Concentrations of Eis inhibitors were 100× their IC50 when IC50 < 1 μM or 100 μM for IC50 > 1 μM.
Activity of KAN against Mtb H37Rv.
Activity of KAN against Mtb K204.
For scaffold 2, compounds 2g–2i, which displayed Eis_Mtb inhibition in the HTS, were freshly synthesized. The p-fluoro substituted 2g displayed good Eis inhibitory activity (IC50 = 0.09 ± 0.03 μM) and, when used in combination with KAN, resulted in MICKAN of 5 μg/mL against K204 Mtb. The p-chloro substituted 2h was less active (IC50 = 2.2 ± 0.7 μM) than the p-fluoro substituted 2g and did not sensitize Mtb K204 to KAN (MICKAN = 5–10 g/mL). The p-bromo substituted 2i was found to be completely inactive (IC50 > 200 μM and MICKAN ≥ 10 μg/mL against Mtb K204), while it displayed limited Eis inhibition in the HTS, which could indicate that the compound in the HTS library was not completely pure. We also found that the p-methyl derivative 2j displayed a 100-fold decrease in Eis inhibitory activity (IC50 = 8.7 ± 2.2 μM) from 2g and, in combination with KAN, resulted in almost the same KAN MIC (5–10 μg/mL) against KAN-resistant Mtb as 2g did. The nonsubstituted counterpart of parent 2g, derivative 2a, displayed weaker Eis inhibitory activity (IC50 = 0.33 ± 0.16 μM) and improved the activity of KAN against Mtb K204 (MICKAN = 5 μg/mL). We also synthesized the m-fluoro, m-chloro, and m-bromo derivatives 2c, 2d, and 2e. The m-chloro substituted 2d showed the same inhibitory activity as that of 2g but did not sensitize Mtb K204 to KAN (MICKAN ≥ 10 μg/mL). The m-fluoro and m-bromo substituted 2c and 2e resulted in a 3- and 5-fold worse Eis inhibitory activity (IC50 = 0.30 ± 0.08 and 0.54 ± 0.25 μM), respectively. When used with the m-bromo-substituted 2e, KAN had a MIC of 5–10 μg/mL against Mtb K204. However, when used with the m-fluoro substituted 2c, KAN displayed a better MIC value of 2.5–5 μg/mL. As observed with scaffold 1, the presence of m-methoxy-phenyl, naphthyl, ethyl, and t-butyl groups in scaffold 2 resulted in molecules that were completely inactive (IC50 > 200 μM and MICKAN ≥ 10 μg/mL against Mtb K204). For scaffold 2, we also synthesized a m-nitro substituted compound (2n) to investigate the potential effect of a strong electron-withdrawing group on Eis inhibitory activity. Compound 2n was less active (IC50 = 1.2 ± 0.4 μM) than 2g, but it sensitized Mtb K204 to KAN (MICKAN = 5–10 μg/mL). The absence of antibacterial activity of these compounds when used alone along with a general correlation between IC50 and MIC values indicated that inhibition of Eis by these compounds is the main mechanism of sensitization to KAN.
To investigate the selectivity of our inhibitors toward Eis_Mtb, we tested two of our derivatives, one from each series, 1c and 2c, against three other AAC enzymes: AAC(2′)-Ic,5 AAC(3)-IV,12 and AAC(6′)-Ie/APH(2″)-Ia.12 Similarly to other known non-Eis AACs, these three enzymes were previously shown to be strictly regiospecific, but like Eis, each enzyme was capable of acetylating structurally distinct AGs. Neither 1c nor 2c inhibited KAN acetylation by these three AACs at concentrations as high as 200 μM, which indicated that our compounds were highly selective against Eis_Mtb.
To explain the results of our SAR study, we used previously published crystal structures of ternary Eis_Mtb-CoA-compound A (13g in ref (14); PDB ID 5EC4) and B (11c in ref (14); PDB ID 5EBV) complexes to model our inhibitors 1a and 2g in a position similar to that of inhibitors A and B (Figure S4). Without the crystal structures, de novo computational modeling of and screening for Eis inhibitors, including pharmacophore-based computer modeling, are invalidated by significant conformational changes in the Eis active site upon inhibitor binding.14 The inhibitors occupy the site overlapping with the AG-binding site of Eis. The models show that the cores of inhibitors 1a and 2g are surrounded by the side chains of hydrophobic amino acid residues (Trp36, the aliphatic part of Glu401, Ile28, Phe24, and Val400). The cores stack with the indole of Trp36, and in the orthogonal direction they are sandwiched between Glu401, the Eis C-terminus on one side and Ile28 on the other. The acetophenone rings of both series 1 and 2 with different substituents stack with Phe84, explaining why replacing these aromatic rings with alkyl chains resulted in a loss of activity for 1l, 1m, 2l, and 2m. The acetophenone rings are also surrounded by several hydrophobic amino acid residues (Phe84, Trp36, Met65, Ala33). Thus, putting a polar methoxy group in this hydrophobic environment would likely destabilize Eis binding, explaining the IC50 values of >200 μM for 1f and 2f. The para position of the acetophenone rings is flanked by Phe84 and Trp36, and it is ∼5 Å away from Trp13 and Met65, explaining why the bulkier bromo substituents of 1i and 2i resulted in lower Eis inhibitory activity, whereas the small fluoro substituents of 1g and 2g improved Eis inhibitory activity. The ortho position of the acetophenone rings is flanked by Phe402, explaining why an ortho substituent, as in 1b and 2b, resulted in a loss of Eis inhibition. The models shows that there is space for small substitution in the meta position of the acetophenone rings (a ∼5 Å gap). Small substituents such as the fluoro and chloro of 1c, 1d, 2c, and 2d fit well in the cavity, explaining why these compounds displayed good Eis inhibition. However, bulkier substituents such as the bromo of 1e and 2e or the nitro of 2n are too big to be accommodated at this site and would clash with Eis residues, accounting for the poor Eis inhibition by these compounds. We also determined that the calculated LogP values of all compounds are in the desirable range (0.98–3.75; Table S1).
In sum, we have discovered two scaffolds with Eis inhibitory activity. From 27 synthesized analogues of these scaffolds with the variable acetophenone appendage, we identified potent inhibitors of Eis. Growth inhibition studies of our inhibitors in combination with KAN in KAN-susceptible Mtb H37Rv (MICKAN ≤ 1.25 μg/mL) and KAN-resistant Mtb K204 (MICKAN ≥ 10 μg/mL) showed that some of our inhibitors were able to sensitize Mtb K204 to KAN. Smaller substituents, like hydrogen and fluorine, yielded the best compounds. In contrast, larger substituents, such as bromo or methoxy dramatically decreased the potency of the compounds. The best compound identified was 2c with the 3-(1,3-dioxolano)-2-indolinone core and a m-fluoro-phenyl substituent. This compound when used in combination with KAN reduced the MICKAN for KAN-resistant Mtb to 2.5–5 μg/mL. While CLSI recommends MICKAN of 5 μg/mL on Middlebrook 7H10 agar, it has no recommendation for susceptibility testing by Alamar Blue. One study suggests a critical MICKAN of 2.5 μg/mL for Alamar Blue testing. Since our inhibitors are able to return KAN-resistant isolates to an MICKAN below the critical concentration, essentially making resistant Mtb isolate KAN-susceptible, such inhibitors could play a crucial role in recovering KAN as a treatment option. However, clinical studies to support this hypothesis are yet to be undertaken. We are actively pursuing these avenues.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00261.
Structures, models, experimental procedures, and characterization data (PDF)
This work was supported by a NIH Grant AI090048 (S.G.-T.), a grant from the CCG at the U. Michigan (S.G.-T.), a grant from the Firland Foundation (S.G.-T.), and by startup funds from the College of Pharmacy at the U. Kentucky (S.G.-T. and O.V.T.). We thank Steve Vander Roest, Martha Larsen, and Paul Kirchhoff (CCG, UM) for help with HTS.
The authors declare no competing financial interest.
Supplementary Material
References
- Zaunbrecher M. A.; Sikes R. D. Jr.; Metchock B.; Shinnick T. M.; Posey J. E. Overexpression of the chromosomally encoded aminoglycoside acetyltransferase eis confers kanamycin resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2009, 106, 20004–20009. 10.1073/pnas.0907925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Green K. D.; Tsodikov O. V.; Garneau-Tsodikova S. Aminoglycoside multiacetylating activity of the enhanced intracellular survival protein from Mycobacterium smegmatis and its inhibition. Biochemistry 2012, 51, 4959–4967. 10.1021/bi3004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton J. L.; Green K. D.; Pricer R. E.; Mayhoub A. S.; Garneau-Tsodikova S. Unexpected N-acetylation of capreomycin by mycobacterial Eis enzymes. J. Antimicrob. Chemother. 2013, 68, 800–805. 10.1093/jac/dks497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Biswas T.; Porter V. R.; Tsodikov O. V.; Garneau-Tsodikova S. Unusual regioversatility of acetyltransferase Eis, a cause of drug resistance in XDR-TB. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 9804–9808. 10.1073/pnas.1105379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. D; Pricer R. E.; Stewart M. N.; Garneau-Tsodikova S. Comparative study of Eis-like enzymes from pathogenic and non-pagthogenic bacteria. ACS Infect. Dis. 2015, 1, 272–283. 10.1021/acsinfecdis.5b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. D.; Biswas T.; Chang C.; Wu R.; Chen W.; Janes B. K.; Chalupska D.; Gornicki P.; Hanna P. C.; Tsodikov O. V.; Joachimiak A.; Garneau-Tsodikova S. Biochemical and structural analysis of an Eis family aminoglycoside acetyltransferase from Bacillus anthracis. Biochemistry 2015, 54, 3197–3206. 10.1021/acs.biochem.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. D.; Chen W.; Garneau-Tsodikova S. Identification and characterization of inhibitors of the aminoglycoside resistance acetyltransferase Eis from Mycobacterium tuberculosis. ChemMedChem 2012, 7, 73–77. 10.1002/cmdc.201100332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. D.; Chen W.; Houghton J. L.; Fridman M.; Garneau-Tsodikova S. Exploring the substrate promiscuity of drug-modifying enzymes for the chemoenzymatic generation of N-acylated aminoglycosides. ChemBioChem 2010, 11, 119–126. 10.1002/cbic.200900584. [DOI] [PubMed] [Google Scholar]
- Willby M. J.; Green K. D.; Gajadeera C. S.; Hou C.; Tsodikov O. V.; Posey J. E.; Garneau-Tsodikova S. Potent inhibitors of acetyltransferase Eis overcome kanamycin resistance in Mycobacterium tuberculosis. ACS Chem. Biol. 2016, 11, 1639–1646. 10.1021/acschembio.6b00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


