Abstract
The Ret receptor tyrosine kinase plays a crucial role in the development of the enteric nervous system and the kidney. Tyrosine 1062 in Ret represents a binding site for the phosphotyrosine-binding domains of several adaptor and effector proteins that are important for the activation of intracellular signaling pathways, such as the RAS/ERK, phosphatidylinositol 3-kinase/AKT, and Jun-associated N-terminal kinase pathways. To investigate the importance of tyrosine 1062 for organogenesis in vivo, knock-in mice in which tyrosine 1062 in Ret was replaced with phenylalanine were generated. Although homozygous knock-in mice were born normally, they died by day 27 after birth and showed growth retardation. The development of the enteric nervous system was severely impaired in homozygous mutant mice, about 40% of which lacked enteric neurons in the whole intestinal tract, as observed in Ret-deficient mice. The rest of the mutant mice developed enteric neurons in the intestine to various extents, although the size and number of ganglion cells were significantly reduced. Unlike Ret-deficient mice, a small kidney developed in all knock-in mice, accompanying a slight histological change. The reduction of kidney size was due to a decrease of ureteric bud branching during embryogenesis. Thus, these findings demonstrated that the signal via tyrosine 1062 plays an important role in histogenesis of the enteric nervous system and nephrogenesis.
The RET proto-oncogene encodes a receptor tyrosine kinase that has diverse roles in development and disease (40, 60). It has been demonstrated that Ret is a functional receptor for members of the glial cell line-derived neurotrophic factor (GDNF) family, including GDNF, neurturin, artemin, and persephin (1). However, these neurotrophic factors do not bind to the extracellular domain of Ret directly but require glycosylphosphatidylinositol-linked cell surface proteins called GFRαs for complex formation with Ret. GDNF, neurturin, artemin, and persephin preferentially bind to GFRα1, GFRα2, GFRα3, and GFRα4, respectively, and show specific biological roles in vivo (1). Gene knockout studies revealed that Ret, Gdnf, and Gfrα1 are indispensable for the development of the kidney and the enteric nervous system (ENS). Ret−/− (55), Gdnf−/− (44, 52, 54), and Gfrα1−/− (10) mice died within 12 to 24 h after birth, lacked enteric neurons posterior to the stomach, and showed kidney aplasia or severe hypoplasia.
To date, intracellular signaling via activated Ret has been studied extensively, with a variety of cell lines and primary culture cells. Specific tyrosine residues in the Ret intracellular domain are phosphorylated by GDNF stimulation and have been identified as docking sites for many adaptor or effector proteins. For example, Tyr905, Tyr1015, and Tyr1096 represent docking sites for GRB7/GRB10, phospholipase-Cγ, and GRB2, respectively (2, 9, 49). Intriguingly, Tyr1062 is a multidocking site for several signaling molecules, including SHC, FRS2, DOK family proteins, IRS1/2, Enigma, and protein kinase Cα (3-5, 15, 22, 35, 38, 42, 43, 45). Consistent with these findings, it turned out that representative intracellular signaling pathways, such as the RAS/mitogen-activated protein kinase, phosphatidylinositol 3-kinase/AKT, Jun-associated N-terminal kinase (JNK), and ERK5 pathways are activated via phosphorylated Tyr1062 (7, 14, 23, 24, 57). Activation of these pathways leads to Ret-mediated biological responses such as cell transformation, survival and migration of neuronal cells, and tubule formation of kidney-derived cells (5, 17, 46, 61).
RET loss-of-function mutations have been identified in human Hirschsprung's disease, a congenital malformation associated with the absence of intrinsic ganglion cells in the distal gastrointestinal tract (16, 53). The mutations cause Ret dysfunction by various mechanisms (8, 11, 12, 20, 29, 31, 32, 50, 51). We and other investigators reported that a few mutations identified in Hirschsprung's disease could affect signaling via Tyr1062 in Ret (20, 28). In this study, to elucidate the in vivo role of signaling via Tyr1062, knock-in mice in which Tyr1062 was replaced with phenylalanine were generated. Our results reveal that signaling via Tyr1062 is necessary for normal development of the ENS and the kidney. In addition, because the abnormalities of homozygous mutant mice were milder than those of Ret-deficient mice, it is likely that signaling via other tyrosine residues also plays a role in organogenesis.
MATERIALS AND METHODS
Transgene construction.
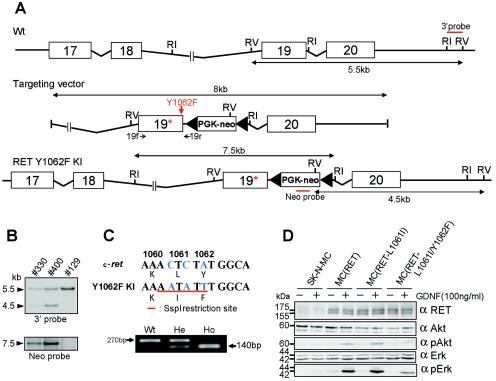
A genomic DNA fragment encompassing exon 17 to exon 20 of the mouse Ret gene was isolated by screening a mouse genomic DNA library. To introduce a mutation at the Tyr1062 codon in exon 19 and an SspI restriction enzyme site, PCR mutagenesis was used, resulting in two amino acid replacements (KLY1062 to KIF1062). The insertion-type targeting vector pUC118 contained an approximately 8-kb mouse Ret genomic DNA fragment in which a phosphoglycerokinase-neo cassette flanked by loxP sites was inserted into intron 19 (Fig. 1A). The mutation and absence of polymerase errors were verified by sequencing the construct.
FIG. 1.
Generation of Ret Y1062F knock-in mice by homologous recombination of embryonic stem cells. (A) Schematic diagram of the targeted Y1062 allele. A red arrow indicates the mutated tyrosine 1062 (changed to phenylalanine). 19f and 19r with small black arrows indicate the forward and reverse primers for genomic sequencing, respectively. RI, EcoRI; RV, EcoRV. (B) Southern blot analysis of ES clones. Genomic DNA was extracted from each clone, digested with EcoRV or EcoRI, and analyzed by Southern blotting with the 3′ or Neo probe; 5.5-kb and 4.5-kb bands detected by the 3′ probe represent the wild-type and mutant alleles, respectively. (C) Genotyping by SspI digestion of an amplified genomic DNA fragment. Wild-type mice (Wt) gave an undigested single band of 270 bp, and Y1062F homozygous mutant mice (Ho) gave a digested band of ≈140 bp. Heterozygous mice (He) gave both bands. (D) Analysis of intracellular signaling via Ret with the L1061I or L1061I/Y1062F mutation. Lysates from GDNF-treated (100 ng/ml, 15 min) and untreated SK-N-MC cells that were transiently transfected with wild-type RET (designated MC [RET]), L1061I mutant RET, or L1061I/Y1062F mutant RET were immunoblotted with anti-Ret, anti-Akt, anti-phospho-Akt, anti-Erk, and anti-phospho-Erk antibodies.
Generation of knock-in mice.
The targeting vector was linearized by KpnI digestion and electroporated into embryonic stem (ES) cells derived from 129svj mice. Neomycin-resistant ES cells were obtained by G418 selection, and their genomic DNAs were extracted for Southern blotting. Genomic DNA was digested with EcoRV and EcoRI and hybridized to the Neo probe and 3′ probe, respectively. The Neo probe was derived from the neo coding region and the 3′ probe from intron 20 of the mouse Ret gene (Fig. 1A). Two ES clones, 330 and 400, with an expected recombinant allele, were obtained and injected into blastocysts of C57BL/6J mice. Mutant mice were generated by crossing chimeric males with C57BL/6J female mice.
Genotype screening.
The genomic DNA of the offspring was extracted from their tails. Mice with the mutant Ret allele were screened by genomic PCR followed by SspI digestion. PCR was performed with the forward primer in exon 19 (19f; 5′-ACTACTTGGACCTGGCTGCATCCA-3′) and the reverse primer in intron 19 (19r; 5′-AGAAAAGGGTTCGGAGGAGGCTTTGGTGTC-3′) (Fig. 1A).
Transfection.
A plasmid containing the entire coding sequence of the human RET gene (long isoform) was produced as described previously (5). Each mutation (isoleucine for leucine at codon 1061 and phenylalanine for tyrosine at codon 1062) was introduced by the QuikChangeII site-directed mutagenesis kit (Stratagene) according to the manufacturer's instruction. Plasmid DNAs (4 μg) were transfected into SK-N-MC human primitive neuroectodermal tumor cells (5 × 105) with the Lipofectamine 2000 reagent (Invitrogen). The cells were maintained in a humidified incubator with 95% air and 5% CO2 at 37°C. After 24 h of incubation, the medium was replaced with serum-free Dulbecco's modified Eagle's medium (DMEM) and kept for 3 h. Then the transfected cells were treated with GDNF (100 ng/ml) for 15 min, lysed in sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris-HCl [pH 6.8], 5 mM EDTA, 2% SDS, 10% glycerol, 20 μg of bromophenol blue per ml) containing 80 mM dithiothreitol and subjected to Western blot analysis as described below.
Tissue preparation for protein and RNA extraction.
After body weight measurement, mice were sacrificed under general anesthesia. A complete autopsy was performed, and organs were resected, cut into 5-mm3 specimens, and quickly frozen for protein and RNA extraction.
Gene expression analysis.
Total cellular RNA was isolated from frozen tissues with the RNeasy minikit (Qiagen). RNA was reverse transcribed with avian myeloblastosis virus reverse transcriptase XL (TaKaRa) for 30 min at 55°C. The resulting cDNA was subjected to 30 cycles of PCR consisting of 30 s at 94°C, 30 s at 56°C, and 30 s at 72°C. The PCR primer sets were as follows: mouse Ret short isoform (Ret9) (forward primer, 5′-CCAGCTATGTGTCCACAGCCGTGC-3′, and reverse primer, 5′-CATGATGGGGGTAGGGTGCAAAGG-3′); mouse Ret long isoform (Ret51) (forward primer, 5′-CCGATGGCACTAGCACTGGGTTCC-3′, and reverse primer, 5′-ATTTTGCCGCTGAGGGTGAAACCA-3′); and mouse β-actin (forward primer, 5′-AGCTGCCTGACGGCCAGGTC-3′, and reverse primer, 5′-GCTCAGGAGGAGCAATGATC-3′).
Antibodies.
Anti-Ret rabbit polyclonal antibody was developed against the carboxyl-terminal 19 amino acids as described previously (47). Polyclonal anti-Akt, anti-phospho-Akt, anti-Erk, and anti-phospho-Erk antibodies were purchased from Cell Signaling Technology. Monoclonal antiphosphotyrosine antibody was purchased from Upstate Biotechnology. Monoclonal anti-β-actin antibody was purchased from Sigma.
Protein analysis.
Frozen mouse tissues were homogenized in SDS sample buffer containing 80 mM dithiothreitol. The lysates, containing 30 μg of protein, were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene fluoride membranes (Immobilon-P, Millipore). Membranes were blocked for 1 h at room temperature in 3% ovalbumin in TPBS (phosphate-buffered saline containing 0.05% Tween 20) with gentle shaking and incubated with the primary antibody overnight at 4°C. After washing the membranes with TPBS three times, they were incubated with the secondary antibody conjugated to horseradish peroxidase (goat anti-rabbit immunoglobulin G- horseradish peroxidase; Dako) for 1 h at room temperature. The reaction was examined by an enhanced chemiluminescence detection kit (ECL; Amersham Biosciences) according to the manufacturer's instructions.
Primary culture of dorsal root ganglia.
Dorsal root ganglia were obtained from mice at postnatal day 13.5 under a stereoscopic microscope and washed twice with ice-cold physiological saline and twice with Ham's F-12 (Gibco). They were digested with 0.15% collagenase and 0.05% trypsin-EDTA, and mechanically triturated. Isolated cells were suspended in F-12, and 1.5 volumes of Dulbecco's modified Eagle's medium supplemented with 8% calf serum were added. The cells were plated on 35-mm-diameter tissue culture dishes coated with filtrated 0.01% poly-l-lysine (Sigma) and maintained in a humidified incubator with 95% air and 5% CO2 at 37°C. The medium was replaced with serum-free Dulbecco's modified Eagle's medium/F-12 (at a ratio of 1.5 to 1) 48 h after the initial plating and kept for 24 h. Then cultured cells were treated with GDNF (100 ng/ml) for 15 min, lysed in SDS sample buffer, and subjected to Western blot analysis.
Histology and immunohistochemistry.
Organs were resected as described above. The stomach was incised along the greater curvature. The intestine was linearized and sectioned in 20 to 30 pieces of 1-cm-long fragments. The kidney was cut along the longitudinal axis to yield the greatest section.
Embryos were taken from female mice at gestational day 13.5 (E13.5) and E17.5 and sacrificed by cervical joint dislocation. Genomic DNA was extracted from the amnion, and the genotype was screened as described above.
All tissues and embryos were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5 μm for light microscopic analysis. Longitudinal sections of the stomach, transverse sections of the colon and small intestine, and sagittal sections of the kidney and embryos were prepared. Hematoxylin and eosin staining was performed by a conventional method. For immunohistochemistry, slides were deparaffinized in xylene and rehydrated in a graded series of ethanol (100, 90, and 70%). For antigen retrieval, they were incubated in 50 mM Tris-HCl (pH 7.6) containing 5% trypsin at 37°C for 10 min. Nonspecific binding sites were blocked with 10% normal goat serum for 10 min. The sections were incubated with the primary antibody (rabbit anti-peripherin antibody, Chemicon) overnight at 4°C, and endogenous peroxidase was inhibited with 0.3% hydrogen peroxide in methanol for 15 min. The slides were incubated with the secondary antibody conjugated to peroxidase-labeled polymer (EnVision+, rabbit; Dako) for 30 min at room temperature. The reaction products were visualized with diaminobenzidine. Counterstaining was performed with hematoxylin.
RESULTS
Generation of Ret Y1062F knock-in mice.
To elucidate the in vivo role of signal transduction via tyrosine 1062 in Ret, knock-in mice carrying a mutant Ret gene in which tyrosine 1062 the codon was replaced with a phenylalanine codon (named Y1062F mice) were generated. In addition, leucine 1061 was replaced with isoleucine (L1061I mutation) to create an SspI restriction enzyme site in the mutated allele for rapid analysis (Fig. 1C). To confirm that the L1061I mutation does not affect intracellular signaling via Ret, the wild-type RET or L1061I mutant RET gene was transiently transfected into SK-N-MC human primitive neuroectodermal tumor cells. After GDNF stimulation, Erk and Akt activation was detected at similar levels in wild-type Ret and L1061I mutant Ret-expressing cells (Fig. 1D). As expected, Erk and Akt activation was impaired in L1061I/Y1062F mutant Ret-expressing cells.
The targeting construct contained a neomycin resistance cassette for positive selection (Fig. 1A). Genomic DNA from ES clones obtained by G418 selection was analyzed by Southern blotting with the 3′ and Neo probes (Fig. 1A). From these results, two ES clones (330 and 400) were expected to harbor a targeted mutant Ret allele (Fig. 1B). Both clones were used successfully to generate chimeric mice, and germ line transmission of the mutant allele was achieved. Genomic DNA fragments containing tyrosine 1062 codons were amplified from tail DNAs of wild-type, heterozygous, and homozygous mutant mice and digested with SspI. As expected, approximately 270-bp and 140-bp fragments were obtained from the wild-type and mutant alleles, respectively (Fig. 1C). The mutations introduced at codons 1061 and 1062 in the mutant allele were confirmed by sequencing the amplified fragments.
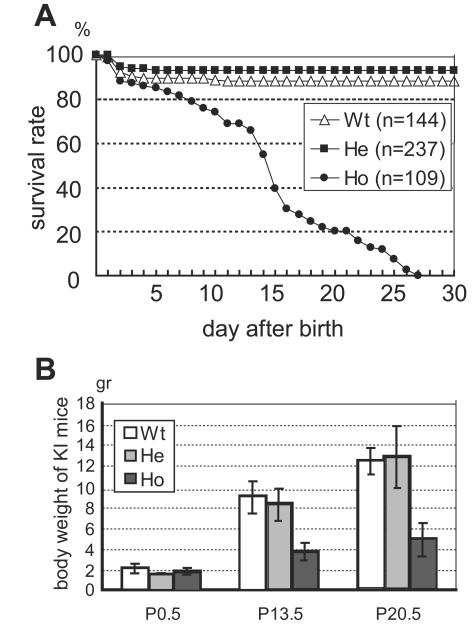
When heterozygous mutant mice were mated, wild-type, heterozygous, and homozygous mice were born at the expected frequencies (data not shown). Homozygous mice exhibited obvious abdominal distension around day 10 and died by 27 days of age (Fig. 2A). There was no significant difference in body weight among the three genotypes at birth, although homozygous mice displayed severe growth retardation at day 13.5 (Fig. 2B). No difference in gross appearance, body weight, or survival rate was observed between wild-type and heterozygous mutant mice at least up to 18 months of age (data not shown).
FIG. 2.
Survival rate and body weight of Ret Y1062F knock-in mice. (A) We analyzed 144 wild-type (Wt), 237 heterozygous (He), and 109 homozygous (Ho) mice. The survival rate is expressed as a percentage of surviving mice for the total number of mice of each genotype analyzed. (B) Average body weights of Y1062F knock-in mice at postnatal days 0.5, 13.5, and 20.5. Bars indicate standard deviation.
Ret mRNA and protein expression in Y1062F knock-in mice.
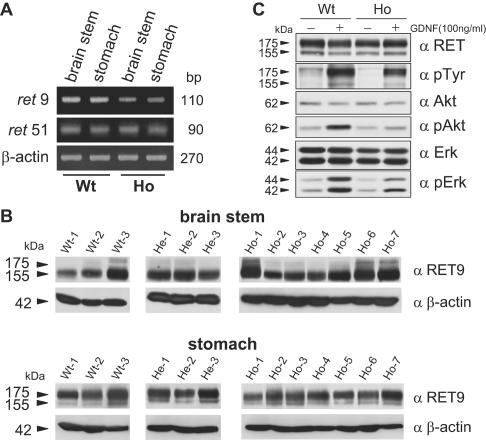
The Ret gene encodes two major isoforms (short and long isoforms, designated Ret9 and Ret51), resulting from alternative splicing in the 3′ region (36). Ret51 has 51 amino acids in the carboxyl-terminal tail that are replaced by nine unrelated amino acids in Ret9 (59). Because Tyr1062 is located just before the splicing site, reverse transcription-PCR analysis was performed to confirm that splicing occurred normally in Ret Y1062F knock-in mice. With the primer sets specific for each isoform, cDNAs were amplified from RNAs of the brain stem and stomach. Ret9 and Ret51 transcripts were detected in both tissues from both wild-type and homozygous Y1062F mice (Fig. 3A), indicating normal splicing of Ret in the latter.
FIG. 3.
Ret mRNA and protein expression in tissues and dorsal root ganglia of Y1062F mutant mice. (A) mRNA expression of two splice variants (Ret9 and Ret51) in knock-in mice. Two main Ret splice variants were detected in the brain stem and stomach of both wild-type and homozygous mutant mice by reverse transcription-PCR. (B) Lysates (30 μg of proteins) of the brain stem and stomach from three wild-type (Wt), three heterozygous (He), and seven homozygous (Ho) mice were subjected to immunoblotting with anti-Ret9 or anti-β-actin antibodies. The anti-Ret9 antibody was prepared as described previously (47). (C) Ret expression and activation of Erk and Akt in dorsal root ganglia cells. Dorsal root ganglia were dissected from postnatal day 13.5 wild-type and homozygous Y1062F knock-in mice and cultured for 72 h. The lysates (20 μg of proteins) from GDNF-treated (100 ng/ml, 15 min) and untreated dorsal root ganglia cells were immunoblotted with anti-Ret, antiphosphotyrosine, anti-Akt, anti-phospho-Akt, anti-Erk, and anti-phospho-Erk antibodies.
Because it was reported that the presence of the neo cassette caused a reduction in protein expression (34, 39), we compared Ret9 protein expression in the brain stem and stomach among wild-type, heterozygous, and homozygous Y1062F mice. As shown in Fig. 3B, we did not observe a significant difference in Ret9 expression among the three genotypes, although individual mice showed some differences. In addition, the levels of Ret expression in dorsal root ganglia cells were almost the same between wild-type and homozygous Y1062 mice (Fig. 3C), indicating that the presence of the neo cassette in intron 19 does not significantly affect Ret expression in homozygous mutant mice. However, Erk and Akt activation by GDNF was impaired in dorsal root ganglia cells from Y1062F mice (Fig. 3C), as observed in GDNF-treated SK-N-MC (RET-L1061I/Y1062F) cells (Fig. 1D).
Defects of the ENS in Ret Y1062F knock-in mice.
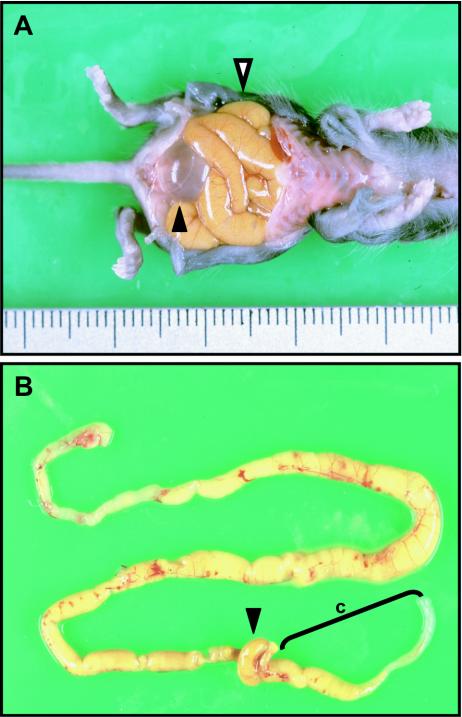
The abdomen of homozygous Y1062F mice was distended macroscopically. Autopsy revealed severe dilation of the small intestine in all homozygous mutant mice (Fig. 4A). About 25% of the homozygous mice showed total colon stenosis, whereas the remaining mice displayed dilation of the oral part of the colon and stenosis of its anal part (Fig. 4B).
FIG. 4.
Macroscopic abdominal and intestinal findings for a homozygous Y1062F mouse. (A) Incised abdomen. The abdomen was markedly distended, and the small intestine was dilated (white arrowhead). The urinary bladder was filled with urine (black arrowhead). (B) Macroscopic appearance of the intestine. The distal part of the colon was stenotic, and its proximal part was dilated. The small intestine was mostly dilated. c, colon; black arrowhead, cecum.
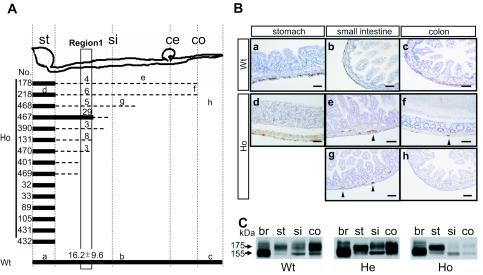
To determine the intestinal ganglion cell distribution, the small intestine and colon were sectioned (1-cm long), and transverse sections were prepared for each fragment. From 20 to 30 fragments per mouse were stained with antiperipherin antibody and compared for the presence of ganglion cells among seven wild-type, eight heterozygous, and 15 homozygous mice. As shown in Fig. 5A and B, mature ganglion cells were observed throughout the gastrointestinal tract in all of the wild-type and heterozygous mutant mice. The homozygous mice, on the other hand, showed various maturation and distribution patterns of ganglion cells (Fig. 5A and Bd, e, f, g, and h). Six of 15 homozygous mice (nos. 32, 33, 89, 105, 431, and 432 in Fig. 5A) displayed a complete absence of ganglion cells in both the small intestine and colon, as observed in Ret-deficient mice. Nine homozygous mutant mice, however, displayed ganglion cells to various extents in the intestine, although their number and/or size was significantly reduced (Fig. 5A and Be, f, g, and h). For example, when the peripherin-positive ganglion cells in the intramuscular layer of the small intestine were counted in the transverse sections of region 1 (Fig. 5A), their numbers (per section) in six homozygous Y1062F mice (nos. 178, 218, 468, 390, 131, and 470) were 18 to 50% of the average number in wild-type mice (Fig. 5A). The size of the ganglion cells was also small in some homozygous Y1062F mice (Fig. 5Bf and g). Although the ganglion cells were well preserved in the stomachs of homozygous mice (Fig. 5Bd), the number decreased slightly (approximately 60 to 80% of that in wild-type mice). We found no significant difference in neuron number and distribution between wild-type and heterozygous mice (data not shown).
FIG. 5.
Impairment of ENS development in Y1062F mutant mice. (A) Distribution pattern of enteric neurons in 15 homozygous Y1062F mutant mice. Bold solid lines indicate the region where the number of neurons was more than 50% of that in wild-type mice. Broken lines indicate the region with fewer neurons (less than 50% of the average number in wild-type mice). Six homozygous mutant mice (nos. 32, 33, 89, 105, 431, and 432) showed a complete absence of enteric neurons throughout the intestinal tract posterior to the stomach. The numbers above the lines in region 1 indicate the number of peripherin-positive ganglion cells (per section) in the intramuscular layer of the intestine. In all wild-type and heterozygous Y1062F mutant mice, ganglion cells were present from the stomach to the end of the colon (six wild-type and nine heterozygous mice were analyzed). The letters a to h represent the regions analyzed in panel B. (B) Immunohistochemical analysis of the intestinal tract with antiperipherin antibody. Representative sections of wild-type (a, b, and c) and homozygous Y1062F mice (d, e, f, g, and h) were stained with antiperipherin antibody. (a and d) stomach; (b, e, and g) small intestine; (c, f, and h) colon. Arrowheads denote peripherin-immunoreactive ganglion cells. In homozygous Y1062F mice, the ganglion cells in the small intestine and colon were absent (h) or their number and/or size were markedly reduced (e, f, and g). Bars, 50 μm. (C) Ret protein expression in Ret Y1062F knock-in mice. Total cell lysates (30 μg of protein) from the brain stem (br), stomach (st), small intestine (si), and colon (co) of wild-type (Wt), heterozygous (He), and homozygous (Ho) mutant mice were analyzed by Western blotting with anti-Ret antibody. The 175-kDa and 155-kDa Ret proteins are shown. Ret expression in the small intestine and the colon of homozygous Y1062F mice was noticeably reduced.
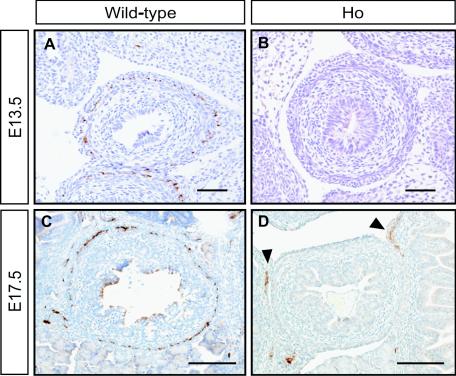
We further compared the presence of ENS progenitors between E13.5 and E17.5 wild-type and homozygous Y1062F embryos. As shown in Fig. 6A and C, ENS progenitors stained with antiperipherin antibody were detected in the intestines of wild-type embryos in a ring-like fashion. In contrast, they were undetectable (Fig. 6B) or few (Fig. 6D) in the homozygous Y1062F embryos examined. These findings suggested that the signal through tyrosine 1062 in Ret is crucial for the migration and proliferation of ENS progenitors during embryogenesis.
FIG. 6.
Immunohistochemistry of the embryonic (E13.5 and E17.5) intestine of Y1062F knock-in mice. In wild-type embryos, ENS progenitors were stained with antiperipherin antibody in a ring-like fashion (A and C). In homozygous mutant embryos, positive cells were undetectable (B) or few (arrowheads in panel D). Bars, 50 μm.
Ret protein expression in the gastrointestinal tract.
To compare Ret protein expression in the enteric nervous system among the three genotypes, Western blot analysis was performed with lysates from the brain stem, stomach, small intestine, and colon of wild-type, heterozygous, and homozygous mutant mice. Ret expression was identified at high levels in all four organs from the wild-type and heterozygous mutant mice (Fig. 5C). Although high levels of Ret expression were observed in the brain stems and stomachs of homozygous mutant mice, its expression decreased markedly in their small intestines and colons (Fig. 5C). A marked reduction in Ret expression was observed in the colon of all homozygous Y1062F mice examined (data not shown). The results are consistent with the histological findings showing lack or remarkable reduction in the number of enteric neurons in the small intestine and colon of homozygous Y1062F mice.
Renal hypoplasia in Ret Y1062F knock-in mice.
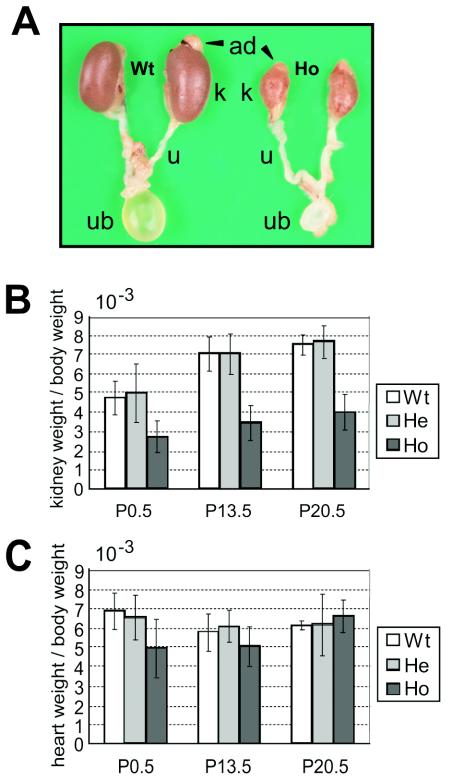
Intriguingly, kidneys developed in all homozygous Y1062F knock-in mice, although their sizes were significantly smaller than those of wild-type and heterozygous mice (Fig. 7A and B). The adrenal gland, ureter, and urinary bladder developed normally in homozygous mutant mice, and urine production was observed. Because homozygous mice exhibited growth retardation after birth, the kidney weight-to-body weight ratio was analyzed to evaluate the degree of kidney hypoplasia. The ratio in homozygous mice was about half of that in the wild-type and heterozygous mutant mice from birth onward (Fig. 7B). No significant difference in the heart weight-to-body weight ratio was observed among the three genotypes (Fig. 7C).
FIG. 7.
Kidney hypoplasia in Y1062F knock-in mice. (A) Macroscopic analysis of the kidney at postnatal day 13.5. The shape was mostly normal in homozygous Y1062F mice, and the adrenals (ad), ureter (u), and urinary bladder (ub) developed normally. (B) The kidney weight-to-body weight of homozygous mice was about half of that of wild-type mice at each time point. Wild-type (Wt), heterozygous (He), and homozygous (Ho) mutant mice were tested. (C) The heart weight-to-body weight ratio was not significantly different among the three genotypes. Bars indicate the standard deviation.
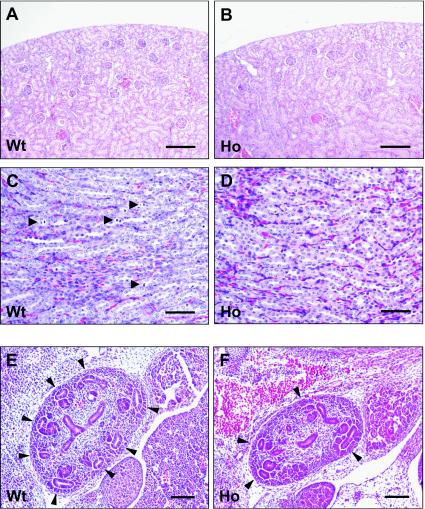
Despite kidney hypodysplasia, glomeruli, proximal and distal convoluting tubules, and collecting ducts developed in homozygous Y1062F mice (Fig. 8A to D), but in some homozygous mice, a cystic change of the tubules was observed (data not shown). Mitotic figures of the collecting duct epithelia in 2-week-old wild-type mice were seen more frequently than in homozygous mutant mice (Fig. 8C and D).
FIG. 8.
Histological findings for Y1062F knock-in mouse kidneys. The cortical (A and B) and medullary (C and D) regions are shown. Despite kidney hypodysplasia, the basic structures of glomeruli, proximal and distal tubules, and collecting ducts were maintained in homozygous Y1062F mice. Mitotic figures of collecting duct cells (arrowheads in panel C) were more frequently detected in the wild-type kidney. Bars indicate 100 μm (A and B) and 50 μm (C and D). (E and F) Histology of E13.5 metanephric kidneys of wild-type and homozygous Y1062F knock-in mice. Sagittal sections of embryos were prepared to yield the greatest section of the metanephric kidneys. Normal branching of ureteric buds was observed in wild-type mice (E) and heterozygous mice (data not shown). In homozygous Y1062F mice, the number of branching ureteric buds was reduced. Bars, 50 μm.
Finally, we investigated the abnormality of renal development during embryogenesis of Y1062F mutant mice. The evagination and initial branching of the ureteric bud occurred normally in E11.5 and E12.5 homozygous mutant mice (data not shown). However, the number of ureteric buds in five E13.5 homozygous mice examined was significantly reduced compared with that in wild-type mice (5.2 ± 0.9 versus 8.0 ± 1.0 ureteric buds per section) (Fig. 8E and F), suggesting that the signal via Tyr1062 plays a role in ureteric bud branching during the late embryogenesis stage. Branching occurred normally in the heterozygous mice (data not shown).
DISCUSSION
It has been established that GDNF/Ret signaling plays a pivotal role in renal development and histogenesis of the ENS (1, 40, 60). Ret activation by GDNF mediates a variety of intracellular signaling such as the RAS/ERK, phosphatidylinositol 3-kinase/AKT, JNK, and PLCγ pathways (60). These pathways are activated via phosphorylated tyrosine residues in the Ret intracellular domain. For example, Tyr1015 is a binding site for PLCγ that is responsible for protein kinase C activation (9). Phosphorylated Tyr1062 binds several adaptor and effector proteins including SHC, FRS2, IRS1/2, and the DOK protein family, resulting in activation of RAS/ERK, phosphatidylinositol 3-kinase/AKT, and JNK (7, 22-24, 35, 42, 43, 45, 57). In addition, Tyr1096, which is only present in the carboxyl-terminal sequence of the long Ret isoform (Ret51), is a binding site for GRB2 and appears to activate partially, but not fully, the RAS/ERK and phosphatidylinositol 3-kinase/AKT pathways (7).
The Y1062F knock-in mice that we generated in this study confirmed the importance of the signal via Tyr1062 in the development of the ENS and the kidney. However, significant differences in phenotype were observed between Y1062F knock-in and Ret-deficient mice. First, although about 40% of the knock-in mice exhibited a lack of enteric neurons posterior to the stomach, as observed in Ret-deficient mice, the rest of them had enteric neurons in the small intestine and colon to various extents, accompanying the reduction in the size and number of ganglion cells. Many reports suggested that GDNF/Ret signaling is required for the proliferation, migration, and survival of the ENS progenitors (18, 21, 25, 27, 30, 46, 62, 64, 65). The fact that the ENS progenitors were few in the intestines of E13.5 and E17.5 homozygous Y1062F embryos supported this view. In addition, these findings are consistent with a report showing that the phosphatidylinositol 3-kinase and ERK pathways play crucial roles in the migratory response of enteric neural crest cells (ENCC) to GDNF (46). However, the signal via Tyr1062 was not necessarily a sole residue for complete migration and proliferation of ENCC, because the development of enteric neurons was detected in the intestine of some homozygous Y1062F mice. Thus, signaling via distinct tyrosine residues such as Tyr1015 or Tyr1096 may also play a role in the migration and proliferation of ENCC.
Maina et al. (39) reported knock-in mice in which the multifunctional docking sites of Met receptor tyrosine kinase were replaced with specific binding motifs for phosphatidylinositol 3-kinase, Src or Grb2. These mutant mice retained normal signaling via Gab1, but differentially recruited specific effectors. While the mutants with optimal Grb2 binding motifs developed normally, the mutants with phosphatidylinositol 3-kinase or Src binding motifs resulted in severe loss of function but displayed different phenotypes and rescue of distinct tissues. The rescue of placenta and myoblast proliferation versus axon growth by Src and phosphatidylinositol 3-kinase binding sites, respectively, indicated that Met-mediated developmental events require the activation of specific pathways. The introduction of these types of mutations in the surrounding sequence of Tyr1062 in Ret may elucidate specific roles for each signaling in organogenesis.
In addition to GDNF, neurturin that is another member of the GDNF protein family that activates Ret though binding to GFRα2. Although GDNF and neurturin have similar effects on ENS progenitors in vitro (27), Gdnf−/− (44, 52, 54) and Neurturin−/− (26) mice have strikingly different phenotypes. Recently, it was reported that neurturin is essential for maintaining the size of enteric neurons, whereas GDNF availability determines neuron number by controlling ENS progenitor proliferation (21). Our findings that both enteric neuron number and size were markedly reduced in homozygous Y1062F mice suggested that the action of both GDNF and neurturin was impaired in them.
The difference in the extent of aganglionosis observed in individual mutant mice suggests the involvement of factors other than Ret in the development of enteric neurons. The spatial and temporal regulation of Gdnf expression along the developing bowel has recently been reported (46, 66). Gdnf mRNA upregulation was detected in the stomach of E9.0 to 9.5 and the cecum of E11.5 to 13.5 embryos and appeared to function as a chemoattractant of ENS progenitors in vivo, leading to the ordered colonization of the esophagus, stomach, and small intestine (46). Thus, the difference in the level of Gdnf upregulation in the developing bowel of individual Y1062F mice may affect the proliferation of Ret-expressing ENS progenitors in the stomach as well as their migration towards the intestine during embryogenesis.
In addition, it is possible that other signals produced by the microenvironment of the developing gut play some role in the migration of Ret-expressing ENS progenitors in Y1062F mice. In this regard, endothelin-3 (ET-3) is a candidate molecule because ET-3 itself is involved in the pathogenesis of aganglionosis in human Hirschsprung's disease and a high level of ET-3 expression was observed in the cecum and the colon during embryogenesis (33, 37, 41, 58, 63). In fact, it was recently demonstrated that ET-3 regulates GDNF-induced ENCC proliferation and migration by inhibiting protein kinase A activity (6). ET-3 promoted GDNF-induced ENCC proliferation but inhibited their migration. In addition, we recently reported that serine 696 phosphorylation in the juxtamembrane region of Ret by protein kinase A promoted lamellipodium formation in neuroectodermal cells, which is a critical event for neuritegenesis (19). Thus, it is likely that modulation of Ret function by ET-3 is crucial for normal development of the ENS.
Unlike Ret-deficient mice, kidneys developed in all of the homozygous Y1062F mice, although the kidney weight-to-body weight ratio of the homozygous mice was about half that of the wild-type and heterozygous mutant mice. Although the early stages of metanephric development in homozygous Y1062F mice, such as evagination of the ureteric bud and initial branching, occurred normally, defects in ureteric bud branching at later stages of nephrogenesis were observed. It was reported that half of the Ret-deficient mice had the defects in ureteric bud formation from the Wolffian duct (56). Approximately 15% formed a ureteric bud, but it failed to grow into the metanephric mesenchyme. In the rest of the Ret-deficient mice, the ureteric bud succeeded in reaching the metanephric mesenchyme, but the growth and branching of the ureteric bud were severely retarded and often abnormal (56). These findings suggested that signaling other than that via Tyr1062 is critical for ureteric bud formation and evagination during early nephrogenesis stages.
Ret is translated into two major isoforms, Ret9 and Ret51, by alternative splicing (36). The Ret isoforms differ only in their carboxyl-terminal amino acid sequences. The carboxyl-terminal 51 amino acids in Ret51 are replaced with nine unrelated amino acids in Ret9 (59). Recently, de Graaff et al. (13) generated mutant mice that expressed single Ret isoforms and showed that Ret9 and Ret51 have different signaling properties in vivo. Monoisomeric Ret9 mice, which lack Ret51, appeared normal, whereas monoisomeric Ret51 animals, which lack Ret9, exhibited kidney hypodysplasia and lacked enteric ganglia from the colon. As in homozygous Y1062F mice, ureteric bud branching was impaired in monoisomeric Ret51 mice, although histologic abnormalities in the kidney, such as cystic changes of the tubules, appeared to be found more frequently in the latter. On the other hand, most of the homozygous Y1062F mice had more severe defects in the ENS than monoisomeric Ret51 mice.
Because the sequence divergence of the two isoforms occurs one amino acid after Tyr1062, one hypothesis to explain the differential signaling properties of Ret9 and Ret51 is that the sequence differences that are carboxyl terminal to Tyr1062 may modulate the efficiency of the complex formation of signaling molecules. The fact that monoisomeric Ret51 mice and homozygous Y1062F mice had similar kidney phenotypes supported the view that the signal via Tyr1062 in Ret51 is not sufficient for ureteric bud branching at late embryogenesis. It is interesting that the specific sequence that is carboxyl terminal to Tyr1062 observed in each isoform can affect the binding ability of the phosphotyrosine-binding and SH2 domains of SHC (48).
In this study, we elucidated the in vivo role of signaling via Tyr1062 by targeted mutagenesis. This signaling appeared to play a crucial role in the migration and/or proliferation of ENS progenitors because the neuron number and distribution in the intestine of Y1062F homozygous mice were markedly reduced. In addition, signaling via Tyr1062 was required for ureteric bud branching at later stages of nephrogenesis but not for ureteric bud formation, evagination, and first branching at its early stages. To explain the difference in phenotype between Ret-deficient mice and Y1062F mutant mice, it would be interesting to generate mutant mice in which other tyrosine residues, such as Tyr1015 and Tyr1096, are replaced.
Acknowledgments
We are grateful to T. Morinaga for designing the Ret isoform-specific primers and to K. Kiuchi and M. Kusakabe for encouragement. We thank K. Uchiyama, K. Imaizumi, N. Misawa, C. Kawaguchi, Y. Ito, and S. Kawai for technical support.
This work was supported in part by Grants-in-Aid for COE Research, Scientific Research (A), and Scientific Research on Priority Areas Cancer from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a grant from the Uehara Memorial Foundation.
REFERENCES
- 1.Airaksinen, M. S., and M. Saarma. 2002. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 3:383-394. [DOI] [PubMed] [Google Scholar]
- 2.Alberti, L., M. G. Borrello, S. Ghizzoni, F. Torriti, M. G. Rizzetti, and M. A. Pierotti. 1998. Grb2 binding to the different isoforms of Ret tyrosine kinase. Oncogene 17:1079-1087. [DOI] [PubMed] [Google Scholar]
- 3.Andreozzi, F., R. M. Melillo, F. Carlomagno, F. Oriente, C. Miele, F. Fiory, S. Santopietro, M. D. Castellone, F. Beguinot, M. Santoro, and P. Formisano. 2003. Protein kinase Calpha activation by RET: evidence for a negative feedback mechanism controlling RET tyrosine kinase. Oncogene 22:2942-2949. [DOI] [PubMed] [Google Scholar]
- 4.Arighi, E., L. Alberti, F. Torriti, S. Ghizzoni, M. G. Rizzetti, G. Pelicci, B. Pasini, I. Bongarzone, C. Piutti, M. A. Pierotti, and M. G. Borrello. 1997. Identification of Shc docking site on Ret tyrosine kinase. Oncogene 14:773-782. [DOI] [PubMed] [Google Scholar]
- 5.Asai, N., H. Murakami, T. Iwashita, and M. Takahashi. 1996. A mutation at tyrosine 1062 in MEN2A-Ret and MEN2B-Ret impairs their transforming activity and association with shc adaptor proteins. J. Biol. Chem. 271:17644-17649. [DOI] [PubMed] [Google Scholar]
- 6.Barlow, A., E. de Graaff, and V. Pachnis. 2003. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron 40:905-916. [DOI] [PubMed] [Google Scholar]
- 7.Besset, V., R. P. Scott, and C. F. Ibanez. 2000. Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J. Biol. Chem. 275:39159-39166. [DOI] [PubMed] [Google Scholar]
- 8.Bordeaux, M. C., C. Forcet, L. Granger, V. Corset, C. Bidaud, M. Billaud, D. E. Bredesen, P. Edery, and P. Mehlen. 2000. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 19:4056-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrello, M. G., L. Alberti, E. Arighi, I. Bongarzone, C. Battistini, A. Bardelli, B. Pasini, C. Piutti, M. G. Rizzetti, P. Mondellini, M. T. Radice, and M. A. Pierotti. 1996. The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase Cγ. Mol. Cell. Biol. 16:2151-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cacalano, G., I. Farinas, L. C. Wang, K. Hagler, A. Forgie, M. Moore, M. Armanini, H. Phillips, A. M. Ryan, L. F. Reichardt, M. Hynes, A. Davies, and A. Rosenthal. 1998. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 21:53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlomagno, F., G. De Vita, M. T. Berlingieri, V. de Franciscis, R. M. Melillo, V. Colantuoni, M. H. Kraus, P. P. Di Fiore, A. Fusco, and M. Santoro. 1996. Molecular heterogeneity of RET loss of function in Hirschsprung's disease. EMBO J. 15:2717-2725. [PMC free article] [PubMed] [Google Scholar]
- 12.Cosma, M. P., M. Cardone, F. Carlomagno, and V. Colantuoni. 1998. Mutations in the extracellular domain cause RET loss of function by a dominant negative mechanism. Mol. Cell. Biol. 18:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Graaff, E., S. Srinivas, C. Kilkenny, V. D'Agati, B. S. Mankoo, F. Costantini, and V. Pachnis. 2001. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 15:2433-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Vita, G., R. M. Melillo, F. Carlomagno, R. Visconti, M. D. Castellone, A. Bellacosa, M. Billaud, A. Fusco, P. N. Tsichlis, and M. Santoro. 2000. Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res. 60:3727-3731. [PubMed] [Google Scholar]
- 15.Durick, K., R. Y. Wu, G. N. Gill, and S. S. Taylor. 1996. Mitogenic signaling by Ret/ptc2 requires association with enigma via a LIM domain. J. Biol. Chem. 271:12691-12694. [DOI] [PubMed] [Google Scholar]
- 16.Edery, P., S. Lyonnet, L. M. Mulligan, A. Pelet, E. Dow, L. Abel, S. Holder, C. Nihoul-Fekete, B. A. Ponder, and A. Munnich. 1994. Mutations of the RET proto-oncogene in Hirschsprung's disease. Nature 367:378-380. [DOI] [PubMed] [Google Scholar]
- 17.Encinas, M., M. G. Tansey, B. A. Tsui-Pierchala, J. X. Comella, J. Milbrandt, and E. M. Johnson, Jr. 2001. c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J. Neurosci. 21:1464-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Focke, P. J., A. R. Swetlik, J. L. Schilz, and M. L. Epstein. 2003. GDNF and insulin cooperate to enhance the proliferation and differentiation of enteric crest-derived cells. J. Neurobiol. 55:151-164. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda, T., K. Kiuchi, and M. Takahashi. 2002. Novel mechanism of regulation of Rac activity and lamellipodia formation by RET tyrosine kinase. J. Biol. Chem. 277:19114-19121. [DOI] [PubMed] [Google Scholar]
- 20.Geneste, O., C. Bidaud, G. De Vita, R. M. Hofstra, S. Tartare-Deckert, C. H. Buys, G. M. Lenoir, M. Santoro, and M. Billaud. 1999. Two distinct mutations of the RET receptor causing Hirschsprung's disease impair the binding of signalling effectors to a multifunctional docking site. Hum. Mol. Genet. 8:1989-1999. [DOI] [PubMed] [Google Scholar]
- 21.Gianino, S., J. R. Grider, J. Cresswell, H. Enomoto, and R. O. Heuckeroth. 2003. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development 130:2187-2198. [DOI] [PubMed] [Google Scholar]
- 22.Grimm, J., M. Sachs, S. Britsch, S. Di Cesare, T. Schwarz-Romond, K. Alitalo, and W. Birchmeier. 2001. Novel p62dok family members, dok-4 and dok-5, are substrates of the c-Ret receptor tyrosine kinase and mediate neuronal differentiation. J. Cell Biol. 154:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi, H., M. Ichihara, T. Iwashita, H. Murakami, Y. Shimono, K. Kawai, K. Kurokawa, Y. Murakumo, T. Imai, H. Funahashi, A. Nakao, and M. Takahashi. 2000. Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene 19:4469-4475. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi, Y., T. Iwashita, H. Murakamai, Y. Kato, K. Kawai, K. Kurokawa, I. Tohnai, M. Ueda, and M. Takahashi. 2001. Activation of BMK1 via tyrosine 1062 in RET by GDNF and MEN2A mutation. Biochem. Biophys. Res. Commun. 281:682-689. [DOI] [PubMed] [Google Scholar]
- 25.Hearn, C. J., M. Murphy, and D. Newgreen. 1998. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev. Biol. 197:93-105. [DOI] [PubMed] [Google Scholar]
- 26.Heuckeroth, R. O., H. Enomoto, J. R. Grider, J. P. Golden, J. A. Hanke, A. Jackman, D. C. Molliver, M. E. Bardgett, W. D. Snider, E. M. Johnson, Jr., and J. Milbrandt. 1999. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 22:253-263. [DOI] [PubMed] [Google Scholar]
- 27.Heuckeroth, R. O., P. A. Lampe, E. M. Johnson, and J. Milbrandt. 1998. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev. Biol. 200:116-129. [DOI] [PubMed] [Google Scholar]
- 28.Ishiguro, Y., T. Iwashita, H. Murakami, N. Asai, K. Iida, H. Goto, T. Hayakawa, and M. Takahashi. 1999. The role of amino acids surrounding tyrosine 1062 in ret in specific binding of the shc phosphotyrosine-binding domain. Endocrinology 140:3992-3998. [DOI] [PubMed] [Google Scholar]
- 29.Ito, S., T. Iwashita, N. Asai, H. Murakami, Y. Iwata, G. Sobue, and M. Takahashi. 1997. Biological properties of Ret with cysteine mutations correlate with multiple endocrine neoplasia type 2A, familial medullary thyroid carcinoma, and Hirschsprung's disease phenotype. Cancer Res. 57:2870-2872. [PubMed] [Google Scholar]
- 30.Iwashita, T., G. M. Kruger, R. Pardal, M. J. Kiel, and S. J. Morrison. 2003. Hirschsprung disease is linked to defects in neural crest stem cell function. Science 301(Suppl. 32):972-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwashita, T., K. Kurokawa, S. Qiao, H. Murakami, N. Asai, K. Kawai, M. Hashimoto, T. Watanabe, M. Ichihara, and M. Takahashi. 2001. Functional analysis of RET with Hirschsprung mutations affecting its kinase domain. Gastroenterology 121(Suppl. 30):24-33. [DOI] [PubMed] [Google Scholar]
- 32.Iwashita, T., H. Murakami, N. Asai, and M. Takahashi. 1996. Mechanism of ret dysfunction by Hirschsprung mutations affecting its extracellular domain. Hum. Mol. Genet. 5:1577-1580. [DOI] [PubMed] [Google Scholar]
- 33.Kenny, S. E., R. M. Hofstra, C. H. Buys, C. R. Vaillant, D. A. Lloyd, and D. H. Edgar. 2000. Reduced endothelin-3 expression in sporadic Hirschsprung disease. Br. J. Surg. 87:580-585. [DOI] [PubMed] [Google Scholar]
- 34.Kissel, H., I. Timokhina, M. P. Hardy, G. Rothschild, Y. Tajima, V. Soares, M. Angeles, S. R. Whitlow, K. Manova, and P. Besmer. 2000. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 19:1312-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurokawa, K., T. Iwashita, H. Murakami, H. Hayashi, K. Kawai, and M. Takahashi. 2001. Identification of SNT/FRS2 docking site on RET receptor tyrosine kinase and its role for signal transduction. Oncogene 20:1929-1938. [DOI] [PubMed] [Google Scholar]
- 36.Lee, K. Y., E. T. Samy, M. H. Sham, P. K. Tam, and V. C. Lui. 2003. 3′ splicing variants of ret receptor tyrosine kinase are differentially expressed in mouse embryos and in adult mice. Biochim. Biophys. Acta 1627:26-38. [DOI] [PubMed] [Google Scholar]
- 37.Leibl, M. A., T. Ota, M. N. Woodward, S. E. Kenny, D. A. Lloyd, C. R. Vaillant, and D. H. Edgar. 1999. Expression of endothelin 3 by mesenchymal cells of embryonic mouse caecum. Gut 44:246-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenzo, M. J., G. D. Gish, C. Houghton, T. J. Stonehouse, T. Pawson, B. A. Ponder, and D. P. Smith. 1997. RET alternate splicing influences the interaction of activated RET with the SH2 and PTB domains of Shc, and the SH2 domain of Grb2. Oncogene 14:763-771. [DOI] [PubMed] [Google Scholar]
- 39.Maina, F., G. Pante, F. Helmbacher, R. Andres, A. Porthin, A. M. Davies, C. Ponzetto, and R. Klein. 2001. Coupling Met to specific pathways results in distinct developmental outcomes. Mol. Cell 7:1293-1306. [DOI] [PubMed] [Google Scholar]
- 40.Manie, S., M. Santoro, A. Fusco, and M. Billaud. 2001. The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet. 17:580-589. [DOI] [PubMed] [Google Scholar]
- 41.McCallion, A. S., and A. Chakravarti. 2001. EDNRB/EDN3 and Hirschsprung disease type II. Pigment Cell Res. 14(Suppl. 43):161-169. [DOI] [PubMed] [Google Scholar]
- 42.Melillo, R. M., F. Carlomagno, G. De Vita, P. Formisano, G. Vecchio, A. Fusco, M. Billaud, and M. Santoro. 2001. The insulin receptor substrate (IRS)-1 recruits phosphatidylinositol 3-kinase to Ret: evidence for a competiton between Shc and IRS-1 for the binding to Ret. Oncogene 20:209-218. [DOI] [PubMed] [Google Scholar]
- 43.Melillo, R. M., M. Santoro, S. H. Ong, M. Billaud, A. Fusco, Y. R. Hadari, J. Schlessinger, and I. Lax. 2001. Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol. Cell. Biol. 21:4177-4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore, M. W., R. D. Klein, I. Farinas, H. Sauer, M. Armanini, H. Phillips, L. F. Reichardt, A. M. Ryan, K. Carver-Moore, and A. Rosenthal. 1996. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382:76-79. [DOI] [PubMed] [Google Scholar]
- 45.Murakami, H., Y. Yamamura, Y. Shimono, K. Kawai, K. Kurokawa, and M. Takahashi. 2002. Role of Dok1 in cell signaling mediated by RET tyrosine kinase. J. Biol. Chem. 277:32781-33290. [DOI] [PubMed] [Google Scholar]
- 46.Natarajan, D., C. Marcos-Gutierrez, V. Pachnis, and E. de Graaff. 2002. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development 129:5151-5160. [DOI] [PubMed] [Google Scholar]
- 47.Nozaki, C., N. Asai, H. Murakami, T. Iwashita, Y. Iwata, K. Horibe, R. D. Klein, A. Rosenthal, and M. Takahashi. 1998. Calcium-dependent Ret activation by GDNF and neurturin. Oncogene 16:293-299. [DOI] [PubMed] [Google Scholar]
- 48.Ohiwa, M., H. Murakami, T. Iwashita, N. Asai, Y. Iwata, T. Imai, H. Funahashi, H. Takagi, and M. Takahashi. 1997. Characterization of Ret-Shc-Grb2 complex induced by GDNF, MEN 2A, and MEN 2B mutations. Biochem. Biophys. Res. Commun. 237:747-751. [DOI] [PubMed] [Google Scholar]
- 49.Pandey, A., H. Duan, P. P. Di Fiore, and V. M. Dixit. 1995. The Ret receptor protein tyrosine kinase associates with the SH2-containing adapter protein Grb10. J. Biol. Chem. 270:21461-21463. [DOI] [PubMed] [Google Scholar]
- 50.Pasini, B., M. G. Borrello, A. Greco, I. Bongarzone, Y. Luo, P. Mondellini, L. Alberti, C. Miranda, E. Arighi, R. Bocciardi, et al. 1995. Loss of function effect of RET mutations causing Hirschsprung disease. Nat. Genet. 10:35-40. [DOI] [PubMed] [Google Scholar]
- 51.Pelet, A., O. Geneste, P. Edery, A. Pasini, S. Chappuis, T. Atti, A. Munnich, G. Lenoir, S. Lyonnet, and M. Billaud. 1998. Various mechanisms cause RET-mediated signaling defects in Hirschsprung's disease. J. Clin. Investig. 101:1415-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pichel, J. G., L. Shen, H. Z. Sheng, A. C. Granholm, J. Drago, A. Grinberg, E. J. Lee, S. P. Huang, M. Saarma, B. J. Hoffer, H. Sariola, and H. Westphal. 1996. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382:73-76. [DOI] [PubMed] [Google Scholar]
- 53.Romeo, G., P. Ronchetto, Y. Luo, V. Barone, M. Seri, I. Ceccherini, B. Pasini, R. Bocciardi, M. Lerone, H. Kaariainen, et al. 1994. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung's disease. Nature 367:377-378. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez, M. P., I. Silos-Santiago, J. Frisen, B. He, S. A. Lira, and M. Barbacid. 1996. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382:70-73. [DOI] [PubMed] [Google Scholar]
- 55.Schuchardt, A., V. D'Agati, L. Larsson-Blomberg, F. Costantini, and V. Pachnis. 1994. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367:380-383. [DOI] [PubMed] [Google Scholar]
- 56.Schuchardt, A., V. D'Agati, V. Pachnis, and F. Costantini. 1996. Renal agenesis and hypodysplasia in ret-k- mutant mice result from defects in ureteric bud development. Development 122:1919-1929. [DOI] [PubMed] [Google Scholar]
- 57.Segouffin-Cariou, C., and M. Billaud. 2000. Transforming ability of MEN2A-RET requires activation of the phosphatidylinositol 3-kinase/AKT signaling pathway. J. Biol. Chem. 275:3568-3576. [DOI] [PubMed] [Google Scholar]
- 58.Sidebotham, E. L., M. N. Woodward, S. E. Kenny, D. A. Lloyd, C. R. Vaillant, and D. H. Edgar. 2002. Localization and endothelin-3 dependence of stem cells of the enteric nervous system in the embryonic colon. J. Pediatr. Surg. 37:145-150. [DOI] [PubMed] [Google Scholar]
- 59.Tahira, T., Y. Ishizaka, F. Itoh, T. Sugimura, and M. Nagao. 1990. Characterization of ret proto-oncogene mRNAs encoding two isoforms of the protein product in a human neuroblastoma cell line. Oncogene 5:97-102. [PubMed] [Google Scholar]
- 60.Takahashi, M. 2001. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 12:361-373. [DOI] [PubMed] [Google Scholar]
- 61.Tang, M. J., Y. Cai, S. J. Tsai, Y. K. Wang, and G. R. Dressler. 2002. Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev. Biol. 243:128-136. [DOI] [PubMed] [Google Scholar]
- 62.Taraviras, S., C. V. Marcos-Gutierrez, P. Durbec, H. Jani, M. Grigoriou, M. Sukumaran, L. C. Wang, M. Hynes, G. Raisman, and V. Pachnis. 1999. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development 126:2785-2797. [DOI] [PubMed] [Google Scholar]
- 63.Woodward, M. N., E. L. Sidebotham, M. G. Connell, S. E. Kenny, C. R. Vaillant, D. A. Lloyd, and D. H. Edgar. 2003. Analysis of the effects of endothelin-3 on the development of neural crest cells in the embryonic mouse gut. J. Pediatr. Surg. 38:1322-1328. [DOI] [PubMed] [Google Scholar]
- 64.Worley, D. S., J. M. Pisano, E. D. Choi, L. Walus, C. A. Hession, R. L. Cate, M. Sanicola, and S. J. Birren. 2000. Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Develop-ment 127:4383-4393. [DOI] [PubMed] [Google Scholar]
- 65.Young, H. M., C. J. Hearn, P. G. Farlie, A. J. Canty, P. Q. Thomas, and D. F. Newgreen. 2001. GDNF is a chemoattractant for enteric neural cells. Dev. Biol. 229:503-516. [DOI] [PubMed] [Google Scholar]
- 66.Young, H. M., and D. Newgreen. 2001. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat. Rec. 262:1-15. [DOI] [PubMed] [Google Scholar]