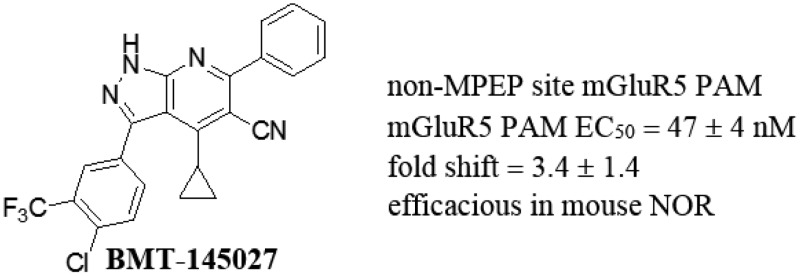
Abstract
The metabotropic glutamate receptor 5 (mGluR5) is an attractive target for the treatment of schizophrenia due to its role in regulating glutamatergic signaling in association with the N-methyl-d-aspartate receptor (NMDAR). We describe the synthesis of 1H-pyrazolo[3,4-b]pyridines and their utility as mGluR5 positive allosteric modulators (PAMs) without inherent agonist activity. A facile and convergent synthetic route provided access to a structurally diverse set of analogues that contain neither the aryl-acetylene-aryl nor aryl-methyleneoxy-aryl elements, the predominant structural motifs described in the literature. Binding studies suggest that members of our new chemotype do not engage the receptor at the MPEP and CPPHA mGluR5 allosteric sites. SAR studies culminated in the first non-MPEP site PAM, 1H-pyrazolo[3,4-b]pyridine 31 (BMT-145027), to improve cognition in a preclinical rodent model of learning and memory.
Keywords: mGluR5, positive allosteric modulator, schizophrenia, novel object recognition, glutamate
Schizophrenia is a debilitating psychiatric disorder and afflicts approximately 1% of the general population. Schizophrenia-related health care costs are measured in the tens of billions of dollars in the US market alone. More importantly, the chronic nature of schizophrenia leads to a 2–3-fold increased rate of all-cause mortality, which translates into a 10 year decrease in life expectancy for those suffering from this disease.1 Symptoms of schizophrenia are characterized as positive, negative, or cognitive in nature.2 Currently available antipsychotics can successfully treat positive symptoms; however, negative and cognitive symptoms remain areas of significant unmet medical need. Treatment is further complicated by poor patient compliance due to extrapyramidal and metabolic side effects associated with existing medications.
Metabotropic glutamate receptor 5 (mGluR5) is a member of the mGluR family of G-protein-coupled receptors that contains a large extracellular glutamate-binding domain.3,4 When mGluR5 binds to endogenous glutamate, enhanced N-methyl-d-aspartate receptor (NMDAR) function occurs with neuronal signal transduction.5 Antagonism of NMDAR with ketamine or phencyclidine recapitulates all three symptomatic domains associated with schizophrenia and suggests NMDAR hypofunction may contribute to the disease.6 Direct activation of NMDAR generates excitotoxic increases in levels of neuronal calcium and subsequent neuronal death. Allosteric mGluR5 activation could provide a potentially advantageous approach to modulation of mGluR5 because NMDAR function is only enhanced in the presence of an mGluR5 positive allosteric modulator (PAM) and an orthosteric agonist.7,8 Such activation should maintain both spatial and temporal components of glutamate signaling.
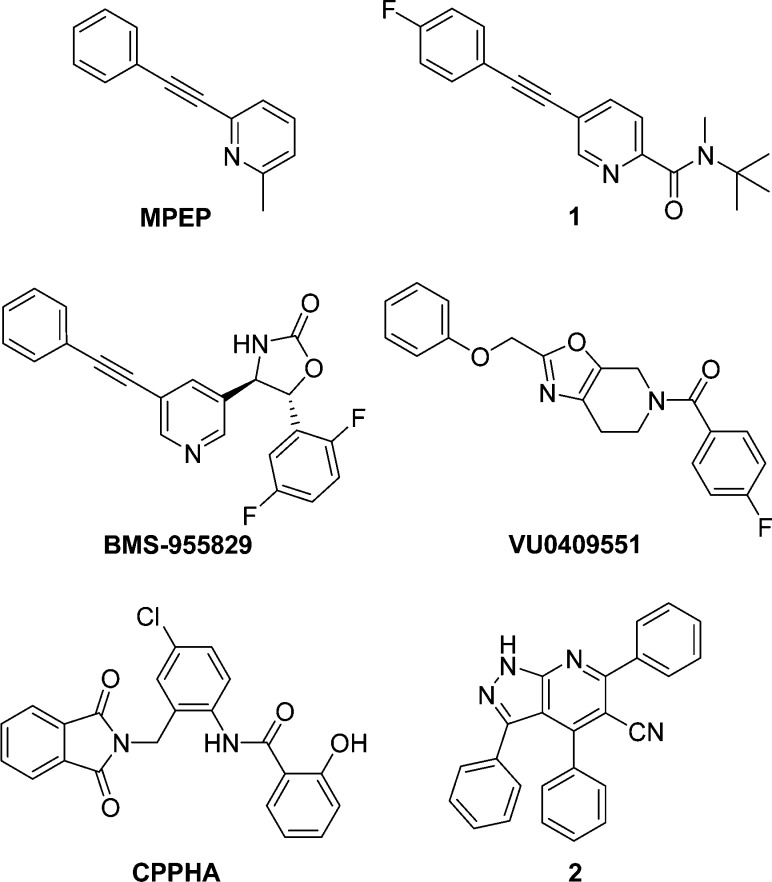
Most reported mGluR5 allosteric modulators contain an aryl-acetylene-aryl element, exemplified by 1 and MPEP, the negative allosteric modulator (NAM) for which the first and most-studied mGluR5 allosteric binding site is named,9,10 or the aryl-methyleneoxy-aryl motif found in the positive allosteric modulator VU0409551 (Figure 1).12 Our initial development candidate, oxazolidinone BMS-955829, contained the former motif and provided a low fold shift (2.3) without agonism.14 This compound progressed into a one month pre-IND toxicology study, during which it demonstrated drug-induced liver injury at all doses studied (3–30 mg/kg QD). While biotransformation studies failed to demonstrate involvement of the acetylene moiety, this functionality has been repeatedly linked with CYP-mediated bioactivation in vivo.15 In an effort to discover a new, structurally divergent chemotype, we performed a functional high-throughput screen (HTS) of more than 1.1 million compounds in the presence of an EC10 of glutamate. This effort identified 1H-pyrazolo[3,4-b]pyridine 2 (Figure 1), which represented a novel mGluR5 chemotype with promising submicromolar PAM activity. Importantly, this compound was a pure PAM, devoid of inherent mGluR5 agonist activity. We quickly generated functional SAR aimed at the identification of a suitable analogue to progress into the novel object recognition (NOR) cognition assay. Herein, we report these data in addition to studies designed to elucidate the allosteric site to which this chemotype binds.
Figure 1.
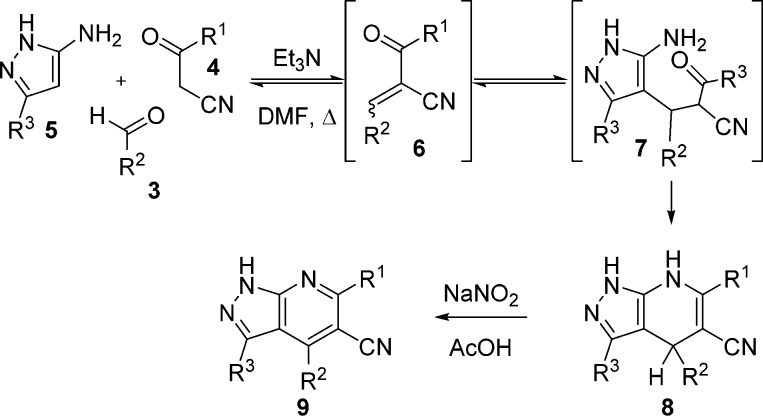
1H-Pyrazolo[3,4-b]pyridines were prepared using several methods, but the most practical procedure proved to be a two-step, one-pot dehydrative cyclization process developed for this program (Scheme 1).16,17 In a typical reaction, components 3, 4, and 5 were heated together with triethylamine in DMF to afford 4,7-dihydro-1H-pyrazolo[3,4-b]pyridine 8. Presumably, this sequence progressed first through a Knoevenagel condensation between aldehyde 3 and 3-oxopropanenitrile 4 to form an acrylonitrile intermediate 6. Subsequent Michael addition of 1H-pyrazol-5-amine 5 to the acrylonitrile followed by dehydrative cyclization completed the molecular framework. Removal of volatiles in situ, followed by oxidation with sodium nitrite in acetic acid provided final compound 9. Notably, this procedure was used to synthesize more than one gram of our HTS hit 2 in 80% yield.
Scheme 1. Two-Step, One-Pot Synthetic Route to 1H-Pyrazolo[3,4-b]pyridines 9.
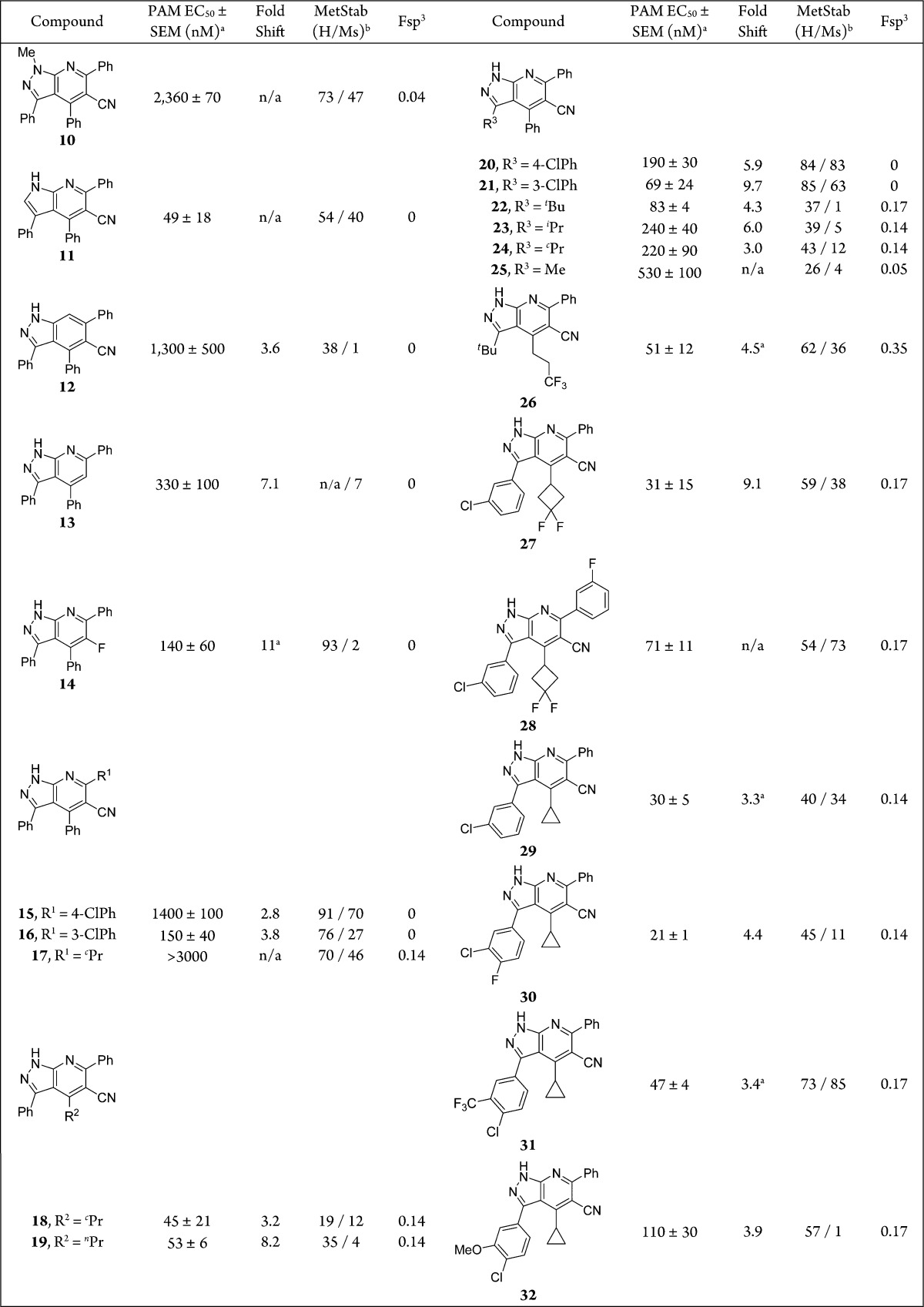
Our primary program goals included increasing the potency and mouse (Ms) liver microsomal (LM) stability relative to HTS hit 2 in order to facilitate in vivo efficacy studies. In parallel with this effort, we sought opportunities to reduce planarity (measured as fraction of sp3-hybridized carbons, Fsp3) of the series using alkyl replacements for aryl substituents (R1, R2, and R3; Scheme 1).18 We targeted compounds with mGluR5 EC50s under 50 nM (Table 1) as measured in a Ca2+-based FLIPR (Fluorescence Image Plate Reader) assay.17 HTS hit 2 demonstrated a PAM EC50 of 120 nM and moderate human (H) LM and poor MsLM stability (75% and 17% remaining, respectively). N-Methylation (10) led to a 19-fold loss in potency, which suggested the free pyrazole −NH– might participate in a hydrogen-bond interaction with the allosteric pocket. In an effort to define the minimum pharmacophore, core nitrogens were deleted. Removal of N2 gave 7-azaindole 11, which was about twice as potent as HTS hit 2 but with reduced LM stability. Elimination of N7 (indazole 12) led to a 10-fold loss in potency. This suggested that N7 might engage the binding pocket through a hydrogen bond. Omitting the C5 nitrile (13) or replacing it with substituents such as fluorine (14) offered no advantage.
Table 1. mGluR5 PAM Potency, Fold Shift, Metabolic Stability, and Fsp3 Data for Selected 1H-Pyrazolo[3,4-b]pyridines.
Values represent mean of n ≥ 2 replicates.
Percentage remaining after 10 min incubation with human or mouse liver microsomes.17
Changes to R1 in 9 demonstrated very dramatic mGluR5 PAM SAR. Aryl elaboration (e.g., 4-chlorophenyl 15) reduced potency while alkyl substitution (e.g., cPr 17) eliminated PAM activity all together, but LM data suggested this group could be used to affect MsLM stability (Table 1). Simple R2 alkyl groups (e.g., analogues 18 and 19) provided a potency advantage over aryl substituents, a finding that was critical to our goal of reducing planarity (18 and 19, Fsp3 = 0.14); however, this came at the cost of LM stability. Importantly, we found that R3 aryl modifications could be used to modulate oxidative stability and offered potential to offset the loss associated with R2 alkyl moieties. Specifically, 4-chloro- (20) and 3-chloro-analogues (21) provided a significant MsLM stability advantage compared to unsubstituted phenyl analogue 2. Moreover, these examples demonstrated the effect of substitution pattern on potency. R3 alkyl groups were also tolerated (examples 22–25), although potency and Fsp3 appeared inversely related.
Combined, these SAR lessons led to compounds 26–32 with potencies within our desired range; however, the identification of very potent analogues with high levels of LM stability was still challenging. Metabolite identification studies conducted on 29 revealed extensive oxidation on R1 and R3 phenyl groups (data not shown), consistent with the finding of increased LM stability upon substitution of these moieties.17 Various aryl substitutions (R3) were used to tune LM stability. This exercise afforded 1H-pyrazolo[3,4-b]pyridine 31 (BMT-145027), a compound with high MsLM stability (85% remaining), acceptable potency (EC50 = 47 nM), and a modest decrease in planarity (Fsp3 = 0.17). Importantly, 31 lacked inherent mGluR5 agonist activity when tested at concentrations up to 16 μM.17,19
PAMs function by increasing the affinity and/or functional efficacy of an endogenous ligand (e.g., glutamate) for the orthosteric binding site, a process that affords a leftward shift of the ligand concentration–response curve. Most reported highly potent mGluR5 PAMs provide concentration-dependent fold shift values ≥7;20 however, in the context of the oxazolidinone chemotype (e.g., BMS-955829), we found that compounds with low glutamate fold shift were devoid of mechanism-based convulsions,14 a phenomenon we observed with higher fold shift PAMs.21,22 Ideally, we wanted a 1H-pyrazolo[3,4-b]pyridine with a low glutamate fold shift, a goal that was achieved when R2 = cPr (e.g., analogue 31 fold shift = 3.4, Table 1).
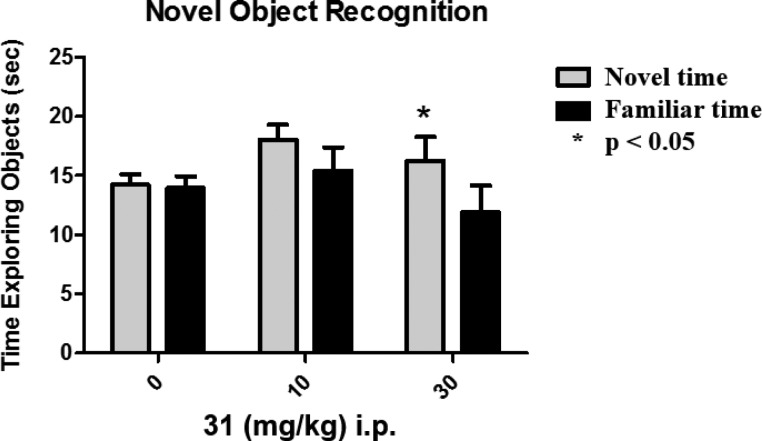
1H-Pyrazolo[3,4-b]pyridine 31 was chosen to advance into a mouse NOR behavioral study.17 This model measures visual recognition memory, which is an impaired cognitive domain in schizophrenia patients.23,24 In this model, drug-treated and control mice are shown two identical objects. After a 24-h natural forgetting period, the mice were reintroduced to a familiar object while simultaneously presented with a novel object. Since mice spend more time exploring unfamiliar objects, improved memory was measured as time spent exploring the novel object. 1H-Pyrazolo[3,4-b]pyridine 31 led to a significant (p < 0.05) increase in time spent with the novel object when dosed at 30 mg/kg, with an apparent trend in novel object preference at 10 mg/kg. Satellite animals indicated a total plasma concentration = 2800 nM at 30 mg/kg (B/P = 0.5, Figure 2).25
Figure 2.
Pretreatment with 30 mg/kg 1H-pyrazolo[3,4-b]pyridine 31 60 min prior to training significantly increased the time spent exploring the novel object compared to the familiar object when tested 24 h later.
We evaluated representative analogues in a competition-binding assay using a known mGluR5 ligand, 3H-MPEPy, which binds to the MPEP allosteric modulator site on the receptor.9 Data presented in Table 2 show that compounds from the 1H-pyrazolo[3,4-b]pyridine series (9) displaced MPEPy with nanomolar potency in membranes prepared from both HEK cells expressing mGluR5 and primary rat astrocytes, indicating that the overexpressing system mimicked endogenously expressed mGlu5 receptor binding.17,26 Interestingly, the displacement of MPEPy was partial and only reached about 50% for all 1H-pyrazolo[3,4-b]pyridines tested. This observation suggested that this PAM chemotype did not bind at the MPEP binding site and instead disrupted MPEPy binding through an allosteric interaction with a non-MPEP binding site. These results were consistent with those described for several non-MPEP site PAMs including CPPHA.27 To confirm this, we performed a dissociation binding assay, which indicated that both 21 (Koff = 114% of control) and 28 (Koff = 116% of control) increased the dissociation rate of 3H-MPEPy relative to the known MPEP site ligand (p < 0.05), BMS-955829 (Koff = 100% of control), using HEK cell membrane expressing mGluR5.17 The altered dissociation rates of 21 and 28 unequivocally demonstrated binding to a non-MPEP site28 Additionally, compound 21 was inactive up to 10 μM in PAM mode against group I receptor, mGluR1, and group II receptors up to 30 μM.29
Table 2. Competition Binding Studies That Demonstrated Partial MPEPy Displacement.
| HEK-mGluR5a |
primary astrocytesa |
|||
|---|---|---|---|---|
| compd | IC50 ± SEM (nM) | Ymax ± SEM (%) | IC50 ± SEM (nM) | Ymax ± SEM (%) |
| BMS-955829 | 3.0 ± 0.6 | 99 ± 0.3 | 2.0 ± 0.1 | 100 ± 2 |
| CPPHA | >10,000b | <20 | 930 ± 40 | 63 ± 9 |
| 28 | 87b | 44 | 19 ± 2 | 63 ± 4 |
| 29 | 66 ± 26 | 45 ± 5 | 27 ± 6 | 52 ± 3 |
| 32 | 77b | 60 | 240 ± 110 | 65 ± 2 |
Values represent mean of n ≥ 2 replicates.
Single run.
To further understand how this chemotype (9) binds to the mGlu5 receptor, we screened compounds in a functional assay that measured potentiated glutamate-induced intracellular calcium increases using HEK cells that expressed mGluR5 with mutations in the MPEP and CPPHA30 allosteric binding sites. Binding results demonstrated that mutation of either site failed to impact PAM activity of our 1H-pyrazolo[3,4-b]pyridines (16, 20, and 24) relative to wild type (Table 3),17 suggesting that the 1H-pyrazolo[3,4-b]pyridine chemotype (9) did not bind at either the MPEP or CPPHA sites. As expected, BMS-955829 and MPEP are inactive in the mutant MPEP site cells, whereas both compounds retain efficacy in the CPPHA mutant cells. Conversely, CPPHA retains efficacy in the mutated MPEP site cells, but not the mutated CPPHA site cells (Table 3).
Table 3. Impact of Point Mutations on FLIPR Activity.
| mutant/wild type EC50 ratio |
||
|---|---|---|
| compd | MPEP mutant | CPPHA mutant |
| BMS-955829 | >10 | 1.1 |
| MPEP | >10 | 0.5 |
| CPPHA | 1.0 | 7.2 |
| 16 | 1.4 | n.d. |
| 20 | 0.66 | 1.9 |
| 24 | 0.73 | 1.4 |
In summary, a structurally distinct and potent 1H-pyrazolo[3,4-b]pyridine-based series (9) of mGluR5 PAMs was developed for the potential treatment of schizophrenia. Analogues were prepared using a convergent synthesis that provided significant structural diversity and permitted rapid SAR investigations, which focused on allosteric potency and microsomal stability. 1H-Pyrazolo[3,4-b]pyridine 31 emerged as a low fold-shift and potent mGluR5 PAM with no inherent mGluR5 agonist activity. This compound advanced into the NOR behavioral model in which it demonstrated an improvement in memory retention. Data from parallel binding studies suggests that members of this chemotype do not engage the receptor at the MPEP and CPPHA allosteric sites. To the best of our knowledge, 31 is the first example of a non-MPEP site PAM to demonstrate in vivo efficacy.31 Ultimately, our company’s strategic shift away from neuroscience research prevented further advancement of this promising series.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.6b00292.
Experimental procedures and characterization data for key compounds, and details of in vitro and in vivo assays (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Saha S.; McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time?. Arch. Gen. Psychiatry 2007, 64, 1123–1131. 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Van Os J.; Kapur S. Schizophrenia. Lancet 2009, 374, 635–645. 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- Conn P. J.; Pin J.-P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 205–237. 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Vanejevs M.; Jatzke C.; Renner S.; Müller S.; Hechenberger M.; Bauer T.; Klochkova A.; Pyatkin I.; Kazyulkin D.; Aksenova E.; Shulepin S.; Timonina O.; Haasis A.; Gutcaits A.; Parsons C. G.; Kauss V.; Weill T. Positive and Negative Modulation of Group I Metabotropic Glutamate Receptors. J. Med. Chem. 2008, 51, 634–647. 10.1021/jm0611298. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L.; Bregestovski P.; Ben-Ari Y. Selective activation of quisqualate metabotropic receptor potentiates NMDA but not AMPA responses. Eur. J. Pharmacol. 1991, 205, 327–328. 10.1016/0014-2999(91)90921-C. [DOI] [PubMed] [Google Scholar]
- Coyle J. T. NMDA Receptor and Schizophrenia: A Brief History. Schizophrenia Bull. 2012, 38, 920–926. 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell K. A. Metabotropic glutamate receptor 5 in schizophrenia: emerging evidence for the development of antipsychotic drugs. Future Med. Chem. 2013, 5, 1471–1474. 10.4155/fmc.13.137. [DOI] [PubMed] [Google Scholar]
- Matosin N.; Newell K. A. Metabotropic glutamate receptor 5 in the pathology and treatment of schizophrenia. Neurosci. Biobehav. Rev. 2013, 37, 256–268. 10.1016/j.neubiorev.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Gasparini F.; Lingenhohl K.; Stoehr N.; Flor P. J.; Heirich M.; Vranesic I.; Biollaz M.; Allgeier H.; Heckendorn R.; Uwyler S.; Varney M. A.; Johnson E. C.; Hess S. D.; Rao S. P.; Sacaan A. I.; Santori E. M.; Velicelebi G.; Kuhn R. 2-Methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology 1999, 38, 1493–1503. 10.1016/S0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Malherbe P.; Kratochwil N.; Zenner M. T.; Piussi J.; Diener C.; Kratzeisen C.; Fischer C.; Porter R. H. Mutational Analysis and Molecular Modeling of the Binding Pocket of the Metabotropic Glutamate 5 Receptor Negative Modulator 2-Methyl-6-(phenylethynyl)-pyridine. Mol. Pharmacol. 2003, 64, 823–832. 10.1124/mol.64.4.823. [DOI] [PubMed] [Google Scholar]
- Compound 1:Jaeschke G.; Jolidon S.; Lindemann L.; Ricci A.; Rueher D.; Stadler H.; Vieira E.. (Hoffmann-La Roche Inc., USA). Preparation of phenyl or pyridinyl-ethynyl derivatives. US Patent 8,772,300. July 8, 2014.
- VU0409551:Conde-Ceide S.; Martínez-Viturro C. M.; Alcázar J.; Garcia-Barrantes P. M.; Lavreysen H.; Mackie C.; Vinson P. N.; Rook J. M.; Bridges T. M.; Daniels J. S.; Megens A.; Langlois X.; Drinkenburg W. H.; Ahnaou A.; Niswender A. M.; Jones C. K.; Macdonald G. J.; Steckler T.; Conn P. J.; Stauffer S. R.; Bartolomé-Nebreda J. M.; Lindsley C. W. Discovery of VU0409551/JNJ-46778212: An mGlu5 Positive Allosteric Modulator Clinical Candidate Targeting Schizophrenia. ACS Med. Chem. Lett. 2015, 6, 716–720. 10.1021/acsmedchemlett.5b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CPPHA:O’Brien J. A.; Lemaire W.; Wittmann M.; Jacobson M. A.; Ha S. N.; Wisnoski D. D.; Lindsley C. W.; Schaffhauser H. J.; Sur C.; Duggan M. E.; Pettibone D. J.; Conn J.; Williams D. L. A novel selective allosteric modulator potentiates the activity of the native metabotropic glutamate receptor subtype 5 (mGluR5) in rat forebrain. J. Pharmacol. Exp. Ther. 2004, 309, 568–577. 10.1124/jpet.103.061747. [DOI] [PubMed] [Google Scholar]
- Yang F.; Snyder L. B.; Balakrishnan A.; Brown J. M.; Sivarao D. V.; Easton A.; Fernandes A.; Gulianello M.; Hanumegowda U. M.; Huang H.; Huang Y.; Jones K. M.; Li Y.-W.; Matchett M.; Mattson G.; Miller R.; Santone K. S.; Senapati A.; Shields E. E.; Simutis F. J.; Westphal R.; Whiterock V. J.; Bronson J. J.; Macor J. E.; Degnan A. P. Discovery and Preclinical Evaluation of BMS-955829, A Potent Positive Allosteric Modulator of mGluR5. ACS Med. Chem. Lett. 2016, 7, 289–293. 10.1021/acsmedchemlett.5b00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. F. Designing drugs to avoid toxicity. Prog. Med. Chem. 2011, 50, 1–47. 10.1016/B978-0-12-381290-2.00001-X. [DOI] [PubMed] [Google Scholar]
- Hill M. D. A Multicomponent Approach to Highly Substituted 1H-Pyrazolo[3,4-b]pyridines. Synthesis 2016, 48, 2201–2204. 10.1055/s-0035-1562230. [DOI] [Google Scholar]
- For experimental details, please see Supporting Information.
- Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- This chemotype lacked inherent mGluR5 agonist activity up to 16 μM.
- Zhao Z.; Wisnoski D. D.; O’Brien J. A.; Lemaire W.; Williams D. L. Jr.; Jacobson M. A.; Wittman M.; Ha S. N.; Schaffhauser H.; Sur C.; Pettibone D. J.; Duggan M. E.; Conn P. J.; Hartman G. D.; Lindsley C. W. Challenges in the development of mGluR5 positive allosteric modulators: The discovery of CPPHA. Bioorg. Med. Chem. Lett. 2007, 17, 1386–1391. 10.1016/j.bmcl.2006.11.081. [DOI] [PubMed] [Google Scholar]
- Rook J. M.; Noetzel M. J.; Pouliot W. A.; Bridges T. M.; Vinson P. N.; Cho H. P.; Zhou Y.; Gogliotti R. D.; Manka J. T.; Gregory K. J.; Stauffer S. R.; Dudek F. E.; Xiang Z.; Niswender C. M.; Daniels J. S.; Jones C. K.; Lindsley C. W.; Conn P. J. Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol. Psychiatry 2013, 73, 501–509. 10.1016/j.biopsych.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier-Batteur S.; Hutson P. H.; Menzel K.; Uslaner J. M.; Mattson B. A.; O’Brien J. A.; Magliaro B. C.; Forest T.; Stump C. A.; Tynebor R. M.; Anthony N. J.; Tucker T. J.; Zhang X.-F.; Gomez R.; Huszar S. L.; Lambeng N.; Fauré H.; Le Poul E.; Poli S.; Rosahl T. W.; Rocher J.-P.; Hargreaves R.; Williams T. M. Mechanism based neurotoxicity of mGlu5 positive allosteric modulators – Development challenges for a promising novel antipsychotic target. Neuropharmacology 2014, 82, 161–173. 10.1016/j.neuropharm.2012.12.003. [DOI] [PubMed] [Google Scholar]
- McGuire K. A.; Blahnik M. M.; Sponheim S. R. Discrimination within Recognition Memory in Schizophrenia. Behav. Sci. 2013, 3, 273–297. 10.3390/bs3020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiasen J. R.; DiCamillo A. Novel Object Recognition in the Rat: A Facile Assay for Cognitive Function. Curr. Protoc. Pharmacol. 2010, 5, 1–15. 10.1002/0471141755.ph0559s49. [DOI] [PubMed] [Google Scholar]
- No adverse events were observed at either dose.
- Binding studies were performed prior to the NOR study with 31, so its significance to the program was as of yet unknown.
- Gregory K. J.; Conn P. J. Molecular insights into metabotropic glutamate receptor allosteric modulation. Mol. Pharmacol. 2015, 88, 188–202. 10.1124/mol.114.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostenis E.; Mohr K. Two-point kinetic experiments to quantify allosteric effects on radioligand dissociation. Trends Pharmacol. Sci. 1996, 8, 280–283. 10.1016/0165-6147(96)10034-1. [DOI] [PubMed] [Google Scholar]
- All compounds tested lacked mGluR1,2,3 PAM activity. The series was not screened against other mGlu receptors.
- Noetzel M. J.; Gregory K. J.; Vinson P. N.; Manka J. T.; Stauffer S. R.; Lindsley C. W.; Niswender C. M.; Xiang Z.; Conn P. J. A Novel Metabotropic Glutamate Receptor 5 Positive Allosteric Modulator Acts at a Unique Site and Confers Stimulas Bias to mGlu5 Signaling. Mol. Pharmacol. 2013, 83, 835–847. 10.1124/mol.112.082891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A. L.; Zhou Y.; Williams R.; Weaver C. D.; Vinson P. N.; Dawson E. S.; Steckler T.; Lavreysen H.; Mackieg C.; Bartolomé J. M.; Macdonald G. J.; Daniels J. S.; Niswender C. M.; Jones C. K.; Conn P. J.; Lindsley C. W.; Stauffer S. R. Discovery and SAR of a novel series of non-MPEP site mGlu5 PAMs based on an aryl glycine sulfonamide scaffold. Bioorg. Med. Chem. Lett. 2012, 22, 7388–7392. 10.1016/j.bmcl.2012.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.