Abstract
A recently developed proteomics strategy, designated tagging-via-substrate (TAS) approach, is described for the detection and proteomic analysis of farnesylated proteins. TAS technology involves metabolic incorporation of a synthetic azido-farnesyl analog and chemoselective derivatization of azido-farnesyl-modified proteins by an elegant version of Staudinger reaction, pioneered by the Bertozzi group, using a biotinylated phosphine capture reagent. The resulting protein conjugates can be specifically detected and/or affinity-purified by streptavidin-linked horseradish peroxidase or agarose beads, respectively. Thus, the technology enables global profiling of farnesylated proteins by enriching farnesylated proteins and reducing the complexity of farnesylation subproteome. Azido-farnesylated proteins maintain the properties of protein farnesylation, including promoting membrane association, Ras-dependent mitogen-activated protein kinase kinase activation, and inhibition of lovastatin-induced apoptosis. A proteomic analysis of farnesylated proteins by TAS technology revealed 18 farnesylated proteins, including those with potentially novel farnesylation motifs, suggesting that future use of this method is likely to yield novel insight into protein farnesylation. TAS technology can be extended to other posttranslational modifications, such as geranylgeranylation and myristoylation, thus providing powerful tools for detection, quantification, and proteomic analysis of posttranslationally modified proteins.
More than 200 known posttranslational modifications have been reported (1), and yet there is no efficient and convenient method for detection, quantification, and proteomic analysis of most of these modifications. Because molecular biological technologies and genetic methods are not directly applicable to the study of posttranslational modifications, there is a compelling need to develop biochemical or chemical methods to characterize the functions of such modifications.
Protein farnesylation is a posttranslational modification involving the covalent attachment of a 15-carbon farnesyl isoprenoid through a thioether bond to a cysteine residue near the C terminus of proteins in a conserved farnesylation motif designated the “CAAX box,” where “C” is a cysteine residue, “A” as an aliphatic residue, and “X” is usually serine, methionine, glutamine, alanine, or threonine (2–5). Previous studies have identified a number of farnesylated proteins including nuclear lamins, the γ subunit of heterotrimeric G proteins such as transducin, and the Ras superfamily G proteins (6), and enzymes such as some protein tyrosine phosphatases, inositol polyphosphate phosphatases, and phospholipase A2 (6–8). Clearly, many other farnesylated proteins are yet to be identified, because genome sequences predict the presence of a variety of proteins ending with the CAAX motif. However, not all proteins containing a CAAX motif are farnesylated. A proteomics approach is sorely needed for not only identifying farnesylated proteins but also examining changes in modification caused by farnesyltransferase inhibitors currently under clinical trials as anticancer agents (6, 8). Unfortunately, extant proteomics methods are unable to routinely identify farnesylated proteins because of low-to-medium abundance of the proteins and limited dynamic range of the methods.
Here we report a recently developed approach, designated tagging-via-substrate (TAS) technology, for the detection and enrichment of farnesylated proteins based on metabolic incorporation of a synthetic azido-farnesyl analog and chemoselective conjugation between azide-farnesyl (F-azide)-modified proteins and a biotinylated phosphine capture reagent (bPPCR). Affinity purification and proteomic analysis of the conjugated proteins led to the identification of 18 farnesylated proteins. The concept of TAS technology can be extended to other protein modifications as long as cellular enzymatic pathways are flexible to the addition of an azide. Thus, the TAS technology would provide a highly effective approach for the detection and proteomic analysis of a variety of posttranslationally modified proteins, solving a long-standing problem toward molecular characterization of these protein modifications.
Experimental Procedures
Materials. The reagents used in this work include Bio-Rad DC protein assay kit; BSA and DTT from Fisher Scientific; FTI-277 from Calbiochem; Immobilon transfer membrane [poly(vinylidene difluoride)] from Millipore (Bradford, MA); immunoPure d-biotin from Pierce; lovastatin, trans,transfarnesol, and all-trans-geranylgeraniol from Sigma; DMEM, FBS, trypsin, and penicillin/streptomycin from Life Technologies (Gaithersburg, MD); protease inhibitor mixture from Roche Molecular Biochemicals; streptavidin–horseradish peroxidase (HRP) from Amersham Pharmacia; and Western Lightning Chemiluminescence Reagent Plus from Perkin–Elmer. Recombinant farnesyltransferase was expressed and purified as described (9).
Antibodies used in this work included anti-Ras antibody from Upstate Biotechnology (Lake Placid, NY); HRP-conjugated anti-mouse IgG from Sigma; anti-Hdj-2 antibody from NeoMarkers (Fremont, CA); and anti-Rap1 antibody and anti-goat IgG from Santa Cruz Biotechnology.
Chemical Synthesis. Detailed information about the synthesis of substrates for protein farnesylation and bPPCR is found in Supporting Text and Fig. 6, which are published as supporting information on the PNAS web site.
Labeling Proteins With Azido-Farnesyl Substrates. One dish of COS-1 cells (40–50% confluence) was grown in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, and labeling compounds including lovastatin (25 μM), GG-OH (20 μM), and an azido-farnesyl substrate [either azido farnesyl diphosphate (FPP-azide) or azido farnesyl alcohol (F-azide-OH), 20 μM]. The cells were allowed to grow for 24 h to reach 80–90% confluence. The cells were washed with cold Dulbecco's PBS (Sigma) twice, and 400 μl of PBS solution (0.1 M Na2HPO4, pH 7.2/0.15 M NaCl) containing 2% SDS was added. The cell lysate was harvested and sonicated three times for 10 s each with 30-s intervals between sonications. The lysate was centrifuged at 100,000 × g for 20 min. The pellet was discarded, and the supernatant was precipitated by using trichloroacetic acid/acetone.
Gel-Mobility Shift Assay for Farnesylated Proteins. A dish (15 cm) of cells (70% confluence) were harvested and lysed in 0.5 ml of 1× SDS sample buffer (2% SDS/62.5 mM Tris·HCl, pH 6.8). The protein lysate was resolved in an 8% or 12% SDS/polyacrylamide gel and transferred to a poly(vinylidine difluoride) membrane. The membrane was washed with TBST buffer (0.1% Tween 20/150 mM NaCl/25 mM Tris·HCl, pH 7.5), blocked for 2 h with a solution containing 5% (wt/vol) dried nonfat milk in TBST, and immunoblotted for 2 h with antibody in blocking buffer (5% dried nonfat milk in TBST). After washing four times with TBST with changes every 15 min, the membrane was incubated with HRP-conjugated secondary antibody in TBST (1:5,000 dilution) containing 5% dried nonfat milk. The membrane was washed again four times in TBST and visualized by enhanced chemiluminescence.
Detection and Affinity Purification of Azido-Farnesylated Proteins. The protein pellet obtained above was resolubilized in PBS solution containing 2% SDS and then conjugated with 1 mM bPPCR (compound 10) for 10 h at room temperature. The unreacted capture reagent was removed by trichloroacetic acid/acetone precipitation. To detect the biotinylated, azido-farnesyl-modified proteins, the protein pellet was resolubilized in 1× SDS sample buffer and subjected to SDS/PAGE separation. The biotinylated azido-farnesyl-modified proteins were detected by Western blotting analysis using HRP-conjugated streptavidin. To isolate the biotinylated azido-farnesyl-modified proteins, the protein pellet was resolubilized in PBS/0.1% SDS and incubated with streptavidin beads at 4°C for 2 h with shaking. The supernatant was removed, and the beads were washed with 2% SDS/PBS three times, 8.0 M urea three times, and 50 mM NH4HCO3 (pH 8.0) three times. The proteins bound to the beads were then digested in 50 mM NH4HCO3 (pH 8.0) with sequencing-grade trypsin overnight.
Protein Identification by Nano-HPLC/MS. The tryptic peptides were subjected to nano-HPLC/MS analysis for protein identification as described (10). The tandem MS (MS/MS) data were used to identify protein candidates in the NCBI nonredundant protein sequence database with the mascot search engine (Matrix Science, London). The procedures for protein identification using mascot searches and manual verification of protein hits are described in detail in Supporting Text.
The detailed experimental procedures for subcellular fractionation of soluble and membrane fractions and caspase activity assay are also described in Supporting Text.
Results
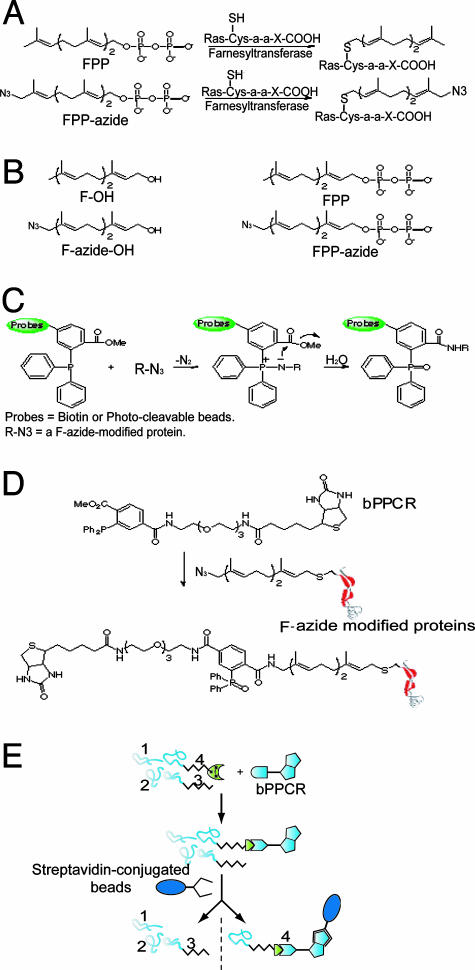
Overview of TAS Technology for the Detection and Affinity Purification of Farnesylated Proteins. TAS technology consists of three steps. First, farnesylated proteins are metabolically labeled by feeding cells with azido-containing synthetic substrates, either FPP-azide or F-azide-OH (Fig. 1). These compounds act as replacements for FPP, the natural substrate of protein farnesyltransferase, while inhibiting the endogenous synthesis of FPP. Second, the F-azide-modified proteins are selectively ligated with a phosphine capture reagent via the Staudinger reaction. Finally, the conjugated products are detected or affinity purified by means of the covalently attached probe (Fig. 1).
Fig. 1.
Schematic representation of the TAS technology for solid-phase affinity isolation of azide-labeled farnesylated proteins. (A) Farnesyltransferase-catalyzed modification of Ras. (B) Chemical structures of F-OH, F-azide-OH, natural FPP, and an FPP-azide. (C) The Staudinger conjugation reaction between bPPCR and an azide-containing molecule. (D) Schematic diagram for the conjugation reaction between a F-azide-modified protein and bPPCR. (E) Experimental procedure for the detection of F-azide modified proteins. Proteins 1 and 2 represent unmodified proteins; protein 3 represents a protein modified by a natural farnesyl group; protein 4 represents an F-azide-modified protein; and bPPCR represents the biotinylated phosphine capture reagent. Only F-azide-modified protein 4 is captured and subsequently detected.
The synthetic substrates, FPP-azide or F-azide-OH, were designed to be similar to natural substrates so that endogenous enzymatic pathways would use them efficiently for protein modification. Previous studies have indicated that minor modification of FPP has little effect on protein farnesylation (11, 12). Therefore, we anticipated that addition of azide would not affect the enzymatic farnesylation reaction. The synthesis of these azido-farnesyl compounds is described in detail in Supporting Text.
Azido-farnesylated proteins containing an azide tag can be chemoselectively conjugated by an improved version of Staudinger reaction, a revolutionary chemistry for biocompatible conjugation pioneered by Bertozzi and coworkers (13, 14). The reaction is very specific between azide and phosphine. Like azide, arylphosphines are comparatively inert in biological milieu on the time scale of a typical experiment (<12 h) (14, 15).
The organic azide has several features that make it well suited to tagging biological molecules. Azide provides minimum structural changes because of its small size and nonpolar nature. It is not present in any known naturally produced molecules, including carbohydrates, proteins, or nucleotides. Moreover, azides are only slowly reduced to amines by thiols (16) under physiological conditions and are otherwise relatively inert in the biological milieu. Although inorganic azide is toxic, organic azides are tolerated during the time course required for labeling experiments; notably, azide-containing molecules have been used in therapeutics [e.g., the anti-AIDS drug 3′-azido-3′-deoxythymidine (AZT)] and various biological studies (13, 17).
FPP-Azide Is an Efficient Substrate for Protein Farnesylation in Vitro. To test whether farnesyltransferase can use FPP-azide as a substrate for protein farnesylation, we carried out an in vitro enzymatic reaction between a CAAX-box containing peptide (RC peptide, KKFFCAIS) and FPP-azide. In vitro farnesylation of this peptide with FPP has been demonstrated (18).
The molecular masses of the peptide before and after enzymatic reaction were determined by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) MS. F-azide-modified peptide was detected after the enzymatic reaction (m/z 1189.6, a mass increase of 245.4 Da over RC peptide) (Fig. 7, which is published as supporting information on the PNAS web site). This experiment suggests that FPP-azide is a good substrate for the farnesyl transfer reaction in vitro.
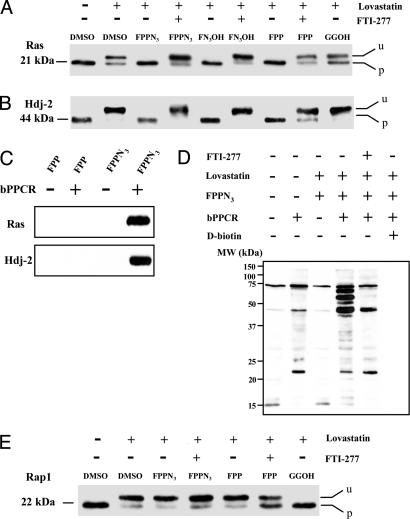
FPP-Azide and F-Azide-OH Are Substrates for Protein Farnesylation in Vivo. To determine whether the cell will process the exogenous azido-farnesyl substrates for protein farnesylation, we carried out a metabolic labeling experiment using either FPP-azide or F-azide-OH. As test proteins, we selected Ras and Hdj-2, two proteins known to be farnesylated in vivo (2, 19). Because unprocessed Ras and Hdj-2 migrate more slowly during SDS/PAGE than their farnesylated isoforms, a mobility-shift assay consisting of SDS/PAGE followed by Western blotting was used to determine whether the proteins had been successfully modified (19, 20).
Lovastatin, an HMG-CoA reductase inhibitor, blocks mevalonate synthesis, and therefore leads to inhibition of FPP synthesis and protein farnesylation (21, 22). As expected, lovastatin induced shifting of both Ras and Hdj-2 to higher molecular weight regions of the gel, suggesting inhibition of endogenous farnesylation (Fig. 2 A and B). Metabolic labeling of the cells with either FPP-azide or F-azide-OH, however, reversed the mobility shift, suggesting that protein farnesylation was restored. The azido-farnesylation was blocked by compound FTI-277, an inhibitor for protein farnesyltransferase (23). As a control, addition of all trans-geranylgeraniol (GG-OH) to the cells did not affect the mobilities of the proteins (Fig. 2 A and B). In contrast, the mobility-shift of a known geranylgeranylated protein, Rap1, could not be reversed by FPP azide in the lovastatin-treated cells, whereas GGOH could. The result suggests that FPP-azide could not be incorporated into this known geranylgeranylated protein (Fig. 2E).
Fig. 2.
Detection and verification of F-azide modification in vivo. F-azide-modified Ras (A) and Hdj-2 (B) were detected by mobility-shift assays. COS-1 cells were labeled with the indicated compounds for 24 h; the cell lysate was resolved by SDS/PAGE and probed by using anti-Ras or anti-Hdj-2 antibodies. Unmodified proteins are indicated by “u,” and farnesylated proteins are indicated by “p.” (C) Confirmation of F-azide modification by reciprocal immunoprecipitation. (D) Global detection of F-azide-modified proteins by Western blotting analysis. The protein lysates from cells with or without metabolic incorporation of FPP-azide were conjugated to bPPCR; the resulting biotinylated proteins were resolved by SDS/PAGE and detected by Western blotting analysis using HRP-conjugated streptavidin. (E) Mobility-shift assay of Rap1. COS-1 cells were labeled with the indicated compounds for 24 h; the cell lysate was resolved by SDS/PAGE and probed by using anti-Rap1 antibody. Unmodified proteins are indicated by “u,” and prenylated proteins are indicated by “p.” The results suggest that FPP azide could not be incorporated into Rap1, a known geranylgeranylated protein.
To further confirm F-azide-modification of Ras and Hdj-2, we carried out a reciprocal immunoprecipitation experiment, where either Ras or Hdj-2 was immunoprecipitated by an antibody. The immunoprecipitated complex was subjected to the conjugation reaction by using the biotinylated capture reagent shown in Fig. 1C. The resulting protein conjugate was subjected to Western blotting analysis using HRP-conjugated streptavidin for detection. As expected, Ras and Hdj-2 were detected when cells were dosed with FPP-azide, whereas signals were detected for neither Ras nor Hdj-2 when cells were dosed with FPP, the natural substrate for protein farnesylation (Fig. 2C). These results confirmed that both Ras and Hdj-2 were modified by F-azide and subsequently by bPPCR.
Global Detection of F-Azide-Modified Proteins. To test whether other cellular proteins could be modified by F-azide in vivo and subsequently detected, we carried out Western blotting analysis (Fig. 2D). The protein whole-cell lysate from cells dosed with lovastatin and azido-farnesyl substrate was subjected to the conjugation reaction by using bPPCR. The resulting mixture was resolved by SDS/PAGE; the biotinylated proteins were detected by Western blotting analysis using HRP-conjugated streptavidin. Dosing the cells with FPP-azide (Fig. 2D) resulted in the detection of multiple proteins. The signal could be competed away by 0.1 mM d-biotin and farnesyltransferase inhibitor, FTI-277 (Fig. 2D), suggesting that the signals detected in lane 4 of Fig. 2D were azide- and farnesyltransferase-specific. Overall, this experiment indicated that multiple proteins other than Ras and Hdj-2 could be farnesylated in vivo with F-azide and detected by Western blotting analysis. Importantly, comparison of blots from reactions using FPP-azide and F-azide-OH revealed that labeling efficiency was similar for both substrates (data not shown).
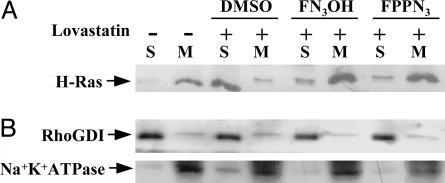
F-Azide Modification Supports Biological Function of Ras. F-azide modification of Ras restores lovastatin-inhibited membrane association. Farnesylated Ras is mainly localized to the plasma membrane, whereas the unprocessed isoform is primarily located in the cytosol. As expected, H-ras was mainly detected in the membrane fraction in untreated cells, but was detected in the soluble fraction when cells were treated with lovastatin (Fig. 3A). Either FPP-azide or F-azide-OH was able to restore the membrane association of Ras (Fig. 3A). As a control, the membrane association of RhoGDI (a cytosolic protein marker) and Na+/K+ ATPase (a membrane protein marker) remained unchanged regardless of treatment with lovastatin or azido-farnesylation substrate (Fig. 3B). These results suggested that Ras modified by F-azide retained its membrane association.
Fig. 3.
Restoration of membrane association of H-Ras to lovastatin-treated COS-1 cells. COS-1 cells were treated with 20 μM lovastatin alone or together with F-azide-OH (20 μM, lanes 5 and 6) or FPP-azide (20 μM, lanes 7 and 8) for 48 h. Membrane (M) or soluble (S) fractions were separated by ultracentrifugation. Equivalent proportions of membrane and soluble fractions were loaded in each lane. (A) Blotted proteins were probed with H-Ras antibody. (B) Another blot of the same samples was probed with RhoGDI antibody and Na+/K+ATPase antibody to demonstrate separation of soluble and membrane proteins, respectively.
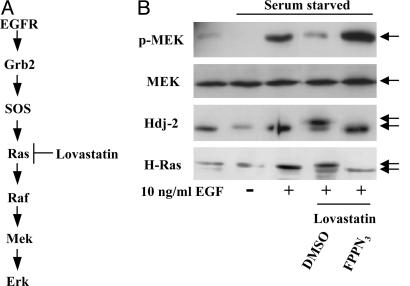
Incorporation of F-azide into Ras maintains its ability to activate mitogen-activated protein (MAP) kinase kinase (MEK). One biological function of Ras is to activate the Raf/MAP kinase pathway (Fig. 4A). We assessed the ability of Ras to maintain this activity when modified with F-azide by examining activation of MEK using an antibody specific to the phosphorylated form of MEK (Fig. 4B). COS-1 cells were serum starved, and then the Raf/MEK/ERK (extracellular-signal related kinase) pathway was activated by the addition of epidermal growth factor (EGF). Lovastatin treatment abrogated this EGF-stimulated MEK activation. However, addition of FPP-azide restored MEK activation (Fig. 4B).
Fig. 4.
Azido-farnesyl substrates restore the ability of Ras to activate Raf/MAP kinase signaling. (A) Signal transduction cascade of EGF stimulation. (B) Western blotting analysis of Hdj-2, H-Ras, MEK, and phosphorylated MEK using antibodies. COS-1 cells were starved for 48 h in serum-free DMEM. At the same time, the cells were treated with 20 μM lovastatin alone or together with 20 μM azido-farnesyl substrate. After starvation, the COS-1 cells were stimulated with 10 ng/ml EGF, which activates the Raf/MAP kinase cascade through a Ras-dependent pathway. MEK activation was detected by using an antiphospho-specific MEK antibody.
F-azide compounds block apoptosis induced by lovastatin in H-ras transformed NIH 3T3 cells. It has been shown that the H-rasVal–12 oncogene sensitizes NIH 3T3 fibroblasts to lovastatin cytotoxicity and that this process involves apoptosis (24). As shown in Fig. 8, which is published as supporting information on the PNAS web site, treatment of H-rasQ61L-transformed NIH 3T3 cells with lovastatin led to the activation of caspase-3. However, addition of F-OH or mevalonate blocked this caspase-3 activation. Similar inhibition of caspase-3 activation was observed when F-azide-OH or FPP-azide was added, suggesting that these azide compounds restored the function of H-Ras in a manner similar to the endogenous substrate.
MS Analysis of F-Azide-Modified Proteins Identifies Farnesylated Proteins. To identify farnesylated proteins, F-azide-modified proteins were subjected to the Staudinger reaction using bPPCR and subsequently purified by streptavidin–agarose beads. The affinity-purified proteins were digested with trypsin, and the resulting tryptic peptides were subjected to nano-HPLC/LCQ MS analysis for protein identification (Fig. 5). A protein sequence database search using the MS/MS data led to the identification of 21 proteins, including 17 CAAX box-containing proteins, three endogenously biotinylated proteins (propionyl-CoA carboxylase α subunit, 3-methylcrotonyl-CoA carboxylase subunit, acetyl-CoA carboxylase α) (25), and annexin A2 containing an unprecedented CXXXX terminus (Table 1).
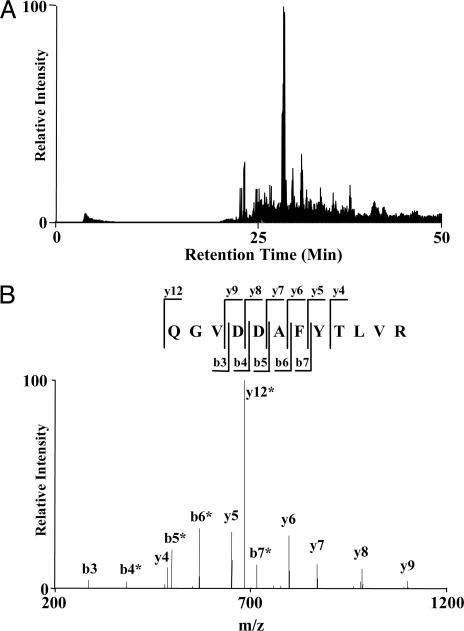
Fig. 5.
An example of nano-HPLC/MS/MS analysis for protein identification. (A) Total ion current chromatogram of a nano-HPLC/MS/MS of the beads digest. (B) MS/MS spectrum of 692.9 m/z at the retention time of 40.67 min, which identified the peptide QGVDDAFYTLVR, unique to K-Ras.
Table 1. A list of identified proteins.
| Protein name | Gl no. | Mass, kDa | No. of peptides identified | CAAX motif |
|---|---|---|---|---|
| H-Ras | 190891 | 21.3 | 1 | CVLS |
| K-Ras | 186764 | 21.4 | 2 | CVIM |
| N-Ras | 4505451 | 21.2 | 1 | CVVM |
| Rap2C, Ras family protein | 13097300 | 20.7 | 1 | CVVQ |
| Cdc42, Ras family protein | 4557920 | 21.3 | 3 | CVLL |
| TC21, Ras family protein | 21361416 | 23.4 | 3 | CVIF |
| Rab21, Ras family protein | 7661922 | 24.3 | 1 | CSSG |
| Rheb, Ras family protein | 1772345 | 20.6 | 3 | CSVM |
| Peroxisomal farnesylated protein | 4506339 | 32.8 | 2 | CLIM |
| DnaJ (HDJ-2) subfamily A member 1 | 4504511 | 44.8 | 9 | CQTS |
| DnaJ subfamily A member 2 | 5031741 | 45.7 | 9 | CAHQ |
| Dnj3/Cpr3 | 2352904 | 46.3 | 6 | CAHQ |
| Nucleosome assembly protein 1-like 1 | 4758756 | 45.3 | 4 | CKQQ |
| Lamin B1 | 5031877 | 66.3 | 14 | CAIM |
| Lamin B2 | 27436951 | 67.6 | 14 | CYVM |
| Hypothetical protein | 11277141 | 84.8 | 8 | CALV |
| Peroxisome biogenesis factor 1 | 14289177 | 71.2 | 1 | CKAL |
| Annexin A2 | 12314197 | 38.6 | 1 | CGGDD |
The identification of a number of previously known farnesylated proteins confirms the ability of TAS technology for efficient isolation and identification of farnesylated proteins. In addition to lamins and DnaJ proteins, several Ras superfamily G proteins, including H-ras, K-ras, N-ras, Rheb, and Rap2, were identified. TC 21 is reported to serve as a good substrate for farnesyltransferase as well as geranylgeranyltransferase (26). Because these G proteins are much less abundant than lamins, our method should enable identification of low abundance farnesylated proteins. The identified farnesylated proteins fall into a wide molecular mass range, starting from high molecular mass proteins of 142.8 and 84.8 kDa to low molecular mass proteins of ≈20–25 kDa. It is interesting to point out that the distribution of farnesylated proteins shown here is similar to that observed when farnesylated proteins were detected by using [3H]farnesol labeling (27), again confirming that our method is effective in detecting farnesylated proteins.
Some insight into farnesylated proteins can be gleaned from the list of farnesylated proteins identified by the MS analysis. Our analysis suggests that nucleosome assembly protein 1-like protein (NAP-1) ending with CKQQ is farnesylated. Indeed, we confirmed that a GST fusion protein containing a C-terminal 11-aa peptide of NAP-1 can be farnesylated by farnesyltransferase in vitro (data not shown). NAP-1 is a highly conserved protein that is involved in nucleosome remodeling (28). Furthermore, involvement of NAP-1 in mitosis is suggested from the study of yeast protein (29). Interestingly, the yeast protein ends with CKQS, suggesting that this family of proteins share a similar C-terminal motif. Another protein of interest is Dnj3/Cpr3. This protein was initially identified in a screen to overcome G1 arrest of yeast cells (30). Identification of two proteins involved in peroxisome biogenesis may point to the significance of farnesylation in peroxisome function. Identification of annexin A2 was a surprise, because this protein ends with the motif CGGDD. Prenylation of these putative farnesylated proteins need to be further investigated. The reason a known geranylgeranylated protein Cdc 42 was detected in our analysis is unclear at the moment, but may be due to a small amount of GGPP-azide arising from conversion of FPP-azide or the low level usage of FPP-azide as an alternative substrate by geranylgeranyltransferase under conditions of isoprene starvation.
Discussion
We describe the design and synthesis of azido-farnesyl substrates for protein farnesylation. The compounds were able to efficiently enter into cells and become incorporated into proteins containing the CAAX motif, which is known to be targeted by farnesyltransferases. Metabolic incorporation of the azido-farnesyl group into proteins could be increased by inhibition of endogenous synthesis of FPP using the HMG-CoA reductase inhibitor, lovastatin. The metabolically labeled, azido-farnesyl-modified proteins were chemoselectively conjugated to bPPCR for detection and affinity purification.
Several lines of evidence suggest that F-azide-modified proteins maintained the major characteristics of naturally farnesylated proteins. First, F-azide-modified Ras retained GTPase activity and was able to activate EGF-dependent MEK phosphorylation. Second, F-azide modification induced membrane association of Ras. Third, F-azide compounds block Ras-dependent apoptosis induced by lovastatin in H-ras-transformed NIH 3T3 cells. Finally, cells cultured in DMEM containing lovastatin together with FPP-azide/F-azide-OH and GG-OH had a growth rate comparable to cells cultured in the absence of lovastatin (unpublished data).
There are a few unique features of the TAS technology. First, it allows uniformly chemoselective detection of proteins containing an azide moiety by phosphine capture reagents. Chemo-selective conjugation is based on a reaction between the phosphine capture reagent and the azide, and does not depend on surrounding structural elements. Any protein with the F-azide modification will be conjugated, as long as the azide group is accessible to the chemoselective conjugation reagent. In contrast, the binding affinities of antibodies to farnesylated proteins are peptide sequence-dependent and can only recognize a subset of farnesylated proteins. Second, the Staudinger reaction is performed under such mild conditions that proteins and their modifications will not be changed. Finally, the conjugation reaction is very specific between azide and phosphine capture reagents.
It appears that FPP-azide retains specificity and is not used by geranylgeranyltransferases. Western blotting analysis indicated that almost all of the azido-farnesyl-specific signal detected in Western blotting analysis were blocked by FTI-277 (Fig. 2D), suggesting that little, if any, FPP azide was used by geranylgeranyltransferase for protein prenylation. In addition, the azide substrate could not become incorporated into a GGTase substrate, Rap1 (Fig. 2E). Nevertheless, we could not completely exclude the possibility that geranylgeranylated proteins would use FPP-azide for protein prenylation under stressed conditions (e.g., lovastatin treatment). However, such modification will not be blocked by FTI. Thus, the method described here could still be used to perform proteomics study to identify true protein targets for FTIs, because azido-farnesylation of FTI-targets will be blocked due to FTI-treatment, whereas geranylgeranyltransferase-catalyzed azido-farnesylated proteins will remain unchanged.
TAS technology allows purification of proteins containing the azide moiety with minimal contamination. Because of the covalent nature of the bond between azide and the phosphine capture reagent and the high binding affinity between biotin and streptavidin, both nonspecifically binding proteins and proteins interacting noncovalently with F-azide-modified proteins can be thoroughly removed by stringent washing buffers, such as (i) high-detergent buffer, (ii) denatured zwitterionic buffer, or (iii) high-salt buffer. Thus, the TAS technology in combination with existing proteomics methods [e.g., 2D gel MS (31), multipledimensional MS (32), and/or isotope coded affinity tagging/MS (33, 34)] will expedite efficient analysis of dynamic farnesyl modifications.
Given that human genome contain only ≈100 genes with the known CAAX-box sequence for protein farnesylation and there might be only 30–50% of genes expressed in a specific cell type, 18 farnesylated proteins identified here might account for 36–60% farnesylation subproteome. Thus, although the total number of farnesylated proteins identified in this study is moderate, it represents one of most comprehensive analysis of a specific type of modification subproteomes to date.
If we assume that 20,000 proteins are expressed in a cell, the TAS technology can easily achieve a >200-fold purification of the modified proteins. In combination with other existing proteomics technologies, this enrichment should allow practical identification and quantification of low-abundance farnesylated proteins in the range of a few hundred copies per cell. We believe the described technology is well suited to the functional characterization of farnesylated proteins, and to the identification of changes in protein farnesylation in response to drugs that have current clinical applications or are under clinical evaluation, such as farnesyltransferase inhibitors and statins.
Proteomic analysis of dynamic posttranslational modifications is notoriously challenging because of the paucity of high-affinity methods for enriching modified proteins and the limited dynamic range of existing proteomic methods. The TAS technology is likely to be applicable to other protein modifications whose cellular enzymatic pathways can tolerate the addition of an azide moiety. Indeed, we recently showed that the TAS technology worked well for protein geranylgeranylation (data not shown). We anticipate that the TAS technology will find a wide application toward the efficient proteomic analysis of posttranslationally modified proteins by reducing the dynamic range and complexity of the proteome.
Supplementary Material
Acknowledgments
We thank Patrick J. Casey for helpful discussions. Y.Z. is supported by the Robert A. Welch Foundation (I-1550) and National Institutes of Health Grant CA 85146. J.F. is supported by the Robert A. Welch Foundation and National Institutes of Health Grant GM 31278. F.T. is supported by National Institutes of Health Grants CA32737 and CA41996. C.W. is supported by National Institutes of Health Grant GM56372 (through Prof. Patrick J. Casey).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TAS, tagging-via-substrate; F-azide, azide-farnesyl; FPP, azido farnesyl diphosphate; bPPCR, biotinylated phosphine capture reagent; HRP, horseradish peroxidase; F-azide-OH, azido farnesyl alcohol; MAP, mitogen-activated protein; MEK, MAP kinase kinase; EGF, epidermal growth factor; MS/MS, tandem MS.
References
- 1.Gudepu, R. G. & Wold, F. (1998) in Proteins: Analysis and Design, ed. Angeletti, R. H. (Academic, San Diego), pp. 121–207.
- 2.Reiss, Y., Goldstein, J. L., Seabra, M. C., Casey, P. J. & Brown, M. S. (1990) Cell 62, 81–88. [DOI] [PubMed] [Google Scholar]
- 3.Moores, S. L., Schaber, M. D., Mosser, S. D., Rands, E., O'Hara, M. B., Garsky, V. M., Marshall, M. S., Pompliano, D. L. & Gibbs, J. B. (1991) J. Biol. Chem. 266, 14603–14610. [PubMed] [Google Scholar]
- 4.Seabra, M. C., Reiss, Y., Casey, P. J., Brown, M. S. & Goldstein, J. L. (1991) Cell 65, 429–434. [DOI] [PubMed] [Google Scholar]
- 5.Spence, R. A. & Casey, P. J. (2000) Mechanism of Catalysis by Protein Farnesyltransferase (Academic, San Diego).
- 6.Tamanoi, F., Gau, C. L., Jiang, C., Edamatsu, H. & Kato-Stankiewicz, J. (2001) Cell. Mol. Life Sci. 58, 1636–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jenkins, C. M., Han, X., Yang, J., Mancuso, D. J., Sims, H. F., Muslin, A. J. & Gross, R. W. (2003) Biochemistry 42, 11798–11807. [DOI] [PubMed] [Google Scholar]
- 8.Sebti, S. M. & Der, C. J. (2003) Nat. Rev. Cancer 3, 945–951. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W. J., Moomaw, J. F., Overton, L., Kost, T. A. & Casey, P. J. (1993) J. Biol. Chem. 268, 9675–9680. [PubMed] [Google Scholar]
- 10.Zhao, Y., Zhang, W., White, M. A. & Zhao, Y. (2003) Anal. Chem. 75, 3751–3757. [DOI] [PubMed] [Google Scholar]
- 11.Chehade, K. A., Kiegiel, K., Isaacs, R. J., Pickett, J. S., Bowers, K. E., Fierke, C. A., Andres, D. A. & Spielmann, H. P. (2002) J. Am. Chem. Soc. 124, 8206–8219. [DOI] [PubMed] [Google Scholar]
- 12.Micali, E., Chehade, K. A., Isaacs, R. J., Andres, D. A. & Spielmann, H. P. (2001) Biochemistry 40, 12254–12265. [DOI] [PubMed] [Google Scholar]
- 13.Saxon, E., Luchansky, S. J., Hang, H. C., Yu, C., Lee, S. C. & Bertozzi, C. R. (2002) J. Am. Chem. Soc. 124, 14893–14902. [DOI] [PubMed] [Google Scholar]
- 14.Kiick, K. L., Saxon, E., Tirrell, D. A. & Bertozzi, C. R. (2002) Proc. Natl. Acad. Sci. USA 99, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soellner, M. B., Dickson, K. A., Nilsson, B. L. & Raines, R. T. (2003) J. Am. Chem. Soc. 125, 11790–11791. [DOI] [PubMed] [Google Scholar]
- 16.Staros, J. V., Bayley, H., Standring, D. N. & Knowles, J. R. (1978) Biochem. Biophys. Res. Commun. 80, 568–572. [DOI] [PubMed] [Google Scholar]
- 17.Chin, J. W., Cropp, T. A., Anderson, J. C., Mukherji, M., Zhang, Z. & Schultz, P. G. (2003) Science 301, 964–967. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama, K., Goodwin, G. W., Ghomashchi, F., Glomset, J. A. & Gelb, M. H. (1991) Proc. Natl. Acad. Sci. USA 88, 5302–5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adjei, A. A., Davis, J. N., Erlichman, C., Svingen, P. A. & Kaufmann, S. H. (2000) Clin. Cancer Res. 6, 2318–2325. [PubMed] [Google Scholar]
- 20.James, G. L., Brown, M. S., Cobb, M. H. & Goldstein, J. L. (1994) J. Biol. Chem. 269, 27705–27714. [PubMed] [Google Scholar]
- 21.Sinensky, M., Beck, L. A., Leonard, S. & Evans, R. (1990) J. Biol. Chem. 265, 19937–19941. [PubMed] [Google Scholar]
- 22.Kim, R., Rine, J. & Kim, S. H. (1990) Mol. Cell. Biol. 10, 5945–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lerner, E. C., Qian, Y., Blaskovich, M. A., Fossum, R. D., Vogt, A., Sun, J., Cox, A. D., Der, C. J., Hamilton, A. D. & Sebti, S. M. (1995) J. Biol. Chem. 270, 26802–26806. [DOI] [PubMed] [Google Scholar]
- 24.Chang, M. Y., Jan, M. S., Won, S. J. & Liu, H. S. (1998) Biochem. Biophys. Res. Commun. 248, 62–68. [DOI] [PubMed] [Google Scholar]
- 25.Chapman-Smith, A. & Cronan, J. E., Jr. (1999) Biomol. Eng. 16, 119–125. [DOI] [PubMed] [Google Scholar]
- 26.Carboni, J. M., Yan, N., Cox, A. D., Bustelo, X., Graham, S. M., Lynch, M. J., Weinmann, R., Seizinger, B. R., Der, C. J., Barbacid, M., et al. (1995) Oncogene 10, 1905–1913. [PubMed] [Google Scholar]
- 27.Corsini, A., Farnsworth, C. C., McGeady, P., Gelb, M. H. & Glomset, J. A. (1999) Incorporation of Radiolabeled Prenyl Alcohols and their Analogs into Mammalian Cell Proteins (Humana, Totowa, NJ). [DOI] [PubMed]
- 28.Ishimi, Y., Hirosumi, J., Sato, W., Sugasawa, K., Yokota, S., Hanaoka, F. & Yamada, M. (1984) Eur. J. Biochem. 142, 431–439. [DOI] [PubMed] [Google Scholar]
- 29.Miyaji-Yamaguchi, M., Kato, K., Nakano, R., Akashi, T., Kikuchi, A. & Nagata, K. (2003) Mol. Cell. Biol. 23, 6672–6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards, M. C., Liegeois, N., Horecka, J., DePinho, R. A., Sprague, G. F., Jr., Tyers, M. & Elledge, S. J. (1997) Genetics 147, 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanash, S. (2003) Nature 422, 226–232. [DOI] [PubMed] [Google Scholar]
- 32.Wu, C. C., MacCoss, M. J., Howell, K. E. & Yates, J. R. (2003) Nat. Biotechnol. 21, 532–538. [DOI] [PubMed] [Google Scholar]
- 33.Aebersold, R. & Mansion, M. (2003) Nature 422, 198–207. [DOI] [PubMed] [Google Scholar]
- 34.Gygi, S. P., Rist, B., Gerber, S. A., Turecek, F., Gelb, M. H. & Aebersold, R. (1999) Nat. Biotechnol. 17, 994–999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.