Abstract
Cellular adhesions are modulated by cytoskeletal forces or external stresses and adapt to the mechanical properties of the extracellular matrix. We propose that this mechanosensitivity can be driven at least in part by the elastic, cell-contractility-induced deformations of protein molecules that form the adhesion. The model accounts for observations of anisotropic growth and shrinkage of focal adhesions in the direction of the force and predicts that focal adhesions only grow within a range of force that is determined by the composition and matrix properties. This prediction is consistent with the observations of a force threshold for the appearance of elongated focal adhesions and the disruption of adhesions into fibrils on a mobile extracellular matrix. The growth dynamics is calculated and the predicted sliding of focal adhesions is consistent with several experiments.
Adhesion of live cells to external surfaces (1) plays an important role in many cellular processes such as cell growth, differentiation, motility, and apoptosis (programmed cell death) (2). Cell adhesion is not a passive process, restricted to the formation of bonds between membrane receptors and extracellular ligands. Adhering cells actively probe the physical properties of the extracellular matrix; their cellular contractile machinery participates in the formation of the adhesive junctions. It has been shown that rigid surfaces give rise to large and stable adhesions, termed focal adhesions (FAs), that are associated with the termini of actin stress fibers and trigger signaling activity that affects gene expression, cell proliferation, and cell survival. On the other hand, soft surfaces mainly support the development of relatively small, transient dot-like or fibrillar adhesions that are involved in cell motility and matrix reorganization, respectively. In addition, observation of the early phase in the assembly of FAs shows small, primordial adhesions, termed focal complexes (FXs), as precursors of FAs. FXs are formed close to the edge of the advancing membrane protrusions of cells and can grow in some cases into FAs when subjected to mechanical stress caused by either cell contractility or external perturbations (3). Indeed, the adhesion process has been shown to be mechanosensitive; cells can probe the physical properties of their environment and respond by modulating their adhesions or migratory activity (4–6).
The nature and localization of the “mechanosensor” are key issues. Although the molecular identity of the mechanosensitive system is still unclear, several candidate target systems were considered, including the contractile fibers attached to the adhesion sites. We propose here a mechanism that focuses on the very region of the adhesion that is shear-sensitive. Indeed, several clues lead to the conclusion that some proteins inside the adhesion site respond to force (see ref. 7 for a review), because the growth of the adhesion site is a local process and is mediated by signaling (8–10): the strength and size of adhesions adapt to the local stiffness of the extracellular matrix (11) and to the local internal tension applied to the adhesion by the cytoskeleton (8) (see Movie 1, which is published as supporting information on the PNAS web site). The molecular basis for the response to force and its regulation by various signaling mechanisms are beginning to be understood (see ref. 12 for a review). However, the transformation of the elastic properties of the extracellular matrix into biochemical signals by cytoskeletal forces remains a mystery. In this article, we propose that this force-dependent biochemical response is driven by the elastic deformation of the adhesive junctions by stresses that originate in the cytoskeleton.
The theory presented here has important consequences for the growth and translocation of FAs. In this article we assume that primary adhesions already exist, and we focus on their response to force. The nucleation of the primary adhesions is beyond the scope of our work, because we focus on a scenario that accounts for the observed behavior of stressed FAs. We base our model on the assumption that the mechanosensors responsible for initiating the biochemical signaling cascade involved in the growth of FAs are proteins located in the junction itself that respond to the compression induced by lateral stresses. We thus predict the anisotropic growth of FAs in the direction of the force, as observed. In addition, we show that the growth of FAs is a process that releases energy and is driven by the balance between the exothermic adsorption of new proteins and the mechanical cost of elastic deformations of the junction induced by cytoskeletal forces. Our model also accounts for the observed sensitivity to the elastic properties of the extracellular matrix: reducing the strength of adhesion of the extracellular matrix transforms the growth process from favorable to unfavorable, which we associate with FAs and fibrillar adhesions, respectively. Finally, the kinetic analysis of our model reproduces some of the observed features of FAs such as the apparent sliding of the adhesions and the sensitivity of their size to force. Several predictions for additional experiments are discussed at the end of the article.
Model and Methods
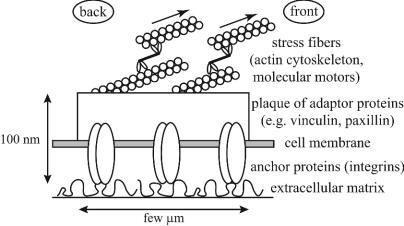
Deformation of Stressed FAs as the Origin of the Mechanosensitivity. Live cells exert directional, lateral forces on adhesive junctions; these forces originate from the organization of actin filaments and their associated molecular motors into stress fibers (Fig. 1). Adhesions respond dynamically to the local stresses: increased contractility leads locally to larger adhesions, whereas FAs disrupt when myosin II is inhibited (8).
Fig. 1.
Schematic representation of a FA.
We assume in our model that the biochemical response of adhesions to cytoskeletal stresses originates from the stress-induced elastic deformation of the adhesion site. We propose that these force-induced deformations modify the local density of the proteins found in and around the adhesive junction and thus their interactions. This local change in density, in turn, may trigger a conformational change or a molecular reorganization that initiates the biochemical cascade responsible for the aggregation of new proteins and the directional, anisotropic growth of the adhesion. As a specific example, transmembrane, integrin proteins could be the mechanosensor (3), because they are connected to the extracellular matrix and are elastically deformed by cytoskeletal forces; this deformation can activate conformational or organizational changes that enhance their binding with plaque proteins (2).
In addition to the fact that adhesion to an anchoring surface makes a junction sensitive to shear (even if the latter is composed of fluid-like assemblies of molecules), several observations support the view we propose. First is the observation that independent of the origin of the stress [e.g., internal contractility (13), shear flow (14), micropipette-induced shear stress (8)], small adhesions grow into FAs that elongate in the direction of the force. This anisotropy is predicted by our theory, because stress-induced deformations are correlated with the applied force and exert different effects on the proximal (front) and distal (back) aspects of the adhesive junctions.§ In our model, in which we relate the biochemical response to deformations, this anisotropy also leads to an anisotropic addition (or loss) of proteins. Moreover, the size and strength of the adhesive junctions depend on the elastic properties of the extracellular matrix (9, 11). We predict in our model that the extent and nature of the adhesion are sensitive to the mechanical properties of the underlying matrix: a local force applied to an adhesive junction induces a pure translation of the junction (and thus no deformation) if the extracellular matrix is very soft, whereas strongly grafted adhesions with rigid matrix are elastically deformed by lateral, local stresses.
Description of the Model. The total force transmitted by FAs has been proved to be proportional to their surface (13), suggesting that the region over which stress fibers act grows with the junction. As a model, we consider the adhesion and its membrane-associated integrins as an elastic, thin film grafted at its bottom surface and stressed by a local lateral force on its top surface; “local” means here that the force only acts on a finite region of the adhesive junction, which, as explained below, needs only to be somewhat smaller than the total area of the adhesion by a few thicknesses of the FA (typically, a few tens of nanometers). One possible scenario is that the integrin layer forms in advance of the adhesion protein plaque and the associated stress fibers such that the stressed region is smaller than the adhering integrin cluster.¶ The localization of the force is a crucial aspect of our model, because a homogeneously applied stress would give rise to a uniform shear of the adhesive junction and a translational motion of the surrounding lipid membrane. Because the latter is not grafted to the surface, no anisotropic density changes in the protein layer occur and no directional force-induced biochemical response can arise from a homogeneous lateral stress. On the contrary, a local tangential stress induces a compression at the front edge and an expansion at the back of the stressed region, because the unstressed part is also grafted and cannot slide. In this case, a lateral force induces anisotropic density changes in proteins located inside or close to the stressed region. The resulting stress-induced deformation profile can be calculated by using continuum elasticity theory (15). Because adhesions are thin films [on the order of 100 nm, compared to their width, ranging from a few hundred nanometers (FXs) to several micrometers (FAs)], their elastic response is expected to be similar to that of an infinite, thin plate. Therefore, variation of density caused by a local stress is only significant close to the edges of the stressed area. For large adhesions (compared to their thickness), these effects are independent of the size of the adhesion. We assume in our model that this change in density triggers the biochemical response responsible for the growth or dissolution of FAs. Anisotropic variations of density then give rise to different molecular dynamics of the front and back regions near the edge and can result in either overall growth or shrinking of the FAs as shown below. We therefore predict that this biochemical response occurs only at the edges of the stressed region and affects both the front and back regions, as explained below.
In this article we propose an approach that is simpler than the full three-dimensional elastic treatment (15) and present a quasi-two-dimensional model of FAs in which the elastic thin film is represented by particles that interact through springs of stiffness k0 (see Fig. 2). The particles are bound to the anchoring surface by springs of stiffness kb that measure the resistance to normal shear and therefore are related to the normal shear modulus (in contrast to the in-plane shear modulus). Each particle in our model is a protein complex consisting of one integrin molecule and the associated intracellular proteins that connect it to the actin cytoskeleton; this we define as the minimal complex of proteins needed for the adhesion to grow. The elastic properties of the extracellular matrix and its connection to the anchoring surface are included by fixing the anchors to the surface through a sinusoidal potential V(x) = kma2/(2π)2cos(2πx/a). In the limit of small displacements, this potential reduces to a spring-like interaction in which km is the stiffness of the anchorage close to the equilibrium position. The case of a grafted layer is found by taking the limit km → ∞.
Fig. 2.
One-dimensional chain of interacting particles bound to anchors as a model for FAs grafted to an extracellular matrix. The potential V(x) accounts for the grafting properties of the extracellular matrix. The distance a between the anchors is the integrin–integrin spacing, and un is the displacement of the nth particle from its position in the absence of force.
In a one-dimensional version of this approach (which we later generalize to a two-dimensional layer; Fig. 2), the interaction of the particle n with its neighbors is
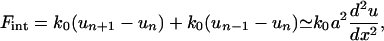 |
where u(x) is the displacement with respect to the equilibrium position in a continuum approximation, and a is the equilibrium distance between the particles. The connection to a fixed and rigid extracellular matrix gives rise to a restoring force Fb = –kbu(x) such that the mechanical equilibrium for a such a system of particles pulled by a force F(x) is, in the continuum limit,
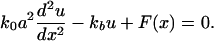 |
[1] |
Although the overall phenomenon is dynamic, we assume local mechanical and thermodynamical equilibrium, because the diffusion times of proteins in the cytosol or in the cell membrane are much faster than the observed time scales for the growth of an FA [on the order of seconds (8)]. The local force balance gives rise to deformations near the edge that modulate the associations of new proteins and thus control the growth of the adhesion. We solve Eq. 1 for a gate-shaped field of force of amplitude, f, per unit length and width L; that is, the force is only nonzero for –L/2 < x < L/2. This force profile corresponds to the local pulling force caused by stress fibers and induces the following spatial variation of the local, average density, δΦ(x), relative to the undeformed density Φ:
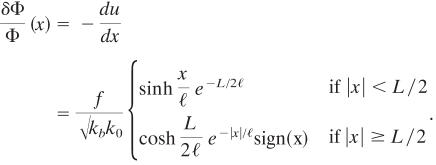 |
[2] |
Eq. 2 is derived in the case of a stiff matrix (km → ∞) and shows that the deformation decreases exponentially with a range 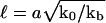 . Remembering that an actual adhesion has a finite thickness fixes the order of magnitude of
. Remembering that an actual adhesion has a finite thickness fixes the order of magnitude of 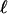 : force-induced deformations must vanish at the bottom surface because of the grafting boundary condition appropriate to the case of an immobilized extracellular matrix. The range
: force-induced deformations must vanish at the bottom surface because of the grafting boundary condition appropriate to the case of an immobilized extracellular matrix. The range 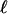 is thus on the order of the thickness of the junction (≈100 nm). The representation of the adhesion as an infinite elastic medium pulled by a force acting on a region of size L is therefore valid once the actual length of the adhesion is larger than L + 2
is thus on the order of the thickness of the junction (≈100 nm). The representation of the adhesion as an infinite elastic medium pulled by a force acting on a region of size L is therefore valid once the actual length of the adhesion is larger than L + 2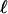 , where
, where  ≈ 100 nm « L.
≈ 100 nm « L.
Results
Anisotropic Response of FAs in the Direction of the Force. Eq. 2 predicts a polarized response to stress: the medium is compressed at the front edge of the stressed area and expanded at the back. Flow (14) and micropipette (8) experiments both observe that the growth of FAs occurs in the direction of the force. Our model therefore leads to the conclusion that the biochemical response of a stressed FA occurs only in a narrow strip (on the order of the thickness of the adhesion) at its front rim, where the density is increased. The variations of density induced by the stress dominate thermal fluctuations:
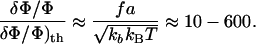 |
This estimate assumes that each complex that defines the particle of our model contains one integrin so that a ranges from 20 nm (close packing) to 80 nm (16). The stiffness kb of each protein complex is calculated from the Young modulus E of the assembly of proteins: kb = Ea2/h, where h is the vertical thickness of the adhesion. E is taken in the range of 103 to 106 Pa (17), and the rigidity of a particle of area a2 is therefore in the range of 10–3 to 102 mN/m. The force per unit length, f, on each particle is extracted from the measurement in ref. 13: f = 5.5 nN/μm2 × a.
Until now, we have shown that a shear force induces a polarized deformation of the adhesive junction by using a one-dimensional model. This approach can be easily extended to two dimensions. Although highly anisotropic along its thickness, the elastic properties of an FA are expected to be isotropic in the plane, because the plaque of proteins is largely similar to an isotropic gel that connects the transmembrane integrins. Our model (Fig. 2) is therefore extended to two dimensions by adding an in-plane shear modulus, due to either the cross-linking in the gel or the effects of the anchoring surface (15) that couples displacements of both in-plane directions. However, despite this shear modulus, the displacement mainly takes place along the force (18). We find here again the trend seen in the one-dimensional approach: the force induces an in-plane compression at the front edge and an expansion at the rear. There is no change in density along the axis of symmetry of the stressed area perpendicular to the force. Changes in density therefore are much smaller on the sides of the stressed area perpendicular to the force. Rigorous calculation within continuum elasticity theory (15) confirms this expectation. This prediction agrees with mechanically induced growth of FAs (8, 3) and leads to the conclusion that the mechanosensor is activated at the front edge of the adhesion and is sensitive to compression.
The Growth of FAs Is an Exothermic Process. The main hypothesis of our model is that force-induced deformations trigger the biochemical response of FAs through conformational (e.g., activation of integrins) and/or organizational (e.g., binding of plaque proteins) changes of molecules embedded in a narrow region of size  in which the density varies. This local activation increases the rate of association of free proteins within this area. The addition of new proteins triggers a complex biochemical cascade that results in the growth of the stressed region by an amount proportional to the number of added units. Thus, on the one hand, an exchange of energy takes place because of the association of new proteins to the deformed layer. On the other hand, the cell has to invest more energy to maintain the stress on the larger FAs. Within our model, the addition of a protein complex of size a2 to the FAs results in a deformation with an elastic energy cost, ΔEel, given by
in which the density varies. This local activation increases the rate of association of free proteins within this area. The addition of new proteins triggers a complex biochemical cascade that results in the growth of the stressed region by an amount proportional to the number of added units. Thus, on the one hand, an exchange of energy takes place because of the association of new proteins to the deformed layer. On the other hand, the cell has to invest more energy to maintain the stress on the larger FAs. Within our model, the addition of a protein complex of size a2 to the FAs results in a deformation with an elastic energy cost, ΔEel, given by
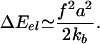 |
[3] |
f/a is the force per unit surface pulling on the junction, which is the relevant quantity in the real, two-dimensional problem. We can generalize Eq. 3 to include the elastic properties of the extracellular matrix. In the limit of small displacements, the sinusoidal potential of adhesion shown in Fig. 2 can be approximated by a set of springs of stiffness km. Particles embedded in the adhesion then are bound to the anchoring surface by two successive springs, km and kb. The effective stiffness of the bond therefore is
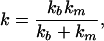 |
[4] |
and k replaces kb in Eq. 3. We now suppose that the association of a free protein complex to the front edge of the adhesion releases an energy ΔEa. The reaction can be either endothermic (ΔEa > 0) or exothermic (ΔEa < 0). However, ΔEa depends on the change in density at the front edge of the stressed region, δΦ/Φ at x = L/2: in the absence of density variations, the probability of attaching a new particle is unchanged, and ΔEa = 0. For small variations of the density, ΔEa can be taken to vary linearly with the change in density
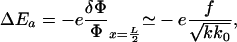 |
[5] |
where e is the free energy of reaction of one protein complex. Adding a protein complex to an adhesive junction thus involves an overall variation of free energy:
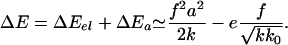 |
[6] |
We first discuss the case in which e < 0: aggregation of new particles to an existing adhesion is thermodynamically unfavorable (ΔE > 0), and an additional input of energy is required to stabilize the protein cluster. Such input of energy may come from fluctuations arising from active processes in the surroundings, as opposed to thermal fluctuations that are small compared to the required energies. However, in the situation in which a limited amount of external energy is available, the adhesions can only grow to a finite size. If the input of energy is E0, an adhesive junction with initial length L0 and width w reaches a maximal size:
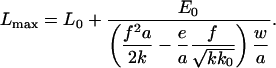 |
[7] |
For this thermodynamically unfavorable growth process, increasing the force f per unit length thus leads to smaller adhesions.
An endothermic process leading to an adhesion with a maximal size that decreases with the applied force (Eq. 7) and has a transient nature (because of its dependence on fluctuations) cannot describe the directional deterministic growth of FAs. We therefore conclude that FAs correspond to the case in which e > 0 in Eq. 6. We thus predict that the process of molecular aggregation of new proteins to an FA is energetically favorable (e > 0) and releases energy (ΔE < 0). However, processes for which e < 0 may account for the origin of the small, transient, thermodynamically unfavorable FXs, which are important in motile cells in which the adhesions must disassemble in a time scale that is short enough for the cell to move (see Movie 1). The different values of e that characterize the growth of FAs and transient FXs therefore suggests that the two types of junctions are biochemically different. This biochemical distinction between FXs and FAs is supported by the experimental evidence that some proteins, such as Zyxin, only link to the adhesion when it is stressed (3).
Large Force and Soft Matrix Arrest the Growth Process. FAs, however, can only grow when the addition of a new protein complex is favorable: ΔE < 0 in Eq. 6. Reduced immobilization of the extracellular matrix (km → 0) or very large forces lead to a positive value for ΔE and will arrest the growth process even if e > 0. This regime in which the anchorage of the extracellular matrix on the anchoring surface or of the integrin molecules on the extracellular matrix cannot resist the pulling force corresponds to fibrillar adhesion. For such large displacements, the description of the anchorage as a spring of stiffness km is no longer valid, and the actual shape of the adhesion potential must be considered. For the sinusoidal potential described above (Fig. 2), we find that when the force per unit length exceeds the threshold f* = km/(2π), the displacement of the anchors is large and the junction undergoes a stick-slip motion (19). In this force regime, the adhesion moves rather than deforms, and changes in density around it are negligible; thus, no further adsorption of proteins to the FA is expected. Indeed, protein aggregates involved in fibrillar adhesions are tension-insensitive (20), as we predict here.
Growth Dynamics of FAs. We emphasized above that the growth of FAs is a process that releases energy: ΔE < 0 as defined by Eq. 6. The addition of proteins to the existing adhesion is therefore favorable. Freely diffusing protein complexes that come in close contact with the activated (compressed) region near the front edge of an adhesive junction will have a higher rate of attachment, although the molecules are elastically deformed (21):
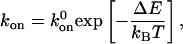 |
where 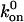 is the rate of attachment in the absence of force. The velocity of the front edge of the adhesion is proportional to the number of newly adsorbed proteins, and we suppose that it depends linearly on the concentration, cf, of free protein complexes. The adsorption of such elementary units causes the front edge of the FAs to advance with a velocity v+ given by
is the rate of attachment in the absence of force. The velocity of the front edge of the adhesion is proportional to the number of newly adsorbed proteins, and we suppose that it depends linearly on the concentration, cf, of free protein complexes. The adsorption of such elementary units causes the front edge of the FAs to advance with a velocity v+ given by
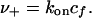 |
In addition to the motion of the front edge, we must consider the effects of the stress on the back edge. Because of the stress, the back edge is expanded (δΦ/Φ< 0), which deactivates the proteins in this region and the kinetics of attachment is therefore very slow. Assuming that the rate of detachment is not affected by a variation of the local density, the dynamics of the back region consists mainly in the detachment of proteins, with a rate that only depends on the gain of elastic energy,
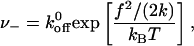 |
where 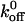 is the rate of detachment in the absence of stress.
is the rate of detachment in the absence of stress.
FAs Only Grow Within a Range of Stress. As long as the ratio v+/v– > 1, the adhesion grows in the direction of the force. This condition fixes a minimal value for the force, fm, that enables the growth of the FA. Forces smaller than fm cannot induce growth of the adhesions but shrink. This force threshold depends on the precise chemical nature of the adhesion, because 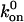 and
and  are sensitive to the molecular architecture. Increased phosphorylation, for example, would modify the force threshold. Forces larger than fm thus drive the growth of FAs. However, there is an upper limit to the force: very large stresses or reduced immobilization of the extracellular matrix (k → 0) lead to positive values for ΔE during the process of growth. We therefore predict that FAs cannot grow under such conditions, consistent with the observation of the transformation of FAs into fibrillar adhesions (11). The maximal force that enables the growth of an FA is then
are sensitive to the molecular architecture. Increased phosphorylation, for example, would modify the force threshold. Forces larger than fm thus drive the growth of FAs. However, there is an upper limit to the force: very large stresses or reduced immobilization of the extracellular matrix (k → 0) lead to positive values for ΔE during the process of growth. We therefore predict that FAs cannot grow under such conditions, consistent with the observation of the transformation of FAs into fibrillar adhesions (11). The maximal force that enables the growth of an FA is then
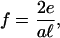 |
where the adsorption of a new protein complex no longer compensates for the energy cost of the mechanical, stress-induced deformation. Stress fibers have been observed to pull on mature FAs with a stress f/a = 5.5 nN/μm2 (13), which fixes a minimal range of values for the enthalpy of reaction, e, of one protein complex of area a2 such that our model will reproduce the observed growth of stressed FAs (as opposed to their shrinking as the force is increased) (8, 10):
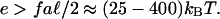 |
The consumption of one molecule of ATP releases ≈10 kBT. The addition of a single additional protein complex to an FA involves several chemical reactions (22); the required range of values for the energy, e, for growth with increasing force seems then to be realistic.
Concomitant growth of FAs at the front end and its disruption at its back edge give rise to an apparent sliding of the FA (see Movie 1). Our analysis shows that the movement of FAs corresponds to an exchange of molecules and not a simple translational motion of the structure. This result is consistent with several observations that focused on integrins (23) or adaptor proteins (24). Nevertheless, a larger-scale model of the kinetics of aggregation of the additional protein complexes is needed to extend the dynamic analysis to late times to account for the observed saturation effects (8).
Experimental Correlations and Predictions
We propose a model that assumes that mechanosensitivity of FAs is directly related to the local deformation of molecules that form the adhesive junction (e.g., the integrin layer) as a result of directional, cytoskeletal forces. This assumption allows us to use elasticity theory to predict that stressed FAs grow in the direction of the applied force, as observed. The growth process is shown to be sensitive to both the elastic rigidity of the extracellular matrix and the strength of its adhesion onto the underlying matrix. We conclude that the apparent observed sliding of FAs is caused by a combination of the attachment of new molecules at the energetically favorable front end of the adhesion site and the symmetrical detachment of others at the back, where the deformations favor desorption. All these predictions are in agreement with observations.
We predict in our model that the growth of FAs is a thermodynamically favorable process associated with a large enthalpy of reaction (several tens of kBT) and does not rely on fluctuations of energy. We distinguish two other types of adhesions that are transient, and may describe two types of FXs: (i) transient adhesions that are thermodynamically unstable and only exist because of fluctuations of energy (induced by the chemical activity of the cell); the chemical composition of such adhesions is different from FAs and they do not resist forces (i.e., their size decreases with increasing force), and (ii) transient adhesions that have chemical compositions similar to growing FAs but in which the lack of stability is related to the fact that the cytoskeletal forces pulling on them are too weak to cause growth; these adhesions are thermodynamically stable but kinetically unstable, because the desorption of units from the back region outpaces the adsorption of new units in front. For such adhesions, we predict that the amplitude of the local cytoskeletal forces determines the growth process: forces that are too small lead to shrinking adhesions, whereas forces that are too large translocate FAs with a stick-slip motion. We associate this latter situation with fibrillar adhesion, because the evolution of the size of the adhesion becomes independent of the force. Finally, we predict in our model that the initial speed of growth of FAs is exponentially dependent on the local force.
Several experiments can be performed that are crucial tests of our model. First is a test of the assumption that cytoskeletal stresses are applied only to a finite area of the adhesion. A measurement of the relative extent of the integrin layer and the stress fibers in the plane of the adhesion could validate this hypothesis. A second test of our theory would be a measurement of the exponential dependence of the initial speed of growth with the local force; time-resolved experiments on cells plated onto patterned substrates should enable such observations. Finally, varying cell contractility should lead to the observation of the three types of adhesions (FXs, FAs, and fibrillar adhesion) when increasing the force. Finally, one can check whether the integrin layer precedes the formation of the protein plaque when an FA grows. Time-resolved experiments in which integrins and a plaque protein are tagged separately would test this scenario directly. However, although separation of the integrin layer dynamics from the protein plaque dynamics certainly simplifies the interpretation of our model, one can still imagine our model being applicable to the growth of units that include both the integrins and plaque proteins.
Our basic hypothesis is the presence of a mechanosensor within the adhesion. Alternate locations of the mechanosensor can also be imagined. One hypothesis is that the response of the adhesion to stress is triggered by the opening of ion channels. Glogauer et al. (25) show that a normal force induces F-actin recruitment preceded by calcium influx. Ion channels, however, cannot account for the polarized response of FAs to force. The diffusion of such small ions as calcium is very fast (D ≈ 10–10 to 10–12 m2/s), and thus they become isotropically distributed in a very short time; the growth of FAs should then be isotropic on the usual time scales of experiments (several minutes). We therefore conclude that ion channels cannot be the initiators of the response of FAs to force. This conclusion does not contradict the results shown in ref. 25, because they demonstrate that, in their system, normal forces are not involved in the growth of FAs. Nevertheless, we do not exclude ion channels from participating in other stages of growth, for example in the regulation of the adhesion size. Another scenario could be that the mechanosensor is a protein localized in the actin cytoskeleton (M. P. Sheetz, personal communication). This effect could indeed be responsible for FX formation. However, it cannot account for the local and directional growth of stressed FAs; the same argument as above shows that mechanisms based on diffusion should lead to an isotropic response, because diffusion times are much faster than the time scales involved in FA maturation (8). Experiments with cells plated onto very force-sensitive pattern substrates (16) should allow one to measure the size of FAs as a function of the cytoskeletal forces. Such experiments could discriminate between these different possibilities.
Supplementary Material
Acknowledgments
We thank C. Ballestrem for providing the additional material and M. Elbaum for comments. We are grateful to M. P. Sheetz, L. Addadi, and A. Bershadsky for useful discussions. B.G. and S.A.S. are grateful to the Israel Science Foundation and the Clore Center for Biological Physics for support. B.G. is the E. Neter Professor for Cell and Tumor Biology and S.A.S. is the Fern and Manfred Steinfeld Professor (both at the Weizmann Institute of Science).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: FA, focal adhesion; FX, focal complex.
Footnotes
The front and back edges of the adhesion are defined relative to the direction of the force induced by stress fibers. They should not be confused with the front and back of a moving cell (see Fig. 1).
This assumption can be tested experimentally by time-resolved experiments in which integrins and a plaque protein are tagged separately (3). Double immunofluorescence of actin and vinculin already shows that the density of the stress fibers decreases at the edges of the adhesion (B. Zimerman and B.G., unpublished data).
References
- 1.Geiger, B. (2001) Science 294, 1661–1663. [DOI] [PubMed] [Google Scholar]
- 2.Giancotti, F. G. & Ruoslahti, E. (1999) Science 285, 1028–1032. [DOI] [PubMed] [Google Scholar]
- 3.Zaidel-Bar, R., Ballestrem, C., Kam, Z. & Geiger, B. (2003) J. Cell Sci. 116, 4605–4613. [DOI] [PubMed] [Google Scholar]
- 4.Pelham, R. J., Jr., & Wang, Y.-L. (1997) Proc. Natl. Acad. Sci. USA 94, 13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo, C.-M., Wang, H.-B., Dembo, M. & Wang, Y.-L. (2000) Biophys. J. 79, 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischofs, I. B. & Schwarz, U. S. (2003) Proc. Natl. Acad. Sci. USA 100, 9274–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiger, B. & Bershadsky, A. D. (2002) Cell 110, 139–142. [DOI] [PubMed] [Google Scholar]
- 8.Riveline, D., Zamir, E., Balaban, N. Q., Schwarz, U. S., Ishizaki, T., Narumiya, S., Kam, Z., Geiger, B. & Bershadsky, A. D. (2001) J. Cell Biol. 153, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choquet, D., Felsenfeld, D. P. & Sheetz, M. P. (1997) Cell 88, 39–48. [DOI] [PubMed] [Google Scholar]
- 10.Sawada, Y. & Sheetz, M. P. (2002) J. Cell Biol. 18, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz, B. Z., Zamir, E., Bershadsky, A. D., Kam, Z., Yamada, K. M. & Geiger, B. (2000) Mol. Cell. Biol. 11, 1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zamir, E. & Geiger, B. (2001) J. Cell Sci. 114, 3583–3590. [DOI] [PubMed] [Google Scholar]
- 13.Balaban, N. Q., Schwarz, U. S., Riveline, D., Goichberg, P., Tzur, G., Sabanay, I., Mahalu, D., Safran, S. A., Bershadsky, A. D., Addadi, L. & Geiger, B. (2001) Nat. Cell Biol. 3, 466–472. [DOI] [PubMed] [Google Scholar]
- 14.Davies, P. F., Robotewskyj, A. & Griem, M. L. (1994) J. Clin. Invest. 93, 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolas, A. & Safran, S. A. (2004) Phys. Rev. E Stat. Nonlin. Soft. Matter Phys. 69, 051902. [DOI] [PubMed] [Google Scholar]
- 16.Arnold, M., Cavalcanti-Adam, E. A., Glass, R., Bluemmel, J., Eck, W., Kantlehner, M., Kessler, H. & Spatz, J. (2004) Chemphyschem 3, 383–388. [DOI] [PubMed] [Google Scholar]
- 17.Bausch, A. R., Ziemann, F., Boulbitch, A. A., Jacobson, K. & Sackmann, E. (1998) Biophys. J. 75, 2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landau, L. D. & Lifshitz, E. M. (1970) Theory of Elasticity (Pergamon, New York), 2nd Ed.
- 19.Caroli, C. & Nozières, P. (1998) Eur. Phys. J. B 4, 233–246. [Google Scholar]
- 20.Zamir, E., Katz, M., Posen, Y., Erez, N., Yamada, K. M., Katz, B.-Z., Lin, S., Lin, D. C., Bershadsky, A. D., Kam, Z. & Geiger, B. (2000) Nat. Cell Biol. 2, 191–197. [DOI] [PubMed] [Google Scholar]
- 21.Bell, G. I. (1978) Science 200, 618–627. [DOI] [PubMed] [Google Scholar]
- 22.Zamir, E. & Geiger, B. (2001) J. Cell Sci. 114, 3577–3579. [DOI] [PubMed] [Google Scholar]
- 23.Ballestrem, C., Hinz, B., Imhof, B. A. & Wehrle-Haller, B. (2001) J. Cell Biol. 155, 1319–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuruta, D., Gonzales, M., Hopkinson, S. B., Otey, C., Khuon, S., Goldmann, R. D. & Jones, J. C. R. (2002) FASEB J. 16, 866–868. [DOI] [PubMed] [Google Scholar]
- 25.Glogauer, M., Arora, P., Yao, G., Sokhlov, J., Ferrier, J. & McCulloch, C. A. G. (1997) J. Cell Sci. 110, 11–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.