Abstract
Oocytes of most vertebrates arrest at metaphase of the second meiosis (meta-II) to await fertilization, thus preventing parthenogenetic activation. This arrest is caused by a cytoplasmic activity called cytostatic factor (CSF), which was first identified in the frog Rana pipiens oocyte >30 years ago. CSF arrest is executed by maintaining the activity of cyclin B-Cdc2 at elevated levels largely through prevention of cyclin B destruction. Although CSF arrest is established by the Mos-mitogen-activated protein kinase pathway and is released by the Ca-calmodulin kinase II pathway, it remains unclear precisely how cyclin B destruction is regulated. Recently, an early mitotic inhibitor, Emi1, was reported to be a critical component of CSF. This report has been expected to provide a final resolution to the CSF problem because Emi1 inhibits the anaphase-promoting complex/cyclosome, a ubiquitin ligase for cyclin B destruction, through sequestration of Cdc20, an activator for the anaphase-promoting complex/cyclosome. In mitotic cycles, however, Emi1 is destroyed in every pro-metaphase, and accordingly, it is unclear why Emi1 should be required for CSF activity, which is seen only in meta-II. Here, we show that Emi1 is absent in unfertilized mature Xenopus eggs and that exogenous Emi1 is destroyed in meta-II and mitotic metaphase. The expression of Emi1 in oocytes hinders meiotic progression. Although both Emi1 and Mos can inhibit progression through M phase, the Emi1-mediated arrest does not require mitogen-activated protein kinase activity and is not released by Ca. Together, our results indicate that Emi1 is unlikely to be a component of CSF.
Meiosis is comprised of two consecutive M phases, meiosis I and II, which result in the production of haploid gametes. In most metazoan oocytes, the cell cycle arrests twice during meiosis. The first arrest occurs at prophase of meiosis I and its resumption is generally controlled by a maturation-inducing hormone. The second arrest occurs after the hormone-initiated meiotic resumption but before fertilization. Because failure in the second arrest often leads to initiation of embryonic cell cycles in the absence of fertilization, the second arrest is implicated in the prevention of parthenogenesis (see ref. 1 for review). In most vertebrates, the second arrest is at metaphase of the second meiosis (meta-II), and the activity responsible for the meta-II arrest has been termed cytostatic factor (CSF) (see ref. 2 for review), whose essential components are Mos (3) and the downstream effector, mitogen-activated protein kinase (MAPK) (4, 5). Although CSF was originally defined in the meta-II arrest of amphibian Rana pipiens oocytes (6), the same Mos-MAPK pathway causes the second arrest at G1 phase after completion of meiosis II in starfish Asterina pectinifera eggs (7), and MAPK is responsible for preventing DNA replication in unfertilized sea urchin eggs arrested at the G1 phase (8). Consequently, CSF can be considered as a cell cycle arrest factor that prevents parthenogenetic activation, regardless of the arrest phase during the meiotic cycles (see ref. 9 for review). However, it is largely unknown how the Mos-MAPK pathway brings about the CSF activity in any arrest.
CSF has been studied most extensively in Xenopus oocyte system (see ref. 10 for review). Throughout meta-II, CSF maintains the activity of cyclin B-Cdc2 kinase at elevated levels through prevention of the proteasome-dependent degradation of cyclin B that is mediated by the anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin ligase (see ref. 11 for review). Immediately downstream of Mos-MAPK, p90Rsk is activated (12, 13) to phosphorylate and activate the spindle checkpoint kinase, Bub1, resulting in inhibition of the APC/C (14, 15). Although Mad1 and Mad2, the spindle checkpoint proteins downstream of Bub1, are required for CSF activity, they appear to be involved in the APC/C inhibition in a manner distinct from the spindle checkpoint (16). However, CSF arrest is cancelled by the release of Ca ions at fertilization or during artificial egg activation. The CSF-mediated inhibition of the APC/C is overridden by Ca through activation of calmodulin kinase II (17), although how calmodulin kinase II releases the inhibition of the APC/C is unknown. Thus, it remains unclear how CSF inhibits the APC/C and how the inhibition is released.
Reimann and Jackson (18) recently reported that an early mitotic inhibitor, Emi1, constitutes an essential component of CSF as an inhibitor of the APC/C. In fact, Emi1 inhibits the APC/C by sequestrating Cdc20, an activator of the APC/C (19, 20), and Ca addition causes the dissociation of Cdc20 from Emi1 (18). Nonetheless, Emi1 is usually destroyed during prometaphase of the mitotic cycle through the SKP1/Cul1/F-box protein (SCF) ubiquitin ligase-proteasome system (21, 22). Hence, the question of why Emi1 is not destroyed in meta-II oocytes that are arrested by CSF remains to be addressed.
To investigate this question, we have monitored the levels of Emi1 during Xenopus oocyte maturation and examined the effects of Emi1 on cell cycle progression. Our observations revealed that Emi1 is undetectable throughout the meiotic cycles of oocytes and that exogenously introduced Emi1 is destroyed before each meiotic metaphase, excluding the possibility that Emi1 is a component of CSF.
Materials and Methods
Oocytes, Eggs, Embryos, and Cultured Cells. Xenopus laevis oocytes at stages I and VI (23) were obtained, and meiosis was resumed as described (24). To determine the meiotic stage of maturing oocytes after germinal vesicle breakdown (GVBD), oocytes were fixed and stained with 3.7% formaldehyde in MMR (100 mM NaCl/2 mM KCl/1 mM MgCl2/2 mM CaCl2/0.1 mM EDTA/5 mM Hepes-KOH, pH 7.8) (25) containing 10 μg/ml Hoechst 33342, and their chromosomes at the animal pole region were observed through an epifluorescence microscope. Mature eggs and embryos were obtained as described (26). Xenopus A6 cells were cultured in 70% Leibovitz-15 medium supplemented with antibiotics at 27°C.
Egg Extracts. CSF-arrested extracts (27) were prepared from unfertilized eggs as described (28). Interphase egg extracts were prepared by adding 0.4 mM CaCl2 along with 50 μg/ml cycloheximide to CSF-arrested extracts and subsequently incubating for 30 min at 22°C. Mitotic cycling extracts were prepared according to Murray (27) with modifications as described (24). Demembranated sperm nuclei were added to all extracts to monitor the cell cycle phase (24).
Recombinant Proteins and mRNA Synthesis. Full-length Emi1 cDNA (19) was isolated from Xenopus total egg cDNAs by PCR. An undegradable, β-TrCP-binding site mutant (AA-Emi1) was produced by substituting S95 and S99 in the WT-Emi1 with alanine, as described (22). Emi1-ΔZBR mutant (amino acids 1–338 of AA-Emi1; ΔZ-Emi1) was obtained as described (19). The WT and mutant Emi1 cDNAs were cloned into pBS-RNT3, and mRNAs were synthesized by using a mMESSAGE mMACHINE T3 kit (Ambion, Austin, TX). For maltose-binding protein (MBP)-Emi1 fusion proteins, WT and mutant Emi1 cDNAs were cloned into a plasmid vector pMAL-c2X (New England Biolabs) and transformed into Escherichia coli BL21. The full-length Emi1 cDNA was cloned into pGEX-5X-2 plasmid vector (Amersham Pharmacia). Fusion proteins were expressed, purified, and concentrated on Vivaspin 30K PET (Vivascience, Hanover, Germany) as described (24). The concentration of fusion proteins was determined by using Coomassie protein assay reagent (Pierce), or by SDS/PAGE, with BSA as a standard. GST-Xenopus Mos was bacterially expressed, purified, and used at a final concentration of 30 μg/ml.
Emi1 Abs, Immunoblot, and Immunoprecipitation. Antisera for Xenopus Emi1 were obtained by immunizing four rabbits with MBP-Emi1 produced by E. coli. Emi1 Abs were affinity-purified with GST-Emi1 from the antiserum showing the highest titer for MBP-Emi1 in immunoblot. In brief, GST-Emi1 produced by E. coli was separated by SDS/PAGE and transferred to nitrocellulose membranes. The membranes were stained with Ponceu S, and the GST-Emi1 band was cut out. After blocking with skimmed milk, the membranes were incubated with the Emi1 serum, one-half diluted with TBS (150 mM NaCl/50 mM Tris·HCl, pH 7.5) for 12 h at 4°C. After washing thoroughly with TBS and 0.15 M NaCl, the membranes were treated with 0.2 M glycine·HCl (pH 2.8) for 10 min, and the glycine·HCl solution was removed and immediately neutralized with 1 M Tris·HCl (pH 9.0). BSA and NaN3 were added to the Ab solution to final concentrations of 0.1% and 0.02%, respectively. For immunoblots, four oocytes, eggs, or embryos were homogenized with 16 μl of ice-cold βGP-EB (240 mM β-glycerophosphate/20 mM EGTA/5 mM MgCl2/20 mM Hepes-KOH, pH 7.5) by pipetting and immediately centrifuged at 15,000 × g for 1 min at 2°C. The supernatant (12 μl) was mixed with 4 μl of 4 × SDS sample buffer and boiled for 5 min. SDS/PAGE and immunoblot analysis was performed as described (24) by using Abs for Emi1, Xenopus cyclin B2 (a gift from J. L. Maller, University of Colorado, Denver), Xenopus Mos, and MAPK (Upstate Biotechnology, Lake Placid, NY). For immunoprecipitation, Protein G CL4B-beads (Sigma) were covalently conjugated with Emi1 Abs. Lysates (50 μl) were incubated for 90 min on ice with 10 μl of Emi1 Ab beads equilibrated with EB (100 mM KCl/2 mM MgCl2/20 mM Hepes-KOH, pH 7.4), with gentle stirring at every 10 min. After washing three times with wash buffer (0.5 M NaCl/5 mM EDTA/0.5% Triton X-100/20 mM Tris·HCl, pH 7.4) and once with EB, immunocomplexes were eluted with 2× SDS sample buffer and separated by SDS/PAGE for immunoblot.
Histone H1 Kinase Assay. Extracts of oocytes and eggs were prepared by the same method as for immunoblotting and immediately frozen in liquid nitrogen. After thawing, histone H1 kinase activity was measured as described (24).
Results and Discussion
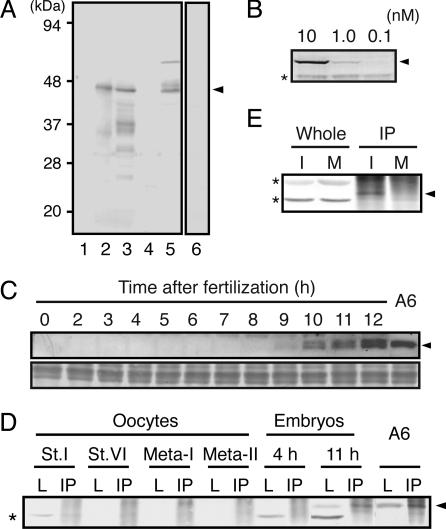
Expression Levels of Emi1 Protein in Xenopus Oocytes and Embryos. Based on Reimann et al. (19), we have isolated a cDNA of Xenopus Emi1 by PCR and confirmed that its sequence is the same as that reported. Polyclonal Abs against an MBP full-length Emi1 fusion protein were raised in rabbits and were affinity-purified with a GST full-length Emi1 fusion protein. The specificity of the Emi1 Abs was examined by immunoblotting of a reticulocyte lysate expressing Emi1 protein and of extracts from immature oocytes that had been injected with Emi1 mRNA at 12 h before extraction (Fig. 1A). The Abs showed high specificity, reacting predominantly with exogenously expressed Emi1 at 44 K; this band was not detected with Abs preabsorbed with GST-Emi1. The bands <36 K, seen in oocyte extract, are likely to be abortive products of Emi1 or possibly the products of partial degradation during extraction of oocytes. However, no band was seen at the expected position of endogenous Emi1 in the immunoblot of uninjected oocytes, even though the titer of the Abs was high enough to detect as little as 1 nM MBP-Emi1 contained in egg extracts (I-phase extract) (Fig. 1B).
Fig. 1.
Emi1 expression in Xenopus oocytes, embryos, and cultured cells. (A and B) Characterization of affinity-purified Emi1 Abs used in the present study. (A) Reticulocyte lysate added with water (lane 1) or with mRNA for Xenopus Emi1 (lane 2), lysates of fully grown immature oocytes injected with Emi1 mRNA (lane 3) or water (lane 4), and A6 culture cells (lane 5) were immunoblotted with Emi1 Abs. The sample of lane 3 was immunoblotted with the control Emi1 Abs that had been preabsorbed by MBP-Emi1 (lane 6). (B) Egg extracts containing MBP-WT-Emi1 at the concentrations of 10 (lane 1), 1 (lane 2), and 0.1 nM (lane 3) were immunoblotted with Emi1 Abs. (C–E) Emi1 expression in oocytes, early embryos, and cultured cells. (C Upper) Lysates of A6 cells and embryos at various times after fertilization were immunoblotted with Emi1 Ab. (Lower) A part of the same transferred membrane was stained with amide black to support that equal levels of proteins were applied. (D) Lysates of growing oocytes at stage I (St.I), stage VI (St.VI), maturing oocytes at Meta-I and Meta-II, embryos at 4 h (4h; early blastula) and 11 h (11 h; early gastrula) after fertilization, and A6 cells (A6) were prepared and either directly immunoblotted (L) or immunoprecipitated with Emi1 Ab beads before immunoblotting (IP). (E) Mitotic cycling extracts from activated eggs at 40 min (at the end of interphase, I) and 60 min (M phase, M) after incubation were either directly immunoblotted (Whole) or immunoprecipitated with Emi1 Ab beads before immunoblotting (IP). Arrowheads and asterisks indicate the position of Emi1 proteins and nonspecific signals, respectively.
To examine whether our Emi1 Abs can detect endogenous Emi1, we performed immunoblotting for growing oocytes, developing embryos, and Xenopus-cultured cells. As shown in Fig. 1 C and D, extracts from embryos at gastrula stage (11 h after fertilization) and A6 cells (see also Fig. 1 A) gave a clear signal at the anticipated position, but those from oocytes at the all stages examined and early embryos until 9 h after fertilization did not. The same protein was contained in anti-Emi1 immunoprecipitates (IPs) from lysates of gastrula and A6 cells. It is unlikely that endogenous Emi1 in oocytes and early embryos was undetectable because of degradation during extraction procedure, because total protein levels were made equal and the dose-dependent accumulation of Emi1 was detectable upon injection of its mRNA into immature oocytes (see Fig. 3A). These observations strongly suggest that Emi1 expression is first activated after the gastrula stage of development.
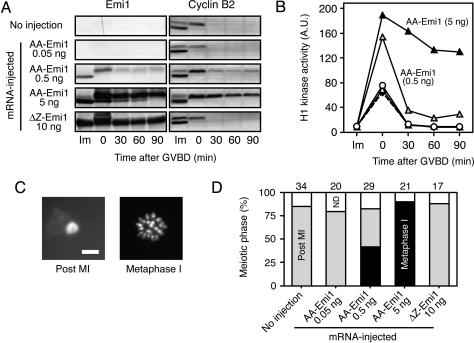
Fig. 3.
Persistence of Emi1 protein in maturing oocytes causes meiotic arrest at meta-I. Fully grown immature oocytes were injected with mRNA for AA-Emi1 or ΔZ-Emi1, incubated for 12 h, and stimulated with progesterone. Maturing oocytes were treated with cycloheximide after GVBD. (A and B) Oocytes injected with none (white circles) or AA-Emi1 mRNA of 0.05 (white triangles), 0.5 (gray triangles), and 5.0 (black triangles) ng or 10 ng of ΔZ-Emi1 mRNA (diamonds with broken line) were examined for Emi1 and cyclin B2 expression by immunoblotting (A) and for histone H1 kinase activity (B) before progesterone treatment (Im) and at the indicated times after GVBD. A.U., arbitrary units. (C) Oocyte chromosomes at the animal pole region were stained with Hoechst 33342 and observed at 180 min after GVBD. Either a chromatin mass of the extruded polar body (Post MI) or metaphase chromosomes without chromatin mass (Meta-I) was observed. (Scale bar, 10 μm.) (D) Meiotic phases of oocytes at 180 min after GVBD were examined for each oocyte group. Numbers above the graph indicate the numbers of examined oocytes. ND, not determined.
To further examine whether or not Emi1 is expressed in eggs, we performed immunoblotting of IPs obtained from various egg extracts with Emi1 Abs. We reasoned that IPs were likely to concentrate Emi1, if contained in extracts. As shown in Fig. 1E, a small amount of Emi1 was detected in the IP from cycling egg extract at the stage just before entry into M phase, but no Emi1 was seen in IPs from other extracts including extracts from immature (stage VI), meta-I and meta-II oocytes, and cycling extract that had entered M phase (Fig. 1 D and E). Although we cannot exclude the possibility that Emi1 is present at a very low level in fertilized eggs, it never accumulated to a concentration >1 nM during oocyte maturation and cleavage period, as judged by the titer of our Abs. These observations contrast to previous studies (18, 19) reporting that Emi1 is present at levels of 300 nM both in mature oocytes and interphase egg extracts and that Emi1 protein levels do not change significantly until the first mitosis after fertilization.
Fate of Emi1 Protein During Meiotic and Mitotic Cycles. Because the discrepancy in protein levels of Emi1 was more than two orders of magnitude between previous reports (18, 19) and our present observations, we examined the stability of Emi1 protein in oocytes during maturation (Fig. 2). For comparison, we prepared a stable mutant of Emi1 (AA-Emi1) (see refs. 19 and 22), in which the two serine residues required for recognition by SCF have been altered to alanine. When immature oocytes were injected with 1 ng of mRNA for WT-Emi1, ≈100 nM WT-Emi1 protein accumulated in the oocytes by 12 h after injection. However, after progesterone treatment to induce the resumption of meiosis, the amount of WT-Emi1 was greatly reduced at the GVBD stage and completely disappeared in mature eggs (180 min after GVBD) (Fig. 2 A). In contrast, AA-Emi1 protein that had been expressed in immature oocytes was stable at the GVBD stage and remained even 180 min later. Both Emi1 proteins had a reduced mobility from the GVBD stage onward, probably caused by phosphorylation. This observation indicates that Emi1 is very unstable at meta-I.
Fig. 2.
Emi1 protein is unstable in meiotic and mitotic M phases. (A and B) Stability of Emi1 in M phase of meiosis I and II in maturing oocytes. Fully grown immature oocytes were injected with mRNA for WT-Emi1 or AA-Emi1, incubated for 12 h, and stimulated with progesterone (A), and maturing oocytes were injected with mRNA for WT-Emi1 or an AA-Emi1 at 60–90 min after GVBD (B). Oocytes before progesterone treatment (Im), those at the indicated times after GVBD (A), and maturing oocytes at the indicated times after mRNA injection (B) were examined for expressed Emi1 by immunoblotting with Emi1 Abs. (C–E) Stability of Emi1 protein in egg extracts. CSF extracts (C), interphase extracts (D), and mitotic cycling extracts (E) were added with MBP-WT-Emi1 or MBP-AA-Emi1 fusion proteins to a final concentration of 100 nM and immunoblotted with Emi1 Abs at various times after incubation. The same CSF extracts were immunoblotted with cyclin B Abs (C). Mitotic cycling extracts entered M phase 40 min after incubation (E).
We also examined the stability of Emi1 in meiosis II, which is more important to CSF arrest in mature oocytes. We injected Emi1 mRNA into maturing oocytes at the interkinesis stage (60–90 min after GVBD) and examined the change in the amount of expressed Emi1 protein by immunoblotting (Fig. 2B). These oocytes were confirmed to be free from activation by the absence of the capping of the pigmented area. The results showed that the amount of WT-Emi1 decreased with time, whereas AA-Emi1 steadily accumulated, indicating that Emi1 is also unstable in meiosis II. Similarly, when added to CSF-arrested extracts to a final concentration of 100 nM, MBP-WT-Emi1 was unstable, whereas MBP-AA-Emi1 was stable; cyclin B was stably present in both cases, confirming that the extracts stayed in M phase (Fig. 2C).
To examine Emi1 stability in mitotic cycles, we added MBP-Emi1 proteins to mitotic cycling extracts (100 nM). When added to cycling extracts treated with cycloheximide (I-phase extracts), MBP-WT-Emi1 was stable for at least 2 h (Fig. 2D), but when added to cycling extracts without cycloheximide treatment, it was mostly degraded by 40 min after incubation, at which time the extract entered M phase (Fig. 2E). These observations are consistent with detection of endogenous Emi1, albeit at very low levels, in cycling extracts in interphase but not in M phase (Fig. 1E). In contrast, MBP-AA-Emi1 added to cycling extracts was stable when the extract entered M phase; that the band shifted up at 40 min after incubation and remained up-shifted afterward suggested the cell cycle of the extract was arrested in M phase (Fig. 2E).
Together, we find that WT-Emi1 is destroyed at every M phase during both meiotic cycles and during cleavage cycles. Whereas these observations confirm previous study in mitotic cycles (19), they exclude the possibility that Emi1 persists in each metaphase of meiotic cycles, particularly in meta-II, and thus are incompatible with the previous idea (18) that Emi1 functions in meta-II as CSF.
Effects of Emi1 on Meiotic Cell Cycle Progression. Although the results so far strongly suggest that Emi1 is not present in oocytes and unfertilized eggs, we investigated what would happen to meiotic progression in maturing oocytes if Emi1 had been present. To do this, it was necessary to use AA-Emi1 because WT-Emi1 was unstable in M phase. For experiments in which AA-Emi1 was used, we also prepared an AA-Emi1 derivative (ΔZ-Emi1) that lacked the C terminus domain required to inhibit Cdc20 (see refs. 19 and 22); this Emi1 mutant served as a negative control for AA-Emi1.
It was expected that the presence of Emi1 in maturing oocytes during meiosis I would cause a meiotic arrest at meta-I. Therefore, we examined meiotic progression in oocytes that had expressed the stable Emi1 mutants, paying particular attention to whether meta-I arrest took place or not. To distinguish between meta-I arrest and normal meta-II arrest, oocytes that had been induced to resume meiosis were treated with cycloheximide at the GVBD stage to suppress the transition to meiosis II; normal maturing oocytes in which protein synthesis was inhibited after GVBD enter S phase after exit from meiosis I, with a polar body appearing instead of metaphase chromosomes well aligned on the spindle at the animal pole region (see ref. 26). In the experiment presented in Fig. 3, immature oocytes were injected with ΔZ-Emi1 mRNA or various amounts of AA-Emi1 mRNA and after incubation for 12 h, treated with progesterone to induce the resumption of meiosis. Before progesterone treatment, the amounts of AA-Emi1 protein that accumulated in oocytes were proportional to the amount of injected mRNA. When GVBD occurred, a portion of Emi1 was destroyed, but the remaining Emi1 persisted for at least 90 min (Fig. 3A). In oocytes expressing the greatest amount of AA-Emi1 protein, the degradation of cyclin B at the meiosis I exit was mostly inhibited (Fig. 3A), with Cdc2 activity being maintained at a high level (Fig. 3B), and meiosis was arrested at metaphase; this metaphase was unambiguously meiosis I because no polar body was observed at the animal pole region of the oocytes (Fig. 3 C and D). In contrast, cyclin B degradation in meiosis I was not inhibited by ΔZ-Emi1.
When AA-Emi1 was expressed in maturing oocytes after the meiosis I exit, the oocytes arrested at meta-II, as in normal oocytes. However, the cell cycle remained arrested at metaphase even after the oocytes were activated by Ca-ionophore treatment (see also below), despite the occurrence of other activation responses including the capping (data not shown).
Thus, the presence of Emi1 protein is detrimental to the progression of meiosis, contrary to the previous report that meiosis I proceeds normally whereas Emi1 remains constant at levels of 300 nM after GVBD (18).
Emi1-Mediated Meta-II Arrest Is Distinct from CSF Arrest. Finally, we asked whether Emi1 induces M-phase arrest when introduced to extracts from mature and activated eggs. As shown in Fig. 4A, when added to cycling extract, Emi1 protein caused an M-phase arrest with elevated levels of H1 kinase activity and the persistence of cyclin B; to induce M-phase arrest in cycling extract, 100 nM AA-Emi1 was sufficient, whereas as much as 300 nM WT-Emi1 was required, probably because of degradation of WT-Emi1 in M phase. The occurrence of M-phase arrest in extracts was also indicated by the morphology of sperm nuclei incubated in the extracts (data not shown). Thus, we confirmed that Emi1 effectively causes M-phase arrest in mitotic cycles, just as Mos does.
Fig. 4.
Emi1 causes M-phase arrest in mitotic cycling extracts independently of MAPK activity. (A) Mitotic cycling extracts were added with 100 nM MBP-AA-Emi1 (squares), 100 nM (triangles with broken line), 300 nM MBP-WT-Emi1 (triangles with solid line), or 600 nM MBP-ΔZ-Emi1 (circles) and examined for histone H1 kinase activity (Left) and for Emi1 (Center) and cyclin B2 (Right) proteins by immunoblotting at various times after incubation. (B) Mitotic cycling extracts were added with GST-Mos (Left), 200 nM MBP-AA-Emi1 (Center), or 600 nM MBP-ΔZ-Emi1 (Right) along with either control DMSO (circles) or 100 μM U0126 to inhibit MAPK activation (triangles). The changes in histone H1 kinase activity (Upper) and cyclin B2 (cyc B) and MAPK (Lower) were examined at various times after incubation. Filled and open symbols in the graphs represent the extracts where sperm chromatin was in M phase and interphase, respectively. Asterisks beside MAPK immunoblots indicate the position of active MAPK. A.U., arbitrary units.
However, as described above, mature eggs expressing AA-Emi1 did not leave meta-II after activation, a clear distinction from normal activated eggs. Therefore, the M-phase arrest induced by Emi1 may be different from that induced by Mos. To examine this further, we compared two criteria of CSF arrest in Emi1-induced and Mos-induced M-phase arrest, the requirement of MAPK activity to establish the arrest and the dependence on Ca ions to terminate the arrest. As shown in Fig. 4B, Mos failed to induce M-phase arrest in the absence of MAPK activity, as previously demonstrated (4). In contrast, when added to cycling extracts, 200 nM AA-Emi1 caused an M-phase arrest even in the absence of MAPK activity. It was noted, however, that the Cdc2 activity level in AA-Emi1-arrested extracts was significantly higher in extracts with MAPK activity than those without MAPK activity. Interestingly, Cdc2 activity levels in extracts arrested at M phase with Emi1 were remarkably higher than those in extracts arrested with Mos (see also Fig. 5). The 600 nM ΔZ-Emi1 did not cause any effect on mitotic cycles in extracts.
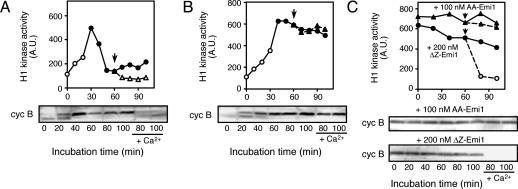
Fig. 5.
M-phase arrest in egg extracts caused by Emi1 is not terminated by Ca addition. (A and B) The response to Ca addition of mitotic cycling extracts arrested in M phase with either Mos or Emi1. Mitotic cycling extracts were added with either GST-Mos (A) or 200 nM MBP-AA-Emi1 (B) at the beginning of incubation and after a 60-min incubation, added with CaCl2 (arrows). (C) The response to Ca addition of CSF extracts added with Emi1. CSF extracts were added with either 100 nM MBP-AA-Emi1 (triangles) or 200 nM MBP-ΔZ-Emi1 (circles), and after a 60-min incubation, added with CaCl2 (arrows). Extracts with (symbols with broken lines) or without (symbols with solid lines) Ca addition were examined for histone H1 kinase activity and for cyclin B2 (cyc B) by immunoblotting at various times after incubation. Filled and open symbols in the graphs represent the extracts in which sperm chromatin was in M phase and interphase, respectively. A.U., arbitrary units.
To examine the sensitivity of Emi1- and Mos-arrested extracts to Ca ions, CaCl2 was added to cycling extracts that had been arrested in M phase with AA-Emi1 or Mos. Emi1-arrested extracts did not show any response to Ca addition (Fig. 5B), whereas Mos-arrested extracts exited M phase in response to Ca addition, with the accompanying cyclin B degradation and Cdc2 inactivation (Fig. 5A). The same results were obtained with CSF-arrested extracts. CSF-arrested extracts to which 100 nM AA-Emi1 had been added failed to degrade cyclin B in response to Ca addition, whereas those having been added with 200 nM ΔZ-Emi1 did so (Fig. 5C). The ability of AA-Emi1 to prevent the inactivation of cyclin B-Cdc2 after Ca addition was not caused by the mutation introduced to Emi1. CSF-arrested extracts that received 300 nM WT-Emi1 also failed to exit M phase when Ca was added within 15 min of the WT-Emi1 addition but exited M phase when Ca was added 60 min after the WT-Emi1 addition, presumably because of the partial degradation of WT-Emi1 during incubation (data not shown; see also Fig. 4A). This observation is again incompatible with a previous report (18) that Emi1 is stably present at the level of 300 nM in normally meta-II-arrested eggs, which can respond to Ca. Taken together, the present results strongly suggested that despite the ability of Emi1 to cause M-phase arrest both in mitotic cycling and CSF-arrested extracts, the mechanisms for establishing and terminating the arrest are different from those operating in CSF arrest.
In summary, we have shown that Emi1 is absent from mature eggs, that Emi1 hinders the meiotic progression in maturing oocytes, and that Emi1-induced M-phase arrest is different from CSF arrest in its independence of MAPK activity and its insensitivity to Ca ions. All of these results argue against the notion that Emi1 is responsible for CSF arrest in Xenopus eggs.
From where does the inconsistency between the previous study (18) and the present one arise? Both studies agree with the issues that Emi1 inhibits the APC/C and is destroyed every M phase in mitotic cycles. Based on these results, to function as CSF, it would be necessary for Emi1 to be exceptionally stable in meta-II. The present study, however, indicates that Emi1 is unstable both in meta-I and meta-II, as well as in the other mitotic metaphases. Nonetheless, the previous study presented immunoblot data of the presence of Emi1 during oocyte maturation and meta-II arrest (figure 2A in ref. 18). If these bands reflect Emi1, the protein levels would be much lower, possibly two orders lower than the estimated 300 nM. Unfortunately, no basal data in support of this estimation were presented anywhere. Low levels of Emi1 could not cause metaphase arrest because in our observations, even AA-Emi1 is not able to maintain metaphase arrest at 25 nM in CSF extracts. We are also unable to explain why in the previous study immunodepletion of Emi1 from CSF extracts caused the release from the arrest. Although we cannot exclude the possibility that the protein recognized as Emi1 by immunoblots in the previous study (18) may be related to Emi1 and may function as CSF, our results indicate that it is unlikely to be the product of the Emi1 clone that was originally reported and to which we have raised specific Abs.
Acknowledgments
We thank J. L. Maller for cyclin B Abs, N. Sagata and K. Uto for unpublished results, K. Tachibana and E. Okumura for suggestions, and M. J. Lohka for reading the manuscript. This work was supported by grants from the Ministry of Education, Science, and Culture of Japan and the Human Frontier Science Program (to T.K.).
Abbreviations: APC/C, anaphase-promoting complex/cyclosome; CSF, cytostatic factor; Emi1, early mitotic inhibitor 1; GVBD, germinal vesicle breakdown; MAPK, mitogen-activated protein kinase; meta-I and -II, metaphase of the first and second meiosis; IP, immunoprecipitate; MBP, maltose-binding protein.
References
- 1.Sagata, N. (1996) Trends Cell Biol. 6, 22–28. [DOI] [PubMed] [Google Scholar]
- 2.Masui, Y. (2000) Nat. Rev. Mol. Cell Biol. 1, 228–232. [DOI] [PubMed] [Google Scholar]
- 3.Sagata, N., Watanabe, N., Vande Woude, G. F. & Ikawa, Y. (1989) Nature 342, 512–518. [DOI] [PubMed] [Google Scholar]
- 4.Haccard, O., Sarcevic, B., Lewellyn, A., Hartley, R., Roy, L., Izumi, T., Erikson, E. & Maller, J. L. (1993) Science 262, 1262–1265. [DOI] [PubMed] [Google Scholar]
- 5.Shibuya, E. K. & Ruderman, J. V. (1993) Mol. Biol. Cell 4, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masui, Y. & Markert, C. L. (1971) J. Exp. Zool. 177, 129–145. [DOI] [PubMed] [Google Scholar]
- 7.Tachibana, K., Tanaka, D., Isobe, T. & Kishimoto, T. (2000) Proc. Natl. Acad. Sci. USA 97, 14301–14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll, D. J., Albay, D. T., Hoang, K. M., O'Neill, F. J., Kumano, M. & Foltz, K. R. (2000) Dev. Biol. 217, 179–191. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto, T. (2003) Curr. Opin. Cell Biol. 15, 654–663. [DOI] [PubMed] [Google Scholar]
- 10.Tunquist, B. J. & Maller, J. L. (2003) Genes Dev. 17, 683–710. [DOI] [PubMed] [Google Scholar]
- 11.Peters, J. M. (2002) Mol. Cell 9, 931–943. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt, R. R. & Ferrell, J. E., Jr. (1999) Science 286, 1362–1365. [DOI] [PubMed] [Google Scholar]
- 13.Gross, S. D., Schwab, M. S., Lewellyn, A. L. & Maller, J. L. (1999) Science 286, 1365–1367. [DOI] [PubMed] [Google Scholar]
- 14.Schwab, M. S., Roberts, B. T., Gross, S. D., Tunquist, B. J., Taieb, F. E., Lewellyn, A. L. & Maller, J. L. (2001) Curr. Biol. 11, 141–150. [DOI] [PubMed] [Google Scholar]
- 15.Tunquist, B. J., Schwab, M. S., Chen, L. G. & Maller, J. L. (2002) Curr. Biol. 12, 1027–1033. [DOI] [PubMed] [Google Scholar]
- 16.Tunquist, B. J., Eyers, P. A., Chen, L. G., Lewellyn, A. L. & Maller, J. L. (2003) J. Cell Biol. 163, 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorca, T., Cruzalegui, F. H., Fesquet, D., Cavadore, J. C., Mery, J., Means, A. & Doree, M. (1993) Nature 366, 270–273. [DOI] [PubMed] [Google Scholar]
- 18.Reimann, J. D. R. & Jackson, P. K. (2002) Nature 416, 850–854. [DOI] [PubMed] [Google Scholar]
- 19.Reimann, J. D. R., Freed, E., Hsu, J. Y., Kramer, E. R., Peters, J. M. & Jackson, P. K. (2001) Cell 105, 645–655. [DOI] [PubMed] [Google Scholar]
- 20.Reimann, J. D. R., Gardner, B. E., Margottin-Goguet, F. & Jackson, P. K. (2001) Genes Dev. 15, 3278–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guardavaccaro, D., Kudo, Y., Boulaire, J., Barchi, M., Busino, L., Donzelli, M., Margottin-Goguet, F., Jackson, P. K., Yamasaki, L. & Pagano, M. (2003) Dev. Cell 4, 799–812. [DOI] [PubMed] [Google Scholar]
- 22.Margottin-Goguet, F., Hsu, J. Y., Loktev, A., Hsieh, H.-M., Reimann, J. D. R. & Jackson, P. K. (2003) Dev. Cell 4, 813–826. [DOI] [PubMed] [Google Scholar]
- 23.Dumont, J. N. (1972) J. Morphol. 136, 153–179. [DOI] [PubMed] [Google Scholar]
- 24.Iwabuchi, M., Ohsumi, K., Yamamoto, T. M., Sawada, W. & Kishimoto, T. (2000) EMBO J. 19, 4513–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newport, J. & Kirschner, M. (1982) Cell 30, 675–686. [DOI] [PubMed] [Google Scholar]
- 26.Ohsumi, K., Sawada, W. & Kishimoto, T. (1994) J. Cell Sci. 107, 3005–3013. [DOI] [PubMed] [Google Scholar]
- 27.Murray, A. W. (1991) Methods Cell Biol. 36, 581–605. [PubMed] [Google Scholar]
- 28.Iwabuchi, M., Ohsumi, K., Yamamoto, T. M. & Kishimoto, T. (2002) Dev. Biol. 243, 34–43. [DOI] [PubMed] [Google Scholar]