Abstract
The goat (Capra hircus) is one of the first farm animals that have undergone domestication and extensive natural and artificial selection by adapting to various environments, which in turn has resulted in its high level of phenotypic diversity. Here, we generated medium-coverage (9–13×) sequences from eight domesticated goat breeds, representing morphologically or geographically specific populations, to identify genomic regions representing selection signatures. We discovered ~10 million single nucleotide polymorphisms (SNPs) for each breed. By combining two approaches, ZHp and di values, we identified 22 genomic regions that may have contributed to the phenotypes in coat color patterns, body size, cashmere traits, as well as high altitude adaptation in goat populations. Candidate genes underlying strong selection signatures including coloration (ASIP, KITLG, HTT, GNA11, and OSTM1), body size (TBX15, DGCR8, CDC25A, and RDH16), cashmere traits (LHX2, FGF9, and WNT2), and hypoxia adaptation (CDK2, SOCS2, NOXA1, and ENPEP) were identified. We also identified candidate functional SNPs within selected genes that may be important for each trait. Our results demonstrated the potential of using sequence data in identifying genomic regions that are responsible for agriculturally significant phenotypes in goats, which in turn can be used in the selection of goat breeds for environmental adaptation and domestication.
The goat (Capra hircus) is believed to be one of the first livestock species that underwent domestication approximately 10,000 years ago1,2, and therefore has been a witness to the historical progress of human civilization. A variety of natural or artificial factors (e.g., environmental changes, human migration, and socioeconomic influences) have shaped the phenotypic diversity of goats, leading to 557 registered goat breeds worldwide (FAO)3. Since the Neolithic age, goats have played economically important roles by providing various products (e.g., fiber, milk, meat, and hide) to the human population.
Artificial selection during domestication and production-oriented breeding has greatly shaped the level of genomic variability in goats. The genome of goats, which have diverse production potentials and extensive adaptation to diverse environments, provides a unique opportunity for identifying signatures associated with selection. Array- or sequencing-based detection of signatures during the selection process has been described in cattle4,5,6, chicken7, pigs8,9,10, sheep11,12, and recently in goats13,14,15. By using whole-genome resequencing (WGS) data, Benjelloun et al. identified positive selection sweeps of three indigenous goat populations in Morocco14, whereas Dong et al. described selection signals during goat domestication by analyzing the sequences of domestic goats and its wild progenitor, bezoar15. However, investigations on selection signatures with respect to selection purposes and breed formation of goats are limited. WGS using a group of DNA samples (Pool-seq) from the same individuals permits molecular biologists to simultaneously examine sequence divergence among populations, morphs, or breeds for hundreds of genes and gene families in a cost-efficient manner, especially for well-studied species with large genome sizes7,16.
The aim of this study was to detect evidence of signatures of recent selection among goats for different selection objectives. To do this, we investigated selection signatures using pooling sequencing of eight distinct goat populations (Supplementary Table S1), including a black coated breed (Taihang Black), a highland breed (Tibetan goat), two cashmere goat breeds (Inner Mongolia Cashmere and Shaanbei Cashmere) a breed for mohair (Angora), a dairy goat population (Saanen), a meat goat breed (Boer), and a mini goat breed with small body size (Guizhou Small) (Fig. 1a and b). By performing combined calculations for the ZHp and di values of all autosome SNPs, we observed a small number of strong selection signatures near known artificial selective genes in other animals. Our findings can be used to better understand genomic signatures under selection, as well as shed some light on genomic regions that harbor genes controlling production or adaptive traits in goats.
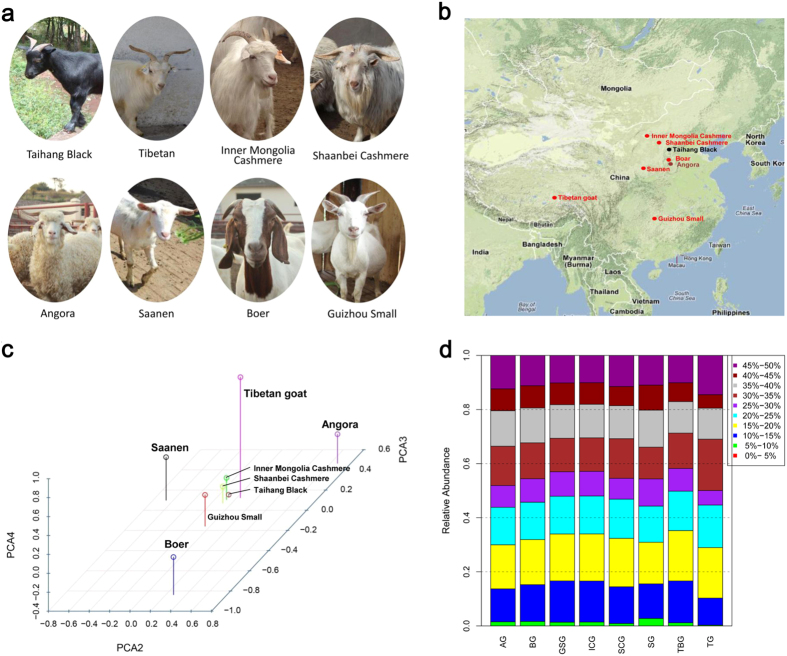
Figure 1. Summary of eight goat breeds.
(a) The eight goat breeds included in this study (Photographs were taken by Xiaolong Wang and Xingui Tian). (b) Geographic map indicating the distribution of the goats sampled in this study. Each point represents the location of sampling. The map was generated using the ‘ggmap’ package in R (version 3.1.0)60. (c) Principal components analysis (PCA) of eight goat breeds using components PC2, PC3, and PC4. (d) A schematic representation of MAF plotted as a function of distance for each goat population. Angora (AG), Boer (BG), Guizhou Small (GSG), Inner Mongolia Cashmere (ICG), Shaanbei Cashmere (SCG), Saanen (SG), Taihang Black (TBG), and Tibetan (TG).
Results and Discussion
Genome resequencing of eight goat breeds
Eight genetically diverse domestic goat breeds with different production purposes were used to systematically investigate the selection signatures in goats (Fig. 1b, Supplementary Table S1). WGS was performed on an Illumina HiSeq 2000 platform by using the pooled DNA from each breed. Genome sequencing yielded a total of 203 Gb raw data, and produced 219 to 350 million sequence reads per breed (Table 1). Over 97.5% of the generated sequence reads mapped to approximately 93.73% (93.19–94.07%) of the newly annotated goat reference genome (CHIR_2.0), indicating that high quality sequences were obtained. Our efforts yielded an average sequence coverage of 10.5× per breed, within a range of 9- to 13-fold. Single-nucleotide polymorphisms (SNPs) varied from 8–9 million for each population (Table 2).
Table 1. Results of Illumina sequencing and assembly.
| Breed | Raw data (G) | Clean data (G) | Reads number (M) | Reads for alignment (%) | Sequence coverage |
|---|---|---|---|---|---|
| TBG | 22.43 | 22.02 | 224.37 | 220.13 (98.11) | 9.35× |
| TG | 35.01 | 34.13 | 350.10 | 341.46 (97.53) | 13.95× |
| ICG | 23.30 | 22.86 | 233.03 | 228.62 (98.10) | 9.67× |
| SCG | 21.90 | 21.43 | 219.07 | 214.28 (97.81) | 9.13× |
| AG | 25.90 | 25.44 | 259.06 | 254.47 (98.23) | 10.65× |
| SG | 26.55 | 26.00 | 265.58 | 259.98 (97.89) | 10.86× |
| BG | 24.08 | 23.59 | 240.37 | 236.02 (98.20) | 9.95× |
| GSG | 24.17 | 23.72 | 241.61 | 237.34 (98.23) | 10.0× |
Table 2. Summary and annotation of SNPs and indels in the goat genome.
| Breed | Number of animals | #SNPs | SNPs |
Coding SNPs |
||
|---|---|---|---|---|---|---|
| Homo. | Hete. (%) | Non- syn. (%) | Syn. | |||
| SCG | 29 | 10519612 | 1226326 | 9293286 (88.3) | 26845 (43.4) | 35010 |
| TG | 25 | 9469142 | 1659401 | 7809741 (82.5) | 23720 (43.5) | 30802 |
| ICG | 28 | 10282737 | 1332165 | 8950572 (87.0) | 25732 (43.5) | 33381 |
| TBG | 21 | 9896309 | 1508571 | 8387738 (84.8) | 25107 (43.5) | 32613 |
| AG | 20 | 9121167 | 2329265 | 6791902 (74.5) | 24042 (43.9) | 30775 |
| SG | 21 | 9813582 | 2112018 | 7701564 (78.5) | 25104 (43.5) | 32631 |
| BG | 22 | 9735835 | 1961090 | 7774745 (79.9) | 25229 (43.7) | 32473 |
| GSG | 21 | 9866776 | 1151745 | 8715031 (88.3) | 24917 (43.7) | 32136 |
Principal components analysis (PCA) was performed to examine the genetic separation of eight goat breeds (Fig. 1c, Supplementary Fig. S1). PCA clearly classified the three introduced breeds (Saanen, Boer, and Angola) and other Chinese indigenous goat breeds. Analysis of breeding history of these five Chinese indigenous breeds, confirmed the results of the PCA; for example, two cashmere goat breeds (Inner Mongolia Cashmere and Shaanbei Cashmere) were clustered together, and were closely related to Taihang Black, both genetically and geographically. The cluster results were in agreement with the findings of a previous study on the genetic diversity of goat breeds in China17.
Identification of coding SNPs and short insertions/deletions
More than nine million SNPs for each breed that confidently remained after filtering were used in the subsequent analyses. Around 74–88% of all the SNPs were heterozygous, and Shaanbei Cashmere and Guizhou Small have the largest proportion of heterozygous SNPs, indicating these two underwent recent intensive selection. Only a few SNPs (~0.5%) were located within coding regions. The non-synonymous and synonymous variants were also identified in the goat genome (Table 2), and there were more synonymous variants than non-synonymous substitutions. The proportion of non-synonymous SNPs in each breed was stable (~43.5%) (Supplementary Fig. S2), which is close to the proportion of non-synonymous SNPs in cattle18. We also identified a large number of SNPs with large effects (premature stop codons, start codon to non-start codon, stop codon to non-stop, and splice site) across the goat genome (Supplementary Fig. S3).
The distribution of minor allelic frequency (MAF) with 10 continued classes from 0–0.05 to 0.45–0.50 for each breed was observed (Fig. 1d). The largest group of the SNPs had a MAF within the range of 0.15–0.20 (16–18%), whereas the proportion of rare alleles (MAF < 0.05) only accounted for <0.01% of the total SNPs, because most of the rare alleles were removed during the SNP calling process.
Identification of selective loci and candidate genes
To detect genomic regions related to selection in domesticated animals, several statistical methods have been developed such as overall low heterozygosity7,9, genetic diversity patterns19, haplotype homozygosity20, and integrated haplotype score (|iHS|)21. To detect putative selective loci in the present study, we first calculated the pooled heterozygosity (Hp) and its Z transformations, ZHp, in sliding 150-kb windows along the autosomes as previously described7,9. We further selected the top 1% of the SNPs with the highest di values as differentiated genomic regions among breeds22. Putative genomic regions that overlapped between these two approaches were defined as candidate selective loci.
Coat Color
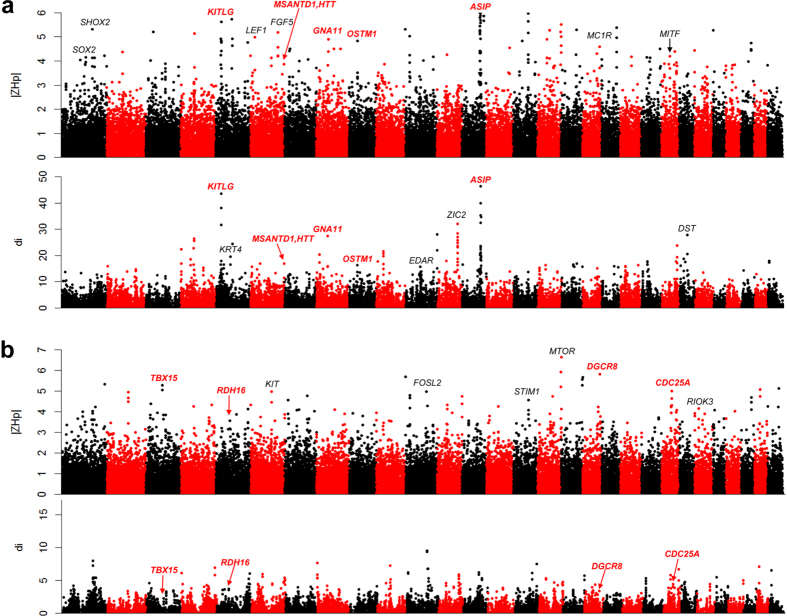
By analyzing the heterozygosity of Taihang Black, 48 distinct loci specific for the Taihang Black breed were identified (ZHp ≤ −4), including well known coat color genes ASIP, MC1R, MITF, and KITLG (Fig. 2a, Supplementary Dataset S1 and S2). MC1R plays key roles in the regulation of eumelanin (black/brown) and phaeomelanin (red/yellow) synthesis in mammalian melanocytes. Mutations in the MC1R gene have been associated with coat colors variation in pigs23,24, cattle25, and goats26. As an antagonist to MC1R to stimulate pheomelanin synthesis, ASIP has been implicated as a strong candidate gene that controls coat color patterns in goats and sheep27,28,29. MITF is a key regulator of melanocyte development and is associated with various coat patterns in mammals30. KITLG, which encodes for the ligand of c-Kit, plays a role in the melanocyte production pathway, and variations in the KITLG locus have been associated with coat color patterns in pigs31, and cattle32. In addition, a total of 54 genomic regions were found within the top 1% distribution (di > 10.82), and a list of candidate genes was generated. Among these, 29 genes were listed by the European Society for Pigment Cell Research (http://www.espcr.org/micemut) (only 150 genes were well annotated in autosomes of goat genome), suggesting that these genes might also play important roles in coat color formation in domestic goats.
Figure 2. Overview of selective sweeps in the Taihang Black and Guizhou Samll breeds plotted by ZHp and di values.
(a) Taihang Black goat breed. (b) Guizhou Samll goat. Functional genes are highlighted, red and bold characters represent overlapping genes that were generated by using the two methods. Absolute values of ZHp were used for plotting.
Six loci overlapped between the genetic regions with the lowest ZHp values and highest di values. Five overlapped regions contained strongest candidate genes (ASIP, KITLG, MSANTD1, HTT, GNA11, and DST) that were specific to Taihang black (Table 3). A locus encompassing the MSANTD1 and HTT genes was recently identified as the strongest selective sweep in European black goat populations14, thereby highlighting the importance of this locus in the determination of black coat color in goats. Given that the HTT gene plays an important role in nerve cells (neurons) in the brain and takes part in pigments associated with aging and diseases, such as Huntington disease33, thus it is likely that HTT is the candidate gene in this locus that is responsible for coat color. GNA11 and OSTM1 were listed as coat color genes in mice (http://www.espcr.org/micemut). These results further indicate the reliability to identify strong selective genes using this approach.
Table 3. Overlapped genes that identified by both ZHp and di for different goat breeds.
| Candidate gene | Chr | Annotation | ZHp | di |
|---|---|---|---|---|
| Taihang Black | ||||
| ASIP | 13 | agouti signaling protein | −5.60 | 55.46 |
| KITLG | 5 | KIT ligand | −5.83 | 43.56 |
| MSANTD1, HTT | 6 | Myb/SANT DNA binding domain containing 1, huntingtin | −4.96 | 16.85 |
| GNA11 | 7 | G protein subunit alpha 11 | −5.30 | 12.87 |
| OSTM1 | 9 | G protein subunit alpha 11 | −4.94 | 16.35 |
| Guizhou Small | ||||
| TBX15 | 3 | T-box 15 | −5.04 | 24.67 |
| DGCR8 | 17 | DGCR8 Microprocessor Complex Subunit | −6.06 | 32.60 |
| CDC25A | 22 | cell division cycle 25A | −4.99 | 37.99 |
| RDH16 | 5 | retinol dehydrogenase 16 | −6.06 | 37.14 |
| Inner Mongolian Cashmere | ||||
| LHX2 | 11 | LIM homeobox 2 | −4.98 | 23.34 |
| FGF9 | 12 | fibroblast growth factor 9 | −4.93 | 13.80 |
| WNT2 | 4 | Wnt family member 2 | −4.29 | 19.04 |
| MC1R | 18 | melanocortin 1 receptor | −5.52 | 13.73 |
| FGF5 | 6 | fibroblast growth factor 5 | −6.12 | 15.68 |
| Tibetan goat | ||||
| CDK2 | 5 | cyclin dependent kinase 2 | −6.45 | 39.75 |
| SOCS2 | 5 | suppressor of cytokine signaling 2 | −5.25 | 33.18 |
| NOXA1 | 11 | NADPH oxidase activator 1 | −4.53 | 22.83 |
| ENPEP | 6 | glutamyl aminopeptidase | −5.65 | 17.41 |
| KITLG | 5 | KIT ligand | −5.82 | 15.06 |
| FGF5 | 6 | fibroblast growth factor 5 | −6.29 | 16.77 |
Body size
The Guizhou Small goats originated from the remote mountain area of the Guizhou Province in southwest China. To maintain its small physical figure and meat taste, intercrosses are often made and the population size of the Guizhou Small has become smaller34. Compared to the body weight of larger meat goat breeds, e.g. Boer, which could weigh over 100 kg, the average body weight of the Guizhou Small is as low as ~20 kg in females and ~25 kg in males. Therefore, body size trait of Guizhou Small could be beneficial in increasing carcass weight, and should be considered in meat goat breeding programs.
A total of 49 regions related to Guizhou Small breeds were mapped with a ZHp value of <−4. Strong selection signals including known genes FOSL2, DGCR8, MTOR, and TBX15 were localized. We discovered 56 regions that were within the top 1% distribution of the di values. Only four functional genes (TBX15, DGCR8, CDC25A, and RDH16) within four loci overlapped showed low ZHp values and high divergence (Fig. 2b, Supplementary Dataset S1 & S2). TBX15 controls the number of mesenchymal precursor cells and chondrocytes, and is essential to skeletal development35. Osteoclast-specific deletion of DGCR8 results in impaired osteoclastic development and bone resorbing activity, indicating that the DGCR8 gene is essential for bone development36. CDC25A plays important roles in G1 quiescence and myogenic differentiation of myoblasts in mice37. The RDH16 gene is involved in energy and metabolism processes in adipose tissues in pigs38,39, and rats40.
Cashmere traits
In mammals, coat hair acts as a protective material against environmental changes. Unlike other mammals, cashmere-producing goats have a double coat consisting of the outer coarse hair produced by primary hair follicles (PHF) and the inner fine coat (cashmere) produced by secondary hair follicles (SHF). In contrast, the coat hair of the Angora goat exclusively produces a fleece of fibers named mohair, which is generated by SHF with limited proportion of guard hair from PHF41. In the case of cashmere fibers, selection for an optimal fiber diameter with an increased fiber length is the long-term goal of cashmere goat breeding programs. Although earlier studies have assessed only a few candidate genes [e.g., POU1F142, and PRL43] that associated with cashmere traits (cashmere yield, cashmere diameter and length), the genetic determinants controlling cashmere traits in goats have remained largely elusive at a genome level.
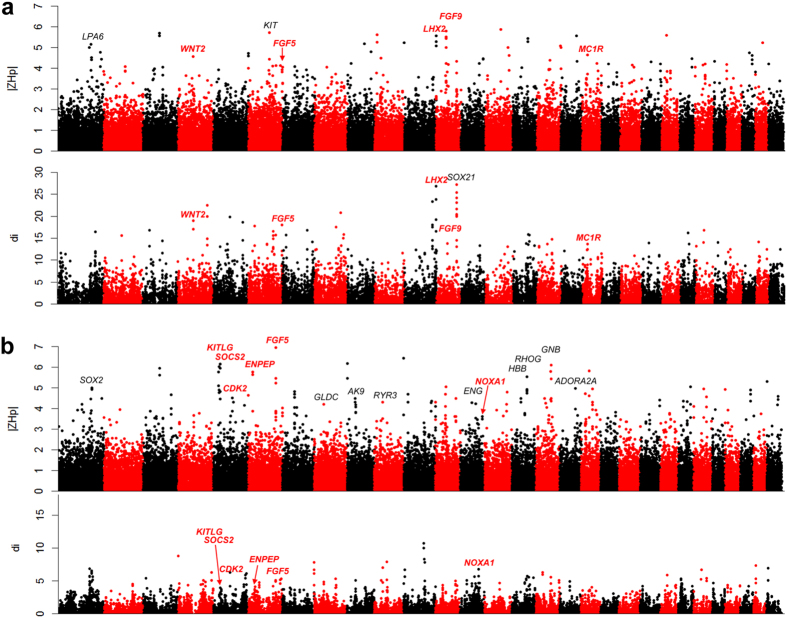
By analyzing the sequence heterozygosity and divergence of a well-known cashmere goat breed, Inner Mongolia Cashmere, with other goat breeds, 40 and 37 genomic regions were implicated to be cashmere goat-specific regions using ZHp and di methods, respectively (Fig. 3a, Supplementary Dataset S1 & S2). We merged the regions that were generated by these two approaches to identify the strongest signature of selection. Five regions encompassing the LHX2, FGF9, WNT2, MC1R, and FGF5 were detected. LHX2, a LIM homeobox gene, regulates the generation and regeneration of hair44. We have previously shown that the cyclic expression of LHX2 is involved in the development of SHF in cashmere goats45. FGF9 is able to promote hair follicle regeneration after wounding46, and the Wnt-related genes WNT2 is a key mediator and regulator of Wnt signaling, and is involved in hair follicle initiation47. These two genes may explain the cyclic growth of cashmere fibers in cashmere goats. Furthermore, the coat color gene MC1R controls white coat color and FGF5 that regulates hair length were also mapped, consistent with the selection purposes (such as white and longer fibers) of cashmere goats.
Figure 3. Overview of selective sweeps in the Inner Mongolian Cashmere and Tibetan goats plotted by ZHp and di values.
(a) Inner Mongolian Cashmere breed. (b) Tibetan goats breed.
High altitude adaptation
The Tibetan goat, together with the yak and Tibetan sheep, are the three major livestock species that serve as sources of meat and fibers for Tibetan inhabitants. Endemic to the Tibetan Plateau, the Tibetan goats are well adapted to high altitudes, often inhabiting open alpine and cold steppe environments located between 4,000 and 5,500 m elevation.
In the genome of the Tibetan goat, 49 loci were regarded as selected regions for adaptation to highland altitude environment based on the calculated ZHp values (Fig. 3b, Supplementary Dataset S1 & S2). Several genes within these regions were previously implicated in the adaptation of Tibetan dwellers, including HMOX2 and HBB in Tibetans48, AK9 in Tibetan chickens49, GLDC and RHOG in Tibetan pigs10, ATP12A, PIK3C2A, ADORA2A, and ENG in Tibetan antelope50, GNB1 in Tibetan dogs51. In addition, a total of 53 regions were within the top 1% of the distribution (di > 12.79), which included highland adaptation related genes such as ANGPTL4 in Tibetans48, ENO3 and KIF1C in Tibetan dogs51, and PKLR in Tibetan antelope50. We overlapped the genomic regions generated by these two approaches and identified seven regions that showed the strongest signature of selection by displaying both high di and low ZHp values. Six genes (CDK2, SOCS2, NOXA1, ENPEP, KITLG, and FGF5) within these seven regions have plausible biological functions that are associated with high altitude adaptation or breed features. CDK2 is a selected gene in the Tibetan mastiff52, and is involved in hypoxia-induced apoptosis in cardiomyocytes53. SOCS2 is implicated as a selective gene in Tibetan sheep54. NOXA1 is the activator of NOX1, which is associated with HIF-1 response under intermittent hypoxia conditions55. ENPEP is a candidate gene for high-altitude adaption in Andeans56. Moreover, FGF5, a key regulator that controls hair length in mammals, was also identified and may explain the longer hair of Tibetan goats as an adaptation of cold environments of highlands. KITLG, a coat color gene, may be associated with white color in Tibetan goats.
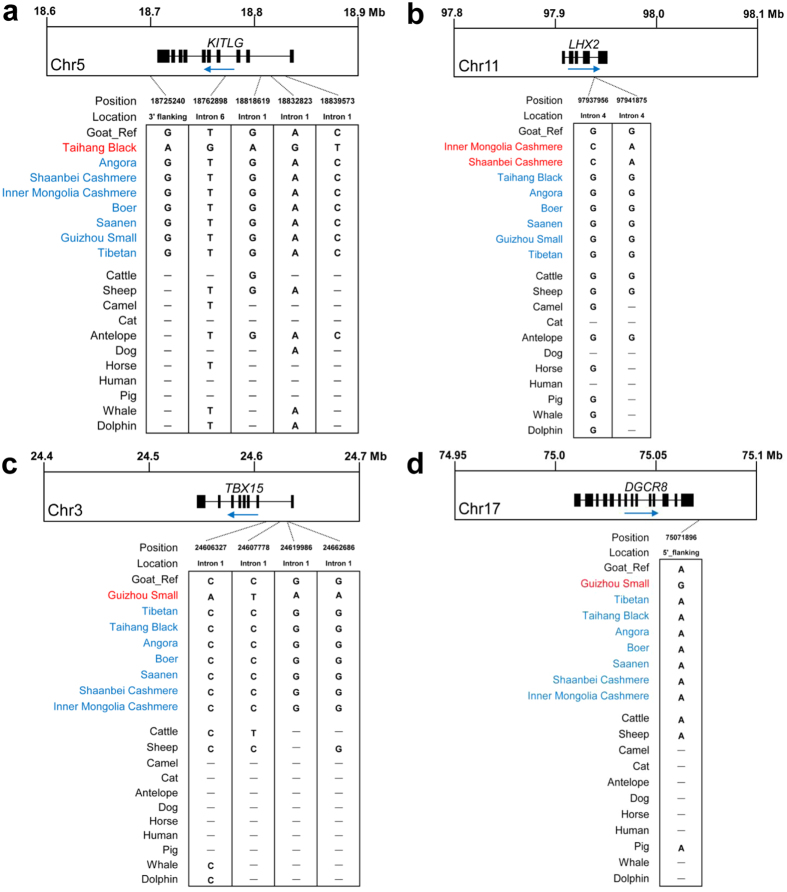
We further examined the functional importance of SNPs that were within seven representative selected genes (KITLG, ASIP, LHX2, TBX15, DGCR8, CDK2, and SOCS2), breed-specific SNPs among four genes (KITLG, LHX2, TBX15, and DGCR8) were localized to evolutionary conserved regions in mammals (Fig. 4a–d), suggesting that these SNPs might be functional. Consistent with a previous finding in rabbit domestication57, none of these conserved SNPs in these four genes were located within coding regions that lead to amino acid exchanges, thereby indicating that the genetic basis of goat production and adaptive traits are complex, and are rather regulatory variants.
Figure 4. The conservation analyses identified candidate functional mutations that are specific to goat breeds.
Breed-specific SNPs within or close to the key selected genes KITLG (a), LHX2 (b), TBX15 (c), and DGCR8 (d) were first screened, and the SNPs localized to evolutionary conserved sites were remained. The location and position of the candidate SNPs are indicated.
Taken together, we conducted a comprehensive study to identify selection signatures in goats based on resequencing data. A total of 22 strong candidate regions with respect to distinct breeds were identified, which comprised genes involved in coat color patterns, body size, cashmere selection-specific phenotypes, as well as adaptation to low-oxygen environments in the highlands (Supplementary Table S2). Because no systematic mapping studies in goats at a genome-wide scale are currently available, the genes we highlighted may be regarded as the major candidate genes that are involved in shaping the particular characteristics of goat populations. For instance, we confirmed the importance of the HTT locus as a strong signal in the determination of black coat color in goats. Similarly, fine-scale mapping of selection signatures in goats may also facilitate in the interpretation and establishment of the molecular basis of economically important goat traits. For example, the availability of the Illumina goat SNP50 array11,58, as well as the rapid decrease in sequencing cost permit the identification of candidate economically important traits in goats. Our findings will facilitate the genetic dissection of phenotypic variation and aid in the future genetic improvement of production and adaptive traits in goats.
Materials and Methods
Ethics statement
The sampling procedures were compliance with the “Guidelines on Ethical Treatment of Experimental Animals” (2006) No. 398 established by the Ministry of Science and Technology, China. The sampling procedures in the present study had received prior approval from the Experimental Animal Manage Committee of Northwest A&F University (Approval ID: 2012ZX08008–002).
Animals and whole genome sequencing
Over 20 animals from each breed were combined into a pool for high-throughput resequencing (Supplementary Table S1). DNA was extracted from whole blood samples by using the Qiagen DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) and was used in the generation of paired-end libraries by using the Genomic DNA Sample Preparation Kit (Illumina, San Diego, CA). Briefly, 5 μg of DNA were sheared with a nebulizer and after end repair, A-tailing, and ligation of paired-end adapters, the library was size-selected on an agarose gel (300 bp) and amplified using 10 PCR cycles. Cluster amplification was performed using the Illumina Paired-End Cluster Generation Kit v2. Sequences were generated with the Illumina Sequencing Kit v3 and the Illumina Genome AnalyzerIIx System at Novogene (http://www.novogene.com). Image analysis was performed with Firecrest, Bustard, and Gerald modules of the Illumina pipeline v. 1.4. In total, 5 paired-end lanes were sequenced, which produced 80 mio PE fragments (160 mio individual reads), with an average read length of 74 bp.
Reads alignment and variations calling
Reads were aligned to the goat reference genome CHIR_2.0 (http://www.ncbi.nlm.nih.gov/assembly/GCA_000317765.2/)13 using BWA (0.6.2-r126 version) followed by duplicate removal using Picard-Tools-1.55 (http://broadinstitute.github.io/picard/). The Genome Analysis Toolkit (GATK-2.6)59 was used to perform local realignment around existing indels and base quality score recalibration. Variant detection was performed using the GATK Unified Genotyper. To filter SNPs for flowing analysis, at least three reads with different start sites supporting the non-reference allele had to be present.
Detection of selective loci
To identify regions that were likely to be or have been under selection, the “Z transformed heterozygosity” (ZHp) approach was used, as previously described7,9. In brief, in an overlapping sliding window, Hp was calculated as follows: 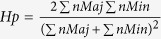 is the sum of major allele frequencies, and ΣnMin is the sum of MAF within each window. Individual Hp values were Z transformed as follows:
is the sum of major allele frequencies, and ΣnMin is the sum of MAF within each window. Individual Hp values were Z transformed as follows: 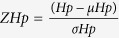 , where μHp is the overall average heterozygosity, and σHp is the standard deviation for all windows within each group. We calculated the ZHp value in sliding 150-kb windows along the autosomes from sequence reads corresponding to the most and least frequently observed alleles at all SNP positions as previously described7,9. Because sex chromosomes and autosomes are subjected to different selective pressures and have different effective population sizes, we decided to calculate the ZHp for autosomes of specific breeds only. Genetic differentiation between every pairwise comparison was measured by means of the fixation index (FST) and di values were calculated as described by Akey et al.22 to evaluate population differentiation among specific breeds. Putatively selected loci were defined as genetic regions in overlapped windows with extremely low ZHp values (<−4) and extremely high di values (top 1% level).
, where μHp is the overall average heterozygosity, and σHp is the standard deviation for all windows within each group. We calculated the ZHp value in sliding 150-kb windows along the autosomes from sequence reads corresponding to the most and least frequently observed alleles at all SNP positions as previously described7,9. Because sex chromosomes and autosomes are subjected to different selective pressures and have different effective population sizes, we decided to calculate the ZHp for autosomes of specific breeds only. Genetic differentiation between every pairwise comparison was measured by means of the fixation index (FST) and di values were calculated as described by Akey et al.22 to evaluate population differentiation among specific breeds. Putatively selected loci were defined as genetic regions in overlapped windows with extremely low ZHp values (<−4) and extremely high di values (top 1% level).
Bioinformatics analysis of breed specific SNPs
Seven genes representing breed-specific selection signatures in Taihang Black (KITLG and ASIP), Guizhou Small (TBX15 and DGCR8), cashmere breeds (LHX2), and Taibetan (CDK2, and SOCS2) were chosen for further analysis. We specifically focused on SNPs within genes and 1000-bp upstream and downstream flanking regions. We defined the breed-specific SNPs that differed from the goat reference sequence (CHI2.0) and other seven goat breeds used in the present study, and were localized to evolutionary conserved regions among mammal species.
Additional Information
How to cite this article: Wang, X. et al. Whole-genome sequencing of eight goat populations for the detection of selection signatures underlying production and adaptive traits. Sci. Rep. 6, 38932; doi: 10.1038/srep38932 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by grants from National Science Foundation Council (31372279, 31402038, and 31572369), Fundamental Research Funds for the Central Universities (2014YB008), Major Projects for New Varieties of Genetically Modified Organisms of China (2014ZX08008-002), Natural Science Foundation (2014JM3068) and International Sci-Tech Cooperation Program (2014KW14-01) of Shaanxi Province.
Footnotes
Author Contributions Conceived and designed the experiments: X.W., J.S., Y.C. Performed the experiments: J.L., G.Z., C.Y., H.Y., R.G., Y.N. Analyzed the data: X.W., J.L., Y.J., J.G., H.Z. Prepared the samples: Y.L., Y.N., X.L., X.A., X.T. Wrote the manuscript: X.W., Y.J., J.Z., Y.C. All authors read and approved the final manuscript.
References
- Zeder M. A. & Hesse B. The Initial Domestication of Goats (Capra hircus) in the Zagros Mountains 10,000 Years Ago. Science. 287, 2254–2257 (2000). [DOI] [PubMed] [Google Scholar]
- Naderi S. et al. The goat domestication process inferred from large-scale mitochondrial DNA analysis of wild and domestic individuals. PNAS. 105, 17659–17664 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO Status and trends of animal genetic resources. Commission on Genetic Resources for Food and Agriculture. Rome, 15–19 April 2013 (2012).
- Daetwyler H. D. et al. Whole-genome sequencing of 234 bulls facilitates mapping of monogenic and complex traits in cattle. Nat Genet. 46, 858–865 (2014). [DOI] [PubMed] [Google Scholar]
- Qanbari S. et al. Classic Selective Sweeps Revealed by Massive Sequencing in Cattle. PLoS Genet. 10, e1004148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. et al. Genomic signatures reveal new evidences for selection of important traits in domestic cattle. Mol Biol Evol. 32, 711–725 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C.-J. et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature. 464, 587–591 (2010). [DOI] [PubMed] [Google Scholar]
- Axelsson E. et al. The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature. 495, 360–364 (2013). [DOI] [PubMed] [Google Scholar]
- Rubin C.-J. et al. Strong signatures of selection in the domestic pig genome. PNAS. 109, 19529–19536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. et al. Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars. Nat Genet. 45, 1431–1438 (2013). [DOI] [PubMed] [Google Scholar]
- Kijas J. W. et al. Genetic diversity and investigation of polledness in divergent goat populations using 52 088 SNPs. Anim Genet. 44, 325–335 (2013). [DOI] [PubMed] [Google Scholar]
- Lv F.-H. et al. Adaptations to Climate-Mediated Selective Pressures in Sheep. Mol Biol Evol. 31, 3324–3343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. et al. Sequencing and automated whole-genome optical mapping of the genome of a domestic goat (Capra hircus). Nat Biotech. 31, 135–141 (2013). [DOI] [PubMed] [Google Scholar]
- Benjelloun B. et al. Characterizing neutral genomic diversity and selection signatures in indigenous populations of Moroccan goats (Capra hircus) using WGS data. Front Genet. 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. et al. Reference genome of wild goat (Capra aegagrus) and sequencing of goat breeds provide insight into genic basis of goat domestication. BMC Genomics. 16, 431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlötterer C., Tobler R., Kofler R. & Nolte V. Sequencing pools of individuals — mining genome-wide polymorphism data without big funding. Nat Rev Genet. 15, 749–763 (2014). [DOI] [PubMed] [Google Scholar]
- Qi Y. et al. Genetic diversity and relationships of 10 Chinese goat breeds in the Middle and Western China. Small Ruminant Res. 82, 88–93 (2009). [Google Scholar]
- Eck S. H. et al. Whole genome sequencing of a single Bos taurus animal for single nucleotide polymorphism discovery. Genome Biol. 10, R82 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener P. & Pong-Wong R. A regression-based approach to selection mapping. J. Hered. 102, 294–305 (2011). [DOI] [PubMed] [Google Scholar]
- Depaulis F. & Veuille M. Neutrality tests based on the distribution of haplotypes under an infinite-site model. Mol. Biol. Evol. 15, 1788–1790 (1998). [DOI] [PubMed] [Google Scholar]
- Voight B. F., Kudaravalli S., Wen X. & Pritchard J. K. A map of recent positive selection in the human genome. PLoS Biol. 4, e72 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey J. M. et al. Tracking footprints of artificial selection in the dog genome. PNAS 107, 1160–1165 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M., Larson G., Soares Ribeiro H., Li N. & Andersson L. Contrasting Mode of Evolution at a Coat Color Locus in Wild and Domestic Pigs. PLoS Genet. 5, e1000341 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijas J. M. H. et al. Melanocortin Receptor 1 (MC1R) Mutations and Coat Color in Pigs. Genetics. 150, 1177–1185 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo V. et al. Analysis of melanocortin 1 receptor (MC1R) gene polymorphisms in some cattle breeds: their usefulness and application for breed traceability and authentication of Parmigiano Reggiano cheese. Italian J Anim Sci. 6, 257–272 (2009). [Google Scholar]
- Fontanesi L. et al. Missense and nonsense mutations in melanocortin 1 receptor (MC1R) gene of different goat breeds: association with red and black coat colour phenotypes but with unexpected evidences. BMC Genet. 10, 47 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanesi L. et al. Copy number variation and missense mutations of the agouti signaling protein (ASIP) gene in goat breeds with different coat colors. Cytogenet. Genome Res. 126, 333–347 (2009). [DOI] [PubMed] [Google Scholar]
- Adefenwa M. A. et al. Identification of single nucleotide polymorphisms in the agouti signaling protein (ASIP) gene in some goat breeds in tropical and temperate climates. Mol. Biol. Rep. 40, 4447–4457 (2013). [DOI] [PubMed] [Google Scholar]
- Norris B. J. & Whan V. A. A gene duplication affecting expression of the ovine ASIP gene is responsible for white and black sheep. Genome Res. 18, 1282–1293 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy C., Khaled M. & Fisher D. E. MITF: master regulator of melanocyte development and melanoma oncogene. Trends in Molecular Medicine 12, 406–414 (2006). [DOI] [PubMed] [Google Scholar]
- Hadjiconstantouras C. et al. Characterization of the porcine KIT ligand gene: expression analysis, genomic structure, polymorphism detection and association with coat colour traits. Anim Genet. 39, 217–224 (2008). [DOI] [PubMed] [Google Scholar]
- Pausch H. et al. Identification of QTL for UV-Protective Eye Area Pigmentation in Cattle by Progeny Phenotyping and Genome-Wide Association Analysis. PLoS ONE. 7, e36346 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. et al. Neuronal pigmented autophagic vacuoles: lipofuscin, neuromelanin, and ceroid as macroautophagic responses during aging and disease. Journal of Neurochemistry 106, 24–36 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.-H. et al. Genetic relationships among twelve Chinese indigenous goat populations based on microsatellite analysis. Genet Sel Evol. 34, 729 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M. K. et al. The T-box transcription factor Tbx15 is required for skeletal development. Mechanisms of Development 122, 131–144 (2005). [DOI] [PubMed] [Google Scholar]
- Sugatani T. et al. Expression of DGCR8-Dependent MicroRNAs is Indispensable for Osteoclastic Development and Bone-Resorbing Activity. J Cell Biochem. 115, 1043–1047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Dey B. K. & Dutta A. MiR-322/424 and -503 Are Induced during Muscle Differentiation and Promote Cell Cycle Quiescence and Differentiation by Down-Regulation of Cdc25A. Mol. Biol. Cell 21, 2138–2149 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodhi S. S. et al. Comparative transcriptomic analysis to identify differentially expressed genes in fat tissue of adult Berkshire and Jeju Native Pig using RNA-seq. Mol Biol Rep 41, 6305–6315 (2014). [DOI] [PubMed] [Google Scholar]
- Kim S.-S. et al. Transcriptional alteration of p53 related processes as a key factor for skeletal muscle characteristics in Sus scrofa. Mol. Cells 28, 565–573 (2009). [DOI] [PubMed] [Google Scholar]
- Hariya N., Miyake K., Kubota T., Goda T. & Mochizuki K. Putative PPAR Target Genes Express Highly in Skeletal Muscle of Insulin-Resistant MetS Model SHR/NDmc-cp Rats. Journal of Nutritional Science and Vitaminology 61, 28–36 (2015). [DOI] [PubMed] [Google Scholar]
- Dreyer J. H. & Marincowitz G. Some observations on the skin histology and fibre characteristics of the Angora goat. S. Afr. J. Agric. Sci. 10, 477 (1967). [Google Scholar]
- Lan X. Y. et al. A PstI polymorphism at 3′UTR of goat POU1F1 gene and its effect on cashmere production. Mol Biol Rep. 36, 1371–1374 (2009). [DOI] [PubMed] [Google Scholar]
- Lan X.-Y. et al. Novel SNP of the goatprolactin gene (PRL) associated with cashmere traits. J Appl Genet. 50, 51–54 (2009). [DOI] [PubMed] [Google Scholar]
- Törnqvist G. et al. Cyclic Expression of Lhx2 Regulates Hair Formation. PLoS Genet. 6, e1000904 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng R. et al. Cyclic expression of Lhx2 is involved in secondary hair follicle development in cashmere goat. Gene Expr Patterns. 16, 31–35 (2014). [DOI] [PubMed] [Google Scholar]
- Gay D. et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med 19, 916–923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadau S. et al. Early stages of hair follicle development: a step by step microarray identity. Eur J Dermatol, doi: 10.1684/ejd.2013.1972 (2013). [DOI] [PubMed] [Google Scholar]
- Simonson T. S. et al. Genetic Evidence for High-Altitude Adaptation in Tibet. Science. 329, 72–75 (2010). [DOI] [PubMed] [Google Scholar]
- Wang M.-S. et al. Genomic analyses reveal potential independent adaptation to high altitude in Tibetan chickens. Mol Biol Evol msv071, doi: 10.1093/molbev/msv071 (2015). [DOI] [PubMed] [Google Scholar]
- Ge R.-L. et al. Draft genome sequence of the Tibetan antelope. Nat Commun. 4, 1858 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou X. et al. Whole-genome sequencing of six dog breeds from continuous altitudes reveals adaptation to high-altitude hypoxia. Genome Res. 24, 1308–1315 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. et al. Population Variation Revealed High-Altitude Adaptation of Tibetan Mastiffs. Mol Biol Evol 31, 1200–1205 (2014). [DOI] [PubMed] [Google Scholar]
- Adachi S. et al. Cyclin A/cdk2 Activation Is Involved in Hypoxia-Induced Apoptosis in Cardiomyocytes. Circulation Research 88, 408–414 (2001). [DOI] [PubMed] [Google Scholar]
- Wei C. et al. Genome-wide analysis reveals adaptation to high altitudes in Tibetan sheep. Sci Rep 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec V. et al. HIF-1α signaling is augmented during intermittent hypoxia by induction of the Nrf2 pathway in NOX1-expressing adenocarcinoma A549 cells. Free Radical Biology and Medicine 48, 1626–1635 (2010). [DOI] [PubMed] [Google Scholar]
- Eichstaedt C. A. et al. Genetic and phenotypic differentiation of an Andean intermediate altitude population. Physiol Rep 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro M. et al. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science. 345, 1074–1079 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosser-Klopp G. et al. Design and Characterization of a 52K SNP Chip for Goats. PLoS ONE. 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A. et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org (2013).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.