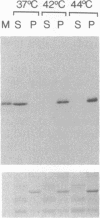
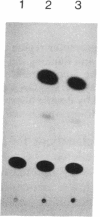
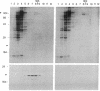
Abstract
Sequence similarity between alpha B-crystallin and small heat shock proteins (HSPs) has prompted us to investigate whether alpha B-crystallin expression is induced by heat shock. Indeed, accumulation of alpha B-crystallin was detected immunologically in NIH 3T3 cells after incubation at elevated temperatures and after addition of Cd2+ or sodium arsenite to these cells. Two-dimensional gel electrophoresis revealed identity between alpha B-crystallin from eye lenses and from heat-treated fibroblasts. The promoter of the alpha B-crystallin gene was fused to the bacterial chloramphenicol acetyltransferase gene and was shown to confer heat inducibility on this reporter gene in transient transfection assays. A perfect heat shock element within the promoter region is likely to mediate this response. Small HSPs and alpha B-crystallin were shown to share the following two physical properties: (i) they form supramolecular structures with sedimentation values around 17 S and (ii) they are associated with the nucleus at high temperatures and are localized in the cytoplasm under normal conditions. We conclude that alpha B-crystallin has to be considered a member of the class of small HSPs.
Full text
PDF


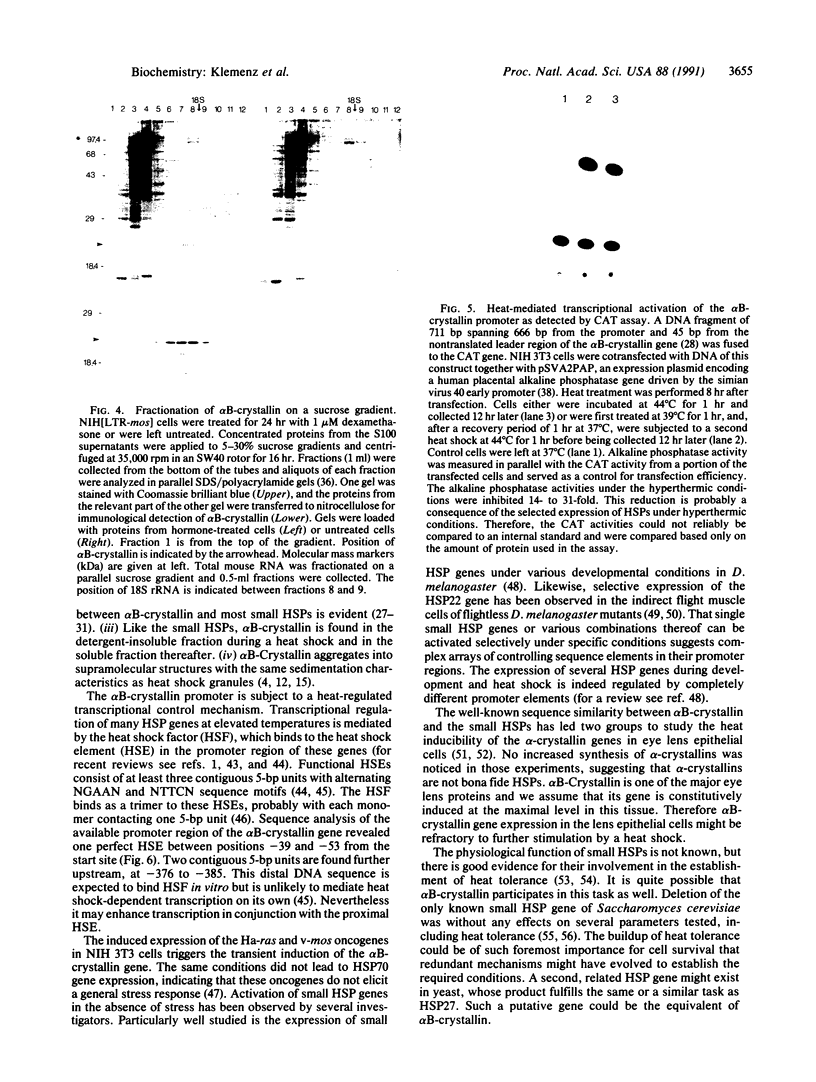

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Darlix J. L., Khandjian E. W., Simon M., Spahr P. F. Characterization of the prosome from Drosophila and its similarity to the cytoplasmic structures formed by the low molecular weight heat-shock proteins. EMBO J. 1985 Feb;4(2):399–406. doi: 10.1002/j.1460-2075.1985.tb03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Suhan J. P., Welch W. J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988 Dec;8(12):5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Welch W. J. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987 Nov 15;262(32):15359–15369. [PubMed] [Google Scholar]
- Berger E. M., Woodward M. P. Small heat shock proteins in Drosophila may confer thermal tolerance. Exp Cell Res. 1983 Sep;147(2):437–442. doi: 10.1016/0014-4827(83)90225-2. [DOI] [PubMed] [Google Scholar]
- Bhat S. P., Nagineni C. N. alpha B subunit of lens-specific protein alpha-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989 Jan 16;158(1):319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- Collier N. C., Heuser J., Levy M. A., Schlesinger M. J. Ultrastructural and biochemical analysis of the stress granule in chicken embryo fibroblasts. J Cell Biol. 1988 Apr;106(4):1131–1139. doi: 10.1083/jcb.106.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier N. C., Schlesinger M. J. Induction of heat-shock proteins in the embryonic chicken lens. Exp Eye Res. 1986 Jul;43(1):103–117. doi: 10.1016/s0014-4835(86)80049-5. [DOI] [PubMed] [Google Scholar]
- Collier N. C., Schlesinger M. J. The dynamic state of heat shock proteins in chicken embryo fibroblasts. J Cell Biol. 1986 Oct;103(4):1495–1507. doi: 10.1083/jcb.103.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces V., Holmgren R., Freund R., Morimoto R., Meselson M. Four heat shock proteins of Drosophila melanogaster coded within a 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5390–5393. doi: 10.1073/pnas.77.9.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F. Lens proteins. More molecular opportunism. Nature. 1988 Nov 3;336(6194):18–18. doi: 10.1038/336018a0. [DOI] [PubMed] [Google Scholar]
- Dubin R. A., Wawrousek E. F., Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989 Mar;9(3):1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P., Zervos P., Raducha M., Harris H., Kadesch T. Expression of a human placental alkaline phosphatase gene in transfected cells: use as a reporter for studies of gene expression. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6342–6346. doi: 10.1073/pnas.85.17.6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey E. D., Weber L. A. Modulation of heat-shock polypeptide synthesis in HeLa cells during hyperthermia and recovery. Biochemistry. 1982 Mar 30;21(7):1513–1521. doi: 10.1021/bi00536a008. [DOI] [PubMed] [Google Scholar]
- Hickey E., Brandon S. E., Potter R., Stein G., Stein J., Weber L. A. Sequence and organization of genes encoding the human 27 kDa heat shock protein. Nucleic Acids Res. 1986 May 27;14(10):4127–4145. doi: 10.1093/nar/14.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Hotta Y. Actin gene mutations in Drosophila; heat shock activation in the indirect flight muscles. EMBO J. 1985 Jul;4(7):1681–1687. doi: 10.1002/j.1460-2075.1985.tb03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A. Four small Drosophila heat shock proteins are related to each other and to mammalian alpha-crystallin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Goldman J. E. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990 Jan;38(1):31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Kume-Iwaki A., Liem R. K., Goldman J. E. Alpha B-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell. 1989 Apr 7;57(1):71–78. doi: 10.1016/0092-8674(89)90173-6. [DOI] [PubMed] [Google Scholar]
- Jaggi R., Salmons B., Muellener D., Groner B. The v-mos and H-ras oncogene expression represses glucocorticoid hormone-dependent transcription from the mouse mammary tumor virus LTR. EMBO J. 1986 Oct;5(10):2609–2616. doi: 10.1002/j.1460-2075.1986.tb04541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlik C. C., Coutu M. D., Fyrberg E. A. A nonsense mutation within the act88F actin gene disrupts myofibril formation in Drosophila indirect flight muscles. Cell. 1984 Oct;38(3):711–719. doi: 10.1016/0092-8674(84)90266-6. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Shuman J., Sette M., Przybyla A. Nuclear localization and phosphorylation of three 25-kilodalton rat stress proteins. Mol Cell Biol. 1984 Mar;4(3):468–474. doi: 10.1128/mcb.4.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Aoyama A., Hoffmann S., Simpson R. J., Moritz R. L., Schäfer R. Alpha B crystallin accumulation is a specific response to Ha-ras and v-mos oncogene expression in mouse NIH 3T3 fibroblasts. Mol Cell Biol. 1991 Feb;11(2):803–812. doi: 10.1128/mcb.11.2.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Hoffmann S., Jaggi R., Werenskiold A. K. The v-mos and c-Ha-ras oncoproteins exert similar effects on the pattern of protein synthesis. Oncogene. 1989 Jun;4(6):799–803. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Longoni S., James P., Chiesi M. Cardiac alpha-crystallin. I. Isolation and identification. Mol Cell Biochem. 1990 Dec 3;99(1):113–120. [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. A. Chromatin-associated heat shock proteins of Dictyostelium. Dev Biol. 1982 Apr;90(2):412–418. doi: 10.1016/0012-1606(82)90390-6. [DOI] [PubMed] [Google Scholar]
- Mansfield M. A., Key J. L. Synthesis of the low molecular weight heat shock proteins in plants. Plant Physiol. 1987 Aug;84(4):1007–1017. doi: 10.1104/pp.84.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni C. N., Bhat S. P. Alpha B-crystallin is expressed in kidney epithelial cell lines and not in fibroblasts. FEBS Lett. 1989 May 22;249(1):89–94. doi: 10.1016/0014-5793(89)80022-5. [DOI] [PubMed] [Google Scholar]
- Nover L., Scharf K. D., Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol Cell Biol. 1983 Sep;3(9):1648–1655. doi: 10.1128/mcb.3.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nover L., Scharf K. D. Synthesis, modification and structural binding of heat-shock proteins in tomato cell cultures. Eur J Biochem. 1984 Mar 1;139(2):303–313. doi: 10.1111/j.1432-1033.1984.tb08008.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Perisic O., Xiao H., Lis J. T. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989 Dec 1;59(5):797–806. doi: 10.1016/0092-8674(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens crystallins and their genes: diversity and tissue-specific expression. FASEB J. 1989 Jun;3(8):1933–1940. doi: 10.1096/fasebj.3.8.2656357. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Wistow G. J. Enzyme/crystallins: gene sharing as an evolutionary strategy. Cell. 1989 Apr 21;57(2):197–199. doi: 10.1016/0092-8674(89)90956-2. [DOI] [PubMed] [Google Scholar]
- Russnak R. H., Jones D., Candido E. P. Cloning and analysis of cDNA sequences coding for two 16 kilodalton heat shock proteins (hsps) in Caenorhabditis elegans: homology with the small hsps of Drosophila. Nucleic Acids Res. 1983 May 25;11(10):3187–3205. doi: 10.1093/nar/11.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Nelson H. C. Trimerization of a yeast transcriptional activator via a coiled-coil motif. Cell. 1989 Dec 1;59(5):807–813. doi: 10.1016/0092-8674(89)90604-1. [DOI] [PubMed] [Google Scholar]
- Southgate R., Ayme A., Voellmy R. Nucleotide sequence analysis of the Drosophila small heat shock gene cluster at locus 67B. J Mol Biol. 1983 Mar 25;165(1):35–57. doi: 10.1016/s0022-2836(83)80241-1. [DOI] [PubMed] [Google Scholar]
- Spector A., Chiesa R., Sredy J., Garner W. cAMP-dependent phosphorylation of bovine lens alpha-crystallin. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4712–4716. doi: 10.1073/pnas.82.14.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek R. E., Lindquist S. L. hsp26 of Saccharomyces cerevisiae is related to the superfamily of small heat shock proteins but is without a demonstrable function. Mol Cell Biol. 1989 Nov;9(11):5265–5271. doi: 10.1128/mcb.9.11.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis P. A., Manes T., Millan J. L., Goetinck P. F. CAT constructs with convenient sites for cloning and generating deletions. Nucleic Acids Res. 1988 Aug 11;16(15):7745–7745. doi: 10.1093/nar/16.15.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Ouderaa F. J., De Jong W. W., Hilderink A., Bloemendal H. The amino-acids sequence of the alphaB2 chain of bovine alpha-crystallin. Eur J Biochem. 1974 Nov 1;49(1):157–168. doi: 10.1111/j.1432-1033.1974.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Voorter C. E., Mulders J. W., Bloemendal H., de Jong W. W. Some aspects of the phosphorylation of alpha-crystallin A. Eur J Biochem. 1986 Oct 1;160(1):203–210. doi: 10.1111/j.1432-1033.1986.tb09958.x. [DOI] [PubMed] [Google Scholar]
- Welch W. J. Phorbol ester, calcium ionophore, or serum added to quiescent rat embryo fibroblast cells all result in the elevated phosphorylation of two 28,000-dalton mammalian stress proteins. J Biol Chem. 1985 Mar 10;260(5):3058–3062. [PubMed] [Google Scholar]
- Wistow G. J., Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- Wistow G. Domain structure and evolution in alpha-crystallins and small heat-shock proteins. FEBS Lett. 1985 Feb 11;181(1):1–6. doi: 10.1016/0014-5793(85)81102-9. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hendriks W., Mulders J. W., Bloemendal H. Evolution of eye lens crystallins: the stress connection. Trends Biochem Sci. 1989 Sep;14(9):365–368. doi: 10.1016/0968-0004(89)90009-1. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Hoekman W. A., Mulders J. W., Bloemendal H. Heat shock response of the rat lens. J Cell Biol. 1986 Jan;102(1):104–111. doi: 10.1083/jcb.102.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sa C. M., Rollet E., de Sa M. F., Tanguay R. M., Best-Belpomme M., Scherrer K. Prosomes and heat shock complexes in Drosophila melanogaster cells. Mol Cell Biol. 1989 Jun;9(6):2672–2681. doi: 10.1128/mcb.9.6.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]