Abstract
Natural wetlands form the largest source of methane (CH4) to the atmosphere. Emission of this powerful greenhouse gas from wetlands is known to depend on climate, with increasing temperature and rainfall both expected to increase methane emissions. This study, combining our field and controlled environment manipulation studies in Europe and North America, reveals an additional control: an emergent pattern of increasing suppression of methane (CH4) emission from peatlands with increasing sulfate (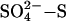 ) deposition, within the range of global acid deposition. We apply a model of this relationship to demonstrate the potential effect of changes in global sulfate deposition from 1960 to 2080 on both northern peatland and global wetland CH4 emissions. We estimate that sulfur pollution may currently counteract climate-induced growth in the wetland source, reducing CH4 emissions by ≈15 Tg or 8% smaller than it would be in the absence of global acid deposition. Our findings suggest that by 2030 sulfur pollution may be sufficient to reduce CH4 emissions by 26 Tg or 15% of the total wetland source, a proportion as large as other components of the CH4 budget that have until now received far greater attention. We conclude that documented increases in atmospheric CH4 concentration since the late 19th century are likely due to factors other than the global warming of wetlands.
) deposition, within the range of global acid deposition. We apply a model of this relationship to demonstrate the potential effect of changes in global sulfate deposition from 1960 to 2080 on both northern peatland and global wetland CH4 emissions. We estimate that sulfur pollution may currently counteract climate-induced growth in the wetland source, reducing CH4 emissions by ≈15 Tg or 8% smaller than it would be in the absence of global acid deposition. Our findings suggest that by 2030 sulfur pollution may be sufficient to reduce CH4 emissions by 26 Tg or 15% of the total wetland source, a proportion as large as other components of the CH4 budget that have until now received far greater attention. We conclude that documented increases in atmospheric CH4 concentration since the late 19th century are likely due to factors other than the global warming of wetlands.
Atmospheric methane (CH4) is a powerful greenhouse gas (GHG) that is responsible for an estimated 22% of the present anthropogenically enhanced greenhouse effect (1). Natural (nonrice agriculture) wetlands are the world's largest single CH4 source and are estimated to currently contribute between 110 and 260 Tg (Tg = 1012 g) to the global methane budget (2), of which one-third is derived from temperate and boreal northern wetlands (3). CH4 emissions from wetlands are climate-sensitive, responding positively to increases in temperature and rainfall as microbial activity and anaerobic conditions increase and negatively to cool temperatures and drought (4, 5). Like many other ecosystems, wetlands are also subject to the effects of aerial pollution and increasing CO2 levels. The stimulatory effects of increased atmospheric CO2 concentrations on CH4 emission (by enhancement of net primary productivity) is well reported (6–8), although a similar stimulatory effect of nitrogen pollution on wetland CH4 emission has not always been identified (8–10) because of differing effects nitrogen has on the ecosystem, e.g., plant species composition is an important factor in determining the effect of experimental N additions on CH4 fluxes (10).
CH4 is produced by two different groups of methanogenic archaea (MA); one group obtains energy by the fermentation of simple organic compounds, such as acetate to CO2 and CH4, and the other obtains energy by oxidizing molecular hydrogen to H2O by using CO2, which is reduced to CH4. Acetate-fermenting MA tend to dominate in more nutrient-rich peatlands and in summer, when the supply of labile organic carbon is relatively high. However, it has been recently demonstrated that climate, depth of the acrotelm, and acetate concentrations add a fair degree of plasticity over controls on acetate-fermenting MA (11). Both groups of microorganisms are strictly anaerobic, and both are suppressed by another group of anaerobic microorganisms, sulfate-reducing bacteria (SRB) (12).
SRB have a higher affinity for both hydrogen and acetate than MA, which, under ideal conditions, enables them to maintain the pool of these substrates at concentrations too low for MA to use (13, 14). In wetlands, however, the balance between sulfate reduction and methanogenesis is affected by factors such as the temperature [warmer temperatures favor methanogenesis (15)], the rate of 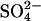 and acetate supply [lower concentrations of sulfate or higher concentrations of acetate reduce the intensity of competition (13)], and the availability of noncompetitive substrates [some low molecular weight hydrocarbons may be preferentially used over acetate by SRB (16, 17) and some substrates such as methanol, methanethiol, and dimethyl sulfide may be used by MA but are poorly used by SRB (18, 19)]. As a consequence, sulfate reduction in wetlands partially, rather than completely, inhibits methane production (19). Stimulation of sulfate reduction has been exploited as a mechanism to reduce GHG emissions from rice paddies; in field trials, CH4 emissions have been reduced by as much as 72% with doses of gypsum (CaSO4) ranging from several hundred to thousands of kilograms of
and acetate supply [lower concentrations of sulfate or higher concentrations of acetate reduce the intensity of competition (13)], and the availability of noncompetitive substrates [some low molecular weight hydrocarbons may be preferentially used over acetate by SRB (16, 17) and some substrates such as methanol, methanethiol, and dimethyl sulfide may be used by MA but are poorly used by SRB (18, 19)]. As a consequence, sulfate reduction in wetlands partially, rather than completely, inhibits methane production (19). Stimulation of sulfate reduction has been exploited as a mechanism to reduce GHG emissions from rice paddies; in field trials, CH4 emissions have been reduced by as much as 72% with doses of gypsum (CaSO4) ranging from several hundred to thousands of kilograms of 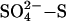 per hectare (ha) (20, 21).
per hectare (ha) (20, 21).
A Synthesis of Recent Studies
Recently, we conducted independent field and controlled environment experiments to test whether the far lower levels of sulfate “naturally” present in acid deposition have the potential to reduce the emission of methane from natural wetlands. In these separate studies (9, 22–24), we dosed peatlands or peat columns in the United States, the United Kingdom, and Sweden with levels of sulfate [(NH4)2SO4, Na2SO4, and Na2SO4, respectively] that span the range of global acid deposition (10–150 kg 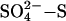 ha–1·yr–1) in small, regular pulses that mimic rainfall/snowfall deposition rates. The peatlands differ in hydrology, vegetation, climate, and methane emission.
ha–1·yr–1) in small, regular pulses that mimic rainfall/snowfall deposition rates. The peatlands differ in hydrology, vegetation, climate, and methane emission.
Taken together, our experiments reveal an emergent pattern of suppression of methane emission by sulfate (Fig. 1, filled circles). With respect to control sites where no sulfate was added, suppression of CH4 increases from 12% to ≈30% as the experimental sulfate additions increase from 10 to 20 kg 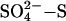 ha–1·yr–1. Above this level of experimental sulfate deposition, CH4 suppression increases to ≈30–45%. All our studies also show that the level of suppression is significantly reduced (CH4 emission began to recover, in some cases approaching control levels) during times when the peatlands are warmer. This reduction in the sulfur effect may be due to enhanced decomposition rates at higher temperatures, allowing more dissolved carbon to become available to the whole microbial community, or to temperature sensitivity in the competition between acetoclastic methanogens and SRB (15).
ha–1·yr–1. Above this level of experimental sulfate deposition, CH4 suppression increases to ≈30–45%. All our studies also show that the level of suppression is significantly reduced (CH4 emission began to recover, in some cases approaching control levels) during times when the peatlands are warmer. This reduction in the sulfur effect may be due to enhanced decomposition rates at higher temperatures, allowing more dissolved carbon to become available to the whole microbial community, or to temperature sensitivity in the competition between acetoclastic methanogens and SRB (15).
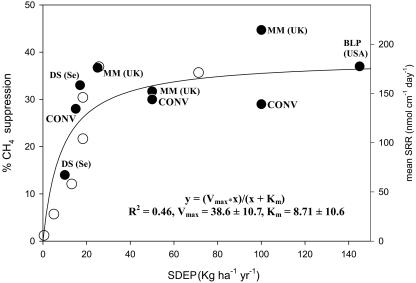
Fig. 1.
Percentage change in suppression of CH4 flux and change in sulfate-reduction rates with SDEP. Percentage of CH4 flux suppression and sulfate reduction rates (SRR) as a function of SDEP. •, changes in suppression of CH4 emission with manipulated 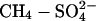 input; ○, changes in sulfate reduction rates with SDEP (ref. 26 and references therein). DS (Se), Degerö Stormyr, Västerbotten, Sweden, 1996 (9); MM (UK), Moidach More, Morayshire, Scotland (23); BLP (USA), Bog Lake Peatland, MN (22); CONV, laboratory peat monolith study under controlled conditions (24). Calculations of annual SDEP for CH4 studies are based on multiplying weekly addition rates by 52.
input; ○, changes in sulfate reduction rates with SDEP (ref. 26 and references therein). DS (Se), Degerö Stormyr, Västerbotten, Sweden, 1996 (9); MM (UK), Moidach More, Morayshire, Scotland (23); BLP (USA), Bog Lake Peatland, MN (22); CONV, laboratory peat monolith study under controlled conditions (24). Calculations of annual SDEP for CH4 studies are based on multiplying weekly addition rates by 52.
Our interpretation of the pattern shown in Fig. 1 is that, given a strong relationship between sulfate deposition and sulfate concentration in porewater (25), SRB in peatlands receiving <≈15 kg 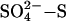 ha–1·yr–1 are limited by sulfate availability (18, 19). This hypothesis is strongly supported by the observation that, for a field survey across North America and Europe, the rate of sulfate reduction in peatland soils increases with increasing sulfate deposition up to a rate of 15–20 kg
ha–1·yr–1 are limited by sulfate availability (18, 19). This hypothesis is strongly supported by the observation that, for a field survey across North America and Europe, the rate of sulfate reduction in peatland soils increases with increasing sulfate deposition up to a rate of 15–20 kg 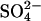 -S ha–1·yr–1 (26). Above this level of sulfate deposition, sulfate reduction rates stabilize, presumably limited by factors other than sulfate supply (26) (Fig. 1, open circles). These factors include the availability of carbon, the temperature, and competition with MA (which results in the pattern of suppression of some, but not all, of CH4 emission represented by the filled circles in Fig. 1). The distinct pattern that arises on combining both the sulfate-reduction data with the acid rain manipulation experiments (Fig. 1) leads us to conclude that not only does the potential exist for suppression of methane emission by sulfate deposition, but this suppression is currently occurring and has occurred in the past.
-S ha–1·yr–1 (26). Above this level of sulfate deposition, sulfate reduction rates stabilize, presumably limited by factors other than sulfate supply (26) (Fig. 1, open circles). These factors include the availability of carbon, the temperature, and competition with MA (which results in the pattern of suppression of some, but not all, of CH4 emission represented by the filled circles in Fig. 1). The distinct pattern that arises on combining both the sulfate-reduction data with the acid rain manipulation experiments (Fig. 1) leads us to conclude that not only does the potential exist for suppression of methane emission by sulfate deposition, but this suppression is currently occurring and has occurred in the past.
Given that sulfur pollution varies both spatially and temporally across the planet, and that projections of population growth and energy consumption in Asia suggest that the problem of sulfur pollution will continue to increase in the first half of this century (27), it is important to examine how this mechanism may have an impact on the wetland CH4 source in the future. To date, no study has quantitatively examined the extent to which areas experiencing enhanced sulfur deposition (SDEP) spatially coincide with CH4-producing wetlands. Here, we estimate the potential for this mechanism to affect both the northern wetland methane source and global wetlands as a whole and estimate the changing importance of atmospheric 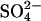 pollution on CH4 emission during both the 20th and 21st centuries.
pollution on CH4 emission during both the 20th and 21st centuries.
Methods
Modeled Global Wetland CH4 Emission. To estimate the significance of this pollution effect, we combined model output of SDEP from a tropospheric sulfur simulation by using the Goddard Institute for Space Studies General Circulation model (GISS GCM) (28) with global CH4 emission estimates. Global, spatially explicit predictions of CH4 emission from natural wetlands for the years 1960, 1970, 1980, 1990, 2000, 2030, and 2080 were used. A1° × 1° process-based and climate-sensitive model of daily CH4 emission from wetlands (29, 30) was simplified by multiple linear regression analysis of modeled monthly CH4 anomalies, monthly anomalies of temperature, and a 2-week lag in precipitation over 12 years by using climate data from the European Centre for Medium-Range Weather Forecasts (31). This analysis was performed for the entire global 1° × 1° gridded data set of natural wetlands (32). The regression model captured the majority of the variability in CH4 emissions induced through changes in temperature and precipitation (r2 = 0.8) and successfully reproduced observed atmospheric CH4 concentration anomalies in the 1990s (31).
New CH4 anomalies were calculated by using this regression model for the time under investigation (1960–2080) by using 20-year running means of two climate scenarios generated by the GISS GCM-coupled ocean–atmosphere model as climate input (temperature and precipitation) (33). One of these scenarios estimated the effect of changes in atmospheric GHG concentration on climate from 1960 to 2080; the second included the combined impact of GHGs and the relative cooling effect of atmospheric 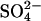 aerosol (GHG+AERO) on climate (33). Distributions of CH4 from wetlands were produced for decadal intervals by using the mean of emissions for the reference year ±3 years (e.g., average of emissions from 1967 to 1973 for 1970).
aerosol (GHG+AERO) on climate (33). Distributions of CH4 from wetlands were produced for decadal intervals by using the mean of emissions for the reference year ±3 years (e.g., average of emissions from 1967 to 1973 for 1970).
Modeled Global SDEP. Total SDEP fields for 1960, 1970, 1980, 1990, 2000, 2030, and 2080 from the tropospheric sulfur simulation in the GISS GCM (28) were used in this study. Model runs for 1960–1990, relied on SO2 emissions data (34); the remaining runs (2000, 2030, and 2080) used emissions data from the Special Report on Emissions Scenarios (SRES scenario A2, which is high compared with other estimates; ref. 35). For 2080 SDEP, was scaled between 2030 and 2,100 Special Report on Emissions Scenario runs. For a full description of the model, see Koch (28). Modeled SDEP (wet and dry) is at the standard GISS GCM 4° latitude × 5° longitude resolution. It is assumed that all sulfur is deposited either in the 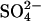 form, or, in the case of dry deposition, is oxidized to
form, or, in the case of dry deposition, is oxidized to 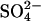 when deposited on wetlands.
when deposited on wetlands.
Combining CH4 and SDEP Data Sets. Spatially explicit estimates of CH4 emission and SDEP were combined for the study period by using a Michaelis–Menton model of the response of CH4 emission to 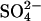 deposition (Fig. 1). This model characterizes findings from our synthesis of experimental S manipulation studies in wetlands and accurately reflects both the results from the low
deposition (Fig. 1). This model characterizes findings from our synthesis of experimental S manipulation studies in wetlands and accurately reflects both the results from the low 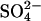 deposition experiment in Sweden (10) and the field survey of
deposition experiment in Sweden (10) and the field survey of 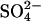 reducing activity relative to
reducing activity relative to 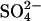 deposition (26) (Fig. 1). Findings were compared against a control where wetland methane fluxes were affected by GHG-forced climate change alone; i.e.,
deposition (26) (Fig. 1). Findings were compared against a control where wetland methane fluxes were affected by GHG-forced climate change alone; i.e., 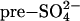 aerosol was not included. Our approach enables the wetland contribution to the global CH4 budget to be estimated with sensitivity to past and predicted changes in both climate and sulfur pollution.
aerosol was not included. Our approach enables the wetland contribution to the global CH4 budget to be estimated with sensitivity to past and predicted changes in both climate and sulfur pollution.
Model Assumptions. A number of simplifications and uncertainties are related to our approach. Our major assumption is that the only source of sulfate to these freshwater wetlands is from the atmosphere (the SDEP model includes all sources of sulfate, including natural sources from volcanoes and biogenic sulfur production). Although we eliminate saltwater marshes and mangrove swamps (both in high-sulfate marine environments) from our database, it is certain that some wetlands have significant local sulfate sources from bedrock, sediments, or groundwater/throughflow. We have no way of estimating the magnitude of this existing sulfate effect, although globally, sulfide-rich rocks are much rarer than silicate or carbonate rocks. Although this would suggest that our findings should be considered as maximum effects, the trajectory of recovery in CH4 fluxes when SDEP is relieved and the factors governing recovery remain poorly understood. The estimates reported here assume an immediate return to normal, 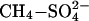 rates of CH4 emission. However, peatlands are net sinks of sulfur, which undergoes reduction and oxidation over short and long timescales (hours and years), moving between pools of varying biological availability, resulting in a likely extension to the duration of its impact on CH4 emission (24, 36, 37). In this respect, any extrapolation is likely to be underestimating the size and duration of the effect of sulfur on wetland methane fluxes.
rates of CH4 emission. However, peatlands are net sinks of sulfur, which undergoes reduction and oxidation over short and long timescales (hours and years), moving between pools of varying biological availability, resulting in a likely extension to the duration of its impact on CH4 emission (24, 36, 37). In this respect, any extrapolation is likely to be underestimating the size and duration of the effect of sulfur on wetland methane fluxes.
Results and Discussion
Adjustment to CH4 Model Output. Global estimates of CH4 emission from wetlands from the control GHG model run are ≈250 Tg for 1960, which is larger than the estimate of the preindustrial wetland source of ≈165 Tg, derived from a 3D chemistry-transport model in combination with isotopic analysis of CH4 trapped in polar ice cores (38). Observed climate warming of 0.3°C (33) between 1860 and 1960 has offset an estimated reduction in the wetland source during this time [which is thought to have resulted from anthropogenic drainage and agricultural conversion of wetlands (38)] but has contributed little more. Moreover, it is likely that the model overestimates the absolute amount of CH4 emitted from natural wetlands because of a bias of high-CH4-emitting intensively studied sites on which the model (on which our simple regression adaptation is based) was generated and validated (29).
To compensate for these effects the modeled global CH4 emission from 1960 (under the GHG or “S-clean” scenario) was scaled back by 33% so that the estimated annual output of CH4 was equivalent to the estimated preindustrial source strength (38). In doing so we took into account both the likely effects of climate change and the influence of anthropogenic disturbance of wetlands on the source size during the preceding 100 years. The same scaling factor was applied to modeled CH4 emissions for each subsequent year of the study (i.e., 1970–2080). The scaling factor resulted in a more conservative estimate of the effect of sulfate on global CH4 emissions from wetlands than if no scaling factor had been applied.
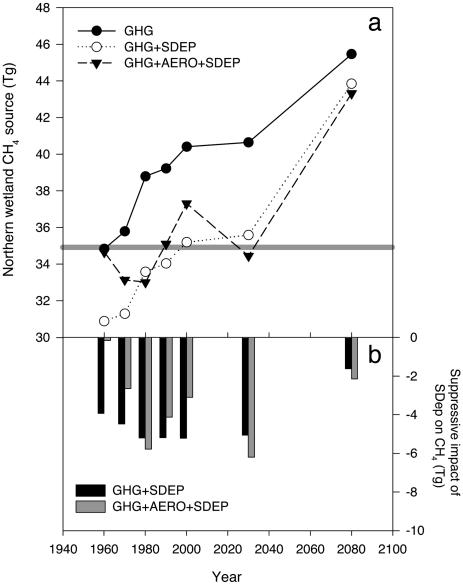
Northern Wetlands. We estimate that northern wetlands (40°N–90°N) were emitting 5 Tg more CH4 in 2000 under the GHG control scenario than is estimated for the preindustrial source strength of 35 Tg (Fig. 2). Without the moderating influence of sulfur pollution, northern wetlands are predicted to continue to increase CH4 output until reaching a predicted source strength of 45.5 Tg, an 11-Tg enhancement or 30% larger than estimated preindustrial emission levels (Fig. 2). Under both sulfur pollution scenarios [GHG+SDEP and GHG+ AERO+SDEP] northern wetland CH4 emission is maintained either below (1960–1990) or at about (2000 and 2030) the preindustrial level, offsetting predicted GHG-forced growth in emissions by between 5.2 and 6.2 Tg or suppressing emissions by between 13% (GHG+SDEP) and 15% (GHG+AERO+ SDEP) by 2030 (Fig. 2). Between 2030 and 2080 the importance of sulfur pollution in relation to GHG warming decreases such that both sulfur pollution scenarios come within 2 Tg of the GHG control CH4 emission scenario (Fig. 2b). Because of spatial differences in the climate input between the separate GHG and GHG+AERO model runs, the suppressive effect of SDEP is not always larger in the GHG+AERO model, as one would expect (Figs. 1 and 2), e.g., for 1960 and 1970.
Fig. 2.
Effect of SDEP on northern wetland CH4 emission with time. (a) Change in northern wetland CH4 source (>40° North) with time under three different climate/sulfate scenarios. Gray line indicates the size of the estimated preindustrial northern wetland methane source (Tg) (38). (b) Relative impact of the 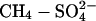 interaction on northern wetland CH4 emissions for two climate scenarios (GHG and GHG+AERO).
interaction on northern wetland CH4 emissions for two climate scenarios (GHG and GHG+AERO).
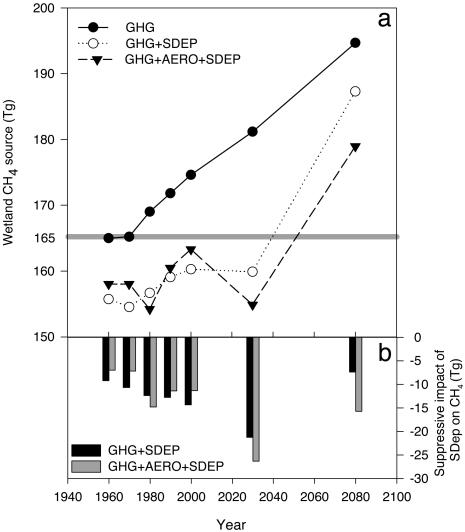
Global Wetlands. For global natural wetlands under the control GHG scenario, we estimate that the 2000 CH4 source is enhanced over the estimated preindustrial source strength (165 Tg) by 10 Tg (Fig. 3). Future climate change will increase this source strength to a 16- and 30-Tg enhancement by 2030 and 2080, respectively (Fig. 3). Suppression of CH4 emissions by sulfate deposition decreases the estimate of the 2000 wetland CH4 source to 5 Tg below preindustrial levels, with the CH4 emission from wetlands not exceeding preindustrial levels until ≈2050 (Fig. 3). In 2030 the amount of methane predicted from the model with the 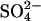 suppression included will be 21 Tg less than the amount that would be estimated under current estimates of climate change alone. By 2080, a combination of global warming and stabilizing SO2 emissions will cause wetland CH4 emissions to finally exceed preindustrial levels by 15 Tg (Fig. 3a).
suppression included will be 21 Tg less than the amount that would be estimated under current estimates of climate change alone. By 2080, a combination of global warming and stabilizing SO2 emissions will cause wetland CH4 emissions to finally exceed preindustrial levels by 15 Tg (Fig. 3a).
Fig. 3.
Effect of SDEP on the global wetland CH4 source with time. (a) Change in wetland CH4 source with time under three different climate/sulfate scenarios. Gray line indicates the size of the estimated preindustrial wetland methane source (Tg) (38). (b) Relative impact of the 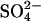 interaction on the wetland CH4 source for two climate scenarios (GHG) and (GHG+AERO).
interaction on the wetland CH4 source for two climate scenarios (GHG) and (GHG+AERO).
Our estimates of the combined effects of climate change, sulfate aerosol radiative effects, and SDEP (GHG+AERO+SDEP) on CH4 emissions show that anthropogenic SDEP may have been sufficient to have decreased the global wetland CH4 source to a level below preindustrial estimates by ≈10–15 Tg during the second half of the 20th century (Fig. 3). The combined effect of 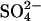 aerosols (cooling) and
aerosols (cooling) and 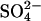 deposition (limiting methane production at the source by microbial competition) are predicted to offset the effect of GHG warming on CH4 emissions by 26 Tg in 2030 and by ≈15 Tg in 2080. In this scenario, CH4 emissions will exceed preindustrial emissions by 14 Tg by 2080. The influence of production and deposition of oxidized sulfur compounds through economic growth in North America and Europe between 1960 and 1980, followed by increases in the economic growth in South America, Africa, and (primarily) Asia, are responsible for this pattern. Beyond 2030, however, a decline is predicted in sulfur pollution because of anticipated cleaner technologies. Together with the additional effect of enhanced greenhouse warming, we predict this reduction in sulfur pollution will result in a rapid increase in CH4 emission (15% enhancement between 2030 and 2080) that may exacerbate climate warming during that time.
deposition (limiting methane production at the source by microbial competition) are predicted to offset the effect of GHG warming on CH4 emissions by 26 Tg in 2030 and by ≈15 Tg in 2080. In this scenario, CH4 emissions will exceed preindustrial emissions by 14 Tg by 2080. The influence of production and deposition of oxidized sulfur compounds through economic growth in North America and Europe between 1960 and 1980, followed by increases in the economic growth in South America, Africa, and (primarily) Asia, are responsible for this pattern. Beyond 2030, however, a decline is predicted in sulfur pollution because of anticipated cleaner technologies. Together with the additional effect of enhanced greenhouse warming, we predict this reduction in sulfur pollution will result in a rapid increase in CH4 emission (15% enhancement between 2030 and 2080) that may exacerbate climate warming during that time.
Our findings are based on research in peatlands, and so to extrapolate these results to all natural wetlands, including peatlands, swamps, and marshes, necessitates the assumption that the relationship between SDEP and CH4 suppression holds equally for these systems across the globe. Although to our knowledge no similar low-dose sulfate manipulation experiments have been carried out in other wetlands, studies from low-latitude marshes have shown far smaller methane fluxes from sulfate-rich sites such as salt marshes (39) and sulfate-rich freshwater marshes (40) when compared with low-sulfate sites in the same locality. Rice paddies also respond with reductions in methane flux of ≈40–70% when fertilized with sulfate (20). Indeed, relatively small individual fertilization additions of 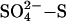 (75–140 kg of S ha–1) have been demonstrated to suppress CH4 emissions by 16–40% (28). This mode of application, although at the top end of annual rates of sulfate deposited in highly polluted regions, may be less efficient at reducing CH4 emissions than continuous pollutant deposition of low levels of sulfate applied at the same annual rate (41).
(75–140 kg of S ha–1) have been demonstrated to suppress CH4 emissions by 16–40% (28). This mode of application, although at the top end of annual rates of sulfate deposited in highly polluted regions, may be less efficient at reducing CH4 emissions than continuous pollutant deposition of low levels of sulfate applied at the same annual rate (41).
Given these uncertainties, the potential size of this CH4 suppression places the role of sulfur pollution in limiting wetland CH4 emissions in a size category similar to the major terrestrial sink in the CH4 budget, that of oxidation in dry soils (42) (25–30 Tg). Furthermore, the implications of this study in globally characterizing the effect of acid rain are important for improving our understanding of factors controlling the largest CH4 source. Moreover, our results indicate that the observed increase in atmospheric CH4 concentration throughout the 20th century is the result of factors other than growth in the contribution from wetlands (43).
Acknowledgments
We thank R. Kelman Weider, E. Dlugokencky, S. Chapman, and T. Christensen for helpful comments on an earlier version of this article and two anonymous reviewers for their constructive critiques. This work was funded by a National Aeronautics and Space Administration Planetary Biology Internship. Additional support was received from the U.K. Natural Environment Research Council (Grant GR9/04644), the U.S. Department of Agriculture Forest Service, and the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (Contract 21.4/2002-1510).
Abbreviations: MA, methanogenic archaea; SRB, sulfate-reducing bacteria; GISS GCM, Goddard Institute for Space Studies General Circulation model; GHG, greenhouse gas; SDEP, sulfur deposition; AERO, atmospheric 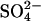 aerosol; ha, hectare.
aerosol; ha, hectare.
See Commentary on page 12400.
References
- 1.Lelieveld, J., Crutzen, P. & Dentener, F. J. (1998) Tellus 5B, 128–150. [Google Scholar]
- 2.Intergovernmental Panel on Climate Change (IPCC) (2001) Climate Change 2001: The Scientific Basis—Contribution of Working Group I to the Third Assessment Report of IPCC (Cambridge Univ. Press, Cambridge, U.K.).
- 3.Matthews, E. (2000) in Atmospheric Methane: Its Role in the Global Environment, ed. Kahlil, M. A. K. (Springer, New York), pp. 202–233.
- 4.Dise, N. B., Gorham, E., Verry, E. S. (1993) J. Geophys. Res. Atmos. 98, 10583–10594. [Google Scholar]
- 5.Freeman, C., Lock, M, A. & Reynolds, B. (1993) Biogeochemistry 19, 51–60. [Google Scholar]
- 6.Hutchin, P. R., Press, M. C., Lee, J. A. & Ashenden, T.W. (1995) Global Change Biol. 1, 125–128. [Google Scholar]
- 7.Megonigal, J. P. & Schlesinger, W. H. (1997) Biogeochemistry 37, 77–88. [Google Scholar]
- 8.Saarnio, S., Saarinen, T., Vasander, H. & Silvola, J. (2000) Global Change Biol. 6, 137–144. [Google Scholar]
- 9.Granberg, G., Sundh, I., Svensson, B. & Nilsson, M. (2001) Ecology 82, 1982–1998. [DOI] [PubMed] [Google Scholar]
- 10.Nykanen, H., Vasander, H., Huttunen, J. T. & Martikainen, P. J. (2002) Plant Soil 242, 147–155. [Google Scholar]
- 11.Hines, M. E., Duddleston, K. N. & Kiene, R. P. (2001) Geophys. Res. Lett. 28, 4251–4254. [Google Scholar]
- 12.Lovley, D. R. & Klug, M. J. (1983) Appl. Environ. Microbiol. 45, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lovley, D. R., Dwyer, D. F. & Klug, M. J. (1982) Appl. Environ. Microbiol. 43, 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winfrey, M. R. & Zeikus, J. G. (1977) Appl. Environ. Microbiol. 33, 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Bodegom, P.M. & Stams, A. J. M. (1999) Chemosphere 39, 167–182. [Google Scholar]
- 16.Westermann, P. & Ahring, B. (1987) Appl. Environ. Microbiol. 53, 2554–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oude Elferink, S. J. W. H., Luppens, S. B. I., Marcelis, C. L. M. & Stams A. J. M. (1998) Appl. Environ. Microbiol. 64, 2301–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lomans, B. P., Op den Camp, H., Pol, A., van der Drift, C. & Vogels, G. (1999) Appl. Environ. Microbiol. 65, 2116–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyimo, T. J., Pol, A. & Op den Camp, H. (2002) Ambio 31, 614–616. [PubMed] [Google Scholar]
- 20.Denier van der Gon, H. A. C., van Bodegom, P. M., Wassmann, R., Lantin, R. S. & Metra-Corton, T. M. (2001) Mitigation and Adaptation Strategies for Global Change 6, 71–89. [Google Scholar]
- 21.Lindau, C. W., Wickersham, P., DeLaune, R. D., Collins, J. W., Bollick, P. K., Scott, L. M. & Lambremont, E. N. (1998) Agric. Ecosyst. Environ. 68, 165–173. [Google Scholar]
- 22.Dise, N. B. & Verry, E. S. (2001) Biogeochemistry 53, 143–160. [Google Scholar]
- 23.Gauci, V., Dise, N. B. & Fowler, D. (January 19, 2002) Global Biogeochem. Cycles 16, 10.1029/2000GB001370.
- 24.Gauci, V, Fowler, D., Chapman, S. J. & Dise, N. B. (2004) Biogeochemistry, in press.
- 25.Urban, N. R., Eisenreich, S. J. & Gorham, E. (1987) in Effects of Atmospheric Pollutants on Forests, Wetlands and Agricultural Ecosystems, eds. Hutchinson, T. C. & Meema, K. M. (Springer, Berlin), pp. 577–598.
- 26.Vile, M. A., Bridgham, S. D., Wieder, R. K. & Novak, M. (2003) Global Biogeochem. Cycles 17, 1058. [Google Scholar]
- 27.Streets, D. G. & Waldhoff, S. T. (2000) Atmos. Environ. 34, 363–374. [Google Scholar]
- 28.Koch, D. (2001) J. Geophys. Res. Atmos. 106, 20311–20332. [Google Scholar]
- 29.Walter, B. P. Heimann, M. & Matthews, E. (2001) J. Geophys. Res. 26, 34189–34206. [Google Scholar]
- 30.Walter, B. P. & Heimann, M. (2000) Global Biogeochem. Cycles 14, 745–765. [Google Scholar]
- 31.Dlugokencky, E. J, Walter, B. P., Masarie, K. A., Lang, P. M. & Kasischke, E. S. (2001) Geophys. Res. Lett. 28, 499–502. [Google Scholar]
- 32.Matthews, E. & Fung, I. (1987) Global Biogeochem. Cycles. 1, 61–86. [Google Scholar]
- 33.Russell, G. L., Miller, J. R., Rind, D., Ruedy, R. A., Schmidt, G. A. & Sheth, S. (2000) J. Geophys. Res. Atmos. 105, 14891–14898. [Google Scholar]
- 34.Lefohn, A. S., Husar, J. D. & Husar, R. B. (1999) Atmos. Environ. 33, 3435–3444. [Google Scholar]
- 35.Nakicenovic, N. (2000) in Special Report on Emissions Scenarios: A Special Report of the Intergovernmental Panel on Climate Change, eds. Alcamo, J., Davis, G. & De Vries, B. (Cambridge Univ. Press, Cambridge, U.K.).
- 36.Wieder, R. K., Lang, G. E. & Granus, V. A. (1987) Soil Biol. Biochem. 19, 101–106. [Google Scholar]
- 37.Chapman, S. J. (2001) Water Air Soil Pollut. 1–2, 23–39. [Google Scholar]
- 38.Houweling, S., Dentener, F. & Lelieveld, J. (2000) J. Geophys. Res. Atmos. 105, 17243–17255. [Google Scholar]
- 39.Bartlett, K. B., Bartlett, D. S., Harriss, R. C. & Sebacher, D. I. (1987) Biogeochemistry 4, 183–202. [Google Scholar]
- 40.Rejmankova, E. & Post, R. A. (1996) Biogeochemistry 34, 57–70. [Google Scholar]
- 41.Arah, J. R. M. & Stephen K. D. (1998) Atmos. Environ. 32, 3257–3264. [Google Scholar]
- 42.Kahlil, M. A. K., Shearer, M. J. & Rasmussen, R. A. (2000) in Atmospheric Methane: Its Role in the Global Environment, ed. Khalil, M. A. K. (Springer, New York), pp. 86–97.
- 43.Stern, D. I. & Kaufmann, R. K. (1996) Chemosphere 33, 159–176. [Google Scholar]