Abstract
Long-lived organisms tend to be more resistant to various forms of environmental stress. An example is the Drosophila longevity mutant, methuselah, which has enhanced resistance to heat, oxidants, and starvation. To identify genes regulated by these three stresses, we made a cDNA library for each by subtraction of “unstressed” from “stressed” cDNA and used DNA hybridization to identify genes that are regulated by all three. This screen indeed identified 13 genes, some already known to be involved in longevity, plus candidate genes. Two of these, hsp26 and hsp27, were chosen to test for their effects on lifespan by generating transgenic lines and by using the upstream activating sequence/GAL4 system. Overexpression of either hsp26 or hsp27 extended the mean lifespan by 30%, and the flies also displayed increased stress resistance. The results demonstrate that multiple-stress screening can be used to identify new longevity genes.
Keywords: aging, heat-shock proteins hsp26 and hsp27, chaperones, paraquat, starvation
Genetic or environmental manipulations to extend lifespan in various organisms have been found to correlate with increases in resistance to environmental stress (1, 2). Drosophila selected for delay in age of reproduction have increased longevity and higher resistance to many stresses, including desiccation, heat, starvation, and oxidants (3, 4), and the long-lived Drosophila methuselah and ecdysone receptor (EcR) mutants show enhanced resistance to paraquat, starvation, and heat (5, 6). The long-lived Caenorhabditis elegans mutants age-1 and daf-2 have higher resistance to oxidative stress (7), ultraviolet light (8), and heat (9, 10). Skin fibroblasts taken from long-lived p66shc knockout mice show increased resistance to oxidative stress and UV light (11). Skin fibroblasts from mammals with varying lifespan show a positive correlation between lifespan and resistance to a variety of stressors (12). Hormesis, the beneficial effect of exposure to sublethal stress, can extend longevity in C. elegans (13) and in Drosophila (14).
The correlation between stress resistance and lifespan has prompted efforts to screen for lifespan extension mutants via increased stress resistance. Several such screens have been successful, including selection for paraquat resistance in Drosophila (15) and for increased thermotolerance in C. elegans (16, 17). A screen for resistance to both heat and paraquat in yeast identified mutations in adenylate cyclase and Akt/PKB that extended stationary-phase survival 3-fold (18).
The link between stress resistance and lifespan extension is further strengthened by findings that manipulation of stress-responsive genes can extend lifespan. For example, Drosophila lifespan is increased by overexpression of the antioxidant Cu-Zn superoxide dismutase (SOD) (19–21) or by overexpression of the heat-shock protein (HSP) gene hsp70 (22). In the C. elegans mutant age-1, up-regulation of Hsp16 extends lifespan (23), and heat-shock factor 1 promotes longevity as well as resistance to heat and oxidative stress (24, 25). In Saccharomyces cerevisiae, increased expression of pyrazinamidase/nicotinamidase 1, which responds to a variety of low-intensity stresses, extends lifespan (26).
Although longevity-extending mutations often confer increased resistance to stress, the converse is often not true; in our hand, mutants isolated for resistance to a single form of stress may not have extended longevity (unpublished data). We therefore designed an approach to the identification of genes that are up-regulated by all three stresses, paraquat, heat, and starvation, using cDNA subtraction libraries and DNA microarray methodology. This method indeed turned up some already known genes that increase longevity, including hsp70, Cu/Zn sod, and catalase. Among the new candidates found were heat-shock genes hsp26 and hsp27. Here we report that overexpression of these fly genes indeed increases lifespan.
Materials and Methods
Generation of Stress-Subtracted cDNA Libraries and Microarray Membranes. Three different stresses, paraquat, starvation, and heat, were used, as described by Lin et al. (5). Twenty 2- to 4-day-old w1118 adult male flies were placed in each vial. For paraquat, control flies were fed with 5% sucrose for 42 h, whereas the treated flies were fed with 5% sucrose containing 20 mM paraquat. For starvation, control flies were given normal fly food for 58 h, whereas starved flies had only wet filter paper. For heat, control flies were maintained at 25°C for 4 h on 5% sucrose/1% agar, whereas treated flies were incubated at 36°C. mRNA was prepared with the Qiagen (Valencia, CA) mRNA isolation kit. For each form of stress, a subtractive hybridization was done with the PCR-Select cDNA subtraction kit from Clontech, thus generating three subtracted cDNA libraries, each enriched for cDNA up-regulated by one of the three stresses. In each case, subtracted cDNA was ligated to the pGEM-T vector (Promega) and Escherichia coli transformed by electroporation. Under isopropyl β-d-thiogalactoside/5-bromo-4-chloro-3-indolyl β-d-galactoside selection, colonies of white transformed cells were picked and used to spot two identical microarray membranes on Hybond nylon filter membranes, at the California Institute of Technology Genome Technology Facility. One of these membranes was hybridized with the starvation-subtracted cDNA labeled with 32P, the other with labeled heat-subtracted cDNA, and exposed to x-ray film. By comparing the spots on the two hybridized assays, we identified clones corresponding to up-regulation by both heat and starvation. Because the arrays were prepared by using a paraquat library, these clones were candidates for up-regulation by all three stressors. Those cDNA candidates were sequenced and identified by a blast search.
Northern Blot Analysis. To test for the abundance of candidate RNAs in the flies, total RNA was prepared from control and stress-treated flies by Trizol extraction. Equal amounts (10 μg) were subjected to formaldehyde RNA gel electrophoresis and transferred to a nylon membrane. The membrane was hybridized with specific labeled probes, as specified in Fig. 1C, washed with high-stringency buffer, and autoradiographed on x-ray film. The membrane was stripped, then rehybridized with a 16S rRNA DNA probe as a loading control.
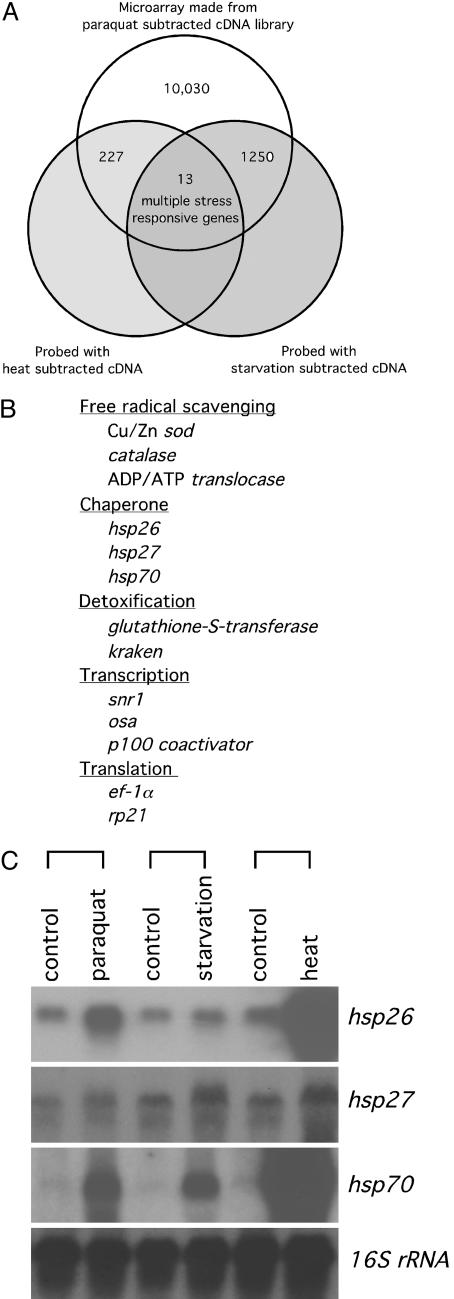
Fig. 1.
The screen for multiple-stress-responsive genes. (A) The strategy. Three subtracted cDNA libraries were prepared, one for each stress. The paraquat stress-subtracted cDNA library was used to make duplicate microarray membranes. Each microarray membrane was probed separately with one of the other two stress-subtracted cDNA libraries. Triple-stress up-regulated genes were identified by comparison of the two membranes. (B) Triple-stress-responsive candidate genes were identified by DNA sequencing and blast search. (C) Confirmation of up-regulation of three heat-shock genes by Northern blot. Ten micrograms of total RNA from either control or stressed flies was run on the gel and the blots hybridized with 32P-labeled clones as indicated. 16S rRNA was used as an internal loading control.
Construction of hsp26 and hsp27 Transgenic Flies. PstI/XhoI cDNA fragments containing full-length hsp26 from Drosophila EST clone GM08850 or full-length hsp27 from clone LD06811 were subcloned into the P element transformation vector pINDY6 (27), resulting in pINDY6-hsp26 and pINDY-hsp27. The constructs were injected into w1118 embryos. Transgenic lines were selected by w+ expression in the eye, and insertions mapping to the second or third chromosomes were isolated. The hs-GAL4 strain (Bloomington stock number 1799) used carries a third chromosome enhancer trap that drives the GAL4 transcriptional activator ubiquitously in adult flies (28).
RT-PCR Analysis. Total RNA was prepared from transgenic flies containing one copy of the transgene alone and from flies containing also the GAL4 driver and treated with DNase I to eliminate any DNA contamination. Total RNA from w1118 flies was used as a negative control. Equal amounts (5 μg) were used to synthesize first-strand cDNA with oligo dT15 in the reverse transcription reaction. Equal volumes (10 μl) of the cDNA preparations from each of the different reactions were subjected to PCR. The primers used for hsp26 were 5′-CAAGCGCAGCTGAACAAGCTAAACAATCTG-3′ (here named HDW54) and 5′-GCATGATGTGACCATGGTCGTCCTGG-3′ (here named HDW55) shown in Fig. 2A. The primers for hsp27 were HDW54 and 5′-CCATCTACCACCACGGTGT TGTCCACCA-3′ (named HDW57). HDW54 primes transcripts expressed from the pINDY6 vector. The transgenic constructs pINDY6-hsp26 and pINDY-hsp27 were also used as positive controls in the PCR reaction, to indicate the sizes of the expected amplified DNA fragments. One-tenth (10 μl) of each PCR product was run by 1.5% agarose gel electrophoresis, as shown in Fig. 2B.
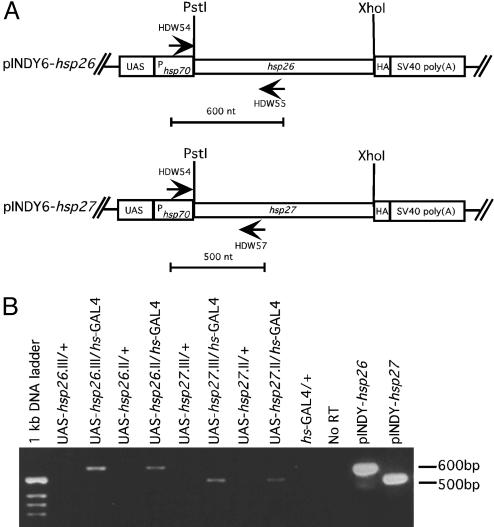
Fig. 2.
Generation of hsp26 and hsp27 transgenic flies. (A) The transgenic constructs. Full-length hsp26 and hsp27 cDNA were subcloned into expression vector pINDY6, driven by the yeast USA enhancer linked with an hsp70 minimal promoter, to generate pINDY6-hsp26 and pINDY6-hsp27. Each construct was used to make transgenic flies, as described in Materials and Methods. Two different insertion lines, one on the second chromosome and the other on the third chromosome, were obtained for each transgene. These are named UAS-hsp26.II, UAS-hsp26.III, UAS-hsp27.II, and UAS-hsp27.III. The primer set HDW54 and HDW55 detects the hsp26 transcripts, generating a 600-bp DNA fragment. The primer set HDW54 and HDW57 amplifies the hsp27 transcript, generating a 500-bp DNA fragment. (B) RT-PCR to detect expression of hsp26 and hsp27 in the transgenic flies. Five micrograms of total RNA was used in the RT-PCR reaction, as described in Materials and Methods. Equal amounts (10 μl) of each PCR product were applied to 1.5% agarose gel for electrophoresis.
Lifespan Measurement and Stress Test. Male flies were maintained at 25°C in vials with normal fly food, transferred to fresh food every 3–4 days, and dead flies counted until all died. The mean lifespan was calculated for each strain. Four independent experiments were carried out with ≈75 flies per experiment for a total of ≈300 flies. For stress tests, male flies were subjected to the same conditions used in preparations of the subtraction cDNA, using paraquat, starvation, and heat. Three independent experiments of 90 flies were performed for each stress test.
Fecundity Measurements. Virgin female transgenic flies with or without hs-GAL4 and ones with hs-GAL4 alone were collected. In each case, 20 5-day-old females were mated to 20 w1118 males for 2 days, then transferred to a cage, and grape juice plates were used to collect eggs at 24-h intervals, up to 96 h. The number of eggs on each plate was counted. Each plate was kept at 25°C for 2 extra days and examined for the number of larvae hatched. Each experiment was done in three independent replicates.
Results
Isolation of Longevity Candidate Genes by Multiple Stresses. As described in Materials and Methods, for each of the three forms of stress, paraquat, starvation, and heat, cDNA libraries were prepared; one using stressed flies, the other unstressed controls. In each case, cDNA from control flies was subtracted from cDNA from stressed flies. The paraquat-subtracted cDNA library was used to make duplicate microarray membranes, each containing 11,520 clones. One of the membranes was hybridized with labeled starvation-subtracted cDNA probe, the other with heat-subtracted cDNA probe. By comparing the results from the two different hybridizations, we could identify genes for which the cDNA was enriched by both paraquat and starvation, by both paraquat and heat, or by all three stresses (Fig. 1 A). Thirteen triple stress-responsive candidate genes were found, their functions known to include free radical scavenging, chaperoning, detoxification, transcription, and translation (Fig. 1B). Among these, Cu/Zn sod, catalase, and hsp70 have been reported to extend lifespan when overexpressed (19–22). hsp26 as well was found in a screen, using a bidirectional upstream activating sequence (UAS) construct, to extend lifespan, but overexpression of the gene was not documented (29). The expression level of hsp26 was found elevated in 61-day-old Drosophila and also in 100% hyperoxia-treated flies in an Affymetrix genechip analysis (30). This confirmed the validity of this approach in screening for candidate longevity genes.
Based on their responses to all three stresses, as confirmed by Northern blot (Fig. 1C), two candidate genes, hsp26 and hsp27, were chosen for further study. To generate hsp26 and hsp27 transgenic flies, their full-length cDNAs were subcloned and driven by the yeast UAS enhancer, as shown in Fig. 2 A. For each gene, two independent inserts, one in the second chromosome, the other in the third, were obtained. RT-PCR confirmed overexpression of the transgenes when crossed with hs-GAL4 drivers (lanes 3, 5, 7, and 9 in Fig. 2B). Without GAL4, no expression was detected (lanes 2, 4, 6, and 8 in Fig. 2B).
Flies containing the heat-shock transgene alone or the transgene plus the GAL4 driver were used to prepare total RNA for RT-PCR analysis. By using the primer HDW54 derived from the pINDY6 injection vector, we detected the expression of hsp26 and hsp27 in both the second and third chromosome insertion flies when GAL4 was present (lanes 3, 5, 7, and 9 in Fig. 2B). Without GAL4, there was no detectable expression of hsp26 and hsp27 (lanes 2, 4, 6, and 8 in Fig. 2B). Total RNA from w1118 flies was used in the RT-PCR as a control, to confirm that the amplification was not from endogenous hsp26 and hsp27 (lanes 10 and 11 in Fig. 2B). The injection constructs, pINDY-hsp26 and pINDY6-hsp27, were used to show the size of the amplified cDNA.
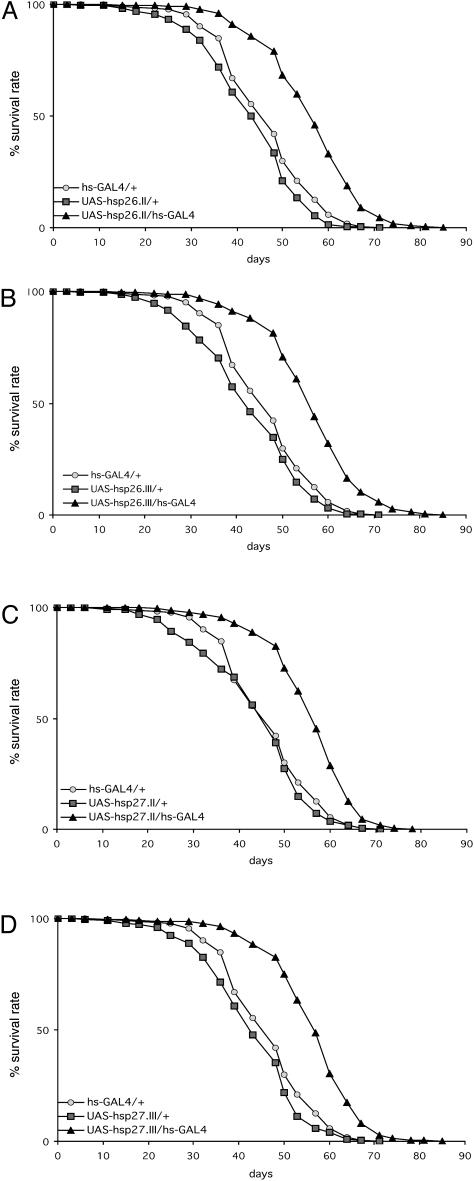
Overexpression of hsp26 or hsp27 Increases Lifespan of Drosophila. We next examined the lifespans at 25°C of flies overexpressing hsp26 or hsp27, each in its two different transgenic alleles (Fig. 3 and Table 1). Compared with transgenic flies without GAL4, there was a 30% increase in mean lifespan for flies overexpressing the hsp26 transgene on the second chromosome (Fig. 3A) and a 31% increase in those with the transgene on the third chromosome (Fig. 3B). For the hsp27 transgenic flies, 27% mean lifespan increase was observed in the flies with the transgene on the second chromosome (Fig. 3C) and 31% on the third (Fig. 3D). In additional experiments at 29°C (data not shown), lifespan extension was also found for the UAS-hsp26 and UAS-hsp27 transgenic flies using two different drivers, daughterless-GAL4 and hs-GAL4.
Fig. 3.
Lifespan extension by overexpression of hsp26 and hsp27 in transgenic flies. Each experiment was repeated four times with ≈75 flies per trial. (A) UAS-hsp26.II. (B) UAS-hsp26.III. (C) UAS-hsp27.II. (D) UAS-hsp27.III. Each of the four transgenic strains was tested with and without the hs-GAL4 driver, as compared with the driver alone.
Table 1. Increased lifespan in transgenic flies overexpressing HSP26 or HSP27 under a hs-GAL4 driver.
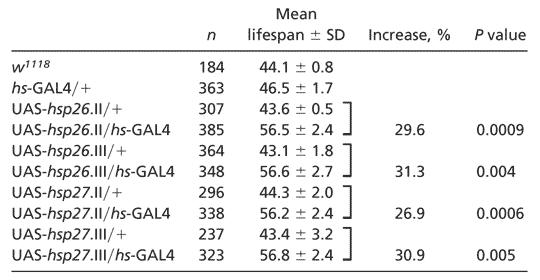 |
The mean ± SD (days) is calculated from four independent lifespan measurements; P value is determined by Student's t test: n is the total number of flies from four independent experiments (+ designates the w1118chromosome).
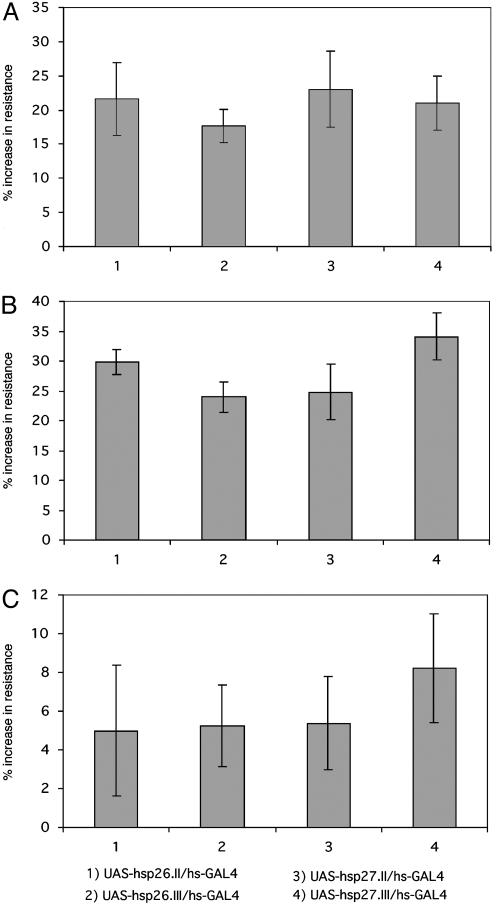
Overexpression of hsp26 or hsp27 Increases Stress Resistance. For resistance to paraquat, the hsp26 overexpressing transgenic flies, compared with controls with UAS alone, showed increases of 21% for the insertion on chromosome II, and 18% for the one on chromosome III. The hsp27 overexpressing transgenics showed, for insertions on II and III, 23% and 21%, respectively (Fig. 4A). Similar results were found for heat stress (Fig. 4B). The effects for starvation resistance (Fig. 4C) were only marginal, which may reflect the lesser enhancement of transcription of these genes by starvation, as compared to the other two forms of stress (Fig. 1C).
Fig. 4.
Increased stress resistance in transgenic flies overexpressing hsp26 or hsp27. Three- to 5-day-old adult male transgenic flies overexpressing hsp26 or hsp27, driven by hs-GAL4, as compared with controls without the driver, were tested with the three separate stresses, as described in Materials and Methods. The measure of stress resistance is mean survival time under paraquat (A), heat (B), or starvation (C).
Overexpression of hsp26 or hsp27 Reduces Fecundity. Increased longevity is often correlated with a decline in fecundity, as defined by the number of eggs laid (31, 32). Extension of lifespan in hsp70 transgenic flies showed such a reproductive cost (33). We measured fecundity and egg hatching of the hsp26 or hsp27 overexpressing transgenic flies, as compared to controls. The cumulative numbers of eggs are shown in Table 2. Flies overexpressing hsp26 showed, for the insertions on chromosome II and III, respectively, 44% and 60% reduction in fecundity. For hsp27, there were reductions of 33% and 42%. However, there was no significant difference in egg hatching between the overexpressing transgenic flies and controls (Table 2). Female transgenic flies first aged to 28 days, then mated for 2 days, showed similar reductions in fecundity and egg hatching (not shown). Our data indicate a tradeoff between lifespan and reproduction in the overexpression of hsp26 and hsp27.
Table 2. Reduced fecundity in transgenic flies overexpressing HSP26 or HSP27 under a hs-GAL4 driver.
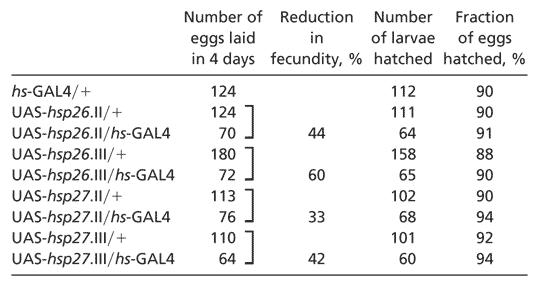 |
Discussion
In addition to hsp26 and hsp27 described here, others of the multiple stress-responsive genes found in our screen are known to have varying functions in stress response and aging. Some help to counteract oxidative stress: Cu/Zn sod and catalase serve as free-radical scavengers. Overexpression of Cu/Zn sod and catalase combined increased lifespan in the fly. ADP/ATP translo-case (the mouse Ant gene) exchanges ATP with cytosolic ADP across the mitochondrial inner membrane. Mutational inactivation of the Ant1 gene in the mouse results in an elevated level of reactive oxygen species, in association with multiple deletions in senescent mitochondrial DNA (34, 35), and the ant1 null mutant mouse is sensitive to oxidative stress and displays shortened lifespan. Glutathione-S-transferases detoxify toxic electrophiles, both xenobiotic and endobiotic, by conjugation with glutathione, and the resultant products can be metabolized and excreted (36), and transgenic expression of a glutathione S-transferase gene in C. elegans increases resistance to oxidative stress. Decreasing its expression by RNA interference results in decreased resistance to oxidative stress (37).
A second group of the multiple-stress-responsive genes involves gene expression mechanisms. snr1 and osa are components of the Drosophila Brahma ATP-dependent chromatin remodeling complex, which is involved in cell proliferation and pattern formation. Null mutants of snr1 in Drosophila display shortened lifespan (38). Several chromatin modifiers have been shown to extend lifespan (39–41). Elongation factor 1α (ef-1α) plays a role in protein synthesis, which decreases during aging (42). Transgenic flies of overexpressing ef-1α were reported to extend lifespan in Drosophila (43), but whether ef-1α was overexpressed in these flies is controversial (44). In our screening, the level of ef-1α was up-regulated by all three stresses. Recently, EF-1α was found to associate with some HSPs in polyglutamine aggregates, and transient expression of ef-1α or hsp84 improved the viability of the polyglutamine-aggregate-containing cells (45). Overexpression of ef-1α also resulted in resistance to apoptosis induced by growth factor withdrawal or estrogen receptor stress (46). Feeding Drosophila with 4-phenylbutyrate, which extended their lifespan, caused a 4-fold up-regulation of ef-1α, and also up-regulated CuZn sod and glutathione S-transferase (40). All three of these genes also appeared in our screen.
The set of HSPs is one of the intracellular defense systems in response to different stressful conditions (47). HSPs function as molecular chaperones to enhance protein folding, prevent protein denaturation and aggregation, and facilitate proteolysis of damaged proteins. A decrease in the response of HSPs to stress occurs during aging (2, 48). Deterioration of the cell's capacity to produce active HSPs could lead to the accumulation of damaged proteins or lipofuscin (47, 49). HSPs could prevent age-associated protein damage and aggregation. Small HSPs, including HSP26, form large oligomers, become phosphorylated under stress, and dissociate from the oligomers to bind nonnative proteins (50–52). HSP27 increases glucose-6-phosphate dehydrogenase activity, maintaining the cellular glutathione level against oxidative stress (53). It protects cells against apoptosis by binding to cytochrome c released from mitochondria (54–57) and counteracts polyglutamine toxicity by inhibiting the increase of reactive oxygen species caused by aggregation of Huntingtin protein (58).
A body of evidence supports the idea that the expression of HSPs regulates lifespan. As mentioned above, that is true for Hsp70 in Drosophila (22). Flies selected for longer life display an elevated expression level of hsp22 (59), and transgenic flies overexpressing hsp22 also increase mean lifespan by 30% (60). Increased lifespan was observed by the insertion of a P element containing bidirectional UAS upstream of hsp26 genomic DNA (29). In C. elegans, up-regulation of Hsp16, a homolog of Drosophila Hsp22, extended lifespan (23), and overexpression of heat-shock transcription factor hsf-1, which regulates expression of an ensemble of HSPs acting in multiple tissues, increases lifespan (24, 25).
Conclusion
We used a multiple-stress screen strategy, combined with cDNA subtraction and microarray, to isolate longevity candidates genes, and demonstrated that the overexpression of two candidate genes, hsp26 or hsp27, extended lifespan, and increased stress resistance in the transgenic flies. This approach can be used to identify candidate genes down-regulated by stress by reversing cDNA subtraction by subtracting cDNA of stressed flies from cDNA of unstressed flies. We have also applied multiple stress in a forward genetic screen on adult flies, which yielded numerous stress-resistant mutants with increased longevity (H.-D.W and S.B., unpublished data).
Acknowledgments
We thank members of the Benzer laboratory, including P. Kapahi, T. Brummel, D. Walker, D. Tracey, and B. Al-Ansi, for helpful discussions and comments on the manuscript. We are grateful for technical support from T. Gill, G. Kwan, C. Lai, P. Karayan, A. Lam, A. Dinh, U. Zimmermann, J. Silverlake, R. Young, and V. Sapin. We also thank A. Cameron and E. Davidson for use of the California Institute of Technology Genome Technology facility. This work was supported by a National Research Service Award from the National Institute of Aging (Grant AG05890 to H.-D.W.), grants from Hereditary Disease Foundation and the Wills Foundation (to P.K.-E.), and grants to S.B. from the National Institutes of Health (Grant AG16630), the National Science Foundation (Grant MCB-9907939), and the Ellison Foundation.
Abbreviations: SOD, superoxide dismutase; EF, elongation factor; HSP, heat-shock protein; UAS, upstream activating sequence.
References
- 1.Martin, G. M., Austad, S. N. & Johnson, T. E. (1996) Nat. Genet. 13, 25–34. [DOI] [PubMed] [Google Scholar]
- 2.Finkel, T. & Holbrook, N. J. (2000) Nature 408, 239–247. [DOI] [PubMed] [Google Scholar]
- 3.Arking, R., Buck, S., Berrios, A., Dwyer, S. & Baker, G. T., III (1991) Dev. Genet. 12, 362–370. [DOI] [PubMed] [Google Scholar]
- 4.Rose, M.R., Vu, L. N., Park, S. U. & Graves, J. L., Jr. (1992) Exp. Gerontol. 27, 241–250. [DOI] [PubMed] [Google Scholar]
- 5.Lin, Y. J., Seroude, L. & Benzer, S. (1998) Science 282, 943–946. [DOI] [PubMed] [Google Scholar]
- 6.Simon, A. F., Shih, C., Mack, A. & Benzer, S. (2003) Science 299, 1407–1410. [DOI] [PubMed] [Google Scholar]
- 7.Larsen, P. L. (1993) Proc. Natl. Acad. Sci. USA 90, 8905–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murakami, S. & Johnson, T. E. (1996) Genetics 143, 1207–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lithgow, G. J., White, T. M., Hinerfeld, D. A. & Johnson, T. E. (1994) J. Gerontol. 49, B270–B276. [DOI] [PubMed] [Google Scholar]
- 10.Lithgow, G. J., White, T. M., Melov, S. & Johnson, T. E. (1995) Proc. Natl. Acad. Sci. USA 92, 7540–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migliaccio, E., Giorgio, M., Mele, S., Pelicci, G., Reboldi, P., Pandolfi, P. P., Lanfrancone, L. & Pelicci, P. G. (1999) Nature 402, 309–313. [DOI] [PubMed] [Google Scholar]
- 12.Kapahi, P., Boulton, M. E. & Kirkwood, T. B. L. (1999) Free Radical Biol. Med. 26, 495–500. [DOI] [PubMed] [Google Scholar]
- 13.Cypser, J. R. & Johnson, T. E. (2002) J. Gerontol. A Biol. Sci. Med. Sci. 57, 109–114. [DOI] [PubMed] [Google Scholar]
- 14.Hercus, M. J., Loeschcke, V. & Rattan, S. I. (2003) Biogerontology 4, 149–156. [DOI] [PubMed] [Google Scholar]
- 15.Vettraino, J., Buck, S. & Arking, R. (2001) J. Gerontol. A Biol. Sci. Med. Sci. 56, B415–B425. [DOI] [PubMed] [Google Scholar]
- 16.Sampayo, J. N., Jenkins, N. L. & Lithgow, G. J. (2000) Ann. N.Y. Acad. Sci. 908, 324–326. [DOI] [PubMed] [Google Scholar]
- 17.Munoz, M. J. & Riddle, D. L. (2003) Genetics 163, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabrizio. P., Pozza, F., Pletcher, S. D., Gendron, C. M. & Longo, V. D. (2001) Science 292, 288–290. [DOI] [PubMed] [Google Scholar]
- 19.Orr, W. C. & Sohal, R. S. (1994) Science 263, 1128–1130. [DOI] [PubMed] [Google Scholar]
- 20.Sun, J. & Tower, J. (1999) Mol. Cell. Biol. 19, 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkes, T. L., Elia, A. J., Dickinson, D., Hilliker, A. J., Phillips, J. P. & Boulianne, G. L. (1998) Nat. Genet. 19, 171–174. [DOI] [PubMed] [Google Scholar]
- 22.Tatar, M., Khazaeli, A. A. & Curtsinger, J. W. (1997) Nature 390, 30 (lett.). [DOI] [PubMed] [Google Scholar]
- 23.Walker, G. A. & Lithgow, G. J. (2003) Aging Cell 2, 131–139. [DOI] [PubMed] [Google Scholar]
- 24.Hsu, A. L., Murphy, C. T. & Kenyon, C. (2003) Science 300, 1142–1145. [DOI] [PubMed] [Google Scholar]
- 25.Morley, J. F. & Morimoto, R. I. (2004) Mol. Biol. Cell. 15, 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson, R. M., Bitterman, K. J., Wood, J. G., Medvedik, O. & Sinclair, D. A. (2003) Nature 423, 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazemi-Esfarjani, P. & Benzer, S. (2000) Science 287, 1837–1840. [DOI] [PubMed] [Google Scholar]
- 28.Brand, A. H. & Perrimon, N. (1993) Development (Cambridge, U.K.) 118, 401–415. [DOI] [PubMed] [Google Scholar]
- 29.Seong, K. H., Ogashiwa, T., Matsuo, T., Fuyama, Y. & Aigaki, T. (2001) Biogerontology 2, 209–217. [DOI] [PubMed] [Google Scholar]
- 30.Landis, G. N., Abdueva, D., Skvortsov, D., Yang, J., Rabin, B. E., Carrick, J., Tavare, S. & Tower, J. (2004) Proc. Natl. Acad. Sci. USA 101, 7663–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sgro, C. M. & Partridge, L. (1999) Science 286, 2521–2524. [DOI] [PubMed] [Google Scholar]
- 32.Partridge, L. & Gems, D. (2002) Nat. Rev. Genet. 3, 165–175. [DOI] [PubMed] [Google Scholar]
- 33.Silbermann, R. & Tatar, M. (2000) Evol. Int. J. Org. Evol. 54, 2038–2045. [DOI] [PubMed] [Google Scholar]
- 34.Esposito, L. A., Melov, S., Panov, A., Cottrell, B. A. & Wallace, D. C. (1999) Proc. Natl. Acad. Sci. USA 96, 4820–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melov, S., Schneider, J. A., Coskun, P. E., Bennett, D. A. & Wallace, D. C. (1999) Neurobiol. Aging 20, 565–571. [DOI] [PubMed] [Google Scholar]
- 36.Salinas, A. E. & Wong, M. G. (1999) Curr. Med. Chem. 6, 279–309. [PubMed] [Google Scholar]
- 37.Kampkotter, A., Volkmann, T. E., de Castro, S. H., Leiers, B., Klotz, L. O., Johnson, T. E., Link, C. D. & Henkle-Duhrsen, K. (2003) J. Mol. Biol. 325, 25–37. [DOI] [PubMed] [Google Scholar]
- 38.Marenda, D. R., Zraly, C. B., Feng, Y., Egan, S. & Dingwall, A. K. (2003) Mol. Cell. Biol. 23, 289–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tissenbaum, H. A. & Guarente, L. (2001) Nature 410, 227–230. [DOI] [PubMed] [Google Scholar]
- 40.Kang, H. L., Benzer, S. & Min, K. T. (2002) Proc. Natl. Acad. Sci. USA 99, 838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogina, B., Helfand, S. L. & Frankel, S. (2002) Science 298, 1745. [DOI] [PubMed] [Google Scholar]
- 42.Webster, G. C. & Webster, S. L. (1983) Mech. Ageing Dev. 22, 121–128. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd, J. C., Walldorf, U., Hug, P. & Gehring, W. J. (1989) Proc. Natl. Acad. Sci. USA 86, 7520–7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shikama, N., Ackermann, R. & Brack, C. (1994) Proc. Natl. Acad. Sci. USA 91, 4199–4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitsui, K., Nakayama, H., Akagi, T., Nekooki, M., Ohtawa, K., Takio, K., Hashikawa, T. & Nukina, N. (2002) J. Neurosci. 22, 9267–9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talapatra, S., Wagner, J. D. & Thompson, C. B. (2002) Cell Death Differ. 9, 856–861. [DOI] [PubMed] [Google Scholar]
- 47.Verbeke, P., Clark, B. F. & Rattan, S. I. (2000) Exp. Gerontol. 35, 787–794. [DOI] [PubMed] [Google Scholar]
- 48.Rattan, S. I. (1995) Mol. Aspects Med. 5, 439–508. [DOI] [PubMed] [Google Scholar]
- 49.Terman, A. & Brunk, U. T. (1998) APMIS 106, 265–276. [DOI] [PubMed] [Google Scholar]
- 50.Rogalla, T., Ehrnsperger, M., Preville, X., Kotlyarov, A., Lutsch, G., Ducasse, C., Paul, C., Wieske, M., Arrigo, A. P., Buchner, J., et al. (1999) J. Biol. Chem. 274, 18947–18956. [DOI] [PubMed] [Google Scholar]
- 51.Stromer, T., Ehrnsperger, M., Gaestel, M. & Buchner, J. (2003) J. Biol. Chem. 278, 18015–18021. [DOI] [PubMed] [Google Scholar]
- 52.Haslbeck, M., Walke, S., Stromer, T., Ehrnsperger, M., White, H. E., Chen, S., Saibil, H. R. & Buchner, J. (1999) EMBO J. 18, 6744–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Preville, X., Salvemini, F., Giraud, S., Chaufour, S., Paul, C., Stepien, G., Ursini, M. V. & Arrigo, A. P. (1999) Exp. Cell Res. 247, 61–78. [DOI] [PubMed] [Google Scholar]
- 54.Garrido, C., Bruey, J. M., Fromentin, A., Hammann, A., Arrigo, A. P. & Solary, E. (1999) FASEB J. 13, 2061–2070. [DOI] [PubMed] [Google Scholar]
- 55.Bruey, J. M., Ducasse, C., Bonniaud, P., Ravagnan, L., Susin, S. A., Diaz-Latoud, C., Gurbuxani, S., Arrigo, A. P., Kroemer, G., Solary, E., et al. (2000) Nat. Cell Biol. 2, 645–652. [DOI] [PubMed] [Google Scholar]
- 56.Pandey, P., Farber, R., Nakazawa, A., Kumar, S., Bharti, A., Nalin, C., Weichselbaum, R., Kufe, D. & Kharbanda, S. (2000) Oncogene 19, 1975–1981. [DOI] [PubMed] [Google Scholar]
- 57.Takayama, S., Reed, J. C. & Homma, S. (2003) Oncogene 22, 9041–9047. [DOI] [PubMed] [Google Scholar]
- 58.Wyttenbach, A., Sauvageot, O., Carmichael, J., Diaz-Latoud, C., Arrigo, A. P. & Rubinsztein, D. C. (2002) Hum. Mol. Genet. 11, 1137–1151. [DOI] [PubMed] [Google Scholar]
- 59.Kurapati, R., Passananti, H. B., Rose, M. R. & Tower, J. (2000) J. Gerontol. A Biol. Sci. Med. Sci. 55, B552–B559. [DOI] [PubMed] [Google Scholar]
- 60.Morrow, G., Samson, M., Michaud, S. & Tanguay, R. M. (2004) FASEB J. 18, 598–599. [DOI] [PubMed] [Google Scholar]