Abstract
Sjögren's syndrome (SS) is an incurable, autoimmune exocrinopathy that predominantly affects females and whose pathogenesis remains unknown. Like rheumatoid arthritis, its severity increases after menopause, and estrogen deficiency has been implicated. We have reported that estrogen receptor-α and -β-knockout mice develop autoimmune nephritis and myeloid leukemia, respectively, but neither develops SS. One model of estrogen deficiency in rodents is the aromatase-knockout (ArKO) mouse. In these animals, there is elevated B lymphopoiesis in bone marrow. We now report that ArKO mice develop severe autoimmune exocrinopathy resembling SS. By 1 year of age, there is B cell hyperplasia in the bone marrow, spleen, and blood of ArKO mice and spontaneous autoimmune manifestations such as proteinuria and severe leukocyte infiltration in the salivary glands and kidney. Also, as is typically found in human SS, there were proteolytic fragments of α-fodrin in the salivary glands and anti-α-fodrin antibodies in the serum of both female and male ArKO mice. When mice were raised on a phytoestrogen-free diet, there was a mild but significant incidence of infiltration of B lymphocytes in WT mice and severe destructive autoimmune lesions in ArKO mice. In age-matched WT mice fed a diet containing normal levels of phytoestrogen, there were no autoimmune lesions. These results reveal that estrogen deficiency results in a lymphoproliferative autoimmune disease resembling SS and suggest that estrogen might have clinical value in the prevention or treatment of this disease.
Keywords: autoimmune exocrinopathy, salivary gland, B lymphocytes, autoantibody, bone marrow
There is a higher incidence of many autoimmune diseases, including Sjögren's syndrome (SS) and systemic lupus erythematosus (SLE), in women than in men (1, 2). Estrogen, thought to be the most important factor regulating sex differences in the immune system, is a Janus-like molecule with contradictory effects on autoimmune diseases. It seems to offer protection in experimental autoimmune encephalomyelitis (3), collagen-induced arthritis (4), multiple sclerosis (5), and rheumatoid arthritis (6), but it promotes autoimmunity and worsens the symptoms of SLE (7). In lupus-prone mice, a model of SLE, estrogen treatment increases the incidence of autoimmune disease, whereas tamoxifen, an estrogen receptor (ER)-α antagonist, seems to suppress SLE (8).
In mice, ovariectomy results in splenomegaly (9), increased production of colony-forming units–granulocyte-erythroid-macrophage-megakaryocytes, burst-forming units–erythroid cells, (10–12) and B lymphocytes in bone marrow (13). Conversely, pregnancy or administration of estrogen decreases the bone marrow B lymphocyte population (14, 15). Recently, it was shown that, in the bone marrow, estrogen represses differentiation of multipotent hematopoietic stem cells into both lymphoid and myeloid lineages (16, 17). We have shown that there are distinct immunological phenotypes in mice in which ERα or ERβ has been inactivated (ERα–/– and ERβ–/– mice, respectively). ERα–/– mice develop autoimmune nephritis with spontaneous formation of splenic germinal centers (18), whereas ERβ–/– mice develop myeloproliferative disease with enhanced B cell proliferation in bone marrow (19). In addition, mice that are estrogen-free because of inactivation of the aromatase gene (ArKO mice) have higher levels of B220+ B lymphocytes in bone marrow (20).
SS is a chronic, incurable autoimmune exocrinopathy that occurs 10 times more frequently in women than in men (1, 21). The symptoms of primary SS are salivary gland inflammation, lacrimal gland dysfunction manifested by xerostomia (dry mouth), keratoconjuctivitis sicca (dry eye), and other extraglandular abnormalities. In secondary SS, there is, in addition, SLE, rheumatoid arthritis, or other connective tissue diseases. SS is regarded as an autoimmune disease and is characterized by initial infiltration of exocrine glands with CD4+ CD45RO+ T cells; the presence of B cell hyperactivity, including germinal center formation; and the presence in serum of various autoantibodies such as rheumatoid factors, anti-Ro (SS-A), anti La (SS-B), and anti-α-fodrin autoantibodies (21).
Very recently, Hayashi and his colleagues (22) have shown that in normal mice, estrogen suppresses the development of SS, whereas ovariectomy leads to a condition mimicking SS. In addition, estrogen can ameliorate T cell-mediated sialoadenitis (23) and prevent cell death in the lacrimal gland (24). ERα mRNA has been detected in salivary tissue (25) and cultured human nonneoplastic salivary gland epithelial cells (26), but ERβ is the predominant ER subtype in human salivary glands (27). Because ovariectomy induces apoptosis in epithelial cells of the mouse salivary gland (24), it seems likely that estrogen, by means of ERs, plays a role in survival of epithelial cells in the salivary gland.
However, no evidence of an SS-like syndrome has been observed in either ERα–/– or ERβ–/– mice. In the present study, we investigated whether lifetime loss of estrogen, as in ArKO mice, leads to development of autoimmune disease different from that in the ER mutant mice. We have found that ArKO mice spontaneously develop signs of lymphoproliferative autoimmunity, which particularly resembles SS. Our results indicate that long-term estrogen deficiency causes autoimmune exocrinopathy resembling SS and, sometimes, renal failure. Estrogen and estrogenic compounds like phytoestrogens might prevent SS.
Materials and Methods
Mouse Strains and Housing. ArKO mice on a C57BL/6J/129 background were kept in the animal facility at Huddinge University Hospital under specific pathogen-free conditions, and were fed phytoestrogen-free laboratory chow. All experimental procedures were approved by the local animal ethics committee.
Peripheral Blood Analysis. Peripheral blood and cells were prepared as described in ref. 19. The blood smears were prepared and stained with May–Grünwald and Giemsa solutions (Fluka).
Bone Marrow Cells and Splenocytes. The femurs and tibiae were surgically removed from the animals. The bones were cut, and marrow was flushed out with 10 ml of PBS containing 1 mM EDTA. The spleen was minced and suspended in PBS. Bone marrow cells and splenocytes were centrifuged at 515 × g for 5 min. Pelleted spleen cells were resuspended in PBS. After washing in PBS, the total number of leukocytes from the organs was calculated by using a hemocytometer.
Flow-Cytometric Analysis. FITC-conjugated antibodies (anti-Gr-1, T cell antigen receptor-β, syndecan-1, and anti-IgM) and phycoerythrin-conjugated antibodies (anti-Mac-1 and anti-B220) were purchased from Pharmingen. Single-cell suspensions were prepared from bone marrow and spleens, and 1 × 106 cells were incubated with 10% rat serum (Sigma) for 30 min at 4°C and stained with the monoclonal antibodies described above. After a 30-min incubation on ice, cells were washed twice and resuspended in PBS with 1% paraformaldehyde. The analyses were carried out by using a fluorescence-activated cell sorter (FACS-Calibur, Becton Dickinson).
Scoring of Mouse Salivary Glands for Degree of Inflammation and Tissue Destruction. Tissue sections of mouse submaxillary glands stained with hematoxylin/eosin were examined at ×100 under the microscope and scored. The degree of inflammatory infiltrates was graded as follows: A grade of 1 indicated that 1–5 foci of mononuclear cells were seen (>20 cells per focus); a grade of 2 indicated that >5 foci of mononuclear cells were seen but without significant parenchymal destruction; a grade of 3 indicated that multiple confluent foci were seen, with moderate degeneration of parenchymal tissue; and a grade of 4 indicated extensive infiltration of the gland with mononuclear cells and extensive parenchymal destruction.
Proteinuria. The presence of proteins in mouse urine was measured by using a Combur 5 test strip (Roche Diagnostics).
Histological Methods. Histological studies including immunohistochemistry were performed as described in refs. 18 and 19. Tissue sections were incubated for 1 h at 4°C with normal goat serum diluted at 1:10 in PBS. Antibodies were diluted individually in PBS containing 3% BSA. Sections were incubated with antibodies overnight at 4°C. For negative controls, the primary antibody was replaced with PBS. Before the addition of a secondary antibody, the sections were rinsed in PBS. The avidin–biotin complex method was used to visualize the signal according to the manufacturer's instructions (Vector Laboratories). Sections were incubated in biotinylated goat anti-rabbit or goat anti-mouse IgG (1:200 dilution) for 2 h at room temperature, followed by washing with PBS and incubation in avidin-biotin–horseradish peroxidase for 1 h. After thorough washing in PBS, sections were developed with 3,3′-diaminobenzidine tetrahydrochloride (DAKO), slightly counterstained with Mayer's hematoxylin, dehydrated through an ethanol series, exposed to xylene, and mounted.
Measurement of Anti-α-Fodrin and Anti-DNA Antibodies by ELISA. The serum level of anti-α-fodrin antibody was determined by ELISA; positive mouse serum and human recombinant α-fodrin protein were provided by Y. Hayashi (Tokushima University School of Dentistry, Tokushima, Japan) (22). Serial dilutions (1:50–1:5,000) of test serum samples were used. Levels of circulating antibodies reactive with single-stranded (ss) and double-stranded (ds) DNA were determined with a mouse anti-ssDNA and -dsDNA ELISA kit (Alpha Diagnostic International, San Antonio, TX).
Western Blot. For α-fodrin detection by Western blot, total protein of salivary glands was applied on an 8% polyacrylamide gel (NOVEX, San Diego). Blots were probed with specific primary antibodies, followed by appropriate secondary antibodies conjugated with horseradish peroxidase. Detection was by enhanced chemiluminescence.
Results
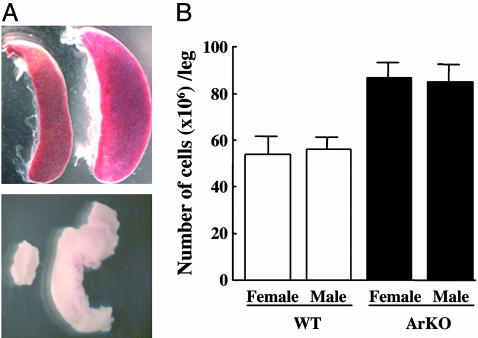
Aged ArKO Mice Show Enlarged Spleen, Mesenteric Lymph Nodes, and Hypercellularity of Bone Marrow. We studied long-term effects of estrogen deficiency by using aging ArKO mice. All female and male ArKO mice >1 year of age developed a mild splenomegaly (Fig. 1A Upper) and lymphoadenopathy (Fig. 1 A Lower), although the degree of enlargement varied between mice. In nonhematopoietic organs such as liver and lung, there was no significant infiltration of leukocytes (data not shown). The cellularity of bone marrow from the femurs and tibiae was determined by using a hemocytometer. The total cellularity was higher in ArKO mice than in WT mice (Fig. 1B). This finding indicates that the splenomegaly is a consequence of the hypercellularity in the bone marrow.
Fig. 1.
Enlarged spleen, mesenteric lymph nodes, and bone marrow hyperplasia in ArKO mice. (A) Photograph of spleen (Upper) and mesenteric lymph nodes (Lower) of WT (left) and ArKO (right) mice. Pictures are representative of at least six mice killed for each sex and genotype. (B) Twelve- to 16-month-old ArKO (black columns) and age-matched WT (white columns) male and female mice were killed and, from each mouse, one femur and one tibia were flushed with PBS. The cells were washed and resuspended in 10 ml of PBS. The total cells per leg were calculated based on hemocytometer counts (data are shown as mean; n = 6 per group).
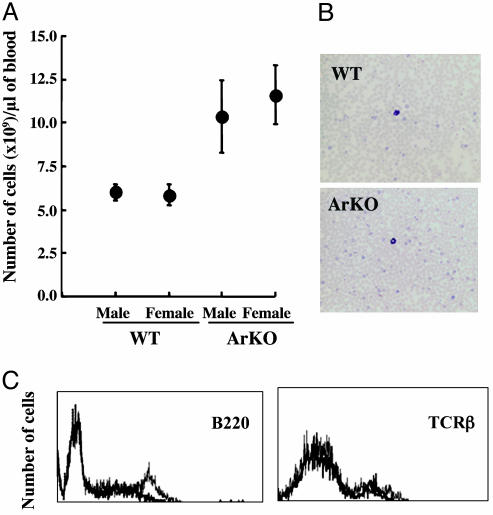
Blood Profiling of Aged ArKO Mice. The number of leukocytes, particularly lymphocytes, in peripheral blood was significantly increased by 1.5- to 2-fold in ArKO mice (Fig. 2 A and B). Lymphoid malignancy, however, was not evident in 12- to 16-month-old ArKO mice. Flow cytometric analysis confirmed a significant increase in the population of B220+ B lymphocytes in blood (Fig. 2C), whereas the number of T cell antigen receptor-β positive T cells was normal.
Fig. 2.
Increased blood lymphocyte counts and proportion of B cells in peripheral blood lymphocyte from ArKO mice. (A) Number of white blood cells. The graph compares six control littermates with six ArKO mice for each sex group. Blood was drawn from the mice by cardiac puncture and smeared on microscope slides. (B) The slides were stained with May–Grünwald and Giemsa solutions. (C) After lysis of RBC, peripheral blood lymphocytes were stained with anti-B220-phycoerythrin or anti-T cell antigen receptor-β-FITC (TCRβ) for flow cytometry analysis. Graphs for WT (bold line) and ArKO (light line) mice are overlaid for comparison.
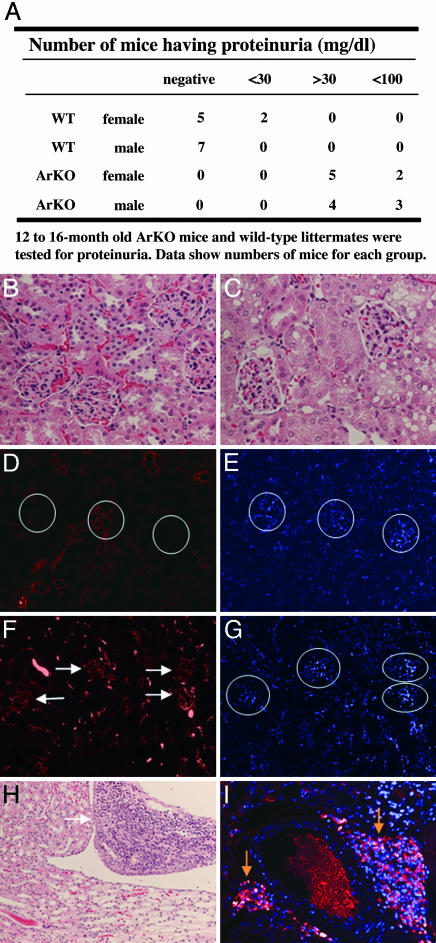
ArKO Mice Show Mild Proteinuria and B Lymphocyte Infiltration in the Kidney. Impaired renal function was suggested by the presence of mild proteinuria in ArKO mice (Fig. 3A). Two of 14 WT mice had urinary protein levels of >30 mg/dl and 12 of 14 WT mice had levels of <30 mg/dl, whereas all female and male ArKO mice had protein levels of >30 mg/dl; 36% of ArKO mice had levels of >100 mg/dl. By 12–16 months of age, male and female WT and ArKO mice did not show pathological features of glomerulonephritis with hematoxylin/eosin staining (Fig. 3 B and C). Only ArKO mice showed mild depositions of IgG in renal glomeruli (Fig. 3 D and F), but no positive signals for IgM or IgA were detected when the appropriate antibodies were used (data not shown). Histological sections of kidneys from ArKO mice showed the presence of massive infiltration of B lymphocytes (Fig. 3H), many of which were identified as plasma cells on the basis of the presence of IgG in their cytoplasm (Fig. 3I). These abnormalities were not seen in age-matched WT mice, suggesting that lifetime deficiency of estrogen may result in development of renal dysfunction.
Fig. 3.
Presence of proteinuria and infiltrating leukocytes in the perirenal area of the kidney from ArKO mice. (A) Impaired renal function was confirmed by the presence of significant proteinuria in ArKO mice. (B, C, and H) Kidney sections from old WT (B) and ArKO (C and H) mice stained with hematoxylin/eosin. (D–G) Deposition of IgG was detected by immunofluorescence using kidney sections from WT (D and E) and ArKO (F and G) mice. Nuclei counter-stained with 4′,6-diamidino-2-phenylindole are shown in E and G. White circles, location of glomeruli; white arrows, IgG deposition in the glomeruli. (H and I) There is a severe infiltration of B lymphocytes in kidneys from ArKO mice (white arrow, area of infiltration; yellow arrows, plasma B cell-related phenotype, showing intracellular Ig), whereas WT littermates are normal.
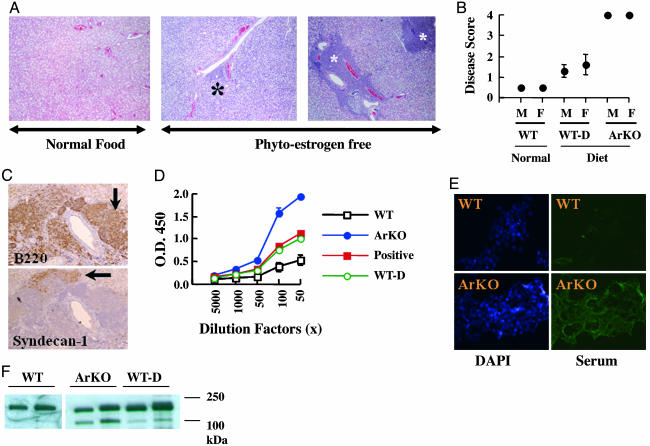
ArKO Mice Develop a SS-Like Phenotype as They Age. After routine dissection of ArKO mice, we observed that there was an incidence of enlarged salivary glands. However, comparing the weight of salivary glands, normalized with whole body weight, between WT and ArKO mice was difficult, because ArKO mice developed severe obesity with age. Histologically, we observed massive leukocytic infiltration with destruction of acinar cells in the salivary glands of ArKO mice (Fig. 4A Right). We analyzed histological preparations of 12 ArKO mice, 12–17 months old, and 12 WT age-matched mice, both raised on a phytoestrogen-free diet, as well as 10 WT age-matched mice that were raised on standard diet. When salivary glands were scored for severity of disease, we found that ArKO mice, both females and males, spontaneously developed SS-like disease, unlike age-matched WT mice (Fig. 4B). Our results indicated an increased incidence of SS-like symptoms in WT mice fed a phytoestrogen-free diet, whereas age-matched mice fed a standard diet were normal (Fig. 4B). Immunohistological sections of affected salivary glands from ArKO mice showed the presence of massive infiltration of B220+ B lymphocytes (Fig. 4C), some of which were identified as plasma cells on the basis of the presence of syndecan-1 positivity (Fig. 4C). Abnormal B cell activity is a predominant feature of SS, which is manifested by massive polyclonal B cell activation and elevated secretion of autoantibodies such as rheumatoid factors, anti-Ro (SS-A), anti-La (SS-B), or anti-α-fodrin autoantibodies. To test the presence of a typical autoantibody, we measured the level of anti-α-fodrin antibody in serum. In three of five female ArKO mice, there were strong positive signals, and in two of five ArKO mice, there were weakly positive signals. WT mice fed a phytoestrogen-free diet were all weakly positive, whereas WT mice fed a normal diet were all negative (Fig. 4D). Fig. 4D shows representative results. Two ArKO mice showed extremely high levels of α-fodrin autoantibodies that were higher than in α-fodrin-immunized mice.
Fig. 4.
ArKO mice develop a SS-like phenotype as they age. (A) Paraffin sections of salivary glands from WT mice fed a normal diet (Left), WT mice fed a phytoestrogen-free diet (Center, WT-D), and ArKO mice fed a phytoestrogen-free diet (Right) were stained with hematoxylin/eosin. Asterisks, periductal infiltrates (foci). (B) Paraffin sections of salivary glands from six mice from each group were prepared as shown in A and scored for disease as described in Materials and Methods. (C) B220+ B lymphocytes are dominant in the lymphoepithelial lesions in ArKO mice, and some of these lymphocytes are characterized as plasma B cells showing syndecan-1 positivity. (D) Anti-α-fodrin antibodies were detected in sera from WT and ArKO mice by ELISA. (E) Sera from ArKO mice show immunoreactivity against cytoskeletal proteins in cultured MCF-7 cells. DAPI, 4′,6-diamidino-2-phenylindole. (F) Detection of α-fodrin cleavage products in salivary gland from WT (lanes 1 and 2, from left to right), ArKO (lanes 3 and 4), and WT-D (lanes 5 and 6) mice.
The protein α-fodrin belongs to a family of widely distributed filamentous cytoskeletal proteins. Proteolytic cleavage of this protein leads to tissue destruction in primary SS. The presence of an α-fodrin fragment of 120 kDa molecular mass is characteristic of SS. Immunohistochemical staining of MCF-7 human breast cancer cells with all sera (five of five) from ArKO mice revealed that ArKO mice had autoantibodies against filamentous cytoskeletal proteins (Fig. 4E). No antinuclear antibodies were detected. Western blotting showed an intense band of the 120-kDa α-fodrin fragment in the salivary gland from ArKO mice and a less intense but clearly positive band in WT mice fed a phytoestrogen-free diet. In WT mice fed a normal diet, only intact 250-kDa α-fodrin was detected (Fig. 4F lanes 1 and 2).
ArKO Mice Show an Increased Number of B Lymphocytes in Lymphoid Tissues. Bone marrow cells were prepared from 12- to 16-monthold ArKO and age-matched WT mice. Cells were analyzed by flow cytometry on side scatter/forward light scatter plots. After analysis of 106 cells from ArKO mice, the gated area, which represents leukocytes including lymphoid and myeloid cells, was increased, whereas the ungated area, representing RBC and dead cells, was decreased (Fig. 5). Flow cytometric analysis confirmed a 2-fold increase in B220+ B lymphocyte population in bone marrow (Fig. 5 Middle Left), and, furthermore, there was a 2-fold increase in the percentage of granulocytes in this tissue (Fig. 5 Bottom Left). There was an overall hypercellularity in bone marrow from ArKO mice (Fig. 1B), and the absolute number of leukocytes in bone marrow was >3-fold that in WT mice. These results indicate that ArKO mice develop a progressive bone marrow hyperplasia with overproduction of mature granulocytes and B cells.
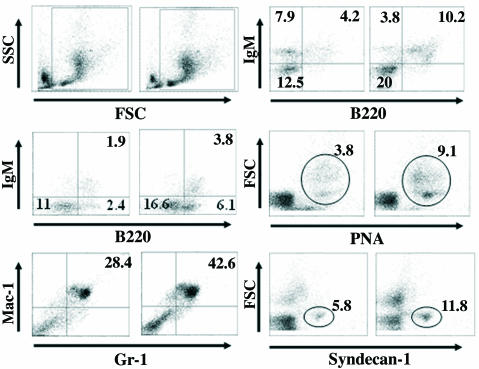
Fig. 5.
Increased number of B lymphocytes in ArKO mice. (Top Left) Side scatter/forward light scatter plots of bone marrow cells from ArKO (Right) and WT (Left) mice are shown. Leukocytes were gated, as shown in the plots for each analysis. (Middle Left) Increased B220+ B cells from bone marrow of ArKO mice. (Bottom Left) Increased Gr-1+/Mac-1+ myeloid cells from bone marrow of ArKO mice. (Top Right) Freshly prepared splenocytes were stained with antibody against IgM and B220 and significant increase in B220+ B cells was observed, whereas the percentage of IgM+/B220– B cells was decreased in ArKO mice. Increased percentages of peanut agglutinin-positive germinal center B cells (Middle Right) and plasma B cells (syndecan-1 positive) (Bottom Right) are shown. Percentages of each cell type from a total of 106 cells are indicated in each box. The figures are representative of five different female mice.
The mature B cell compartment was analyzed by dual staining with anti-B220 and -IgM antibodies. The mature B cell compartment (IgM+/B220+) was increased, whereas the proportion of IgM+/B220– B cells was decreased in the spleen of ArKO mice. Because in autoimmune diseases there is frequently germinal center formation and accumulation of plasma B cells in the spleen, we analyzed splenocytes with peanut agglutinin (indicates germinal centers) and anti-syndecan-1 antibodies (plasma B cells). In ArKO mice, there was a 2-fold increase in the percentage of peanut agglutinin-positive and syndecan-1-positive cells, suggesting immunological activation in the spleen. However, in ArKO mice, the percentage of T lymphocytes in the spleen was not different from or only slightly increased over that of WT mice (data not shown), but the total number of T cells was larger because splenic cellularity was increased by 2- to 4-fold.
Discussion
Autoimmune diseases occur more frequently in women than in men (1, 2), and estrogen alters the course of these diseases (3–7). So far, the exact role of estrogen in autoimmune diseases is not understood. The purpose of this study was to determine whether lifetime loss of estrogen leads to autoimmune disease and, if so, to characterize the abnormalities in the immune system.
We used mice that, because of the inactivation of the aromatase gene, do not synthesize estrogen (20). We found that by 1 year of age, ArKO mice spontaneously exhibited signs of autoimmunity with severe lymphoproliferative phenotypes in bone marrow and spleen. In addition, there was destructive infiltration of B lymphocytes in the salivary glands, resembling human SS. WT littermates were normal. There was also mild but significant nephropathy as evidenced by the presence of proteinuria. This report presents the previously unrecognized phenomenon that upon estrogen deprivation, there is development of spontaneous exocrinopathy in aging mice.
It has been well demonstrated that bone marrow is an estrogen-regulated organ and that estrogen modulates B lymphopoiesis, myelopoiesis (10–17), and immune responses. After ovariectomy in mice, there is a significant increase in the number of pro/pre B lymphocytes (B220low/IgM–) in bone marrow (28). Interestingly, in ERα–/– mice, there is a slight increase in cellularity of bone marrow even in young (4-month-old) mice but a decrease in the number of pro/pre B lymphocytes (B220low/IgM–) and mature B lymphocytes (B220high/IgM+) in bone marrow (29). In contrast, in ERβ–/– mice, there is an increase in the total number of pro/pre B lymphocytes (19). Thus, in mice, with respect to B lymphocytes, loss of ERβ mimics loss of estrogen. One other characteristic of estrogen deficiency is splenomegaly (9), which is caused by enhanced B lymphopoiesis and myelopoiesis.
As in ovariectomized mice, increases in the number of mature B lymphocytes in bone marrow and enlargement of spleen also were found in ArKO mice. The effect of estrogen on multipotent hematopoietic stem cells has been well demonstrated. Estrogen treatment in vivo decreases the percentage of c-Kit+/Sca-1+ cells, i.e., early hematopoietic stem cells that can differentiate into myeloid and lymphoid lineages (16, 29). Estrogen can still partially decrease the percentage of c-Kit+/Sca-1+ cells in the absence of ERα (in ERα–/– mice) (29), suggesting that both ERα and ERβ are mediators of estrogenic control of c-Kit+/Sca-1+ cells. This negative function of estrogen by means of ERα and ERβ in Kit+/Sca-1+ cells may explain the increased B lymphopoiesis in ArKO mice. Even though it is clear that estrogen can regulate B cell differentiation, the primary defect in SS, whether in humans or ArKO mice, is still unclear. We hypothesize that differentiation of B lymphocytes in the bone marrow is a critical step for deletion of autoreactive B lymphocytes and that, in this way, estrogen may be involved in B cell tolerance. In ArKO mice, excess B cell survival may be caused by up-regulation of the estrogen-regulated antiapoptotic protein Bcl-2 (30). There is previous evidence that excess B cell survival is a key event in SS-related disorders: In transgenic mice overexpressing Bcl-2 (31) and in mice overexpressing the B cell-activating factor of the TNF family (BAFF), (32) there are lymphocytic infiltrates and an SS-like phenotype in the salivary glands.
Insufficient estrogen signaling in ERβ-deficient mice leads to bone marrow hyperplasia resembling myeloproliferative disease (19). Remarkably, however, there is only mild bone marrow hyperplasia in ArKO mice, and there was no sign of myeloproliferative disease even though ArKO mice have complete loss of estrogen. Because androgen and estrogen have similar effects on bone marrow and proliferation of myeloid cells in vitro, it is possible that the increased concentration of testosterone in ArKO mice (33) may compensate for the loss of estrogen signaling, resulting in a milder bone marrow phenotype.
Renal protective effects of estrogen have been demonstrated in both cultured renal proximal tubular cells (34) and whole animals (35). In glomerular sclerosis-prone mice, estrogen treatment results in a reduced prosclerotic response (35) and ovariectomy accelerates its progression (36). Recently, we also have reported that ERα–/– mice develop an autoimmunity with severe nephritis (18). The nephritis in ArKO mice, observed in the present study, was much less severe than that seen in ERα–/– mice. Because there are other estrogenic steroids besides estradiol-17β that may activate ERs (37), loss of aromatase may not result in complete loss of estrogen signaling.
In addition, because ERα is expressed in the kidney, some of the renal dysfunction in ERα–/– mice may be due to loss of a protective effect of estrogen in the kidney itself. In fact, tissue-selective expression of ER might explain why some tissues are more severely damaged than others in autoimmune diseases that are exacerbated by estrogen deficiency.
In this study, we have demonstrated that estrogen deficiency spontaneously leads to SS-like phenotypes in ArKO mice. In view of recent reports that show involvement of estrogen in SS and the existence of ERα in the salivary gland (25, 26), estrogen seems to play an important role in the survival of epithelial cells. We have found no evidence of SS-like disease in ERα–/– mice at 1 year of age (18) but have found that ERβ is the predominant ER in mouse and human salivary glands (27). Clearly, further studies are needed for a deeper understanding of the role of ERs in salivary glands.
There are two different immune responses, called T helper (TH)1 and TH2. TH1 response is associated with multiple sclerosis and rheumatoid arthritis, and there is decreased severity of these TH1-related autoimmune diseases with estrogen treatment (38). In contrast, TH2 immune response, which is also called antibody-promoting response, is associated with SLE and is worsened after estrogen treatment (38). SS is regarded as an autoimmune disease characterized by infiltration of CD4+ CD45RO+ T cells and also by the presence of B cell hyperactivity and various serum autoantibodies (21, 39, 40). The percentage of the T cell population in ArKO mice was not dramatically changed, but the absolute number of T cells seemed to be increased, as judged from the 2- to 4-fold higher splenic cellularity of ArKO mice. The involvement of T cells in SS of ArKO mice remains to be investigated.
SS is an incurable autoimmune disorder, and the association of SS with lymphoproliferative disease has been confirmed by several studies (21). There is evidence for an increased risk of developing lymphomas (41), especially non-Hodgkin's lymphoma. Specifically, the proportion of SS patients who develop lymphomas is 4.3%; the majority of these malignancies are low-grade marginal zone B cell lymphomas, mainly of mucosa-associated lymphoid tissue origin (42). Our results suggest that, in mice, phytoestrogens may help to prevent the development of SS. If phytoestrogens do offer protection in humans, the implication would be that ERβ is involved in this protection, and, therefore, it may be suggested that ERβ-selective agonists could be useful in prevention or treatment of autoimmune exocrinopathies.
Acknowledgments
We thank Dr. Y. Hayashi for the gift of recombinant human α-fodrin protein and antiserum; Patricia Humire and Ann-Marie Witte for excellent animal care and genotyping; and Christina Tuhlin Andersson for protein work. This study was supported by grants from the Swedish Cancer Fund, National Institutes of Health (AG 08174), and Karo Bio AB.
Abbreviations: ArKO, aromatase-knockout; ER, estrogen receptor; SS, Sjögren's syndrome; SLE, systemic lupus erythematosus.
References
- 1.Whitacre, C. C. (2001) Nat. Immunol. 2, 777–780. [DOI] [PubMed] [Google Scholar]
- 2.Beeson, P. B. (1994) Am. J. Med. 96, 457–462. [DOI] [PubMed] [Google Scholar]
- 3.Ito, A., Bebo, B. F., Jr., Matejuk, A., Zamora, A., Silverman, M., Fyfe-Johnson, A. & Offner, H. (2001) J. Immunol. 167, 542–552. [DOI] [PubMed] [Google Scholar]
- 4.Jansson, L. & Holmdahl, R. (1998) Inflamm. Res. 47, 290–301. [DOI] [PubMed] [Google Scholar]
- 5.Kim, S., Liva, S. M., Dalal, M. A., Verity, M. A. & Voskuhl, R. R. (1999) Neurology 52, 1230–1238. [DOI] [PubMed] [Google Scholar]
- 6.Schuna, A. A. (2002) J. Am. Pharmacol. Assoc. 42, 612 [Google Scholar]
- 7.Carlsten, H., Tarkowski, A., Holmdahl, R. & Nilsson, L. A. (1990) Clin. Exp. Immunol. 80, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu, W. M., Lin, B. F., Su, Y. C., Suen, J. L. & Chiang, B. L. (2000) Scand. J. Immunol. 52, 393–400. [DOI] [PubMed] [Google Scholar]
- 9.Zhang, J., Pugh, T. D., Stebler, B., Ershler, W. B. & Keller, E. T. (1998) Calcif. Tissue Int. 62, 219–226. [DOI] [PubMed] [Google Scholar]
- 10.Erben, R. G., Raith, S., Eberle, J. & Stangassinger, M. (1998) Am. J. Physiol. 274, E476–E483. [DOI] [PubMed] [Google Scholar]
- 11.Jilka, R. L., Passeri, G., Girasole, G., Cooper, S., Abrams, J., Broxmeyer, H. & Manolagas, S. C. (1995) Exp. Hematol. 23, 500–506. [PubMed] [Google Scholar]
- 12.Jilka, R. L., Hangoc, G., Girasole, G., Passeri, G., Williams, D. C., Abrams, J. S., Boyce, B., Boxmeyer, H. & Manolagas, S. C. (1992) Science 257, 88–91. [DOI] [PubMed] [Google Scholar]
- 13.Masuzawa, T., Miyaura, C., Onoe, Y., Kusano, K., Ohta, H., Nozawa, S. & Suda, T. (1994) J. Clin. Invest. 94, 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medina, K. L., Smithson, G. & Kincade, P. W. (1993) J. Exp. Med. 94, 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smithson, G., Medina, K., Ponting, I. & Kincade, P. W. (1995) J. Immunol. 155, 3409–3419. [PubMed] [Google Scholar]
- 16.Medina, K. L., Garret, K. P., Thompson, L. F., Rossi, M. I. D., Payne, K. J. & Kincade, P. W. (2001) Nat. Immunol. 2, 718–724. [DOI] [PubMed] [Google Scholar]
- 17.Kouro, T., Medina, K. L., Oritani, K. & Kincade, P. W. (2001) Blood 97, 2708–2715. [DOI] [PubMed] [Google Scholar]
- 18.Shim, G.-J., Kis, L. L., Warner, M. & Gustafsson, J.-Å. (2004) Proc. Natl. Acad. Sci. USA 101, 1720–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shim, G.-J., Wang, L., Andersson, S., Nagy, N., Kis, L. L., Zhang, Q., Makela, S., Warner, M. & Gustafsson, J.-Å. (2003) Proc. Natl. Acad. Sci. USA 100, 6694–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oz, O. K., Hirasawa, G., Lawson, J., Nanu, L., Constantinescu, A., Antich, P. P., Mason, R. P., Tsyganov, E., Parkey, R. W., Zerwekh, J. E. & Simpson, E. R. (2001) J. Steroid Biochem. Mol. Biol. 79, 49–59. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto, K., (2003) Autoimmun. Rev. 2, 13–18. [DOI] [PubMed] [Google Scholar]
- 22.Ishimaru, N., Arakaki, R., Watanabe, M., Kobayashi, M., Miyazaki, K. & Hayashi, Y. (2003) Am. J. Pathol. 163, 1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlsten, H., Nilsson, N., Jonsson, R., Backman, K., Holmdahl, R. & Tarkowski, A. (1992) Cell. Immunol. 144, 190–202. [DOI] [PubMed] [Google Scholar]
- 24.Ishimaru, N., Saegusa, K., Yanagi, K., Haneji, N., Saito, I. & Hayashi, Y. (1999) Am. J. Pathol. 155, 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leimola-Virtanen, R., Salo, T., Toikkanen, S., Pulkkinen, J. & Syranen, S. (2000) Maturitas 36, 131–137. [DOI] [PubMed] [Google Scholar]
- 26.Kassi, E., Moutsatsou, P, Sekeris, C. E., Moutsopoulos, H. M. & Manoussakis, M. N. (2003) Rheumatology 42, 1120–1122. [DOI] [PubMed] [Google Scholar]
- 27.Valimaa, H., Savolainen, S., Soukka, T., Silvoniemi, P., Makela, S., Kujari, H., Gustafsson, J.-Å. & Laine, M. (2004) J. Endocrinol. 180, 55–62. [DOI] [PubMed] [Google Scholar]
- 28.Miyaura, C., Onoe, Y., Inada, M., Maki, K., Ikuta, K., Ito, M. & Suda, T. (1997) Proc. Natl. Acad. Sci. USA 94, 9360–9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurmond, T. S., Murante, F. G., Staples, J. E., Silverstone, A. E., Korach, S. K. & Gasiewicz, T. A. (2000) Endocrinology 141, 2309–2318. [DOI] [PubMed] [Google Scholar]
- 30.Medina, K. L., Strasser, A. & Kincade, P. W. (2000) Blood 95, 2059–2067. [PubMed] [Google Scholar]
- 31.Strasser, A. (1991) Proc. Natl. Acad. Sci. USA 88, 8661–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groom, J. (2002) J. Clin. Invest. 109, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher, C. R., Graves, K. H., Parlow, A. F. & Simpson, E. R. (1998) Proc. Natl. Acad. Sci. USA 95, 6965–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han, H. J., Park, S. H., Park, H. J., Park, K. M., Kang, J. W., Lee. J. H., Lee, B. C. & Hwang, W. S. (2002) Clin. Exp. Pharmacol. Physiol. 29, 60–67.11906461 [Google Scholar]
- 35.Potier, M., Karl, M., Zheng, F., Elliot, S. J., Striker, G. E. & Striker, L. J. (2002) Am. J. Pathol. 160, 1877–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elliot, S. J., Karl, M., Berho, M., Potier, M., Zheng, F., Leclercq, B., Striker, G. E. & Striker, L. J. (2003) Am. J. Pathol. 162, 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuiper, G. G., Lemmen, J. C., Carlsson, B., Corton, J. C., Safe, S. H., van der Saag, P. T., van der Burg, B. & Gustafsson, J.-Å. (1998) Endocrinology 139, 4252–4263. [DOI] [PubMed] [Google Scholar]
- 38.Whitacre, C. C., Reingold, S. C. & O'Looney, P. A. (1999) Science 283, 1277–1278. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi, M., Mimura, Y., Hamano, H., Haneji, N., Yanagi, K. & Hayashi, Y. (1996) Cell. Immunol. 170, 54–62. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi, Y., Haneji, N., Hamano, H., Yanagi, K., Takahashi, M. & Ishimaru, N. (1996) Cell. Immunol. 171, 217–225. [DOI] [PubMed] [Google Scholar]
- 41.Kassan, S., T., Thomas, H., Moutsopoulos, H. M., Hoover, R., Kimberly, R. P., Budman, D. R., Costa, J., Decker, J. L. & Chused, T. M. (1978) Ann. Intern. Med. 89, 888–892. [DOI] [PubMed] [Google Scholar]
- 42.Isaacson, P. G. & Spencer, J. (1987) Histopathology 11, 445–462. [DOI] [PubMed] [Google Scholar]