Abstract
Transformation of natural polymers to three-dimensional (3D) scaffolds for biomedical applications faces a number of challenges, viz., solubility, stability (mechanical and thermal), strength, biocompatibility, and biodegradability. Hence, intensive research on suitable agents to provide the requisite properties has been initiated at the global level. In the present study, an attempt was made to engineer chitosan and collagen macromolecules using sebacic acid, and further evaluation of the mechanical stability and biocompatible property of the engineered scaffold material was done. A 3D scaffold material was prepared using chitosan at 1.0% (w/v) and sebacic acid at 0.2% (w/v); similarly, collagen at 0.5% (w/v) and sebacic acid at 0.2% (w/v) were prepared individually by freeze-drying technique. Analysis revealed that the engineered scaffolds displayed an appreciable mechanical strength and, in addition, were found to be biocompatible to NIH 3T3 fibroblast cells. Studies on the chemistry behind the interaction and the characteristics of the cross-linked scaffold materials suggested that non-covalent interactions play a major role in deciding the property of the said polymer materials. The prepared scaffold was suitable for tissue engineering application as a wound dressing material.
Electronic supplementary material
The online version of this article (doi:10.1186/2194-0517-2-11) contains supplementary material, which is available to authorized users.
Keywords: Chitosan, Collagen, Sebacic acid, Mechanical strength, Biocompatible
Background
Polymers, in general, (whether natural or synthetic) play major roles in biomaterial preparations. However, natural polymers or polymers derived from living creatures are of greater interest, and most research publications majorly discussed chitosan and collagen Parenteau-Bareil et al. (2010; Iwasaki et al. 2011). Further, Ko et al. (2010) suggested the use of naturally derived polymers as three-dimensional (3D) scaffolds, which received much attention due to their low cost, ease of processing, and biocompatibility.
With regard to chitosan and collagen, after extraction, both these materials did not have much stability to act as a biomaterial for clinical applications and demanded stabilizers in the form of cross-linkers Friess (1998; Austero et al. 2012). Diisocyanates, Resimene Ligler et al. (2001), N, N-disuccinimidyl suberate Schauer et al. (2003), epichlorohydrin Wei et al. (1992), Genipin Jin et al. (2004), and glutaraldehyde Tual et al. (2000) were the stabilizers studied for chitosan and chromium Usha and Ramasami (2000), while aldehydes Sheu et al. (2001), hexamethylene diisocyanate Miles et al. (2005), carbodiimide Nam et al. (2008), acyl azides Petite et al. (1990), citric acid, maleic acid derivatives Saito et al. (2008), and various other physical treatments, such as UV Weadock et al. (1995) and gamma irradiation Olde Damink et al. (1995a), were the stabilizers studied for type I collagen. All the said exogenous cross-linkers cross-linked with chitosan or with type I collagen through (1) covalent amide/imine linkage, (2) metal-protein complex formation (chromium cross-linking with type I collagen), and (3) H-bond formation (between polyphenolic -OH group with different types of amino acids of type I collagen molecule and amino group of chitosan). Although the resultant biomaterial upon cross-linking with these agents was acceptable, the complete utilization of these materials was restricted because of low mechanical strength Schiffman and Schauer (2007). The biocompatibility of the scaffold material is also questionable because of the release of toxic components from some of the cross-linkers upon usage Gough et al. (2002).
In general, the mechanical property of any biomaterial depends on the interaction between the cross-linkers/stabilizers and the parent molecule (here, it is chitosan and collagen type I) Rinaudo (2010). As summarized above, reports on bonding interaction of the said cross-linkers suggest the predominance of covalent interactions which ultimately restrict the molecule to attain the desired mechanical strength.
Hence, in order to engineer the macromolecules and also to obviate the problems associated with the mechanical property and biocompatibility of biomaterials, we attempted to cross-link the parent molecule with a suitable cross-linker through non-covalent interactions. In our previous study, we detailed the cross-linking chemistry between malonic acid (MA) with chitosan/collagen Mitra et al. (2012). Observations with short-chain dicarboxylic acid (MA, three carbons) cross-linked chitosan/collagen scaffold gave impetus to carry out further work with long-chain dicarboxylic acids, namely sebacic acid (SA) (ten carbons).
Thus, in the present study, SA was chosen to cross-link with natural polymers, and to this day, no reports are available on the non-covalent interaction of SA with chitosan and type I collagen. Sebacic acid (decanedioic acid), a C-8 dicarboxylic acid (HOOC-(CH2)8-COOH), is a white flake or powdered crystal generally used as a component in metalworking fluids, surfactants, lubricants, detergents, oiling agents, emulsifiers, etc. Sebacic acid is the natural metabolic intermediate in ω-oxidation of medium- to long-chain fatty acids Liu et al. (1996). According to Tamada and Langer (1992), it is safe under in vivo condition.
The present study emphasizes the detailed chemistry behind the engineering of chitosan and collagen using SA and the evaluation of the thermal, mechanical, and biocompatible properties of the engineered chitosan and collagen scaffolds.
Results and discussion
Scaffold preparation using sebacic acid: understanding the cross-linking chemistry
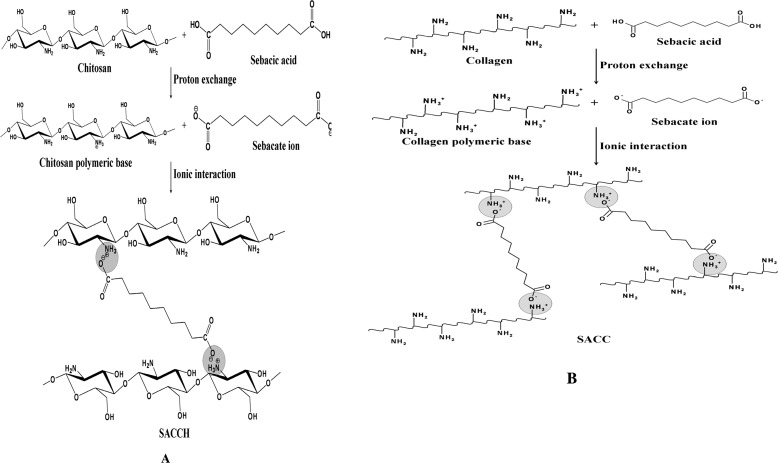
For the preparation of any scaffold materials, the solution form of the parent compound/polymer is required to proceed further. However, in the case of chitosan and collagen, acetic and formic acids were generally used for dissolution Ohkawa et al. (2004; El-Tahlawy et al. 2006). The ‘proton exchange’ between the -COOH groups of acid molecule and free -NH2 groups of chitosan and collagen could be the reason for the dissolution in the said acids.
Therefore, it has been expected that like acetic acid, sebacic acid is also able to donate protons to dissolve chitosan and type I collagen. Further, similar to the interaction of TPP Bhumkar and Pokharkar (2006) with chitosan, sebacic acid may also interact with both natural polymers through ionic interaction. Because of the said proton exchange, chitosan and type I collagen dissolve in the presence of sebacic acid in water; the following schematic representation (Scheme 1a,b) illustrates the nature of proton exchange between sebacic acid with chitosan and with collagen for better understanding.
Scheme 1.
Possible reaction mechanisms. (A) Possible reaction mechanism between chitosan and sebacic acid. (B) Possible reaction mechanism between collagen and sebacic acid.
Because of the said interaction, both natural polymers were completely dissolved in water in the presence of sebacic acid. With the resulting solution, scaffolds were prepared and subjected to characterization studies. Figure 1 shows the morphological features of the cross-linked scaffolds, namely sebacic acid cross-linked chitosan (SACCH) and sebacic acid cross-linked collagen (SACC). The 3D scaffold material was highly porous, and the pore structures of the membranes were well distributed and interconnected. It was obvious that most of the membrane volume was taken up by interconnecting pore space. The high porosity suggests the suitability of this scaffold for biomedical applications, including serving as absorption sponges and matrices for cell proliferation.
Figure 1.
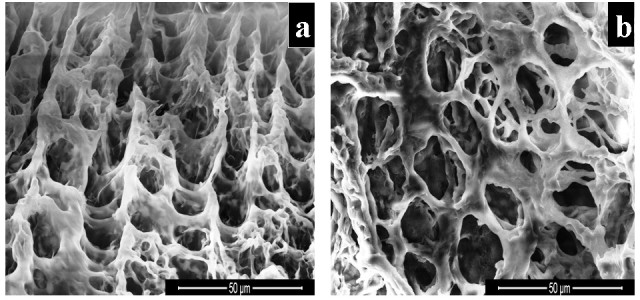
SEM micrographs of (a) sebacic acid cross-linked chitosan and (b) sebacic acid cross-linked collagen scaffolds.
Fourier transform infrared spectroscopy (FT-IR) studies were conducted to monitor chemical modifications in the chitosan and collagen structures upon cross-linking with SA. Figure 2 illustrates the FT-IR spectral details of SA, chitosan, collagen, SACCH, and SACC. Table 1 demonstrates the FT-IR peak assignments of SA, chitosan, and collagen. In the SACCH spectrum, few significant changes were observed. A broad, strong absorption peak in the region of 3,433 to 2,928 cm-1 resulted from the superimposed -OH and -NH3+ stretching bands. Absorption in 1,640 and 1,557 cm-1 corresponded to the presence of asymmetric N-H (-NH3+) bends and asymmetric -COO- stretching, respectively. A peak observed at 1,403 cm-1 was due to symmetric -COO- stretching. Other absorption peaks around 1,257, 1,157, and 899 cm-1 observed in the SACCH spectrum were similar to the native chitosan spectrum which exhibits that there was no change in the main backbone of the chitosan structure Lopez et al. (2008).
Figure 2.
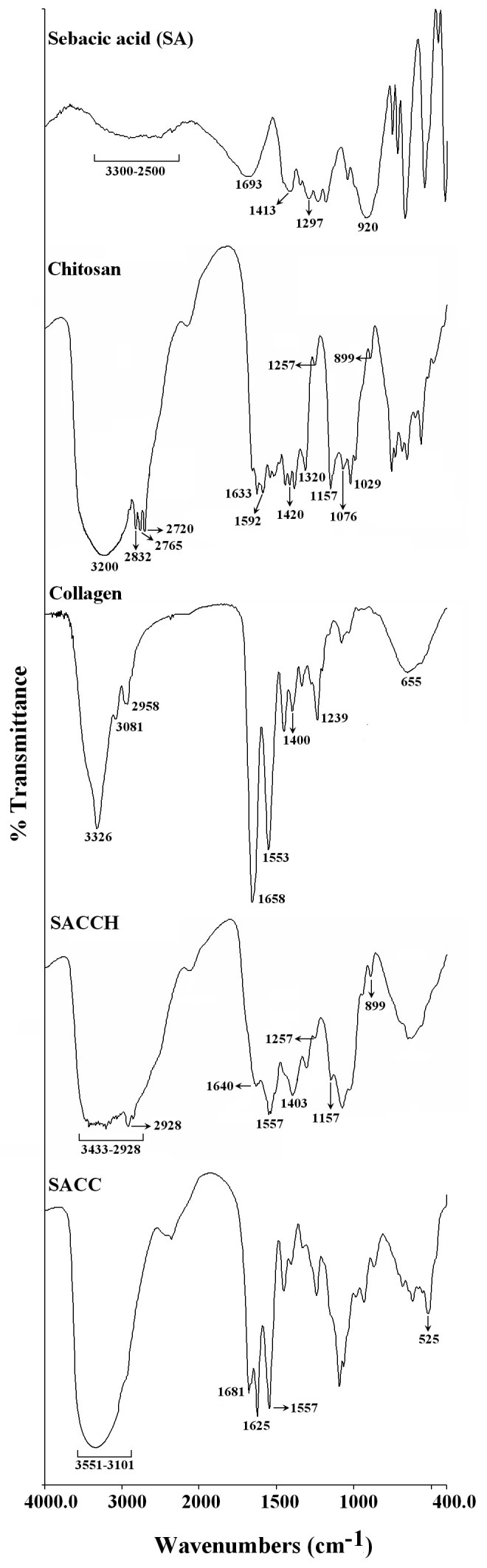
FT-IR spectra of SA, chitosan, type I collagen, SACCH, and SACC scaffolds. SA, sebacic acid; SACCH, sebacic acid cross-linked chitosan; SACC, sebacic acid cross-linked collagen.
Table 1.
FT-IR analysis of SA, chitosan, and collagen
| Material | Wave number (cm-1) | Peak assignment |
|---|---|---|
| SA | 3,300 to 2,500 | Overlapping stretching vibration of C-H and O-H groups (νC-H-νOH) |
| 1,693 | -C=O group (νC=O) | |
| 1,413 | C-O-H in-plane bending ( δC-O-H) | |
| 1,297 | C–O stretching vibration (νC-O) | |
| 920 | Out-of-plane bending of the bonded O-H (δO-H) | |
| Chitosan | 3,200 | -NH2 stretching vibration (νNH) |
| 2,832, 2,765, 2,720 | Symmetric or asymmetric -CH2 stretching vibration attributed to the pyranose ring (νC-H) | |
| 1,633 | -C=O in acetamide group (amide I band) | |
| 1,592 | -NH2 bending vibration in amino group (δNH) | |
| 1,420, 1,320 | Vibrations of OH, CH in the ring | |
| 1257 | C-O group | |
| 1157 | -C-O-C in the glycosidic linkage | |
| 1,076, 1,029 | C-O stretching in acetamide (νC-O) | |
| 899 | Corresponds to the saccharide structure | |
| Collagen | 3,326 | -NH2 stretching vibration (νNH) |
| 3,081 | Fermi resonance overtone of 1,553 band | |
| 2,958 | C-H stretching (νC-H) | |
| 1,658 | Amide I band (νC=O) | |
| 1,553 | Amide II band (δNH) | |
| 1,400 | Amide III band (νC-N) | |
| 1,239 | C-N stretching of amine (νC-N) | |
| 655 | Out-of-plane N-H wagging of amide and amine (δNH) |
ν, stretching; δ, bending.
In the SACC spectrum, few changes were observed when compared with native type I collagen. A broad, strong absorption peak in the region of 3,551 to 3,101 cm-1 resulted from the superimposed -OH and -NH3+ stretching bands. In the type I collagen spectrum, a sharp intense amide I band observed around 1,658 cm-1 disappeared with the appearance of two new bands in 1,681 and 1,625 cm-1 in the SACC spectrum; these bands were supposed to be caused by -NH3+ and -COO-, respectively. Moreover, when compared with native type I collagen spectrum, there was a reduction in the region of 1,557 cm-1 (overlapped band of amide II and free primary amines) in the SACC spectrum, which may be due to the reduction of free -NH2 group in the SACC. In the SACC spectrum, the observed band around 525 cm-1 was ascribed to the N-H oscillation of -NH3+. Results from FT-IR analysis reflected that SA was ionically cross-linked with chitosan and type I collagen Pavia et al. (2001; Lawrie et al. 2007).
Though FT-IR analysis displayed the ionic interaction between the cross-linker and the natural polymers, results on the percentage of cross-linking degree calculations suggested that increasing the concentration of SA increases the degree of cross-linking up to 0.4% and confirmed the interaction. About 60% to 65% cross-linking was observed with 0.2% SA with chitosan and collagen. However, in the case of experiments with glutaraldehyde, about 88% to 93% of cross-linking was observed with 0.2% concentration.
With regard to the mechanical property of the scaffold materials, it is a fundamental property for any scaffold material in the application point of view. From the results, we observed that the mechanical strength of the scaffold increased with the increase in sebacic acid concentration up to 0.2%. Further increase in SA concentration leads to the decrease in mechanical strength (results not shown). Table 2 illustrates the tensile strength, Young's modulus, and stiffness of native and sebacic acid (0.2%) cross-linked scaffolds. High tensile strength (MPa) values were observed for both the cross-linked scaffolds (SACCH 8.94; SACC 2.96) than for the native polymers (chitosan 0.37; type I collagen 0.13). Moreover, the Young's modulus of SACCH and SACC were 200.8 and 18.61, respectively. The stiffness values (SACCH 9.53 N/mm; SACC 0.69 N/mm) were also greater than those of the native polymers (chitosan 0.79 N/mm; type I collagen 0.3 N/mm).
Table 2.
Assessment of mechanical properties of chitosan, SACCH, collagen and SACC
| Samples | Maximum loada | Tensile strengtha | Elongation at breaka | Extension at maximum loada | Young's modulus/tensile modulusa | Stiffness ( κ)a |
|---|---|---|---|---|---|---|
| (N) | (MPa) | (%) | (mm) | (MPa) | (N/mm) | |
| Chitosan | 1.32 ± 0.08 | 0.37 ± 0.03 | 8.33 ± 0.6 | 1.67 ± 0.04 | 4.43 ± 0.5 | 0.790 ± 0.07 |
| SACCH | 5.61 ± 0.5 | 3.21 ± 0.2 | 8.63 ± 1 | 1.73 ± 0.04 | 37.1 ± 2.2 | 3.24 ± 0.4 |
| Collagen | 0.37 ± 0.03 | 0.13 ± 0.02 | 6.17 ± 0.8 | 1.23 ± 0.05 | 2.1 ± 0.7 | 0.3 ± 0.01 |
| SACC | 2.22 ± 0.5 | 2.96 ± 0.5 | 31.84 ± 2 | 3.18 ± 0.4 | 18.61 ± 1.5 | 0.698 ± 0.02 |
The assessment is in terms of tensile strength, elongation at break, Young's modulus, and stiffness. aMean ± SD
All these observations on mechanical properties suggest that sebacic acid cross-linked materials demonstrated appreciable mechanical strength compared to glutaraldehyde, wherein we observed brittleness similar to the observation made by Schiffman and Schauer (2007). Further, when the concentration of SA was increased > 0.2%, a decrease in mechanical strength was observed, and this could be reasoned to the high degree of cross-linking of SA with the polymers, as evidenced from the 2,4,6-trinitrobenzenesulfonic acid (TNBS) assay Wang et al. (2002).
Further, the reason for the brittle nature of the glutaraldehyde cross-linked material is that glutaraldehyde could covalently cross-link with chitosan and collagen through the formation of a double bond (C=N, imine bond) between the -CHO group of glutaraldehyde and the -NH2 group of natural polymers (chitosan and collagen), which results in the large energy barrier for the rotation of associated groups linked by a double bond (C=N), and finally, this provided the brittle nature to the material.
Thermogravimetric analyses for the experimental samples SA, chitosan, type I collagen, SACCH, SACC, glutaraldehyde cross-linked chitosan (GACCH), and glutaraldehyde cross-linked collagen (GACC) were illustrated in Figure 3a,b, and the corresponding thermal degradation values were displayed in Table 3. From the results, we observed that the incorporation of SA with chitosan and type I collagen tended to shift the thermal region into higher temperature, and such a shift is attributed to an increase in thermal stability.
Figure 3.
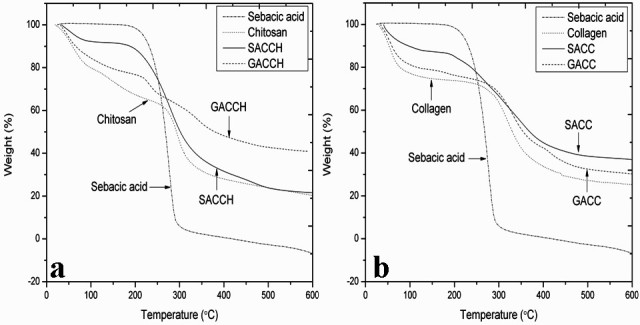
Thermogravimetric analyses for experimental samples. (a) Thermogravimetric analysis of sebacic acid (SA), chitosan, sebacic acid cross-linked chitosan (SACCH), and glutaraldehyde cross-linked chitosan (GACCH) scaffolds. (b) Thermogravimetric analysis of SA, collagen, sebacic acid cross-linked collagen (SACC), and glutaraldehyde cross-linked collagen (GACC) scaffolds.
Table 3.
Thermal analyses of SA, chitosan, SACCH, GACCH, collagen, SACC, and GACC under N 2 air atmosphere
| Temperature (°C) | Percentage of weight loss (heating rate 20°C/min) | ||||||
|---|---|---|---|---|---|---|---|
| SA | Chitosan | SACCH | GACCH | Collagen | SACC | GACC | |
| 100 | 0 | 21 | 8 | 16 | 23 | 11 | 20 |
| 200 | 1 | 33 | 12 | 24 | 27 | 15 | 24 |
| 300 | 95 | 56 | 50 | 38 | 37 | 34 | 33 |
| 400 | 100 | 73 | 69 | 52 | 67 | 55 | 58 |
| 500 | 100 | 77 | 77 | 58 | 73 | 62 | 68 |
| 600 | 100 | 81 | 79 | 60 | 75 | 63 | 70 |
SA, sebacic acid; SACCH, sebacic acid cross-linked chitosan; GACCH, glutaraldehyde cross-linked chitosan; SACC, sebacic acid cross-linked collagen; GACC, glutaraldehyde cross-linked collagen.
Differential scanning calorimetry (DSC) studies were performed to understand the behavior of SACCH and SACC on the application of thermal energy. The thermogram values of SA, chitosan, type I collagen, SACCH, SACC, GACCH, and GACC were shown in Figure 4a,b. DSC studies recorded the melting temperature of SA (135°C) and the degradation temperature differences among chitosan (107°C), type I collagen (92°C), SACCH (119°C), and SACC (125°C), whereas in GACCH and GACC, it was observed at 149°C and 151°C, respectively. The higher transition temperature suggests that SACCH and SACC have high stability at high-temperature environment. Thermal stability also influences on the durability of the scaffolds. A similar kind of observation was reported by Bhumkar and Pokharkar (2006).
Figure 4.
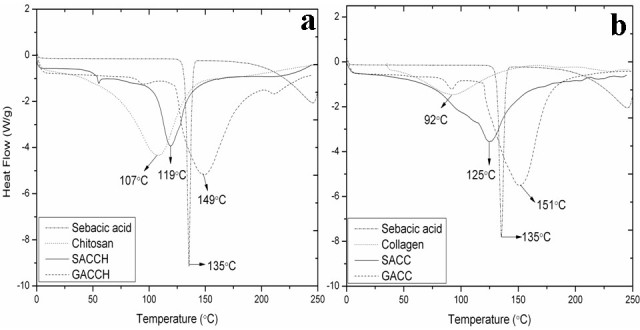
DSC analyses of SA, chitosan, type I collagen, SACCH, SACC, GACCH, and GACC. (a) DSC analysis of sebacic acid (SA), chitosan, sebacic acid cross-linked chitosan (SACCH), and glutaraldehyde cross-linked chitosan (GACCH) scaffolds. (b) DSC analysis of sebacic acid (SA), collagen, sebacic acid cross-linked collagen (SACC), and glutaraldehyde cross-linked collagen (GACC) scaffolds.
Results on binding energy calculations based on the bioinformatics tool for the cross-linking of SA with chitosan and type I collagen using AutoDock software (The Scripps Research Institute La Jolla, CA 92037, USA) proved that chitosan and type I collagen cross-links with sebacic acid not only through ionic interaction, but also through multiple intermolecular hydrogen bonding. AutoDock is an automated procedure for predicting the interaction of ligands with biomacromolecular targets. Hundred runs were given for docking SA with chitosan and type I collagen. The best binding energy values and their corresponding rank and run numbers were depicted in Table 4. The binding energy of -4.21 and -4.49 (kcal/mol) was observed when SA interacted with chitosan and type I collagen, respectively. These interactions were made by multiple intermolecular hydrogen bonds between the -COOH group of SA and the -NH2 group of chitosan and the free ε-NH2 group of lysine from type I collagen (Figure 5a,b). In addition to ionic cross-linking, hydrogen bonding interaction also improves the mechanical property of the scaffold. The ionic interaction and hydrogen bonding between the -COOH group of cross-linker and the -NH2 group of natural polymers (chitosan/collagen) has already been reported Milosavljevic et al. (2010; Vijayaraghavan et al. 2010). The details of these intermolecular hydrogen bonding sites are given in the following sections.
Table 4.
Binding energy values of SACCH and SACC scaffolds
| Rank | Binding energy (Kcal/mol) | Number of runs | |
|---|---|---|---|
| Binding energy calculation between chitosan and sebacic acid based on AutoDock tool software | 1 | -4.21 | 2 |
| 2 | -4.17 | 24 | |
| 3 | -3.92 | 65 | |
| 4 | -3.73 | 29 | |
| Binding energy calculation between type I collagen and sebacic acid based on AutoDock tool software | 1 | -4.49 | 49 |
| 2 | -4.36 | 20 | |
| 3 | -4.04 | 58 | |
| 4 | -3.94 | 21 | |
| 5 | -3.79 | 51 | |
| 6 | -3.71 | 63 | |
| 7 | -3.63 | 15 | |
| 8 | -3.20 | 85 | |
| 9 | -3.13 | 53 |
Figure 5.
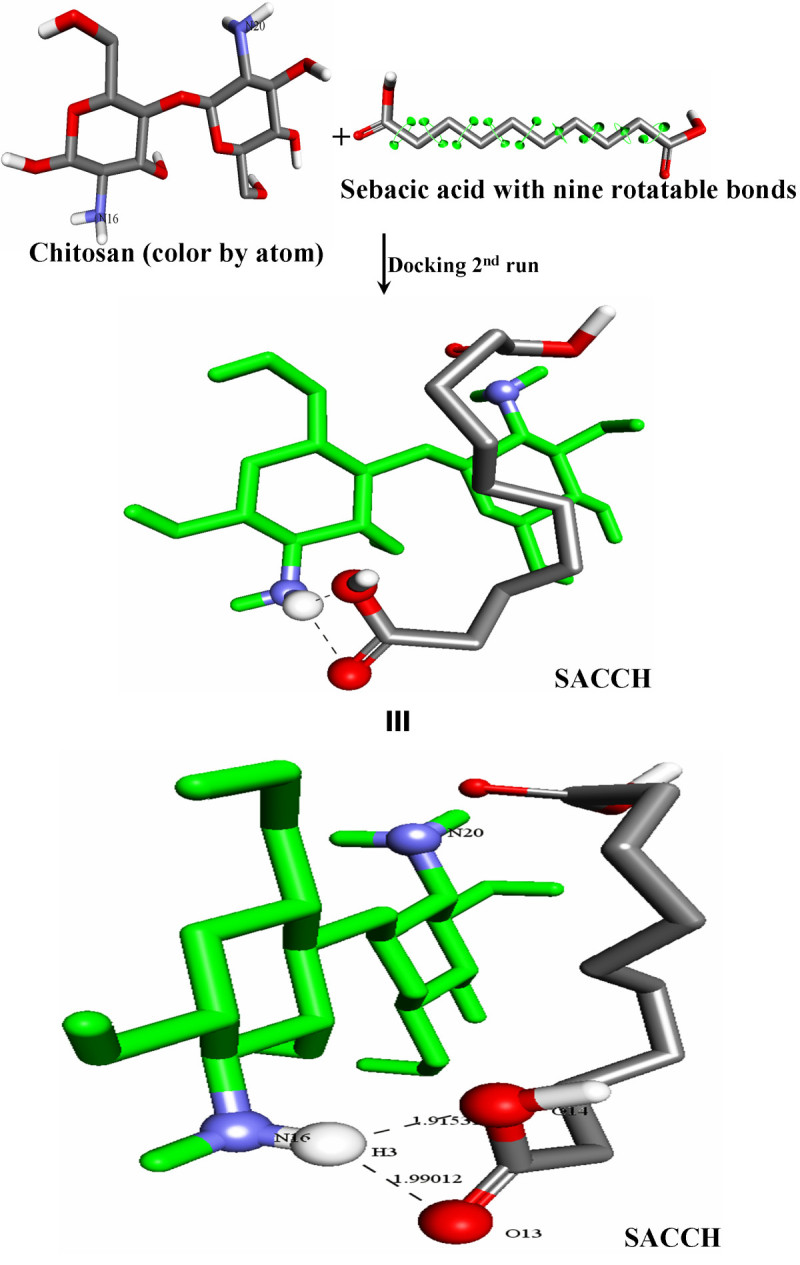
Multiple hydrogen bonding interactions. (a) Multiple hydrogen bonding interaction between chitosan and sebacic acid. Images of SACCH are displayed in two different orientations. The first orientation demonstrates the interaction, and the second illustrates the details on hydrogen bonding. (b) Multiple hydrogen bonding interaction between collagen and sebacic acid. The black dotted line indicates the hydrogen bond. Various colors denote the different atoms to be recognized: white color is for hydrogen atom (H), red color indicates oxygen atom (O), gray is for carbon atom (C), and blue corresponds to nitrogen atom (N).
Intermolecular hydrogen bond details between SA and chitosan
H (3) of chitosan is linked to O (14) of SA with a bond distance of 1.91531, and H (3) of chitosan is linked to O (13) of SA with a bond distance of 1.99012.
Intermolecular hydrogen bond details between SA and type I collagen
Lysine amino acid (LYS) (12) H2 of type I collagen is linked with O (7) of SA with a bond distance of 2.06994, LYS (12) H2 of type I collagen is linked with O (8) of SA with a bond distance of 2.09251, LYS (12) H3 of type I collagen is linked with O (13) of SA with a bond distance of 2.11339, and LYS (12) H3 of type I collagen is linked with O (14) of SA with a bond distance of 1.91386.
Concerning the biocompatibility of the resulting polymers, cell attachment and viability assays were carried out. 3-[4,5-Dimethylthiazol-2-yl]-2,5-dephenyltetrazolium bromide (MTT) assay was done to check the toxicity profile of the prepared scaffolds (SACCH, SACC, GACCH, and GACC). Only cells that are metabolically normal can turn the tetrazolium salts into purple crystals. Compared with the native chitosan and type I collagen, SACCH and SACC showed no significant differences in absorbance (Figure 6) and inferred that scaffolds being in direct contact with fibroblast did not lead to apoptosis or necrosis. MTT results clearly indicated that NIH 3T3 cells are viable on the surface of the SA cross-linked scaffolds (SACCH and SACC). However, only 10% cells were viable with the samples of GACCH and GACC. Though glutaraldehyde (GA) has been widely used as chemical cross-linking agent Jorge-Herrero et al. (1999) because of stabilizing the collagen efficiently, and the cross-linking is thought to involve the formation of Schiff bases Olde Damink et al. (1995b), the poor biocompatibility of GA-cross-linked biomaterials with some other cell lines, including human fibroblasts, osteoblasts, Chang cells, and endothelial cells, had been reported Gough et al. (2002). The side effects of GA treatment were attributed to the degradation of the GA-derived cross-links and the continuous release of aldehydes contributing to prolong toxic effects Schmidt and Baier (2000).
Figure 6.
MTT analysis at 24-, 48-, and 72-h time interval. The analysis was for control, chitosan, collagen, sebacic acid cross-linked chitosan (SACCH), glutaraldehyde cross-linked chitosan (GACCH), sebacic acid cross-linked collagen (SACC), and glutaraldehyde cross-linked collagen (GACC) scaffolds.
In the cell viability assay, we observed intense fluorescence of the cells on the surface of the native and SA cross-linked scaffolds and suggest the viability of the cells as illustrated in Figure 7.
Figure 7.
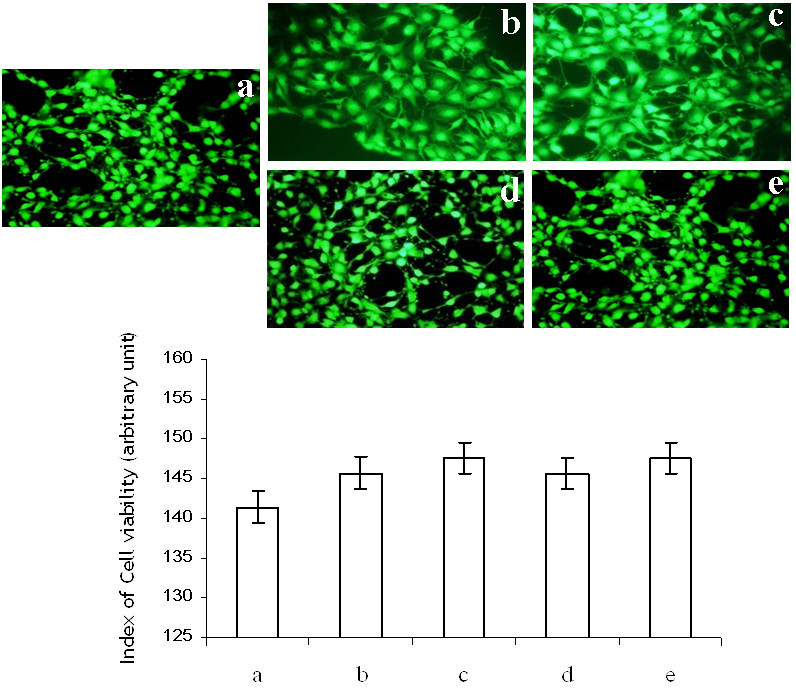
Cell viability index (arbitrary unit) assessed in SACCH and SACC compared with parent molecules and control. (a) Control, (b) chitosan, (c) collagen, (d) sebacic acid cross-linked chitosan (SACCH), and (e) sebacic acid cross-linked collagen (SACC). The assay was carried out using a cell tracker kit. NIH3T3 cells were treated on the surface of native and cross-linked scaffolds for 6 h followed by incubation with cell viable dye cell tracker for 30 min. Fluorescence images of the cells were acquired using DP71 camera adapted to an Olympus IX71 microscope (Olympus Corporation, Shinjuku, Tokyo, Japan). Intensity of green positive cells were counted and plotted. Next, fluorescence intensities of the images were calculated using Adobe Photoshop version 7.0.
The SEM images of the cell seeded SACCH and SACC scaffolds displayed in Figure 8a,b demonstrated that after being cultured for a prolonged time (12 days), fibroblasts were detected in the scaffolds (SACCH and SACC) with typical spindle-shaped morphology, suggesting that the cells were infiltrated into the scaffolds.
Figure 8.
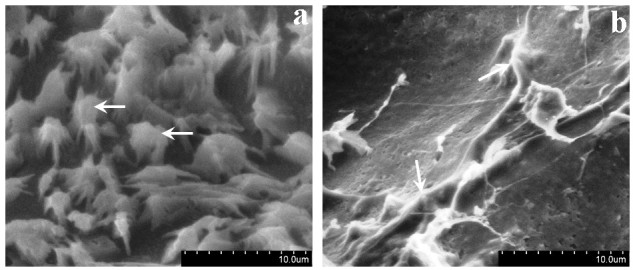
Attachment of fibroblast cells on the (a) SACCH and (b) SACC scaffolds. In (a), white arrows indicate the adhered cells on the scaffold. In (b), porous SACC scaffold was completely covered by fibroblast cells which are indicated by the white arrow.
When comparing the present results with the previous work carried out with malonic acid (three carbons) Mitra et al. (2012), no significant differences in mechanical and thermal stability were observed. However, when comparing the pore size (16 to 18 μm) determination from SEM results of malonic acid cross-linked chitosan/collagen, an increased pore size (27 to 30 μm) was observed in SACCH and SACC scaffolds. This could be due to the long chain length of sebacic acid (ten carbons) compared with malonic acid (three carbons). In addition, the docking studies suggested that there was stronger intermolecular hydrogen bonding interaction between sebacic acid and chitosan/collagen compared with the interaction between malonic acid and chitosan/collagen.
Conclusions
The present study explicitly demonstrates sebacic acid acting as suitable cross-linker for the preparation of biocompatible scaffolds from natural polymers (chitosan and collagen) with appreciable mechanical properties compared to short-chain dicarboxylic acids. The interactions between SA and chitosan or collagen were identified as non-covalent, i.e., both for ionic and multiple intermolecular hydrogen bonding interactions. These non-covalent interactions offered high mechanical strength to the resultant material. All the instrumental analyses and bioinformatics tool authenticated the non-covalent interactions. The scaffold material prepared upon cross-linking of SA with chitosan or collagen was the green method of preparation. Sebacic acid not only dissolves the above-said natural polymers, but also improves the property of the material through its non-covalent interactions with natural polymers. No toxic compounds were involved in this preparation, and the resultant material can be used as a wound dressing material or as an implant in clinical applications.
Methods
Materials
Chitosan from shrimp shells (≥75% deacetylated), sebacic acid, and Picrylsulfonic acid (TNBS) were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA). MTT and dexamethasone were purchased from HiMedia Laboratories (Mumbai, India). All the other reagents were of analytical grade and used without further purification. Type I collagen from bovine skin was extracted according to the procedure followed by Mitra et al. (2011).
Preparation of a 3D scaffold
In the powder form of chitosan (1%), sebacic acid at different concentrations was mixed, and 20 ml of water was added to the mixture which was stirred at room temperature until the solution became homogenous. Similarly, collagen (0.5%) and sebacic acid at different concentrations were mixed in the presence of 20 ml of water and stirred at 4°C to obtain a clear homogenous solution. The concentration of SA varied between 0.05% and 0.5% (w/v). The samples were subjected to centrifugation in order to remove any nonreactive molecules, and a clear solution obtained from centrifugation at 5,000 rpm for 10 min was poured in Tarsons vials (Tarsons Product Pvt. Ltd., Kolkata, India), which have an inner diameter of 4.5 cm and frozen at -4°C for 2 h, -20°C for 12 h, and -80°C for another 12 h according to Peng et al. (2006). The frozen samples were lyophilized for 48 h at a vacuum of 7.5 mTorr (1 Pa) and a condenser temperature of -70°C (PENQU CLASSIC PLUS, Lark, India). The resultant 3D scaffold material was neutralized with repeated washings with 0.05 N of NaOH and ethanol mixture, followed by washings with water and ethanol mixture; finally, it was again lyophilized for 24 h. The scaffolds obtained during this procedure were designated as SACCH and SACC. For comparative analysis, GACCH and GACC were prepared according to the method described previously using 0.2% glutaraldehyde.
SEM analysis of SACCH and SACC
The physical texture and the morphology of the scaffold of SACCH and SACC were assessed using a scanning electron micrograph. SEM micrograph analysis was made using FEI Quanta (FEI Company, Hillsboro, OR, USA) FEG 200 high-resolution scanning electron microscope under a high voltage at 20 kV.
FT-IR analysis
Functional group analysis for SA, chitosan, type I collagen, SACCH, and SACC scaffolds were made using Spectrum One FT-IR (PerkinElmer Instruments, Branford, CT, USA). All spectra were recorded with the resolution of 4 cm-1 in the range of 400 to 4,000 cm-1 with 20 scans.
Cross-linking degree (2,4,6-trinitrobenzenesulfonic acid assay) determination
Degree of cross-linking was quantified using TNBS assay according to the procedure summarized by Bubnis and Ofner (1992). In brief, native and cross-linked (SACCH and SACC) biopolymer materials were cut into small pieces at 4.5 mm. Cut pieces (6 mg) were immersed in a 2-ml solution (1 ml of 4% disodium hydrogen orthophosphate (w/v) and 1 ml of 0.5% TNBS (v/v)) and incubated at 40°C for 2 h. Pure sebacic acid at respective percentages was also treated with a 2-ml solution in separate test tubes. Termination of reaction was by the addition of 3 ml of 6 M HCl (v/v), and the incubation was continued at 60°C for 90 min. The absorbance of the resulting solution was measured at 345 nm using a UV-visible spectrophotometer (UV-2450, Shimadzu Corporation, Nakagyo-ku, Kyoto, Japan), and the percentage of cross-linking was calculated from the difference in the absorbance divided by the absorbance of the native material and then multiplied by 100.
Mechanical properties of SACCH, SACC, GACCH, and GACC scaffolds
Mechanical properties, viz., Young's modulus, ultimate tensile strength, stiffness, and percentage of elongation of the dried scaffold materials, were measured using a universal testing machine (model 1405, Instron Corporation, Norwood, MA, USA) at a crosshead speed of 5 mm min-1 at 25°C and 65% relative humidity. Length and width of the dumbbell-shaped test samples were maintained at 20 and 5 mm, respectively, according to Shanmugasundaram et al. (2004). All the mechanical tests were performed with dried samples and were examined in triplicates.
Thermogravimetric analysis
Thermal decomposition analysis of SA, native, and cross-linked scaffolds (chitosan, type I collagen, SACCH, SACC, GACCH, and GACC) was carried out under nitrogen flow (40 and 60 ml min-1) with ramp at 20°C min-1 using TGA Q 50 (TA Instruments, New Castle, DE, USA) with an isothermal temperature accuracy of ±1°C.
Differential scanning calorimetry
DSC analysis for SA, native, and cross-linked scaffolds (chitosan, type I collagen, SACCH, SACC, GACCH, and GACC) was analyzed using a differential scanning calorimeter (model DSC Q 200, TA Instruments) with standard mode at nitrogen (50 ml min-1) atmosphere with ramp at 10°C min-1.
Docking and binding energy calculations
For the docking study, chemical structures of chitosan and SA were generated using ACD/ChemSketch ACD (2009), and the 3D structure of type I collagen was generated using the gencollagen program. The docking technique is useful to find the binding efficiency with a ligand and a chemical compound. To find out the interaction between chitosan and type I collagen with SA, AutoDock 4.2 was used to calculate Morris et al. (2009) the free energy of binding of SA with chitosan and type I collagen.
Biocompatibility of SACCH and SACC scaffold an in vitro assessment
Cell viability study (MTT assay)
NIH 3T3 fibroblast cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (v/v) and 1% antibiotics and were incubated at 37°C in 5% CO2 humidified atmosphere. Polystyrene 96-well culture plates (Tarsons) were coated with native chitosan, type I collagen, SA cross-linked chitosan (SACCH), GA cross-linked chitosan, SA cross-linked type I collagen (SACC), and GA cross-linked collagen samples. The plates were dried using a laminar air flow hood, followed by 30 min of UV sterilization. The cells were seeded at the density of 0.5 × 106 per well and incubated at 37°C in humidified atmosphere containing 5% CO2. At scheduled time points of 24, 48, and 72 h, the supernatant of each well was replaced with MTT diluted in serum-free medium, and the plates incubated at 37°C for 4 h. After removing the MTT solution, acid isopropanol (0.04 N HCl in isopropanol) was added to each well, was then pipetted up and down to dissolve all the dark-blue crystals, and was left at room temperature for a few minutes to ensure the dissolution of all crystals. Finally, absorbance was measured at 570 nm using a UV spectrophotometer Mossmann (1983). Each experiment was performed at least three times. The sets of three wells for the MTT assay were used for each experimental variable.
Cell tracker assay to detect live cells
Cell viability was measured using 5-chloromethylfluorescein diacetate probe (CMFDA) (Invitrogen Life Technologies, Carlsbad, CA, USA). NIH 3T3 cells were subjected to respective treatment conditions. Cells were probed with 5 μM CMFDA and incubated for 2 h. Cells were then washed with sterile PBS, and images were taken using DP71 camera adapted to an Olympus IX71 (Olympus Corporation) microscope Majumder et al. (2011). The assay was carried out using a cell tracker kit. NIH3T3 Cells were treated on the surface of native and cross-linked scaffolds for 6 h, followed by the incubation with cell viable dye cell tracker for 30 min. Fluorescence images of the cells were acquired using a DP71 camera adapted to an Olympus IX71 microscope. The intensity of green positive cells was counted and plotted. Next, fluorescence intensities of the images were calculated using Adobe Photoshop version 7.0. No cell tracker assay was carried out for GACCH and GACC based on the observations made with the MTT assay.
Cell morphology of NIH 3T3 cells in SACCH and SACC scaffolds
SACCH and SACC scaffolds (2 × 2 × 1 cm) were placed individually in six-well culture plates (Tarsons, India) and sterilized ETO. Culture media were added to the scaffolds overnight. NIH 3T3 fibroblast cells were seeded onto the scaffolds at a density of 5 × 104 cells and incubated in an atmosphere of 5% CO2 at 37°C. The medium was changed every 24 h. The morphology of cells was examined after 12 days according to the following procedure:
The cells-and-scaffold constructs were fixed in 2.5% glutaraldehyde and dehydrated through a graded ethanol series.
The dried cells-and-scaffold were coated with gold (E-1010 Ion sputter, Hitachi High-Tech, Minato-ku, Tokyo, Japan) and examined under SEM (S-3400 N Hitachi High-Tech).
Experiments for GACCH and GACC were not conducted.
Statistical analysis
When necessary, the experimental results were expressed as the mean ± SD values of triplicates.
Acknowledgments
One of the authors Mr. Tapas Mitra acknowledges CSIR, New Delhi for the financial assistance provided in the form of CSIR-SRF. The authors thank Dr. Swaraj Sinha and Dr. Suvro Chatterjee, AU-KBC Research Centre, MIT Campus, Anna University, Chennai for their help and lab facility to perform cell line studies.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The work was designed by TM, GS and AG and performed by TM and GS. Manuscript was drafted by TM & GS and written and corrected by AG. All authors read and approved the final manuscript.
Contributor Information
G Sailakshmi, Email: sailakshmi84@gmail.com.
Tapas Mitra, Email: tapasbiochem1@gmail.com.
A Gnanamani, Email: gnanamani3@gmail.com.
References
- ACD . ChemSketch Version 12. Toronto: Advanced Chemistry Development, Inc.; 2009. [Google Scholar]
- Austero MS, Donius AE, Wegst UGK, Schauer CL. New crosslinkers for electrospun chitosan fibre mats. I. Chemical analysis. J R Soc Interface. 2012;9:2551–2562. doi: 10.1098/rsif.2012.0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhumkar DR, Pokharkar VB. Studies on effect of pH on crosslinking of chitosan with sodium tripolyphosphate: a technical note. AAPS Pharm Sci Tech. 2006;7:1–6. doi: 10.1208/pt070250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubnis WA, Ofner CM. The determination of ε-amino groups in soluble and poorly soluble proteinaceous materials by a spectrophotometric method using trinitrobenzenesulfonic acid. Anal Biochem. 1992;207:129–133. doi: 10.1016/0003-2697(92)90513-7. [DOI] [PubMed] [Google Scholar]
- El-Tahlawy K, Gaffar MA, El-Rafie S. Novel method for preparation of cyclodextrin/grafted chitosan and its application. Carbohydr Polym. 2006;63:385–392. doi: 10.1016/j.carbpol.2005.08.057. [DOI] [Google Scholar]
- Friess W. Collagen—biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45:113–136. doi: 10.1016/S0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- Gough JE, Scotchford CA, Downes S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly (vinyl alcohol) films is by the mechanism of apoptosis. J Biomed Mater Res. 2002;61:121–130. doi: 10.1002/jbm.10145. [DOI] [PubMed] [Google Scholar]
- Iwasaki N, Kasahara Y, Yamane S, Igarashi T, Minami A, Nisimura SI. Chitosan-based hyaluronic acid hybrid polymer fibers as a scaffold biomaterial for cartilage tissue engineering. Polymers. 2011;3:100–113. doi: 10.3390/polym3010100. [DOI] [Google Scholar]
- Jin J, Song M, Hourston DJ. Novel chitosan-based films cross-linked by Genipin with improved physical properties. Biomacromolecules. 2004;5:162–168. doi: 10.1021/bm034286m. [DOI] [PubMed] [Google Scholar]
- Jorge-Herrero E, Fernandez P, Turnay J, Olmo N, Calero P, Garcia L, Freile I, Olivares JL. Influence of different chemical cross-linking treatments on the properties of bovine pericardium and collagen. Biomaterials. 1999;20:539–545. doi: 10.1016/S0142-9612(98)90205-8. [DOI] [PubMed] [Google Scholar]
- Ko HF, Feir CS, Kumta PN. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Phil Trans R Soc A. 2010;368:1981–1997. doi: 10.1098/rsta.2010.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie G, Keen I, Drew B, Temple AC, Rintoul L, Fredericks P, Grondahl L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules. 2007;8:2533–2541. doi: 10.1021/bm070014y. [DOI] [PubMed] [Google Scholar]
- Ligler FS, Lingerfelt BM, Price RP, Schoen PE. Development of uniform chitosan thin-film layers on silicon chips. Langmuir. 2001;17:5082–5084. doi: 10.1021/la010148b. [DOI] [Google Scholar]
- Liu G, Hinch B, Beavis AD. Mechanisms for the transport of α, β-dicarboxylates through the mitochondrial inner membrane. J Biol Chem. 1996;271:25338–25344. doi: 10.1074/jbc.271.41.25338. [DOI] [PubMed] [Google Scholar]
- Lopez FA, Merce ALR, Alguacil FJ, Delgado AL. A kinetic study on the thermal behaviour of chitosan. J Therm Anal Cal. 2008;91:633–639. doi: 10.1007/s10973-007-8321-3. [DOI] [Google Scholar]
- Majumder S, Siamwala JH, Srinivasan S, Sinha S, Sridhara SRC, Soundararajan G, Seerapu HR, Chatterjee S. Simulated microgravity promoted differentiation of bipotential murine oval liver stem cells by modulating BMP4/Notch1 signaling. J Cell Biochem. 2011;112:1898–1908. doi: 10.1002/jcb.23110. [DOI] [PubMed] [Google Scholar]
- Miles CA, Avery NC, Rodin VV, Bailey AJ. The increase in denaturation temperature following cross-linking of type-I collagen is caused by dehydration of the fibres. Mol Biol. 2005;346:551–556. doi: 10.1016/j.jmb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Milosavljevic NB, Kljajevic LM, Popovic IG, Filipovic JM, Krusic MTK. Chitosan, itaconic acid and poly(vinyl alcohol) hybrid polymer networks of high degree of swelling and good mechanical strength. Polym Int. 2010;59:686–694. [Google Scholar]
- Mitra T, Sailakshmi G, Gnanamani A, Raja ST, Thiruselvi T, Mangala Gowri V, Selvaraj NV, Ramesh G, Mandal AB. Preparation and characterization of a thermostable and biodegradable biopolymers using natural cross-linker. Int J Biol Macromol. 2011;48:276–285. doi: 10.1016/j.ijbiomac.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Mitra T, Sailakshmi G, Gnanamani A, Mandal AB. Preparation and characterization of malonic acid cross-linked chitosan and collagen 3D scaffolds: an approach on non-covalent interactions. J Mater Sci Mater Med. 2012;23:1309–1321. doi: 10.1007/s10856-012-4586-6. [DOI] [PubMed] [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Immunol Met. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nam K, Kimura T, Kishida A. Controlling coupling reaction of EDC and NHS for preparation of type-I collagen gels using ethanol/water co-solvents. Macromol Biosci. 2008;8:32–37. doi: 10.1002/mabi.200700206. [DOI] [PubMed] [Google Scholar]
- Ohkawa K, Cha DI, Kim H, Nishida A, Yamamoto H. Electrospinning of chitosan. Macromol Rapid Commun. 2004;25:1600–1605. doi: 10.1002/marc.200400253. [DOI] [Google Scholar]
- Olde Damink LHH, Dijkstra M, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J. Glutaraldehyde as a cross-linking agent for collagen-based biomaterials. J Mater Sci Mater Med. 1995;6:460–472. doi: 10.1007/BF00123371. [DOI] [Google Scholar]
- Olde Damink LHH, Dijkstra PJ, van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J. Influence of ethylene oxide gas treatment on the in vitro degradation behavior of dermal sheep type-I collagen. J Biomed Mater Res. 1995;29:149–155. doi: 10.1002/jbm.820290203. [DOI] [PubMed] [Google Scholar]
- Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–1887. doi: 10.3390/ma3031863. [DOI] [Google Scholar]
- Pavia DL, Lampman GM, Kriz GS. Introduction to spectroscopy. 3. USA: Thomson Learning, Inc; 2001. [Google Scholar]
- Peng L, Cheng XR, Wang JW, Xu DX, Wang GE. Preparation and evaluation of porous chitosan/collagen scaffolds for periodontal tissue engineering. J Bioact Compat Polym. 2006;21:207–220. doi: 10.1177/0883911506065100. [DOI] [Google Scholar]
- Petite H, Rault I, Huc A, Menasche P, Herbage D. Use of the acyl azide method for cross-linking type-I collagen-rich tissues such as pericardium. J Biomed Mater Res. 1990;24:179–187. doi: 10.1002/jbm.820240205. [DOI] [PubMed] [Google Scholar]
- Rinaudo M. New way to crosslink chitosan in aqueous solution. Eur Polym J. 2010;46:1537–1544. doi: 10.1016/j.eurpolymj.2010.04.012. [DOI] [Google Scholar]
- Saito H, Murabayashi S, Mitamura Y, Taguchi T. Characterization of alkali-treated type-I collagen gels prepared by different crosslinkers. J Mater Sci Mater Med. 2008;19:1297–1305. doi: 10.1007/s10856-007-3239-7. [DOI] [PubMed] [Google Scholar]
- Schauer CL, Chen MS, Chatterley M, Eisemann K, Welsh ER, Price R, Schoen PE, Ligler FS. Color changes in chitosan and poly(allyl amine) films upon metal binding. Thin Solid Films. 2003;434:250–257. doi: 10.1016/S0040-6090(03)00055-5. [DOI] [Google Scholar]
- Schiffman JD, Schauer CL. Cross-linking chitosan nanofibers. Biomacromolecules. 2007;8:594–601. doi: 10.1021/bm060804s. [DOI] [PubMed] [Google Scholar]
- Schmidt CE, Baier JM. Acellular vascular tissues: natural biomaterials for tissue repair and tissue engineering. Biomaterials. 2000;21:2215–2231. doi: 10.1016/S0142-9612(00)00148-4. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram N, Ravikumar T, Babu M. Comparative physico-chemical and in vitro properties of fibrillated collagen scaffolds from different sources. J Biomater Appl. 2004;18:247–264. doi: 10.1177/0885328204040945. [DOI] [PubMed] [Google Scholar]
- Sheu MT, Huang JC, Yeh GC, Ho HO. Characterization of type-I collagen gel solutions and type-I collagen matrices for cell culture. Biomaterials. 2001;22:1713–1719. doi: 10.1016/S0142-9612(00)00315-X. [DOI] [PubMed] [Google Scholar]
- Tamada J, Langer R. The development of polyanhydrides for drug delivery applications. J Biomater Sci Polym Ed. 1992;3:315–353. doi: 10.1163/156856292X00402. [DOI] [PubMed] [Google Scholar]
- Tual C, Espuche E, Escoubes M, Domard AJ. Transport properties of chitosan membranes: influence of crosslinking. Polym Sci Part B: Polym Phys. 2000;38:1521–1529. doi: 10.1002/(SICI)1099-0488(20000601)38:11<1521::AID-POLB120>3.0.CO;2-#. [DOI] [Google Scholar]
- Usha R, Ramasami T. Effect of crosslinking agents (basic chromium sulfate and formaldehyde) on the thermal and thermomechanical stability of rat tail tendon type-I collagen fibre. Thermochim Acta. 2000;356:59–66. doi: 10.1016/S0040-6031(00)00518-9. [DOI] [Google Scholar]
- Vijayaraghavan R, Thompson BC, MacFarlane DR, Ramadhar K, Surianarayanan M, Aishwarya S, Sehgal PK. Biocompatibility of choline salts as crosslinking agents for collagen based biomaterials. Chem Comm. 2010;46:294–296. doi: 10.1039/B910601D. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- Weadock KS, Miller EJ, Bellincampi LD, Zawadsky JP, Dunn MG. Physical crosslinking of type-I collagen fibers: comparison of ultraviolet irradiation and dehydrothermal treatment. J Biomed Mater Res. 1995;29:1373–1379. doi: 10.1002/jbm.820291108. [DOI] [PubMed] [Google Scholar]
- Wei YC, Hudson SM, Mayer JM, Kaplan DL. The cross linking of chitosan fibers. Polym Sci Part A: Polym Chem. 1992;30:2187–2193. doi: 10.1002/pola.1992.080301013. [DOI] [Google Scholar]