Abstract
Th17 cells, a CD4+ T-cell subset, produce interleukin (IL)-17, a pro-inflammatory cytokine that has been shown to be involved in several forms of infectious and noninfectious uveitis. Here, we explore the roles of this IL in uveitic disorders as well as in experimental autoimmune uveitis, the possible pathogenic implications of several cytokines associated with IL-17 and analyze the current outcomes and goals for drugs aiming for the IL-17 pathway.
Key words: Interferon-γ, interleukin-6, interleukin-17, interleukin-23, interleukin-27, secukinumab, transforming growth factor-β, uveitis
Th17 cells are a subset of CD4+ T-cells responsible for the production of interleukin (IL)-17. This T-cell subset is responsible for the production of IL-17A, a pro-inflammatory cytokine involved in several autoimmune diseases.
Both IL-6 and transforming growth factor (TGF)-β are necessary for Th17 differentiation and IL-17 expression; they feature complementary roles since the sole presence of TGF-β induces T-regulatory (Treg) cell production from CD4+ T-cells.[1,2,3] IL-6 needs to be present to promote the differentiation of a Th17 cell subset in addition to concurrent Treg cell inhibition.[4] Although IL-6 and TGF-β have synergistic roles in Th17 cell differentiation, IL-23 is also important for Th17 cell expansion and activation.[1] By contrast, interferon (IFN)-γ and IL-27 show a regulatory role in uveitis induction through the suppression of Th17 differentiation [Fig. 1].[5,6]
Figure 1.
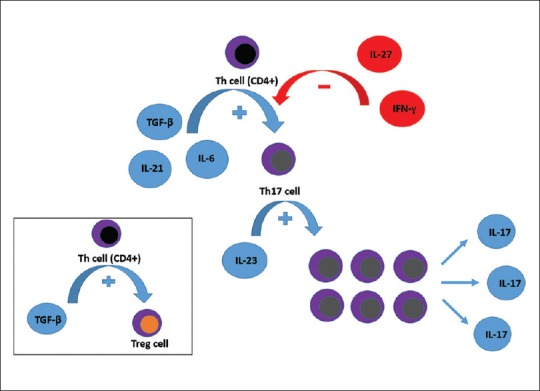
Both interleukin-6 and interleukin-21 can cooperate with transforming growth factor-β to promote Th17 differentiation from CD4+ Th-cells while interleukin-23 is important for Th17 cell expansion and activation. Interferon-γ and interleukin-27 have regulatory roles through the suppression of Th17 differentiation. Note that the sole presence of transforming growth factor-β induces Treg cell production from CD4+ Th-cells
IL-17 induces the production of other inflammatory cytokines such as IL-6, granulocyte colony-stimulating factor (CSF), granulocyte-macrophage-CSF, IL-1, TGF-β, and tumor necrosis factor (TNF)-α; chemokines such as monocyte chemoattractant protein-1, cytokine-induced neutrophil chemoattractant, and macrophage inflammatory protein-2; prostaglandin E2; intercellular adhesion molecule-1; and matrix metalloproteinases. It is also involved in the recruitment of neutrophils, monocytes, and Th1 cells acting with other inflammatory cytokines to induce inflammation in target tissues.[7]
Th1 cells have been previously implicated in the induction of uveitis in experimental autoimmune uveitis (EAU), but since then, several authors have reported the crucial role of IL-17-producing Th17 cells in the genesis of experimental uveitis in mice. They have pointed that treatment with anti-IL-17 antibodies reduces the induction and severity of uveitis in EAU. Moreover, the degree of intraocular inflammation was reduced in IL-17 knockout mice,[8] and although both Th1 and Th17 cells are activated during EAU,[6,8] it has been proposed that Th17 cells are responsible for retinal inflammation in the early stages of uveitis whereas Th1 expression is increased during the late phases and resolution of the disease.[6] It has thus been shown that Th1 and Th17 cells are implicated in the genesis of EAU and may be synergistic with each other, having definitive functions at different stages of the disease.
In addition to its obvious pro-inflammatory role in the induction of EAU, IL-17 expression is also increased in the human peripheral blood of patients suffering from autoimmune and infectious uveitis.
This review aims to explore the roles of this IL in uveitic disorders as well in EAU. The possible pathogenic implications of several cytokines associated with IL-17 induction and suppression will also be discussed, and the current outcomes and goals for drugs aiming for the IL-17 pathway are analyzed. A systematic literature search was carried out using the PubMed and EMBASE databases with the search terms, “uveitis,” “interleukin-17,” and IL-17,” until July 2015. Bibliographies of the retrieved literature were manually searched.
Experimental Autoimmune Uveitis
IL-17 is crucial in the development of EAU in mice, as well as in other models of experimental autoimmune disorders.[6,8] In EAU, the intraocular expression of IL-17 is elevated in mice with uveitis and it promotes the release of inflammatory mediators from ARPE-19 cells, disrupting the retinal pigment epithelium barrier function.[9]
There is abundant information regarding the role of Th17 cells and IL-17, its main pro-inflammatory cytokine, in the induction and clinical severity of EAU, although some studies have inconsistently determined the specific timing of increased IL-17 expression in this condition.
The Th1 cytokine, IFN-γ, has a complex role in EAU and can be protective, suggesting that IL-17 has an important inflammatory effect given that IFN-γ can inhibit IL-17 expression through Th17 suppression. In a study addressing the different functions of Th1 and Th17 cells in EAU, IL-17-/- mice showed no difference in terms of uveitis severity concerning the early stages of the disease, and after anti-IFN-γ and anti-IL-4 antibody treatment and concomitant increase in Th17 expression, only the late stages of the disease were affected, showing the aforementioned differential response of Th1 and Th17 cell subsets during the clinical course of EAU.[8]
In another study concerning the monophasic and relapsing phases of EAU, the authors concluded that IFN-γ-producing cells may be responsible for initiating recurrence in the relapsing form of EAU. Conversely, IL-17-producing cells might be implicated in the primary mechanisms related to intraocular inflammation, thus exhibiting different roles in the monophasic and relapsing forms of the disease.[10]
Previous data have also shown that even though a Th1- or Th17-driven response can initiate uveitis in an animal model, IL-17 plays a critical role in the induction of EAU; moreover, anti-IL-17 treatment can reduce the severity of antigen-induced autoimmune uveitis in mice. The same authors suggested that IL-23 may be a key element in the early stages of intraocular inflammation, and that its function may even surpass the expansion and activation of Th17 cells. Furthermore, there seems to be an exacerbation of the Th17 response and increased disease severity with inhibition of the IL-12-IFN-γ pathway, reinforcing the previously described regulatory activities of these Th1 cytokines in Th17 differentiation.[11]
Noninfectious Uveitis
Increased IL-17 expression has been proven in several studies measuring its aqueous humor or peripheral blood concentration in noninfectious uveitic disorders [Table 1], as well as in other autoimmune diseases such as rheumatoid arthritis,[23] ankylosing spondylitis,[24] inflammatory bowel disease,[25] systemic lupus erythematosus,[26] and psoriasis,[27] in which circulating IL-17 was accessed.
Table 1.
Studies demonstrating increased interleukin-17 expression in several autoimmune uveitic disorders that are not cited in the text
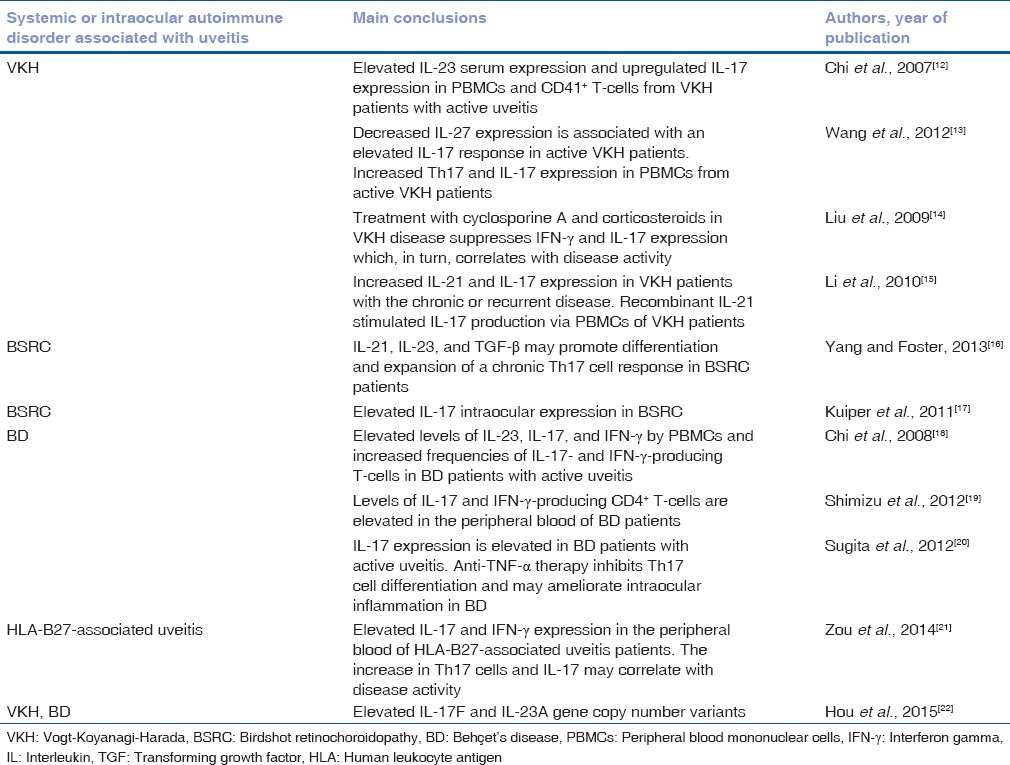
In a study measuring IL-15, IL-17, IFN-γ, TNF-α, and IL-10 levels in the aqueous humor of patients with active autoimmune uveitis from different etiologies, including Behçet's disease (BD), Vogt–Koyanagi–Harada (VKH) disease, and human leukocyte antigen-B27-associated-uveitis, IL-17 levels were found to be higher in these patients than in control subjects, and these levels correlated with disease activity.[28] These results were confirmed by another study that measured IL-17 levels in the peripheral blood of a large group of patients with autoimmune uveitis with and without associated systemic disease. IL-17 levels were elevated in the serum of uveitis patients when compared to controls, and they also served as a marker for disease activity.[29] When comparing intraocular and serum IL-17 levels, one study used a multiplex immunoassay to determine IL-17 levels in paired aqueous humor and serum samples of birdshot retinochoroidopathy (BSRC) patients.[17] The authors not only found an increased intraocular IL-17 expression in BSRC patients when compared to age-related cataract controls but also found that IL-17 levels in aqueous humor were higher than their concurrent serum levels.
All of these studies suggest that IL-17 may be used as a possible biomarker in autoimmune uveitis. Moreover, a recent study revealed a novel association between IL-17A locus polymorphisms and panuveitis, suggesting that IL-17A may also be a possible genetic risk factor for panuveitis.[30]
Infectious Uveitis
IL-17 has been implicated in the development of toxoplasmic encephalitis in an animal model, and since then, there have been studies that have analyzed its role in the induction and maintenance of intraocular inflammation caused by an infectious agent. In a previous study, upregulation of IL-17 levels in mice chronically infected with Toxoplasma gondii that lacked the IL-27 receptor was observed, and the authors suggested a protective role of IL-27 in the inflammation that follows toxoplasmic infection.[31] Moreover, the inflammatory roles of Th1 and Th17 cytokines and the regulatory roles of IL-10, TGF-β, and IL-27 in the immunologic responses following toxoplasmic infection have been reinforced in a later study concerning cytokine regulation in T. gondii infection.[32]
One study analyzed the inflammatory cytokine and chemokine levels in the aqueous humor of patients with toxoplasmic and viral uveitis. The authors found that IL-17 was upregulated in the majority of screened patients with toxoplasmic uveitis when compared to cataract patients without uveitis or with those with noninfectious intermediate uveitis. The authors suggested that the presence of elevated levels of IL-17 in the intraocular fluids of these patients may be a clue to a possible autoimmune mechanism that contributes to ocular inflammation following infection.[33] These findings were reinforced in a study addressing various cytokine levels in the aqueous humor of patients with uveitis from various etiologies – infectious and noninfectious. Patients with toxoplasmic infection and subsequent uveitis had significantly increased intraocular levels of IL-17A, whereas patients with viral uveitis showed increased IL-1β and IL-10 expression.[34] The authors postulate that this different cytokine pattern in intraocular fluids may help understand disease pathogenesis and even be used as a specific diagnostic marker for each etiology.
Another recent study highlighted the upregulation of IL-17 expression in patients with acute ocular toxoplasmosis and demonstrated a pathogenic role for this cytokine in the development of intraocular inflammation after T. gondii infection. The authors proposed a possible in vivo therapeutic approach for toxoplasmic retinochoroiditis based on the use of local anti-IL-17 antibodies.[35]
Interleukin-17 Induction
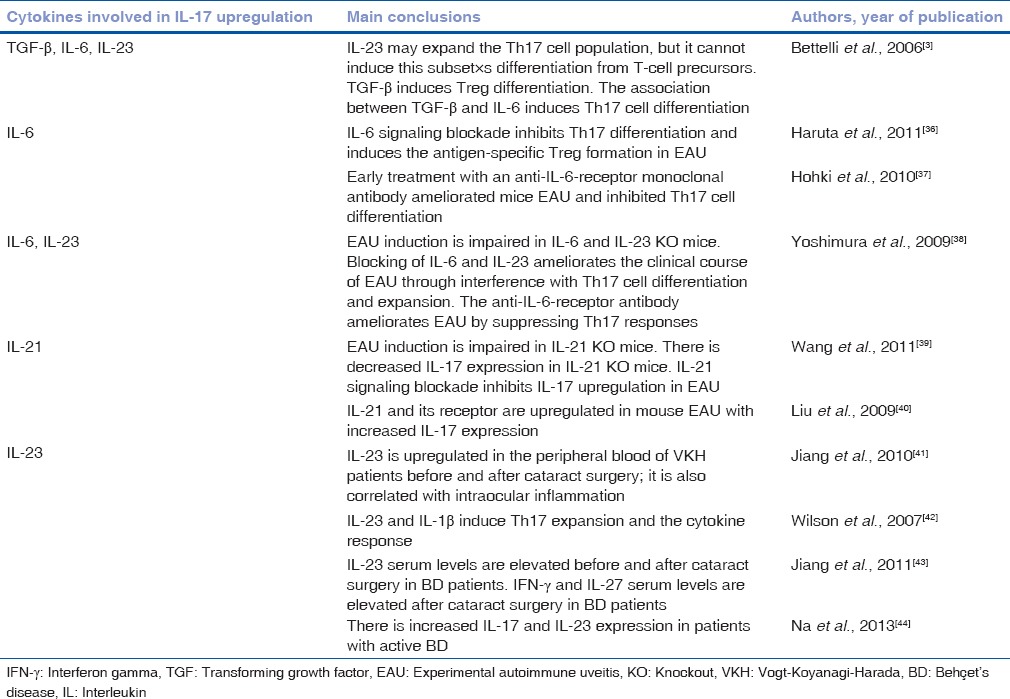
Several studies have shown that TGF-β and IL-6 are important cytokines in Th17 differentiation, and that IL-23 and IL-21 may also be crucial in Th17 cell expansion and activation [Table 2 and Fig. 1].
Table 2.
Cytokines involved in interleukin-17 induction
Interleukin-17 suppression
Interferon-γ
Antigen-specific IL-17 production is exacerbated in the absence of IFN-γ increasing the severity of uveitis in a murine model of spondyloarthritis. In this model, IL-17 blocking ameliorated intraocular inflammation in IFN-γ knockout mice, suggesting the regulatory function that this cytokine plays in IL-17 production.[45] Moreover, IL-17 expression was increased in the peripheral blood of patients with uveitis and scleritis and also in an EAU model. In the animal model used in this study, IFN-γ upregulated IL-27 expression by retinal cells which, in turn, inhibited Th17 cell proliferation, reducing uveitis severity and altering its clinical course.[6] Another study involving an EAU animal model also showed that IFN-γ ameliorated uveitis through Th1 and Th17 cell inhibition and IL-10 upregulation.[46] IFN-γ may also play a key role in infectious disease pathogenesis since the presence of anti-IFN-γ autoantibodies seems to be associated with nontuberculous mycobacterial infections.[47]
Interleukin-27
IL-27 is known as a regulatory cytokine that is capable of inhibiting the differentiation of precursor cells into their Th17 phenotype. By blocking the production of Th17 cells, this cytokine has been implicated in the suppression of experimental autoimmune encephalomyelitis and EAU.[6,48] A previous investigation addressing the regulatory cytokines, IL-27 and IL-10, in the development of uveitis found that mice retinal microglia and ganglion cells constitutively expressed IL-27, and that IL-27 production was elevated during uveitis.[49]
IL-27 expression was found to be decreased in BD patients with active uveitis,[50] and another study demonstrated elevated levels of IL-27 after cataract surgery in VKH patients, indicating that the upregulation of this cytokine during the 1st month following surgery might serve a protective function in postoperative inflammation.[41] Similar results were found in BD patients after cataract surgery as increased IL-27 serum levels were also evident during the postoperative period. These IL-27 levels correlated both with uveitis severity and IFN-γ levels.[43]
Therapeutic Targets
Anti-interleukin-17
An anti-IL-17 monoclonal antibody was used in the treatment of chronic noninfectious uveitis in patients with posterior and anterior segment disease. The treatment featured effects comparable to those of historical control patients with chronic noninfectious uveitis that were treated with infliximab. Specifically, this treatment was associated with improvements in visual acuity and the reduction of intraocular inflammation.[51]
Another study conducted of an animal model of spondyloarthritis demonstrated that IL-17 blockade reduced intraocular inflammation and peripheral arthritis although there was suspicion of retinal toxicity.[45] Treatment of EAU with the anti-IL-17 antibody in rats also showed a reduction in intraocular inflammation and of T-cell proliferation during disease onset.[52]
Secukinumab
Secukinumab, a human monoclonal antibody against IL-17, was used in a Phase III trial for the treatment of chronic noninfectious uveitis associated with BD; however, the study's primary outcome was not met (SHIELD study). Some authors have since claimed that the use of secukinumab in chronic uveitis has not been correctly assessed to date, since the other two trials enrolling patients with active and inactive noninfectious uveitis not associated with BD (INSURE and ENDURE) were interrupted following the termination of SHIELD. In the SHIELD study, although there was no significant difference between the treated patients and controls, there was a reduction in the use of concomitant immunosuppressant drugs and a trend toward a reduction in recurrence.[53]
Recently, another study demonstrated good results, in terms of both efficacy and safety, using intravenous secukinumab in the treatment of 37 patients with active noninfectious intermediate, posterior, or panuveitis who required corticosteroid-sparing immunosuppressive therapy.[48] This may suggest that there is still an opportunity for Phase III clinical trials using this or other monoclonal antibodies against IL-17 (or the IL-17 receptor) for noninfectious uveitis treatment.
Interleukin-17 Pathway
In addition to IL-17, there are other possible candidates for the treatment of noninfectious uveitis.
Ustekinumab, a monoclonal antibody directed at the IL-23 and IL-12 p40 subunit, was already approved for the treatment of psoriasis and it may be a future option for the treatment of uveitis patients, since a different study addressing the therapeutic effect of STA-5326 (another IL-12/IL-23 inhibitor) showed EAU clinical improvement and suppressed IL-17 production.[54]
Tocilizumab, a monoclonal antibody directed against the IL-6 receptor, has been successfully used in the treatment of refractory uveitis[55] and was approved for the treatment of rheumatoid and systemic juvenile idiopathic arthritis. It is currently being studied for use in noninfectious and juvenile idiopathic arthritis-associated uveitis (clinicaltrials.gov). Another monoclonal antibody against the IL-6 receptor, sarilumab, is also currently being studied in a Phase II trial accessing subcutaneous administration in patients with active noninfectious intermediate, posterior, or panuveitis (clinicaltrials.gov).
Conclusions
Various studies have highlighted the importance of the IL-17 pathway in the development of various forms of infectious and noninfectious uveitis. Although the main Phase III trial addressing the use of a human anti-IL-17 monoclonal antibody in Behçet's-associated uveitis has failed to meet its primary outcomes, it seems adequate to continue the investigation concerning neutralization of the primary IL-17 pathway cytokines in the treatment of chronic noninfectious uveitis. Furthermore, the IL-17 pathway may also serve as a therapeutic target for infectious uveitis like toxoplasmic retinochoroiditis although always in addition to infection targeted therapy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T (H) 17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 2.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 4.Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, et al. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105:18460–5. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, et al. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–8. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 7.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimura T, Sonoda KH, Miyazaki Y, Iwakura Y, Ishibashi T, Yoshimura A, et al. Differential roles for IFN-gamma and IL-17 in experimental autoimmune uveoretinitis. Int Immunol. 2008;20:209–14. doi: 10.1093/intimm/dxm135. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Yang P, Li F, Kijlstra A. The effects of Th17 cytokines on the inflammatory mediator production and barrier function of ARPE-19 cells. PLoS One. 2011;6:e18139. doi: 10.1371/journal.pone.0018139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufmann U, Diedrichs-Möhring M, Wildner G. Dynamics of intraocular IFN-γ, IL-17 and IL-10-producing cell populations during relapsing and monophasic rat experimental autoimmune uveitis. PLoS One. 2012;7:e49008. doi: 10.1371/journal.pone.0049008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J Exp Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi W, Yang P, Li B, Wu C, Jin H, Zhu X, et al. IL-23 promotes CD4+T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol. 2007;119:1218–24. doi: 10.1016/j.jaci.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Tian Y, Lei B, Xiao X, Ye Z, Li F, et al. Decreased IL-27 expression in association with an increased Th17 response in Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci. 2012;53:4668–75. doi: 10.1167/iovs.12-9863. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Yang P, Lin X, Ren X, Zhou H, Huang X, et al. Inhibitory effect of Cyclosporin A and corticosteroids on the production of IFN-gamma and IL-17 by T cells in Vogt-Koyanagi-Harada syndrome. Clin Immunol. 2009;131:333–42. doi: 10.1016/j.clim.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Yang P, Liu X, Wang C, Hou S, Kijlstra A. Upregulation of interleukin 21 and promotion of interleukin 17 production in chronic or recurrent Vogt-Koyanagi-Harada disease. Arch Ophthalmol. 2010;128:1449–54. doi: 10.1001/archophthalmol.2010.265. [DOI] [PubMed] [Google Scholar]
- 16.Yang P, Foster CS. Interleukin 21, interleukin 23, and transforming growth factor ß1 in HLA-A29-associated birdshot retinochoroidopathy. Am J Ophthalmol. 2013;156:400–6.e2. doi: 10.1016/j.ajo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Kuiper JJ, Mutis T, de Jager W, de Groot-Mijnes JD, Rothova A. Intraocular interleukin-17 and proinflammatory cytokines in HLA-A29-associated birdshot chorioretinopathy. Am J Ophthalmol. 2011;152:177–82.e1. doi: 10.1016/j.ajo.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 18.Chi W, Zhu X, Yang P, Liu X, Lin X, Zhou H, et al. Upregulated IL-23 and IL-17 in Behçet patients with active uveitis. Invest Ophthalmol Vis Sci. 2008;49:3058–64. doi: 10.1167/iovs.07-1390. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu J, Takai K, Fujiwara N, Arimitsu N, Ueda Y, Wakisaka S, et al. Excessive CD4+ T cells co-expressing interleukin-17 and interferon-γ in patients with Behçet's disease. Clin Exp Immunol. 2012;168:68–74. doi: 10.1111/j.1365-2249.2011.04543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugita S, Kawazoe Y, Imai A, Yamada Y, Horie S, Mochizuki M. Inhibition of Th17 differentiation by anti-TNF-alpha therapy in uveitis patients with Behçet's disease. Arthritis Res Ther. 2012;14:R99. doi: 10.1186/ar3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou W, Wu Z, Xiang X, Sun S, Zhang J. The expression and significance of T helper cell subsets and regulatory T cells CD4+ CD25+ in peripheral blood of patients with human leukocyte antigen B27-positive acute anterior uveitis. Graefes Arch Clin Exp Ophthalmol. 2014;252:665–72. doi: 10.1007/s00417-014-2567-9. [DOI] [PubMed] [Google Scholar]
- 22.Hou S, Liao D, Zhang J, Fang J, Chen L, Qi J, et al. Genetic variations of IL17F and IL23A show associations with Behçet's disease and Vogt-Koyanagi-Harada syndrome. Ophthalmology. 2015;122:518–23. doi: 10.1016/j.ophtha.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Cascão R, Moura RA, Perpétuo I, Canhão H, Vieira-Sousa E, Mourão AF, et al. Identification of a cytokine network sustaining neutrophil and Th17 activation in untreated early rheumatoid arthritis. Arthritis Res Ther. 2010;12:R196. doi: 10.1186/ar3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei Y, Pan F, Gao J, Ge R, Duan Z, Zeng Z, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–73. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 25.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: Implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–93. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Chen X, Liu Z, Yue Q, Liu H. Expression of Th17 cytokines in skin lesions of patients with psoriasis. J Huazhong Univ Sci Technolog Med Sci. 2007;27:330–2. doi: 10.1007/s11596-007-0329-1. [DOI] [PubMed] [Google Scholar]
- 28.El-Asrar AM, Struyf S, Kangave D, Al-Obeidan SS, Opdenakker G, Geboes K, et al. Cytokine profiles in aqueous humor of patients with different clinical entities of endogenous uveitis. Clin Immunol. 2011;139:177–84. doi: 10.1016/j.clim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Jawad S, Liu B, Agron E, Nussenblatt RB, Sen HN. Elevated serum levels of interleukin-17A in uveitis patients. Ocul Immunol Inflamm. 2013;21:434–9. doi: 10.3109/09273948.2013.815786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mucientes A, Márquez A, Cordero-Coma M, Martín-Villa JM, Gorroño-Echebarría MB, Blanco R, et al. Specific association of IL17A genetic variants with panuveitis. Br J Ophthalmol. 2015;99:566–70. doi: 10.1136/bjophthalmol-2014-306106. [DOI] [PubMed] [Google Scholar]
- 31.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 32.Gaddi PJ, Yap GS. Cytokine regulation of immunopathology in toxoplasmosis. Immunol Cell Biol. 2007;85:155–9. doi: 10.1038/sj.icb.7100038. [DOI] [PubMed] [Google Scholar]
- 33.Lahmar I, Abou-Bacar A, Abdelrahman T, Guinard M, Babba H, Ben Yahia S, et al. Cytokine profiles in toxoplasmic and viral uveitis. J Infect Dis. 2009;199:1239–49. doi: 10.1086/597478. [DOI] [PubMed] [Google Scholar]
- 34.Sauer A, Villard O, Creuzot-Garcher C, Chiquet C, Berrod JP, Speeg-Schatz C, et al. Intraocular levels of interleukin 17A (IL-17A) and IL-10 as respective determinant markers of toxoplasmosis and viral uveitis. Clin Vaccine Immunol. 2015;22:72–8. doi: 10.1128/CVI.00423-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauer A, Pfaff AW, Villard O, Creuzot-Garcher C, Dalle F, Chiquet C, et al. Interleukin 17A as an effective target for anti-inflammatory and antiparasitic treatment of toxoplasmic uveitis. J Infect Dis. 2012;206:1319–29. doi: 10.1093/infdis/jis486. [DOI] [PubMed] [Google Scholar]
- 36.Haruta H, Ohguro N, Fujimoto M, Hohki S, Terabe F, Serada S, et al. Blockade of interleukin-6 signaling suppresses not only Th17 but also interphotoreceptor retinoid binding protein-specific Th1 by promoting regulatory T cells in experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 2011;52:3264–71. doi: 10.1167/iovs.10-6272. [DOI] [PubMed] [Google Scholar]
- 37.Hohki S, Ohguro N, Haruta H, Nakai K, Terabe F, Serada S, et al. Blockade of interleukin-6 signaling suppresses experimental autoimmune uveoretinitis by the inhibition of inflammatory Th17 responses. Exp Eye Res. 2010;91:162–70. doi: 10.1016/j.exer.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura T, Sonoda KH, Ohguro N, Ohsugi Y, Ishibashi T, Cua DJ, et al. Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatology (Oxford) 2009;48:347–54. doi: 10.1093/rheumatology/ken489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Yu CR, Kim HP, Liao W, Telford WG, Egwuagu CE, et al. Key role for IL-21 in experimental autoimmune uveitis. Proc Natl Acad Sci U S A. 2011;108:9542–7. doi: 10.1073/pnas.1018182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Xu Y, Wang J, Li H. Upregulated IL-21 and IL-21 receptor expression is involved in experimental autoimmune uveitis (EAU) Mol Vis. 2009;15:2938–44. [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang S, Liu X, Luo L, Qu B, Huang X, Xu L, et al. Elevated serum IL-23 correlates with intraocular inflammation after cataract surgery in patients with Vogt-Koyanagi-Harada disease. Br J Ophthalmol. 2010;94:1078–82. doi: 10.1136/bjo.2009.169052. [DOI] [PubMed] [Google Scholar]
- 42.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–7. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 43.Jiang S, Liu X, Luo L, Qu B, Huang X, Lin Y, et al. Serum levels of Th17-related cytokines in Behcet disease patients after cataract surgery. Mol Vis. 2011;17:1425–30. [PMC free article] [PubMed] [Google Scholar]
- 44.Na SY, Park MJ, Park S, Lee ES. Up-regulation of Th17 and related cytokines in Behçet's disease corresponding to disease activity. Clin Exp Rheumatol. 2013;31(3 Suppl 77):32–40. [PubMed] [Google Scholar]
- 45.Kezic JM, Glant TT, Rosenbaum JT, Rosenzweig HL. Neutralization of IL-17 ameliorates uveitis but damages photoreceptors in a murine model of spondyloarthritis. Arthritis Res Ther. 2012;14:R18. doi: 10.1186/ar3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun M, Yang Y, Yang P, Lei B, Du L, Kijlstra A. Regulatory effects of IFN-ß on the development of experimental autoimmune uveoretinitis in B10RIII mice. PLoS One. 2011;6:e19870. doi: 10.1371/journal.pone.0019870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, et al. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175:4769–76. doi: 10.4049/jimmunol.175.7.4769. [DOI] [PubMed] [Google Scholar]
- 48.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 49.Lee YS, Amadi-Obi A, Yu CR, Egwuagu CE. Retinal cells suppress intraocular inflammation (uveitis) through production of interleukin-27 and interleukin-10. Immunology. 2011;132:492–502. doi: 10.1111/j.1365-2567.2010.03379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang C, Tian Y, Ye Z, Kijlstra A, Zhou Y, Yang P. Decreased interleukin 27 expression is associated with active uveitis in Behçet's disease. Arthritis Res Ther. 2014;16:R117. doi: 10.1186/ar4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med. 2010;2:52ra72. doi: 10.1126/scitranslmed.3001107. [DOI] [PubMed] [Google Scholar]
- 52.Zhang R, Qian J, Guo J, Yuan YF, Xue K. Suppression of experimental autoimmune uveoretinitis by Anti-IL-17 antibody. Curr Eye Res. 2009;34:297–303. doi: 10.1080/02713680902741696. [DOI] [PubMed] [Google Scholar]
- 53.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, et al. Secukinumab in the treatment of noninfectious uveitis: Results of three randomized, controlled clinical trials. Ophthalmology. 2013;120:777–87. doi: 10.1016/j.ophtha.2012.09.040. [DOI] [PubMed] [Google Scholar]
- 54.Keino H, Watanabe T, Sato Y, Niikura M, Wada Y, Okada AA. Therapeutic effect of the potent IL-12/IL-23 inhibitor STA-5326 on experimental autoimmune uveoretinitis. Arthritis Res Ther. 2008;10:R122. doi: 10.1186/ar2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Papo M, Bielefeld P, Vallet H, Seve P, Wechsler B, Cacoub P, et al. Tocilizumab in severe and refractory non-infectious uveitis. Clin Exp Rheumatol. 2014;32(4 Suppl 84):S75–9. [PubMed] [Google Scholar]


