Abstract
We make several eye movements per second when we explore a visual scene. Each eye movement sweeps the scene's projection across the retina and changes its representation in retinotopic areas of the visual cortex, but we nevertheless perceive a stable world. Here we investigate the neuronal correlates of visual stability in the primary visual cortex. Monkeys were trained to make two saccades along a single curve and to ignore another, distracting curve. Attention enhanced neuronal responses to the entire relevant curve before the first saccade. This response enhancement was rapidly reestablished after the saccade, although the image was shifted across the primary visual cortex. We argue that this fast postsaccadic restoration of the attentional response enhancement contributes to the stability of vision across eye movements, and reduces the impact of saccades on visual cognition.
It is still unclear how visual information acquired before a saccade is integrated with information acquired after the saccade. Early theories of transsaccadic integration postulated that perceptual stability across eye movements is achieved by use of a transsaccadic memory buffer. Information from successive fixations can be accumulated in such a buffer if the changes in eye position, which are reflected by oculomotor signals, are taken into account (1-3). Subsequent theories, however, argued that such a memory buffer is unnecessary and suggested that the visual system can afford to be amnesic about the presaccadic scene, because visual representations can be rebuild rapidly after each saccade (4, 5). This view has received some support from neurophysiological data indicating that visual processing indeed proceeds rapidly. High-level visual areas and even frontal areas, which are separated from the retina by many synapses, are activated within 100 ms after stimulus presentation (6-8). However, some tasks that involve shifts of visual attention, like visual search (9) or curve tracing (10-12), require more time than the typical intersaccadic interval. For these tasks, a complete interruption of processing by saccades would be detrimental. Therefore, some recent theories take a less extreme view and suggest that the visual system is not entirely amnesic across saccades, because information about attended objects is maintained (13-17).
These psychophysical theories yield different predictions about the fate of object representations in areas of the visual cortex during saccades. Studies in the lateral intraparietal area (18, 19) and the frontal eye fields (20, 21) provide support for theories that include a transsaccadic buffer by demonstrating that the retinotopic coordinates of objects are remapped across saccades. Neurons in these areas signal that a saccade will bring a visual object into their receptive field (RF) shortly before saccade execution, a phenomenon that has been called “predictive remapping.” They also respond if a saccade brings the RF onto a location of an object that has just been removed from sight. Thus, neurons in these areas maintain a spatially accurate representation of the retinotopic location of visual objects and even of their memory traces across saccades. The percentage of neurons with these properties is high in the lateral intraparietal area, but decreases in earlier visual areas (22).
It is not clear at present, however, whether remapping across saccades occurs for all objects or whether it occurs only for objects that receive attention (as suggested by, for example, ref. 19). Therefore, in this study, we compare the fate of attended and unattended objects across saccades by recording from the primary visual cortex (V1) of monkeys. The monkeys are trained to trace a curve through the visual field. This curve-tracing task has a number of advantages for the study of transsaccadic integration. First, there are many versions of the task that take more time than the interval between saccades; this makes it unlikely that saccades reset processing. Second, the natural strategy is to make a sequence of saccades along the path that has to be traced (23). Third, the spatial distribution of attention has been measured during this task in human subjects. Subjects solve it by directing attention to all segments of the curve that has to be traced (11, 12).
A neurophysiological correlate of curve-tracing has been found in area V1, where responses to a traced curve are enhanced relative to responses to other, distracting curves (24, 25). This attentional response enhancement does not occur during the initial stimulus-driven neuronal responses, which have a latency of ≈40 ms (8), but occur after a delay of >100 ms (24, 26), which suggests the involvement of feedback from higher visual areas. In this study, we exploit this temporal separation between bottom-up and top-down influences to investigate transsaccadic integration.
Methods
Behavioral Task. Two macaque monkeys took part in the experiments. They were trained on a curve-tracing task in which they had to make two saccades along a single curve (Fig. 1A). A trial started once the monkey's eye position was within a 1° square window centred on a 0.2° fixation point (FP1) in the middle of a cathode ray tube (CRT) monitor (70 Hz). After 300 ms, a stimulus appeared that consisted of two white curves (luminance, 24 cd/m2) on a black background (luminance, 0.5 cd/m2) and three red circles subtending 0.4° of visual angle. One of the curves connected FP1 to two of the circles, and this curve will be called target curve. The other curve was connected to the third circle and served as a distractor. The animals were rewarded for making a sequence of two saccades into 1.5° square windows around FP2 and around the circle at the end of the target curve.
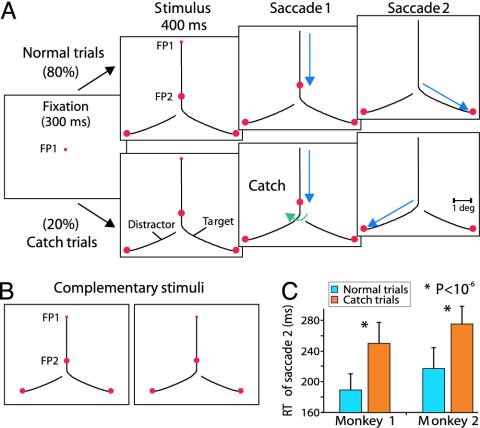
Fig. 1.
Curve-tracing task with two eye movements and behavioral performance. (A) Sequence of events during the normal (Upper) and catch (Lower) trials. The monkey had to fixate on FP1 until the FP disappeared. Then, a saccade had to be made to FP2, followed by a second saccade to the circle located at the end of the same curve (target curve). On catch trials, the first saccade triggered a small change in the stimulus that switches the connection to the previously unconnected curve (green arrow). Visual events for the complementary stimulus are not shown. (B) A pair of complementary stimuli. (C) Mean reaction time of the second saccade on normal (blue) and catch (orange) trials for both monkeys, pooled across complementary stimuli. Error bars indicate standard deviation.
Recording Technique and Data Analysis. All experimental procedures complied with the National Institutes of Health Guide for Care and Use of Laboratory Animals, and were approved by the institutional animal care and use committee of the Royal Netherlands Academy of Arts and Sciences. Standard surgical and electrophysiological techniques were used to record multiunit activity in area V1 (refs. 24 and 25; see also supporting information, which is published on the PNAS web site). Eye movements were monitored with the double magnetic induction method (27), which has a resolution better than 0.l°, and digitized at a rate of >500 Hz. RFs are small in area V1, and therefore, differences in eye position between conditions may influence firing rates. In case of multiunit recording, it is possible that individual neurons contribute differently to the recording at different eye positions. We applied a stratification procedure to factor out these eye position effects and ensure that we compared the same cells between conditions (supporting information).
In some experiments, the RF of a recording site was brought onto one of the curves by a saccade. The onset of visual stimulation in this saccade condition depends on the timing and trajectory of the saccade, and was determined on a trial-by-trial basis by computing the moment at which the leading edge of the RF came in contact with one of the curves (see supporting information). Note that this analysis takes the shape of the RF, the shape of the curves, and the eye trajectory in each individual trial into account. Neuronal responses in the saccade condition were always normalized to the onset condition, which is why the peak responses are not always equal to 1.
The target curve usually evoked stronger responses than the distractor curve. This effect was quantified with the modulation index (MI). MI was defined as the difference in response strength normalized to the average response: (T - D)/([T + D]/2), where T and D are responses to the target and distractor curve, respectively, after subtraction of spontaneous firing rate.
To determine the latency of attentional response enhancement, a curve was fitted to the difference between responses to the target and distractor curve. The significance of the difference in latencies between conditions was determined by using a Monte Carlo procedure (see supporting information).
Results
Psychophysical Performance. We first investigated whether the monkeys traced the entire target curve, including segments that were only relevant for the second saccade, while they fixated on FP1. Monkeys had to fixate on FP1 for 400 ms, then FP1 was extinguished and a first saccade was made to the second point on the target curve (FP2) (Fig. 1 A). At the end of this saccade, FP2 was turned off and a second saccade had to be made to the circle that was at the end of the target curve. The monkeys could make this second saccade immediately. Complementary stimuli, for which the animals had to make a different sequence of saccades, were randomly interleaved (Fig. 1B). Saccadic reaction time for the second saccade was defined as the time interval between the offset of FP2 and the onset of the saccade (see supporting information). The animals were successful in >95% of the trials, and the mean reaction time across monkeys for the second saccade was 203 ms (blue bars, Fig. 1C). To probe whether the monkeys traced the entire target curve before the first saccade, catch trials were included (20% of the trials). On these catch trials, the stimulus changed during the first saccade because, unexpectedly, the connection between FP2 and the original target for the second eye movement was switched (Fig. 1 A Lower). To respond correctly on such a catch trial, the monkeys had to make the second saccade to the other circle, which was now connected to FP2. Catch trials introduce a mismatch between visual information before and after the saccade, which should have no effect if the monkeys are amnesic about the presaccadic stimulus. However, on catch trials, average performance dropped to 70%, and both monkeys fixated significantly longer at FP2 than on normal trials, with an average increase in saccadic reaction time of 60 ms (P < 10-6, Wilcoxon test for both monkeys; mean latency, 263 ms; orange bars, Fig. 1C). This is direct evidence for transsaccadic integration, because some information regarding the identity of the target curve is apparently maintained across the first saccade. One possibility is that the monkeys “mentally” trace the entire target curve before the first saccade and that attention is also directed to segments of this curve that are distal to FP2. Furthermore, if attention can remain on these curve segments across the saccade, this would explain why subsequent performance is superior if the stimulus remains the same.
When attention is directed to the target curve, neuronal responses to this curve are enhanced in area V1 (24, 25). The psychophysical results therefore yield two predictions regarding the pattern of neuronal activity in area V1. The first prediction is that neuronal responses to the entire target curve are enhanced before the first saccade. The second prediction is that attention is maintained on the target curve during the saccade, although its representation is shifted in area V1 and other retinotopic areas.
Neuronal Activity in Area V1 Before the First Saccade. A first electrophysiological experiment investigated whether attention enhances neuronal firing rates in area V1 evoked by the entire target curve before the first saccade. Again, the monkeys made two saccades along the target curve, but in this experiment they had to hold fixation for 500 ms on each FP (performance was >95% correct). Stimuli were configured in such a way that the neurons' RF was located on a curve segment distal to FP2, while the monkey was still fixating on FP1 (Fig. 2A). Thus, the segment in the RF was only relevant for the planning of the second saccade. Fig. 2 B and C compares the responses of two simultaneously recorded groups of V1 neurons during fixation at FP1. Activity evoked by the target curve was significantly stronger than activity evoked by the distractor curve at both recording sites (site a, P < 0.005; site b, P < 10-6, U test). A total of 55 recording sites with a RF distal to FP2 were tested. Likewise, at the population level, responses to the target curve were stronger than those to the distractor curve (Fig. 2D; P < 10-7, paired t test). Note that the curve segment in the RF was always identical for the two stimuli and that the enhanced responses therefore presumably reflect visual attention that is directed to the target curve (11, 12, 24, 25). This finding suggests that, while the monkey fixates on FP1, attention is directed to all contour segments of the target curve, including those that are only relevant for the second saccade.
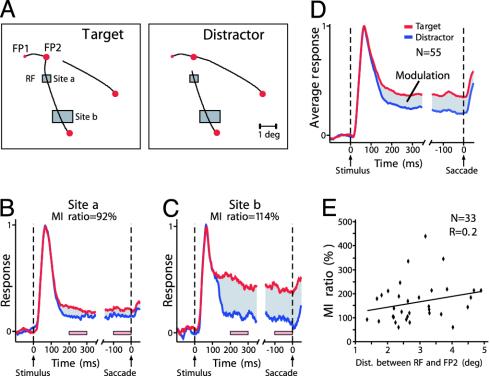
Fig. 2.
Attentional modulation of V1 responses evoked by curve segments relevant for the second saccade, while the monkey fixates on FP1. (A) Complementary stimuli. Rectangles depict the locations of V1 receptive fields (RFs) at two recording sites. At stimulus onset, the RFs were located on segments of the target curve (Left) or the distractor curve (Right). (B and C) Responses to target and distractor curve at these recording sites as the monkey fixated on FP1. Responses are aligned on stimulus onset and the start of the saccade. Red and blue traces show the responses to target and distractor curve, respectively. Gray area, difference between these responses. Pink bars, time windows used to compute the modulation index. The distance between the RF and FP2 was 1.3° for site a and 3.7° for site b. (D) Population responses averaged across 55 recording sites. (E) Distribution of the modulation index ratio of sites (33 of 55) with a significant response modulation as a function of the distance between the RF and the first saccade target (FP2).
A number of psychophysical studies (28-30) suggested that attention needs to be focused onto the endpoint of a saccade, just before saccade execution. In the context of the curve-tracing task, this finding might imply that attention constricts on FP2 in an interval preceding the saccade. We therefore investigated whether the attentional response enhancement to the target curve was reduced before the saccade, especially at RF locations far from FP2. We quantified the strength of the attentional effect in two time intervals by computing the MI. The MI from 200 to 300 ms after stimulus onset (MIstim) was compared with that in a 100-ms interval preceding the saccade (MIpresacc), by computing the MI ratio = MIpresacc/MIstim × 100%. Thus, values of the MI ratio <100% indicate that the attentional effect decreases before the saccade. The MI ratios of the recording sites in Fig. 2 B and C were both close to 100% (site a, 92%; site b, 114%), although the RF of site b was much further from FP2 than that of site a. We repeated this analysis for all sites (n = 33 of 55) that discriminated between the target and distractor curve (P < 0.05) (Fig. 2E). The geometric mean of the MI ratios was 146%, but there was substantial variability of MI ratios across the population. Unexpectedly, the MI ratio did not depend on the distance between the RF and FP2. If anything, the MI ratio tended to increase with increasing distance between RF and FP2, but this trend was not significant (linear regression, R = 0.2, two-tailed t test, t = 1.14, df = 31, P > 0.1). Thus, these results indicate that attentional modulation in area V1 is maintained on the entire target curve and does not constrict onto the endpoint of the upcoming saccade.
Postsaccadic Remapping of Attentional Modulation. We have shown that the monkeys trace the entire target curve while fixation is at FP1, and the first, psychophysical experiment suggested that attention can remain on this curve during the first saccade. We next investigated how transsaccadic integration influences neuronal responses in area V1 in a further electrophysiological experiment with two conditions (four stimuli) that were randomly interleaved. In the first condition (onset condition), a segment of a curve appeared in the RF because of stimulus onset, while the monkey fixated on FP1 (Fig. 3A Upper). In the second condition (saccade condition), the stimulus was outside the RF while the monkey fixated on FP1. The first saccade brought the RF onto one of the curves (Fig. 3A Lower). Thus, in the saccade condition, the contour segment that entered into the RF could have been assigned to either target or distractor curve during the previous fixation. If information is carried across a saccade, it is expected that the visual response or the attentional modulation of this response occurs earlier in the saccade condition than in the onset condition.
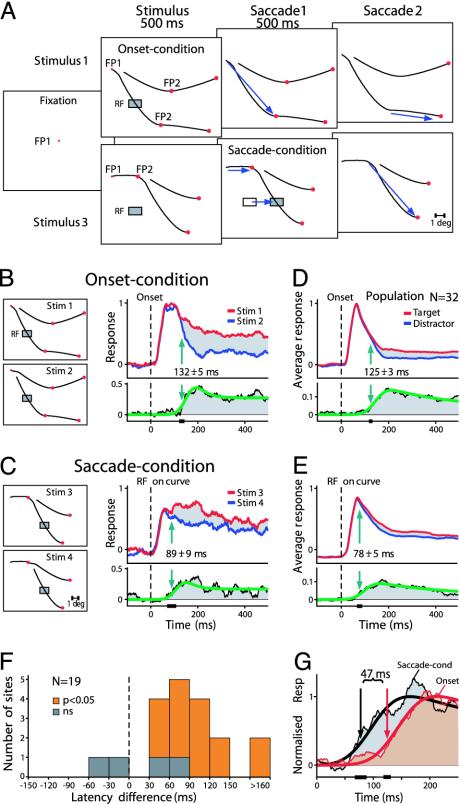
Fig. 3.
Remapping of attentional modulation in area V1. (A) Sequence of events in the onset and saccade condition. When the stimulus appeared, fixation had to be maintained on FP1 for 500 ms, and then a saccade was made to FP2. A second saccade was made after 500 ms of fixation at FP2. In the onset condition (Upper), a curve appeared in the RF (shaded rectangle) during fixation at FP1. In the saccade condition (Lower), the RF was unstimulated during fixation at FP1, but was shifted onto a contour segment by the first saccade. Note that the shape of the curve segment that entered into the RF was identical to that in the onset condition. FP2 was located at a distance of 3° or 3.3° from FP1. Blue arrows illustrate the saccades and corresponding RF shift. (B) Complementary stimuli in the onset condition. (Left) The RF (eccentricity, 4.2°) was on the target curve for stimulus 1 and on the distractor curve for stimulus 2. (Right) Response to the target curve (red) was stronger than the response to the distractor curve (blue). Green arrow, latency of modulation. (Lower) A curve that was fitted to the response difference, to determine the latency of response enhancement. Black bar on x axis, 95% confidence interval of the latency. (C) Complementary stimuli in the saccade condition. The RF shifted onto the target (stim 3) or distractor curve (stim 4) during the first saccade to FP2. Responses are aligned on the moment that the RF touches a contour segment because of the first saccade (see Methods). (D and E) Population response (n = 32) in the onset (D) and saccade (E) conditions. Note different scaling in D Lower and E Lower. (F) Distribution of the difference in latency of the response enhancement between the onset and saccade condition for sites that showed a significant response enhancement in both conditions. Positive values indicate that the latency was earlier in the saccade compared with the onset condition. Cases with a significant latency difference (P < 0.05) are shown in orange. (G) Comparison of the temporal profile of the enhancement of the population response in the onset (red) and saccade condition (gray). To facilitate the comparison, response differences have been normalized similarly. Best fitting curves to the response differences are superimposed.
Fig. 3B depicts the location of the RF of a recording site in area V1 relative to the stimuli while the animal fixated on FP1 in the onset condition. The response to the target curve of stimulus 1 was stronger than the response to the distractor curve of stimulus 2 (P < 10-6, U test). The latency of attentional modulation was 132 ± 5 ms (SD), a value that was derived by fitting a function to the difference between the two responses (Fig. 3B Lower; see also supporting information).
In the saccade condition (Fig. 3C), the neurons started to respond when their RF was brought onto the target (stimulus 3) or distractor curve (stimulus 4) by the first saccade. This response was somewhat (17%) weaker than the response in the onset condition and had a latency that was 8 ms shorter (33 ms vs. 41 ms; see supporting information). This slight difference may be caused by the different way of RF stimulation in the saccade condition (31). After the saccade, the response to the target curve was stronger than the response to the distractor curve (P < 2.10-5, U test). Remarkably, attentional modulation in the saccade condition started 43 ms earlier than in the onset condition (89 ± 9 vs. 132 ± 5 ms; P < 0.01), suggesting that some information about the identity of the target curve is indeed maintained across the saccade.
Similar results were also observed across the population of recording sites. Fig. 3 D and E shows the population response (n = 32) in the onset and saccade condition, respectively. In both conditions, the response to the target curve was significantly stronger than the response to the distractor curve (P < 10-5, paired t test). We note that the spontaneous activity in the saccade condition before the RF falls on a curve is negative (Fig. 3E); this effect is caused by the appearance of a stimulus outside the RF while the monkey is fixating on FP1 (Fig. 3A), which causes a slight suppression of the spontaneous activity (see supporting information). The visual response in the saccade condition has a latency of 40 ms at the population level, a value close to the response latency in the onset condition (37 ms). Thus, transsaccadic integration does not influence the latency of visual responses in area V1. In contrast, there is a clear influence on the onset of attentional response modulation. In the onset condition, attentional modulation occurred at a latency of 125 ± 3 ms, whereas it occurred significantly earlier in the saccade condition, at a latency of 78 ± 5 ms (P < 0.01). Indeed, when the time course of the attentional effect is directly compared between conditions (Fig. 3G), its earlier appearance in the saccade condition is evident. To gain insight into the consistency of these effects, we analyzed the differences in the latency of attentional modulation at individual recording sites (Fig. 3F). In this analysis, only cases that exhibited a significantly (P < 0.05) stronger response to the target curve than to the distractor curve in both conditions (n = 19 of 32) were included. The latency of the attentional effect was significantly earlier (P < 0.05) in the saccade condition for the majority of these sites (15 of 19).
One difference between the two conditions is that the monkey always made his first saccade to the same target in the saccade condition, but chose between two targets in the onset condition. Can this difference account for the timing of the attentional effects? To investigate this possibility, we computed the latency of the response enhancement in Fig. 2D. Here, the stimulus was similar to the saccade condition of the present experiment, but the RFs were stimulated during fixation at FP1. The attentional effect occurred 123 ± 9 ms after the onset of the stimulus, which is similar to the timing of the effect in the onset condition of the present experiment (125 ± 3 ms). Thus, the timing of the attentional effect does not depend on the number of targets from which the monkey chooses while planning his first saccade. This finding implies that the difference in the timing of the attentional effect between the onset and saccade condition in Fig. 3 is not caused by the difference in the number of potential saccade targets.
Neuronal Activity on Catch Trials. The early attentional modulation after a saccade may reflect a trace of the attentive processing that was carried out during the previous fixation. However, the way in which the RFs were stimulated differed between the two conditions of the previous experiment. In the saccade condition, the RFs were brought onto a curve segment by a saccade, whereas the segment abruptly appeared within the RF in the onset condition. We therefore carried out an additional experiment in which we manipulated the utility of the information that could be maintained across the saccade; however, the stimulus in the RF was held constant. Specifically, we used catch trials (20% of trials) to introduce a mismatch between the pre- and postsaccadic stimuli. To our knowledge, the neurophysiological consequences of changes in the stimulus during saccades have not been investigated previously. Fig. 4A illustrates the procedure for a recording site in area V1. On normal trials (Fig. 4A Upper), the first saccade brought the RF onto a contour segment that had been assigned to either the target or distractor curve during the first fixation at FP1. Attentional modulation (P < 10-6, U test) appeared early, at a latency of 81 ± 4 ms after the RF came in contact with one of the curves (Fig. 4B Upper). Fig. 4A Lower illustrates the sequence of events during catch trials. On “catch-tar” trials, the RF fell on a contour segment that had been assigned to the distractor curve but that unexpectedly became part of the target curve because of a change in the stimulus during the first saccade. In contrast, on “catch-dist” trials, the RF fell on a contour segment that unexpectedly became part of the distractor curve. The early and late part of the neuronal responses differed on these catch trials (Fig. 4B Lower). Early activity was strongest on catch-dist trials (orange area, P < 0.05, U test); this finding is consistent with the presaccadic stimulus configuration, which predicted that the saccade would bring the RF onto the target curve. Thus, this early modulation reflects a trace of the attentive processing carried out during the previous fixation. However, at a latency of 163 ± 8 ms, neuronal activity started to reflect the changed configuration, because responses became strongest on catch-tar trials (P < 10-6, U test).
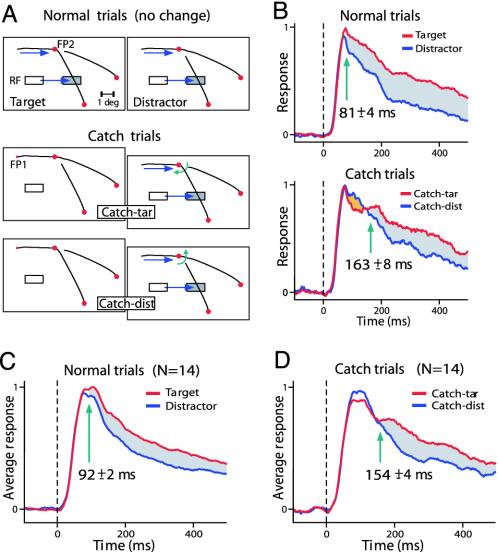
Fig. 4.
Early postsaccadic response modulation is determined by the presaccadic stimulus configuration. (A) The saccade to FP2 brought the RF onto the target or distractor curve. On normal trials, no change occurred in the stimulus. On catch trials, however, a change triggered by the first saccade introduced a mismatch between the pre- and the postsaccadic stimulus. White and shaded rectangles depict pre- and postsaccadic location of the RF, respectively. RF eccentricity, 3°. (B) Response to the target and distractor curve on normal (Upper) and catch (Lower) trials. Green arrow, latency of modulation. Note that on catch trials, the initial response is strongest on catch-dist trials (orange area). After some delay, the response on catch-tar trials (red line) becomes strongest. (C and D) Population response (n = 14) on normal trials (C) and on catch trials (D).
Similar effects were observed across a population of V1 recording sites (n = 14) tested in this paradigm. On normal trials, the response was strongest if the first saccade brought the RF onto the target curve (P < 2.10-4, paired t test) (Fig. 4C). The latency of modulation was 92 ± 2 ms. On catch trials, however, the early response was strongest if it was evoked by a contour segment that unexpectedly became part of the distractor curve during the first saccade (Fig. 4D). The response to the curve that changed into the target curve became strongest (P < 5.10-4, paired t test) at a longer latency of 154 ± 4 ms, 62 ms later than on normal trials (P < 0.001). It is remarkable that the early part of the response after a saccade depends on the presaccadic, not on the postsaccadic, stimulus. This finding implies that contours that receive attention before a saccade are still attended immediately after it. The relatively long latency of the switch in the response enhancement on catch trials presumably reflects the time required to reallocate attention to the relevant curve (see also ref. 32).
Preview Effects Within a Single Fixation. Some theories pose that the mechanisms that transfer information across saccades overlap with other memory systems that maintain information in the absence of a stimulus (33). We tested this possibility in an additional experiment (fully described in supporting information) where the monkeys saw a target and a distractor curve for 200 ms. The stimulus disappeared for 500 ms and then reappeared at the same location, while the monkey held fixation. When the stimulus appeared for the first time, the response enhancement evoked by the target curve in area V1 had a latency of 133 ms, but when the same stimulus reappeared for the second time, the response enhancement had a significantly shorter latency of 104 ms (P < 0.05). Thus, attentional modulation of V1 responses also occurs earlier when advance information about the stimulus is provided within the same fixation. This finding suggests that transsaccadic memory indeed overlaps with other memory systems. Nevertheless, in the saccade task, the pre- and postsaccadic stimuli activate different V1 neurons. Our results therefore imply that transsaccadic integration is equipped with a mechanism that takes the saccade metrics into account, so that it can route information about the location of the target curve to the correct neurons in area V1. The retinotopic coordinates of attended objects are thereby updated across the saccade.
Discussion
In normal vision, it is safe to assume that images do not change abruptly during eye movements (4). Here we have shown that monkeys also take this regularity into account, because changes in the image during a saccade deteriorate their performance and increase the reaction time of subsequent saccades. This finding implies that monkeys do not only process information for the upcoming saccade, but also for saccades that will be carried out thereafter (34, 35). We confirmed this finding by recording from area V1, where neuronal responses to contour segments that have to be traced for the second saccade are enhanced before the first saccade is made (Fig. 2). Processing of information that is relevant for the second saccade is useful, because this information is not lost during the first saccade.
Transsaccadic integration provides a mechanism that rapidly redirects attention to the relevant curve segments after the saccade, which is reflected by a fast restoration of attentional modulation in area V1. Attentional modulation after a saccade occurs 47 ms earlier than if a new stimulus is presented. We hypothesize that the early postsaccadic response enhancement is mediated by feedback connections from higher-order visual areas that maintain a trace of the presaccadic stimulus. This explains why early postsaccadic V1 responses reflect attentive processing of the presaccadic stimulus, even if this stimulus changed during the saccade. On such catch trials, it takes an additional 62 ms before responses to the target curve are enhanced in area V1 (Fig. 4D). Interestingly, this value matches the lengthening of the subsequent fixation duration in the psychophysical experiment (Fig. 1C). Our data also demonstrate that transsaccadic integration does not have a systematic influence on the latency of the visual responses. This finding implies that transsaccadic integration primarily affects top-down but not bottom-up inputs to area V1.
There are a number of potential sources of feedback that may be responsible for the rapid postsaccadic restoration of the response enhancement in area V1. Recent studies demonstrated that the retinotopic location of salient (18-20) and behaviorally relevant (19, 21, 36) objects is remapped across saccades in areas of the parietal and frontal cortex. Neurons in these areas have predictive responses, i.e., they discharge before a saccade that will bring their RF onto an item of interest. Thus, these neurons maintain a spatially accurate representation of stimuli across saccades and may provide feedback to area V1, thereby allowing a fast restoration of attentional effects after the saccade. The present results show that attentional enhancement of neuronal responses in area V1 is not predictive, because it does not occur before the saccade. Instead, attentional effects appear to be gated by the visual input and only occur once the RFs are brought onto one of the curves.
There are further potential sources of feedback that may rescue additional features of the target curve across saccades, and that thereby may contribute to the earlier expression of attentional effects in area V1. Some neurons in the parietal and premotor cortex, for example, encode the location of visual objects relative to the body or head, irrespective of eye position (37, 38). These cells need not update their activity across a saccade, because saccades do not change the stimulus' location relative to the body. They may therefore store the location of the target curve across a saccade and provide feedback to lower visual areas after its completion. A similar argument holds for neurons in areas that are involved in object recognition, such as area V4 and the inferotemporal cortex. These neurons enhance their activity if attention is directed to their preferred shape (39-41), and they can store this shape during a saccade (42, 43). Moreover, inferotemporal neurons have large RFs, and their shape-tuning is relatively independent of the object's location on the retina (44). Thus, neurons that are tuned to the shape of the target curve may maintain their activity across saccades and provide feedback to those V1 cells whose RFs are brought onto this curve. Attention to the shape of the target curve would explain why the whole curve is remapped in retinotopic coordinates (Fig. 2), and not only the locations that serve as saccade targets, as might have been predicted from psychophysical studies (28-30). We note that feedback can also account for the early attentional modulation if the same stimulus appears twice during a single fixation. Neurons in higher visual areas maintain activity that is evoked by attended stimuli across memory delays, and they can therefore rapidly feed back to area V1 when the stimulus reappears.
The present study goes beyond earlier work by directly measuring the effects of presaccadic attentional processing on postsaccadic processing, both behaviorally and physiologically. Our results rule out theories that suggest that the visual system is entirely amnesic about the presaccadic scene (4, 5), but support other theories that propose that information about attended objects is maintained across saccades (13-16). The data show that the spatial profile of attention in retinotopic coordinates is rapidly restored after a saccade. We propose that this mechanism is useful for the construction of a stable representation of the visual scene despite eye movements, and is essential for cognitive tasks that require more time than the typical intersaccadic interval. The early postsaccadic attentional modulation would allow the subject to continue curve-tracing immediately after the saccade.
Supplementary Material
Acknowledgments
We thank Drs. F. Bremmer and V. A. F. Lamme for providing valuable comments on the manuscript. We thank K. Brandsma and J. C. de Feiter for technical assistance. This study was supported by a grant of the McDonnell Pew Program in Cognitive Neuroscience and a Human Frontier Science Program grant (to P.R.R.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RF, receptive field; V1, primary visual cortex; FP, fixation point; MI, modulation index.
References
- 1.McConkie, G. W. & Rayner, K. (1976) in Theoretical Models and Processes in Reading, eds. Singer, H. & Ruddell, R. B. (International Reading Institute, Newark, DE), pp. 137-162.
- 2.Jonides, J., Irwin, D. E. & Yantis, S. (1982) Science 215, 192-194. [DOI] [PubMed] [Google Scholar]
- 3.Breitmeyer, B. G. (1984) Visual Masking: An Interactive Approach (Oxford Univ. Press, New York).
- 4.O'Regan, J. K. (1992) Can. J. Psychol. 46, 461-488. [DOI] [PubMed] [Google Scholar]
- 5.Bridgeman, B., Van der Heijden, A. H. C. & Velichkovsky, B. M. (1994) Behav. Brain Sci. 17, 247-292. [Google Scholar]
- 6.Oram, M. W. & Perrett, D. I. (1992) J. Neurophysiol. 68, 70-84. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe, S. J., Fize, D. & Marlot, C. (1996) Nature 381, 520-522. [DOI] [PubMed] [Google Scholar]
- 8.Lamme, V. A. F. & Roelfsema, P. R. (2000) Trends Neurosci. 23, 571-579. [DOI] [PubMed] [Google Scholar]
- 9.Treisman, A. M. & Gelade, G. (1980) Cognit. Psychol. 12, 97-136. [DOI] [PubMed] [Google Scholar]
- 10.McCormick, P. A. & Jolicoeur, P. (1992) J. Exp. Psychol. Hum. Percept. Perform. 18, 72-89. [DOI] [PubMed] [Google Scholar]
- 11.Roelfsema, P. R., Lamme, V. A. F. & Spekreijse, H. (2000) Vision Res. 40, 1385-1411. [DOI] [PubMed] [Google Scholar]
- 12.Scholte, H. S., Spekreijse, H. & Roelfsema, P. R. (2001) Vision Res. 41, 2569-2580. [DOI] [PubMed] [Google Scholar]
- 13.McConkie, G. W. & Currie, C. B. (1996) J. Exp. Psychol. Hum. Percept. Perform. 22, 563-581. [DOI] [PubMed] [Google Scholar]
- 14.Deubel, H., Bridgeman, B. & Schneider, W. X. (1998) Vision Res. 38, 3147-3159. [DOI] [PubMed] [Google Scholar]
- 15.Irwin, D. E. & Gordon, R. (1998) Vis. Cognit. 5, 127-155. [Google Scholar]
- 16.Currie, C. B., McConkie, G. W., Carlson-Radvansky, L. A. & Irwin, D. E. (2000) Percept. Psychophys. 62, 673-683. [DOI] [PubMed] [Google Scholar]
- 17.Posner, M. I. & Cohen, Y. (1984) in Attention and Performance, eds. Bouma, H. & Bouwhuis, D. (Erlbaum, Hillsdale, NJ), Vol. 10, pp. 531-557. [Google Scholar]
- 18.Duhamel, J. R., Colby, C. L. & Goldberg, M. E. (1992) Science 255, 90-92. [DOI] [PubMed] [Google Scholar]
- 19.Gottlieb, J. P., Kusunoki, M. & Goldberg, M. E. (1998) Nature 391, 481-484. [DOI] [PubMed] [Google Scholar]
- 20.Umeno, M. M. & Goldberg, M. E. (1997) J. Neurophysiol. 78, 1373-1383. [DOI] [PubMed] [Google Scholar]
- 21.Tian, J., Schlag, J. & Schlag-Rey, M. (2000) Exp. Brain Res. 130, 433-440. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura, K. & Colby, C. L. (2002) Proc. Natl. Acad. Sci. USA 99, 4026-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowe, D. A., Averbeck, B. B., Chafee, M. V., Anderson, J. H. & Georgopoulos, A. P. (2000) J. Cognit. Neurosci. 12, 813-827. [DOI] [PubMed] [Google Scholar]
- 24.Roelfsema, P. R., Lamme, V. A. F. & Spekreijse, H. (1998) Nature 395, 376-381. [DOI] [PubMed] [Google Scholar]
- 25.Roelfsema, P. R. & Spekreijse, H. (2001) Neuron 31, 853-863. [DOI] [PubMed] [Google Scholar]
- 26.Posner, M. I. & Gilbert, C. D. (1999) Proc. Natl. Acad. Sci. USA 96, 2585-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bour, L. J., van Gisbergen, J. A., Bruijns, J. & Ottes, F. P. (1984) IEEE Trans. Biomed. Eng. 31, 419-427. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman, J. E. & Subramaniam, B. (1995) Percept. Psychophys. 57, 787-795. [DOI] [PubMed] [Google Scholar]
- 29.Kowler, E., Anderson, E., Dosher, B. & Blaser, E. (1995) Vision Res. 35, 1897-1916. [DOI] [PubMed] [Google Scholar]
- 30.Deubel, H. & Schneider, W. X. (1996) Vision Res. 36, 1827-1837. [DOI] [PubMed] [Google Scholar]
- 31.Gawne, T. G. & Woods, J. M. (2003) NeuroReport 14, 105-109. [DOI] [PubMed] [Google Scholar]
- 32.Motter, B. C. (1994) J. Neurosci. 14, 2190-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irwin, D. E. (1996) Curr. Dir. Psychol. Sci. 5, 94-100. [Google Scholar]
- 34.McPeek, R. M. & Keller, E. L. (2001) Vision Res. 41, 785-800. [DOI] [PubMed] [Google Scholar]
- 35.McPeek, R. M. & Keller, E. L. (2002) J. Neurophysiol. 87, 1805-1815. [DOI] [PubMed] [Google Scholar]
- 36.Mazzoni, P., Bracewell, R. M., Barash, S. & Andersen, R. A. (1996) J. Neurophysiol. 76, 1439-1456. [DOI] [PubMed] [Google Scholar]
- 37.Duhamel, J. R., Bremmer, F., BenHamed, S. & Graf, W. (1997) Nature 389, 845-848. [DOI] [PubMed] [Google Scholar]
- 38.Graziano, M. S. A., Yap, G. S. & Gross, C. G. (1994) Science 266, 1054-1057. [DOI] [PubMed] [Google Scholar]
- 39.Moran, J. & Desimone, R. (1985) Science 229, 782-784. [DOI] [PubMed] [Google Scholar]
- 40.Connor, C. E., Preddie, D. C., Gallant, J. L. & Van Essen, D. C. (1997) J. Neurosci. 17, 3201-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chelazzi, L., Miller, E. K., Duncan, J. & Desimone, R. (1993) Nature 363, 345-347. [DOI] [PubMed] [Google Scholar]
- 42.Moore, T., Tolias, A. S. & Schiller, P. H. (1998) Proc. Natl. Acad. Sci. USA 95, 8981-8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tolias, A. S., Moore, T., Smirnakis, S. M., Tehovnik, E. J., Siapas, A. G. & Schiller, P. H. (2001) Neuron 29, 757-767. [DOI] [PubMed] [Google Scholar]
- 44.Tovée, M. J., Rolls, E. T. & Azzopardi, P. (1994) J. Neurophysiol. 72, 1049-1060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.