Summary
In this work total anthocyanin content (TAC), total flavonoid content (TFC), total phenolic content (TPC) and minerals found in five black glutinous rice cultivars (MS, SK, PY, PC and KK) from Thailand were analyzed. The antioxidant activity of anthocyanin-rich black glutinous rice bran extracts against nitric oxide radical (NO˙), superoxide radical (O2˙Ż) and lipid peroxyl radical (LOO˙) was also determined. Potential chemopreventive property of rice bran extract was screened based on cellular bioassays for phase II detoxification enzyme induction. Quinone reductase (QR) induction in murine hepatoma cells was used as a marker for this effect. Rice bran extract of cultivar KK had the highest TAC, of SK the highest TFC and of PC the highest TPC. The best antioxidants against NO˙, O2˙Ż and LOO˙ were cultivars KK, MS, and SK, respectively. Overall, TAC, TFC and TPC had a combinatorial effect on the antioxidant activities of all extracts; none of them dominated. Minerals may not play a role in the antioxidant activity of the extracts because most correlations between them and the antioxidant activity were unpredictable. However, rice bran contained high mass fractions of some essential minerals on dry mass basis, including Zn (103–133 µg/g), Se (11–18 µg/g) and Cu (3.8–7.1 µg/g). Chemopreventive study indicated that PC cultivar was the most potent chemopreventor with the lowest concentration of an inducer needed to double the QR activity (CD value) of 0.7 µg/mL. These findings showed that black glutinous rice bran is rich in phytochemicals and some essential minerals, and has a potential chemopreventive property.
Key words: anthocyanins, black glutinous rice bran, mineral content, antioxidant activity, quinone reductase induction
Introduction
Health concerns have led to an increased trend in the adoption of phytochemical-rich diets. Phytochemicals such as phenolics and flavonoids (including anthocyanins) have been reported to have potential of preventing reactive oxygen species (ROS)-induced diseases like diabetes, cancer and cardiovascular diseases (1–3). Phenolics, flavonoids and anthocyanins have been found in various plants and rice (Oryza sativa L.) is one of the predominant sources of phytochemicals consumed daily, especially in Asian countries.
Glutinous rice with different bran colours, such as brown, red and black is commonly consumed in Thailand (4, 5) as a regular steamed rice or in sweet dishes. Among these rice types, black glutinous rice has received more interest as it has been found to contain higher contents of phenolics, flavonoids and especially anthocyanins than others (6). These phytochemicals showed potential for inducing phase II detoxification enzymes, a group of important cell defense enzymes that convert reactive and harmful molecules into inactive water-soluble ones which are more easily eliminated (7). Hence, consumption of black rice or black rice products increases cell defensive actions and prevents ROS-induced diseases.
Rice is also a rich source of minerals, including Ca, Cu, Fe, K, Mg, Mn, Se and Zn (8, 9). It was found in an in vitro study that Mn contributes to the antioxidant activity of rice (10). Concentrations of phenolics, flavonoids, anthocyanins and minerals are different depending on variety, growing conditions and soil components (11, 12). Various cultivars of black glutinous rice are grown in the northern region of Thailand and they may have different antioxidant components that need to be investigated. For these reasons, antioxidant activities of rice bran may not only depend on the contents of phenolics, flavonoids and anthocyanins, but also on the mineral content. However, few studies reporting the correlation between antioxidant activity and mineral content have been published (10, 13).
In this study, the contents of phytochemicals, including phenolics, flavonoids and anthocyanins, and minerals in black glutinous rice bran samples from Thailand were identified. In vitro antioxidant activities against NO˙, O2˙Ż and LOO˙ of black glutinous rice bran extracts were examined. The relationship between antioxidant activity and the content of phytochemicals and some trace minerals was evaluated. Potential chemopreventive properties of the extracts were tested in order to evaluate the health--protective benefits of black glutinous rice bran.
Materials and Methods
Chemicals and reagents
Mineral standards were purchased from Merck (Darmstadt, Germany). Commercial and natural antioxidant standards were of reagent grade and were purchased from Sigma-Aldrich (St. Louis, Mo, USA), together with Folin-Ciocalteu reagent, phenazine methosulphate (PMS), β-nicotinamide adenine dinucleotide (NADH), sodium nitroprusside dihydrate (SNP), Griess-Ilosvay’s nitrite reagent, nitroblue tetrazolium chloride (NBT) and all other reagents.
Samples
Five black glutinous rice cultivars grown in the northern Thailand: Kaw Kum Doi Mooser (MS) from Tak Province, Kaw Kum Doi Saked (SK) from Chiang Mai Province, Kaw Kum Phayao (PY) from Phayao Province, Kaw Neaw Dum Phichit (PC) from Phichit Province and Kaw Kum Khao Kho (KK) from Phetchabun Province were used. Rough rice samples harvested from October to December 2011 were purchased directly from farmers. All samples were stored at (4±1) °C until milling.
Extraction of active compounds from black glutinous rice bran
Black glutinous rice bran powder was defatted with hexane and dried at 40 °C in a hot air oven for 3 h. Moisture content of the defatted rice bran powder was analyzed using the loss on drying test. A mass of 0.1 g of the bran powder was extracted with 1 M HCl methanol/water (15:85, by volume) using magnetic stirrer (C-MAG HS7; IKA® Werke GmbH & Co. KG., Staufen, Germany). Anthocyanin-rich black glutinous rice bran extract solution was concentrated using a rotary evaporator (Rotavapor® R-100; BÜCHI Labortechnik AG, Flawil, Switzerland) at (40±2) °C and the volume was adjusted to 25 mL with methanol before storage at (–20±1) °C for further study.
Total anthocyanin content analysis
Total anthocyanin content (TAC) was quantified using the pH differential method reported by Giusti and Wrolstad (14). Briefly, 100 µL of rice bran extract were filled up to 1 mL either with 0.025 M potassium chloride buffer (pH=1.0) or 0.4 M sodium acetate buffer solution (pH=4.5). Absorbance of each mixture was measured at 510 and 700 nm using a UV-Vis spectrophotometer (DR/ 4000 U; Hach, Loveland, CO, USA) and total absorbance was calculated using the following equation:
where A510 nm and A700 nm are the absorbances measured at 510 and 700 nm, respectively. TAC (in mg/L) was calculated using the following equation:
where A is the absorbance from Eq. 1, M is the molecular mass of cyanidin-3-O-glucoside (M=449.2), DF is the dilution factor (100 µL of sample is diluted to 1 mL, DF=10), and ε is the molar absorption coefficient of cyanidin-3-O- -glucoside (ε=26 900 L/(mol·cm).
Total flavonoid content analysis
Total flavonoid content (TFC) was determined following the method of Pękal and Pyrzynska (15) with some modifications. Briefly, 0.5 mL of rice bran extract was mixed with 2 mL of distilled water, 0.15 mL of 5% NaNO2, 0.15 mL of 10% AlCl3·6H2O and 1 mL of 1 M NaOH. The absorbance was determined by a UV-Vis spectrophotometer (DR/4000 U; Hach) at 415 nm after 15 min of incubation. Flavonoid content was expressed in gram of catechin equivalent (CE) per kg of rice bran on dry mass (dm) basis.
Total phenolic content analysis
Total phenolic content (TPC) of rice bran extract was measured using Folin-Ciocalteu method with some modifications (16). Briefly, 0.1 mL of rice bran extract was mixed with 0.5 mL of Folin-Ciocalteu reagent. After 5 min, 1.5 mL of 7.5% sodium carbonate were added. The mixture was diluted with 5 mL of distilled water and left to react for 2 h before measuring the absorbance at 765 nm by a UV-Vis spectrophotometer (DR/4000 U; Hach). Gallic acid was used as a standard and the TPC was reported in gram of gallic acid equivalent (GAE) per kg of rice bran on dm basis.
Mineral content analysis
To quantify the mineral content, 500 mg of rice bran powder were digested with 5 mL of 60% HClO4 and 62% HNO3 (1:3 by volume). The solution was diluted with deionized water before analysis. Mineral content was analyzed using atomic absorption spectrophotometer (model GBC Avanta; GBC Scientific Equipment, Hampshire, IL, USA) with the detection limit of 4.4 µg/kg.
Antioxidant analyses
Nitric oxide scavenging activity
The experiment was conducted by mixing 0.5 mL of different concentrations of rice bran extract (4–90 mg/mL) with 125 µL of 5 mM SNP solution in phosphate-buffered saline (PBS), 50 mM, pH=7.4 (17). After 2 h, 150 µL of Griess-Ilosvay’s nitrite reagent were added and the absorbance was measured at 550 nm by a UV-Vis spectrophotometer (DR/4000 U; Hach). Quercetin was used as a positive control. Percentage of nitric oxide scavenging activity was calculated by the following equation:
where A0 is the absorbance of the control and A1 is the absorbance of the analyte in the presence of rice bran extract or quercetin. Antioxidant activity was reported as the effective concentration needed to scavenge NO˙ free radicals by 50% compared to the control (EC50).
Superoxide scavenging activity
Scavenging activity against O2˙Ż was performed using PMS/NADH system (18). A volume of 3 mL of Tris-HCl buffer (100 mM, pH=7.4), 750 µL of NBT (300 µM), 750 µL of NADH (936 µM), 750 µL of PMS (120 µM) and 300 µL of different concentrations of rice bran extract (15–250 mg/mL) were mixed together. The absorbance of the solution was measured after 15 min of incubation at 560 nm by a UV-Vis spectrophotometer (DR/4000 U; Hach). l-Ascorbic acid was used as a positive control. Percentage of scavenging was calculated using the following equation:
 |
where A0 is the absorbance of the control and A1 is the absorbance of the analyte in the presence of rice bran extract or l-ascorbic acid. Antioxidant activity was reported as EC50.
Inhibition of lipid peroxidation
Lipid peroxidation (LOO˙) was determined using ammonium thiocyanate method with some modifications (19). Linoleic acid emulsion (20 mM) was prepared in PBS using Tween 20 as an emulsifier. The emulsion was mixed with various concentrations of rice bran extract (4–45 mg/mL). After incubation for 20 h in the dark, 100 µL of the mixture were mixed with 1 mL of absolute methanol, 100 µL of ammonium thiocyanate (30% in distilled water), and 100 µL of ferrous chloride solution (0.02 M in 3.5% HCl). The developed red colour was measured at 500 nm by a UV-Vis spectrophotometer (DR/4000 U; Hach). Butylated hydroxytoluene (BHT) was used as a positive control. LOO˙ inhibition activity (in %) was calculated using the following equation:
 |
where A0 is the absorbance of the control and A1 is the absorbance in the presence of rice bran extract or BHT. The activity was reported as EC50.
Quinone reductase induction assay
The quinone reductase (QR) induction was used as a biomarker for the induction of phase II detoxifying enzymes (20). Murine hepatoma cell line (Hepa 1c1c7 cells; ATCC, Rockville, MD, USA) were cultured with α-MEM (Gibco® by Life TechnologyTM, Thermo Fisher Scientific, Carlsbad, CA, USA) in identical duplicate 96-well plates (Falcon®; Corning, Tewksbury, MA, USA) for 24 h. Then, the culture medium was replaced with fresh medium containing the rice bran extract and the cells were incubated for an additional 48 h. The QR induction activity was analyzed on a 96-well plate by dissolving the treated cells with 50 µL of digitonin solution per well and shaking for 30 min. A volume of 200 µL of reactant cocktail (21 mL of Tris-HCl enzyme buffer, pH=7.4, 1.5 mL of MTT (3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide) and 22.5 µL of menadione solution) per well was added (20). The development of dark purple colour was measured as the absorbance at 490 nm using a microplate reader (SpectraMax Plus 384; Molecular Devices, Sunnyvale, CA, USA). Protein assay was measured on another identical 96-well plate (Falcon®; Corning). The culture medium was discarded and the treated cells were stained with 100 µL of crystal violet stain per well. The stained protein was dissolved with 150 µL of sodium dodecyl sulfate per well and the absorbance was measured at 610 nm by a UV-Vis spectrophotometer (DR/4000 U; Hach). The relative QR activity can be determined from the ratio of ΔA490 nm (QR assay) and A610 nm (protein assay) with the ratio for control cells set to 1.0.
The results of QR induction were expressed as CD value, IC50 and chemopreventive index (CI, calculated as IC50/CD).
Statistical analysis
Results were expressed as mean value±standard deviation of three replications. Statistical differences were analyzed using one-way ANOVA followed by Duncan’s test at 95% confidence level (p≤0.05). Correlation analysis was obtained using bivariate correlations and expressed as Pearson’s correlation coefficient (r). Statistical analysis was performed using SPSS v. 16.0 (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Total anthocyanin, total flavonoid and total phenolic contents
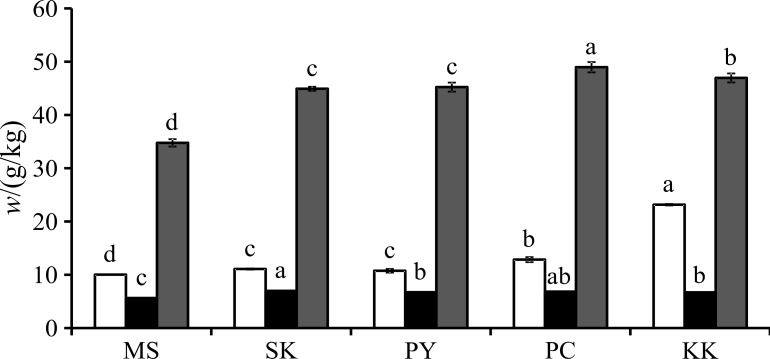
The moisture content of the defatted rice bran powder ranged from 8.5 to 9.4%. Fig. 1 shows the results of total anthocyanin content (TAC), total flavonoid content (TFC) and total phenolic content (TPC) measurements. The value of TAC in rice bran extract samples ranged between 10 (in MS cultivar) and 23 (in KK cultivar) g/kg. The content of TFC expressed as CE ranged between 0.6 (in MS cultivar) and 6.9 (in KK cultivar) g/kg. TPC expressed as GAE was found in the range of 3.4 (in MS cultivar) to 4.7 (in PC cultivar) g/kg. Overall, the extract of MS cultivar had the lowest TAC, TFC and TPC.
Fig. 1.
Total anthocyanin (white column), phenolic (black column) and flavonoid (grey column) contents on dry mass basis of black glutinous rice bran samples (MS, SK, PY, PC and KK; for sample abbreviations see Materials and Methods). Different letters in the same test indicate statistically significant difference (p≤0.05) according to Duncan’s multiple range test (N=3)
Among the five rice bran samples, KK cultivar had the highest TAC, which was about two times higher than that in the other rice bran extracts. The bioactive components in the rice bran extract were different due to several factors, including cultivation techniques, ripening environments and growing conditions (11, 12).
Antioxidant activities of black glutinous rice bran crude extracts
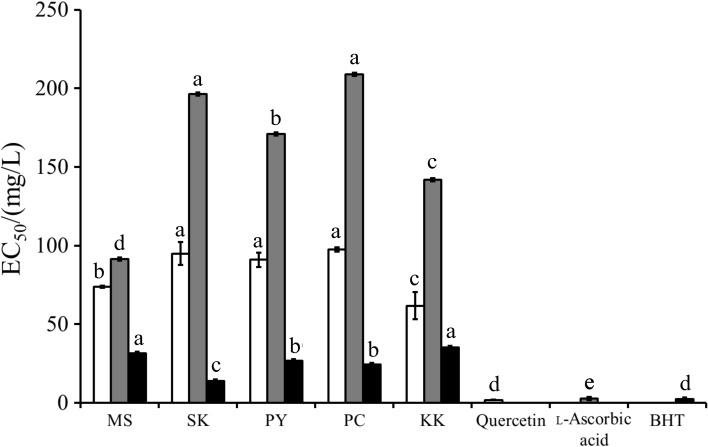
Fig. 2 shows the results of antioxidant activity tests and the EC50 values of rice bran extracts. A lower EC50 value indicates higher antioxidant capacity. The best NO˙ scavenger was quercetin, indicated by the lowest EC50 value (1.9 mg/L), followed by the extracts of KK (62 mg/L), MS (74 mg/L), PY (91 mg/L), SK (95 mg/L) and PC cultivars (98 mg/L). Among the rice bran extracts, KK cultivar had the best antioxidant activity (p≤0.05), while MS cultivar had better activity than SK, PY and PC cultivars, although it had lower TAC, TFC and TPC.
Fig. 2.
Antioxidant activities of black glutinous rice bran crude extracts (MS, SK, PY, PC and KK; for sample abbreviations see Materials and Methods). Quercetin, l-ascorbic acid and BHT were positive controls for nitric oxide (white column), superoxide (grey column), and lipid peroxidation (black column) assays, respectively. EC50=effective concentration needed to scavenge the free radicals by 50% compared to the control. Different letters in the same test indicate statistically significant difference (p≤0.05) according to Duncan’s multiple ranges test (N=3)
l-Ascorbic acid had the best O2˙Ż scavenging activity (EC50=2.8 mg/L). The extract of MS cultivar had significantly better activity (p≤0.05) with regard to EC50 value (31 mg/L) than the extracts of KK (142 mg/L), PY (171 mg/L), SK (196 mg/L) and PC cultivars (208 mg/L).
The best inhibitor against LOO˙ was BHT (EC50=1.9 mg/L). The extract of SK cultivar had significantly lower EC50 value (14 mg/L) with p≤0.05, indicating stronger LOO˙ inhibition than by PC (24 mg/L), PY (26 mg/L), MS (32 mg/L) and KK extracts (35 mg/L).
All rice bran extracts had significantly lower (p≤0.05) antioxidant activities than the corresponding positive controls. Our preliminary study results (data not shown) indicated that acidified methanol/water rice bran extracts contained various compounds, both active (phenolic acids, anthocyanins (5) and proanthocyanidins (21)) and inactive (sugars and soluble oligosaccharides (22)). The active components in rice bran extracts were more dilute than in the highly pure positive controls. Different rice bran extracts show different dominant antioxidant activities. In general, TAC does not seem to play a major antioxidant role against NO˙, LOO˙ and O2˙Ż, as indicated by the extracts of KK, which had the highest TAC but did not show dominant antioxidant activity in all tested assays. It has been reported previously that the chemical structure of anthocyanins is changed or possibly degraded at pH higher than 4.0 (23). The structurally changed anthocyanins may lose their ability to donate proton (24). Protocatechuic acid is the major product of cyanidin-3-O-glucoside (major anthocyanin in rice bran extract) degradation at low pH values (25). However, in vitro antioxidant activity of protocatechuic acid was the weakest compared to other phenolic acids (26). This indicates that antioxidant activities of rice bran extracts are affected by the ratios and types of other active components (TFC, TPC and minerals) and the products of anthocyanin degradation.
Mineral profile of black glutinous rice bran
Three major minerals (Ca, K and Mg) and six trace elements (Cu, Fe, Mn, Na, Se and Zn) were quantified in black rice bran powder (Table 1). Mineral contents (µg/g) in black rice bran were found in the following order: K>Mg>Zn>Na>Fe>Se or Mn>Cu>Ca. The high content of K (16642 µg/g in KK to 21884 µg/g in PY cultivars) and Mg (1894 µg/g in MS to 2411 µg/g in KK cultivars) was found in all the rice bran samples. Some essential minerals were found in black rice bran: Zn (103–133 µg/g), Se (11–18 µg/g) and Cu (3.8–7.1 µg/g). This result is in agreement with the report of Parengam et al. (9), who found that the two preventing minerals in various samples were K or Mg. However, their contents varied even in the same rice cultivar due to many factors and growing conditions, including soil type, fertilizers, herbicides and fungicides (27, 28).
Table 1. Mineral contents on dry mass basis in different black glutinous rice bran extracts (MS, SK, PY, PC and KK; for sample abbreviations see Materials and Methods).
| Mineral | w/(µg/g) | ||||
|---|---|---|---|---|---|
| MS | SK | PY | PC | KK | |
| Ca | (0.8±0.1)b | (1.6±0.1)a | (1.9±0.2)a | (1.9±0.3)a | (1.9±0.1)a |
| Cd | (0.7±0.1)a | N.D. | (0.6±0.1)b | (0.9±0.2)a | N.D. |
| Cr | N.D. | N.D. | N.D. | N.D. | N.D. |
| Cu | (5.9±0.6)b | (3.8±0.2)c | (3.9±0.3)c | (5.2±0.6)b | (7.1±0.3)a |
| Fe | (16.0±1.4)c | (17.0±0.6)c | (17.0±2.2)c | (24.0±1.1)b | (30.0±0.9)a |
| K | (17300±738)b | (20796±2020)a | (21885±451)a | (21823±854)a | (16643±242)b |
| Mg | (1894±63)b | (2123±6)b | (1979±41)b | (2409±188)a | (2412±191)a |
| Mn | (12.0±0.3)e | (15±1)c | (16.0±0.4)b | (21.0±0.6)a | (13.0±0.1)d |
| Na | (97±13)a | (85±14)ab | (84±6)ab | (73±12)b | (84±12)ab |
| Se | (12.0±1.3)bc | (14.0±1.2)b | (14.0±0.4)b | (18.0±1.4)a | (11.0±1.5)c |
| Zn | (133±3)a | (113±8)b | (120±3)b | (103±1)c | (117±2)b |
The data are presented as mean value±standard deviation of triplicate analyses. Different letters in the same row indicate statistically significant values (p≤0.05). N.D.=not detected, below detection limit of the instrument at 0.04 ppm
Although rice bran is a by-product of rice production, this study shows that it is a promising source of concentrated essential minerals. Deficiency of some minerals is an important problem that has led to a study of mineral fortification, such as with Fe, which has been found at low concentrations (6–10 µg/g) in some brown rice cultivars in Thailand (29). However, higher concentrations of Fe were found in the five black glutinous rice bran samples used in this study in the range of 16–30 µg/g.
Some trace elements such as Zn, Se, Cu and Mn were reported to be included in human cell defense mechanisms against reactive oxygen species (ROS) (30, 31). These minerals act as cofactors of several antioxidant enzymes. For example, superoxide dismutase works by incorporating Zn, Cu and Mn in order to convert O2˙Ż to a less harmful H2O2 (31). Consequently, glutathione peroxidase eliminates H2O2 by conjugating with selenium (32). Since high contents of Zn, Se and Cu were found in all of the rice samples, black glutinous rice bran can be an important source of essential minerals that could promote health benefits to consumers.
Correlation between antioxidant activities and TAC, TFC, TPC and mineral content
Pearson’s correlation coefficient (r) (Table 2) was obtained from bivariate correlation analysis and used to describe the correlation between the antioxidant activities against NO˙, O2˙Ż and LOO˙ and the content of antioxidant components (TAC, TFC, TPC, Cu, Fe, Mn and Zn). It needs to be noted that antiradical values (calculated as 1/EC50) were employed for the correlation analysis instead of EC50 value since direct analysis using EC50 gave negative r value, which confused the interpretation of the results. Three correlation levels were defined as strong (r=(+/–)0.600–1.000), moderate (r=(+/–)0.400–0.599), and weak (r=(+/–)0.000–0.399) (33).
Table 2. Pearson’s correlation coefficients (r) between TAC, TFC, TPC and mineral content and antioxidant activity (1/EC50) of rice bran extracts (MS, SK, PY, PC and KK; for sample abbreviations see Materials and Methods).
| Extract | 1/EC50 | r (N=3) | ||||||
|---|---|---|---|---|---|---|---|---|
| TAC | TFC | TPC | Cu | Fe | Mn | Zn | ||
| MS | NO˙ | 0.994 | 0.842 | 0.129 | –0.532 | –0.932 | –1.000* | –0.127 |
| O2˙Ż | –0.380 | –0.874 | 0.811 | –0.493 | 0.762 | 0.475 | 0.926 | |
| LOO˙ | 0.777 | 1.000* | –0.432 | 0.016 | –0.979 | –0.839 | –0.632 | |
| SK | NO˙ | 0.631 | 0.160 | –0.330 | –0.998* | –0.998* | –0.014 | –0.237 |
| O2˙Ż | –0.970 | –0.721 | –0.302 | 0.839 | 0.764 | –0.589 | –0.393 | |
| LOO˙ | –0.949 | –0.666 | –0.230 | 0.877 | 0.811 | –0.527 | –0.323 | |
| PY | NO˙ | 0.153 | 0.724 | –0.981 | –0.176 | –0.933 | 0.024 | –0.688 |
| O2˙Ż | –0.944 | 0.268 | 0.292 | –0.786 | 0.756 | –0.893 | –0.317 | |
| LOO˙ | –0.736 | 0.976 | –0.705 | –0.916 | –0.220 | –0.818 | –0.986 | |
| PC | NO˙ | 0.846 | –0.679 | 0.307 | –0.865 | –0.998* | 0.907 | –0.720 |
| O2˙Ż | –0.227 | –0.916 | –0.799 | –0.757 | –0.392 | 0.694 | 0.420 | |
| LOO˙ | 0.955 | 0.116 | 0.922 | –0.182 | –0.597 | 0.271 | –0.996 | |
| KK | NO˙ | –0.701 | 0.422 | 0.984 | 0.732 | 0.468 | –0.181 | 0.765 |
| O2˙Ż | –0.888 | 0.121 | 0.881 | 0.492 | 0.719 | 0.170 | 0.927 | |
| LOO˙ | 0.913 | 0.702 | –0.177 | 0.376 | –0.992 | –0.878 | –0.870 | |
*correlation is significant at the 0.05 level (2-tailed). TAC=total anthocyanin content, TFC=total flavonoid content, TPC=total phenolic content
Both positive and negative correlations of NO˙ scavenging activity with TAC: strong positive (MS, r=0.994; SK, r=0.631 and PC, r=0.846) and strong negative (KK, r= –0.701), TFC: strong positive (MS, r=0.842 and PY, r=0.724) and strong negative (PC, r=–0.679) and TPC: strong positive (KK, r=0.984) and strong negative (PY, r=–0.981) were found in rice bran extracts from all rice cultivars. These results indicate unpredictable correlation between the antioxidant contents and NO˙ scavenging activity. The content of Cu, Fe, Mn and Zn may not affect the NO˙ scavenging activity as both positive and negative correlations were found. However, low content of Fe tends to promote the NO˙ scavenging activity as most correlations were strong negative in MS (r=–0.932), SK (r=–0.998), PY (r= –0.933) and PC (r=–0.998) cultivars, with the exception of a moderate positive correlation in KK cultivar (r=0.468).
TAC, TFC and TPC may not contribute to the O2˙Ż scavenging since most r values were negative or weak positive. Similarly, Cu, Mn and Zn may also not be involved in the scavenging of O2˙Ż as both positive and negative correlations with the O2˙Ż scavenging activity were found. However, the presence of Fe tends to promote the scavenging of O2˙Ż as most r values were strong positive (r values from 0.719 to 0.764), with only one moderately negative r value in PC cultivar (r=–0.392). Similar results were found in the report of Kaneda et al. (10), where various levels of correlation between the content of trace elements and O2˙Ż scavenging activity were found depending on rice samples.
The inhibition of LOO˙ activity did not correlate with TAC, TFC and TPC since both strong positive and strong negative correlations were found as follows: TAC: strong positive (MS, r=0.777; PC, r=0.955 and KK, r=0.913) and strong negative (SK, r=–0.949 and PY, r=–0.736), TFC: strong positive (MS, r=1.000; PY, r=0.976 and KK, r=0.702) and strong negative (SK, r=–0.666) and TPC: strong positive (PC, r=0.922) and strong negative (PY, r=–0.705). Similar results were also found between LOO˙ inhibition activity and the content of Cu and Fe, which indicated that these two elements did not affect the inhibition of LOO˙. However, low contents of Mn and Zn tended to promote the LOO˙ inhibition activity as negative correlations, except a weak positive correlation between Mn and LOO˙ inhibition activity in PC cultivar (r=0.271), were found in all rice bran extracts.
In general, this correlation study indicated that none of TAC, TFC or TPC solely contributed to the antioxidant activities against NO˙, O2˙Ż and LOO˙, since various levels of both positive and negative correlations were presented. In the case of TAC, antioxidant activity of anthocyanin may be influenced by the pH of the solution in the antioxidant assay, since the chemical structure of anthocyanin is degraded at higher pH values (34). Low and moderate correlations of TFC and TPC with in vitro antioxidant activities were previously reported for a number of plant extracts (6, 13). For these reasons, antioxidant activity of rice bran extracts was considered to be caused by the combined effects of TAC, TFC and TPC. These findings suggest the necessity of choosing a suitable in vitro antioxidant assay for the compounds of interest. The pH of the test solution may influence the antioxidant activity of some compounds, especially anthocyanins, which are susceptible to pH.
Mineral content of rice bran does not seem to affect NO˙, O2˙Ż and LOO˙ antioxidant activities as both positive and negative r values were determined. However, low levels of Mn and Zn had an effect on the inhibition of LOO˙ as the correlations were mostly negative. These results contradict previous studies where these minerals were found to contribute to cell defense against ROS (30, 31). For example, Zn showed protective effects against oxidative damage and lipid peroxidation in cellular environment (35, 36). The results in this study also indicate that high content of Fe tended to promote the scavenging of O2˙Ż. However, excess of Fe was reported to catalyze the generation of ROS in a cellular study (35). These conflicting results could be explained by the protective mechanism of these minerals that was achieved by forming a complex structure with some specific cellular molecules, such as superoxide dismutase, catalase and glutathione peroxidase (30–32, 36). Thus, the chemical reactions in the antioxidant assays used in this experiment may limit the activities of trace elements and prevent their interaction with ROS. This is in agreement with the previous report which suggested that antioxidants investigated in vitro were not affected by the content of trace elements (10, 13).
Induction of quinone reductase
The quinone reductase (QR) activity of rice bran extracts is shown in Table 3. The results were calculated based on the TAC value. The chemopreventive effect of all the crude rice bran extracts expressed as CD values ranged from 0.7 µg/mL in PC cultivar (strongest QR inducer) to 5.6 µg/mL in KK cultivar (weakest QR inducer). The IC50 values, which show the risk of cytotoxicity, ranged from 5.1 µg/mL in SK cultivar (more toxic) to 32.0 µg/mL in KK cultivar (less toxic), whereas MS cultivar was not cytotoxic at the highest concentration used (1500 µg/mL). Overall, the PC extract had the highest QR induction ability indicated by the highest chemopreventive index (CI) value (risk to benefit ratio) of 8.3 (calculated as IC50 (5.9 µg/mL) divided by CD value (0.7 µg/mL)).
Table 3. Quinone reductase (QR) activities of black glutinous rice bran extracts (MS, SK, PY, PC and KK; for sample abbreviations see Materials and Methods).
| Rice bran | CD/(µg/mL) | IC50/(µg/mL) | CI |
|---|---|---|---|
| MS | (3.9±0.1)b | N/A | N/A |
| SK | (0.9±0.1)c | (5.1±0.2)b | 5.7 |
| PY | (0.8±0.1)c | (5.5±0.0)b | 7.2 |
| PC | (0.7±0.1)c | (5.9±0.1)b | 8.3 |
| KK | (5.6±0.2)a | (32.0±1.6)a | 5.5 |
CD=the concentration of anthocyanins required to double the QR activity, IC50=the concentration that causes 50% cell death, CI=chemopreventive index (calculated as IC50/CD), N/A=data not applicable due to the very low toxicity of the extract. Different letters in the same column indicate statistically different values (p≤0.05)
TAC may not play a major role in QR induction since KK cultivar, which had the highest TAC, was not the best QR inducer. Regarding the CD values, PC, PY, SK and MS cultivars were significantly (p≤0.05) stronger QR inducers than KK cultivar. The possible reason is that the assay was conducted in neutral pH environment for two days, during which the anthocyanin fraction in rice bran extract was degraded to cyanidin and protocatechuic acid (25). Consequently, the QR induction activity of anthocyanins decreased. This indicated that anthocyanins were not directly involved in this cell assay. Thus, only the compounds in the rice bran extracts that were more stable at neutral pH (i.e. phenolic acids, with the exception of protocatechuic acid) had the effect on QR induction activity (26). This finding is in agreement with our previously published results (37), which reported that phenolic acid-rich fraction isolated from the crude rice bran extract had stronger QR induction activity than the anthocyanin-rich fraction from the same source.
Conclusions
Crude extracts of anthocyanin-rich black glutinous rice bran have different phytochemical contents. However, the correlation study suggested that the antioxidant activity of these extracts seems to be the combination of activities of these phytochemicals. Minerals did not contribute to the antioxidant activity of the extracts since positive and negative correlations of different strength were found. Although some samples contained significantly high content of anthocyanins, they were not dominant antioxidants because they were affected by the pH values. A screening of chemoprevention effect indicated that anthocyanin-rich black glutinous rice bran extracts had potential anticarcinogenic properties since the products of anthocyanin degradation were considered to be involved in the antioxidant activity and chemopreventive property of the extracts. Further studies on the effects of pH on antioxidant activity of anthocyanins, isolation and identification of products of anthocyanin degradation and their antioxidant activity should be performed.
Acknowledgements
This work was financially supported by the Thailand Research Fund through the Royal Golden Jubilee PhD Program (Grant No. PHD/0292/2552) to Mr. Paradorn Ngamdee and Assoc. Prof. Dr. Sudarat Jiamyangyuen. Laboratory and equipment were supported by Department of Chemistry, Naresuan University, Phitsanulok, Thailand.
References
- 1.Chen PN, Kuo WH, Chiang CL, Chiou HL, Hsieh YS, Chu SC. Black rice anthocyanins inhibit cancer cells invasion via repressions of MMPs and u-PA expression. Chem Biol Interact. 2006;163:218–29. 10.1016/j.cbi.2006.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71S–88S. 10.1016/S0002-9343(01)00995-0 [DOI] [PubMed] [Google Scholar]
- 3.Sancho RAS, Pastore GM. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res Int. 2012;46:378–86. 10.1016/j.foodres.2011.11.021 [DOI] [Google Scholar]
- 4.Chotimarkorn C, Benjakul S, Silalai N. Antioxidant components and properties of five long-grained rice bran extracts from commercial available cultivars in Thailand. Food Chem. 2008;111:636–41. 10.1016/j.foodchem.2008.04.031 [DOI] [Google Scholar]
- 5.Sompong R, Siebenhandl-Ehn S, Linsberger-Martin G, Berghofer E. Physicochemical and antioxidative properties of red and black rice varieties from Thailand, China and Sri Lanka. Food Chem. 2011;124:132–40. 10.1016/j.foodchem.2010.05.115 [DOI] [Google Scholar]
- 6.Shen Y, Jin L, Xiao P, Lu Y, Bao J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size and weight. J Cereal Sci. 2009;49:106–11. 10.1016/j.jcs.2008.07.010 [DOI] [Google Scholar]
- 7.González-Montilla FM, Chávez-Santoscoy RA, Gutiérrez-Uribe JA, Serna-Saldivar SO. Isolation and identification of phase II enzyme inductors obtained from black Shawaya sorghum [Sorghum bicolor (L.) Moench] bran. J Cereal Sci. 2012;55:126–31. 10.1016/j.jcs.2011.10.009 [DOI] [Google Scholar]
- 8.Antoine JMR, Hoo Fung LA, Grant CN, Dennis HT, Lalor GC. Dietary intake of minerals and trace elements in rice on the Jamaican market. J Food Compos Anal. 2012;26:111–21. 10.1016/j.jfca.2012.01.003 [DOI] [Google Scholar]
- 9.Parengam M, Judprasong K, Srianujata S, Jittinandana S, Laoharojanaphand S, Busamongko A. Study of nutrients and toxic minerals in rice and legumes by instrumental neutron activation analysis and graphite furnace atomic absorption spectrophotometry. J Food Compos Anal. 2010;23:340–5. 10.1016/j.jfca.2009.12.012 [DOI] [Google Scholar]
- 10.Kaneda I, Kubo F, Sakurai H. Antioxidative compounds in the extracts of black rice brans. J Health Sci. 2006;52:495–511. 10.1248/jhs.52.495 [DOI] [Google Scholar]
- 11.Mpofu A, Sapirstein HD, Beta T. Genotype and environment variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J Agric Food Chem. 2006;54:1265–70. 10.1021/jf052683d [DOI] [PubMed] [Google Scholar]
- 12.Daiponmak W, Theerakulpisut P, Thanonkao P, Vanavichit A, Prathepha P. Change of anthocyanin cyanidin-3-glucoside content and antioxidant activity in Thai rice varieties under salinity stress. Sci Asia. 2010;36:286–91. 10.2306/scienceasia1513-1874.2010.36.286 [DOI] [Google Scholar]
- 13.Sulaiman SF, Yusoff NAM, Eldeen IM, Seow EM, Sajak AAB. Supriatno, Ooi KL. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). J Food Compos Anal. 2011;24:1–10. 10.1016/j.jfca.2010.04.005 [DOI] [Google Scholar]
- 14.Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Wrolstad RE, editor. Current protocols in food analytical chemistry. New York, NY, USA: John Wiley & Sons; 2001. pp. F1.2.1-13. [Google Scholar]
- 15.Pękal A, Pyrzynska K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal Methods. 2014;7:1776–82. 10.1007/s12161-014-9814-x [DOI] [Google Scholar]
- 16.Shirakawa H, Koseki T, Ohinata K, Hashizume K, Komai M. Rice bran fraction improve blood pressure, lipid profile, and glucose metabolism in stroke-prone spontaneously hypertensive rats. J Agric Food Chem. 2006;54:1914–20. 10.1021/jf052561l [DOI] [PubMed] [Google Scholar]
- 17.Alisi CS, Onyeze GOC. Nitric oxide scavenging ability of ethyl acetate fraction of methanolic leaf extracts of Chromolaena odorata (Linn.). Afr J Biochem Res. 2008;2:145–50. [Google Scholar]
- 18.Liu F, Ooi VEC, Chang ST. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997;60:763–71. 10.1016/S0024-3205(97)00004-0 [DOI] [PubMed] [Google Scholar]
- 19.Yen GC, Hsieh CL. Antioxidant activity of extracts from Du-zhong (Eucommia ulmoides) toward various lipid peroxidation models in vitro. J Agric Food Chem. 1998;46:3952–7. 10.1021/jf9800458 [DOI] [Google Scholar]
- 20.Prochaska HJ. Screening strategies for the detection of anticarcinogenic enzyme inducers. J Nutr Biochem. 1994;5:360–8. 10.1016/0955-2863(94)90067-1 [DOI] [Google Scholar]
- 21.Cuevas-Rodríguez EO, Yousef GG, García-Saucedo PA, López-Medina J, Paredes-López O, Lila MA. Characterization of anthocyanins and proanthocyanidins in wild and domesticated mexican blackberries (Rubus spp.). J Agric Food Chem. 2010;58:7458–64. 10.1021/jf101485r [DOI] [PubMed] [Google Scholar]
- 22.Mukerjea R, Slocum G, Robyt JF. Determination of the maximum water solubility of eight native starches and the solubility of their acidic-methanol and -ethanol modified analogues. Carbohydr Res. 2007;342:103–10. 10.1016/j.carres.2006.10.022 [DOI] [PubMed] [Google Scholar]
- 23.Cevallos-Casals BA, Cisneros-Zevallos L. Stability of anthocyanin-based aqueous extracts of Andean purple corn and red-fleshed sweet potato compared to synthetic and natural colorants. Food Chem. 2004;86:69–77. 10.1016/j.foodchem.2003.08.011 [DOI] [Google Scholar]
- 24.Castańeda-Ovando A, Pacheco-Hernández ML, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA. Chemical studies of anthocyanins: a review. Food Chem. 2009;113:859–71. 10.1016/j.foodchem.2008.09.001 [DOI] [Google Scholar]
- 25.Min SW, Ryu S, Kim D. Anti-inflammatory effects of black rice, cyanidin-3-O-β-d-glucoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010;10:959–66. 10.1016/j.intimp.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 26.Amorati R, Pedulli GF, Cabrini L, Zambonin L, Landi L. Solvent and pH effects on the antioxidant activity of caffeic and other phenolic acids. J Agric Food Chem. 2006;54:2932–7. 10.1021/jf053159+ [DOI] [PubMed] [Google Scholar]
- 27.D’Ilio S, Alessandrelli M, Cresti R, Forte G, Caroli S. Arsenic content of various types of rice and determined by plasma-based techniques. Microchem J. 2002;73:195–201. 10.1016/S0026-265X(02)00064-4 [DOI] [Google Scholar]
- 28.Liao YI, Zheng SX, Nie J, Xie J, Lu YH, Qin XB. Long-term effect of fertilizer and rice straw on mineral composition and potassium adsorption in a reddish paddy soil. J Integr Agric. 2013;12:694–710. 10.1016/S2095-3119(13)60288-9 [DOI] [Google Scholar]
- 29.Prom-U-Thai C . Fukai S, Godwin ID, Rerkasem B, Huang L. Iron-fortified parboiled rice – a novel solution to high iron density in rice-based diets. Food Chem. 2008;110:390–8. 10.1016/j.foodchem.2008.02.043 [DOI] [PubMed] [Google Scholar]
- 30.Negi R, Pande D, Karki K, Kumar A, Khanna RS, Khanna HD. Trace elements and antioxidant enzymes associated with oxidative stress in the pre-eclamptic/eclamptic mothers during fetal circulation. Clin Nutr. 2012;31:946–50. 10.1016/j.clnu.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Spears JW, Weiss WP. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J. 2008;176:70–6. 10.1016/j.tvjl.2007.12.015 [DOI] [PubMed] [Google Scholar]
- 32.Fardet A, Rock E, Rémésy C. Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo? J Cereal Sci. 2008;48:258–76. 10.1016/j.jcs.2008.01.002 [DOI] [Google Scholar]
- 33.Evans JD. Straightforward statistics for the behavioral sciences. Pacific Grove, CA, USA: Brooks/Cole Publishing; 1996. [Google Scholar]
- 34.Moldovan B, David L, Chrişbora C, Cimpoiu C. Degradation kinetics of anthocyanins from European cranberrybush (Viburnum opulus L.) fruit extracts. Effects of temperature, pH and storage solvent. Molecules. 2012;17:11655–66. 10.3390/molecules171011655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Formigari A, Irato P, Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:443–59. 10.1016/j.cbpc.2007.07.010 [DOI] [PubMed] [Google Scholar]
- 36.Cikim G, Canatan H, Gursu MF, Gulcu F, Baydas G, Kilicoglu AE. Levels of zinc and lipid peroxidation in acute coronary syndrome. Biol Trace Elem Res. 2003;96:61–9. 10.1385/BTER:96:1-3:61 [DOI] [PubMed] [Google Scholar]
- 37.Ngamdee P, Jiamyangyuen S, Parkin KL. Phase II enzyme induction and anti-inflammatory effects of crude extracts and secondary fractions obtained from bran from five black glutinous rice cultivars. Int J Food Sci Technol. 2016;51:333–41. 10.1111/ijfs.12967 [DOI] [Google Scholar]