Abstract
This article reports the genetic interaction of two F-box genes, SLEEPY1 (SLY1) and SNEEZY (SNE), in Arabidopsis thaliana gibberellin (GA) signaling. The SLY1 gene encodes an F-box subunit of a Skp1-cullin-F-box (SCF) E3 ubiquitin ligase complex that positively regulates GA signaling. The sly1-2 and sly1-10 mutants have recessive, GA-insensitive phenotypes including delayed germination, dwarfism, reduced fertility, and overaccumulation of the DELLA proteins RGA (Repressor of ga1-3), GAI (GA-Insensitive), and RGL2 (RGA-Like 2). The DELLA domain proteins are putative transcription factors that negatively regulate GA signaling. The requirement for SLY1 in GA-stimulated disappearance of DELLA proteins suggests that GA targets DELLA proteins for destruction via SCFSLY1-mediated ubiquitylation. Overexpression of SLY1 in sly1-2 and sly1-10 plants rescues the recessive GA-insensitive phenotype of these mutants. Surprisingly, antisense expression of SLY1 also suppresses these mutants. This result caused us to hypothesize that the SLY1 homologue SNE can functionally replace SLY1 in the absence of the recessive interfering sly1-2 or sly1-10 genes. This hypothesis was supported because overexpression of SNE in sly1-10 rescues the dwarf phenotype. In addition to rescuing the sly1-10 dwarf phenotype, SNE overexpression also restored normal RGA protein levels, suggesting that the SNE F-box protein can replace SLY1 in the GA-induced proteolysis of RGA. If the C-terminal truncation in the sly1-2 and sly1-10 alleles interferes with SNE rescue, we reasoned that overexpression of sly1-2 might interfere with wild-type SLY1 function. Indeed, overexpression of sly1-2 in wild-type Ler (Landsberg erecta) yields dwarf plants.
Gibberellins (GAs) are a family of tetracyclic diterpenoid phytohormones needed to induce seed germination, stem elongation, transition to flowering, and fertility (1). Many positive and negative regulators of GA signaling have been identified as mutants with increased and decreased GA response (extensively reviewed in refs. 1 and 2). In Arabidopsis, known negative regulators of GA signaling include the O-linked N-acetylglucosamine transferase SPINDLY (SPY) (3) and the following putative transcription factors: SHI (Short Internodes) (4); RGA (Repressor of ga1-3) (5); GAI (GA-Insensitive) (6); and RGL1, RGL2, and RGL3 (RGA-LIKE 1-3) (7-9). Known positive regulators of GA signaling in Arabidopsis include the chromatin remodeling factor PICKLE (PKL) (10) and the F-box domain gene SLEEPY1 (SLY1) (11).
Mutations in the SLY1 gene were originally isolated in screens designed to detect a GA-insensitive increase in seed dormancy. The sly1-2 allele was recovered as a suppressor of the ability of the abi1-1 [abscisic acid (ABA)-insensitive] mutant to germinate on 3 μM ABA (12). The sly1-10 allele was isolated based on brassinosteroid-dependent germination (13). The sly1-2 and sly1-10 mutations result in a recessive GA-insensitive phenotype that includes increased seed dormancy, increased sensitivity to ABA during germination, dwarfism, and reduced fertility. No other recessive alleles have yet been characterized.
Map-based cloning revealed that SLY1 encodes an F-box domain protein of 151 aa (11). F-box proteins are subunits of Skp1-cullin-F-box (SCF) E3 ubiquitin ligases (14). The F-box protein confers specificity on the complex by direct interaction with the substrate through its C terminus. F-box proteins often contain consensus protein-protein interaction domains at the C terminus such as leucine-rich repeats, Kelch repeats, and WD repeats. Whereas SLY1 does not contain such a consensus sequence, it is clear that the C-terminal domain is functionally important. The sly1-2 allele is a frameshift mutation resulting in loss of the last 40 aa, whereas the sly1-10 allele is a complex rearrangement resulting in loss of the last 8 aa and addition of a random 23 aa (11). The sly1-10 mutation results in failure to interact with RGA and GAI proteins in yeast two-hybrid assays (15). The N-terminal F-box domain binds to a Skp1 homologue, which tethers the F-box protein to the N terminus of cullin, the backbone of the SCF complex. The C terminus of cullin binds the RING-finger domain protein Rbx1, which binds the E2 ubiquitin-conjugating enzyme. The SCF complex catalyzes transfer of a ubiquitin moiety from the E2 to the substrate (16). Formation of a polyubiquitin chain on the substrate targets it for proteolysis by the 26S proteasome. The SLY1 gene belongs to the 17-member C2 subfamily of the 694 superfamily of Arabidopsis F-box genes (17). The SLY1 gene is highly conserved in higher plants (11, 18). The two orthologous F-box genes, SLY1 in Arabidopsis and GID2 (GA-insensitive Dwarf2) in rice, are both needed for GA response and for the GA-stimulated disappearance of DELLA proteins (11, 15, 19-22).
GA responses are repressed by the DELLA domain subfamily of the GRAS (GAI, RGA, and SCARECROW) family of putative transcription factors (23). It seems that GA causes GA responses by triggering the disappearance of DELLA proteins (24-26). Whereas only a single DELLA gene is known in barley (SLN1, SLENDER1) (24) and in rice (SLR1, SLENDER RICE1) (27), there are five DELLA genes in Arabidopsis. GAI and RGA act redundantly to negatively regulate stem elongation, leaf expansion, and apical dominance (28, 29). RGL1, RGL2, and RGA seem to be the main negative regulators of floral development (7, 8, 19). RGL1 also seems to be involved in stem elongation and leaf expansion (7). RGL2 is the key DELLA repressing seed germination, but RGL1 also may play a role (7, 9). The precise role of RGL3 in GA signaling remains to be elucidated.
Ubiquitin-proteasome pathway involvement in GA-stimulated disappearance of DELLA proteins was first suggested by the requirement for F-box proteins Arabidopsis SLY1 and rice GID2 in the GA-regulated disappearance of RGA and SLR1 protein, respectively (11, 21). SLY1 and GID2 are each part of an SCF E3 ubiquitin ligase (20, 22). Thus far, SLY1 has been shown to regulate the GA-stimulated disappearance of RGA, GAI, and RGL2 (11, 15, 19, 20). The interaction between rice GID2 and SLR1 depends on phosphorylation of SLR1, suggesting that GA-stimulated phosphorylation is the signal causing SCFGID2 to ubiquitylate SLR1, thereby targeting it for destruction (21, 22). Accumulation of ubiquitylated SLR1 depends on GID2 (21). Recently, Fu et al. (20) showed that the strength of the interaction between SLY1 and the gai-1 mutant protein depends on phosphorylation of gai-1, suggesting that a similar mechanism is at work in Arabidopsis. Fig. 1 summarizes the current model, in which (i) DELLA proteins inhibit GA responses in the absence of GA; (ii) addition of GA causes DELLA phosphorylation causing polyubiquitylation by the SCFSLY1 complex; and (iii) polyubiquitylation targets the DELLA protein for destruction by the 26S proteasome, thereby relieving DELLA inhibition of GA responses.
Fig. 1.
SCFSLY1 inhibits negative regulation of GA responses by the DELLA proteins. In the absence of GA, DELLA proteins inhibit GA responses such as germination, stem elongation, and transition to flowering. Addition of GA stimulates phosphorylation of DELLA proteins by an unidentified kinase. Phosphorylation allows recognition of the DELLA protein by the SCFSLY1 complex. Polyubiquitylation mediated by the SCFSLY1 E3 ubiquitin ligase targets the DELLA protein for degradation by the 26S proteasome. This stimulates GA responses by relieving DELLA inhibition.
There is one homologue of SLY1 in Arabidopsis named SNEEZY (SNE) (11). The predicted SNE protein is 25% identical/42% similar to SLY1 at the amino acid level. The research described here reveals that sly1-2 and sly1-10 are recessive interfering mutations and that SNE can functionally replace SLY1 in GA signal transduction.
Materials and Methods
Plant Growth Conditions. Plants were grown as in McGinnis et al. (11). ABA dose-response curves were generated by using seeds harvested at the same time and stored dry for at least 2 weeks, as described in ref. 12, except that germination was defined as radical emergence.
Plasmid Construction. For use in plasmid construction, SLY1 (At4g24210, NM 118554), sly1-2, and SNE (At5g48170, NM124191) were cloned from either Landsberg erecta (Ler) or sly1-2 genomic DNA by PCR using 2-62f and 2-64r primers for SLY1 and sly1-2, and MIf21.6f and MIf21.6r for SNE. These PCR products were cloned into the pTOPO XL TA vector (Invitrogen) in both orientations. To create pJS53 (35S:SLY1-AS), pJS54 (35S:SLY1-OE), and pJS65 (35S:sly1-2-OE), inserts were excised by using the restriction enzymes XbaI and SstI and ligated into T-DNA vector pBI121 cut with the same enzymes, thereby removing the GUS gene and placing the inserted genes under control of the Cauliflower Mosaic Virus 35S promotor. To create pJS67 (35S:SNE-OE), the SNE insert was excised by using the restriction enzymes BamHI and XbaI and ligated into pBI121, which also had been cut with the same enzymes, removing the GUS gene. When fragments were cloned into the appropriate vectors, the inserts were sequenced to ensure correct orientation and that no mutations were introduced during the PCR and cloning steps.
Transformation and Isolation of Transgenic Lines. By using the floral-dip method (30), Ler, sly1-2, and sly1-10 were transformed with constructs carried by Agrobacterium tumefaciens strain GV3101. Transformants were selected on 0.5× Murashige and Skoog medium (Sigma) and 0.8% agar containing 100 μg/ml kanamycin. Transformation events were confirmed by PCR using the primers sly1r and nosr for pJS53 transformants and the primers 2-63f and nosr for pJS54 and pJS65 transformants.
mRNA Analysis. The SLY1 transcript was analyzed by semiquantitative, directional RT-PCR in SLY1 overexpression and antisense lines. RT-PCR on 100 ng of total RNA was conducted with a LightCycler (Roche) using the LightCycler RNA amplification kit SYBR Green I (Roche), according to the manufacturer's instructions, with 5 mM MgCl2 and an annealing temperature of 56°C. Reverse transcription was performed with the sly1r primer to detect only the SLY1 sense transcript. Primer 2-63f was added before 25 cycles of PCR amplification. The 2-63f and sly1r primers flank the sly1-10 rearrangement, such that there should be no product in sly1-10. This control shows that only SLY1, not SNE, mRNA is being detected. The ACT2 mRNA loading control was amplified (45 cycles) with the ACT2f and ACT2r primers (11, 31).
SNE expression was evaluated by RNA gel blot analysis using 15 μg of total RNA per lane (32). Total RNA was prepared by using the phenol-extraction method (33), except that the phenol extraction was performed at 73°C instead of 80°C. The SNE mRNA was detected by using a random-primed 32P-labeled 1-kb SNE DNA probe (RediPrime II, Amersham Biosciences), encompassing the SNE coding region from 142-bp upstream to 858-bp downstream of the ATG. Signal was detected by using a PhosphorImager (model 445Si, Molecular Dynamics). For a loading control, Northern blots were rehybridized to an 18S rRNA probe generated with the primers 18Sf and 18Sr.
Suppressor Screen. To screen for overexpression, suppressors of sly1-10, sly1-10 plants were first transformed with pVICEn4HPT by the floral-dip method (30). The T-DNA vector pPCVICEn4HPT has the hygromycin resistance gene for selection and four copies of the 35S promoter at the right border (34). The sly1-10 mutant causes increased seed dormancy. After ripened sly1-10 seeds fail to germinate well on 0.6 μM ABA (Fig. 2D). Suppressors were isolated based on their ability to germinate on 0.6 μM ABA. The four copies of the 35S enhancer cause high-level constitutive overexpression of sequences downstream of the insertion site. To select dominant suppressor mutations, T1 seeds were plated on 0.6 μM ABA/15 μg/ml hygromycin in Murashige and Skoog Mes plates (0.5× Murashige and Skoog/0.8% agar/5 mM Mes, pH 5.5). Seedlings that germinated and appeared to be hygromycin-resistant were transferred to soil and retested for suppression of the germination phenotype in the T2 generation.
Fig. 2.
Antisense and overexpression of SLY1. (A) Photograph of 7-week-old plants. (B) Directional RT-PCR analysis of SLY1 and ACT2 mRNA accumulation in antisense and overexpression lines. Primers detect no mRNA in the sly1-10 negative control, because primers were designed around the rearrangement in this mutant. An ethidium bromide-stained 1.5% agarose gel from RT-PCR using 100 ng of total RNA for each sample is shown. The SLY1-AS had a similar effect on SLY1 mRNA accumulation in four independent transformants. (C) Western blot analysis of crude protein extracts of 10-day-old seedlings fractionated on 10% SDS/PAGE, and detected with anti-RGA. Lines shown are Ler and sly1-10 transformed with SLY1-AS, SLY1-OE, SNE-OE, or untransformed, as well as sly1-2/rga-24. (D) Percent germination after 5 days on Murashige and Skoog plates containing 0, 0.3, 0.6, 1.2, or 3.0 μM ABA for sly1-10 (Upper) and sly1-2 (Lower) seeds for the following lines: □, Ler wild-type control; ▪, untransformed sly1-2 or sly1-10; •, SLY1-AS transformant; ▴, SLY1-OE transformant. (E) Photograph of 4-week-old Ler, sly1-10, and suppressor of sly1-10 isolate1.3 (sly1-10 OE SNE).
Immunoblot Analysis. Tissue from 10-day-old seedlings was ground in liquid nitrogen and then homogenized in ice-cold 50 mM Tris·acetate, pH 8.6/5 mM EDTA/5 mM EGTA/5% glycerol/0.5% polyvinylpyrrolidone/5 mM DTT and a Complete Mini protease inhibitor mixture tablet (Roche), according to manufacturer's instructions. The crude protein extract was centrifuged at 20,000 × g for 20 min at 4°C. The supernatant was removed and added to SDS/PAGE sample buffer. Proteins were separated on a 10% SDS/PAGE minigel loaded with ≈20 μg of total protein per lane (mini protean II, Bio-Rad) and blotted (Mini Transblot Electrophoretic Transfer Cell, Bio-Rad) to poly(vinylidene difluoride) membranes, according to the manufacturer's instructions. Protein blots were then incubated for 1 h in 5% (wt/vol) nonfat dry milk in TBS·Tween 20 and for 2 h in anti-RGA (1:15,000) (25). Cross-reacting proteins were visualized with donkey anti-rabbit alkaline phosphatase conjugate (Jackson ImmunoResearch) and visualized with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrate (Roche).
Oligonucleotide Sequences. Sequences are as follows: 2-62f, 5′-AAGGCATCTGAGAAACCC-3′; 2-64r, 5′-GGCTAACCATCGCAAGAATAAC-3′; MIf21.6f, 5′-TCCTCCTCTCTCTCTGCTTCTCAC-3′; MIf21.6r, 5′-TCCCCAAGAGTCAATAACTTGCTC-3′; Sly1r, 5′-CCAGCATTGAACATCACATCTGAC-3′; nosr, 5′-CGGGATCCCCCGATCTAGTAACATAGATGACAC-3′; 2-63f, 5′-TCTCTCTAAACCCAATCCG-3′; Act2f, 5′-CTGGATTCTGGTGATGGTGTGTC-3′; Act2r, 5′-TCTTTGCTCATACGGTCAGCG-3′; 18Sf, 5′-CAGACTGTGAAACTGCGAATGGCTC-3′; and 18Sr, 5′-GACCCATCCCAAGGTTCAACTACGA-3′.
Results
Overexpression and Antisense Expression of SLY1. In the process of characterizing the SLY1 gene, we examined the effect of overexpression and antisense expression of SLY1 in wild-type Ler and in sly1 mutants. Because sly1-2 and sly1-10 mutations in SLY1 are recessive and cause GA insensitivity, we expected overexpression of SLY1 to lead to evidence of increased GA signaling and antisense expression of SLY1 to result in a GA-insensitive phenotype. Overexpression of the SLY1 gene complemented the dwarf and infertility phenotypes in both sly1-2 and sly1-10 and had no apparent effect on growth of wild-type Ler (Fig. 2 A). Surprisingly, antisense expression of SLY1 also rescued the sly1-2 and sly1-10 dwarf and infertility phenotypes, whereas antisense expression in Ler caused no decrease in plant height (Fig. 2 A). PCR analysis confirmed that the lines were transformed with the correct construct (data not shown). Directional RT-PCR showed that lines containing the SLY1-OE (overexpression) construct overproduced the SLY1 transcript, whereas those containing SLY1-AS (antisense) construct produced little or no SLY1 transcript (Fig. 2B). Northern blot analysis showed that the SLY1-AS construct did not eliminate the SNE transcript accumulation, suggesting that the effect of the construct is specific to SLY1 mRNA accumulation (data not shown). Thus, elimination of the SLY1 mRNA by SLY1-AS rescues sly1-2 dwarfism and infertility phenotypes. Whereas sly1-2 and sly1-10 alleles are recessive, they do produce transcript (13). Thus, we postulate that sly1-2 and sly1-10 are recessive interfering mutations such that (i) the SLY1 homologue SNE can functionally replace SLY1 in the complete absence of SLY1 mRNA and (ii) expression of the sly1-2 and sly1-10 alleles containing C-terminal truncations does not interfere with wild-type SLY1 function, but does interfere with the ability of SNE to replace SLY1.
The DELLA protein RGA is subject to GA regulation such that the protein disappears in wild-type Ler upon addition of GA (25). The sly1-2 and sly1-10 mutations lead to overaccumulation of RGA both in the presence and absence of GA (11). Immunoblot analysis of RGA protein accumulation showed that both the SLY1-OE and SLY1-AS constructs reduced the accumulation of RGA protein in sly1-10 (Fig. 2C) and in sly1-2 (data not shown).
The sly1-2 and sly1-10 mutants were isolated in screens for mutants with increased seed dormancy (12, 13). The seed germination phenotype of these GA-insensitive mutants is highly variable from seed lot to seed lot, ranging from 0% to 100% germination after harvest. Those seed lots that are highly dormant do eventually germinate following afterripening in dry storage (K.M.M. and C.M.S., unpublished results). However, afterripened sly1-2 and sly1-10 seeds are hypersensitive to ABA, showing very low germination at 0.6 μM ABA, a concentration that does not inhibit wild-type germination. Neither the SLY1-OE nor SLY1-AS construct had a significant effect on wild-type Ler germination (data not shown). Both SLY1-OE and SLY1-AS significantly increased germination of sly1-2 and sly1-10 on 0.3 and 0.6 μM ABA (Fig. 2D).
Isolation of SNE as an Overexpression Suppressor of sly1-10. SNE was recovered in a screen for genes that could suppress sly1-10 when overexpressed. We screened ≈1,000 pPCVICEn4HPT transformed sly1-10 plants for the ability to germinate on 0.6 μM ABA. Transformation with the T-DNA pPCVICEn4HPT results in overexpression of downstream sequences because of four copies of the 35S promoter at the right border. When suppressor candidates were screened for levels of SNE mRNA accumulation, isolate 1.3 was found to be overexpressing SNE (Fig. 3A). Isolate 1.3 rescues the sly1-10 defects in germination, plant height, and fertility (Fig. 2E). To confirm that these effects are due to SNE overexpression, sly1-10 plants were transformed with SNE under control of the 35S promoter. Transformation of sly1-10 plants with this clone confirmed that SNE overexpression rescues the dwarf phenotype (data not shown). Western blot analysis also shows little or no RGA protein accumulation in sly1-10 plants overexpressing SNE (Fig. 2C). This result suggests that SNE stimulates the disappearance of RGA.
Fig. 3.
Overexpression of SNE and sly1-2. (A) RNA gel blot analysis of SNE mRNA and 18S rRNA accumulation in Ler, sly1-10, and suppressor of sly1-10 isolate 1.3 using 15 μg of total RNA isolated from total aerial tissue of 5-week-old plants. The transcript size from SNE-OE in isolate 1.3 is larger than the natural SNE due to the T-DNA insertion site. (B) RT-PCR analysis of SLY1 mRNA in sly1-2-OE lines, designated as dwarf (D) or tall (T). ACT2 mRNA levels were also monitored for a loading control. An ethidium bromide-stained 1.5% agarose gel is shown. (C) Photograph of 4-week-old Ler, tall (T) and dwarf (D) sly1-2-OE lines, and sly1-2.
Overexpression of sly1-2 in Ler. If the sly1-2 and sly1-10 alleles interfere with the ability of SNE to rescue loss of SLY1 function, it is possible that overexpression of the sly1-2 allele will interfere with wild-type SLY1 activity. To test this hypothesis, wild-type Ler plants were transformed with sly1-2 under control of the 35S promoter (sly1-2-OE). Of eight transformed plants recovered, four showed varying degrees of dwarfism, whereas four appeared to be wild type (Fig. 3C). We expected the variation in phenotype to correspond to the level of sly1-2 overexpression. RT-PCR analysis of sly1-2 expression in two dwarf and two tall sly1-2OE transformants revealed that the dwarf phenotype correlated with higher levels of sly1-2 mRNA accumulation (Fig. 3B).
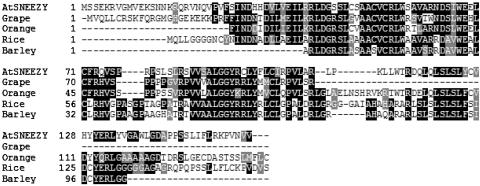
The SNE Gene. Like SLY1, SNE is highly conserved in the plant kingdom. Members of the SNE gene family were identified by a tblastn search of plant ESTs and the rice genomic sequence (ref. 35; see also www.tigr.org) and are distinct from previously identified SLY1 homologues (Fig. 4). No homologues were detected outside of the plant kingdom. SNE homologues ranged from 32.3% amino acid identity in barley to 45.6% in orange.
Fig. 4.
SNE homologues. clustalw alignment of predicted SNE protein (At5g48170) with plant homologues from grape (CF609441), orange (CK665669), rice (AC116426), and barley (CF609441) is shown. The amino acid sequence is predicted from the largest ORF in each sequence.
SNE is a 471-bp gene encoding a predicted 157-aa protein. The SNE transcript is ≈900 bp in length (Fig. 5) and corresponds to GenBank cDNA accession no. NM124191. Like SLY1, SNE contains no intron. clustalw alignment revealed that the SLY1 and SNE genes are 53% identical at the nucleotide level, and they are 25% identical and 42% similar at the amino acid level (36). Developmental Northern blot analysis of the SNE transcript revealed that it accumulates at high levels in stems and flowers and at a low level in seedlings, rosette leaves, cauline leaves, and green siliques (Fig. 5). SLY1, conversely, is expressed at a low level in all examined tissues (11).
Fig. 5.
SNE developmental mRNA expression. A Northern blot analysis of SNE mRNA and 18S rRNA control using 15 μg of total RNA isolated from wild-type Ler stems (ST), rosette leaves (RL), cauline leaves (CL), flowers (F), green siliques (GS), and seedlings (Sdlg).
Discussion
This article describes a genetic interaction between two homologous F-box genes, SLY1 and SNE, in GA signaling. Two lines of evidence converged to indicate that the SNE gene can functionally replace SLY1 and that the sly1-2 and sly1-10 mutations are recessive interfering with respect to SNE. First, antisense expression of the SLY1 gene rescued the sly1-2 and sly1-10 mutations. This result suggests that in the complete absence of SLY1 transcript, the SNE gene is able to functionally substitute for SLY1 in GA signaling. Second, SNE was recovered in a screen for genes that can suppress sly1-10 when overexpressed. These results point to an interesting interaction between the SLY1 and SNE genes in GA signaling.
The sly1-2 and sly1-10 alleles are GA-insensitive recessive mutations causing increased seed dormancy, sensitivity to ABA, dwarfism, and reduced fertility (12, 13). Both mutations result in C-terminal truncation, but neither mutation resulted in a loss of transcript (11), and they are not true knockouts. Because these mutations are not dominant, it is clear that they do not normally interfere with wild-type SLY1 activity. However, it is possible that sly1-2 and sly1-10 can prevent SNE from replacing SLY1 in GA signaling. A number of models might explain these observations. One possibility is that the loss of the last 40 aa in sly1-2 and last 8 aa in sly1-10 prevents the protein from interacting with the DELLA substrate but does not prevent association with the SCF complex through the intact F-box domain. Once sly1-2 and sly1-10 proteins are no longer present, SNE might be able to join the SCF complex in the place of SLY1 for fairly efficient degradation of SCFSLY1 targets. In this case, the affinity of wild-type SLY1 for its SCF complex is greater than the affinity of sly1-2 and sly1-10, and the affinity of sly1-2 and sly1-10 for the SCF complex is greater than the affinity of SNE. Alternatively, SLY1 and SNE proteins may act in a heterodimer such that the truncated sly1-2 or sly1-10 protein can “poison” the complex.
The function of SLY1 and SNE in GA signaling is exquisitely sensitive to changes in dosage. Overexpression of SNE in the sly1-10 mutant background inundates the system with excess SNE, allowing SNE to overcome the interfering effect of sly1-10. Moreover, overexpression of sly1-2 at high levels in Ler pushes the equilibrium in the other direction, allowing sly1-2 to interfere with wild-type SLY1 protein activity causing a dwarf phenotype. The dwarfism in sly1-2-OE lines seems to depend on the level of sly1-2 transcript accumulation (Fig. 3B). Because antisense of SLY1 rescues the sly1-2 and sly1-10 mutants, we predict that a complete knockout of SLY1 will have no phenotype. All of the published alleles of SLY1 and rice GID2 are either point mutations or C-terminal truncations located downstream of the F-box domain (reviewed in ref. 18). Interestingly, the gar2 mutant is a point mutation in the SLY1 C terminus, resulting in a dominant gain-of-function mutation that rescues the gai-1 dwarf (15, 20). This mutation causes increased affinity of SLY1 for its substrates RGA and GAI (15, 20).
Although the fact that SNE-OE reduces RGA accumulation in the sly1-10 background suggests that SNE might normally be involved in GA signaling, the possibility that SNE functions in another pathway has not been ruled out. It is possible that SNE normally regulates a different target and can only regulate RGA in the absence of SLY1. Such cross-reactivity between F-box proteins may provide one mechanism for the crosstalk recently observed between the GA and ethylene or auxin pathways (37, 38). It is interesting to note that F-box proteins involved in hormone signaling have a tendency to cluster in similar F-box families. Whereas SNE and SLY1 are in the C2 family, the auxin F-box TIR1 and the jasmonate F-box COI1 are in the C3 family (17, 39, 40). If SNE normally functions in GA signaling, expression of a truncated SNE gene would be expected to cause a GA-insensitive phenotype.
SNE is the founding member of a new family of F-box proteins in plants (Fig. 4). Like the 151-aa SLY1 protein, the 157-aa SNE protein is small and has an unusually high predicted pI of 10.38. It has recently been shown that both SLY1 and SNE/SLY2 associate with the same Arabidopsis Skp1 homologues (20). SNE transcript accumulation is developmentally regulated, showing the highest level in stems and flowers. This pattern of mRNA accumulation is similar to that seen for GID2 (M. Matsuoka, personal communication). The SNE family members are similar to, but distinct from, the members of the SLY1 protein family in plants (Fig. 4 and refs. 11 and 18). One of the SNE homologues is a GID2-like gene in rice temporarily referred to as OsSNE. The SLY1 amino acid sequence is 30.2% identical/41% similar to GID2 and 25% identical/42% similar to OsSNE, whereas SNE is 21.3% identical/33.3% similar to GID2 and 44.8% identical/57.4% similar to OsSNE. The existence of SNE homologues in other plant species suggests that the function of SNE may be conserved among higher plants.
Acknowledgments
We thank Tai-ping Sun (Duke University, Durham, NC) for the RGA antibody and J. Bean, A. Ardiani, S. Nelson, and K. Johnson for expert technical assistance. This work was supported by U.S. Department of Agriculture National Research Initiative Grant 2002-01351.
Abbreviations: GA, gibberellin; ABA, abscisic acid; Ler, Landsberg erecta; SLY1, SLEEPY1; SNE, SNEEZY; SCF, Skp1-cullin-F-box.
References
- 1.Olszewski, N., Sun, T. P. & Gubler, F. (2002) Plant Cell 14 Suppl., S61-S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomi, K. & Matsuoka, M. (2003) Curr. Opin. Plant Biol. 6, 489-493. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen, S. E., Olszewski, N. E. & Meyerowitz, E. M. (1998) Symp. Soc. Exp. Biol. 51, 73-78. [PubMed] [Google Scholar]
- 4.Fridborg, I., Kuusk, S., Robertson, M. & Sundberg, E. (2001) Plant Physiol. 127, 937-948. [PMC free article] [PubMed] [Google Scholar]
- 5.Silverstone, A. L., Ciampaglio, C. N. & Sun, T. (1998) Plant Cell 10, 155-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng, J., Carol, P., Richards, D. E., King, K. E., Cowling, R. J., Murphy, G. P. & Harberd, N. P. (1997) Genes Dev. 11, 3194-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wen, C. K. & Chang, C. (2002) Plant Cell 14, 87-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, H., Qin, L., Lee, S., Fu, X., Richards, D. E., Cao, D., Luo, D., Harberd, N. P. & Peng, J. (2004) Development 131, 1055-1064. [DOI] [PubMed] [Google Scholar]
- 9.Lee, S., Cheng, H., King, K. E., Wang, W., He, Y., Hussain, A., Lo, J., Harberd, N. P. & Peng, J. (2002) Genes Dev. 16, 646-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogas, J., Kaufmann, S., Henderson, J. & Somerville, C. (1999) Proc. Natl. Acad. Sci. USA 96, 13839-13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGinnis, K. M., Thomas, S. G., Soule, J. D., Strader, L. C., Zale, J. M., Sun, T. P. & Steber, C. M. (2003) Plant Cell 15, 1120-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steber, C. M., Cooney, S. E. & McCourt, P. (1998) Genetics 149, 509-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steber, C. M. & McCourt, P. (2001) Plant Physiol. 125, 763-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng, N., Schulman, B. A., Song, L., Miller, J. J., Jeffrey, P. D., Wang, P., Chu, C., Koepp, D. M., Elledge, S. J., Pagano, M., et al. (2002) Nature 416, 703-709. [DOI] [PubMed] [Google Scholar]
- 15.Dill, A., Thomas, S. G., Hu, J., Steber, C. M. & Sun, T. P. (2004) Plant Cell 16, 1392-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patton, E. E., Willems, A. R. & Tyers, M. (1998) Trends Genet. 14, 236-243. [DOI] [PubMed] [Google Scholar]
- 17.Gagne, J. M., Downes, B. P., Shiu, S. H., Durski, A. M. & Vierstra, R. D. (2002) Proc. Natl. Acad. Sci. USA 99, 11519-11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh, H., Matsuoka, M. & Steber, C. M. (2003) Trends Plant Sci. 8, 492-497. [DOI] [PubMed] [Google Scholar]
- 19.Tyler, L., Thomas, S. G., Hu, J., Dill, A., Alonso, J. M., Ecker, J. R. & Sun, T. P. (2004) Plant Physiol. 135, 1008-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu, X., Richards, D. E., Fleck, B., Xie, D., Burton, N. & Harberd, N. P. (2004) Plant Cell 16, 1406-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki, A., Itoh, H., Gomi, K., Ueguchi-Tanaka, M., Ishiyama, K., Kobayashi, M., Jeong, D. H., An, G., Kitano, H., Ashikari, M. & Matsuoka, M. (2003) Science 299, 1896-1898. [DOI] [PubMed] [Google Scholar]
- 22.Gomi, K., Sasaki, A., Itoh, H., Ueguchi-Tanaka, M., Ashikari, M., Kitano, H. & Matsuoka, M. (2004) Plant J. 37, 626-634. [DOI] [PubMed] [Google Scholar]
- 23.Pysh, L. D., Wysocka-Diller, J. W., Camilleri, C., Bouchez, D. & Benfey, P. N. (1999) Plant J. 18, 111-119. [DOI] [PubMed] [Google Scholar]
- 24.Gubler, F., Chandler, P. M., White, R. G., Llewellyn, D. J. & Jacobsen, J. V. (2002) Plant Physiol. 129, 191-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverstone, A. L., Jung, H. S., Dill, A., Kawaide, H., Kamiya, Y. & Sun, T. P. (2001) Plant Cell 13, 1555-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh, H., Ueguchi-Tanaka, M., Sato, Y., Ashikari, M. & Matsuoka, M. (2002) Plant Cell 14, 57-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikeda, A., Ueguchi-Tanaka, M., Sonoda, Y., Kitano, H., Koshioka, M., Futsuhara, Y., Matsuoka, M. & Yamaguchi, J. (2001) Plant Cell 13, 999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King, K. E., Moritz, T. & Harberd, N. P. (2001) Genetics 159, 767-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dill, A. & Sun, T. (2001) Genetics 159, 777-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clough, S. J. & Bent, A. F. (1998) Plant J. 16, 735-743. [DOI] [PubMed] [Google Scholar]
- 31.An, Y. Q., McDowell, J. M., Huang, S., McKinney, E. C., Chambliss, S. & Meagher, R. B. (1996) Plant J. 10, 107-121. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY).
- 33.Verwoerd, T. C., Dekker, B. M. & Hoekema, A. (1989) Nucleic Acids Res. 17, 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi, H., Czaja, I., Lubenow, H., Schell, J. & Walden, R. (1992) Science 258, 1350-1353. [DOI] [PubMed] [Google Scholar]
- 35.Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. (1990) J. Mol. Biol. 215, 403-410. [DOI] [PubMed] [Google Scholar]
- 36.Smith, R. F., Wiese, B. A., Wojzynski, M. K., Davison, D. B. & Worley, K. C. (1996) Genome Res. 6, 454-462. [DOI] [PubMed] [Google Scholar]
- 37.Vriezen, W. H., Achard, P., Harberd, N. P. & Van Der Straeten, D. (2004) Plant J. 37, 505-516. [DOI] [PubMed] [Google Scholar]
- 38.Fu, X. & Harberd, N. P. (2003) Nature 421, 740-743. [DOI] [PubMed] [Google Scholar]
- 39.Gray, W. M., del Pozo, J. C., Walker, L., Hobbie, L., Risseeuw, E., Banks, T., Crosby, W. L., Yang, M., Ma, H. & Estelle, M. (1999) Genes Dev. 13, 1678-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie, D. X., Feys, B. F., James, S., Nieto-Rostro, M. & Turner, J. G. (1998) Science 280, 1091-1094. [DOI] [PubMed] [Google Scholar]