Abstract
It is known that Escherichia coli K-12 is cryptic (Phn−) for utilization of methyl phosphonate (MePn) and that Phn+ variants can be selected for growth on MePn as the sole P source. Variants arise from deletion via a possible slip strand mechanism of one of three direct 8-bp repeat sequences in phnE, which restores function to a component of a putative ABC type transporter. Here we show that Phn+ variants are present at the surprisingly high frequency of >10−2 in K-12 strains. Amplified-fragment length polymorphism analysis was used to monitor instability in phnE in various strains growing under different conditions. This revealed that, once selection for growth on MePn is removed, Phn+ revertants reappear and accumulate at high levels through reinsertion of the 8-bp repeat element sequence. It appears that, in K-12, phnE contains a high-frequency reversible gene switch, producing phase variation which either allows (“on” form) or blocks (“off” form) MePn utilization. The switch can also block usage of other metabolizable alkyl phosphonates, including the naturally occurring 2-aminoethylphosphonate. All K-12 strains, obtained from collections, appear in the “off” form even when bearing mutations in mutS, mutD, or dnaQ which are known to enhance slip strand events between repetitive sequences. The ability to inactivate the phnE gene appears to be unique to K-12 strains since the B strain is naturally Phn+ and lacks the inactivating 8-bp insertion in phnE, as do important pathogenic strains for which genome sequences are known and also strains isolated recently from environmental sources.
Escherichia coli can use PIII compounds such as phosphite and organophosphonates, e.g., methylphosphonate (MePn) and aminoethylphosphonate (AEPn) as P sources. Their metabolism involves the enzyme C-P lyase, which appears to have a relatively broad substrate specificity (21). Whereas the mechanism of C-P lyase is not well understood, much is known about a cluster of 17 contiguous phn genes in E. coli, required for utilization of PIII compounds (5, 21). The phnGHIJK genes within this cluster are thought to encode the core components of C-P lyase, while phnF and phnO potentially encode regulatory proteins (5). Several phn genes appear to encode components of solute transporters, and it has been deduced that, among these, the phnCDE genes encode an ABC type transporter. In this transporter, phnC encodes the ABC permease component, phnD encodes the periplasmic binding protein, and phnE encodes the integral membrane component.
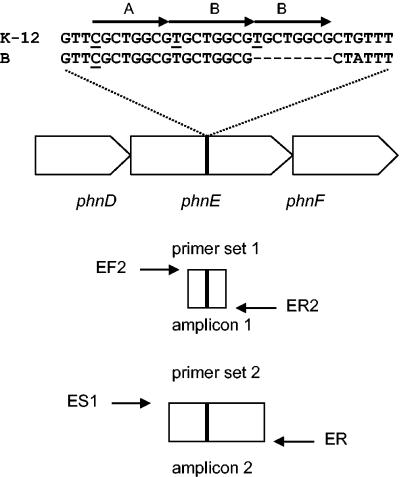
An interesting feature of the genetics of phosphonate metabolism in E. coli is that the B strain can use phosphonates whereas the K-12 strain is cryptic despite containing the entire phn gene cluster (21). The genetic basis for this crypticity was investigated by Makino et al. (14) and traced to an 8-bp insertion in the coding region of the phnE gene in the K-12 strain relative to the B strain, causing truncation of the phnE product. They also observed that the 8-bp sequence is one element in the direct triply repeated sequence in the K-12 strain comprising two types of octamer variants in the arrangement 5′-ABB-3′, where A corresponds to the sequence 5′-CGCTGGCG-3′ and B corresponds to the sequence 5′-TGCTGGCG-3′ (Fig. 1). Makino et al. isolated variants of E. coli K-12 able to use MePn as the sole P source (Phn+), and these were found to have deletions of octamer B, which, they postulated, occurred via a strand slippage event during DNA replication (14). The nature of the variation in the phnE gene in E. coli is investigated in more detail in this work.
FIG. 1.
Repetitive sequence in phnE of E. coli. The figure shows part of the phn gene cluster in E. coli and focuses on phnE and the location and sequences of a direct triple repeat in the K-12 strain and a direct double repeat in the B strain, where A corresponds to the octamer 5′-CGCTGGCG-3′ and B corresponds to the octamer 5′-TGCTGGCG-3′. The relative positions of pairs of primer used in this work to amplify two different segments of phnE of E. coli K-12 are shown under the gene organization. Amplicon 1 was used in the study in the AFLP analysis, and amplicon 2 was used for gene sequencing.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All the strains of E. coli used in this study (Table 1) were routinely cultivated under aerobic conditions at 37°C in Luria-Bertani broth (LB) with agar added to 0.8%, wt/vol, for agar plates except for Phn+ variants of K-12 strains (see below) and temperature-sensitive mutants for which the permissive temperature for growth was 30°C. The minimal medium used was modified Neidhardt’s medium (MNM) and was based on that of Neidhardt et al. (15), but, in order to study the metabolism of different phosphorous sources, the normal phosphate buffer component was replaced with MOPS (morpholinepropanesulfonic acid). MNM contained MOPS (40 mM), glucose (11.1 mM), NH4Cl (9.5 mM), Tricine (4 mM), thiamine · HCl (29.6 μM), FeSO40 · 7H20 (10 μM), CaCl2 (0.5 μM), MnCl2 (0.8 nM), CoCl2 (0.3 nM), CuSO4 (0.16 nM), ZnSO4 (0.1 nM), (NH4)6Mn7O24 (30 pM), and H3BO4 (4 pM). The medium was adjusted to pH 7.4. MNM was solidified for plates (MNM-agarose) with electrophoresis grade agarose (1%, wt/vol) because it is especially low in phosphate compared to purified agars. MNM and MNM-agarose were supplemented with different P sources at 0.5 mM. Phn+ variants of K-12 strains were isolated, purified, and maintained on MNM-agarose containing MePn unless otherwise stated. Culture dilutions were made in MNM lacking added P sources. Phosphorous compounds were obtained from the following sources. MePn was from Fluka Chemie AG; 2-AEPn, aminomethylphosphonate (AMPn), phosphonoacetate, phosphonoformate, phosphonomycin, and o-phospho-l-serine were from Sigma Chemical Co.; 1-AEPn, 1-aminopropylphosphonate (1-APPn), 3-APPn, tert-butylphosphonate, ethylphosphonate (EPn), phosphonomethylglycine (glyphosate), phenylphosphonate (PhPn), and propylphosphonate (PPn) were from Aldrich Chemical Co.; and N-butanephosphonate was from Lancaster Synthesis, Morecambe, United Kingdom
TABLE 1.
E. coli strains used
| Strain | Genotype | Source or reference |
|---|---|---|
| AB1157 | F−thi-1 hisG4 Δ(gpt-proA)62 argE3 thr-1 leuB6 kdgK51 rfbD1 ara-14 lacY-1 galK2 xyl-5 mtl-1 tsx-33 supE44 rpsL-31 Rac− λ− | 1 |
| BL21 (DE3) | F−ompT hsdSB (rB mB) dcm gal λ(DE3) | 19 |
| CD4 | Hfr metD88 proA3 Δ(lacI-Y)6 tsx-76 λ−relAl malA36(λr) metB1 | 7 |
| DH5α | φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 (rk− mk+) supE44 relA1 deoR Δ(lacZYA-argF)U169 | |
| KA796 | ara thi Δpro lac | 7 |
| MC4100 | araD139 Δ(argF-leu)169 LAM− e14−FlhD5301 fruA25 relA1 rpsL150 RbsR22 deoC1 | 4 |
| NR9458 | KA796 mutD5 zaf13::Tn10 | 8 |
| NR9807 | CD4 dnaQ49 | 8 |
| STL1671 | AB1157 sbcB15 Δ(slr-recA)304 | 3 |
| STL2172 | AB1157 mutS201::Tn5 Δ(slr-recA)304 | 13 |
| STL2314 | AB1157 dnaQ Δ(slr-recA)304 | 17 |
| NWA3 | Wild-type isolate from sewerage sludge | North West Water UK |
| NWA4 | Wild-type isolate from sewerage sludge | North West Water UK |
| NWA5 | Wild-type isolate from sewerage sludge | North West Water UK |
| NWD7 | Wild-type isolate from sewerage sludge | North West Water UK |
| NW23341 | Wild-type isolate from raw water | North West Water UK |
| NW211585 | Wild-type isolate from raw water | North West Water UK |
| ST1 | Wild-type isolate from water | Severn Trent Water UK |
| ST8 | Wild-type isolate from water | Severn Trent Water UK |
| ST11 | Wild-type isolate from water | Severn Trent Water UK |
| ST15 | Wild-type isolate from water | Severn Trent Water UK |
Estimation of the frequency of Phn+ variants.
The frequency of Phn+ variants in populations of E. coli strains was estimated by comparing the number of CFU arising when populations were diluted appropriately and plated onto MNM-agarose containing MePn compared to the number on MNM-agarose containing either Pi or o-phospho-l-serine (positive controls) or no added P source (negative control). The inocula for these experiments were first grown to stationary phase on MNM containing Pi and then washed in MNM containing no added P source. Dilutions were performed with MNM containing no added P source. Colonies arising on phosphate or phosphonates were counted at 4 and 10 days, respectively, because growth of colonies on phosphonates was generally slower than growth on Pi.
Molecular biology techniques. (i) Extraction of genomic DNA.
Template DNAs for PCRs were extracted from cultures as follows. One-tenth milliliter of culture was diluted with 0.9 ml of sterile distilled water in a 1.5-ml microcentrifuge tube. The tube was boiled for 5 min and snap-chilled in ice. The boiled cell suspensions were diluted 100-fold in sterile water, and 5 μl was sufficient to set up PCRs.
(ii) AFLP analysis.
Deletions or insertions in phnE were monitored by amplified-fragment length polymorphism (AFLP) analysis as follows. Fragments of phnE of 200 or 192 bp containing the triple or double octameric repeat regions, respectively, were amplified from samples of liquid cultures or colonies by PCR using oligonucleotide primer set 1 (Fig. 1), comprising EcphnEF2 (5′-Cy 5-TTACCAGCCCGTTCGCCGCC-3′) and EcphnER2 (5′-CCTTCCACCGGGCCAGGTTCAAT-3′). Amplifications were carried out with Bio-X-Act DNA polymerase (Bioline UK Ltd.) with the following thermal cycle: 30 cycles of 95°C for 30 s, 60°C for 1 min, and 68°C for 1 min. Products were purified with a PCR fragment purification kit (QIAGEN) and checked by gel electrophoresis in a 1% Tris-acetate-EDTA-agarose gel. Fragments were diluted and run together with standard-size fragments (50-bp ladder; Amersham) on a 6%, wt/vol, polyacrylamide sequencing gel in an ALF-Express automated sequencer (Amersham Pharmacia), and the gel image was visualized with ALF-Express software. Fragments were detected, sized, and quantified with AllelLinks, version 1.00 (Amersham Pharmacia). The relative proportions of the two alleles of phnE were estimated from a standard curve prepared from AFLP analysis of different mixtures of the K-12 strain MC4100 and the B strain BL21(DE3), prepared by mixing individual cultures grown in LB prior to preparation of templates DNA.
DNA sequencing.
To sequence the region of phnE containing the triply or doubly repeated region, a 600-bp fragment was amplified by PCR with oligonucleotide primer set 2 (Fig. 1), consisting of EcphnES1 (5′-GCGGATCCCGCAGCTG-3′) and EcphnER (5′-ACGGTCGCCGAGCGGACGTT-3′). The amplification protocol used was that described for the AFLP analysis above. The DNA was purified with a QIAGEN PCR purification kit and then sequenced with an ALF-Express automated sequencer and a cycle sequencing strategy using Cy 5-labeled EcphnEF and EcphnER primers.
RESULTS
Occurrence of Phn+ variants of E. coli K-12.
The frequencies of occurrence of Phn+ variants in populations of common laboratory E. coli K-12 strains DH5α and MC4100, grown in LB, were estimated by determining the CFU appearing 10 days after plating washed populations at suitable dilutions on MNM-agarose containing MePn and comparing them to those appearing on MNM-agarose containing Pi or o-phospho-l-serine or no added P source, as a negative control. Phn+ variants were observed to be present at the surprisingly high levels of 3.4 and 8.6% of the total CFU of DH5α and MC4100, respectively, of those observed on the plates containing Pi or o-phospho-l-serine. Plates with no added P source contained only pinpoint colonies, presumably growing on traces of utilizable P in the medium.
AFLP analysis of genotypic events in E. coli K-12 Phn variants.
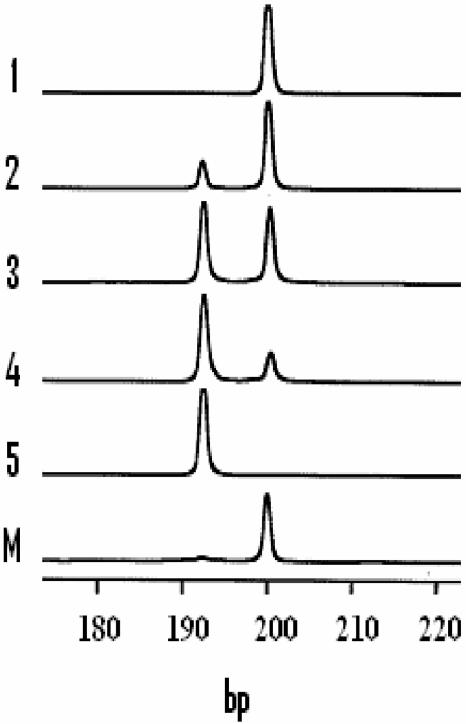
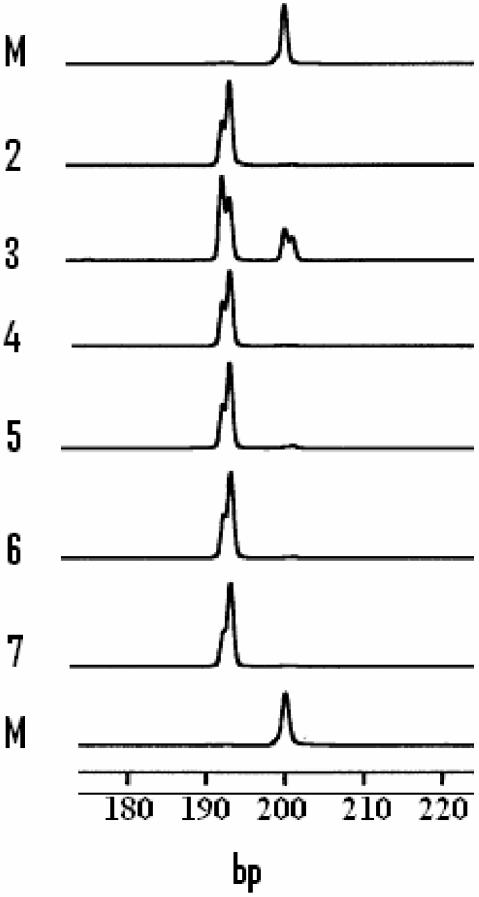
AFLP analysis was used to monitor the status of the phnE gene in different populations of strains. DNA fragments spanning that segment of the phnE gene in K-12 which contains the triple octameric repeat were amplified with primer set 1 (Fig. 1) from culture lysates of organisms grown in various media. When strains were grown in LB, as expected, a 200-bp fragment was amplified from the K-12 strain (DH5α) while a 192-bp fragment was amplified from the B strain [BL21(DE3)] (Fig. 2). When the analysis was performed on various mixtures of cultures of these two strains, both 200- and 192-bp fragments were amplified and the peak areas were approximately proportional to those for the prepared mixtures (Fig. 2). This establishes that AFLP can be used to detect both forms of phnE if present in populations. AFLP analysis was then used to examine phnE in cultures growing in MNM containing alternative P sources. Studies were performed on both MC4100 and DH5α. Results were essentially identical. Fragments of 200 bp were amplified only from cultures grown to stationary phase in MNM containing Pi and o-phospho-l-serine as the sole added P sources. However, populations of Phn+ variants which had grown to stationary phase in MNM with MePn as the sole added P source gave rise to two fragment types, the major species being 192 bp and the minor species being 200 bp. Therefore, stationary-phase populations of purified Phn+ variants unexpectedly contained a mixture of two phnE alleles, of which the major form probably contained the expected 8-bp deletion reported by Makino et al. (14). We extended these studies by examining the state of phnE in cultures in both logarithmic and stationary phases of growth with MePn as the sole P source. In the log-phase populations we could detect only fragments of 192 bp, but, in stationary-phase populations, both 192- and 200-bp fragments were detected (Fig. 3). This suggested that the deletion in phnE may be reversible and of high frequency and that it is potentially linked to later stages of the growth phase.
FIG. 2.
AFLP analysis of phnE in E. coli. Primer pair 1 (Fig. 1) was used to amplify a small fragment containing the octameric repetitive element in phnE from populations of two strains of E. coli grown to log phase in LB. PCR products were analyzed as described in Materials and Methods. Shown are fragments detected as peaks in the AFLP analysis. Tracks 1 and 5, PCR fragments produced from cultures of K-12 strain MC4100 and B strain BL21(DE3), respectively; tracks 2 to 4, artificial mixtures of MC4100 and BL21(DE3), each grown to an optical density at 600 nm of 0.7 and mixed prior to extraction of template and amplification in the ratios 9:1, 6:4, and 2:8; track M, 200-bp marker peak from the 50-bp standard ladder set used to determine fragment lengths.
FIG. 3.
AFLP analysis of phnE in cultures of E. coli K-12 MC4100 growing with different phosphonates. Cultures of K-12 MC4100 were grown in MNM with different phosphonates as sole P sources, which had been inoculated from Phn+ variants isolated and purified on MNM-agarose containing the respective phosphonate. PCR products were analyzed as described in Materials and Methods. Shown are fragments detected as peaks in the AFLP analysis. Tracks correspond to the phosphonates supporting growth as follows: 2, MePn, log phase; 3, MePn, stationary phase; 4, EPn, log phase; 5, AMPn, log phase; 6, 2-AEPn, log phase; 7, 3-APPn, log phase; M, 200-bp marker peak from the 50-bp standard ladder set used to determine fragment lengths.
Reversible switching in the phnE gene.
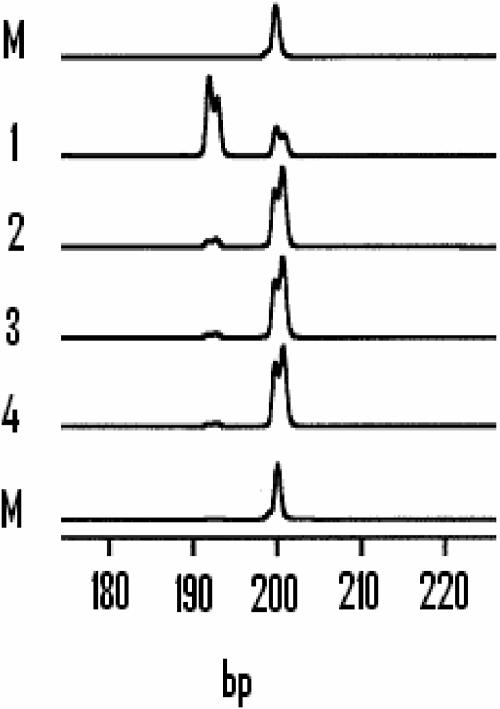
Further evidence for reversibility of the 8-bp deletion in phnE was obtained in the following experiment. Phn+ variants of MC4100 originally selected and purified on MNM containing MePn were first grown in MNM plus MePn and then serially subcultured in LB with a 0.03% inoculum level at each subculture. AFLP analysis was performed on samples removed at stationary phase after each round (Fig. 4). In each set of populations derived from a Phn+ variant, the initial proportion of the 192-bp fragment was high but subculture in LB resulted in a dramatic decrease in the proportion of the 192-bp forms and a reciprocal increase in the proportion of the 200-bp forms (Fig. 4).
FIG. 4.
AFLP analysis of phnE in cultures of E. coli K-12 MC4100 growing with different phosphonates. Shown is AFLP analysis of phnE at the stationary phase of successive subcultures of a Phn+ variant of E. coli K-12 MC4100 selected and purified on MNM-agarose. The inoculum level used for each round of subculture was 0.03%. The tracks show the fragments produced after amplification performed at the end of each subculture in the following succession of media: 1, MNM with MePn as the sole added P source; 2 to 4, LB. Tracks M, 200-bp marker peak from the 50-bp standard ladder set used to determine fragment lengths.
DNA sequencing of amplified fragments.
To confirm that the 8-bp deletion in phnE observed in these experiments was identical to that described by Makino et al. (14), the sequences were determined for 600-bp fragments of phnE amplified directly from boiled lysates of logarithmic-phase cultures of BL21(DE3) and MC4100 grown on Pi or on MePn and also a Phn+ variant of MC4100 which had been repeatedly subcultured on LB and that therefore had apparently undergone reversion. These data (Fig. 5) confirmed that one octamer B sequence had been lost in the Phn+ variants selected on MePn and that the B strain contains only the AB repeat. The data also showed for the first time that the apparent reversion event detected by AFLP when Phn+ variants were cultivated in LB and not selected for MePn utilization involves precise restoration of an octamer B lost during the original selection process.
FIG. 5.
Variation in the sequences of phnE genes in various isolates of E. coli. (Top) Sequences determined in this work for that part of phnE containing the octameric repetitive element for the following strains and conditions: MC4100/Pi, MC4100 grown in MNM with Pi as the sole added P source; MC4100/MePn, a Phn+ variant isolated and purified on MNM-agarose with MePn as the sole P source; MC4100/MePn→LB, revertant obtained after successive subculture of a Phn+ variant in LB. Asterisks, positions of the direct repeat sequences. (Bottom) Sequences published for four strains of E. coli: K-12 and B (14), the enterohemorrhagic strain 0157:H7 (9, 16), and the uropathogenic strain CFT073 (22).
Behavior of the phnE gene in different K-12 strains.
The status and behavior of the phnE gene in K-12 derivatives carrying mutations in recA (STL1671, STL2172, and STL2314), mutS (STL2172), dnaQ (STL2314 and NR9807), mutD (NR9458), and sbcB (STL1671) were examined. When cultures were cultivated in MNM with Pi as the sole added P source, a 200-bp fragment was amplified from all strains with primer set 1. Phn+ variants were then isolated for each strain. All Phn+ variants yielded 192-bp fragments after AFLP analysis using oligonucleotide set 1 (Fig. 5).
Phn+ variants obtained with different phosphonate sources.
A requirement for the 8-bp deletion in phnE for utilization of other organophosphonates was also examined. In this experiment, Phn+ variants of MC4100 were isolated and purified on MNM-agarose with alternative phosphonates provided as sole P sources. AFLP analysis was performed on logarithmically growing populations with primer set 1. As with Phn+ variants selected and grown on MePn, fragments of 192 bp were amplified from all populations which grew well using EPn, AMPn, 2-AEPn, and 3-APPn as P sources (Fig. 3). Also, as with Phn+ variants selected on MePn, all these variants gave rise to mixtures of 192- and 200-bp fragments in stationary phase (data not shown). Very slow growth was observed with PhPn and PPn. In the PhPn cultures, a 200-bp fragment was the sole fragment amplified, but in the PPn-grown organisms traces of the 192-bp forms were detected. No significant growth was observed with the following phosphonates: N-butanephosphonate, tert-buty phosphonate, 1-AEPn, 1-EPn, 1-APPn, phosphonoacetate, phosphonoformate, and phosphonomycin.
Properties and behavior of the phnE gene in non K-12 E. coli strains.
Eleven strains of E. coli originally isolated from raw water or sewerage sludge on nutrient broth or LB were screened for potential crypticity in phosphonate metabolism and for the occurrence of the triple-repeat sequences in phnE. Their ability to use MePn as a sole P source on MNM was tested as for the K-12 strains previously. Of 11 independent isolates, four strains, 1, 8, A3, and D7, gave plating efficiencies significantly below 100% (at 5.7, 39, 35, and 1.3%, respectively) on MePn compared to the controls supplied with Pi. This suggested that these strains may be cryptic and may contain an inactivated phnE gene similar to that observed in K-12 strains. Therefore we determined the nucleotide sequences of the 600-bp fragments of the phnE genes surrounding the octameric repeats amplified from cultures not exposed to added phosphonates (Fig. 5). However, although a few base substitutions affecting only the third base coding positions were observed in the different phnE sequences, all strains contained the double octameric repeat sequence in phnE, as found in the B strain (data not shown).
DISCUSSION
We have established that Phn+ variants appear to be present at a surprisingly high frequency (>10−2) in populations of all the E. coli K-12 strains tested here. AFLP analysis proved useful in monitoring the genotypic behavior of phnE in populations of various strains grown under different conditions, and significantly it revealed the reappearance of the phnE allele in populations of Phn+ variants, especially in stationary-phase cultures. Possible explanations for persistence of the phnE allele include cross-feeding of the Phn− strains by the Phn+ variants and/or genetic heterogeneity in phnE in individual cells carrying more than one chromosome copy. However, because our studies were conducted with purified Phn+ isolates, we conclude that the phnE gene in E. coli K-12 behaves like a reversible gene switch, causing phase variation in phosphonate metabolism. This appears to be the first example of phase variation in a component of an ABC transporter in a gram-negative bacterium (10) although it is interesting that a high-frequency frameshift phase variation event affects the proposed substrate-binding lipoprotein encoded within an ABC transporter operon in Mycoplasma fermentans (20). It is not clear why “off” forms start to accumulate rapidly late in the growth cycle on phosphonates or once the selection for growth on phosphonates is removed. From a large number of such studies with different strains, we estimate that the switch may operate at frequencies as least as high as ∼10−2 per generation in either direction but that the equilibrium of the switch strongly favors the “off” form unless selection for phosphonate utilization is applied.
The octameric sequence involved in this postulated slip strand event is, at 8 bp, relatively long, and interestingly it is the most commonly occurring octamer in the genome of E. coli K-12 (2). It also contains the core trimer 5′-CTG-3′, thought to be the DnaG primase binding site (11, 18, 23, 24). The apparent high instability in phnE in K-12 may be linked to the potential involvement of this octamer in the initiation of DNA replication.
Mutations in DNA replication, repair, and recombination influence instability in tandem repeat sequences (3, 12, 17). All the K-12 mutants examined prior to selection for MePn utilization exhibited the typical K-12 5′-ABB-3′ or “off” form of phnE even though some carry mutations in functions known to increase deletions between repetitive sequences, including mutations in recA and the sbcB-encoded 3′ exonuclease I and the dnaQ49ts mutation, which affects DNA polymerase ɛ-subunit exonuclease activity and the physical interaction of the ɛ-subunit with the polymerizing α-subunit.
The physiological significance of the switch in E. coli K-12 remains unclear. In the “on” direction, the switch allows E. coli K-12 to use not only MePn but also EPn, AMPn, 2-AEPn, and 3-APPn; hence phnE is implicated in transport of all these organophosphonates. Elashvili et al. (6) have shown that the phnE gene is necessary for uptake of some organophosphates since the 8-bp deletion event in phnE enabled the E. coli K-12 strain JA221 to utilize diisopropyl fluorophosphate and its hydrolysis product, diisopropyl phosphate.
Surprisingly, the phnE switch appears to be confined to K-12 strains. E. coli strains for which genomes have been determined appear to contain the “on” form of phnE, including the uropathogenic strain CFT073 (22), where the 5′-AB-3′ repeat is perfectly conserved, and the enterohemorrhagic strain O157:H7 (9, 16), although here T substitutes for C at the seventh base in octamer A in the 5′-AB-3′ sequence (Fig. 5). We also found no evidence for the presence of the “off” form of phnE in several E. coli strains isolated recently from environmental samples.
Although the phnE switch may be an artifact possibly arising from the repeated mutagenesis to which the K-12 strain has been subjected, it is possible that it might protect against the uptake of naturally occurring inhibitory phosphonates present in the natural environment or in some way affect surface receptors required for coliphage or lymphocyte recognition.
Acknowledgments
Samina Iqbal was supported by a grant from The Islamic Development Bank of Saudi Arabia, George Parker was supported by a research grant from Zeneca Agrochemicals, and Helen Davidson and Elham Moslehi-Rahmani were supported by summer vacation studentships from The Society for General Microbiology.
We thank Elizabeth Pontin and Mike Taylor for running the sequencer and help with the analysis software packages. We also most grateful for the provision of strains to Malgorzata Bzymek, Susan Lovett, Roel Schaaper, and Robert Wells and the E. coli Genetic Stock Center at Yale University.
REFERENCES
- 1.Bachmann, B.J. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. AMS Press, Washington, D.C.
- 2.Blattner, F. R., G. Plunkett, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1461. [DOI] [PubMed] [Google Scholar]
- 3.Bzymek, M., C. J. Saveson, V. V. Feschenko, and S. T. Lovett. 1999. Slipped misalignment mechanisms of deletion formation: in vivo susceptibility to nucleases. J. Bacteriol. 181:477-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and mu. J. Mol. Biol. 104:541-555. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C.-M., Q.-Z. Ye, Z. Zhu, B. L. Wanner, and C. T. Walsh. 1990. Molecular biology of carbon-phosphorous bond cleavage. Cloning and sequencing of the phn (psiD) genes involved in alkylphosphonate uptake and C-P lyase activity in Escherichia coli B. J. Biol. Chem. 265:4461-4471. [PubMed] [Google Scholar]
- 6.Elashvili, I., J. D. Joseph, and C. C. Valeria. 1998. phnE and glpT genes enhance utilization of organophosphates in Escherichia coli K-12. Appl. Environ. Microbiol. 64:2601-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fijalkowska, I. J., R. L. Dunn, and R. M. Schaaaper. 1993. Mutants of Escherichia coli with increased fidelity of DNA replication. Genetics 134:1023-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fijalkowska, I. J., P. Joneczyk, M. M. Tkaczyk, M. Biaolskorska, and R. M. Schaaaper. 1998. Unequal fidelity of leading strand and lagging strand DNA replication on the Escherichia coli chromosome. Proc. Natl. Acad. Sci. USA 95:10020-10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C.-G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12 DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 10.Henderson, I. R., P. Owen, and J. P. Nataro. 1999. Molecular switches—the ON and OFF of bacterial phase variation. 33:919-932. [DOI] [PubMed]
- 11.Hiasa, H., H. Sakai, K. Tanaka, Y. Honda, T. Komano, and G. N. Godson. 1989. Mutational analysis of the primer RNA template region in the replication origin (oric) of bacteriophage G4: priming signal recognition by Escherichia coli primase. Gene 84:9-16. [DOI] [PubMed] [Google Scholar]
- 12.Iyer, R. R., A. Pluciennik, W. A. Rosche, R. R. Sinden, and R. D. Wells. 2000. DNA polymerase III proofreading mutants enhance the expansion and deletion of triplet repeat sequences in Escherichia coli. J. Biol. Chem. 275:2174-2284. [DOI] [PubMed] [Google Scholar]
- 13.Lovett, S. T., and V. V. Feschenko. 1996. Stabilization of diverged tandem repeats by mismatch repair: evidence for deletion formation via a misaligned replication intermediate. Proc. Natl. Acad. Sci. USA 93:7120-7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makino, K., S.-K. Kim, H. Shinagawa, M. Amemura, and A. Nakata. 1991. Molecular analysis of the cryptic and functional phn operons for phosphonate use in Escherichia coli K-12. J. Bacteriol. 173:2665-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture media for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. Dimalanta, K. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 17.Saveson, C. J., and S. T. Lovett. 1997. Enhanced deletion formation by aberrant DNA replication in Escherichia coli. Genetics 146:457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swart, J. R., and M. A. Griep. 1993. Primase from Escherichia coli primes single-stranded templates in the absence of single-stranded DNA-binding protein or other auxiliary proteins. Template sequence requirements based on the bacteriophage G4 complementary strand origin and Okazaki fragment initiation sites. J. Biol. Chem. 268:12970-12976. [PubMed] [Google Scholar]
- 19.Tabor, S., and C. C. Richardson. 1985. A bacteriophage-T7 RNA polymerase promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theiss, P., and K. S. Wise. 1997. Localized frameshift mutation generates selective, high-frequency phase variation of a surface lipoprotein encoded by a mycoplasma ABC transporter operon. J. Bacteriol. 179:4013-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanner, B. L. 1994. Molecular genetics of carbon-phosphorous bond cleavage in bacteria. Biodegradation 5:175-184. [DOI] [PubMed] [Google Scholar]
- 22.Welch R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoda, K., H. Yasuda, X. W. Xiang, and T. Okazaki. 1988. RNA-primed initiation sites of DNA replication in the origin region of bacteriophage-lambda genome. Nucleic Acids. Res. 16:6531-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoda, K., and T. Okazaki. 1991. Specificity of recognition sequence for Escherichia coli primase. Mol. Gen. Genet. 227:1-8. [DOI] [PubMed] [Google Scholar]