Abstract
Pathogenic Haemophilus influenzae, Neisseria spp. (Neisseria gonorrhoeae and N. meningitidis), Serratia marcescens, and other gram-negative bacteria utilize a periplasm-to-cytosol FbpABC iron transporter. In this study, we investigated the H. influenzae FbpABC transporter in a siderophore-deficient Escherichia coli background to assess biochemical aspects of FbpABC transporter function. Using a radiolabeled Fe3+ transport assay, we established an apparent Km = 0.9 μM and Vmax = 1.8 pmol/107cells/min for FbpABC-mediated transport. Complementation experiments showed that hFbpABC is dependent on the FbpA binding protein for transport. The ATPase inhibitor sodium orthovanadate demonstrated dose-dependent inhibition of FbpABC transport, while the protonmotive-force-inhibitor carbonyl cyanide m-chlorophenyl hydrazone had no effect. Metal competition experiments demonstrated that the transporter has high specificity for Fe3+ and selectivity for trivalent metals, including Ga3+ and Al3+, over divalent metals. Metal sensitivity experiments showed that several divalent metals, including copper, nickel, and zinc, exhibited general toxicity towards E. coli. Significantly, gallium-induced toxicity was specific only to E. coli expressing FbpABC. A single-amino-acid mutation in the gene encoding the periplasmic binding protein, FbpA(Y196I), resulted in a greatly diminished iron binding affinity Kd = 5.2 × 10−4 M−1, ∼14 orders of magnitude weaker than that of the wild-type protein. Surprisingly, the mutant transporter [FbpA(Y196I)BC] exhibited substantial transport activity, ∼35% of wild-type transport, with Km = 1.2 μM and Vmax = 0.5 pmol/107cells/min. We conclude that the FbpABC complexes possess basic characteristics representative of the family of bacterial binding protein-dependent ABC transporters. However, the specificity and high-affinity binding characteristics suggest that the FbpABC transporters function as specialized transporters satisfying the strict chemical requirements of ferric iron (Fe3+) binding and membrane transport.
To cause disease, many bacterial pathogens must compete for growth-essential iron within the extracellular environment of the human host (30, 33, 45). The majority of pathogenic bacteria employ siderophore-dependent iron acquisition systems in competition for host iron (47). These systems involve the use of nonproteinaceous iron-chelating compounds termed siderophores, which are produced and secreted into the environment (32). In gram-negative bacteria the uptake of iron-bound siderophores involves the expression of siderophore-specific outer membrane receptors and specific inner membrane binding protein-dependent ATP-binding cassette (ABC) transporters (14). These systems offer flexibility in the acquisition of iron from multiple sources; however, the expression of the numerous gene products involved in each specific siderophore-dependent transport pathway may be metabolically demanding.
In contrast, Haemophilus influenzae and pathogenic Neisseria spp. (Neisseria meningitidis and N. gonorrhoeae) utilize a highly conserved siderophore-independent high-affinity iron acquisition system (31). This system employs specific surface receptors that directly bind host iron-binding proteins, transferrin (Tf) or lactoferrin (Lf) (37). Iron is extracted from the host proteins and transported into the periplasm through an energy-dependent TonB-mediated process. Transport of free (naked) iron from the periplasm to the cytosol is mediated via the FbpABC transporter, which is composed of a ferric ion binding protein (FbpA) and an inner membrane ABC transporter consisting of a membrane permease (FbpB) and an ATP-binding protein (FbpC) (1). Although bacteria utilizing this system may express several different host protein-specific outer membrane receptors, FbpABC is a convergence point in the acquisition of iron.
By a strategy similar to that used in cloning the Serratia marcescens sfuABC operon (5), the H. influenzae hitABC and N. gonorrhoeae fbpABC operons were cloned by complementation of a siderophore-deficient (aroB) Escherichia coli strain for growth on nutrient agar containing 200 μM dipyridyl, an iron chelator (1, 2). Expression in this E. coli background has served as a model system with which to study the genetic and biochemical basis of FbpABC iron transport. Results of initial studies demonstrated that the transporter genes from these diverse bacteria exhibit a high level of homology, and a common nomenclature has been devised to designate the genetic and protein components of the transporters: for H. influenzae, the gene name is hitABC and the protein name is hFbpABC; for N. gonorrhoeae, the gene name is fbpABC and the protein name is nFbpABC. The FbpABC transporters are encoded by three-gene operons under negative regulatory control of the ferric uptake regulator (fur) (7, 15). The gene encoding the ferric ion binding protein (FbpA) is separated from the downstream two genes in the operon by a putative stem loop structure indicative of a rho-independent transcriptional terminator. This is consistent with the increase in expression of FbpA by several orders of magnitude compared with that of FbpB and FbpC (26). Biochemical analyses of nFbpA and hFbpA demonstrate that these proteins bind a single ferric (Fe3+) ion with high affinity and exhibit a characteristic spectroscopic profile (13, 34, 35). Interestingly, X-ray structural analyses of these proteins show that they bind iron in a manner remarkably similar to that of mammalian transferrin by using a common set of amino acid residues and employing a synergistic anion (10, 11). This FbpA binding mechanism results in an extremely high affinity for Fe3+, similar in scale to that of Tf (nFbpA, 2.4 × 1018 M−1; N-lobe hTf, 1.8 × 1017 M−1) (44, 49). However, this affinity is 10 to 12 orders of magnitude greater than the affinities exhibited by typical bacterial periplasmic binding proteins (PBPs) for their respective substrates (e.g., maltose binding protein for maltose; Kd = 1.6 × 10−6 M) (18). Based on sequence analysis, the nFbpB and hFbpB proteins are proposed highly hydrophobic proteins that function as membrane permeases within the context of the ABC transporters. The nFbpC and hFbpC proteins are proposed ATP-binding components of these transporters (31). The large number of recent studies on the FbpA ferric binding proteins (3, 8, 10, 16, 19, 22, 38-40, 44, 50) makes a critical investigation of the functional basis of FbpABC transport timely.
This study focuses on functional investigation of the H. influenzae hFbpABC transporter through expression of the hitABC three-gene locus in E. coli. Recombinant expression in this background includes the use of an E. coli strain (H-1443) that has a deletion in the aroB gene, rendering it unable to synthesize the sole E. coli siderophore enterochelin (9). This background allows investigation of the hFbpABC system while controlling for endogenous iron uptake systems (e.g., FepABCD, FecABCDE, FeoABC, and MntH) (17, 25, 28, 41) by the use of high-affinity metal chelators, 2,2,-dipyridyl, and nitrilotriacetic acid. Using this model system, we established an assay for radiolabeled iron uptake in intact cells and generated apparent Michaelis-Menten Km and Vmax constants for hFbpABC transport. We then defined the energy requirements and metal specificity of hFbpABC by monitoring the growth-inhibitory effects of metals competing for hFbpABC-mediated transport. Finally, we investigated the impact of a single-amino-acid hFbpA mutation on hFbpABC-mediated transport and derived the functional basis of this effect. These studies form the basis of continuing investigations aimed at augmenting our understanding of FbpABC iron transport and its contribution to pathogenesis of diverse bacterial species.
MATERIALS AND METHODS
Biochemicals, plasmids, and bacterial strains.
Ampicillin, l-arabinose, 2,2′-dipyridyl, MES (morpholineethanesulfonic acid) and Tris buffers, glucose, carbonyl cyanide m-chlorophenyl hydrazone (CCCP), nitrilotriacetic acid (NTA), cetyltrimethylammonium bromide (CTAB), cupric chloride, aluminum sulfate, zinc chloride, manganese sulfate, nickelous chloride, phenylalanine, tyrosine, tryptophan, and sodium orthovanadate were purchased from Sigma (St. Louis, Mo.). Nutrient broth (NB), Luria broth (LB), Bacto agar, and sterile supplement disks were purchased from Difco Laboratories (Detroit, Mich.). Chelex-100 and sodium dodecyl sulfate-polyacrylamide gel electrophoresis reagents were purchased from Bio-Rad (Hercules, Calif.). Ferric nitrate and gallium nitrate were purchased from Aldrich (Milwaukee, Wis.). 55Ferric chloride was purchased from NEN (Boston, Mass.). 67Gallium citrate was a generous gift from Robert Schork of University of Pittsburgh Medical Center Presbyterian Nuclear Medicine. Oligonucleotides were purchased from Gibco/Life Technologies (Gaithersburg, Md.). Taq polymerase was purchased from Boehringer Mannheim (Mannheim, Germany). Restriction enzymes were purchased from New England Biolabs (Beverly, Mass.). Nitrocellulose filters and scintillation cocktail were obtained from Fisher (Pittsburgh, Pa.). E. coli strains and plasmids were obtained as described in Table 1.
TABLE 1.
Bacterial Strains and Plasmids
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| Strains | ||
| H-1443 | E. coli aroB− | 9 |
| H-1443/pBR322 | H-1443 with pBR322 | This study |
| H-1443/pAHIΔB | H-1443 with hitA, hitC | This study |
| H-1443/pAHIO | H-1443 with hitABC | 2 |
| H-1443/pAHIΔBAY196I | H-1443 with hitA(Y1961), hitC | This study |
| H-1443/pAHIOAY196I | H-1443 with hitA(Y1961)BC | This study |
| H-1443/pACYC184-pBADHIBC | H-1443 with pACYC184 and hitBC | This study |
| H-1443/pACYCHIΔB-pBADHIBC | H-1443 with hitA, hitC, and hitBC | This study |
| H-1443/pACYCHIO-pBADHIBC | H-1443 with hitABC and hitBC | This study |
| Plasmids | ||
| pBR322 | 4.4-kb vector, Apr | Promega |
| pAHIO | 4.3-kb SmaI-BamHI fragment containing intact hitABC sequence cloned into corresponding sites in pBR322, expressing hFbpABC, Apr | 2 |
| pAHIΔB | pAHIO derivative, lacking hitB gene, expressing functional hFbpA, Apr | This study |
| pAHIOAY196I | Site-directed mutant derived from pAHIO, expressing hFbpA(Y196I)BC, Apr | This study |
| pAHIΔBAY196I | pAHIOAY196I derivative lacking hitB gene, expressing functional hFbpA(Y196I), Apr | This study |
| pACYC184 | 4.25-kb vector, Cmr, Tcr | New England Biolabs |
| pACYCHIO | 4.3-kb SmaI-BamHI fragment containing intact hitABC sequence cloned into EcoRV-BamHI sites in pACYC184, expressing hFbpABC, Cmr | This study |
| pACYCHIΔB | pACYCHIO derivative lacking hitB gene, expressing hFbpA, Cmr | This study |
| pBADHIBC | 2.6-kb fragment containing hitBC sequence cloned into pBADtopoTA (Invitrogen), expressing hFbpBC Apr | This study |
Molecular cloning.
The pAHIΔB plasmid was constructed by excision of the hitB gene from the MluI sites of the pAHIO plasmid. The resulting MluI-compatible ends were ligated, and the coding sequence was verified by automated DNA sequencing (Department of Molecular Genetics and Biochemistry Shared Resources Facility, University of Pittsburgh). The pACYCHIΔB and pACYCHIO plasmids were made by PCR amplification of the hitABC genes and ∼250 kb of upstream and downstream sequence from the pAHIO plasmid by the use of the HitO-5′ and HitO-3′ primers described previously (2). The PCR product was restricted with BamHI and SmaI and ligated into compatible BamHI and EcoRV sites of the pACYC184 vector. The pBADHIBC plasmid was constructed by PCR amplification of the hitBC genes from the pAHIO plasmid by the use of the primers HitB5′TA (5′-ATGCCTCGCAGACCGCCATTCTGGCTTAC-3′) and HitC3′TA (5′-AGCGTAAAAAAGCCCTTTTTTATGTAAATA-3′). pBAD-TOPO-TA vector (Invitrogen, Carlsbad, Calif.) was used in direct TA cloning of the PCR product.
Iron transport assay.
NB agar (NBA) and LB agar (LBA) were prepared per manufacturer instructions and supplemented with ampicillin and/or dipyridyl. M9 minimal medium (M9) was prepared, with the following final composition: 6 mg of Na2HPO4/ml, 3 mg of KH2PO4/ml, 0.5 mg of NaCl/ml, 1 mg of NH4Cl/ml, 5 mM MgSO4, and 0.1 mM CaCl2. Prior to sterilization, trace iron was removed from M9 by stirring in the presence of 100 mg of Chelex-100/liter followed by sterile filtration. Transport medium (TM) was prepared by supplementing M9 with 2 mg of glucose/ml, 0.1 mg of phenylalanine/ml, 0.1 mg of tyrosine/ml, and 0.1 mg of tryptophan/ml; radiolabeled Fe(NTA)2 or Ga(NTA)2 was added immediately prior to the assay. Cells were transformed using the heat shock method for chemically competent cells. Fresh transformants were grown to midlog phase in LB supplemented with 100 μg of ampicillin/ml (LBamp100), seeded at 106 CFU/plate on NBA supplemented with 100 μg of ampicillin/ml and 75 μM dipyridyl (NBAamp100dip75), and then grown at 37°C for 18 h. Cells were suspended in M9 and centrifuged at 4°C for 10 min at 4,000 × g. Pelleted cells were brought up in TM and diluted to an optical density at 578 nm of 0.5. The cells were then preincubated at 37°C in 5% CO2 with shaking in 24-well tissue culture plates in a water-jacketed incubator. Following a 10-min preincubation, radiolabeled Fe(NTA)2 (3 × 104 cpm/pmol) was added to the cell suspensions at a final concentration of 1 μM and incubation was continued. At designated time points, 100-μl aliquots were removed and filtered through 0.45-μm-pore-size nitrocellulose filters (filters conditioned with 5 ml of TM) by the use of a vacuum manifold (Millipore, Billerica, Mass.). Filters were immediately washed with 5 ml of 100 mM LiCl, removed, and air dried overnight at 25°C. The filters were dissolved in 4 ml of scintillation cocktail, and counts per minute were measured using a liquid scintillation counter (Packard, Billerica, Mass.).
In complementation experiments, cells were grown as described above except that the growth medium contained 30 μg of chloramphenicol/ml for pACYC vector selection and 0.2% l-arabinose to induce expression of hitBC under the control of the araBAD (pBAD) promoter.
Kinetics of iron transport.
Michaelis-Menten constants for the wild-type hFbpABC and mutant hFbpA(Y196I)BC transporters were determined using the above-described iron transport assay with the following conditions. Cells were used at a concentration of 107 cells per 100 μl, and substrate concentrations were adjusted over a range of 0.1 to 20 μM. Initial velocity measurements were determined by taking 100-μl samples at 5 and 120 s and determining counts per minute for the difference (115-s uptake). Substrate-dependent uptake was determined by subtracting hFbpA-only background uptake (with pAHIΔB or pAHIΔBAY196I) from hFbpABC uptake (with pAHIO or pAHIOAY196I) and plotting initial velocity (in picomoles/107 cells/minute) against substrate concentration (in micromoles). Results were not significantly affected by the use of higher cell concentrations (107 to 109 cells/100 μl). Data were from a single experiment and are representative of data obtained from at least three replicate assays. Nonlinear regression analysis was performed using Origin 7 graphing software.
Complementation studies.
H-1443/pACYC184-pBADHIBC, H-1443/pACYCHIΔB-pBADHIBC, and H-1443/pACYCHIO-pBADHIBC were grown in LBamp100cam30 to midlog phase. Cells were seeded at 106 CFU/plate on NBAamp100cam30dip75 supplemented with 0.2% arabinose and incubated at 37°C for 24 h. The radiolabeled Fe(NTA)2 transport assay was performed as described above.
Energy utilization, metal competition, and metal sensitivity of hFbpABC.
The metabolic inhibitor CCCP or sodium orthovanadate was added 1 or 10 min prior to the addition of radiolabeled Fe(NTA)2, respectively. In metal competition experiments, metal(NTA)2 complexes were added 1 min prior to addition of labeled Fe(NTA)2. Metal sensitivity experiments were performed by growing fresh transformants of H-1443/pBR322, H-1443/pAHIΔB, and H-1443/pAHIO in LBamp100 to midlog phase, diluting, and seeding on NBAamp100 at 104 CFU/plate. Upon drying, sterile disks were placed on the plates to which the following metal salts (200 mM) were applied: NiCl2, MnSO4, ZnCl2, AlSO4, CuCl2, and Ga(NO3)3. The plates were inverted and incubated at 37°C for 18 h. Following incubation the plates were digitally scanned.
Gallium transport assay.
The radiolabeled Ga(NTA)2 transport assay was performed under conditions similar to those for the iron transport assay, using 67Ga(NTA)2 at 3 × 104 cpm/pmol. Dried nitrocellulose filters were subjected to direct counting using a gamma counter (Packard).
Construction and purification of the hFbpA(Y196I) mutant.
Selection of the Tyr196Ile mutation was performed on the basis of the crystal structures of hFbpA and a homologous nFbpA mutant (35). The mutant was constructed using a Gene Editor system (Promega, Madison, Wis.), with the pAHIO plasmid as the template. The mutation was verified by DNA sequencing as described above. The hFbpA(Y196I) protein was purified using a modification of a previously reported protocol (2). Briefly, an overnight culture of JM109/pAHIOAY196I was used to inoculate 1 liter of NBamp100 and the culture was grown at 37°C with shaking for 18 h. Cells were harvested by centrifugation (4,000 × g, 15 min) and washed in phosphate-buffered saline. The cells were then resuspended in a 50-ml solution of 400 mM Tris (pH 8.0)-2% (wt/vol) CTAB and shaken at 37°C for 2 h. Cell debris was removed by centrifugation, and the soluble lysate was diluted to 500 ml in water. The diluted lysate was clarified by filtration and applied to a carboxymethyl-Sepharose CL-6B column equilibrated with 10 mM Tris (pH 7.5). The column was washed with 10 volumes of 10 mM Tris (pH 7.5) and subjected to several step washes: 4 volumes of 10 mM Tris (pH 7.5)-200 mM NaCl, 4 volumes of 400 mM NaCl, and 4 volumes of 500 mM NaCl. Purified hFbpA(Y196I) was collected following a gradient elution of 500 to 1,000 mM NaCl. Fractions were analyzed for protein content by monitoring the A280 and assessed for purity using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. hFbpA(Y196I)-containing fractions were pooled and concentrated in an Amicon concentration cell with a 10-kDa-cutoff Diaflo ultrafiltration membrane and then dialyzed using 20 mM MES (pH 6.5)-200 mM NaCl. Wild-type hFbpA was purified according to a previously published protocol (2). Iron was removed from the hFbpA proteins by incubation with 1,000-fold-molar-excess sodium citrate (pH 6.0) on ice for 30 min. Protein was then dialyzed using 10 volumes of 10 mM sodium citrate followed by exhaustive dialysis using iron-free 20 mM MES (pH 6.5)-200 mM NaCl. Concentrated protein was kept at −80°C.
UV/Vis spectroscopy of hFbpA and hFbpA(Y196I).
The visible absorbances of iron-saturated hFbpA and hFbpA(Y196I) were measured using 30 μM solutions of protein in 20 mM MES (pH 6.5)-200 mM NaCl. Protein was incubated with Fe(NTA)2 (1.2 molar equivalents) for 2 days at 4°C. Absorbance spectra were acquired using an AVIV model 14 UV/Vis spectrophotometer. Three scans of each protein were taken, and the results were averaged. Data were plotted using Cricket Graph III.
Iron binding affinity of hFbpA(Y196I).
The effective Fe3+ dissociation constant (Kd) of hFbpA(Y196I) was determined using equilibrium binding and ultrafiltration methods. Iron-free hFbpA(Y196I) (15 μM) in 20 mM MES (pH 6.5)-100 mM NaCl-150 μM NaPO4 was incubated in the presence of increasing concentrations of Fe(NTA)2 labeled with 55FeCl3 (7 × 104 cpm/μl) for 2 days at 4°C. The solutions were subjected to filtration using BIOMAX ultrafree filter tubes with 10 NMWL membranes centrifuged at 10,000 × g for 5 min at 4°C. Aliquots of both the total protein solution [bound plus free Fe(NTA)2] and the filtrate [free Fe(NTA)2] were removed and measured for radioactivity by the use of a liquid scintillation counter. Bound iron (total minus filtrate) was plotted versus the total iron concentration, and nonlinear regression analysis was performed using Origin 7 graphing software.
RESULTS
hFbpABC iron transport.
Previous work demonstrated that propagation on NBA supplemented with 200 μM dipyridyl allows the selection of H-1443 E. coli complemented with a functional hitABC operon (2). In this study, NBAamp100 supplemented with 75 μM dip (NBAamp100dip75) allowed growth of H-1443 E. coli while forcing upregulation of hitABC derived from the pAHIO plasmid, which is under the transcriptional control of the H. influenzae fur operator. Growth on NBAamp100dip75 medium followed by suspension in iron-free M9 was found to be the optimal condition for measuring iron transport. In this transport assay, 55Fe3+ was provided as a nitrilotriacetate complex for several reasons: (i) previous work has demonstrated that Fe(NTA)2 is very efficient in loading FbpA with Fe3+, as NTA can serve as the synergistic anion (16); (ii) once bound, the NTA anion readily exchanges with other endogenous anions, including PO4, the preferred anion (16); and (iii) the affinity of NTA for Fe3+ is sufficiently high to inhibit competition by E. coli low-affinity iron transport systems. H-1443 E. coli was transformed with pBR322, pAHIΔB, or pAHIO plasmid (pAHIO and mutants used in this study were derived from the pBR322 background and thus have identical copy numbers). Fresh transformants were grown on NBAamp100dip75, washed with M9, and measured via the transport assay (Fig. 1).
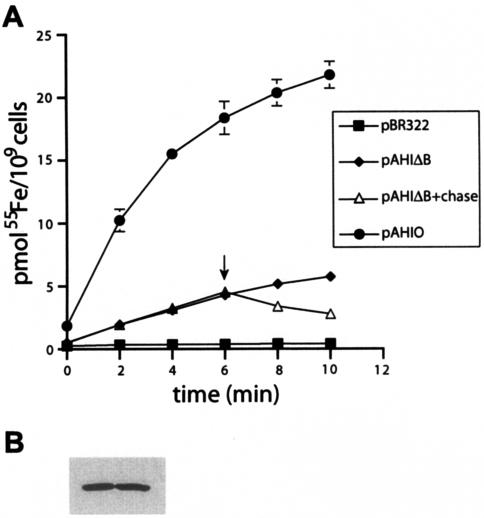
FIG. 1.
hFbpABC 55Fe3+ transport assay. (A) Cells grown on NBAamp100dip75 were resuspended in TM, preincubated for 10 min at 37°C, and supplemented with 1 μM 55Fe(NTA)2. Samples were taken at 2-min intervals and subjected to filtration, and counts per minute were determined. Radiolabeled iron uptake is plotted versus time. Each strain was tested in triplicate; error bars represent standard deviations (SD). pAHIΔB+chase, pAHIΔB in which 100-fold-excess unlabeled Fe(NTA)2 was added at 6 min (arrow). (B) Western blot of lysates from pAHIΔB (left lane) and pAHIO (right lane) probed with anti-hFbpA-specific antibody. Densitometric analysis demonstrated similar levels of hFbpA expression in these cultures (pAHIΔB:pAHIO, 1.0:0.942).
In this assay, H-1443/pBR322 demonstrated minimal iron uptake (∼0.38 pmol Fe/109 cells at time = 6 min [2.1% of pAHIO results]), indicating negligible effects of the E. coli strain under assay conditions with Fe(NTA)2 as a supplement (Fig. 1A). The H-1443/pAHIΔB control demonstrated a low-level time-dependent increase in signal (∼4.29 pmol Fe/109 cells at time = 6 min [23.3% of pAHIO results]) compared to the background (H-1443/pBR322) (Fig. 1A). The signal result is not due to functional iron transport via hFbpABC but rather is due to binding of labeled Fe(NTA)2 to hFbpA within the periplasm of these cells. This is consistent with previous results that showed that pAHIΔC was unable to mobilize iron into the cytosol of H-1443 E. coli (2). Subsequently, the activities of both pAHIΔB and pAHIΔC were measured using the transport assay and shown to be very similar (data not shown). The pAHIΔB control was used in the present study to eliminate any interference the presence of the hFbpB permease may exert on transport analyses. Levels of hFbpA expression by both pAHIΔB and pAHIO were measured by Western blot and densitometric analysis (Kodak Imagestation 1000) and found to be identical (pAHIΔB:pAHIO, 1.0:0.942), indicating that the difference in signal results between pAHIΔB and pAHIO (see below) was not the result of altered levels of hFbpA expression (Fig. 1B).
To address the observation that pAHIΔB does not reach time-dependent saturation, H-1443/pAHIΔB cells were subjected to chasing with 100-fold-excess unlabeled Fe(NTA)2 at the 6-min time point (Fig. 1A). The chase resulted in a >50% loss of signal, indicating release of Fe(NTA)2 from hFbpA within the periplasm of the cells. The nonsaturable pAHIΔB signal may be due to the possibility that hFbpA within the H-1443/pAHIΔB periplasm is nearly saturated with unlabeled iron from the growth medium (the concentration of Fe3+ in NBA is approximately 10 μM [personal observation]). Thus, the rate of binding for 1 μM 55Fe(NTA)2 to hFbpA is likely slow and would require increased time to reach saturation.
The H-1443/pAHIΔB control served as a baseline in the measurement of functional hFbpABC-mediated transport. The full transporter H-1443/pAHIO demonstrated a high-level time-dependent increase in signal (∼18.37 pmol Fe/109 cells at time = 6 min) (Fig. 1A). These results demonstrate that the assay is a specific measure of hFbpABC transport and is of sufficient sensitivity to test multiple parameters of hFbpABC transporter function, including metal specificity, energy requirements, and the effects of mutations.
Kinetics of hFbpABC transport.
Incubation of pAHIO/H-1443 with increasing concentrations of 55Fe(NTA)2 demonstrated saturation of transport characteristic of Michaelis-Menten kinetics. After subtracting the pAHIΔB control results, the pAHIO transport rates were used to calculate estimated values for Km and Vmax. The estimated apparent Km for wild-type hFbpABC Fe3+ transport was 0.9 μM, and the apparent Vmax was 1.8 pmol/107cells/min. These values are within a close range of those derived for other binding protein-dependent ABC transport systems, including the maltose and histidine transporters (4, 29), and demonstrate that the hFbpABC transporter functions with a substrate turnover similar to those of other bacterial small-ligand transporters.
hFbpABC is a binding protein-dependent ABC transporter.
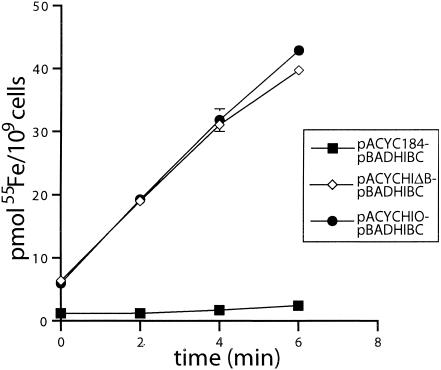
To demonstrate that the hFbpABC transporter is dependent upon the binding protein for function, complementation experiments were performed. Initially, the qualitative assay of growth or absence of growth on NBAamp100cam30 containing 200 μM dipyridyl was used to determine whether hFbpA could complement hFbpBC expressed under the control of an exogenous promoter (PBAD) (data not shown). Results showed that H-1443 cells expressing the hFbpBC proteins only (pACYC184-pBADHIBC) exhibited no growth on NBAamp100cam30dip200 and that cells expressing hFbpBC complemented with hFbpA (pACYCHIΔB-pBADHIBC) exhibited growth on this medium similar to control cells (pACYCHIO-pBADHIBC). The 55Fe(NTA)2 transport assay was used as a more sensitive quantitative measure of complementation. Results demonstrated that the hFbpBC-only cells exhibited a low level of transport similar to background E. coli H-1443 cells observed previously (Fig. 1A and 2). Cells expressing hFbpBC complemented with hFbpA (pACYCHIΔB-pBADHIBC) exhibited a high level of 55Fe(NTA)2 uptake similar in scale to cells expressing the full hFbpABC transporter (Fig. 2). These results verify that the ABC transporter (hFbpBC) is dependent upon the hFbpA binding protein for functional iron transport.
FIG. 2.
hFbpABC is a binding protein-dependent ABC transporter. E. coli H-1443 cells expressing the hFbpBC proteins only (pACYC184-pBADHIBC) demonstrated minimal Fe3+ uptake similar in scale to vector-only cells (Fig. 1A). Cells expressing the hFbpBC proteins complemented with hFbpA (pACYCHIΔB-pBADHIBC) demonstrated a high level of Fe3+ uptake similar in scale to cells expressing the full hFbpABC transporter (pACYCHIO-pBADHIBC). Thus, the ABC transporter (hFbpBC) functions in iron transport only in the presence of the binding protein (hFbpA).
Energy utilization by hFbpABC.
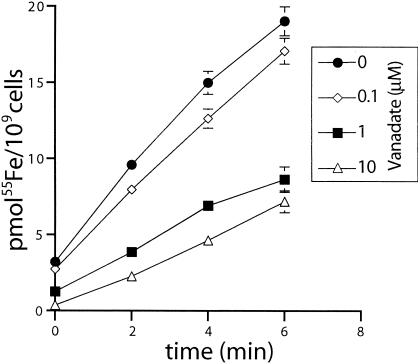
Pretreatment of cells with increasing concentrations of the ATPase inhibitor sodium orthovanadate prior to the addition of 55Fe(NTA)2 resulted in a dose-dependent decrease in iron uptake (Fig. 3). This inhibition leveled off at an amount of signal similar to that seen with pAHIΔB, which is indicative of 55Fe(NTA)2 binding by hFbpA within the periplasm and loss of cytosolic transport (compare Fig. 3 [10 mM vanadate] with Fig. 1A [pAHIΔB]). These results are consistent with hFbpABC functioning as an ATPase-driven transporter, similar to members of the family of bacterial binding protein-dependent ABC transporters. Interestingly, administration of sodium arsenate under similar conditions had notable effects on transport only at high concentrations (data not shown). This result is likely due to the fact that arsenate has previously been shown to function as a suitable ternary anion in FbpA Fe3+ binding. Thus, the presence of arsenate may have the side effect of facilitating hFbpA Fe3+ loading and thereby facilitating Fe3+ transport. Addition of the protonophore CCCP prior to 55Fe(NTA)2 resulted in no apparent effect on iron uptake (data not shown). This is in contrast to the MntH metal permease, which functions as a protonmotive-force-driven symporter and is inhibited by CCCP under similar conditions (28). These results indicate that hFbpABC transport is not driven by protonmotive force.
FIG. 3.
Energy requirements of hFbpABC. The ATPase inhibitor sodium orthovanadate (0.1, 1, or 10 mM) was added 10 min prior to 55Fe(NTA)2. Samples were collected and measured as described in Materials and Methods. Each strain was tested in triplicate; error bars represent SD. The protonophore CCCP (50 or 250 μM) was added 1 min prior to 55Fe(NTA)2, with minimal effects on transport (data not shown).
Metal specificity of hFbpABC.
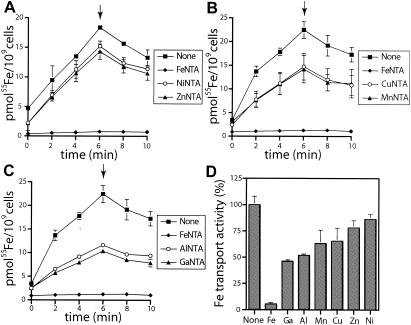
hFbpABC metal specificity was investigated by testing for competition by using trivalent and divalent metals for radiolabeled Fe(NTA)2 transport (Fig. 4). Metal:NTA complexes were added at a 100-fold molar excess to cells 1 min prior to the addition of labeled Fe(NTA)2. Uptake was quenched at the 6-min time point with 100-fold-excess unlabeled Fe(NTA)2. As a control, H-1443/pAHIO to which no competitor was added prior to 55Fe(NTA)2 demonstrated a high-level time-dependent increase in iron uptake similar to that shown in Fig. 1A. The competing metals were grouped into one of four categories, minimal, low, medium, and high, on the basis of their abilities to inhibit Fe3+ uptake. The divalent metals Ni(NTA)2 and Zn(NTA)2 demonstrated minimal transport competition (13.8 and 22% inhibition of 55Fe3+ uptake, respectively), while Cu(NTA)2 and Mn(NTA)2 demonstrated low levels of transport competition (34.5 and 36.8% inhibition) (Fig. 4A and B). The trivalent metals Al(NTA)2 and Ga(NTA)2 demonstrated medium competition (48.1 and 53.7% inhibition) (Fig. 4C). None of the metals, however, exhibited high-level competition on a scale similar to Fe(NTA)2 (94.5% inhibition) (Fig. 4D). Taken together, these results demonstrate that the hFbpABC transporter exhibits high specificity for ferric iron and has general selectivity for trivalent metals, including gallium and aluminum, over divalent metals such as copper, manganese, nickel, and zinc.
FIG. 4.
Metal specificity of hFbpABC. Competing metals [100-fold excess over labeled 55Fe(NTA)2] were added 1 min prior to labeled 55Fe(NTA)2. Samples were collected and measured as described in Materials and Methods. The arrows indicate quenching of uptake through the addition of 100-fold-excess unlabeled Fe(NTA)2. Each strain was tested in triplicate; error bars represent SD. (A) NiNTA and ZnNTA, (B) CuNTA and MnNTA, (D) AlNTA and GaNTA. (D) Inhibition of hFbpABC-mediated Fe transport. Values at the 6-min time points are compared with that for pAHIO with no competitor (None), and percentages of Fe3+ uptake are plotted.
Metal sensitivity of hFbpABC expressed in H-1443 E. coli.
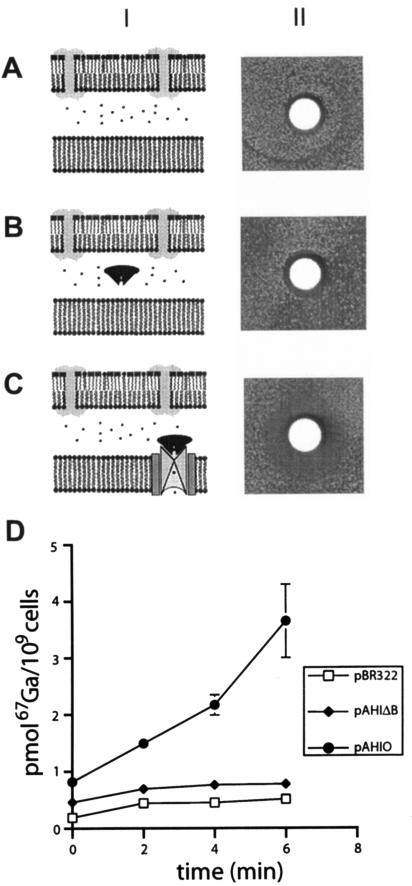
Through the course of these studies, several metals tested for metal specificity of hFbpABC (Fig. 4) were identified as inhibiting the growth of H-1443 E. coli expressing hFbpABC. To assess whether hFbpABC was responsible for the toxicity of these metals, the growth-inhibitory effects of specific metals were tested. The results indicate that the divalent metals Cu2+, Ni2+, and Zn2+ indeed demonstrate general toxicity to H-1443/pBR322, H-1443/pAHIΔB, and H-1443/pAHIO, independent of hFbpABC (data not shown). Interestingly, Ga3+ demonstrated significant toxicity toward H-1443/pAHIO only, indicating a possible correlation between hFbpABC-specific transport and gallium-induced toxicity (Fig. 5). These results, coupled with previous results showing that Ga(NTA)2 is able to compete for binding to hFbpA and form a stable complex (unpublished data), lead to the hypothesis that gallium may act as an iron analog and be bound and transported through hFbpABC. To test this, the iron transport assay was adapted for the use of radiolabeled gallium [67Ga(NTA)2]. Results show that the hFbpABC transporter functions in the direct uptake of gallium (Fig. 5D). Presumably, this transport gives rise to the gallium-induced toxicity which inhibits the growth of H-1443 E. coli expressing hFbpABC.
FIG. 5.
hFbpABC-mediated gallium toxicity. (A) Sterile disks containing 10 μl 500 mM Ga(NO3)3 were applied to the plates. Following incubation, plates were digitally scanned. (A) H-1443/pBR322; (B) H-1443/pAHIΔB; (C) H-1443/pAHIO. (D) The 67Ga(NTA)2 transport assay was conducted as described for 55Fe(NTA)2 transport. Counts per minute for filters were determined using a gamma counter.
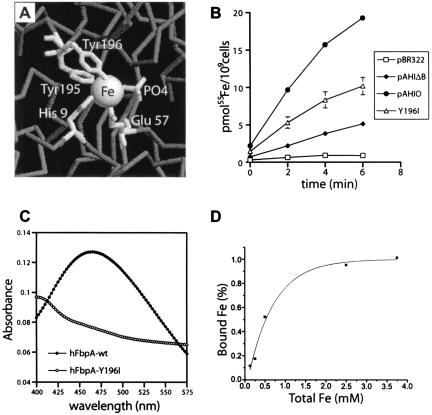
The hFbpA(Y196I)BC mutant transporter.
We investigated the effect of a single-amino-acid mutation on hFbpABC transport activity through the construction and analysis of the hFbpA(Y195I)BC mutant transporter. This mutation, selected through assessment of the hFbpA crystal structure, targeted one of the conserved iron-liganding residues in the Fe3+ binding site of hFbpA (Fig. 6A). Alteration of this site is predicted to affect the ability of hFbpA to bind iron, as previously observed with a homologous nFbpA mutant. Using the radiolabeled iron transport assay, we assessed the impact of the hFbpA(Y196I) mutation on hFbpABC transport (Fig. 6B). The mutant exhibited ∼35% activity of the hFbpABC wild-type transporter under standard conditions. The difference in activity was not due to altered expression levels of the binding proteins, as densitometric analysis demonstrated identical amounts of hFbpA and hFbpA(Y196I) (1:1.05, respectively). These results were unexpected in that one would expect the transport activity of the mutant to be completely lost, equating to that of the pBR322 vector-only control in this assay.
FIG. 6.
The mutant hFbpA(Y196I)BC transporter. (A) View of the hFbpA metal binding site and Tyr196 targeted for mutation. (B) The hFbpA(Y196I)BC mutant transporter was tested via the radiolabeled iron transport assay and compared with pAHIO, pAHIΔB, and pBR322. The mutant demonstrates a rate of transport ∼35% that of wild type. (C) Comparison of the characteristic 480-nm absorption peaks in the hFbpA wild-type and hFbpA(Y196I) mutant proteins. Loss of this peak correlates with a loss in high-affinity iron binding by hFbpA. (D) Equilibrium binding experiments verified the loss of high-affinity Fe3+ binding in hFbpA(Y196I). The protein has an effective dissociation constant (Kd) of 5.2 × 10−4 M−1 for Fe(NTA)2, a drop in affinity of approximately 14 orders of magnitude compared with wild-type results.
We hypothesized that the mutant protein had lost significant binding activity but retained diminished affinity for iron and thus might support lower levels of transport activity. To verify that hFbpA(Y196I) had indeed lost wild-type iron binding activity, the protein was purified and its iron binding characteristics were assessed. One of the signature characteristics that the iron binding proteins share with the transferrins is the presence of a strong visible absorption peak in the 480-nm range. This is attributable to hard ligand electron donors (phenolate tyrosines) that contribute to ligand-to-metal charge transfer upon interaction with Fe3+. Comparison of visible spectra for wild-type hFbpA and mutant hFbpA(Y196I) demonstrated the complete loss of this peak from the mutant (Fig. 6C). Furthermore, trypsin susceptibility analysis of hFbpA(Y196I) resulted in no discernible Fe3+-dependent resistance to trypsin proteolysis (data not shown), in similarity to a result previously observed in the nFbpA mutant (35). Equilibrium binding analysis of the mutant hFbpA(Y196I) protein demonstrated an iron dissociation constant in the submillimolar range (Kd = 5.2 × 10−4 M−1) (Fig. 6D). This affinity is approximately 14 orders of magnitude weaker than the affinity of wild-type FbpA for Fe3+. Substrate-dependent kinetic analysis, using conditions similar to those used with wild-type hFbpABC, demonstrated an estimated apparent Km of 1.2 μM and an apparent Vmax of 0.5 pmol/107cells/min. Although this Km is similar to that of hFbpABC, the Vmax is approximately one-third that of the wild-type transporter. Taken together, these data demonstrate that the hFbpA(Y196I) protein has lost significant iron binding activity, which correlates with a decrease in transport activity-capacity (Vmax). However, this attenuated Fe3+ binding activity still supports a reduced level of transport, indicating that the mutant hFbpA(Y196I) protein is able to present the ABC transporter with Fe3+. These results have interesting implications for the mechanism of hFbpABC-mediated transport in that it appears that high-affinity Fe3+ binding by hFbpA is not an absolute requisite for transport in this model system.
DISCUSSION
Expression of the fbpABC and hitABC operons in E. coli has provided important information regarding the biochemical basis of FbpABC transporter function. Several previous reports have noted the utility of a model system involving the siderophore-deficient E. coli H-1443 strain complemented with functional FbpABC loci from diverse pathogens, including N. gonorrhoeae, S. marcescens, and H. influenzae (1, 2, 6). We have employed this approach to investigate the biochemistry of hFbpABC transport in detail to gain information on the functional physiology of this novel transport system.
The results of this work support the function of hFbpABC as an ATP-dependent transporter with a high specificity for ferric iron. This is consistent with the fact that FbpA is known to bind metals in a manner analogous to that of transferrin and has a clear preference for ferric iron (11) (unpublished results). The fact that transferrin also binds other metals, including aluminum, gallium, copper, and zinc (43), is consistent with our observation that these metals compete for hFbpABC transport. FbpABC-mediated transport is a two-step process, the first step involving the binding of metal to FbpA and the second involving FbpA binding the FbpBC complex and metal transport into the cytosol. It is not clear whether these metals compete for transport at the level of competitive binding to hFbpA or whether they can indeed gain access to the cytosol and thus compete for cytoplasmic iron uptake. The exception to this is gallium. Metal sensitivity experiments (Fig. 5) demonstrate that while gallium may impart low-level toxicity to H-1443/pBR322 and H-1443/pAHIΔB cells (presumably through endogenous metal uptake systems), expression of a functional hFbpABC transporter causes an increased level of toxicity to H-1443/pAHIO. hFbpABC-mediated gallium transport is further demonstrated by the gallium transport assay (Fig. 5D). While gallium has an ionic radius (Ga3+, 0.62 Å; Fe3+, 0.65 Å) and a valence similar to those of ferric iron, it is not a transition metal and therefore cannot replace iron in the redox processes essential to many iron-containing proteins. Thus, the hFbpABC-mediated gallium-induced toxicity is likely the result of the entry of gallium into the cytosol and a general effect of cellular iron deprivation. Gallium toxicity has proven useful in subsequent functional studies of hFbpABC as a selection tool in the identification of hFbpABC mutants (unpublished data).
The hFbpA protein binds iron utilizing six coordinating ligands. This involves four protein side chains (His 9, Glu 57, Tyr 195, and Tyr 196) and two exogenous molecules, a synergistic anion and a water molecule, to complete the binding site (10). This repertoire of iron binding residues is highly conserved, as identical residues are employed by multiple FbpA homologs. Transferrin also utilizes a set of iron binding residues similar to FbpA, differing by only one side chain (glutamate is replaced with aspartate), consistent with the conservation of a highly specialized metal binding site. A functional result of this organization is the remarkable affinity for Fe3+ exhibited by both proteins.
In relation to the proposed hFbpA Fe3+ binding process (10), mutation of one of the tyrosine residues (Tyr 196 in hFbpA) would likely have a dramatic effect on Fe3+ binding, resulting in an altered affinity. Consistent with this, we have measured a large decrease (∼14 orders of magnitude) in the affinity of hFbpA(Y196I) for Fe3+ compared to that of wild type. Although this is a sizeable decrease, the new affinity value is in the overall range of affinities exhibited by PBPs from alternate binding protein-dependent ABC transport systems. This may account for the observation that the rate of iron transport is only moderately diminished (∼35% of wild type) in this mutant transporter.
In other systems, it has been established that the second step of the transport process, binding of the PBP to the ABC transporter and substrate transport across the cytoplasmic membrane, is the rate-limiting step and that the affinity of the PBP for the substrate (Kd) roughly approximates the Km of the transporter (36). Furthermore, mutant PBPs with decreased substrate binding affinities (Kd) do not necessarily correlate with a proportional decrease in transport (Km) (48). These observations are likely explained by fact that the PBP is present at concentrations several orders of magnitude greater than the ABC transporter protein concentrations; PBP concentrations can reach the millimolar range within the periplasm (36). This is consistent with the results seen with the hFbpA(Y196I)BC transporter; the hFbpA(Y196I) protein with a Kd of ∼500 μM gives rise to a transport rate ∼35% that of the wild type. By extrapolation, it is plausible that an hFbpA mutant with a Kd of ∼1 μM could support a rate of transport similar in scale to that of the wild type, although further investigations are required to verify this. If a binding protein with micromolar affinity is indeed sufficient for wild-type transport (in consistency with other systems), why then has FbpA evolved such a high natural Fe3+ binding affinity? This may have to do with several possible factors, including a role in (i) contributing to a thermodynamic driving force in the import of Fe3+ (from transferrin or lactoferrin), (ii) maintaining an available pool of Fe3+ within the periplasm for utilization in times of iron stress, or (iii) satisfying the requirements of maintaining ferric iron in a controlled protein environment to prevent reactivity-toxicity upon transit into the cell (16).
The FbpAs are novel PBPs in that they utilize a ternary anion in binding substrate (iron), they utilize a highly conserved set of iron binding residues, and they exhibit iron binding affinities 10 to 12 orders of magnitude greater than the affinities exhibited by typical PBPs for their respective substrates. These characteristics may be the result of the strict requirements that ferric iron places on proteins that must bind it, perhaps due to the propensity of free (naked) Fe3+ in aqueous, aerobic conditions to undergo hydrolysis and precipitate. The ability of ferric iron to induce cellular damage through hydroxyl-radical catalysis places additional constraints on transport and management. Whether unique characteristics, based on the strict requirements of Fe3+ binding and transport, extend to the membrane permease and ATP binding protein components of the FbpABC transporters is an area of present study.
Although FbpABC is the first identified ferric Fe3+-specific ABC transporter, several alternate systems that have free-iron transport activity, including the ferrous iron transport system feoABC, specific for ferrous iron under anaerobic conditions, and the CorA magnesium transport system, transporting Fe2+ with low affinity under low-magnesium conditions, have been identified (24, 25). Both these systems require free iron in the reduced soluble form, Fe2+. An additional metal transporter, MntH, is a bacterial homolog of the NRAMP (natural resistance against microbial pathogens) family of eukaryotic metal permeases (12, 28) and demonstrates specificity towards manganese and lower selectivity towards ferrous iron, zinc, and other metals. However, it functions as an individual permease independent of a binding protein and is energized through protonmotive force. Recently, a stimulator of Fe transport system has been identified, with homologs thus far identified in humans (23) and yeast (S. cerevisiae) (42) and a putative stimulator of Fe transport system identified in bacteria (B. subtilis) (42). Although the function of these transporters remains largely undescribed, they appear to be specific for ferric iron. Generally speaking, these systems appear to be driven by individual membrane permeases that function in both ATP hydrolysis and metal transport without the interplay of a separate PBP and ATP binding protein. Additionally, they may require the presence of both an iron oxidase and reductase for function. Further detailed investigations of the FbpB and FbpC proteins are required for fuller understanding of the novel structural and functional aspects of ferric iron-specific FbpABC transport.
In conclusion, the FbpABC iron transporters have several characteristics common to the family of bacterial binding protein-dependent ABC transporters, including similar kinetics and the use of ATP hydrolysis as the energy source driving transport. The transporters are highly selective for Fe3+ and have a preference for trivalent metals, including Ga3+. Interestingly, the transporters possess characteristics which may be shared by other iron transport systems and may have additional attributes specifically related to the transport of free ferric iron. Homologs of FbpABC and individual components thereof have recently been identified in numerous bacteria, including Yersinia spp. (21), Campylobacter jejuni (20), Pasteurella haemolytica (27), and Actinobacillus actinomycetemcomitans (46); ongoing genome projects will undoubtedly uncover further homologs. Therefore, the FbpABC transporters may comprise a widely employed iron acquisition system with implications for virulence among many diverse bacterial species.
Acknowledgments
We thank K.G. Vaughan, C. B. Bahr, and S. M. Phadke for technical and editorial assistance.
This work was supported by National Institutes of Health grant R29 A132226 (T.A.M.).
REFERENCES
- 1.Adhikari, P., S. A. Berish, A. J. Nowalk, K. L. Veraldi, S. A. Morse, and T. A. Mietzner. 1996. The fbpABC locus of Neisseria gonorrhoeae functions in the periplasm-to-cytosol transport of iron. J. Bacteriol. 178:2145-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikari, P., S. D. Kirby, A. J. Nowalk, K. L. Veraldi, A. B. Schryvers, and T. A. Mietzner. 1995. Biochemical characterization of a Haemophilus influenzae periplasmic iron transport operon. J. Biol. Chem. 270:25142-25149. [DOI] [PubMed] [Google Scholar]
- 3.Alexeev, D., H. Zhu, M. Guo, W. Zhong, D. J. Hunter, W. Yang, D. J. Campopiano, and P. J. Sadler. 2003. A novel protein-mineral interface. Nat. Struct. Biol. 10:297-302. [DOI] [PubMed] [Google Scholar]
- 4.Ames, G. F. L., C. E. Liu, A. K. Joshi, and K. Nikaido. 1996. Liganded and unliganded receptors interact with equal affinity with the membrane complex of periplasmic permeases, a subfamily of traffic ATPases. J. Biol. Chem. 271:14264-14270. [DOI] [PubMed] [Google Scholar]
- 5.Angerer, A., S. Gaisser, and V. Braun. 1990. Nucleotide sequences of the sfuA, sfuB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J. Bacteriol. 172:572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angerer, A., B. Klupp, and V. Braun. 1992. Iron transport systems of Serratia marcescens. J. Bacteriol. 174:1378-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagg, A., and J. B. Neilands. 1987. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry 26:5471-5477. [DOI] [PubMed] [Google Scholar]
- 8.Boukhalfa, H., D. S. Anderson, T. A. Mietzner, and A. L. Crumbliss. 2003. Kinetics and mechanism of iron release from the bacterial ferric binding protein nFbp: exogenous anion influence and comparison with mammalian transferrin. J. Biol. Inorg. Chem. 8:881-892. [DOI] [PubMed] [Google Scholar]
- 9.Braun, V., R. Gross, W. Koster, and L. Zimmermann. 1983. Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system of Escherichia coli. Use of streptonigrin for selection. Mol. Gen. Genet. 192:131-139. [DOI] [PubMed] [Google Scholar]
- 10.Bruns, C. M., D. S. Anderson, K. G. Vaughan, P. A. Williams, A. J. Nowalk, D. E. McRee, and T. A. Mietzner. 2001. Crystallographic and biochemical analyses of the metal-free Haemophilus influenzae Fe3+-binding protein. Biochemistry 40:15631-15637. [DOI] [PubMed] [Google Scholar]
- 11.Bruns, C. M., A. J. Nowalk, A. S. Arvai, M. A. McTigue, K. G. Vaughan, T. A. Mietzner, and D. E. McRee. 1997. Structure of Haemophilus influenzae Fe(+3)-binding protein reveals convergent evolution within a superfamily. Nat. Struct. Biol. 4:919-924. [DOI] [PubMed] [Google Scholar]
- 12.Cellier, M., G. Prive, A. Belouchi, T. Kwan, V. Rodrigues, W. Chia, and P. Gros. 1995. Nramp defines a family of membrane proteins. Proc. Natl. Acad. Sci. USA 92:10089-10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, C. Y., S. A. Berish, S. A. Morse, and T. A. Mietzner. 1993. The ferric iron-binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol. Microbiol. 10:311-318. [DOI] [PubMed] [Google Scholar]
- 14.Crosa, J. 1989. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol. Rev. 53:517-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Lorenzo, V., M. Herrero, F. Giovannini, and J. B. Neilands. 1988. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur. J. Biochem. 173:537-546. [DOI] [PubMed] [Google Scholar]
- 16.Dhungana, S., C. H. Taboy, D. S. Anderson, K. G. Vaughan, P. Aisen, T. A. Mietzner, and A. L. Crumbliss. 2003. The influence of the synergistic anion on iron chelation by ferric binding protein, a bacterial transferrin. Proc. Natl. Acad. Sci. USA 100:3659-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fecker, L., and V. Braun. 1983. Cloning and expression of the fhu genes involved in iron(III)-hydroxamate uptake by Escherichia coli. J. Bacteriol. 156:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferenci, T., M. Muir, K. S. Lee, and D. Maris. 1986. Substrate specificity of the Escherichia coli maltodextrin transport system and its component proteins. Biochim. Biophys. Acta 860:44-50. [DOI] [PubMed] [Google Scholar]
- 19.Gabricevic, M., D. S. Anderson, T. A. Mietzner, and A. L. Crumbliss. 2004. Kinetics and mechanism of iron(III) complexation by ferric binding protein: the role of phosphate. Biochemistry 43:5811-5819. [DOI] [PubMed] [Google Scholar]
- 20.Galindo, M. A., W. A. Day, B. H. Raphael, and L. A. Joens. 2001. Cloning and characterization of a Campylobacter jejuni iron-uptake operon. Curr. Microbiol. 42:139-143. [DOI] [PubMed] [Google Scholar]
- 21.Gong, S., S. W. Bearden, V. A. Geoffroy, J. D. Fetherston, and R. D. Perry. 2001. Characterization of the Yersinia pestis Yfu ABC inorganic iron transport system. Infect. Immun. 69:2829-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo, M., I. Harvey, W. Yang, L. Coghill, D. J. Campopiano, J. A. Parkinson, R. T. MacGillivray, W. R. Harris, and P. J. Sadler. 2003. Synergistic anion and metal binding to the ferric ion-binding protein from Neisseria gonorrhoeae. J. Biol. Chem. 278:2490-2502. [DOI] [PubMed] [Google Scholar]
- 23.Gutierrez, J. A., J. Yu, S. Rivera, and M. Wessling-Resnick. 1997. Functional expression cloning and characterization of SFT, a stimulator of Fe transport. J. Cell Biol. 139:895-905. (Erratum, 147:204, 1999.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hantke, K. 1997. Ferrous iron uptake by a magnesium transport system is toxic for Escherichia coli and Salmonella typhimurium. J. Bacteriol. 179:6201-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kammler, M., C. Schon, and K. Hantke. 1993. Characterization of the ferrous iron uptake system of Escherichia coli. J. Bacteriol. 175:6212-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khun, H. H., V. Deved, H. Wong, and B. C. Lee. 2000. fbpABC gene cluster in Neisseria meningitidis is transcribed as an operon. Infect. Immun. 68:7166-7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirby, S. D., F. A. Lainson, W. Donachie, A. Okabe, M. Tokuda, O. Hatase, and A. B. Schryvers. 1998. The Pasteurella haemolytica 35 kDa iron-regulated protein is an FbpA homologue. Microbiology 144:3425-3436. [DOI] [PubMed] [Google Scholar]
- 28.Makui, H., E. Roig, S. T. Cole, J. D. Helmann, P. Gros, and M. F. Cellier. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 35:1065-1078. [DOI] [PubMed] [Google Scholar]
- 29.Manson, M. D., W. Boos, P. J. Bassford, Jr., and B. A. Rasmussen. 1985. Dependence of maltose transport and chemotaxis on the amount of maltose-binding protein. J. Biol. Chem. 260:9727-9733. [PubMed] [Google Scholar]
- 30.Mietzner, T. A., and S. A. Morse. 1994. The role of iron-binding proteins in the survival of pathogenic bacteria. Annu. Rev. Nutr. 14:471-493. [DOI] [PubMed] [Google Scholar]
- 31.Mietzner, T. A., S. B. Tencza, K. G. Vaughan, P. A. Adhikari, and A. J. Nowalk. 1998. Periplasm-to-cytosol free Fe(III) transporters of pathogenic gram-negative bacteria. Curr. Top. Microbiol. Immunol. 225:113-135. [DOI] [PubMed] [Google Scholar]
- 32.Neilands, J. B. 1984. Methodology of siderophores. Struct. Bonding 58:1-24. [Google Scholar]
- 33.Neilands, J. B. 1980. Microbial metabolism of iron, p. 529-572. In A. Jacobs and M. Worwood (ed.), Iron in biochemistry and medicine, vol. 2. Academic Press, Inc., New York, N.Y. [Google Scholar]
- 34.Nowalk, A., S. Tencza, and T. Mietzner. 1994. Coordination of iron by the ferric-iron binding protein of pathogenic Neisseria is homologous to the transferrins. Biochemistry 33:12769-12775. [DOI] [PubMed] [Google Scholar]
- 35.Nowalk, A. J., K. J. Vaughan, B. Day, S. B. Tencza, and T. A. Mietzner. 1997. Metal-dependent conformers of the periplasmic ferric ion binding protein. Biochemistry 36:13054-13059. [DOI] [PubMed] [Google Scholar]
- 36.Prossnitz, E., A. Gee, and G. F. Ames. 1989. Reconstitution of the histidine periplasmic transport system in membrane vesicles. Energy coupling and interaction between the binding protein and the membrane complex. J. Biol. Chem. 264:5006-5014. [PubMed] [Google Scholar]
- 37.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 38.Shouldice, S. R., D. R. Dougan, R. J. Skene, L. W. Tari, D. E. McRee, R. H. Yu, and A. B. Schryvers. 2003. High resolution structure of an alternate form of the ferric ion binding protein from Haemophilus influenzae. J. Biol. Chem. 278:11513-11519. [DOI] [PubMed] [Google Scholar]
- 39.Shouldice, S. R., D. R. Dougan, P. A. Williams, R. J. Skene, G. Snell, D. Scheibe, S. Kirby, D. J. Hosfield, D. E. McRee, A. B. Schryvers, and L. W. Tari. 2003. Crystal structure of Pasteurella haemolytica ferric ion-binding protein A reveals a novel class of bacterial iron-binding proteins. J. Biol. Chem. 278:41093-41098. [DOI] [PubMed] [Google Scholar]
- 40.Shouldice, S. R., R. J. Skene, D. R. Dougan, D. E. McRee, L. W. Tari, and A. B. Schryvers. 2003. Presence of ferric hydroxide clusters in mutants of Haemophilus influenzae ferric ion-binding protein A. Biochemistry 42:11908-11914. [DOI] [PubMed] [Google Scholar]
- 41.Staudenmaier, H., B. Van Hove, Z. Yaraghi, and V. Braun. 1989. Nucleotide sequences of the fecBCDE genes and location of the proteins suggest a periplasmic-binding protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J. Bacteriol. 171:2626-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stearman, R., D. S. Yuan, Y. Yamaguchi-Iwai, R. D. Klausner, and A. Dancis. 1996. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 271:1552-1557. [DOI] [PubMed] [Google Scholar]
- 43.Sun, H., M. C. Cox, H. Li, A. B. Mason, R. C. Woodworth, and P. J. Sadler. 1998. [1H,13C] NMR determination of the order of lobe loading of human transferrin with iron: comparison with other metal ions. FEBS Lett. 422:315-320. [DOI] [PubMed] [Google Scholar]
- 44.Taboy, C. H., K. G. Vaughan, T. A. Mietzner, P. Aisen, and A. L. Crumbliss. 2001. Fe3+ coordination and redox properties of a bacterial transferrin. J. Biol. Chem. 276:2719-2724. [DOI] [PubMed] [Google Scholar]
- 45.Weinberg, E. D. 1984. Iron withholding: a defense against infection and neoplasia. Physiol. Rev. 64:65-102. [DOI] [PubMed] [Google Scholar]
- 46.Willemsen, P. T., I. Vulto, M. Boxem, and J. de Graaff. 1997. Characterization of a periplasmic protein involved in iron utilization of Actinobacillus actinomycetemcomitans. J. Bacteriol. 179:4949-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkelmann, F., D. van der Helm, and J. B. Neilands (ed.). 1987. Iron transport in microbes, plants and animals. VCH, Weinheim, Germany.
- 48.Wolf, A., E. W. Shaw, K. Nikaido, and G. F. Ames. 1994. The histidine-binding protein undergoes conformational changes in the absence of ligand as analyzed with conformation-specific monoclonal antibodies. J. Biol. Chem. 269:23051-23058. [PubMed] [Google Scholar]
- 49.Zak, O., A. Leibman, and P. Aisen. 1983. Metal-binding properties of a single-sited transferrin fragment. Biochim. Biophys. Acta 742:490-495. [DOI] [PubMed] [Google Scholar]
- 50.Zhu, H., D. Alexeev, D. J. Hunter, D. J. Campopiano, and P. J. Sadler. 2003. Oxo-iron clusters in a bacterial iron-trafficking protein: new roles for a conserved motif. Biochem. J. 376:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]