Abstract
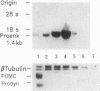
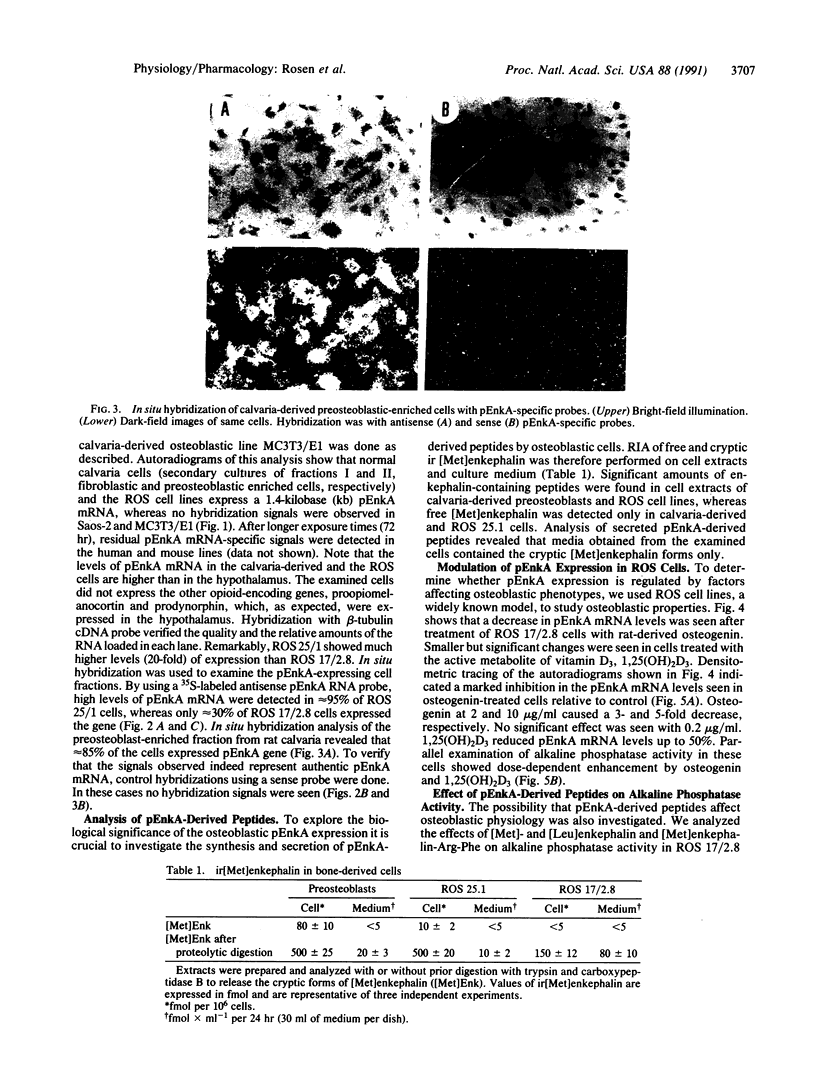
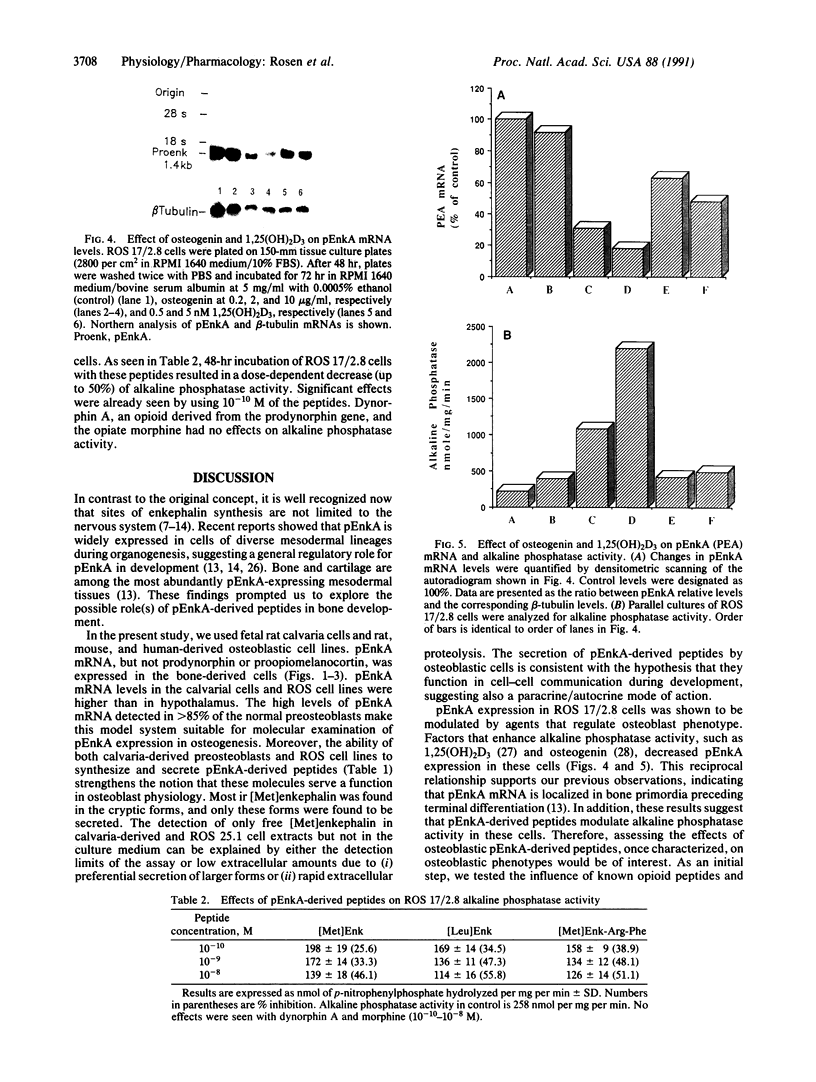
Enkephalins, a group of small peptides with opiate-like activity, have been defined originally as neuropeptides. Recent reports showed, using in situ hybridization, that the enkephalin-encoding gene, proenkephalin A (pEnkA), is expressed in nondifferentiated cells of diverse mesodermal lineages. The transient expression of pEnkA in these tissues during organogenesis suggests that this gene is involved in processes such as differentiation and/or cell proliferation. In situ hybridization revealed that bone and cartilage are among the tissues that express pEnkA most actively during organogenesis. Here we show that pEnkA mRNA is abundant in normal calvaria-derived cells and in osteosarcoma-derived cell lines ROS 17/2.8 and ROS 25/1. In addition, pEnkA-derived peptides are synthesized and secreted by these cells, as revealed by specific RIA. pEnkA expression in ROS cells is decreased by osteogenin, an osteoinductive factor, and by the calcium-regulating hormone, 1,25-dihydroxyvitamin D3, whereas the osteoblastic phenotype marker, alkaline phosphatase, is increased by these factors. These results together with the inhibitory effects of pEnkA-derived peptides on alkaline phosphatase activity in ROS 17/2.8 cells suggest that pEnkA is involved in bone development and provide a model system for further analysis of pEnkA expression during this process.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D. R. Basic methods for the study of phagocytosis. Methods Enzymol. 1986;132:95–180. doi: 10.1016/s0076-6879(86)32005-6. [DOI] [PubMed] [Google Scholar]
- Akil H., Watson S. J., Young E., Lewis M. E., Khachaturian H., Walker J. M. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Comb M., Seeburg P. H., Adelman J., Eiden L., Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982 Feb 25;295(5851):663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bailey L. C., Noe M., Udenfriend S. Proenkephalin mRNA in rat heart. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1960–1963. doi: 10.1073/pnas.83.6.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura H., Kato Y., Nakai Y., Nakao K., Tanaka I., Jingami H., Koh T., Yoshimasa T., Tsukada T., Suda M. Endogenous opioids and related peptides: from molecular biology to clinical medicine. The Sir Henry Dale lecture for 1985. J Endocrinol. 1985 Nov;107(2):147–157. doi: 10.1677/joe.0.1070147. [DOI] [PubMed] [Google Scholar]
- Keshet E., Polakiewicz R. D., Itin A., Ornoy A., Rosen H. Proenkephalin A is expressed in mesodermal lineages during organogenesis. EMBO J. 1989 Oct;8(10):2917–2923. doi: 10.1002/j.1460-2075.1989.tb08441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew D., Kilpatrick D. L. Widespread organ expression of the rat proenkephalin gene during early postnatal development. Mol Endocrinol. 1990 Feb;4(2):337–340. doi: 10.1210/mend-4-2-337. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Noe M., Bailey L. C., Udenfriend S. Expression of preproenkephalin-like mRNA and its peptide products in mammalian testis and ovary. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7467–7469. doi: 10.1073/pnas.82.21.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeska R. J., Rodan G. A. The effect of 1,25(OH)2D3 on alkaline phosphatase in osteoblastic osteosarcoma cells. J Biol Chem. 1982 Apr 10;257(7):3362–3365. [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Maintenance of parathyroid hormone response in clonal rat osteosarcoma lines. Exp Cell Res. 1978 Feb;111(2):465–468. doi: 10.1016/0014-4827(78)90193-3. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Hirose T., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982 Jan 21;295(5846):202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- Polakiewicz R. D., Rosen H. Regulated expression of proenkephalin A during ontogenic development of mesenchymal derivative tissues. Mol Cell Biol. 1990 Feb;10(2):736–742. doi: 10.1128/mcb.10.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Imai Y., Thiede M. A., Wesolowski G., Thompson D., Bar-Shavit Z., Shull S., Mann K., Rodan G. A. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res. 1987 Sep 15;47(18):4961–4966. [PubMed] [Google Scholar]
- Rosen H., Behar O., Abramsky O., Ovadia H. Regulated expression of proenkephalin A in normal lymphocytes. J Immunol. 1989 Dec 1;143(11):3703–3707. [PubMed] [Google Scholar]
- Rosen H., Itin A., Schiff R., Keshet E. Local regulation within the female reproductive system and upon embryonic implantation: identification of cells expressing proenkephalin A. Mol Endocrinol. 1990 Jan;4(1):146–154. doi: 10.1210/mend-4-1-146. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Lorenz R. G., Unanue R. A., Weaver C. T. Nonopiate active proenkephalin-derived peptides are secreted by T helper cells. FASEB J. 1989 Oct;3(12):2401–2407. doi: 10.1096/fasebj.3.12.2529160. [DOI] [PubMed] [Google Scholar]
- Sampath T. K., Muthukumaran N., Reddi A. H. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7109–7113. doi: 10.1073/pnas.84.20.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H., Kodama H. A., Amagai Y., Yamamoto S., Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983 Jan;96(1):191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S., Luyten F. P., Reddi A. H. Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8793–8797. doi: 10.1073/pnas.86.22.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G., Cohn D. V. Separation of parathyroid hormone and calcitonin-sensitive cells from non-responsive bone cells. Nature. 1974 Dec 20;252(5485):713–715. doi: 10.1038/252713a0. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesis. Science. 1986 May 9;232(4751):772–775. doi: 10.1126/science.2938259. [DOI] [PubMed] [Google Scholar]