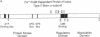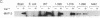Abstract
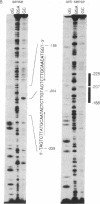
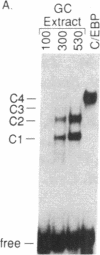
Calcium influx in response to extracellular signals can modulate gene transcription. A constitutive, calcium/calmodulin-independent mutant of type II calcium/calmodulin-dependent protein kinase was capable of increasing the transcription rate of specific genes independently of protein kinase C activation. This increase was mediated by transferable cis-active elements capable of binding the transcription factor CAAT/enhancer binding protein. Therefore, the activation of type II calcium/calmodulin-dependent protein kinase in response to stimuli that increase intracellular calcium is proposed to represent a distinct second messenger pathway in calcium-mediated regulation of gene transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cullen B. R., Raymond K., Ju G. Functional analysis of the transcription control region located within the avian retroviral long terminal repeat. Mol Cell Biol. 1985 Mar;5(3):438–447. doi: 10.1128/mcb.5.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. N., Maurer R. A. Pituitary calcium channel modulation and regulation of prolactin gene expression. Mol Endocrinol. 1990 May;4(5):736–742. doi: 10.1210/mend-4-5-736. [DOI] [PubMed] [Google Scholar]
- Enyeart J. J., Biagi B., Day R. N. Opposing actions of Bay K 8644 enantiomers on calcium current, prolactin secretion, and synthesis in pituitary cells. Mol Endocrinol. 1990 May;4(5):727–735. doi: 10.1210/mend-4-5-727. [DOI] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Montminy M. R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989 Nov 17;59(4):675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hanson P. I., Kapiloff M. S., Lou L. L., Rosenfeld M. G., Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989 Jul;3(1):59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- Hatada Y., Munemura M., Fukunaga K., Yamamoto H., Maeyama M., Miyamoto E. Calmodulin and Ca2+- and calmodulin-dependent protein kinase in rat anterior pituitary gland. J Neurochem. 1983 Apr;40(4):1082–1089. doi: 10.1111/j.1471-4159.1983.tb08096.x. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Jackson A. E., Thompson T. M., Zavacki A. M., Coppola D. A., Bancroft C. Calcium channel agonists and antagonists: effects of chronic treatment on pituitary prolactin synthesis and intracellular calcium. Mol Endocrinol. 1988 Nov;2(11):1132–1138. doi: 10.1210/mend-2-11-1132. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Schorpp M., Wagner U., Ryffel G. U. An estrogen-responsive element derived from the 5' flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell. 1986 Sep 26;46(7):1053–1061. doi: 10.1016/0092-8674(86)90705-1. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Kapiloff M. S., Durgerian S., Tatemoto K., Russo A. F., Hanson P., Schulman H., Rosenfeld M. G. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler J. P., Kley N., Louis J. C., Demeneix B. A. Ca2+ regulates hormone secretion and proopiomelanocortin gene expression in melanotrope cells via the calmodulin and the protein kinase C pathways. J Neurochem. 1989 Apr;52(4):1279–1283. doi: 10.1111/j.1471-4159.1989.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Mangalam H. J., Albert V. R., Ingraham H. A., Kapiloff M., Wilson L., Nelson C., Elsholtz H., Rosenfeld M. G. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989 Jul;3(7):946–958. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Both isoforms of the cAMP-dependent protein kinase catalytic subunit can activate transcription of the prolactin gene. J Biol Chem. 1989 Apr 25;264(12):6870–6873. [PubMed] [Google Scholar]
- Mellon P. L., Clegg C. H., Correll L. A., McKnight G. S. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4887–4891. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986 Aug 7;322(6079):552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Kour G., Marais R. M., Mitchell F., Pears C., Schaap D., Stabel S., Webster C. Protein kinase C--a family affair. Mol Cell Endocrinol. 1989 Aug;65(1-2):1–11. doi: 10.1016/0303-7207(89)90159-7. [DOI] [PubMed] [Google Scholar]
- Patton B. L., Miller S. G., Kennedy M. B. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990 Jul 5;265(19):11204–11212. [PubMed] [Google Scholar]
- Ryden T. A., Beemon K. Avian retroviral long terminal repeats bind CCAAT/enhancer-binding protein. Mol Cell Biol. 1989 Mar;9(3):1155–1164. doi: 10.1128/mcb.9.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H., Kuret J., Jefferson A. B., Nose P. S., Spitzer K. H. Ca2+/calmodulin-dependent microtubule-associated protein 2 kinase: broad substrate specificity and multifunctional potential in diverse tissues. Biochemistry. 1985 Sep 24;24(20):5320–5327. doi: 10.1021/bi00341a008. [DOI] [PubMed] [Google Scholar]
- Schulman H. The multifunctional Ca2+/calmodulin-dependent protein kinase. Adv Second Messenger Phosphoprotein Res. 1988;22:39–112. [PubMed] [Google Scholar]
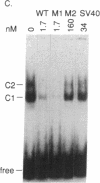
- Sealey L., Chalkley R. At least two nuclear proteins bind specifically to the Rous sarcoma virus long terminal repeat enhancer. Mol Cell Biol. 1987 Feb;7(2):787–798. doi: 10.1128/mcb.7.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R., Hanson P. I., Schulman H. Multifunctional Ca2+/calmodulin-dependent protein kinase made Ca2+ independent for functional studies. Biochemistry. 1990 Feb 20;29(7):1679–1684. doi: 10.1021/bi00459a002. [DOI] [PubMed] [Google Scholar]
- Waterman M. L., Adler S., Nelson C., Greene G. L., Evans R. M., Rosenfeld M. G. A single domain of the estrogen receptor confers deoxyribonucleic acid binding and transcriptional activation of the rat prolactin gene. Mol Endocrinol. 1988 Jan;2(1):14–21. doi: 10.1210/mend-2-1-14. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J., Kelly P. T. Functional analysis of Ca2+/calmodulin-dependent protein kinase II expressed in bacteria. J Biol Chem. 1989 May 5;264(13):7477–7482. [PubMed] [Google Scholar]
- White B. A. Evidence for a role of calmodulin in the regulation of prolactin gene expression. J Biol Chem. 1985 Jan 25;260(2):1213–1217. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]