Abstract
Purpose of review
Sarcoidosis is a chronic granulomatous disease typically affecting the lung, lymph nodes, and other organ systems. Evidence suggests that the morbidity and mortality rates for sarcoidosis in the USA are rising, despite widespread use of anti-inflammatory therapies. In this review, we survey new therapies that target specific inflammatory pathways in other diseases (such as rheumatoid arthritis, Crohn’s disease, and psoriasis) that are similar to pathways relevant to sarcoidosis immunopathogenesis, and therefore, represent potentially new sarcoidosis therapies.
Recent findings
Immunopathogenesis of sarcoidosis has been well elucidated over the past few years. There is abundant evidence for T-cell activation in sarcoidosis leading to activation of both Th1 and Th17 inflammatory cascades. Therapies targeting T-cell activation, Th1 pathways (such as the interleukin-6 inhibitors), Th17 pathway mediators, and others have been Food and Drug Administration approved or under investigation to treat a variety of autoimmune inflammatory diseases, but have not been studied in sarcoidosis. Targeting the p38 mitogen-activated protein kinases and the ubiquitine proteasome system with new agents may also represent a novel therapeutic option for patients with sarcoidosis.
Summary
Rising morbidity and mortality rates for patients with sarcoidosis strongly support the need to develop more effective anti-inflammatory therapies to treat chronic disease.
Keywords: novel sarcoidosis therapies, therapeutic targets, sarcoidosis immunopathogenesis
INTRODUCTION
Sarcoidosis is a chronic, multisystem granulomatous disorder that commonly involves the lungs and lymph nodes [1], but can involve all other organ systems. The disease is thought to affect 10 per 10 000 white Americans and 35 per 10 000 African-Americans [2]. Yet, up to 80% of patients will require treatment [2], and nearly half those who require systemic therapy will still require therapy 5 years after the diagnosis [3]. Since 1951, corticosteroids have been the first line of therapy for patients with symptomatic and progressive disease, but its use, although effective in the short-term, has been limited as a long-term therapy by dose-dependent side effects and a lack of evidence that it favorably alters the natural history of progressive disease [4▪,5–7].
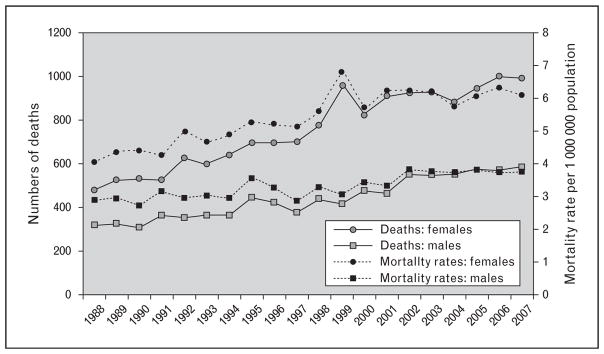
Recent evidence suggests that, despite the widespread use of corticosteroids and other immunosuppressants, mortality rates in sarcoidosis have been steadily increasing [8]. Between 1998 and 2007, sarcoidosis-associated mortality rates increased. They increased more than 50% among women and 30% among men, with an average yearly increase of 3% from 1988 to 2007 [8]. Interestingly, although the greatest relative increase in age-adjusted mortality occurred in non-Hispanic whites compared with non-Hispanic backs, the greatest absolute increase was seen among non-Hispanic black women. The most common cause of death cited in this study was the disease process itself, pointing to the need for more effective therapies for progressive disease [8] (Fig. 1).
FIGURE 1.
Numbers of deaths and age-adjusted mortality rates per 1 000 000 population [8]. Reprinted with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society [8]. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Sarcoidosis pathogenesis begins when an as of yet unidentified antigen is processed by antigen-presenting cells and presented to T-lymphocytes in the context of major histocompatibility complex (MHC) class II surface molecules. The interaction leads to the activation of an inflammatory response with a decidedly Th1 bias, the recruitment of inflammatory cells, and the formation of noncaseating granulomas. More recently, there is compelling evidence for a role of Th17 immune pathways in the generation and maintenance of sarcoidosis granulomas. The prevailing hypothesis is that antigen persistence leads to augmentation and persistence of the inflammatory cascade (with the elevation of tumor necrosis factor), whereas antigen clearance is followed by termination of the inflammatory cascade and disease remission [9].
The decision to initiate chronic immunosuppression for patients with sarcoidosis is often predicated on a number of factors. It may be based on the type of organ involvement (symptomatic cardiac, neurologic, or progressive hepatic disease), whether they are persistently symptomatic on corticosteroids, or whether they are likely to require a long duration of therapy (chronic active disease with advanced organ damage). The Scadding stage of disease correlates loosely with the risk of chronic disease and likelihood for the need for chronic therapy. Those patients who have experienced a pattern of relapsing-remitting disease (often with months or years between relapses) may benefit by chronic immunosuppression to prevent further relapses. Based on these observations, patients can be classified in terms of disease activity as having disease in remission (spontaneous remission may be the norm), acute sarcoidosis (with or without Lofgren’s syndrome), transiently active disease (usually remits within several months), chronic progressive disease (requiring chronic immunosuppression to prevent organ damage), or relapsing-remitting disease (requiring chronic immunosuppression to prevent disease relapse). In our opinion, those with chronic progressive disease and with relapsing-remitting disease most often progress to severe end-organ damage despite chronic immunosuppression. Once the decision to proceed with chronic therapy is made, the next challenge is the choice of agent and the duration of immunosuppression. A recent Delphi study of sarcoidosis experts demonstrates the general lack of consensus and of data to support this decision process [10].
Corticosteroids have been shown to offer short-term benefits (in symptoms, pulmonary function, and radiographically), but early studies suggest that it offers no long-term benefit (radiographically or by pulmonary function testing) compared with placebo [11]. The use of corticosteroid-sparing agents (such as antimetabolites) has improved the treatment options available to patients, but not all benefit from this therapy and some experience adverse effects requiring cessation of therapy [12]. With the emergence of many other biologics targeting specific molecules in the inflammatory cascade, there is a burdening need to explore them as potential therapies for patients with sarcoidosis. There is also a need to develop new therapeutic approaches with the intent of modifying the natural history of active progressive and relapsing-remitting disease, that is, a need for disease modifying antisarcoidosis drug therapies.
IMMUNOPATHOGENESIS OF SARCOIDOSIS
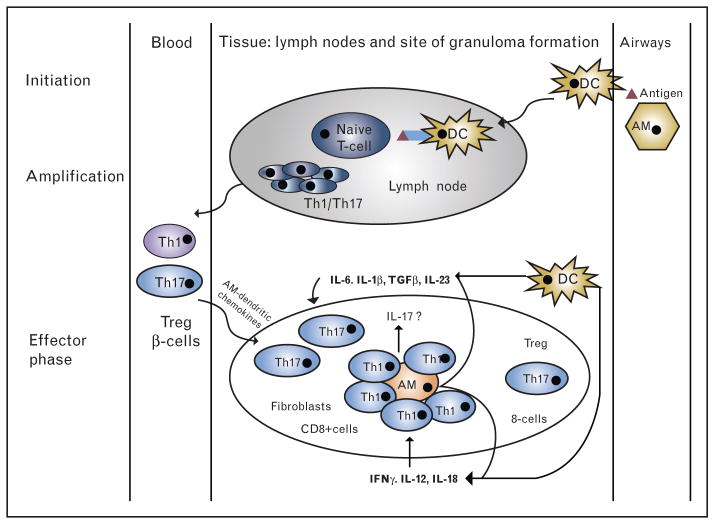
Alveolar macrophages, activated via an aerosol antigen, play a fundamental role in the pathogenesis of sarcoidosis. Alveolar macrophages from patients with sarcoidosis are increased and spontaneously produce tumor necrosis factor that is thought to be the granuloma-promoting factor in sarcoidosis [9]. Antigen processing and presentation by macrophages, primarily via the human leukocyte antigen-antigen D related (HLA-DR) MHC class II cell surface receptor, leads to T-cell activation that is necessary for granuloma formation and maintenance. This T-cell activation has several important characteristics. First, T-cell activation by activated macrophages depends on costimulatory signaling from the macrophage’s CD80 and CD86 which attaches to the T-cell protein receptor cytotoxic T-lymphocyte associated protein 4 (CTLA-4). This T-cell response is biased toward Th1 cytokine release including interleukin (IL)-6 and IL-12. Second, Th17 cells are also increased in sarcoidosis and may play a role in granuloma formation and maintenance, which indicates an important role for IL-17 and IL-23. Finally, the p38 mitogen-activated protein (MAP) kinase tumor necrosis factor signal transduction pathway is dysregulated in sarcoidosis, which leads to excessive Th1 and Th17 immune response, including additional TNFα production [13]. Potential biologic targets for novel sarcoidosis therapies include T-cell activation, Th1-mediated inflammation, Th17-mediated inflammation, and tumor necrosis factor signal transduction pathways such as the p38 MAP kinase (Fig. 2).
FIGURE 2.
Granuloma formation – Th1/Th17 hypothesis. Initiation: alveolar macrophages and dendritic cells are activated by a putative antigen. Dendritic cells migrate to lymph nodes and initiate Th1/Th17-cell amplification. Chemokines, produced by alveolar macrophages, attract Th1/17, Treg, B-cells as well as CD8+ cells and fibroblast and initiate granuloma formation (effector phase). Both dendritic cells and macrophages produce cytokines favoring Th1 and Th17 cells in sarcoidosis [54].
ANTIMETABOLITES: METHOTREXATE, AZATHIOPRINE, LEFLUNOMIDE, AND MYCOPHENOLATE
Among the most utilized steroid-sparing agents, the antimetabolite methotrexate has been shown in a randomized placebo-controlled clinical trial to be an effective therapy for sarcoidosis that allows patients to reduce or completely withdraw systemic corticosteroid therapy [14] (Table 1). Developed as a folate analogue that inhibits dihydrofolate reductase, its anti-inflammatory effects appear to be related to extracellular adenosine release causing inhibition of IL-8 production, IL-6 secretion, and LTB4 synthesis by inflammatory cells [15]. Methotrexate’s efficacy has been limited by its highly variable oral bioavailability, and there are concerns about its adverse effects on the liver and lungs. Azathioprine, a prodrug of mercaptopurine, halts DNA replication and therefore exerts its inhibitory effects on proliferating cells such as lymphocytes [16]. Azathioprine has been found to have similar efficacy as a therapy for sarcoidosis, although the data are more limited [15] and it is discontinued more often than methotrexate due to adverse side effects [16,17]. Leflunomide exerts its immunomodulatory effects by inhibiting mitochondrial dihydroorotate, thereby inhibiting urinidine monophosphate synthesis and cell cycle progression. It has been shown to significantly improve lung function in combination with methotrexate or, occasionally, as monotherapy in patients who do not respond to or tolerate methotrexate therapy [18,19]. Mycophenolate mofetil is an inhibitor of inosine-5-monophosphate dehydrogenase and depletes guanosine nucleotides preferentially from T and B lymphocytes inhibiting proliferation. It has been shown to be useful in case series for those who are intolerant of other steroid-sparing agents, but a recent single-center trial questions its efficacy in sarcoidosis [20,21].
Table 1.
Antimetabolite therapy [4▪]
| Level of evidence in sarcoidosis | Most common toxic effects (>1%) | Rare but important toxic effects | |
|---|---|---|---|
| Methotrexate | Double-blind placebo- controlled trials, prospective case series and case reports | Nausea, mouth ulcers, leucopenia, hepatotoxicity, nausea, and infections | Pneumonitis and teratogenic |
| Azathioprine | Prospective case series and case reports | Leucopenia, nausea, and infections | Severe leucopenia, hepatotoxic effects, pancreatitis, and skin cancer |
| Leflunomide | Double-blind placebo- controlled trials, prospective case series, and case reports | Leucopenia, hepatotoxic effects, infections, and alopecia | Pneumonitis, teratogenic, peripheral neuropathy, and hypertension |
| Mycophenolate mofetil | Case series | Nausea, diarrhea, and infections | Skin cancer |
TNFα INHIBITORS: INFLIXIMAB, ADALIMUMAB, AND NONTARGETED TUMOR NECROSIS FACTOR AGENTS
For patients with systemic disease that is refractory to glucocorticoids or antimetabolites, targeted TNFα inhibition is the next recommended step in treatment [9,13]. The best data are for infliximab, which has shown mixed results in two randomized clinical trials [22,23]. Efficacy data on adalimumab is limited to a single, small open-label study [24]. It may be an effective alternative for patients intolerant to infliximab, showing similar safety and efficacy results [24,25], but the data are limited. Thalidomide, pentoxifylline, and apremilast are three drugs with nontargeted TNFα inhibitory effects and are considered third line because of disappointing data on efficacy and side effects [26–28].
CYTOTOXIC T-LYMPHOCYTE ASSOCIATED BLOCKADE
T-cell activation requires costimulation through connection between the macrophage’s CD80/ CD86 complex and the CTLA protein receptor on the T cell. Blockade of this connection results in downregulation of the T-cell response. Abetacept is a fusion protein composed of the Fc region of the IgG1 fused to the extracellular domain of CTLA-4 that binds to the CD80 and CD86 molecule. This prevents costimulation and results in T-cell down-regulation. Abetacept is currently approved for patients with rheumatoid arthritis who have had an inadequate response to anti-TNFα therapy. Several placebo-controlled trials have been conducted on abetacept in Crohn’s disease and ulcerative colitis [29]. This drug could be a potentially useful immunosuppressant in patients with sarcoidosis.
INHALED VASOACTIVE INTESTINAL PEPTIDE
Vasoactive intestinal peptide (VIP) is a neuropeptide that has pleiotropic effects on smooth muscle contractility and vasodilation as well as anti-inflammatory effects. Inhaled VIP has been shown to have immunoregulatory effects in sarcoidosis, including an increase in regulator T cells resulting in suppressed induction and proliferation of effector T cells and downregulation of HLA-DR, CD86, and CD8+ in bronchoalveolar lavage (BAL) specimens in patients with sarcoidosis [30]. VIP therapy is being explored in a number of inflammatory diseases including sarcoidosis.
EXISTING Th1-TARGETED THERAPIES
There are existing therapies aimed at damping Th1-mediated inflammation through tumor necrosis factor inhibition that have been tested in sarcoidosis. Infliximab is discussed above. Adalimumab has shown therapeutic efficacy in small cohort studies [31]. Golimumab and etanercept are similar agents that have both been shown to be ineffective in sarcoidosis [32,33].
IL-6
IL-6 is an important proinflammatory cytokine secreted by T cells and macrophages that stimulates an immune response leading to granuloma formation. Takizawa et al. [34] demonstrated early on that IL-6 levels were elevated in the BAL fluid of patients with sarcoidosis and correlated strongly with levels of BALF CD3+ lymphocytes. Treatment with corticosteroids led to a significant reduction in the levels of IL-6 cells in this population. The first biologics against IL-6 approved by the Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis were tocilizumab (ACTEMRA; Genentech, San Francisco, California, USA), an anti-IL-6 receptor blocker, and siltuximab, an antibody directed against IL-6 itself. Olokizumab is a human mAb directed at IL-6 that has shown promising results in a randomized phase II study for patients with moderate-to-severe rheumatoid arthritis [35]. There are a number of therapies directed against IL-6 in early development for autoimmune diseases. However, therapies directed against IL-6 or IL-6 receptor have not been studied in patients with sarcoidosis.
IL-12/IL-23P40 AND Th17 PATHWAYS
IL-12/IL-23 is a heterodimeric cytokine composed of the IL-12p40 minor subunit and the IL-23p19 major subunit. IL-12 is a Th1 cytokine produced by macrophages, among other cells, in response to antigenic stimulation and is involved in the differentiation of naïve T cells into Th1 cells. It also stimulates the production of TNFα. The p40 minor subunit of IL-12 (IL-12p40) is elevated in BAL samples from patients with sarcoidosis compared with idiopathic pulmonary fibrosis patients and controls [36]. Gene expression profiles comparing sarcoidosis lesional skin biopsies with nonlesional biopsies and normal controls identified a number of dysregulation genes including IL-12p40 and IL23 receptor, suggesting a role of both Th1 and Th17 pathways in sarcoidosis. Similarly, Ten Berge et al. [37] and Facco et al. [38] both showed elevated levels of IL-17a+ cells in the BAL fluid of patients with sarcoidosis compared with peripheral blood and healthy volunteers and found IL-17a+ T cells within tissue granulomas, indicating a role for Th17 pathways in the induction and maintenance of sarcoidosis granulomas.
There are a plethora of drugs targeting molecules in the Th17 inflammatory pathways. Ustekinumab is a human mAb that binds to the p40 minor subunit of IL-12 (a Th1 cytokine) and IL23 and was recently approved for the treatment of psoriatic arthritis and moderate-to-severe plaque psoriasis. The efficacy of ustekinumab was recently studied in patients with chronic pulmonary sarcoidosis and/or skin sarcoidosis and found to be ineffective in patients with sarcoidosis [33]. However, this lack of effectiveness is at first perplexing as IL-12 is a relevant Th1 cytokine in sarcoidosis. One hypothesis that might explain the findings is that targeting the p40 minor subunit may be less effective than targeting the major subunit of the IL-23 heterodimer complex (IL-23p19). Like IL-12/IL-23p40, IL-23p19 mRNA expression has also been shown to be elevated in sarcoidosis skin lesions [39]. Novel therapies, targeting the major subunit of IL-23, are approved and may provide another opportunity to explore this mechanism. For example, STA 5326 is a potent inhibitor of IL-12/23 production and has been tested in phase I/II studies for moderate-to-severe Crohn’s disease and rheumatoid arthritis with mixed results [40,41]. Tildrakizumab, a mAb that blocks IL-23, is in phase III clinical trials for psoriasis, and secukinumab, a recent FDA-approved human mAb targeting IL-17 for plaque psoriasis, has yielded promising results in rheumatoid arthritis [42] and ankylosing spondylitis [43]. None of these drugs have been studied in sarcoidosis.
P38 MITOGEN-ACTIVATED PROTEIN KINASES, NLRP3 INFLAMMASOME, AND THE UBIQUITIN PROTEASOME SYSTEM
There is a strong link between the p38 group of MAP kinases and production of TNFα [13]. Enhanced p38 activation leads to excessive Th1 and Th17 proinflammatory cytokine production. When BAL cells of sarcoidosis patients are stimulated by sustained p38 phosphorylation, they have been shown to respond with more robust TNFα and IL-12/IL-23p40 production [13]. Targeting p38 MAP kinases has appealing therapeutic potential as it plays an important role both in maintaining tumor necrosis factor mRNA stability as well as in the downstream activation of a variety of cytokines that result from tumor necrosis factor signaling. BIRB 796 and semapimod are p38 MAP kinase inhibitors that have undergone clinical trial testing in Crohn’s disease, but neither has been studied in sarcoidosis [44,45].
First recognized in 2002, inflammasomes are newly recognized pattern-recognition receptors expressed on immune and inflammatory cells that target endogenous and exogenous pathogens. Numerous inflammasomes have been identified, but the best studied is the NLRP3 inflammasome. Activation of this protein complex leads to the activation of caspase-1 and release of the proinflammatory cytokine IL-1β that is involved in both acute and chronic inflammatory responses in a variety of inflammatory diseases including sarcoidosis. Several NLRP3 inhibitors have been approved: rilonocept, an IL-1 blocker for cryopyrin-associated periodic syndrome (CAPS); canakinumab, a mAb inhibitor of IL-1β for CAPS; and anakinra, an IL-1 receptor antagonist for rheumatoid arthritis and CAPS. None have been studied in sarcoidosis [46–48].
The ubiquitin proteasome system (UPS) is a highly regulated component in cells that controls protein degradation and abundance, and therefore regulates many cellular activities and functions [49▪]. Specifically, protein ubiquitination occurs through the activity of protein complexes consisting of ubiquitin protein, the E1 ubiquitin-activating enzyme, E2 ubiquitin-conjugating enzyme, and E3 ubiquitin ligase. The majority of ubiquitinated proteins are degraded by the 26S proteasome [49▪,50]. Interestingly, several components of the UPS, such as E3 ubiquitin ligases, play critical roles in tissue inflammation. For example, patients with mutation of the anti-inflammatory ubiquitin E3 ligase ITCH suffer from syndromic multisystem autoimmunity, inflammatory lung disease, and premature death [51,52]. Also, the major inflammatory signaling pathway, NF-kB activity, is directly regulated by UPS. Many efforts have been made to develop therapeutics targeting components of UPS; however, the only FDA-approved drugs so far are the three 26 proteasome inhibitors – Bortezomib (Millennium Pharmaceuticals, Cambridge, Massachusetts, USA), Carfilzomib (Onyx Pharmaceuticals, Inc., Thousand Oaks, CA, USA), and Ixazomib (Milleneium Pharmaceuticals, Cambridge, MA, USA) [53]. Although all these proteasome inhibitors are only approved for multiple myeloma, many studies had investigated their efficacies in inflammatory diseases. For example, bortezomib and carfilzomib have been shown to be effective in inflammatory models of respiratory syncytial virus infection, muscle wasting in diabetes, adjuvant-induced arthritis, intracerebral hemorrhage, sepsis, acute graft-versus-host diseases, ischemia-reperfusion injury, and acute pancreatitis-induced lung injury [54,55,56▪,57–59]. Moreover, carfilzomib has recently been tested in a clinical trial for antibody-mediated rejection in lung and heart transplant [60]. These proteasome inhibitors hold potential as novel therapies for sarcoidosis.
CONCLUSION
Although there have been significant advances in the understanding and treatment of sarcoidosis, the disease continues to exert an unacceptable burden in terms of morbidity and mortality. Rising mortality from the disease strongly supports the need to develop therapies directed in modifying its natural history, a need for ‘disease modifying antisarcoidosis therapies’ [61]. There is a compelling need to explore the potential benefits of a growing number of therapeutics targeting molecules relevant to T-cell activation, Th1 and Th17 immune pathways, important signal transduction pathways, and the ubiquitin–proteasome system. The growing number of therapeutics to treat diseases that share common pathogenic mechanisms with sarcoidosis – such as rheumatoid arthritis, Crohn’s disease, and psoriasis – offer an excellent opportunity to explore these biologic agents as novel therapies in patients with sarcoidosis.
Acknowledgments
We would like to thank Ms Theresa Heinrich for her help in the preparation of this manuscript.
Footnotes
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
None.
REFERENCES AND RECOMMENDED READING
Articles of particular interest, published within the annual period of review, have been highlighted as
▪ of special interest
▪▪ of outstanding interest
- 1.Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155–1167. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Judson MA, Teirstein A, et al. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM. 2006;99:307–315. doi: 10.1093/qjmed/hcl038. [DOI] [PubMed] [Google Scholar]
- 3.Baughman RP, Nagai S, Balter M, et al. Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:56–64. [PubMed] [Google Scholar]
- 4▪.Baughman RP, Grutters JC. New treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approaches. Lancet Respir Med. 2015;3:813–823. doi: 10.1016/S2213-2600(15)00199-X. This is an excellent review of the current therapeutic strategies for managing chronic and refractory disease. [DOI] [PubMed] [Google Scholar]
- 5.Droughtvourt C, Rybojad M, Porcher R, et al. A Randomized, investigator-masked, double-blind, placebo-controlled trial on thalidomide in severe cutaneous sarcoidosis. Chest. 2014;146:1046–1054. doi: 10.1378/chest.14-0015. [DOI] [PubMed] [Google Scholar]
- 6.Baughman RP, Nunes H, Sweiss NJ, Lower EE. Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J. 2013;41:1424–1438. doi: 10.1183/09031936.00060612. [DOI] [PubMed] [Google Scholar]
- 7.Gibson GJ, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238–247. doi: 10.1136/thx.51.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swigris JJ, Olson AL, Huie TJ, et al. Sarcoidosis-related mortality in the United States from 1988 to 2007. Am J Respir Crit Care Med. 2011;183:1524–1530. doi: 10.1164/rccm.201010-1679OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zissel G, Prasse A, Müller-Quernheim J. Immunologic response of sarcoidosis. Semin Respir Crit Care Med. 2010;31:390–403. doi: 10.1055/s-0030-1262208. [DOI] [PubMed] [Google Scholar]
- 10.Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Resp Med. 2010;104:717–723. doi: 10.1016/j.rmed.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 11.James DG, Carstairs LS, Trowell J, Sharma OP. Treatment of sarcoidosis. Report of a controlled therapeutic trial. Lancet. 1967;2:526–528. doi: 10.1016/s0140-6736(67)90493-x. [DOI] [PubMed] [Google Scholar]
- 12.Baughman RP, Lower EE. Medical therapy of sarcoidosis. Semin Respir Crit Care Med. 2014;35:391–406. doi: 10.1055/s-0034-1376401. [DOI] [PubMed] [Google Scholar]
- 13.Rastogi R, Du W, Ju D, et al. Dysregulation of p38 and MKP-1 in response to NOD1/TLR4 stimulation in sarcoid bronchoalveolar cells. Am J Respir Crit Care Med. 2011;183:500–510. doi: 10.1164/rccm.201005-0792OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60–66. [PubMed] [Google Scholar]
- 15.Cutolo M, Sulli A, Pizzorni C, Seriolo B. Antiinflammaotry mechanisms of methotrexate in rheumatoid arthritis. Ann Rheum Dis. 2001;60:729–735. doi: 10.1136/ard.60.8.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vorselaars ADM, Wuyts Wa, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805–812. doi: 10.1378/chest.12-1728. [DOI] [PubMed] [Google Scholar]
- 17.Maltzman JS, Koretzky GA. Azathioprine: old drug, new actions. J Clin Invest. 2003;111:1122–1124. doi: 10.1172/JCI18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teschler S, Burst V. Leflunomide: a drug with a potential beyond rheumatology. Immunotherapy. 2010;2:637–650. doi: 10.2217/imt.10.52. [DOI] [PubMed] [Google Scholar]
- 19.Galor A, Jabs Da, Leder Ha, et al. Comparison of antimetabolite drugs as corticosteroid-sparing therapy for noninfectious ocular inflammation. Ophthalmology. 2008;115:1826–1832. doi: 10.1016/j.ophtha.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 2005;14:s2–s8. doi: 10.1191/0961203305lu2109oa. [DOI] [PubMed] [Google Scholar]
- 21.Hanzeh N, Voelker A, Forssen A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med. 2014;108:1663–1669. doi: 10.1016/j.rmed.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:201–208. [PubMed] [Google Scholar]
- 23.Judson MA, Baughman RP, Costabel U, et al. Centocor T48 Sarcoidosis Investigators. Efficacy of infliximab in extrapulmonarysarcoidosis: results from a randomised trial. Eur Respir J. 2008;31:1189–1196. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]
- 24.Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:46–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Crommelin HA, Va der Burg LM, Vorselaars AD, et al. Efficacy of adalimumab in sarcoidosis patients who develop intolerance to infliximab. Res Med. 2016;115:72–77. doi: 10.1016/j.rmed.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Baughman RP, Judson MA, Ingledue R, et al. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatol. 2012;148:262–264. doi: 10.1001/archdermatol.2011.301. [DOI] [PubMed] [Google Scholar]
- 27.Park MK, Fontana, Babaali H, et al. Steroid-sparing effects of pentoxifylline in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:121–131. [PMC free article] [PubMed] [Google Scholar]
- 28.Fazzi P, Manni E, Cristofani R, et al. Thalidomide for improving cutaneous and pulmonary sarcoidosis in patients resistant or with contraindications to corticosteroids. Biomed Pharmacother. 2012;66:300–307. doi: 10.1016/j.biopha.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Sandborn WJ, Colombel J-F, Sands BE, et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143:62e4–69.e4. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Prasse A, Zissel G, Lützen N, et al. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am J Respir Crit Care Med. 2010;182:540–548. doi: 10.1164/rccm.200909-1451OC. [DOI] [PubMed] [Google Scholar]
- 31.Kamphuis L, Lam-Tse WK, Dik WA, et al. Efficacy of adalimumab in sarcoidosis. J Transl Med. 2010;8:10. [Google Scholar]
- 32.Utz JP, Limper AH, Kalra S, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003;124:177–185. doi: 10.1378/chest.124.1.177. [DOI] [PubMed] [Google Scholar]
- 33.Judson MA, Baughman RP, Costabel U, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44:1296–1307. doi: 10.1183/09031936.00000914. [DOI] [PubMed] [Google Scholar]
- 34.Takizawa H, Satoh M, Okazaki H, et al. Increased IL-6 and IL-8 in bronchoalveolar lavage fluids (BALF) from patients with sarcoidosis: correlation with the clinical parameters. Clin Exp Immunol. 1997;107:175–181. doi: 10.1046/j.1365-2249.1997.d01-905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese MC, Fleischmann R, Furst D, et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann Rheum Dis. 2014;73:1607–1615. doi: 10.1136/annrheumdis-2013-204760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moller DR, Forman JD, Liu MC, et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol. 1996;156:4952–4960. [PubMed] [Google Scholar]
- 37.Ten Berge B, Paats MS, Bergen IM, et al. Increased IL17A expression in the granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 2012;51:37–46. doi: 10.1093/rheumatology/ker316. [DOI] [PubMed] [Google Scholar]
- 38.Facco M, Cabrelle A, Teramo A, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66:144–150. doi: 10.1136/thx.2010.140319. [DOI] [PubMed] [Google Scholar]
- 39.Judson MA, Marchell RM, Mascelli M, et al. Molecular profiling and gene expression analysis in cutaneous sarcoidosis: the role of interleukin-12, interleukin-23, and the T-helper 17 pathway. J Am Acad Dermatol. 2012;66:910e1–910.e2. doi: 10.1016/j.jaad.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Krausz S, Baumans MJH, Gerlag DM, et al. A phase IIa, randomized, double-blind, placebo-controlled clinical study of apilimod mesylate, an oral IL-12/IL-23 inhibitor, in patients with active rheumatoid arthritis. Arthritis Rheum. 2012;64:1750–1755. doi: 10.1002/art.34339. [DOI] [PubMed] [Google Scholar]
- 41.Burakoff R, Barish CF, Riff D, et al. A phase 1/2A trial of STA 5326, an oral interleukin-12/23 inhibitor, in patients with active moderate to severe Crohn’s disease. Inflamm Bowel Dis. 2006;12:558–565. doi: 10.1097/01.ibd.0000225337.14356.31. [DOI] [PubMed] [Google Scholar]
- 42.Genovese MC, Durez P, Richards HB, et al. One-year efficacy and safety results of secukinumab in patients with rheumatoid arthritis: phase II, dose-finding, double-blind, randomized, placebo-controlled study. J Rheumatol. 2014;41:414–421. doi: 10.3899/jrheum.130637. [DOI] [PubMed] [Google Scholar]
- 43.Baeten D, Baraliakos X, Braun J, et al. Antiinterleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2013;382:1705–1713. doi: 10.1016/S0140-6736(13)61134-4. [DOI] [PubMed] [Google Scholar]
- 44.Schreiber S, Feagan B, D’Haens G, et al. Oral p38 mitogen-activated protein kinase inhibition with BIRB 796 for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol. 2006;4:325–334. doi: 10.1016/j.cgh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Dotan I, Rachmilewitz D, Schreiber S, et al. A randomised placebo-controlled multicentre trial of intravenous semapimod HCl for moderate to severe Crohn’s disease. Gut. 2010;59:760–766. doi: 10.1136/gut.2009.179994. [DOI] [PubMed] [Google Scholar]
- 46.Shao B-Z, Xu Z-Q, Han B-Z, et al. NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol. 2015;6:262. doi: 10.3389/fphar.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott IC, Ibrahim F, Simpson G, et al. A randomised trial evaluating anakinra in early active rheumatoid arthritis. Clin Exp Rheumatol. 2016;34:88–93. [PubMed] [Google Scholar]
- 48.Weathington NM, Mallampalli RK. Emerging therapies targeting the ubiquitin proteasome system in cancer. J Clin Invest. 2014;124:6–12. doi: 10.1172/JCI71602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪.Liu Y, Mallampalli RK. Small molecule therapeutics targeting F-box proteins in cancer. Semin Cancer Biol. 2016;36:105–119. doi: 10.1016/j.semcancer.2015.09.014. This recent review describes the vital role of the ubiquitin–proteasome system in maintaining protein equilibrium and provides a compelling rationale for the development of therapeutics targeting this pathway for cancer treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weathington NM, Sznajder JI, Mallampalli RK. The emerging role of the ubiquitin proteasome in pulmonary biology and disease. Am J Respir Crit Care Med. 2013;188:530–537. doi: 10.1164/rccm.201304-0754PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lohr NJ, Molleston JP, Strauss KA, et al. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am J Hum Genet. 2010;86:447–453. doi: 10.1016/j.ajhg.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teicher BA, Tomaszewski JE. Proteasome inhibitors. Biochem Pharmacol. 2015;96:1–9. doi: 10.1016/j.bcp.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Resmi H. The combination of bortezomib and resveratrol may prevent muscle wasting in diabetes. Med Hypotheses. 2011;76:291–292. doi: 10.1016/j.mehy.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 54.Yannaki E, Papadopoulou A, Athanasiou E, et al. The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis Rheum. 2010;62:3277–3288. doi: 10.1002/art.27690. [DOI] [PubMed] [Google Scholar]
- 55.Sinn DI, Lee ST, Chu K, et al. Proteasomal inhibition in intracerebral hemorrhage: neuroprotective and anti-inflammatory effects of bortezomib. Neurosci Res. 2007;58:12–18. doi: 10.1016/j.neures.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 56▪.Han SH, Kim SJ, Woo JH, et al. The effect of bortezomib on expression of inflammatory cytokines and survival in a murine sepsis model induced by cecal ligation and puncture. Yonsei Med J. 2015;56:112–123. doi: 10.3349/ymj.2015.56.1.112. In this study, bortezomib was shown to improve survival in a murine model of severe sepsis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z, Wu Q, Yan Z, et al. The protection and therapy effects of bortezomib in murine acute graft-versus-host disease. Transplant Proc. 2013;45:2527–2535. doi: 10.1016/j.transproceed.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 58.Chen FT, Yang CM, Yang CH. The protective effects of the proteasome inhibitor bortezomib (velcade) on ischemia-reperfusion injury in the rat retina. PLoS One. 2013;8:e64262. doi: 10.1371/journal.pone.0064262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X, Lee XL, Wu T, et al. Proteasome inhibitor ameliorates severe acute pancreatitis and associated lung injury of rats. World J Gastroenterol. 2008;14:3249–3253. doi: 10.3748/wjg.14.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeevi A, Marrari M, Lunz J, et al. The big picture: a case report of antibody mediated rejection and treatment after lung transplantation illustrating the need to correlate laboratory findings with clinical status. Clin Transpl. 2013:399–405. [PubMed] [Google Scholar]
- 61.Cremers JP, Drent M, Bast A, et al. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: integrating systemic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med. 2013;19:545–561. doi: 10.1097/MCP.0b013e3283642a7a. [DOI] [PubMed] [Google Scholar]