Abstract
Objective: We aimed to systematically evaluate the influence of preoperative transarterial chemoembolization (TACE) for resectable hepatocellular carcinoma (HCC) on long-term prognosis and perioperative safety.
Materials and methods: Databases including PubMed, Embase, Cochrane, Wanfang, CNKI, VIP data were searched, combined with Manual Retrieval and Cited Reference Search to collect the published randomized controlled trial (RCT) about the influence of pre-TACE for curative resection of HCC. The searching cutoff date was 2016/02/25, all the data obtained were statistically analyzed using RevMan5.2 software recommended by Cochrane Collaboration.
Results: A total of 5 RCT including 430 (pre-TACE group: 212, surgery alone group: 218) patients were included. The results of meta-analysis showed that: there was no difference between the 2 groups on overall survival (OS) rate [HR 1.25, 95%CI (0.92–1.68)], disease free survival (DFS) rate [HR 0.95 (0.76–1.19)], perioperative mortality rate [OR 0.70 (0.22–2.30)], or blood loss [SMD 0.07 (−0.14–0.29)], whereas the subgroup analysis revealed that pre-TACE would result in longer operation time [SMD 0.31 (0.06–0.57)], higher postoperative morbidity rate [OR 1.90 (1.02–3.53)] and combined resection rate of perihepatic organs [OR 5.46 (2.73–11.78)] in subgroup with mean tumor diameter >5cm.
Conclusions: According to our study, pre-TACE treatment cannot improve the long-term prognosis of resectable HCC. With the growth of the tumor diameter, especially when it is over 5cm, it might add difficulties to surgery and affect the perioperative safety.
Keywords: Hepatocellular, transarterial, resectable, meta-analysis
Introduction
As one of the most common gastrointestinal malignancies, HCC is the third leading cause of cancer deaths globally.[1] Every year, over 500,000 new cases are diagnosed and more than half of them happen in Asia area.[2,3]
Surgical resection and liver transplantation are recognized as the most effective treatments to patients with HCC. They could provide much higher postoperative DFS than other non-surgical treatments.[4–7] However, donor organ shortage, high cost and the need of lifelong immunosuppressive medications are the major limitations in the field of liver transplantation. For some resectable HCC, surgical resection is comparable with liver transplantation on the long-term prognosis and more affordable on physical, psychological or economic conditions.[8,9] However, the high incidence of tumor recurrence accounts for the major cause of death after operation.[10,11] In order to reduce the recurrence rate, postoperative TACE could remarkably improve the prognosis of patients.[12,13] But, the effect of pre-TACE for curative resection of HCC is still controversial.
Currently, some meta-analyses [14–16] have analyzed the influence of pre-TACE for resectable HCC on long-term prognosis, but hardly of them have analyzed the effect of pre-TACE on operation safety. In our study, we not only evaluated the prognostic indicators of the included studies, but also took indicators such as postoperative morbidity rate, perioperative mortality, blood loss, operation time and combined resection rate of perihepatic organs into consideration. By taking mean tumor size >5 cm or ≤5 cm as a standard to perform subgroup analysis, we effectively solved the problem of large heterogeneity while analyzing some indexes. It ensured the results of our study are under strictly statistical logic.
Methods
Criteria for inclusion: (1) patients: who were diagnosed with resectable primary HCC; (2) intervention: TACE plus operation versus operation alone; (3) type of studies: only published RCT was included; (4) outcome: the reported outcomes including DFS, OS and relevant data of perioperative period; (5) for those repeated publication or overlapping cases, only keep the trials having better quality or more comprehensive information.
Criteria for exclusion: (1) patients with metastatic hepatic carcinoma or unresectable HCC; (2) patients accepted other adjuvant therapy besides TACE before operation; (3) trials did not contain enough data of DFS, OS; (4) abstracts, letters, editorials and expert opinions, reviews, case reports, and uncontrolled studies were being excluded.
Search strategy
Databases including PubMed, Embase, Cochrane, Wanfang data, CNKI, VIP data were searched, combined with Manual Retrieval and Cited Reference Search to collect the published RCT according to the inclusion criteria. The searching cutoff date was 25 February 2016. The following medical subject heading (MeSH) terms were used: liver cancer, hepatocellular carcinoma, transcatheter arterial chemoembolization, transarterial chemoembolization, preoperative, hepatectomy, liver resection.
Data extraction and assessment of risk of bias
Data extraction: two reviewers independently selected the studies according to the criteria described above and extracted the relevant data from each trial. For those repeated publication or overlapping cases, only the trials which had better quality or more comprehensive information were considered.
Assessment of risks bias: the quality of literature depends on the risk of bias. Two reviewers independently assessed the bias of included trials according to the method described in the Cochrane Handbook for Systematic Review of Intervention.[17] The index signs including: ‘low risk’, ‘unclear risk’, ‘high risk’. Reviewers would make the judgment in terms of the following 6 aspects: (1) random sequence generation; (2) allocation concealment; (3) blinding of DFS, OS, and perioperative relevant data; (4) incomplete outcome data; (5) selective reporting; (6) other bias.
During the process of data extraction and assessment of bias, any disagreement was resolved by discussion or with a third reviewer if necessary.
Statistical methods
The evaluation indicators for long-term prognosis were OS and DFS, which were measured by the hazard ratio (HR) with a 95% confidence interval (CI). The overall pooled HR and 95%CI were calculated with the inverse variance method and the method described by Tierney et al.[18] OS and DFS were always analyzed using odds ratio (OR) in most meta-analyses. By contrast, HR is more appropriate for time-to-event outcomes because it not only takes the number and timing of events into consideration but also the time until the last follow-up for each patient who had not experienced an event.
Dichotomous variables were tested by OR with a 95%CI, and continuous variables were tested by the standardized mean difference (SMD) with a 95%CI. A value of p < 0.05 was considered statistically significant. Besides, 95%CI for the pooled HR and OR did not overlap 1 and 95%CI for SMD did not overlap 0 were equivalent to a p value less than 0.05. Heterogeneity between trials was tested by chi-squared test (with significance set at p > 0.1) and I-squared test, trials with a p value less than 0.1 were defined as heterogeneous. Data that were not significantly heterogeneous (p > 0.1) were calculated using a fixed effects model otherwise random effect model was used. Funnel plots would be used to investigate the publication bias if sufficient studies existed. Statistical analyses were performed with Review Manager, version 5.2 recommended by Cochrane Collaboration.
Results
Description of identified studies
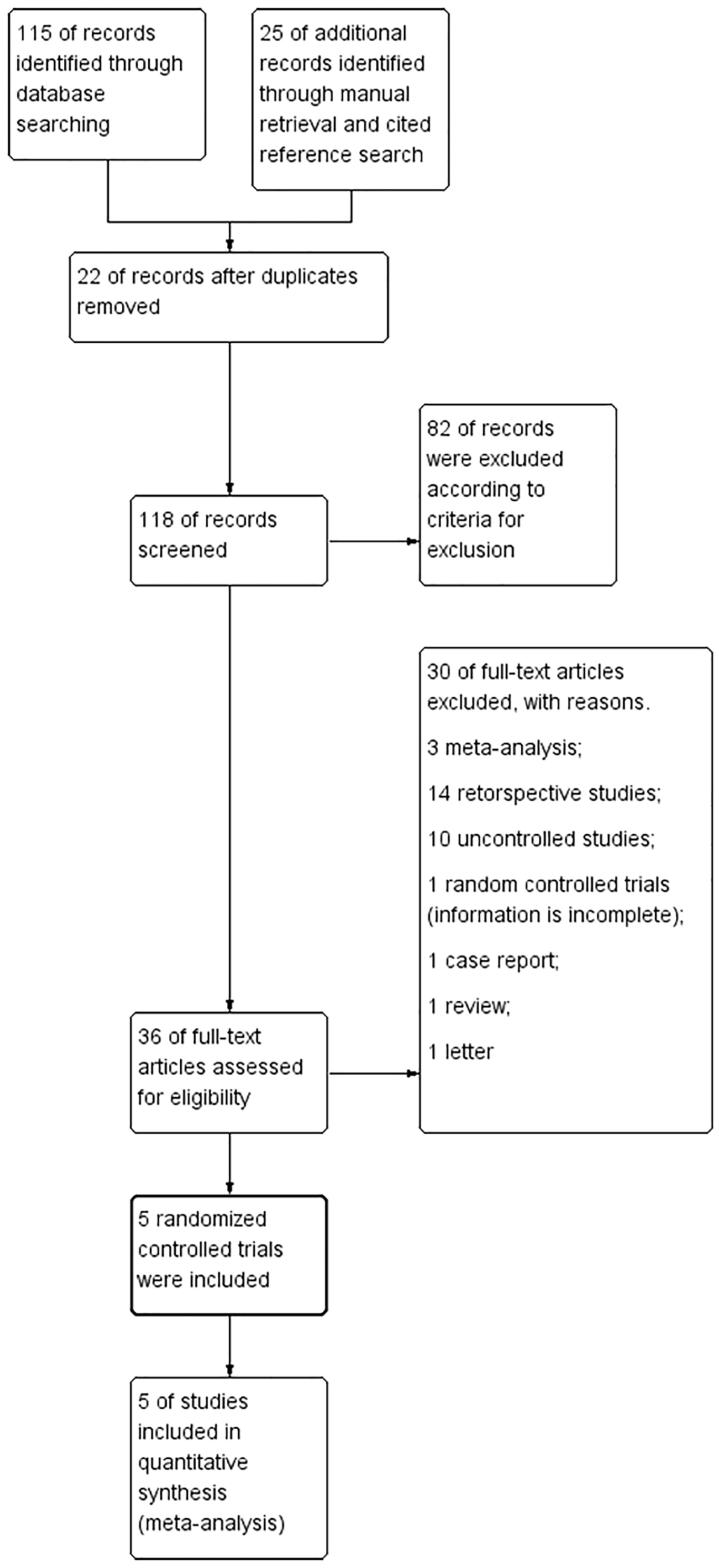
Figure 1 presents the flowchart of the study screening and the detailed selection process. Five RCT [19–23] were included for this systematic review, comprised a total of 430(pre-TACE group: 212; surgery alone group: 218) patients from China, Japan and Taiwan. Two reviewers reached an agreement on the ultimately included and excluded studies. All the included studies used TACE as only neoadjuvant therapy, and the essential characteristics are shown in Table 1 and the details of intervention are shown in Table 2.
Figure 1.
The flowchart of the study screening and the detailed selection process.
Table 1.
Clinical background of studies included in the meta-analysis.
| Author | group | Tumor diameter (cm) | Gender (M/F) | Age (years) | Child-Pugh (A + B/C) | Tumor No. (Single/Multiple) | TNM staging (I + II/III + IV) | Cirrhosis | Matching | Complete necrosis rate |
|---|---|---|---|---|---|---|---|---|---|---|
| Kaibori [19] | Pre-TACE | 4.30 ± 2.13 | 35/7 | 68.1 ± 5.7 | 37 + 5/0 | 32/10 | 31/11 | 14/42 | (1)(2)(3)(4)(5)(6) | 9/42(21.42%) |
| Control | 4.86 ± 4.12 | 32/11 | 66.1 ± 10.6 | 39 + 4/0 | 32/11 | 31/12 | 11/43 | |||
| Zhou [20] | Pre-TACE | 9.0 ± 3.2 | 48/4 | 45.3 ± 9.8 | 44 + 8/0 | NR | 25/22 | 49/52 | (1)(2)(3)(4)(5)(6)(7) | 7/47(14.89%) |
| Control | 9.5 ± 3.9 | 49/7 | 46.8 ± 9.6 | 54 + 2/0 | NR | 31/25 | 50/56 | |||
| Cui [21] | Pre-TACE | 8.33 ± 4.49 | 32/12 | 48.3 ± 8.77 | 44/0 | NR | NR | NR | (1)(2)(3)(4)(5)(6)(7) | 8/44(18.18%) |
| Control | 8.16 ± 5.28 | 30/14 | 47.3 ± 8.91 | 44/0 | NR | NR | NR | |||
| Yamasaki [22] | Pre-TACE | 3.1 ± 0.8 | 50/0 | 54.9 ± 6.4 | NR | 42/4 | NR | NR | (4)(5)(6) | 16/50(32%) |
| Control | 3.3 ± 0.9 | 47/0 | 57.1 ± 4.9 | NR | 38/7 | NR | NR | |||
| Wu [23] | Pre-TACE | 14.3 ± 4.2 | 23/1 | 51.8 ± 12.4 | 22 + 2/0 | NR/4 | 4/20 | 14/24 | (1)(2)(3)(4)(5)(6)(7) | 0/24(0%) |
TACE: transarterial chemoembolization; NR: Not Reported;TNM: Tumor Node Metastasis (1) operation time; (2) blood loss; (3) postoperative morbidity; (4) perioperative mortality; (5) disease free survival; (6) overall survival; (7) combined resection of perihepatic organs.
Numbers are mean value ± standard deviation. Complete necrosis rate are measured by histopathology examination.
Table 2.
Interventions of included trials.
| Author | Drugs and dosage of TACE | Mean numberof courses | Mean interval (d) | Results | Prognosis |
|---|---|---|---|---|---|
| Kaibori [19] | Epirubicin (28.1 ± 5.5 mg) + Lipiodol (2.9 ± 1.4 ml) + gelatin sponge particles in tumor area; epirubicin (22.2 ± 6.2 mg) + Lipiodol (1.9 ± 0.8 ml) into non-cancerous liver | 1.9 | 23.0 | No effect | TACE do not reduce the incidence of postoperative recurrence or prolong survival in patients with resectable HCC |
| Zhou [20] | Emulsion of 5-fluorouracil (1 g) + mitomycin C (20 mg) + cisplatin (5 mg) + lipiodol 10–30 ml (1–2 ml/cm diameter of the tumor) | 1.5 | 58.8 | Suggested unfavourable | TACE did not improve surgical outcome and result in drop-out from definitive surgery. |
| Cui [21] | Mitomycin(20–30 mg) + carboplatin(80–100mg) + fluorouracil(700–1000 mg) + lipiodol 8–30 ml (1ml/diameter cm) + gelatin sponge | NR | NR | Suggested unfavourable | TACE was associated with a higher prevalence of intra-operative adhesion in patients underwent hepatectomy |
| Yamasaki [22] | Doxorubicin(20 mg) + Urografin (2.5 ml) + lipiodol (5 ml) + gelatin sponge (1–3 ml) | 1 | NR | No effect | TACE before hepatectomy is no effective against such HCC accessory lesions and does not influence overall survival and disease-free survival |
| Wu [23] | Doxorubicin(20–30 mg) + cisplatin(2.5–50 mg) + lipiodol(20–30 ml) + gelatin sponge | 2.5 | 112.7 | Suggested unfavourable | TACE does not provide complete necrosis in large tumors and results in delayed surgery and difficulty in the treatment of recurrent lesions, without any benefit. |
Risk of bias in included studies
The risk of bias of the included studies is shown in Table 3. We used the method described in the Cochrane Handbook for Systematic Review of Intervention to evaluate the quality of each retrieved trial. No trial was blinded with regard to participant or caregiver due to the nature of the intervention. No trial gave specific details on the random sequence generation or allocation concealment except Zhou’s study, which was classified as having low risk of selection bias.
Table 3.
Bias assessment of studies included in the meta-analysis.
| Cochrane risk of bias table on the Prognosis for Patients with resectable HCC |
|||||
|---|---|---|---|---|---|
| Kaibori [19] | Zhou [20] | Yamasaki [22] | Wu [23] | Cui [21] | |
| Random sequence generation | Unclear risk (the method of randomizationgeneration was not described) | Unclear risk (the method of randomizationgeneration was not described) | Unclear risk (the method of randomizationgeneration was not described) | Unclear risk (the method of randomizationgeneration was not described) | Unclear risk (the method of randomizationgeneration was not described) |
| Allocation concealment | Unclear risk (whether using sealed, opaque or successive coded envelope was not mentioned) | Low risk (Using sealed, opaque and successive coded envelope) | Unclear risk (whether using sealed, opaque or successive coded envelope was not mentioned) | Unclear risk (information is not complete) | Unclear risk (information is not complete) |
| Blinding of outcomes | Unclear risk (information was not complete) | Unclear risk (information was not complete) | Unclear risk (information was not complete) | Unclear risk (information was not complete) | Unclear risk (information was not complete) |
| Incomplete outcomedata | Low risk (the results were relatively complete, missing data could be ignored) | Low risk (the results were relatively complete, missing data could be ignored) | High risk (incidence of loss to follow-up was up to 10% in pre-TACE group and 20% incontrol group) | Unclear risk (no direct data about overallsurvival and disease free survival) | High risk (the data of overall survival rate was incomplete) |
| Selective reporting | Low risk (predetermined indicators were definite) | Low risk (predetermined indicators were definite) | High risk (incomplete data and uncertainprimary outcome indicators) | High risk (incomplete data and uncertainprimary outcome indicators) | High risk (incomplete data and uncertainprimary outcome indicators) |
| Other bias | High risk (significantly different in the level of AFP and tumor stage among groups) | Unclear risk (information was not complete) | Unclear risk (information was not complete) | Unclear risk (information was not complete) | Unclear risk (information was not complete) |
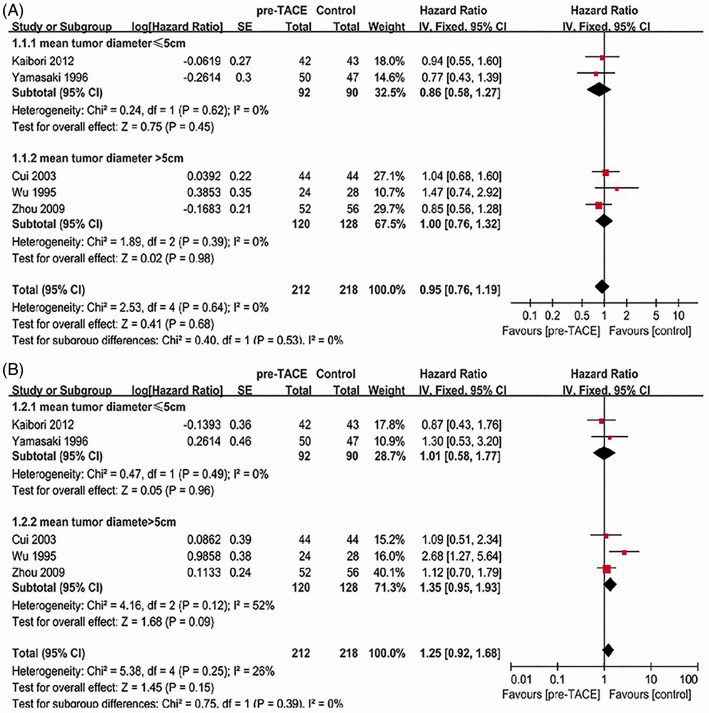
DFS and OS
Five trials [19–23] all reported on DFS and overall OS were considered. The heterogeneity between all five studies was not statistically significant (χ 2 = 2.53, degree of freedom [df] = 4, p = 0.64, I 2 = 0%; χ 2 = 5.38, degree of freedom [df] = 4, p = 0.25, I 2 = 26%) (Figure 2). Pooling of HRs from the five studies showed no significant difference on DFS or OS (HR = 0.95, 95%CI = [0.76–1.19], p = 0.68; HR = 1.25, 95%CI = [0.92–1.68], p = 0.15). When trials were stratified according to mean tumor size >5 cm or ≤5 cm, there was also no significant intervention effect in both subgroups on DFS (HR = 0.86, 95%CI = [0.58–1.27], p = 0.45; HR = 1, 95%CI = [0.76–1.32], p = 0.98) or OS (HR = 1.01, 95%CI = [0.58–1.77], p = 0.96; HR = 1.35, 95%CI = [0.95–1.93], p = 0.09).
Figure 2.
(A) Forest plot of the subgroup analysis on the DFS. (B) Forest plot of the subgroup analysis on the OS.
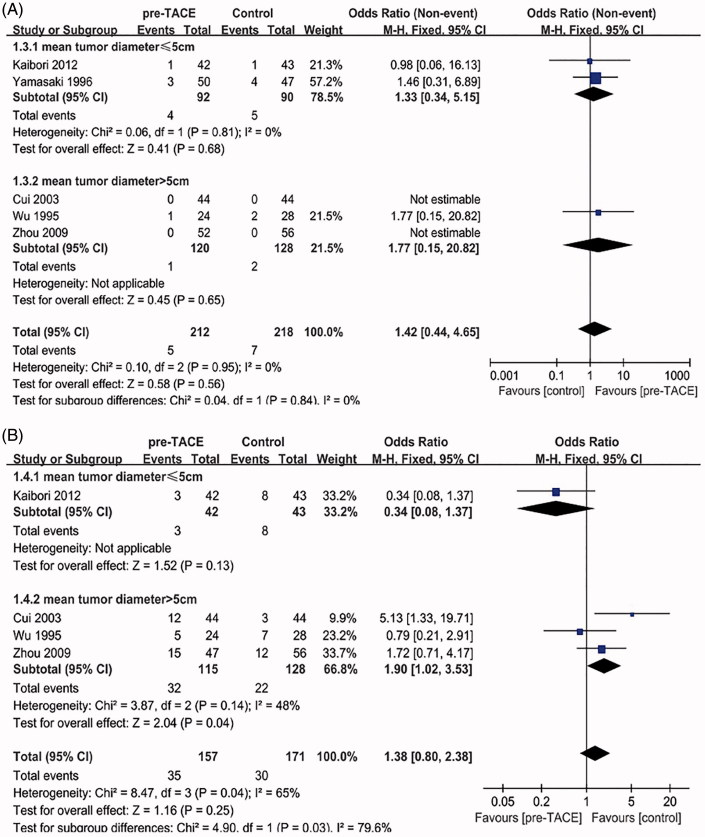
Perioperative mortality and postoperative morbidity
In terms of perioperative mortality, five studies [19–23] reported procedure-related mortality data. In two trials (Cui and Zhou), the number of deaths is 0, so the value of OR could not be calculated. The heterogeneity between the other three trials was not statistically significant (χ 2 = 0.04, degree of freedom [df] = 1, p = 0.84, I 2 = 0%) (Figure 3(A)). Overall meta-analysis of all five trials showed no significant difference on perioperative mortality (OR = 0.70, 95%CI = [0.22–2.30], p = 0.56). When trials were stratified according to mean tumor size >5 cm or ≤5 cm, there was also no significant intervention effect in both subgroups on perioperative mortality (OR = 0.75, 95%CI = [0.19–2.92], p = 0.68; OR = 0.57, 95%CI = [0.05–6.65], p = 0.65).
Figure 3.
(A) Forest plot of the subgroup analysis on the perioperative mortality. (B) Forest plot of the subgroup analysis on the postoperative morbidity
Potential complications after operation mainly include bile leakage, abdominal abscess, wound infection and so on, four studies [19–21,23] reported relevant data. The heterogeneity between all four studies was statistically significant (χ 2 = 8.47, degree of freedom [df] = 3, p = 0.04, I 2 = 65%) (Figure 3(B)); thus, overall meta-analysis of the four trials was not considered. When trials were stratified according to mean tumor size >5 cm or ≤5 cm, there was no significant heterogeneity in tumor size >5 cm subgroup (χ 2 = 3.87, degree of freedom [df] = 2, p = 0.14, I 2 = 48%). Furthermore, Pooling of ORs from mean tumor size >5 cm subgroup showed a significant difference favoring Control group on postoperative morbidity (OR = 1.90, 95%CI = [1.02–3.53], p = 0.04).
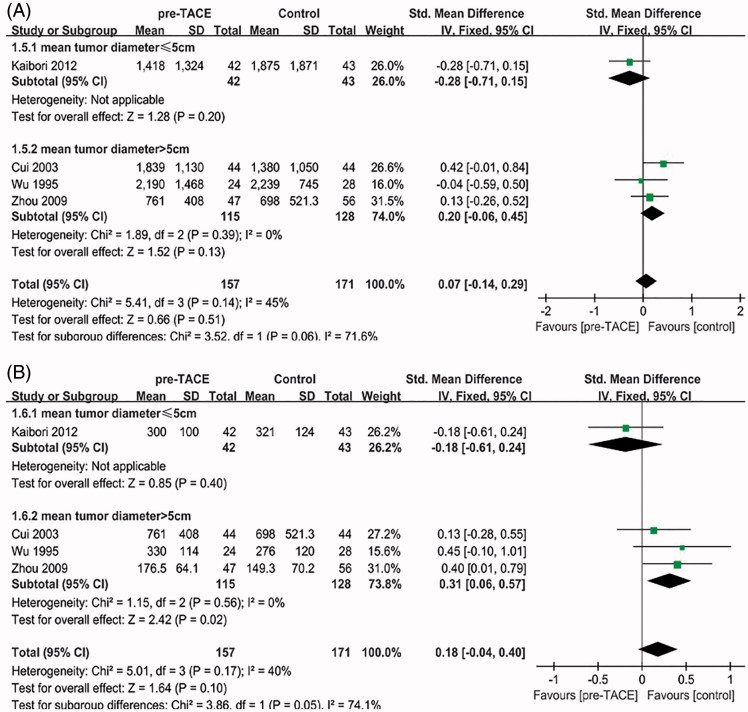
Blood loss and operation time
Four trials [19–21,23] reported on blood loss and operation time. The heterogeneity between all five studies was not statistically significant (χ 2 = 5.41, degree of freedom [df] = 3, p = 0.14, I 2 = 45%; χ 2 = 5.01, degree of freedom [df] = 3, p = 0.17, I 2 = 40%) (Figure 4). Overall meta-analysis of all four studies showed no statistical significance on blood loss (SMD = 0.07, 95%CI = [−0.14–0.29], p = 0.51) or operation time (SMD = 0.18, 95%CI = [−0.04–0.40], p = 0.10). When trials were stratified according to mean tumor size >5 cm or ≤5 cm, there was also no significant intervention effect in both subgroups on blood loss (SMD=−0.28, 95%CI = [−0.71–0.15], p = 0.20; SMD = 0.20, 95%CI = [−0.06–0.45], p = 0.13).
Figure 4.
(A) Forest plot of the subgroup analysis on the blood loss. (B) Forest plot of the subgroup analysis on the operation time
By contrast, pooling of SMDs from three trials in mean tumor size >5 cm subgroup showed a significant difference favoring Control group on operation time (SMD = 0.31, 95%CI = [0.06–0.57], p = 0.02).
Combined resection of perihepatic organs
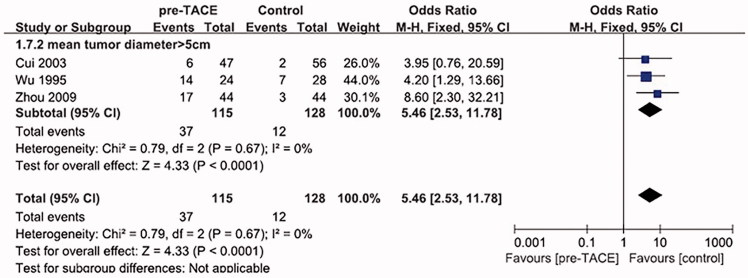
Reasons of combined resection of perihepatic organs are principally related to two aspects: inflammatory peripheral hepatic adhesion caused by TACE and tumor invasion of perihepatic organs. The organs of combined resection included: diaphragm, stomach, adrenal gland, colon, pancreas and spleen. In order to provide convenience for surgical operation, gallbladder was always removed in hepatectomy. Thus, gallbladder was not included in our analyses. Three trials [20,21,23] from mean tumor size >5 cm subgroup reported on combined resection of perihepatic organs.
The heterogeneity between all three studies was not statistically significant (χ 2 = 0.79, degree of freedom [df] = 2, p = 0.67, I 2 = 0%) (Figure 5), pooling of ORs from all three trials showed a significant difference favoring Control group on combined resection of perihepatic organs.
Figure 5.
Forest plot of the subgroup analysis on the combined resection of perihepatic organs.
Publication bias analysis
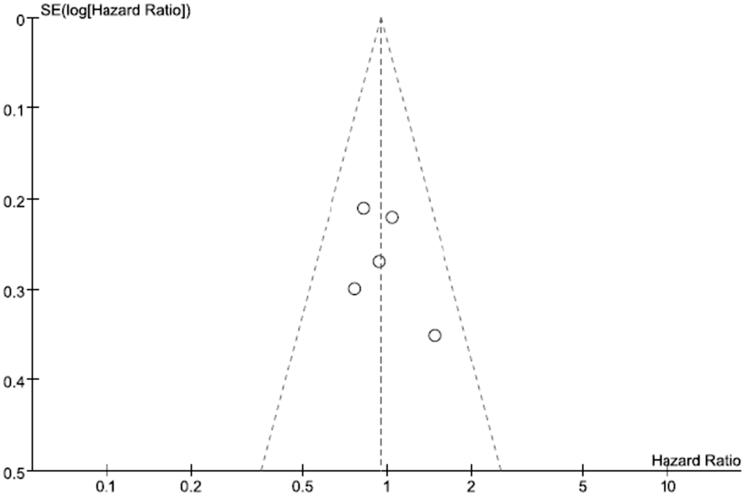
Publication bias of DFS was examined by using a funnel plot. The funnel plot (Figure 6) for overall DFS of the included studies demonstrated basically bilateral symmetry, indicating an insignificant sign of publication bias.
Figure 6.
The funnel plot for overall disease-free survival of the included studies.
Discussion
It has been established in some literatures [12,13] that routine postoperative TACE treatment can remarkably improve the prognosis. But the effect of pre-TACE for curative resection of HCC is still controversial, the debate mainly focuses on whether pre-TACE would improve long-term prognosis or not. As known to all, TACE can reduce the tumor mass by inducing tumor necrosis, thus allow a smaller and safer resection scope and prevent the spread of tumor cells in hepatic artery. Therefore, it is generally believed that TACE could decrease the postoperative recurrence rate and prolong the DFS,[24] but some meta-analyses showed controversial results. This writing revealed that: there was no statistical difference between the pre-TACE and surgery alone group, no matter on OS (HR = 1.25, 95%CI = [0.92–1.68], p = 0.15) or DFS (HR = 0.95, 95%CI = [0.76–1.19], p = 0.68). Pre-TACE did not improve the long-term prognosis and yet worsen the adhesion in some study. Zhou et al. [20] reported that 42 patients from pre-TACE group (47) had the varying degree adhesion, while the number of the control group (n = 56) is only 15 (p = 0.001). Though the severity of adhesion to other organs is influenced by the location of the tumor to a great extent, the adhesion to the other organs after TACE is quite more common. Cui et al. [21] demonstrated that among the patients from pre-TACE group, 20 patients had different degree of adhesion around the hepatic portal area, gallbladder wall thickening or gallbladder shrinking. The diaphragm adhered to diaphragmatic surface of the liver capsular or adhered to tumor existing in 15 patients, respectively. All of these bring huge inconvenience to surgical operation.
Various kinds of pathological response after TACE for hepatocellular carcinoma could predict diverse outcomes. Complete pathological response after chemoembolization has a more favorable outcome compared with partial response.[25,26] Allard et al. reported that a complete or nearly complete pathologic response improves long-term survival after liver resection. Kim et al. also claimed that patients with complete response as the initial response had the longest overall survival. It is worried that if tumors cannot achieve complete pathological response after chemoembolization, the residual intrahepatic tumor cells may develop resistance toward chemotherapeutic agents and become more invasive than those receiving no TACE treatment.[27,28] The intratumoral necrosis induced by TACE may weaken the adhesive potential of the tumor and subsequently facilitate the release of cancer cells from the primary tumor and dislodgment into the bloodstream, thus increasing the risk of postoperative recurrence.[29] Our study showed that: for patients from mean tumor diameter >5 cm subgroup, the complete necrosis rate was all below 20%. The relatively low complete HCC necrosis might weaken adhesiveness within the tumor, thus facilitating the invasion of cancer cells to other perihepatic organs. That may account for the higher combined resection rate of perihepatic organs in pre-TACE group (OR = 5.46, 95%CI = [2.73–11.78], p < 0.0001) from mean tumor diameter >5 cm subgroup.
Considering the high malignant degree and rapid progress of HCC, most patients choose surgical treatment after a clear diagnosis. Therefore, there are less randomized controlled trials about perioperative safety and long-term prognosis of surgery in patients with resectable HCC, except meta-analysis. We performed the meta analysis on five small sample size randomized controlled trials which meet the strict requirements. Compared with other previous reported study, we laid special stress on analyzing the perioperative safety of pre-TACE for resectable HCC and put forward our views. Our subgroup analysis showed that in subgroup with mean tumor diameter above 5 cm, pre-TACE would result in longer operation time (SMD = 0.31, 95%CI = [0.06–0.57], p = 0.02). It was mainly caused by the post-TACE inflammatory reaction which in turn resulted in adhesion around liver and adjacent organs. In order to separate the adhesion, operation time will be extended accordingly. Apart from this, postoperative morbidity is also much higher in tumor size >5 cm subgroup (OR = 1.90, 95%CI = [1.02–3.53], p = 0.04). We believe the drug-induced liver injury caused by chemotherapy drugs of TACE is a major cause of postoperative complication rates rising.
In summary, Pre-TACE does not improve DFS and OS of patients who could receive one-stage resection, for patients with large tumor size (> 5cm), it may even increase the difficulty of operation. But, the five studies included are from Asian region, the regional limitations may limit the applicability of relevant conclusions to other areas.
Conclusion
According to the results of our meta-analysis, there was no statistical difference between pre-TACE group and Control group on perioperative mortality, blood loss, OS or DFS. Pre-TACE treatment cannot improve the long-term prognosis of resectable hepatocellular carcinoma. Thus, the subgroup analysis revealed that in subgroup with tumor diameter above 5 cm, pre-TACE would result in longer operation time, higher postoperative morbidity rate and combined resection rate of perihepatic organs. Based on the analysis mentioned above, we believe that pre-TACE is not suitable to be recommended as the routine therapy for resectable HCC patients.
Disclosure statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service or company that could be construed as influencing the position presented in, or the manuscript entitled ‘Preoperative transarterial chemoembolization for resectable hepatocellular carcinoma: A meta-analysis of randomized controlled trials.’
References
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lope CR, Tremosini S, Forner A, et al. Management of HCC. J Hepatol. 2012;56:75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Hsia CY, Huang YH, et al. Selecting an optimal staging system for hepatocellular carcinoma: comparison of 5 currently used prognostic models. Cancer. 2010;116:3006–3014. doi: 10.1002/cncr.25044. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol. 2013;58:724–729. doi: 10.1016/j.jhep.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Scatton O, Goumard C, Cauchy F, et al. Early and resectable HCC: Definition and validation of a subgroup of patients who could avoid liver transplantation. J Surg Oncol. 2015;111:1007–1015. doi: 10.1002/jso.23916. [DOI] [PubMed] [Google Scholar]
- Proneth A, Zeman F, Schlitt HJ, et al. Is resection or transplantation the ideal treatment in patients with hepatocellular carcinoma in cirrhosis if both are possible? A systematic review and metaanalysis. Ann Surg Oncol. 2014;21:3096–3107. doi: 10.1245/s10434-014-3808-1. [DOI] [PubMed] [Google Scholar]
- Moriguchi M, Takayama T, Higaki T, et al. Early cancer related death after resection of hepatocellular carcinoma. Surgery. 2012;151:232–237. doi: 10.1016/j.surg.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Arnaoutakis DJ, Mavros MN, Shen F, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol. 2014;21:147–154. doi: 10.1245/s10434-013-3211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZR, Zhang PF, Wang CH, et al. Postoperative adjuvant transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond the Milan criteria: a retrospective analysis. Am J Cancer Res. 2015;5:450–457. [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Uchiyama K, Ozawa S, et al. Adjuvant chemolipiodolization reduces early recurrence derived from intrahepatic metastasis of hepatocellular carcinoma after hepatectomy. Ann Surg Oncol. 2011;18:3624–3631. doi: 10.1245/s10434-011-1800-6. [DOI] [PubMed] [Google Scholar]
- Cheng X, Sun P, Hu QG, et al. Transarterial (chemo) embolization for curative resection of hepatocellular carcinoma: a systematic review and meta-analyses. J Cancer Res Clin Oncol. 2014;140:1159–1170. doi: 10.1007/s00432-014-1677-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhang X, Wu L, et al. Meta-analysis: preoperative transcatheter arterial chemoembolization does not improve prognosis of patients with resectable hepatocellular carcinoma. BMC Gastroenterol. 2013;13:51. doi: 10.1186/1471-230X-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Li J, Peng Y, et al. Influence of preoperative transarterial chemoembolization on the prognosis for patients with resectable hepatocellular carcinoma: a meta-analysis of randomized trials. Hepatogastroenterology. 2011;58:869–874. [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibori M, Tanigawa N, Kariya S, et al. A prospective randomized controlled trial of preoperative whole-liver chemolipiodolization for hepatocellular carcinoma. Dig Dis Sci. 2012;57:1404–1412. doi: 10.1007/s10620-012-2029-3. [DOI] [PubMed] [Google Scholar]
- Zhou WP, Lai EC, Li AJ, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195–202. doi: 10.1097/SLA.0b013e3181961c16. [DOI] [PubMed] [Google Scholar]
- Cui H, Gao QQ, Li YY, et al. Influence of preventive effects of transcatheter arterial chemoembolization on primary hepatocellular carcinoma. J Med Forum. 2003;24:1–3. [Google Scholar]
- Yamasaki S, Hasegawa H, Kinoshita H, et al. A prospective randomized trial of the preventive effect of pre-operative transcatheter arterial embolization against recurrence of hepatocellular carcinoma. J Cancer Res. 1996;87:206–211. doi: 10.1111/j.1349-7006.1996.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Ho YZ, Ho WL, et al. Preoperative transcatheter arterial chemoembolization for resectable large hepatocellular carcinoma: a reappraisal. Br J Surg. 1995;82:122–126. doi: 10.1002/bjs.1800820141. [DOI] [PubMed] [Google Scholar]
- Fiore F, Del Prete M, Franco R, et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine. 2014;47:177–182. doi: 10.1007/s12020-013-0130-9. [DOI] [PubMed] [Google Scholar]
- Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol. 2015;63:83–92. doi: 10.1016/j.jhep.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Kim BK, Kim SU, Kim KA, et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62:1304–1310. doi: 10.1016/j.jhep.2015.01.022. [DOI] [PubMed] [Google Scholar]
- Li HL, Ji WB, Zhao R, et al. Poor prognosis for hepatocellular carcinoma with transarterial chemoembolization pre-transplantation: retrospective analysis. World J Gastroenterol. 2015;21:3599–3606. doi: 10.3748/wjg.v21.i12.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Kwon JH, Moon YH, et al. Influence of preoperative transcatheter arterial chemoembolization on gene expression in the HIF-1α pathway in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2014;140:1507–1515. doi: 10.1007/s00432-014-1713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TY, Hwang S, Lee YJ, et al. Absence of benefit of transcatheter arterial chemoembolization (TACE) in patients with resectable solitary hepatocellular carcinoma. World J Surg. 2016;40:1200–1210. doi: 10.1007/s00268-015-3373-1. [DOI] [PubMed] [Google Scholar]