Abstract
Vibrios are ubiquitous and abundant in the aquatic environment. A high abundance of vibrios is also detected in tissues and/or organs of various marine algae and animals, e.g., abalones, bivalves, corals, fish, shrimp, sponges, squid, and zooplankton. Vibrios harbour a wealth of diverse genomes as revealed by different genomic techniques including amplified fragment length polymorphism, multilocus sequence typing, repetetive extragenic palindrome PCR, ribotyping, and whole-genome sequencing. The 74 species of this group are distributed among four different families, i.e., Enterovibrionaceae, Photobacteriaceae, Salinivibrionaceae, and Vibrionaceae. Two new genera, i.e., Enterovibrio norvegicus and Grimontia hollisae, and 20 novel species, i.e., Enterovibrio coralii, Photobacterium eurosenbergii, V. brasiliensis, V. chagasii, V. coralliillyticus, V. crassostreae, V. fortis, V. gallicus, V. hepatarius, V. hispanicus, V. kanaloaei, V. neonatus, V. neptunius, V. pomeroyi, V. pacinii, V. rotiferianus, V. superstes, V. tasmaniensis, V. ezurae, and V. xuii, have been described in the last few years. Comparative genome analyses have already revealed a variety of genomic events, including mutations, chromosomal rearrangements, loss of genes by decay or deletion, and gene acquisitions through duplication or horizontal transfer (e.g., in the acquisition of bacteriophages, pathogenicity islands, and super-integrons), that are probably important driving forces in the evolution and speciation of vibrios. Whole-genome sequencing and comparative genomics through the application of, e.g., microarrays will facilitate the investigation of the gene repertoire at the species level. Based on such new genomic information, the taxonomy and the species concept for vibrios will be reviewed in the next years.
INTRODUCTION: HISTORICAL ASPECTS
In 1854, the Italian physician Filippo Pacini (1812 to 1883) discovered the first Vibrio species i.e., V. cholerae, the causative agent of cholera, while studying outbreaks of this disease in Florence. Records of a cholera-like disease may well be traced back to the times of Hippocrates (460 to 377 BC) (38). Pacini examined the intestinal mucosa of fatal victims of cholera by using a microscope and detected V. cholerae in all samples. He further pointed out that cholera was a contagious disease, but at that time most scientists and physicians believed in the miasmatic theory of disease (the theory of disease causation by bad or pestilential airs). In the same period, John Snow (1813 to 1858) studied the epidemiology of cholera in several cities of England including Birmingham, London, and Manchester (359; available online at http://www.ph.ucla.edu/epi/snow/snowbook.html). Cholera had killed tens of thousands of people in England between the 1830s and 1850s. According to Snow, cholera was propagated by a “morbid poison entering the alimentary canal.” The poison was in (polluted) drinking water. He recommended the provision of pure tap water, free of contamination by sewers and house drains, as an effective means of containing the dissemination of the disease.
Nearly 30 years latter, Robert Koch (1843 to 1910) obtained pure cultures of the deadly V. cholerae on gelatin plates. In August 1883, Koch and his team went to Egypt, where cholera had broken out and caused about 100,000 casualties. In Alexandria, they examined a number of fatal cases and always found a characteristic bacterium in the tissue of the intestine, but they were not able to grow the organism. Subsequently, Koch and his team went to India, and by the end of 1883 they had obtained pure cultures of V. cholerae. They also described some properties of the organism: “It is a little bent resembling a comma or a spiral. It is highly motile and swarms on gelatine plates” and concluded that this organism was indeed the causative agent of cholera (47). In 1893, an outbreak of cholera occurred in Hamburg, Germany, with about 8,000 fatal cases. Koch was requested to study means of providing improved hygiene in that region. He proposed that water supply systems should incorporate filtration of drinking water in order to remove the bacteria. At that time, Koch and his team also realised that vibrios were ubiquitous in aquatic settings and that many “forms” of vibrios were non-pathogenic for humans (47). The first nonpathogenic Vibrio species, i.e., V. fischeri, V. splendidus, and Photobacterium phosphoreum isolated from the aquatic environment, were discovered by the Dutch microbiologist Martinus Beijerinck (1851 to 1931) in the late 1880s.
OCCURRENCE AND IMPORTANCE
According to Bergey's Manual of Determinative Bacteriology (1994) and Bergey's Manual of Systematic Bacteriology (in press), vibrios (Vibrionaceae strains) belong to the Gammaproteobacteria, are gram negative, usually motile rods, are mesophilic and chemoorganotrophic, have a facultative fermentative metabolism, and are found in aquatic habitats and in association with eukaryotes. They are generally able to grow on marine agar and on the selective medium thiosulfate-citrate-bile salt-sucrose agar (TCBS) and are mostly oxidase positive.
Vibrios are highly abundant in aquatic environments, including estuaries, marine coastal waters and sediments, and aquaculture settings worldwide (20, 95, 164, 297, 298, 321, 399, 446). Several cultivation-dependent and independent studies have showed that vibrios appear at particularly high densities in and/or on marine organisms, e.g., corals (331), fish (6, 146, 179, 325), molluscs (345), seagrass, sponges, shrimp (133, 404-406) and zooplankton (163, 194, 365, 408, 409).
Photobacterium leiognathi and P. phosphoreum are found in symbiotic associations with fish, and P. leiognathi, V. logei, and V. fischeri are found in symbiotic associations with squid. These bacteria colonize the light organs of the host and play a role (via emission of light) in communication, prey attraction, and predator avoidance (120, 126, 339). In the light organs of the squid Sepiolla spp., the abundance of vibrios can be as high as 1011 cells/organ (120, 285). Newly hatched squid excrete a mucus matrix from the pores of the light organs whereby V. fischeri cells present in sea water are caught (94, 289, 290). Subsequently, V. fischeri migrates into the organ and colonizes the crypt epithelium. Obviously, the flagella of V. fischeri play a crucial role in the colonization of the light organs, but hyperflagellated V. fischeri cells containing up to 16 flagella are defective in normal colonization (265). V. fischeri, V. logei, and P. leiognathi are apparently the only three organisms colonizing the light organs of squid, but this seemingly specific partnership remains to be confirmed. V. fischeri cells entrapped in the light organs of squid can sense the density of conspecific cells by signaling molecules or pheromones (e.g., N-acyl homoserine lactones) and thereby regulate bioluminescence (436). This cell-to-cell communication, or “quorum sensing,” may play a role in the evolution of symbiotic bacteria (373). It has also been documented for the pathogens V. anguillarum (268), V. cholerae (53, 155, 451), V. harveyi (237, 252), V. parahaemolyticus (165), and V. vulnificus (259). These bacteria monitor cell density and regulate the expression of virulence genes by means of quorum sensing. Luminescence and virulence seem to be coregulated in V. harveyi, and therefore the infections caused by this organism in shrimp could be controlled by signaling antagonists produced by the alga Delisea pulchra (252).
Large numbers of Vibrio and Photobacterium (4.3 × 106/mm2) attached to the external surface of zooplankton have also been reported (163). It has been suggested that a close partnership occurs between these bacteria and zooplankton. The biofilm formation by Vibrio spp. on the exoskeletons of these crustaceans and other marine organisms may in fact constitute a strategy to survive during starvation and/or other environmental stresses (238, 416). In biofilms these bacteria can use trapped and absorbed nutrients, resist antibiotics, and establish favorable partnerships with other bacteria or hosts. Copepods may, in turn, feed on these bacteria. V. cholerae moves along and attaches to surfaces with the aid of the flagellum and pili, which may act as adhesins. In as little as 15 min, V. cholerae forms microcolonies on surfaces; subsequently it produces exopolysacharides, which stabilize the pillars of the biofilm. A 15-μm-thick biofilm, with pillars of cells and water channels, is formed within 72 h (273, 423-425). According to these authors, the strong ability of V. cholerae E1 Tor to form densely packed biofilms in the environment gives a survival advantage to this organism, which is the predominant cause of cholera. Because V. cholerae is closely associated with plankton, it is assumed that cholera outbreaks are linked with planktonic blooms and the sea surface temperature, and so such outbreaks may be predicted by monitoring these parameters by e.g., remote sensing (238). The wide ecological relationships and ability to cope with global climate changes may be a reflection of the high genome plasticity of vibrios (238). Recently, a number of reports have highlighted the pathogenic potential of vibrios toward humans and marine animals (e.g. corals, gorgonians, and shrimp), which may be coupled with rising of sea water temperature due to global warming (215, 253, 331, 350).
Human Pathogens
V. cholerae, V. parahaemolyticus, and V. vulnificus are serious human pathogens (59a, 122, 415). Cholera is a severe disease mainly in developing countries as a result of poor water supplies and sanitation. The main route of contamination is via water and food. Cholera vectors include zooplankton (e.g., copepods), chironomid insects, and cyanobacteria (186, 238). The last three annual reports (2000 to 2002) of the World Health Organization noticed at least 11,399 deaths worldwide (96% of which occurred in Africa) due to cholera (Table 1) (http://www.who.int/wer) (439, 440). In 2003 and 2004, the World Health Organization reported at least 15,000 new cases of cholera in Africa alone, mainly in Mali, Mozambique, and Zambia.
TABLE 1.
World statistics of cholera cases and deaths in the last 3 yearsa
| Location | 2000
|
2001
|
2002b
|
|||
|---|---|---|---|---|---|---|
| No. of cases | No. of deaths | No. of cases | No. of deaths | No. of cases | No. of deaths | |
| Africa | 118,932 | 4,610 | 173,359 | 2,590 | 121,568 | 3,753 |
| Americac | 3,101 (715) | 40 (17) | 535 (7) | 0 (0) | 3 | 0 |
| Asia | 11,246 | 232 | 10,340 | 138 | 2,408 | 10 |
| Europe | 35 | 0 | 58 | 0 | 5 | 0 |
| Oceania | 3,757 | 26 | 19 | 0 | 2 | 0 |
| Total | 137,071 | 4,908 | 184,311 | 2,728 | 123,983 | 3,763 |
Data from World Health Organization (http://www.who.int/en/).
Data for the year 2002 are incomplete.
Numbers in parentheses indicate the numbers of cases and deaths in Brazil.
A Vibrio surveillance system maintained by the Centers for Disease Control and Prevention reported 296 cases of infection caused by vibrios in the United States in 2000 (http://www.cdc.gov/). Most strains (n = 268) were isolated from stool, wound, and blood samples. From this collection, 137, 64, and 27 isolates were identified as V. parahaemolyticus, V. vulnificus, and V. cholerae, respectively. Most patients (n = 22) who died were infected by V. vulnificus. The Centers for Disease Control and Prevention report also stated that most cases occur during the summer months and that seafood, e.g., oysters, shrimp, and fish, had been consumed by the patients. V. cholerae enters the human host via contaminated food and/or water (415). In the intestine, this bacterium adheres to the epithelium and produces an enterotoxin, cholera toxin (CT) (320). This toxin causes an intense watery diarrhea that may lead to death, but it appears to play no role when V. cholerae is in the environment (320). Several virulence genes (n = 30 to 40) within the ToxR regulon are involved in cholera disease (37, 84, 445). In addition to the essential role of CT in cholera, the toxin-coregulated pilus (TCP), encoded by the tcpA to tcpF genes, is pivotal for the colonization of the intestine epithelium. TCP helps in microcolony formation on the epithelial surface. Other colonization factors include mannose-fucose hemagglutinin, regulatory proteins (e.g., ToxR/ToxS and ToxT), outer membrane porins, biotin and purine biosynthetic genes, iron-regulated outer membrane proteins (e.g., IrgA), the O antigen of the lipopolysaccharide, and accessory colonization factors (115, 116, 320). Motility and chemotaxis also play a role in virulence (51, 424, 425). Studies of gene expression in vivo have shown that about 200 genes encoding flagellin synthesis, ribosomal proteins, iron transport, and anaerobic metabolism, as well as more than 700 genes of unknow function, are induced during cholera infection (37, 447).
V. parahaemolyticus causes gastroenteritis in which the hemolysins, thermostable direct hemolysin (TDH) and/or TDH-related hemolysin (TRH), have been considered to play a crucial role (171, 195, 284, 371). It has been suggested that V. parahaemolyticus acquired the genes encoding these hemolysins via horizontal gene transfer (284, 303). Raimondi et al. (317) have proposed that TDH acts as a porin in the enterocyte's plasma membrane and allows the influx of multiple ionic species, e.g., Ca2+, Na+, and Mn2+. A high concentration of TDH increases the number of porin channels, and this, in turn, results in ionic influx, culminating in cell swelling and death due to osmotic imbalance (317).
Whole-genome sequencing of V. parahaemolyticus (251) revealed that the organism possesses two sets of genes for the type III secretion system (TTSS). TTSS is one of the bacterial protein secretion systems that secretes bacterial proteins into the extracellular environment, but it can also inject bacterial proteins directly into target host eukaryotic cells (175). TTSS is essential for the pathogenicity of bacterial pathogens such as Salmonella, Shigella, Yersinia, and plant pathogens, which cause disease by intimate interactions with eukaryotic cells. However, TTSS is absent from the genome of V. cholerae (162). Gene disruption experiments demonstrated that the two TTSSs of V. parahaemolyticus are functional and that they play a role in the pathogenicity of the bacterium (K. S. Park, T. Ono, M. Rokuda, M. H. Jang, K. Okada, T. Iida, and T. Honda, submitted for publication). Other toxins, proteases, cytolysins, and pili may also play a role as virulence factors in both V. parahaemolyticus and V. vulnificus. In the genome of V. parahaemolyticus (251), other genes that may be involved in pathogenicity have been identified. These genes include those used for bacterial adherence and biofilm formation, such as the genes for the biosynthesis of several pili and the tad genes (198) and for several toxin homologues including an RTX toxin. Certain strains of V. parahaemolyticus, probably derived from a common clonal ancestor, have recently caused a pandemic of gastroenteritis (58, 59, 69, 91).
V. vulnificus is an important etiologic agent of wound infections and septicemia in humans (59a, 122). This sort of septicemia occurs mainly in immunosuppressed people and in patients with high levels of serum iron (caused by genetic mutation, e.g., hemochromatosis, or by liver diseases, e.g., cirrhosis). Iron seems to enhance the virulence of vibrios. A capsular polysaccharide (CPS) is the primary virulence factor in V. vulnificus pathogenesis (270, 422, 441). The presence of this factor correlates with the opaque colony phenotype and is thought to play a inflammatory role within the human body. Smith and Siebeling (357) described four essential genes, i.e., wcvA, wcvF, wcvI, and orf4, responsible for the synthesis of CPS. They showed that mutation in any of these genes results in loss of capsule, which is typical of an avirulent translucent colony phenotype (441). Two lytic bacteriophages, i.e., CK-2 and 153A-5, have been successfully used to treat local and systemic infections caused by V. vulnificus in mice (64). A dose of 108 phage/mice significantly reduced the number of V. vulnificus organisms isolated from wounds and liver of mice. Estrogen seems to provide protection against V. vulnificus lipopolysaccharide-induced endotoxic shock in rats, halving the mortality rate of infected animals (263).
Other vibrios, e.g., Grimontia hollisae, P. damselae, V. alginolyticus, V. cincinnatiensis, V. fluvialis, V. furnisii, V. harveyi, V. metschnikovii, and V. mimicus, have been sporadically found in human infections (1, 46, 56, 89, 113, 114, 443). Apparently, they are less important as human pathogens (113, 114).
Coral Pathogens
Corals consist of a coral host, e.g., the reef-building Scleractinia order (Anthozoa class) and symbiotic unicellular algae, the Zooxanthellae (341). Each coral colony may comprise thousands tiny coral polyps that are responsible for production of the calcium carbonate of the reefs. Coralline algae also play a role in the formation of coral reefs by cementing various corals together with compounds of calcium, while other organisms such as tube worms and molluscs donate their hard skeletons. In this partnership, various types of reefs are constructed by these organisms (83). Coral reefs are highly productive and very diverse ecosystems within coastal tropical environments, mainly in oligotrophic regions (83, 169). Brazilian coral reefs have an initial growth as a mushroom-like, relatively low diversity of coral fauna (mostly relics from the Tertiary), with an abundance of incrusting coralline algae and surrounding water rich in muddy siliciclastic sediment and nutrients (228).
Coral reefs are important sources of income for several countries via tourism and fishing, but they also represent protection to coastal areas and may be a source of biologically active compounds e.g., antimicrobials and antivirals. Tourism in the Caribbean generates nearly 90 billion dollars annually (169). Corals reefs may be used as clarifying agents in the sugar industry and as as building materials (228). Some 30 coral diseases (3 of them caused by microbial consortia) have been documented so far, but only 5 have fulfilled the Koch's postulates (370). Coral bleaching, i.e., the paling or the loss of color due to the disruption of symbiosis between the coral host and symbiotic Zooxanthellae, is one of the most serious diseases affecting corals worldwide (332, 370), although it is sometimes reversible in 3 to 6 months (48, 83, 308, 322, 323, 370).
Coral bleaching is thought to be linked to the increased seawater temperature due to recent global climate changes caused by greenhouse gas emissions, although other factors such as seawater eutrophication by sewage and aquaculture, sedimentation, light (UV radiation and photosynthetically active radiation), pollution by heavy metals, and reduction of salinity may also play a role (332). The strongest bleaching episodes have occurred during El Niño years, when surface seawater temperatures reach maxima higher than the summer maximum. The pivotal role of bacteria in coral bleaching and the effect of temperature in bacterial virulence have been studied by Rosenberg and collaborators (32, 33, 331, 333). V. shilonii (also known as V. mediterranei) and V. coralliilyticus have been proven to bleach corals, and their pathogenicity was shown to be temperature dependent.
V. shilonii was identified as an etiological agent of the bleaching of Oculina patagonica, and the main disease steps, i.e., adhesion, penetration, and multiplication (up to 109 CFU/cm3 in 5 days) within the coral tissues have been described in detail (18, 19, 331). Within the coral tissues, most V. shilonii cells become viable but nonculturable (VBNC) but continue to be virulent. According to Sussman et al. (369), the fire worm Hermodice carunculata is a winter reservoir and summer vector of V. shilonii.
V. coralliilyticus, another temperature-dependent pathogen, was shown to cause patchy necrosis of tissues of Pocillopora damicornis when the coral was incubated at temperatures of 27°C or higher (33). Because tropical seawater temperatures have undergone warming in the past 100 years and increases of 1 to 2°C have been predicted by 2100 as a result of increased emission of greenhouse gases, it is expected that bleaching episodes will occur even more frequently (169). Infectious diseases may reduce the global diversity of corals (241).
Recent work on the diversity of vibrios associated with coral bleaching in Davies Reef and Magnetic Island (Great Barrier Reef, Australia) and in the Kaneohe Bay (Hawaii) indicated that different species, i.e., Enterovibrio coralii, P. eurosenbergii, V. fortis, V. campbellii, V. harveyi, V. mediterranei, and V. rotiferianus, may be involved in the process of coral bleaching (F. L. Thompson, D. Gevers, P. Dawyndt, C. C. Thompson, S. Naser, B. Hoste, and J. Swings, submitted for publication; F. L. Thompson, C. C. Thompson, S. Naser, B. Hoste, C. Munn, D. Bourne, and J. Swings, submitted for publication). V. harveyi has been implicated in the disease of a wide range of marine animals, including bleaching in O. patagonica and white band disease in Acropora cervicornis (14, 139, 370).
Nutrient Cycling
There are indications that vibrios play a role in nutrient cycling in aquatic environments by taking up dissolved organic matter (352, 353). Vibrios may provide essential polyunsatured fatty acids to the aquatic food web, which many aquatic organisms cannot produce de novo (88, 282). Vibrios are also able to break down chitin, a homopolymer of N-acetyl-d- glucosamine, which is one of the largest pools of amino sugars in the oceans (85-87, 324). Vibrio harveyi, for instance, excretes at least ten different chitin-degrading enzymes (367, 372). Accordingly, it was suggested that this ability may explain the ubiquitous occurrence of vibrios in aquatic settings (324). Some Vibrio species are able to degrade toxic polycyclic aromatic hydrocarbons within polluted marine sediments (161). Vibrios are important producers of antibiotics among marine bacteria (240). Inhibitory compounds produced by certain Vibrio isolates reduced the number of other community members, e.g., Alfaproteobacteria and Alteromonas. Long and Azam (240) supposed that this strategy accounts for the microscale variations in competing bacterial populations. Because vibrios are selectively grazed by flagellates, it has been suggested that they contribute to the cycling of organic matter in aquatic settings (29).
Role in Aquaculture
Vibrios are important bacterial pathogens for animals reared in aquaculture (14, 35, 168, 236). V. anguillarum, V. salmonicida, and V. vulnificus are among the main bacterial pathogens of several fish species (14), and V. harveyi is a major pathogen of shrimp, e.g., Litopenaeus vannamei and Penaeus monodon (15, 225, 227). Mortality caused by vibrios in reared fish and shellfish is very common during early larval stages and can occur suddenly, leading sometimes to death of the entire population (42, 96, 157, 184, 185, 187, 217, 230, 283, 287, 295). Pathogenicity of V. harveyi is associated with the presence of a bacteriophage (15, 291). A bacteriological survey of cultures of sea bream (Sparus aurata L.) in Spain between 1997 and 2000 detected 25 outbreaks (450). Strains were isolated mainly from the spleen, liver, and kidney of diseased fish, and Vibrio was the dominant group, accounting for about 69% (n = 71) of the total number of isolated strains. It was striking that in different studies several Vibrio isolates had phenotypes different from those of known Vibrio species and thus remained identified only at the genus level (13, 276, 281, 406, 450).
The mode of infection in fish consists of three basic steps (14, 223, 224): (i) the bacterium penetrates the host tissues by means of chemotactic motility; (ii) within the host tissues the bacterium deploys iron-sequestering systems, e.g., siderophores, to “steal” iron from the host; and (iii) the bacterium eventually damages the fish by means of extracellular products, e.g., hemolysins and proteases. Grisez et al. (145) showed that infection of turbot (Scophthalmus maximus) larvae by V. anguillarum occurs in the intestinal epithelium, where the pathogen invades the bloodstream and spreads to different organs, culminating in death of the fish. More recently, Ringo et al. (326) detected bacterial endocytosis in the pyloric ceca and midgut of arctic charr (Salvelinus alpinus L.) adults and suggested that the whole gastrointestinal tract of fish may be subject to infection.
Internal symptoms of disease in fish caused by strains of vibrios include intestinal necrosis, anemia, ascitic fluid, petechial hemorrhages in the muscle wall, liquid in the air bladder, hemorrhaging and/or bloody exudate in the peritoneum, swollen intestine, hemorrhaging in or on internal organs, and pale mottled liver (14). External symptoms include sluggish behavior, twirling, spiral or erratic movement, lethargy, darkened pigment, eye damage/exophthalmia, hemorrhaging in the mouth, gill damage, white and/or dark nodules on the gills and/or skin, fin rot, hemorrhaging at the base of the fins, distended abdomen, hemorrhaging on the surfaces and muscles, ulcers, and hemorrhaging around the vent.
Using a very robust crustacean model organism, i.e., Artemia spp., and with the aid of transmission electron microscopy, Verschuere et al. (410) established the infection route of V. proteolyticus CW8T2. These investigators first infected Artemia nauplii by inoculating the pathogen in the rearing water. One day later, they detected bacteria that had penetrated “in” the gut epithelium, with subsequent tissue damage, qualified by the authors as “devastating,” and had spread toward the host's body cavity. This study illustrates well the infectious capability of certain Vibrio strains and suggests that vibriosis in penaeid shrimp larva rearing systems would be even more devastating, taking into account the fragility of these larvae. Lavilla-Pitogo et al. (225) have reported massive losses in shrimp cultures in Philippines due to a so-called group of “luminous vibrios.” According to these authors, there was a decrease of nearly 60% in the survival of reared shrimp between 1992 and 1994, associated with the presence of luminous vibrios in rearing water. Lavilla-Pitogo et al. (225) recommended to farmers that shrimp rearing should not start unless luminous vibrios were absent. The rationale that all luminous vibrios are invariably associated with disease outbreaks in shrimp rearing contrasts with the results obtained by Fidopiastis et al. (120, 121), McFall-Ngai (260, 261), Oxley et al. (301), and Ruby (339), among others, who have reported beneficial and/or harmless partnership between certain luminous vibrios e.g. V. logei and V. fischeri and host invertebrates. For instance, Oxley et al. (301) examined the bacterial flora of healthy wild and reared Penaeus mergulensis shrimp and found a high abundance of vibrios (including V. logei at ca. 104 to 105 CFU/g of gut). The authors also found that the bacterial floras of wild and reared penaeid shrimp are similar and suggested that shrimp may influence and/or select the composition of their gut microbiota. In the light of the current knowledge about the bacterial population structure of certain human pathogens, e.g., Neisseria spp. (256), it is more likely that under favorable conditions (e.g., high nutrient loads and high animal density) within rearing systems, a certain hypervirulent strain (or clone) dominates the bacterial community and causes disease in fish and shellfish rather than the disease being caused by the whole bacterial species. This view implies that only a minority of Vibrio strains are true pathogens and further underscores the idea that many Vibrio species are opportunistic pathogens.
The pathogenic effects of certain strains of vibrios are critical in aquaculture settings, where organisms, e.g., penaeid shrimps and salmonids, are reared at high densities under very artificial and unstable conditions (14, 35, 295). To maintain the productivity of such an intensive aquaculture, high inputs of fish protein originating from the sea have to be employed for feeding, together with high levels of water exchange and the massive use of antibiotics. It seems that the combination of these conditions favors the proliferation of vibrios and enhances their virulence and disease prevalence. This highly intensive aquaculture has disastrous effects for the environment (132, 279, 280, 431). According to Nailor et al. (279, 280) some of the most serious negative environmental impacts are (i) loss of wild fish (5 kg of wild fish has to be caught to feed 1 kg of carnivorous fish reared), (ii) loss of natural habitats (e.g., mangroves), (iii) use of wild seed to stock ponds, (iv) impact of foreign fish and shellfish introduced in the wild, and (v) effluent discharge and coral reef degradation. The spread of antibiotic resistance from aquaculture settings to natural environments has recently been shown (154, 178, 235, 419). About 70% (n = 100) of the vibrios isolated from aquaculture settings in Mexico are multiple-drug resistant (271, 330). Several Vibrio isolates have acquired resistance to the most commonly employed antibiotics (e.g., enrofloxacin, florfenicol, trimethoprim, and oxytetracycline) in shrimp rearing, suggesting that the recently initiated application of these antimicrobials has led to the generation of resistant strains of vibrios (271, 330). Ben-Haim et al. (34) have advanced the hypothesis that aquaculture settings serve as foci or reservoirs for pathogenic Vibrio strains: during certain periods of the year, pathogenic vibrios would endure adverse environmental conditions within aquaculture settings and when favorable environmental conditions are reestablished, vibrios would be able to cause disease in wild animals.
Alternatives involving more environmentally sound aquaculture have been proposed (35). Vaccination has been successfully used to control V. anguillarum and V. vulnificus infections in fish (49, 124). Because certain Vibrio strains may be potential probiotics and/or symbionts of commercially important organisms such as penacid shrimp, salmonids, flatfish, oysters, and abalones, recent studies have suggested that such strains could act as biocontrol agents in aquaculture, diminishing the need for antibiotics and reducing effluent discharges (99, 327, 411). The normal bacterial community associated with L. vannamei has recently been examined in order to find potential probiotic organisms (133-135, 274, 404, 405). Planktonic and particle-associated vibrios seem to enhance the survival and growth of reared L. vannamei. Moss et al. (274) reported that Vibrio and Aeromonas compose up to 85% of the bacterial flora in the gut of this shrimp (about 109 CFU/g of gut tissue), whereas Gomez-Gil et al. (133) found a wealth of vibrios, i.e., 105 CFU/g and 104 CFU/ml, respectively, in the hepatopancreas and hemolymph of healthy L. vannamei.
Pujalte et al. (313) have reported a dominance of vibrios associated with cultured oysters: up to 6.5 × 105 CFU/g of oyster but only 102 CFU/ml in rearing seawater. Using fluorescence in situ hybridization (FISH), the same authors determined that vibrios accounted for up to 40% (156 cells/ml) of the heterotrophic culturable flora grown on marine agar. In a successful recirculating rearing system for rotifers, the abundance of Vibrio spp. was up to 1.7 × 105 CFU/ml, suggesting that these bacteria were playing a positive role in the health of the rotifers (365). These strains were later classified as a new species, V. rotiferianus (136).
Sawabe et al. (345) estimated the abundance of V. halioticoli strains in the gut of several abalone (Haliotis) species. They reportd that V. halioticoli is the dominant culturable bacterium, representing 40 to 64% of the total heterotrophic community, which varied from 103 to 107 CFU/g of gut. V. halioticoli strains were found to produce large amounts of acetic and formic acids (up to 68.1 mM), which may in turn be used as an energy source or precursor for protein synthesis by the abalones. The authors suggested that a mutual relationship may exist between V. halioticoli and abalones (173).
Because the use of probiotics for humans and domestic animals, e.g., pigs and chickens, has had a certain success (377), several researchers advocate that the use of probiotic bacterial strains or selected mixtures will have a positive impact on health management in marine organisms (10, 295, 411). A considerable difference between the culture of domestic and aquatic animals is that the latter are in constant and intimate contact with a wealth of microrganisms, e.g., viruses, protozoa, and fungi (352, 353). Unfortunately, studies of the use of “probiotic” bacteria have not looked at the interactions with the aquatic microbial food web (16, 352). The so-called probiotic bacterial strains could well be fueling the food web, giving rise to a high abundance of e.g., protozoan flagellates and ciliates, which in turn would be grazed by fish and/or shellfish larvae, improving their survival and growth (383).
ISOLATION AND MAINTENANCE
Vibrios are fairly easy to isolate from both clinical and environmental material, although some species may require growth factors and/or vitamins. There are several commercial media which may be used for the isolation of vibrios, but tryptone soy agar (Oxoid or Difco) supplemented with 1 to 2% NaCl and marine agar (Difco) generally allow the growth of very healthy colonies after 1 to 2 days of incubation at 28°C. Vibrios grow well at temperatures between 15 and 30°C, depending on the strain under analysis. Obviously, psychrophilic vibrios, i.e., V. logei, V. wodanis, and V. salmonicida, grow poorly at temperatures higher than 20°C. It is recommended to grow strains of these species at 15°C in Luria-Bertani broth (Difco) supplemented with 1 to 3% NaCl. Some Vibrio species, e.g., the V. halioticoli group and V. agarivorans, require addition of sodium alginate (0.5%) marine agar (343). Thiosulfate-citrate-bile salts agar (TCBS; Oxoid) is an ideal medium for the selective isolation and purification of vibrios. Strains which are able to use sucrose will form yellow colonies, while the others are green. So far this is the only proven selective medium for isolation of vibrios, although some isolates of Staphylococcus, Flavobacterium, Pseudoalteromonas, and Shewanella may present slight growth on it as well.
Most vibrios (except V. ezurae, V. gallicus, V. pectenicida, V. penaeicida, V. salmonicida, and V. tapetis) withstand the freeze-drying process very well. Coincidentally, these species are also difficult to grow on any culture media. Ampoules containing freeze-dried cultures prepared nearly 30 years ago have yielded viable and healthy colonies on tryptone soy agar. Normally, these ampoules are filled with 0.01 g of bacterial culture previously suspended in 0.5 ml of cryoprotectant mix (horse serum-d- glucose-nutrient broth-MilliQ water, 3:0.3:0.3:1). Alternatively, strains may be kept viable in Microbank vials, which contain 10% glycerol and porous beads, at −80°C for at least 5 years.
GENOTYPIC IDENTIFICATION
An array of phenotypic and genomic techniques have become available for the identification of vibrios in the last three decades (97, 148, 296, 314, 315, 342, 402, 403). Ribotyping and PCR-based techniques, e.g., amplified fragment length polymorphism (AFLP), fluorescence in situ hybridization (FISH), amplified ribosomal DNA restriction analysis (ARDRA), random amplified polymorphic DNA (RAPD), repetitive extragenic palindromes (rep), and restriction fragment length polymorphism (RFLP), along with multilocus enzyme electrophoresis (MLEE) and multilocus sequence typing (MLST), have yielded the most valuable information about and new insights into the population structure of some species of the Vibrionaceae and have also provided a means of identifying these organisms. Below, we discuss some of the methods which have been most commonly used for the identification and typing of vibrios.
Amplified Fragment Length Polymorphism
The AFLP technique consists of three main steps: (i) digestion of total genomic DNA with two restriction enzymes and subsequent ligation of the restriction half-site-specific adaptors to all restriction fragments; (ii) selective amplification of these fragments with two PCR primers that have corresponding adaptor and restriction site sequences as their target sites; and (iii) electrophoretic separation of the PCR products on polyacrylamide gels with selective detection of fragments which contain the fluorescently labelled primer and computer-assisted numerical analysis of the band patterns (177, 193, 413). Originally, Vos et al. (413) used radioactively labeled primers, but now AFLP is performed mainly with fluorescently labeled primers. AFLP measures the variation in the whole genome and thus is considered to give useful information about the short- and long-term evolution of bacterial strains (190). Janssen et al. (188) were the first to use AFLP as a tool in bacterial taxonomy. They examined 147 strains that had a broad range of G+C content (24 to 71%), and focused mainly on Aeromonas (n = 90) and Xanthomonas (n = 36). They also included three V. anguillarum strains and one V. tubiashii strain. The grouping obtained by AFLP corresponded well to that obtained by DNA-DNA similarity data. Janssen et al. (188) also reported that the complexity (i.e., the number and size of the fragments) of the AFLP patterns could be tuned by using different restriction enzymes and selective primers, although in any case the grouping of strains should be very similar. Because each bacterial species had a specific AFLP pattern, they concluded that AFLP could be used as an alternative to bacterial classification and identification. In the following years, AFLP was used to study various vibrios (381), including V. alginolyticus (405), V. cholerae (191, 192, 221), V. harveyi (139, 307), V. vulnificus (7, 8), V. wodanis (31), and P. damselae (391), but most of these studies did not include all the recognized Vibrio species. Arias et al. (7, 8) examined 80 V. vulnificus strains by several phenotypic (Biolog, API, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, serotyping, enzyme-linked immunosorbent assay) and genotypic (AFLP and ribotyping) methods. With AFLP analysis, the authors were able to discriminate strains with identical ribotypes, and thus they concluded that AFLP is the most suitable and discriminatory tool for epidemiological studies of V. vulnificus. Other AFLP analyses of 94 Vibrio strains clearly pointed out that V. carchariae was a synonym of V. harveyi and also indicated that 34 isolates were different from known Vibrio species (307). Vandenberghe et al. (405) discriminated pathogenic and probiotic V. alginolyticus strains by using AFLP and concluded that this technique can be used to authenticate probiotic cultures prior to their use. Thyssen et al. (391) used AFLP to differentiate the two subspecies of P. damselae, i.e., P. damselae subsp. damselae and P. damselae subsp. piscicida.
Jiang et al. (191, 192) discriminated V. cholerae serogroups O1 and O139 by using AFLP with the ApaI and TaqI restriction enzymes. They found that the genetic backgrounds of environmental and clinical V. cholerae strains are quite similar and concluded that pathogenic strains may in fact arise from nontoxigenic strains within the aquatic environment. Jiang et al. (191) demonstrated by AFLP analysis that the population structure of V. cholerae undergoes seasonal shifts. Certain clones are abundant in winter, and others are abundant in summer. More recently, Lan and Reeves (221) examined 45 V. cholerae isolates from the seventh pandemic and partitioned these isolates into 38 AFLP profiles. They concluded that AFLP is the best tool for discriminating clones from the seventh pandemic and suggested the design of PCR primers which target particular AFLP bands that could be used for epidemiological analysis through multiplex PCR or microarays analyses.
Colony Hybridization by Species-Specific Probes
Detection of vibrios on selective media and subsequent colony hybridization with species-specific probes based on variable regions of the 16S rRNA has also been evaluated as a fast screening alternative tool for V. anguillarum (254), V. halioticoli (376), V. harveyi (158), V. parahaemolyticus (356), V. proteolyticus (277), V. scophthalmi (63), and V. vulnificus (61, 62). It was demonstrated that different “selective” media were not quite selective, and species-specific media are yet to be formulated. The specificity of certain probes, e.g., for detection of V. anguillarum, is not sufficient since they also hybridize with V. ordallii, V. diazotrophicus, and V. navarrensis (254). The probe for V. scophthalmi detection has not been evaluated against V. ichthyoenteri. The two species have nearly 100% 16S rRNA similarity, and there is thus a great chance of cross-hybridization. V. scophthalmi and V. ichthyoenteri have been isolated from similar fish hosts, i.e., turbot, but V. scophthalmi is argued to be probiotic while V. ichthyoenteri is a pathogen (60). Misleading conclusions could arise from ecological studies using this probe (63). The probe for V. vulnificus detection seemed to be very reliable (62).
Fluorescence In Situ Hybridization
The application of cultivation-independent techniques such as direct extraction of nucleic acids from environmental samples (e.g., water, gut tissue, and sediment) followed by clone library and 16S rRNA sequencing or alternatively FISH of filter-fixed cells with oligonucleotide probes targeting the 16S rRNA and subsequent visualization by epifluorescence microscopy has provided an efficient means of detecting, identifying, and quantifying marine bacteria, including vibrios (103, 104, 131, 318). These approaches have shed light on the distribution and ecology of vibrios in the marine environment and have overcome the problem of the great plate count anomaly, i.e., the difference of the order of 102 to 103 between direct cell counts by e.g., epifluorescence microscopy and the CFU counts on, e.g., marine agar plates (16). Vibrios may be in a dormant state (VBNC) or may not be able to grow on the selective media employed (82).
What do we know about the nonculturable vibrios? In 1982, Xu et al. showed that certain bacteria, e.g., V. cholerae, although metabolically active, were not able to grow on culture media. At that time, these authors already knew that environmental stresses (e.g., nutrient limitation or starvation and variations in pH, salinity, and temperature) could lead to such a state, for which they proposed the name “viable but nonculturable.” Some researchers hypothesize that this is a ”genetically programmed physiological response” to enable some bacteria to survive in the environment (258). Changes observed in VBNC bacteria include reduction of cell size, increase of cell wall thickness, decrease in the amount of RNA and DNA, and biofilm formation. Several Vibrio species, e.g., V. cholerae, V. shillonii, and V. vulnificus, can be VBNC and virulent. V. shillonii becomes VBNC when entering the cells of O. patagonica, but it remains metabolically active and multiples within its host (331, 333).
Several authors have recently shown that the most abundant prokaryotic groups, e.g., Archaea, cyanobacteria, the Cytophaga-Flavobacterium group, Roseobacter, SAR11, SAR86, SAR116, and SAR202 (α and/or β proteobacteria), found in the marine environment are not readily culturable and that vibrios are rarely found in clone libraries from environmental samples (88, 131, 318). Although a high abundance of vibrios occurs in euthrophic coastal waters and in association with eukaryotes, these authors showed that vibrios represent only a minor fraction of the total bacterioplankton.
Heidelberg et al. (164), in a study of the bacterioplankton in the Chesapeake Bay, showed that γ proteobacteria compose up to 10% (3.1 × 108 cells/liter) of the total bacteria, while Vibrio and Photobacterium compose up to 4% (2.1 × 108 cells/liter) of the total bacteria. In the North Sea, vibrios accounted only for 103 cells/ml (mainly particle associated) when genus-specific probes were used in FISH detection (103). The same authors found that by adding organic substrates (in micromolar concentrations) to the water, vibrios became dominant, reaching up to 65% (9.7 × 105 cells/ml) of the total bacteria in a few hours (104). Vibrios not only could rapidly respond to nutrient-enrichment experiments but also maintained viability for up to 50 days under starved conditions. These authors concluded the high rRNA content of vibrios provide the potential for such rapid responses, which allow them to grow rapidly, outcompeting other members of the bacterial community. The increase in nutrient concentration in the water could lead to an increase in the size of the cells of vibrios, which in turn would escape predation by protozoans. Beardsley et al. (29) have indeed suggested that the low abundance of vibrios observed during certain periods and in some places may be the effect of massive selective grazing by heterothrophic nanoflagellates, which are abundantly found in aquatic environments.
The low fluorescence intensity of marine bacteria is one of the main drawbacks of FISH technology (103, 104). This is not really a problem for vibrios since these organisms have a high content of ribosomes. On the other hand, because several Vibrio species (e.g., V. harveyi, V. campbellii, V. rotiferianus, and other closely phylogenetic neighbours) have very similar 16S rRNA sequences, it may be difficult to perform reliable species identification.
Microarrays
Microarrays have been successfully developed since the middle of the last decade (348). This method may be considered a refinement of Northern and Southern blot techniques and may be an alternative to DNA-DNA hybridizations currently performed in bacterial taxonomy (361, 432). Microarrays have been successfully used for the detection and quantification of bacteria in the environment (41). Dziejman et al. (101) constructed a microarray based on the genome sequence of V. cholerae N16961. They spotted about 3,600 open reading frames (ORFs), corresponding to about 93% of the bacterial genome, and showed that nine different representative strains of V. cholerae have only a 1% difference in gene content. It was also shown that the seventh-pandemic clone of V. cholerae contains two islands, i.e., VSP-I and VSP-II, that were probably acquired via horizontal gene transfer.
Cho and Tiedje (68) successfully designed a microarray, containing up to 96 genomic fragments (about 1 kb long), for the identification of Pseudomonas species. The DNA chip designed showed good correlation with DNA-DNA homology measrurments. It was suggested that a chip containing 100,000 genomic fragments would allow the identification of most gram-negative bacteria (68).
Multilocus Enzyme Electrophoresis and Multilocus Sequence Typing
MLEE was first applied to bacterial systematics in the 1980s and has become the standard technique for studies of population genetics (57, 351) and identification (412). MLST was developed recently as an improved adaptation of MLEE and has been advocated as the most reliable molecular tool for epidemiology (250, 400). Both techniques index the variation in housekeeping genes. MLST assigns alleles directly from the nucleotide sequences, while MLEE compares the electrophoretic mobility of the enzymes encoded by the genes (118). Obviously, MLST has several advantages over MLEE, e.g., higher discriminatory power because it detects synonymous and nonsynonymous changes, accuracy and portability of the data, ease of performance, and reproducibility (250). MLEE analysis of 397 V. cholerae strains isolated from Mexico and Guatemala suggested that horizontal transfer and recombination are important processes in the evolution of clonal complexes of V. cholerae and indicated that successful clonal complexes may persist for decades (30). A high genetic diversity, as assessed by MLEE of 15 enzyme loci, was observed among 107 diverse V. cholerae isolates (109). These isolates displayed 99 different electrophoretic patterns and a large number of alleles (i.e., two to seven) per locus, but no significant clustering between serogroups, biotype, and country of isolation was observed (109). These authors applied MLST of six housekeeping-enzyme loci, i.e., asd, cadA, idh, lap, mdh, and epd, on a subset of 31 V. cholerae serogroup O139 strains and found four distinct groups of strains (110). They concluded that recombination has not occurred among these vibrios. Splits decompostion analysis of 10 representative V. cholerae strains of our collection indicated that epd, asd, and lap may have suffered recombination (unpublished data). The lap, idh, and mdh gene sequence similarity among the 10 V. cholerae strains was >98%, whereas for asd and epd the similarity was >96 and >93%, respectively. A more comprehensive study carried out by Garg et al. (128) analyzed the sequences of dnaE, lap, recA, pgm, gyrB, cat, chi, rstR, and gmd of 96 V. cholerae O139 strains isolated in India between 1992 and 2000. The strains were partitioned into 51 sequence types, whereas the most common alleles were present in at least 77% of the isolates. Overall, the gene sequence similarity for the different loci was higher that 98%, except for recA, for which it was around 97%. This study also indicated that conspecific homologous recombination may have occurred in different loci (e.g., gmd, recA, and lap). Such events would lead to cohesion of the species (128). MLST of four loci, i.e., gyrB, recA, dnaE, and gnd, applied to 81 isolates of V. parahaemolyticus revealed that pandemic strains are clonal (70). The intraspecies gene sequence similarity was >98% for dnaE and gnd, >97% for gyrB, and >96% for recA. Strains of a single serotype had different sequence types, suggesting that genes coding for different serotypes suffered lateral transfer in V. parahaemolyticus. Clearly, MLST data will be useful to delineate species in vibrios.
Random Amplified Polymorphic DNA and Repetitive Extragenic Palindrome PCR
RAPD involves PCR amplification of random fragments of genomic DNA by using arbitrary primers, while rep-PCR amplifies intervening sequences located between highly repetitive DNA motifs (97). This technique has been used mainly with the aim of typing within the species V. alginolyticus (366), V. cholerae (328, 329, 449), V. parahaemolyticus (437), and V. vulnificus (421), and it is thus difficult to determine its taxonomic resolution and value for the whole family Vibrionaceae. According to Sudheesh et al. (366), V. alginolyticus and V. parahaemolyticus have different RAPD profiles and can be reliably separated by this fast screening method. Wong and Lin (437) compared RAPD, rep-PCR, pulsed-field gel electrophoresis, and ribotyping and concluded that rep-PCR is the most discriminatory of the techniques. Rivera et al. (328) analyzed 83 V. cholerae strains by rep-PCR and found that toxigenic and nontoxigenic strains had different patterns. They concluded that this technique could be used in epidemiological studies.
rep-PCR was used to identify presumptive V. harveyi isolates responsible for luminous vibriosis in shrimp (139). These isolates had the major phenotypic features of the species V. harveyi (4, 5, 114, 170). They grew on TCBS agar, were motile, fermented glucose, were oxidase positive, and were sensitive to the vibriostatic agent 0/129 at 150 μg. Presumptive V. harveyi isolates were arginine dihydrolase negative and lysine and ornithine decarboxylase positive. Most isolates were luminescent and utilized d-gluconate, l-glutamate, d-glucuronate, heptanoate, d-galactose, and sucrose and grew at 40 °C, but they did not utilise l-histidine or l-arabinose. Most isolates (n = 31) clustered with the type strain of V. campbellii, LMG 11216T. Because the isolates assigned to V. campbellii and to V. harveyi were very heterogeneous, DNA-DNA hybridizations were performed with representative strains to check the robustness of the clusters based on rep-PCR. The DNA- DNA hybridization experiments clearly showed that the presumptive V. harveyi isolates belong to the species V. campbellii, having at least 71% DNA similarity. In another study, rep-PCR was used to analyze the genomic diversity of vibrios isolated from the abalone gut (Haliotis spp.) (344). rep-PCR patterns using the primer GTG5 showed that each abalone species has a particular population of vibrios which is related to V. halioticoli.
Real-Time PCR
Rapid-cycle real-time PCR is a high-throughput technique and is based on the kinetic reaction paradigm where multiple reactions, i.e., denaturation, annealing, and extension, may occur simultaneously (434). DNA extracted directely from water, food, or fecal samples is used as the template for PCR. PCR products are detected with a double-stranded DNA dye, e.g., SYBR Green I (127, 302), or probes (54, 242) and occurs in about 1 h. The sensitivity of this technique is high, and it can detect as few as 10 V. cholerae CFU per ml of water sample (242). Reliable identification of V. cholerae, V. parahaemolyticus, and V. vulnificus is apparent when a combination of 20 species-specific genes from different human pathogens are used (127). This technique promises to be very useful for rapid detection, identification, and quantification of vibrios, but this remains to be shown in more comprehensive studies including all currently known species.
Restriction Fragment Length Polymorphism
The PCR-RFLP technique consists of PCR amplification of certain genes, e.g., 16S rRNA, gyrB, and rpoD, and subsequent restriction of the PCR products with endonucleases to obtain band patterns (71, 72, 233, 375, 394, 395, 397). According to Urakawa et al. (394), who analyzed the restriction patterns of the 16S rRNA of 35 Vibrionaceae species, this technique is useful for the classification and identification of Vibrionaceae strains. A closer examination of the data presented by these authors reveals that all the Vibrio core group species (i.e., V. alginolyticus, V. parahaemolyticus, V. proteolyticus, V. harveyi, and V. campbellii) and V. vulnificus have the same band pattern and were thus indistinguishable. P. iliopiscarius, P. leignathi and P. phophoreum showed identical genotypes. This is quite striking since the 16S rRNA sequence similarity between these species is <96.5%, clearly proving the low discriminatory power of PCR-RFLP.
Ribotyping
Ribotyping consists of four main steps: (i) restriction of the bacterial chromosome with an endonuclease, (ii) gel electrophoresis of the resulting fragments, (iii) transfer of the fragments to a membrane, and (iv) hybridization of the gel with a labeled probe complementary to the 16S and 23S rRNAs (143). Ribotyping was one of the first fingerprinting techniques to be successfully used in the taxonomy of vibrios, and it has been particularly useful in the study of V. cholerae (143, 144, 218, 310, 311). A standardized ribotyping scheme was proposed as a reliable tool for epidemiological studies of V. cholerae (309). In this scheme, 214 V. cholerae O1 strains isolated from 35 countries were partitioned into 21 different ribotypes. The authors observed that the strains causing the previous fifth and sixth pandemics (from 1881 to 1923) and the current seventh pandemic belong to different ribotypes. They suggested that the wide circulation of different clones might favor the persistence of V. cholerae in the environment but demonstrated that certain clones were restricted to certain regions, e.g., ribotype 8 was restricted to central Africa and ribotype 5 was predominant in the Latin American epidemics. Using ribotyping to analyze the epidemiological relationships of V. cholerae isolates from Latin America, it was concluded that the cholera epidemic which started in Peru in the early 1990s was an extension of the seventh pandemic which started in 1970 in Africa (115). Other studies revealed that V. cholerae strains from different epidemics were clonal and that over the years there has been a continuous emergence of new pathogenic clones (75, 116). More recently, ribotyping has been used to assess the genomic diversity of environmental Vibrio strains associated with fish and oysters (13, 245, 313). According to Austin et al. (11), closely related Vibrio species, e.g., V. anguillarum and V. ordalii, can be differentiated on the basis of ribotyping. Macián et al. (245) analyzed 82 V. splendidus, 25 V. harveyi, and 10 V. tubiashii strains isolated in a 1-year period and found 64, 17, and 9 different ribotypes, respectively. Certain V. splendidus ribotypes were typical for isolates found in summer, while others were typical for isolates found winter.
PHENOTYPIC IDENTIFICATION: THE PITFALLS OF CLASSICAL BIOCHEMICAL IDENTIFICATION AND DICHOTOMOUS KEYS
Classical phenotypic identification techniques, including tests for arginine dihydrolase and lysine and ornithine decarboxylases, were among the most extensively used techniques to screen the diversity of Vibrio strains associated with marine animals and their habitat, and these tests have been proposed as reliable species identification schemes (4, 5, 12, 13, 39, 167, 222, 244, 297, 298). Variable results, e.g., for arginine dihydrolase of some species, have been reported, making their identification on this basis difficult (312). Biolog has been one of the most widely used phenotypic techniques for the identification of Vibrionaceae strains in the last decade (11, 13, 209, 266, 406). Bacterial strains are inoculated onto 95 different compounds which may serve as carbon sources. The identification of strains is based on the pattern of utilization of the 95 carbon sources. A very important diagnostic phenotypic feature for the identification of Vibrio species has always been the presence of flagella and thus motility (3). Nonmotile Vibrio species, e.g., the V. halioticoli group, have described (344, 346, 347), suggesting that the presence of flagella is not an essential diagnostic feature. Likewise, oxidase-negative V. metschnikovii and V. gazogenes strains have been documented, as have Vibrio strains that fail to grow on TCBS (4, 5). Colony variation is also a very common feature among Vibrio species, including the newly described species (12, 166, 425).
Fatty acids methyl ester (FAME) profiling was evaluated for the differentiation of Vibrionaceae species (36, 216, 299). FAME profiling is generally very useful as a chemotaxonomic marker, and apparently, differentiation at the genus level was possible. The similarity of FAME profiles among the different species examined was very striking, and the authors thus concluded that this technique could be used only as an additional phenotypic feature (36, 216). It became clear that the ample phenotypic variability within Vibrionaceae species pointed to the use of classification and identification scheme based on genomic data.
The phenotypic identification of genera and species of the Vibrionaceae is problematic. The main reason is the great variability of diagnostic phenotypic features, e.g., arginine dihydrolase and lysine and ornithine decarboxylases, susceptibility to the vibriostatic agent 0/139, flagellation, indole production, growth at different salinities and temperatures, and carbon utilization as revealed by Biolog (9, 13). Traditionally used as clear-cut tests for identification of species, the latter should thus be interpreted with greatest care. Dichotomous keys (see, e.g., references 4, 5,and 170) are misleading for the identification of Vibrionaceae isolates.
A comparison between a consensus molecular identification, including AFLP, DNA-DNA hybridization, and 16S rRNA sequences, on the one hand, and phenotypic identification (Biolog), on the other, shows that different Vibrio species appear within the same Biolog group. For instance, strains misidentified as V. harveyi by Biolog were later correctly identified as V. campbellii or classified as V. rotiferianus by AFLP and DNA-DNA hybridizations. Indeed, V. campbellii, V. harveyi, and V. rotiferianus have nearly indistinguishable phenotypes (136, 139). Strains misidentified by Biolog as V. campbellii turned out to be V. chagasii, while strains supposed to be V. splendidus were classified as V. kanaloaei. It was also remarked that many strains identified by AFLP as, e.g., V. cincinnatiensis, V. splendidus, and V. tubiashii, corresponded to multiple Biolog groups. Comparing AFLP and Biolog data, (i) a single genotype may correspond to a single phenotype (e.g., A8; V. brasiliensis), (ii) a single genotype may correspond to multiple phenotypes (e.g., A9; V. fortis), or (iii) multiple genotypes may be found in a single phenotype (e.g., A1, A2, A3, A4, and A5; V. coralliilyticus and V. neptunius) (388). It is nearly impossible to distinguish many of the currently known Vibrio species, e.g., V. splendidus-related species, solely on the basis of the phenotype (Table 2).
TABLE 2.
Some important phenotypic and chemotaxonomic features of V. splendidus-related speciesa
| Characteristic | V. splendidus | V. kanaloaei | V. pomeroyi | V. chagasii | V. tasmaniensis | V. lentus | V. cyclitrophicus | V. crassostreae |
|---|---|---|---|---|---|---|---|---|
| Arginine | Vb | V | V | V | − | V | V | + |
| Lysine | − | − | − | − | − | − | − | − |
| Ornithine | − | − | − | − | − | − | − | − |
| Growth at/on: | ||||||||
| 4°C | V | + | + | V | + | V | + | + |
| 8% (wt/vol) NaCl | V | V | + | V | − | V | + | − |
| Nitrate reduction | + | + | + | + | + | + | − | + |
| Fermentation of: | ||||||||
| Sucrose | V | + | V | − | − | − | + | + |
| Arabinose | − | + | − | − | − | − | − | − |
| Utilization of cellobiose | V | − | + | + | + | + | + | + |
| β-Galactosidase activity | V | − | + | − | − | + | − | − |
| Indole production | + | + | + | + | + | V | − | + |
| Fatty acids (%) | ||||||||
| Summed feature 3c | 35 | 39.2 | 32.9 | 38.4 | 35.4 | 41.7 | 35.3 | 39.4 |
| 16:0 | 29.6 | 25.6 | 29.2 | 22.4 | 28.4 | 24.7 | 30.7 | 17.3 |
| 14:0 | 9 | 5.0 | 10.5 | 7.2 | 11.0 | 8.8 | 7.96 | 5.4 |
| 12:0 | 8.7 | 4.2 | 8.9 | 3.8 | 6.6 | 5.3 | 7.7 | 5.5 |
| 18:1 ω7c | 8.2 | 10.2 | 7.6 | 9.7 | 7.6 | 8.7 | 7.5 | 7.0 |
| 12:0 3-OH | 3.41 | 3.4 | 3.9 | 2.7 | 2.8 | 2.5 | 2.1 | 3.3 |
NUMERICAL AND POLYPHASIC TAXONOMY
Today, taxonomy includes the phylogeny, classification, nomenclature, and identification of bacterial isolates (140, 403). One of the main aims of taxonomy is to provide useful classification schemes to be used for various scientific and practical purposes. Ideally, such classifications should be stable, predictive, objective, and highly informative (140).
The genera Vibrio and Photobacterium are among the oldest known bacterial genera (100, 201). The beginning of the taxonomy of vibrios can be traced back to the work of Pacini. Until the middle of the 1900s, the taxonomy of vibrios was dominated by morphological studies that tried to group strains on the basis of very few phenotypic features, e.g., flagellation, morphology, and curvature of the cells, and cultural aspects. These studies led to the description of many new Vibrio species. In the seventh edition of Bergey's Manual of Determinative Bacteriology (45), the genus Vibrio belonged to the family Spirillaceae and consisted of 34 species, which, with the exception of V. cholerae (listed as V. comma) and V. metschnikovii, were later reclassified into other genera, e.g., Campylobacter (C. fetus, C. jejuni, and C. sputorum), Comamonas (C. terrigena), or Pseudomonas (P. fluorescens) or no longer accepted as validly described species according to the Approved List of Bacterial Names (355). The genus Photobacterium, on the other hand, harbored one species, i.e., P. phosphoreum, and was allocated into the genus Bacterium of the family Bacteriaceae (45).
The heterogeneity within the genus Vibrio was highlighted by Davis and Park (90, 305). By examining morphological and biochemical features of most species of the genus Vibrio, they showed that it was quite artificial and concluded that at least three genera existed among the species examined. The foundation of modern Vibrio taxonomy was laid by a number of numerical (phenetic) and/or polyphasic taxonomic studies (17, 21-28, 74, 81, 125, 152, 229, 319, 393, 427, 438). Most of these studies clustered large collections of strains on the basis of their ability to utilize different (ca. 50 to 150) compounds as sources of carbon and/or energy, enzyme activity (e.g., gelatinase, chitinase, and DNase), salt tolerance, luminescence, growth at different temperatures, antibiograms, DNA base composition, morphological features, and other biochemical tests (e.g., oxidase, catalase, Voges-Proskauer, indole, nitrate reduction, arginine dihydrolase, and lysine and ornithine decarboxylases). The clusters defined by phenotypic features were further refined and validated by DNA-DNA hybridization experiments, and phenotypic clusters with about 80% similarity were found to correspond to DNA-DNA homology clusters with more than 80% similarity (24, 28). This suggests that for Vibrionaceae taxonomy, one should use 80% DNA-DNA similarity as the limit for species definition instead of the canonical 70% proposed by Wayne et al. (426).
In the eighth edition of Bergey's Manual of Determinative Bacteriology (50), the family Vibrionaceae, which was proposed by Véron, comprised Vibrio and Photobacterium along with Beneckea, Aeromonas, Plesiomonas, and Lucibacterium. The combination of Vibrio (V. anguillarum, V. cholerae, V. costicola, V. fischeri, and V. parahaemolyticus) and Photobacterium (P. mandapamensis [P. leiognathi] and P. phosphoreum) in a single family was an improvement in the taxonomy of these two related genera, which were thought for a long time to be only distantly related. Baumann et al. (22) proposed the genus Beneckea to encompass vibrios (i.e., B. campbellii, B. neptuna, B. nereida, and B. pelagia) isolated from the marine environment which required Na+ for growth. In subsequent studies, Baumann et al. (24-26) proposed that Beneckea species and Lucibacterium harveyi should be reallocated to the genus Vibrio, Aeromonas and Plesiomonas should be placed into other families, and V. costicola should be placed in another genus. These authors also suspected that the evolution of Vibrio and Photobacterium species was driven mainly by vertical processes (mutations) rather than horizontal gene transfer. The DNA-DNA relatedness studies among Vibrio and Photobacterium species underpinned the taxonomy of these groups (26, 27, 319). These studies disclosed a core group of related vibrios, i.e., the V. harveyi group, consisting of V. harveyi, V. campbellii, V. natriegens, V. alginolyticus, and V. parahaemolyticus. V. harveyi and V. campbellii were found to have 61 to 74% DNA-DNA similarity, while V. parahaemolyticus and V. alginolyticus had 61 to 67% similarity. Reichelt et al. (319) also proposed biotypes I and II for V. splendidus and V. pelagius, but they suspected that these biotypes could be different species. Biotypes I and II of V. splendidus and V. pelagius showed a maximum of 61 and 58% DNA-DNA similarity, respectively. Additionally, the biotypes of both species were clearly distinguishable by phenotypic features. Nevertheless, researchers are still using the biotype designation today (368). Arias et al. (7, 8, 111) have suggested that the two biotypes of V. vulnificus should be abolished. These biotypes should be considered as different species according to the current species definition (361).
Baumann et al. (24-26) compared the amino acid sequence differences of glutamine synthetase, superoxide dismutase, and alkaline phosphatase to distinguish Vibrionaceae species. Because the determination of amino acid sequences was very time- consuming and cumbersome at that time, Baumann and colleagues applied a technique called microcomplement fixation, which is based on the immunological reaction of antigens and antisera of the target proteins. On the basis of this analysis, they concluded that Beneckea species, P. fischeri, and P. logei should be transferred to the genus Vibrio (25) (see also the first edition of Bergey's Manual of Systematic Bacteriology [210]). They also mentioned that they applied a certain “subjective judgement” about the limits of the genus Vibrio because they found that this genus was highly diverse. Several species, e.g., V. cholerae, V. fischeri, V. logei, and V. costicola (now Salinivibrio costicola), were distantly related to each other and to the Beneckea species.
PHYLOGENY OF THE VIBRIOS
In the last two decades, bacterial phylogeny has been enriched with chronometers, e.g., rRNAs (5S, 16S, and 23S), to reconstruct bacterial phylogenies but also to be used as taxonomic markers for identification. In many cases, the phylogenies obtained by 16S rRNA sequencing pointed out the inadequacy of grouping bacteria by the classical criteria, e.g., morphology and biochemical features. The close relationship of Vibrio and Photobacterium was confirmed by this approach, and both genera were placed within the purple bacteria, a diverse group of gram-negative phototrophic and heterotrophic bacteria (435), later renamed Proteobacteria (360). More recently, Proteobacteria has been elevated to the rank of phylum (206; see The Prokaryotes online at http://link.springer-ny.com/link/service/books/10125/index.htm or http://141.150.157.117:8080/prokPUB/index.htm). Proteobacteria is the largest group within Bacteria, having about 1,600 species partitioned in five classes, i.e., Alfaproteobacteria, Betaproteobacteria, Gammaproteobacteria, Deltaproteobacteria, and Epsilonproteobacteria. These classes are phenotypically indistinguishable. More detailed phylogenetic analyses within the family Vibrionaceae, using 5S and 16S rRNA sequences, were performed in the following years, leading to a refinement of this group (98, 208, 243, 312, 340).
Application of 5S rRNA as a First Phylogenetic Attempt
MacDonell and Colwell (243), analyzing the 5S rRNA of superfamily I (Vibrionaceae plus Enterobacteriaceae), correctly concluded that V. marinus (now Moritella marina in the family Alteromonadaceae), V. psychroerythrus (now Colwellia psychoerythrus in the family Alteromonadaceae), and Aeromonas spp. (now in the family Aeromonadaceae) were not authentic members of the Vibrionaceae and should be placed into other families. These authors also proposed the creation of two new genera, Listonella and Shewanella. The genus Shewanella is currently found within the family Alteromonadaceae, but the genus Listonella, which would encompass the species V. anguillarum, V. pelagius, and P. damselae, has not been recognized by many expert taxonomists in the field (11, 246). Further phenotypic and phylogenetic studies clearly showed that these three species should be retained in their original genera (98, 208, 358). The use of 5S rRNA to reconstruct the phylogeny is clearly of limited use (312), probably due to its small size (ca. 120 bp [435]).
Phylogenetic Picture Obtained by the 16S rRNA Chronometer
The 16S rRNA molecule (about 1,500 bp in length) consists of highly conserved regions which may reveal deep-branching (e.g., classes, phyla) relationships but may also demonstrate variable regions which may discriminate species within the same genus. This feature has prompted researchers to use 16S rRNA both as a phylogenetic marker and as an identification tool (428). The Ribosomal Database Project II (http://rdp.cme.msu.edu/html/ [249]) consists of over 75,000 16S rRNA sequences. These entries can be easily queried using publicly available software, e.g., BLAST and FASTA (306).
Dorsch et al. (98) determined the almost complete 16S rRNA sequences of 10 Vibrio species and obtained results for the Vibrio core group which were in agreement with previous DNA-DNA homology data obtained by Baumann et al. (24). Dorsch et al. (98) also indicated that V. hollisae should be allocated into a new genus. Kita-Tsukamoto et al. (208) presented a comprehensive phylogenetic study of the Vibrionaceae. Although this study was based on partial 16S rRNA sequences, Kita-Tsukamoto et al. selected a broad collection of 50 species, including the type species of the family Vibrionaceae, V. cholerae, most Vibrio species, and species of Aeromonas, Deleya, Escherichia, Marinomonas, Pseudomonas, and Shewanella. The main outcomes of this study were (i) the circumscription of species (at least 99.3% 16S rRNA similarity), genus (95 to 96%), and family (90 to 91%) borders within the Vibrionaceae and (ii) the delineation of seven main groups of Vibrionaceae species that would correspond to different genera or families. The suggestions of reclassification proposed by Kita-Tsukamoto et al. (208) were further addressed by Mellado et al. (262), who transferred V. costicola into Salinivibrio costicola, and Urakawa et al. (396, 398), who transferred V. marinus into Moritella marina and V. iliopiscarius into Photobacterium iliopiscarius. According to Kita-Tsukamoto et al. (208), V. cholerae and V. mimicus would correspond to a genus on their own. V. fischeri, V. logei, V. salmonicida, and relatives should be elevated to the genus rank. In both cases, the status of these Vibrio species has not yet been fully determined. If V. cholerae, V. mimicus, and the V. fischeri-related group are to be elevated to the genus level, one might argue for the revival of Beneckea to encompass all other remaining vibrios, an idea which was originally supported by Baumann et al. (22).
New Phylogenetic Insights Obtained by Other Chronometers
Any phylogenetic marker has weaknesses and strengthes because no single gene is completely resistant to lateral transfer, hidden paralogy, and changes in the evolutionary clock among related species (130, 193). Alternative phylogenetic markers should fulfill several criteria as put forward by Zeigler (448): (i) the genes must be widely distributed among genomes, (ii) the genes must be single copy within a given genome, (iii) the individual gene sequences must be long enough to contain sufficient information but short enough to allow sequencing in a convenient way (900 to 2,250 nucleotides), and (iv) the sequences must predict whole-genome relationships with acceptable precision and accuracy that correlates well with the 16S rRNA and with whole-genome similarity measured by, e.g., DNA-DNA hybridizations. recA, 23S rRNA, elongation factors G and Tu/1α, the catalytic subunit of the proton-translocating ATPase, the hsp60 and hsp70 heat shock proteins, glyceraldehyde-3-phosphate dehydrogenase (gap), rpoB, and gyrB are among the most promising alternative markers (52, 117, 247, 248, 272, 414). A comprehensive list containing the so-called bacterial core genes, which may be useful for further phylogenetic analyses, was proposed by Gevers et al. (130).
Zeigler (448) examined the whole-genome sequences of 49 bacterial species from different phyla, including Bacillus spp., Escherichia sp., Mycobacterium spp., and Streptococcus spp., with the aim of finding genes which are phylogenetically informative and may predict genome (taxonomic) similarity. He found high correlation between genes, e.g., recA, thdF, rpoA, ligA, and dnaX, and whole-genome sequences. The genome similarities calculated from whole-genome sequence alignments were very compatible with the values obtained by standard DNA-DNA hybridization experiments. He concluded that the sequences of three or four protein-coding genes can be used to precisely assign new isolates to species. recA- and rpoA-based phylogenies of the vibrios correlate very well with the one based on the 16S rRNA, but recA gene sequences seem to be much more discriminatory (378; Thompson, Gevers, et al., submitted). For 16S rRNA values above 98%, there was a wide range of recA similarities, varying from 83 to 99%. G. hollisae was distantly related to Vibrio (66.3% recA sequence similarity) and Photobacterium (70.5% recA sequence similarity). rpoA and recA gene sequences suggest that V. fischeri-related species are closely related to Photobacterium.
DEFINING TAXA WITHIN THE VIBRIOS
The Families Vibrionaceae, Enterovibrionaceae, Photobacteriaceae, and Salinivibrionaceae
16S rRNA sequencing is considered the most reliable tool for the allocation of genera, species, and strains into the family Vibrionaceae. Following this approach, the outline of Bergey's Manual of Systematic Bacteriology, 2nd ed., 2004 (see http://dx.doi.org/10.1007/bergeysoutline200310 [129]) lists eight genera, i.e., Allomonas (1 species), Catenococcus (1 species), Enterovibrio (1 species), Grimontia (1 species), Listonella (2 species), Photobacterium (7 species), Salinivibrio (1 species), and Vibrio (51 species) within the family Vibrionaceae. The genera Allomonas (199) and Enhydrobacter (363) were tentatively allocated to the family Vibrionaceae based mainly on phenotypic features.
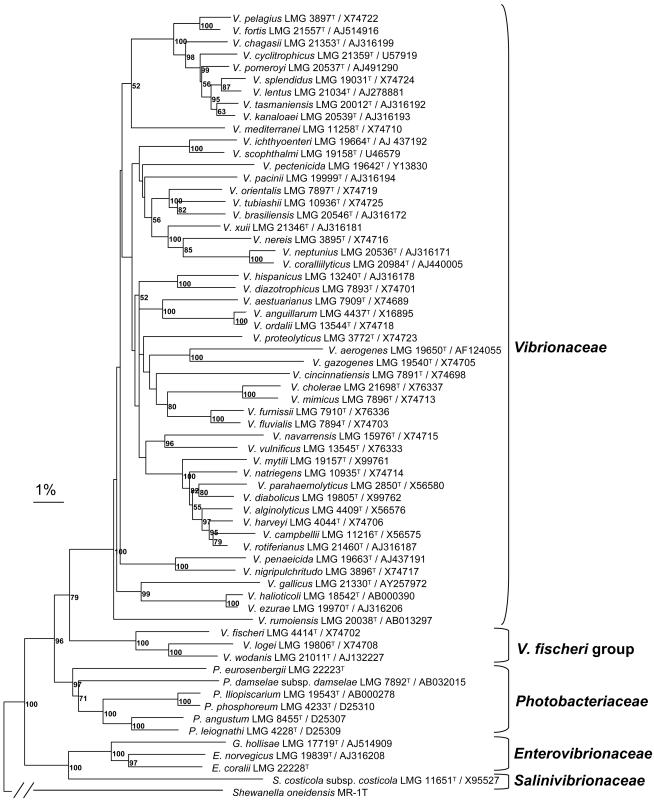
Phylogenetic analyses of concatenated 16S rRNA, recA, and rpoA gene sequences clearly show that vibrios are distributed in four different families, i.e., Salinivibrionaceae (comprising the genus Salinivibrio), Enterovibrionaceae (comprising the genera Enterovibrio and Grimontia), Photobacteriaceae (comprising the genus Photobacterium) and Vibrionaceae (comprising all the Vibrio species expect the V. fischeri group) (Fig. 1; Table 3).
FIG. 1.
Phylogenetic tree based on the neighbor-joining method, using the 16S rRNA, recA and rpoA concatenated gene sequences (2,898 bp), showing the different families of vibrios. Distance estimations were obtained by the model of Jukes and Cantor (196). Bootstrap percentages after 1,000 simulations are shown. Bar, 1% estimated sequence divergence. Sequences originated from the type strains of each species. The rpoA and recA gene sequences are from our own databases.
TABLE 3.
Phenotypic differences among Vibrionaceae-related families
| Characteristic | Enterovibrionaceae | Photobacteriaceae | Salinivibrionaceae | Vibrionaceae |
|---|---|---|---|---|
| d-mannitol fermentation | − | − | V | V |
| PHB accumulation | − | + | − | V |
| Growth in/at: | ||||
| 20% NaCl | − | − | + | − |
| 45°C | − | − | + | − |
| Voges-Proskauer | − | V | + | V |
| Indole | V | − | − | V |
| ADHa | V | + | − | V |
| ODHa | − | − | − | V |
| Presence of fatty acids: | ||||
| 16:1 ω9c | + | − | + | V |
| 18:1 ω9c | + | − | −b | V |
ADH, arginine dihydrolase; ODH, ornithine decarboxylase.
Trace amounts (<1%) occur in Salinivibrio strains.
The proposal to split the family Vibrionaceae into three new families was put forward by Thompson (388) on the basis of 16S rRNA and phenotypic features (Table 3) and was intended to facilitate further studies of vibrios. The creation of these three new families resulted in a more compact Vibrionaceae family. Allocation of strains into different families and genera is done on the basis of 16S rRNA (Fig. 1) and phenotypic analyses, while allocation of strains into species is currently best achieved by using AFLP, rep-PCR, or rpoA, atpA, and recA gene sequences.
Genera within the Vibrionaceae
The newly proposed family Vibrionaceae comprises only the genus Vibrio, with 63 species (Table 4) (388). The number of Vibrio species increased from 20 in 1981 to 47 in 2002 (106; http://www.bacterio.cict.fr/). New species isolated from the marine environment have been described each year, with V. crassostreae (117), Enterovibrio coralii, and P. eurosenbergii (Thompson, Thompson, et al., submitted) being the most recent ones. We have recently described 21 new species of vibrios (Fig. 1). V. fischeri-related species appear together at the outskirts of this diverse taxon, which we proposed to name Vibrionaceae (Fig. 1). Ruimy et al. (340) compared the 16S rRNA sequences of several Aeromonas, Grimontia, Photobacterium, Salinivibrio, Vibrio, and Plesiomonas representatives and concluded that the genus Vibrio is a distinct phylogenetic group which deserved elevation to the family rank. Overall, the analyses of several chronometers, e.g., 16S rRNA (208), 23S rRNA (247, 248), recA (378), and gyrB, and phenotypic features (26) indicate that V. cholerae and V. mimicus should be given genus rank. V. cholerae and V. mimicus are the bona fide members of the genus Vibrio. Whether all the other named Vibrio species should be retained in the same taxon as V. cholerae and V. mimicus or whether Vibrio should be split into different genera remains to be determined by future studies. Many species of the genus Vibrio, particularly those of the V. harveyi group, do not readily show conspicuous phenotypic features to warrant their elevation to the genus rank on this basis.
TABLE 4.
List of species of vibrios
| Species name | Type strain | Place and date of isolationa | Source |
|---|---|---|---|
| Enterovibrio coralii | LMG 22228T | Magnetic Island (Australia) 2002 | Water extract of bleached coral (Merulina ampliata) |
| Enterovibrio norvegicus | LMG 19839T | AARS Austevoll (Norway), 1997 | Gut of turbot larvae (Scophthalmus maximus) |
| Grimontia hollisae | LMG 17719T | Maryland (United States) | Human feces |
| Photobacterium angustum | LMG 8455T | Hawaii (United States) | Sea water |
| P. damselae subsp. damselae | LMG 7892T | United States | Diseased damsel fish (Chromis punctipinnis) |
| P. eurosenbergii | LMG 22223T | Magnetic Island (Australia), 2003 | Tissue extract of bleached coral (Pachyseris speciosa) |
| P. iliopiscarius | LMG 19543T | Norway | Gut of fish |
| P. leiognathi | LMG 4228T | Thailand | Leiognathidae fish (Family Leiognathidae) |
| P. phosphoreum | LMG 4233T | Hawaii (United States) | Seawater |
| P. profundum | LMG 19446T | Ryukyu Trench (Japan) | Sediment |
| Salinivibrio costicola subsp. costicola | LMG 11651T | Australia | Bacon-curing brine |
| Vibrio aerogenes | LMG 19650T | Seagrass bed in Nanwan bay (Taiwan) | Sediment |
| V. aestuarianus | LMG 7909T | Oregon (United States) | Oyster |
| V. agarivorans | LMG 21449T | Valencia (Spain) | Seawater |
| V. alginolyticus | LMG 4409T | Japan | Spoiled horse mackerel (Trachurus trachurus) |
| V. anguillarum | LMG 4437T | Norway | Diseased cod (Gadus morhua) |
| V. brasiliensis | LMG 20546T | LCMM Florianópolis (Brazil), 1999 | Bivalve larvae (Nodipecten nodosus) |
| V. calviensis | LMG 21294T | Bay of Calvi (Mediterranean), France | Seawater |
| V. campbellii | LMG 11216T | Hawaii (United States) | Seawater |
| V. chagasii | LMG 21353T | AARS Austevoll (Norway), 1997 | Gut of turbot larvae (Scophthalmus maximus) |
| V. cholerae | LMG 21698T | Asia | Clinical |
| V. cincinnatiensis | LMG 7891T | Ohio (United States) | Human blood and cerebrospinal fluid |
| V. coralliilyticus | LMG 20984T | Indian Ocean near Zanzibar, 1999 | Diseased Pocillopora damicornis |
| V. crassostreae | LMG 22240T | IFREMER La tremblade (France) | Hemolymph of diseased reared oysters (Crassostera gigas) |
| V. cyclitrophicus | LMG 21359T | Washington (United States) | Creosote-contaminated sediment |
| V. diabolicus | LMG 19805T | East Pacific Rise, 1991 | Dorsal integument of polychaete (Alvinella pompejana) |
| V. diazotrophicus | LMG 7893T | Nova Scotia (Canada) | Sea urchin (Strongylocentrotus) |
| V. ezurae | LMG 19970T | Kanagawa (Japan), 1999 | Gut of abalone (Haliotis diversicolor supertexta) |
| V. fischeri | LMG 4414T | Massachusetts (United States), 1933 | Dead squid |
| V. fluvialis | LMG 7894T | Bangladesh | Human feces |
| V. fortis | LMG 21557T | Ecuador, 1996 | Litopenaeus vannamei larvae |
| V. furnissii | LMG 7910T | Japan | Human feces |
| V. gallicus | LMG 21330T | Brest (France), 2001 | French abalone Haliotis tuberculata |
| V. gazogenes | LMG 19540T | Massachusetts (United States) | Mud from saltmarsh |
| V. halioticoli | LMG 18542T | Kumaishi (Japan); 1991 | Gut of abalone (Haliotis discus hanai) |
| V. harveyi | LMG 4044T | Massachusettes (United States), 1935 | Dead amphipod (Talorchestia sp.) |
| V. hepatarius | LMG 20362T | CENAIM (Ecuador), 2000 | Digestive gland of white shrimp (Litopenaeus vannamei) |
| V. hispanicus | LMG 13240T | Barcelona (Spain), 1990 | Culture water |
| V. ichthyoenteri | LMG 19664T | Hiroshima (Japan) | Gut of diseased Japanase flounder (Paralichtys olivaceus) |
| V. kanaloaei | LMG 20539T | IFREMER (France), 1998 | Diseased oyster larvae (Ostrea edulis) |
| V. lentus | LMG 21034T | Mediterranean coast, Valencia (Spain) | Oysters |
| V. logei | LMG 19806T | United States | Gut of Arctic scallop |
| V. mediterranei | LMG 11258T | Valencia (Spain) | Coastal seawater |
| V. metschnikovii | LMG 11664T | Asia | Diseased fowl |
| V. mimicus | LMG 7896T | North Carolina (United States) | Infected human ear |
| V. mytili | LMG 19157T | Valencia (Spain) | Bivalve (Mytilus edulis) |
| V. natriegens | LMG 10935T | Sapelo Island (United States) | Salt marsh mud |
| V. navarrensis | LMG 15976T | Villa Franca Navarra (Spain), 1982 | Sewage |
| V. neonatus | LMG 19972T | Kanagawa (Japan), 1999 | Gut of abalone (Haliotis discus discus) |
| V. neptunius | LMG 20536T | LCMM Florianópolis (Brazil), 1998 | Bivalve larvae (Nodipecten nodosus) |
| V. nereis | LMG 3895T | Hawaii (United States) | Seawater |
| V. nigripulchritudo | LMG 3896T | Hawaii (United States) | Seawater |
| V. ordalii | LMG 13544T | Washington (United States), 1973 | Diseased coho salmon (Oncorhynchus rhoddurus) |
| V. orientalis | LMG 7897T | Yellow Sea (China) | Seawater |
| V. pacinii | LMG 19999T | Dahua (China), 1996 | Healthy shrimp larvae (Penacus chinensis) |
| V. parahaemolyticus | LMG 2850T | Japan | Diseased human |
| V. pectenicida | LMG 19642T | Brittany (France), 1991 | Diseased bivalve larvae (Pecten maximus) |
| V. pelagius | LMG 3897T | Hawaii (United States) | Seawater |
| V. penaeicida | LMG 19663T | Kagoshima (Japan) | Diseased kuruma prawn (Penaeus japonicus) |
| V. pomeroyi | LMG 20537T | LCMM Florianópolis (Brazil), 1998 | Healthy bivalve larvae (Nodipecten nodosus) |
| V. proteolyticus | LMG 3772T | United States | Intestine of isopod (Limnoria tipunctala) |
| V. rotiferianus | LMG 21460T | ARC Gent (Belgium), 1999 | Rotifer in recirculation system (Brachionus plicatilis) |
| V. ruber | LMG 21676T | Keelung (Taiwan) | Seawater |
| V. rumoiensis | LMG 20038T | Japan | Drain pool of a fish-processing plant |
| V. salmonicida | LMG 14010T | Norway | Diseased Atlantic salmon (Salmo salar) |
| V. scophthalmi | LMG 19158T | Spain | Turbot juvenile (Scophthalmus maximus) |
| V. splendidus | LMG 19031T | North Sea | Marine fish |
| V. superstes | LMG 21323T | Australian Coast | Gut of abalone (Haliotis laevigata and H. rubra) |
| V. tapetis | LMG 19706T | Landeda (France) | Clam (Tapes philippinarum) |
| V. tasmanienis | LMG 20012T | MPL (Tasmania) | Atlantic salmon (Salmo salar) |
| V. tubiashii | LMG 10936T | Milford, Conn. (United States) | Hard clam (Mercenaria mercenaria) |
| V. vulnificus | LMG 13545T | U.S.A. | Human wound infection |
| V. wodanis | LMG 21011T | Norway, 1988 | Salmon with winter ulcer (Salmo salar) |
| V. xuii | LMG 21346T | Dahua (China), 1995 | Shrimp culture water |
AARS, Austevoll, Aquaculture Research Station of Austevoll, Austevoll, Norway; LCMM Florianópolis, Laboratory for Culture of Marine and Molluscs, Florianópolis, Brazil; CENAIM, National Center for Marine and Aquaculture Research, Guayaquil, Ecuador; IFREMER, French Institute for Exploration of the Sea, Brittany, France; MPL, Mount Pleasant Laboratories, Tasmania, Australia.
If V. cholerae and V. mimicus are to be considered the only current members of the genus Vibrio, then one might argue the revival of Beneckea to encompass all other remaining vibrios, an idea which was originally supported by Baumann et al. (22). It is clear that certain Vibrio species, i.e., V. aerogenes, V. ruber, V. gazogenes, V. rumoiensis, and V. halioticoli-related species, form distinct clades, most probably different genera, within the emended Vibrionaceae, whereas V. fischeri-related species represent a genus on its own, between Vibrionaceae and Photobacteriaceae. Striking phenotypic and ecological features of some of these Vibrio species illustrate clearly the biodiversity within this group of vibrios. For instance, V. fischeri and V. logei cells have a yellow pigment and several polar flagella but are generally unable to grow at 35 to 40°C (17, 113, 114). Glutamine synthetase and superoxide dismutase sequence divergence analyses also suggested that V. fischeri and V. logei are separate from the genus Vibrio (25). Also, these two species form a unique symbiotic association with squid (339). Clearly, the analyses of whole-genome sequences of Photobacterium and Vibrio spp. will shed light on the taxonomic position of V. fischeri and related species. V. gazogenes and V. ruber have a red pigment, are indole and oxidase negative, but grow in 10% NaCl and at 40°C. The further expansion of the current databases of, e.g., atpA, obg, pyrH, serS, pheS, and ligA sequences of vibrios and the search for alternative phylogenetic markers will yield a much better view of the heterogeneity within this group of marine organisms. Based on recA and rpoA analyses, V. halioticoli-, V. harveyi-, and V. splendidus-related species formed compact separate groups while V. tubiashii-related species may be scattered within the genus Vibrio. Vibrio strains have at least 84 and 73% rpoA and recA gene sequence similarity, respectively.
Allomonas enterica is almost identical to V. fluvialis on the basis of 16S rRNA, DNA-DNA hybridization and phenotypic features (112). The Subcommittee on the Taxonomy of Vibrionaceae had already concluded that A. enterica is a junior synonym of V. fluvialis (112). Enhydrobacter aerosaccus is located within the family Moraxellaceae and is closely related to Moraxella osloensis (∼100% 16S rRNA similarity) (388).
Species within the Vibrionaceae
Bacterial species can be thought of as “condensed nodes in a cloudy and confluent taxonomic space” (403). This view signifies that “classification is a frame for the condensed nodes where some isolated internodal strains (unclustered strains) also must get a place and name” (403). According to Stackebrandt et al. (361), a species is “a category that circumscribes a genomically coherent group of isolates sharing a high degree of similarity in many independent features.” In this case, DNA-DNA similarity remains the “gold” standard for species delineation. Strains from the same species have at least 70% DNA-DNA similarity under stringent conditions. Other authors suggest a more relaxed limit, i.e., 50 to 70% similarity (334; T. Coenye, D. Gevers, Y. Van de Peer, P. Vandamme, and J. Swings, submitted for publication). Young (444) has pointed out that the phenotypic circumscription of the bacterial species boundaries is the most important criterion for species definition. Other researchers have developed a species definition based only on the 16S rRNA sequences to delineate marine bacterioplankton species (153). These authors accept that species would be entities with at least 97% 16S rRNA similarity. It is our opinion that bacterial taxonomy is moving toward a genomically based concept in which phenotypic data may not have a clear standing.
In this respect, AFLP analysis proved to be valuable for the discrimination of phylogenetically and phenotypically related Vibrio species, e.g., the pairs V. coralliilyticus plus V. neptunius and V. harveyi plus V. rotiferianus (139). For classification and identification purposes, AFLP offers an alternative to DNA-DNA hybridizations by adopting the AFLP pattern similarity levels of 60 to 70%, which correspond to a DNA-DNA similarity higher than 70%. AFLP is one of the most reliable genomic fingerprinting identification tools in the taxonomy of the Vibrionaceae to date. Aside from its high discriminatory power and value for species circumscription, AFLP is easy and fast to perform and is amenable to automation. AFLP data can also be accumulated in databases.
The discrimination of AFLP is indeed very high and is based on a survey of the entire bacterial genome (190). In our experiments with AFLP (381), the average number of fragments obtained for the 506 strains analyzed with the enzyme combination HindIII plus TaqI and with the primer combination H01 (a adenine as selective base) plus T03 (a guanine as selective base) was 107 ± 23 (minimum of 46 and maximum of 164 fragments). The number of fragments obtained corresponded well to the expected number of fragments, i.e., 76 (assuming a average genome size of 5 Mb, a probability-of-cutting frequency of 0.0002 [1/46], and a factor of 0.0625 [1/16, due to the selective bases in each primer]). AFLP proved to be a most valuable tool for the circumscription of new species. With few exceptions (Table 3), strains from the same species were found in a single AFLP cluster. Indeed, AFLP screens a large number of point mutations. For instance, the estimated number of point mutations surveyed among 5 V. rotiferianus and 12 V. harveyi strains is 23,052 (average number of fragments of 113 × 12 nucleotides [6 plus 4 of the restriction sites plus the two selective bases] × number of strains). AFLP has been validated as an alternative to DNA-DNA hybridizations for a number of bacterial genera, including Aeromonas (176), Agrobacterium (275), Acinetobacter (189, 190), Bradyrhizobium (429), Burkholderia (76), Stenotrophomonas (159), and Xanthomonas (315). We compared the AFLP band pairwise simlarities found in the study by Thompson et al. (381) with all subsequent DNA-DNA homology values obtained in the course of the new species descriptions (388). In total, we included 234 values in the regression and correlation analyses. Using Pearson's product-moment correlation and Kendall's tau coefficients, the correlation of FAFLP and DNA-DNA data was found to be high, i.e., 0.80 and 0.50, respectively. The data fit well in a polynomial regression of second degree (r = 0.8) (Fig. 2). The data depicted in Fig. 2 clearly show a close relationship between FAFLP and DNA-DNA homology data. In fact, DNA-DNA homology values can be predicted from the FAFLP similarities; FAFLP band pairwise similarities of about 60% corresponded to DNA-DNA homologies of about 75 to 95%, while FAFLP similarities of about 70% corresponded to DNA- DNA homologies of about 80 to 100%. This level of correlation is very similar to that obtained in Xanthomonas by Rademaker et al. (315).
FIG. 2.
Polynomial regression (second degree) of FAFLP versus DNA-DNA homology data. FAFLP pattern pairwise similarities were calculated with the Dice coefficient, and 0.2% band position tolerance was used to allow technical errors. The diagonal (i.e., 100% theoretical values) of the DNA-DNA hybridization matrices was not included, in order to give a more realistic view of the relationship between the two techniques. The dashed line indicates the threshold (70% DNA-DNA similarity) for species-level circumscription.
Currently, the gold standard DNA-DNA hybridization experiments for delineating new species are performed mainly by the method described by Ezaki et al. (107, 108). Experiments are run in microplates in which DNA is noncovalently adsorbed and subsequently hybridized with photobiotin-labeled probe DNA. This method, which was thoroughly described by Willems et al. (430), is much faster than the classic DNA-DNA hybridization techniques (e.g., initial renaturation, hydroxyapatite, and S1 nuclease), can be performed in quadruplicate and with reciprocal reactions simultaneously, and has high correlation with classic techniques (142). To evaluate the reproducibility of this technique for Vibrionaceae, DNA-DNA hybridizations between various strains were repeated over a certain time period. It was found that a mean variation of 5% (±3.5% [standard deviation]; n = 24) between pairs of strains hybridized up to four times consecutively. This value is in agreement with the error of 7% estimated for this DNA hybridization method previously (142, 430).
Stackebrandt (362) has listed the main shortcomings of DNA-DNA hybridization techniques in the study of bacterial taxonomy: (i) few laboratories can execute this technique; (ii) the method is the slowest and most problematic step in the species description; and (iii) DNA-DNA data are not cumulative, and each new experiment requires the inclusion of reference strains. Finally, Stackebrandt (362) concluded that the method cannot be improved and that researchers should therefore seek new alternatives and new species proposals based on new approaches.
Applying MLST and the principles of bacterial population genetics (255, 256), bacterial taxonomists think that they will formulate an evolutionarily sound species concept (361, 362). A recent ecological and evolutionary theory has for the first time provided a species concept—the ecotype—based on the population genetics principles applied to MLST data (78, 79). According to this concept, an ecotype is a clonal complex, i.e., a cluster of strains that have five or six identical 500-bp gene fragments out of seven housekeeping genes. For nonclonal populations, e.g., Neisseria spp., an ecotype has four identical loci out of seven (250). Cohan (78, 79) also argues that most of the currently recognized species thus harbor several different ecotypes, each of which occupies a different ecological niche. This author concluded that actually recognized species could be regarded as genera. This view has been supported by other population geneticists, who propose a species genome definition based on phylogenetic and genetic analyses of housekeeping genes (219, 220). Because gene loss and acquisition play a role in speciation, some authors have proposed the species genome concept; i.e., strains from the same species would have at least 95% of their genes in common (219, 220). Venter et al. (407) advanced the concept of genomic species as biological unities having >94% sequence similarity in their housekeeping (protein-coding) genes. The idea that a bacterial species concept should be shaped by both evolutionary and ecological grounds has also been argued by other microbiologists (420). According to Ward, each ecologically unique population is in fact a unique species.
Maynard Smith et al. (256) see the Neisseria species as clusters, partially characteristic but having some identity to other clusters through horizontal gene transfer, and consequently they concluded that “there are no such entities as species in these pathogenic bacteria.” Maynard Smith et al. (256) also recommended that “a study of the genetic and phenotypic variation in a (diverse) taxon such as Neisseria should be compulsory for all philosophers who believe in the existence of natural kinds, for all cladists who believe in the universal validity of phylogenetic classification, and for all pheneticists, whatever they believe. In the end, we are forced to adopt a pragmatic approach, and view the Neisseria genus as a kind of commonwealth of phenetic and genetic clusters (which do not quite correspond to each other), each in turn partially characteristic, but also sharing some common identity with other clusters through horizontal gene transfer.” Whether the thoughtful perception of Maynard Smith et al. (256) about Neisseria species holds for other or perhaps all currently circumscribed bacterial species is yet to be proven. Accordingly, in the next few years bacterial taxonomists will probably be faced with the goal of identifying clones and clonal complexes if they intend to verify the hypothesis of Maynard Smith and colleagues.
The advantage of these ideas lies in the fact that microevolution in the nucleotides of the strain level and phylogeny can finally be merged into one consensus classification. The nomenclature can be of only secondary importance and might even be completely revised. A highly accessible and applicable prokaryotic genomic taxonomy will emerge from the analyses of the nucleotide sequences.
GENOMIC DIVERSITY AND PHYLOGENY AS REVEALED BY IDENTIFICATION METHODS: TOWARD A SYNTHESIS
Recent work on vibrios has provided an extended taxonomic framework for the identification and classification of Vibrionaceae isolates abundantly found in aquatic environments (388) (Table 4; Fig. 1). It is clear that these findings will have a positive impact on future studies of the taxonomy and ecology of these organisms. The descriptions of new Vibrionaceae species are in agreement with recommendations by the ad hoc committee for the reevaluation of the species definition in bacteriology (361). The new classification of vibrios is based on genomic data rather than on more traditional phenotypic features (33, 117, 136-139, 160, 346, 347, 378-388). FAFLP and to some extent rep-PCR have been applied to and validated for the classification of the new taxa. For identification purposes, FAFLP band pairwise similarities of about 70% will correspond to DNA-DNA homologies of about 80 to 100%, which equate to the recommended 70% DNA-DNA similarity.
The 16S rRNA gene sequences have elucidated the phylogenetic structure of the vibrios. Clearly, Salinivibrionaceae, Enterovibrionaceae, and Photobacteriaceae species are separate from one another and from the members of the family Vibrionacae. This family is in turn also heterogeneous and may require further splitting. Several Vibrionaceae species have nearly identical 16S rRNA gene sequences. In these cases, the only alternatives for identification are the genomic fingerprinting, e.g., FAFLP and rep-PCR, DNA-DNA hybridizations, or MLST. The genomic taxonomy of vibrios is in a phase of tremendous improvement as a result of the application of the MLST concepts. A more complete view of the genomic diversity of Vibrionacae is emerging with the analysis of the sequences of other housekeeping genes (Thompson, Gevers, et al., submitted). A consensus phylogenetic analysis based on concatenated recA, rpoA, and 16S rRNA gene sequences has revealed different groups, i.e., genera (e.g., the V. splendidus-, V. harveyi-, and V. halioticoli-related species) within the family Vibrionaceae.
It is our intention to identify and classify vibrios by their nucleotide sequences in protein-coding genes, using particularly the genes of the bacterial core genome (119, 130, 232, 448). Data gathered so far show that strains of the same species have >97% 16S rRNA, >99% rpoA, >98% gap, >97% atpA, >98% obg, >95% recA, >98% lap, >98% idh, >98% mdh, >96% asd and >93% epd gene sequence similarity. This type of data will aid in the development of an acceptable and coherent species concept, better reflecting the population structure and its (micro) evolution (79).
PLASTICITY OF VIBRIO GENOMES
Studies of the genome of vibrios, particularly V. cholerae, V. parahaemolyticus, and V. vulnificus, have improved our current knowledge of their ecology, pathogenicity, and epidemiology. The whole-genome sequences of three vibrios, i.e., V. cholerae (162), V. parahaemolyticus (251), and V. vulnificus (C. Chen et al., unpublished data; accession no. NC_004459 and NC_004460) are completed. The genome sequencing of a strain of V. fischeri http://ergointegratedgenomics.com/Genomes/VFI/), V. lentus (F. Le Roux and D. Mazel, unpublished data), and P. profundum (D. Bartlett et al., unpublished data) are under way. The paradigm of cholera epidemics held until recently was that the disease originated in a particular region of the globe and then spread to other places via human contact and/or contaminated material (269, 415). It has now been pointed out that the genetic backgrounds of environmental and clinical V. cholerae strains are quite similar and that pathogenic strains may arise from nontoxigenic strains within the aquatic environment (44, 65, 115, 234, 269, 350, 354). V. cholerae, “once a harmless environmental organism, has become pathogenic via multiple horizontal gene transfers” (162).
Compared with the genome of, for example, pathogenic E. coli, which shows numerous traces of horizontal gene transfer (294), vibrios seem to have fewer mobile genetic elements, e.g., transposons and phages, and DNA regions with a G+C content that differ from the whole-genome average, which are indicative of recent horizontal transfer (162, 251). Nevertheless, it has been demonstrated by studies in the last few years that horizontal gene transfer has contributed to several important characteristics of vibrios, such as pathogenicity and ecological niches (43, 203-205, 335-338, 417).
The genes for CT, the most important virulence factor of V. cholerae, have long been thought to be encoded in the chromosome of the bacterium. In 1996, Waldor and Mekalanos (417) reported that these genes are actually encoded in the genome of a newly identified bacteriophage, CTXφ, and that the phage genome is integrated into the bacterial chromosome as a prophage. Unlike other toxin-converting phages, most of which are double-stranded DNA phages, CTXφ is a filamentous phage with a single-stranded DNA genome, and it shares some features with the well-known filamentous phage, M13. Although the M13 phage does not integrate into the host chromosome, CTXφ is able to do so. CTXφ infects host cholera vibrios through pili, using the TCP as its receptor (417). Phylogenetically related filamentous phages have been identified for V. cholerae and other Vibrio species (55, 66, 172, 183, 202, 278). The genomes of these filamentous phages are composed of conserved regions and distinctive regions. The distinctive regions are considered to be foreign DNA sequences, often containing genes unique to each phage, e.g., the CT genes of CTXφ. Thus, the phages can play a role in genetic transmission between bacterial strains. It has been revealed that this group of phages targets dif-like sites as their integration site (55, 174, 180). The dif site is located in the replication terminus region of bacterial chromosomes and plays a role in resolving dimeric chromosomes formed during replication.
One of the most striking characteristics of the genomes of vibrios is the presence of super-integrons (73). Classical integrons are natural cloning and expression systems that incorporate ORFs and convert them into functional genes. Integrons have been widely identified as the constituents of transferable elements responsible for the evolution of multidrug resistance, i.e., multiresistance integrons (335). The integron platform codes for an integrase (intI) that mediates recombination between a proximal primary recombination site (attI) and a target recombination sequence, called an attC site (59 base elements). The attC site is usually associated with a single ORF in a circular structure, termed a gene cassette. Insertion of the gene cassette at the attI site drives the expression of the encoded proteins. Super-integrons comprise a large ancestral integron which was first discovered in the V. cholerae genome (73, 257). The super-integron spanned 126 kb and harbored 179 cassettes of mainly unassigned function (162). To date, super-integrons have been identified in several Vibrio species, including V. cholerae (335), V. parahaemolyticus (374), and V. vulnificus (357). A comparison of the super-integrons of V. cholerae and V. parahaemolyticus revealed that the contents of the gene cassettes differed substantially between the two (251). This suggests that the genes making up the super-integron are highly diverse between species. Considering the high abundance of Vibrionaceae strains in aquatic environments, it can be said that super-integrons constitute a vast gene pool in these systems. Recently, it was reported that chromosomal super-integrons of vibrios may be a genetic source for the evolution of resistance to clinically relevant antibiotics through integron-mediated recombination events (335-338).
Pathogenicity islands (PAIs) were first described in the genomes of pathogenic E. coli (40). Subsequently, they were found in other pathogens, where they form specific entities associated with bacterial pathogenicity (147, 150). PAIs are DNA regions in bacterial genomes, generally 10 to 200 kb long, that have features characteristic of transferred elements, i.e., different G+C content and codon usage compared with the rest of the genome, and the presence of mobility genes, e.g., insertion sequences and parts of phages. PAIs often accommodate large clusters of genes contributing to a particular virulence phenotype, and they appear exclusively in pathogenic strains of a given species. It is now accepted that the generation of PAIs often starts with the integration of plasmids, phages, or conjugative transposons into specific target genes, preferentially on the chromosomes (149). These targets are often tRNA genes. After integration into the bacterial genome, the integrated elements experience multiple genetic events such as mutations, deletions, and insertions of genes under specific selective pressures (149). In V. cholerae, a cluster of genes for the biogenesis of TCP, which play an important role in human intestinal colonization by the pathogen, are located in a PAI designated VPI (205). The sequencing of the whole genome of V. parahaemolyticus RIMD2210633 revealed a PAI of ca. 80 kb on chromosome 2 (181, 182, 251, 303, 371). This PAI encodes the genes for hemolysins, toxins, enzymes, possible membrane proteins, and the TTSS (175). The involvement of these newly identified genes in the pathogenicity of V. parahaemolyticus is now under investigation.
Sequencing of entire bacterial genomes revealed that PAI-like DNA regions are much more widespread than previously thought and represent a paradigm of more general entities that are present in the genomes of many bacterial species, termed “genomic islands” (150). Functions encoded in genomic islands are not restricted to pathogenicity but include many aspects such as antibiotic resistance, symbiosis, metabolism, degradation, and secretion, which may increase the fitness of bacteria in certain environments. The evolutionary advantage of genomic islands over mutations and other smaller insertions is that a large number of genes (e.g., operons and gene clusters encoding related functions) may be transferred and incorporated en bloc into the recipient genome. This transfer may lead to dramatic changes in the behavior of the organism, resulting ultimately in “evolution in quantum leaps” (147).
Genome Configuration
Several Vibrio species, including V. anguillarum, V. cholerae, V. fluvialis, V. parahaemolyticus, V. vulnificus, V. mimicus, and V. fischeri, contain two chromosomes (392, 442). Most Vibrionaceae species have this feature. How did the two- chromosome structure of vibrios develop? Heidelberg et al. (162) hypothesized that chromosome 2 of V. cholerae was a megaplasmid acquired by an ancestral vibrio. Several lines of evidence support this hypothesis, e.g., the presence of an integron (an element often found on plasmids) (257). In V. parahaemolyticus and V. vulnificus, an integron is present on the chromosome 1, suggesting that the position of integrons does not provide evidence for the megaplasmid hypothesis (67, 251, 374). Other researchers argue that the small chromosome may have arisen by excision from a single, large ancestral genome (418). The scenario has been reported for Brucella spp. (197). Egan and Waldor (102) identified the origins of replication (ori) of the two V. cholerae chromosomes. Their work revealed that the replication origin of chromosome 1 (oriCIVC) largely conforms to the basic features of E. coli oriC, which has been thought to define chromosomal origins of replication in Gammaproteobacteria. Although the replication origin of chromosome 2 (oriCIIVC) shares certain features with E. coli oriC, several unusual features for a bacterial chromosome are also present. oriCIIVC-based replication displays several features that characterize certain plasmid replicons. The authors suggested that these similarities may support the proposal that chromosome 2 was originally acquired as a plasmid. In any case, since the two Vibrio chromosomes appear to have coexisted throughout the speciation of vibrios, the generation of the two-chromosome configuration must have occurred before the diversification of this group.
Why do vibrios have two chromosomes? Why has the small chromosome not been integrated into the large chromosome? Yamaichi et al. (442) suggested that the split of the genome into two replicons would be advantageous for the rapid DNA replication normally observed in V. parahaemolyticus, a species with a doubling time of only 8 to 9 min (2). Heidelberg et al. (162) hypothesized that under certain conditions, differences in the copy numbers of chromosome 1 and chromosome 2 might occur, potentially increasing the effective level of gene expression on the more numerous chromosome, to the organism's advantage. The extreme in chromosome copy number asymmetry is the loss of one chromosome, and nonreplicating, single-chromosome cells (”drone” cells), within a nutrient-stressed population of normal two-chromosomal cells, might contribute to the survival of the community by continuing the secretion of enzymes that break down molecules in the surrounding microenvironment. Heidelberg et al. (162) proposed that the resulting nutrients might be used by normal members of the population, thereby promoting the survival of the species. Although there is no direct evidence for the change in stoichiometry of the two chromosomes, splitting the genome into two replicons might be advantageous for the vibrios that experience changing environmental conditions (80, 349).
The distribution of genes of known function between the two chromosomes of vibrios provides tantalizing clues about how this configuration may confer an evolutionary advantage to vibrios. Although chromosome 1 contains most of the genes that are required for growth (162, 251), chromosome 2 contains more genes for bacterial adaptation to environmental changes, indicating that the chromosomes play different roles (251). The global expression pattern of the genes on the two chromosomes of V. cholerae has been analyzed by using a whole-genome microarray (37, 231, 264, 447) and other methods (156). By using the rabbit ileal-loop model, Xu et al. (447) compared the global transcriptional pattern of in vivo-grown cells with that of cells grown to mid- exponential phase in rich medium under aerobic conditions. Under both conditions, the genes showing the highest levels of expression reside primarily on chromosome 1. During bacterial growth in the intestine, many more genes of the small chromosome are expressed.
A comparison of the genomes of V. cholerae and V. parahaemolyticus revealed that although chromosome 1 does not differ greatly in size between the two genomes (3.0 and 3.3 Mb respectively), chromosome 2 is much larger in V. parahaemolyticus than in V. cholerae (1.9 and 1.1 Mb, respectively) (251, 374). Examination of more species from the Vibrionaceae also demonstrated that while the sizes of chromosome 1 do not differ greatly, those of chromosome 2 are variable (T. Iida et al., unpublished data). The small chromosome seems to have higher proportions of genes unique to each Vibrio species (251). The size differences and the discrepancy in the number of unique genes suggest that the small chromosome of vibrios is more diverse in structure and gene content than is the large chromosome.
The relative position of the conserved genes in V. cholerae and V. parahaemolyticus demonstrates that extensive genome rearrangements have occurred within, and between, the two chromosomes (251, 374). Most of the intrachromosomal rearrangements, particularly in chromosome 1, may have occurred without changing the relative distance from the replication origin (251, 374). Such symmetrical rearrangements have been observed in other bacterial chromosomes (105, 239, 364). Of the 2,293 conserved genes on chromosome 1 of V. cholerae, 2,076 (90.5%) are also found on chromosome 1 in V. parahaemolyticus; and 539 (85.0%) of 634 conserved genes found on V. cholerae chromosome 2 are found on V. parahaemolyticus chromosome 2 (251). Despite extensive genome rearrangements, the locations of most of the conserved genes on both chromosomes are conserved between the two Vibrio species. Apparently, intrachromosomal rearrangements have occurred more often than interchromosomal rearrangements.
Driving Forces in the Evolution of Vibrios
Comparative genome analysis has revealed a variety of genomic events, including mutations, chromosomal rearrangements, loss of genes by decay or deletion, and gene acquisitions through duplication or horizontal transfer (200, 251). All of these events may be driving forces in evolution and speciation of vibrios (149-151, 292, 293). Horizontal gene transfer seems to be an efficient mechanism for introducing new phenotypes into the genome of these bacteria. Several researchers have claimed that this process can even make the delineation of bacterial species difficult (141, 226). Kurland et al. (211) and Daubin et al. (92), however, argued against this view, insisting that horizontal gene transfer events most often have little influence on organismal phylogeny. Cohan argues that mutation is one of the main driving forces in the evolution of bacteria (79). Although its influence on phylogenetic interpretation is controversial, the impact of horizontal gene transfer on the evolution of vibrios is apparent, and it can explain a number of unique genotypes e.g., the V. cholerae seventh-pandemic E1 Tor O1 strains (101, 116).
It has long been thought that the complex interactions between pathogens and the hosts they infect are the primary driving forces that determine the strategies used by bacteria to counter host defenses. However, new evidence suggests that the external environment, including other hosts, might play a greater role in the evolution of certain pathogens than was previously thought (433). In particular, acquisition of virulence traits through horizontal gene transfer might occur at high frequency through microbial contacts in the environment, either as mixed microbial soups in the guts of animals or as aquatic biofilms.
PERSPECTIVES AND EXPLOITATION
Research into many other molecular chronometers, e.g., atpA, obg, pyrH, serS, pheS, and ligA (130, 232, 448), is urgently needed to complete the phylogeny of the Vibrionaceae, as well as to refine the discrimination of closely related species. Analyses of the sequences of many different loci may also shed more light on the role of recombination and mutation in the evolution of vibrios. It is our conviction that the taxonomy and phylogeny of vibrios will be improved by MLST. The delineation of clonal complexes within each recognized Vibrio species by means of MLST may result in an unambiguous and direct method of identifying isolates in the future (119). According to the ecotype concept advanced by Cohan (79), each clonal complex is a new species and each currently recognized bacterial species represents in fact a genus. No more than 100 clonal complexes are expected to exist within a given species (79). The actual number of clonal complexes established for Neisseria meningitidis, Campylobacter jejuni and Streptococcus pneumoniae is about 10 to 15 (see the MLST website at http://www.mlst.net/new/index.htm). If we assume that each species of vibrios comprises 10 clonal complexes, we would, expect a multiplication of species of vibrios from the current 74 to 740 species.
Vibrios have been exploited in various ways. These organisms may be used for environmental monitoring (286, 288, 401), as well as to produce N-acyl homoserine lactone autoinducer molecules for controlling infections and biofilm formation (373). Vibrios are also used in vaccine (207) and probiotic (411) production. Bioremediation of polyaromatic hydrocarbons may be carried out by vibrios (161). Vibrios may also be a source of novel polysaccharides and proteases (93, 316). Further exploration and description of the biodiversity among vibrios in the environment is an important topic for future research. Although there are now already 74 validly described species within this group, the biodiversity of the vibrios has not been fully covered yet. The ecological role, distribution, and abundance of the newly described species of vibrios is another important issue. Several new species were dominant on plates at the time of their isolation and appear to be ubiquitous in aquatic environments. For instance, V. brasiliensis was very abundant in cultures of Nodipecten nodosus larvae, while V. neptunius and V. rotiferianus were dominant in Brachionus plicatilis rearing systems. The pathogenicity of newly described species, including E. norvegicus, V. brasiliensis, V. coralliilyticus, V. fortis, V. kanaloaei, V. pomeroyi, V. rotiferianus, and V. xuii, for fish and crustacean models is now under investigation (B. Austin et al., unpublished data). Newly isolated strains associated with coral bleaching were allocated into the species V. coralliilyticus, V. fortis, and V. rotiferianus (Thompson, Gevers, et al., submitted). Do these culturable microorganisms really represent the dominant microbiota of those sites, or are they just overestimated on marine agar plates? Are there other dominant VBNC vibrios in the same habitats where the new species were isolated? If so, what is the taxonomic allocation and abundance of these VBNC Vibrionaceae strains? Are they related to the new species recently described? Apparently, euthrophic environments, e.g., certain estuarine ecosystems and aquaculture settings, favor the growth of culturable vibrios, and one would expect that these organisms are really dominant over other groups, including VBNC bacteria, but this remains to be proven (300).
Whole-genome sequencing of other vibrios is needed (116). The reduction in the costs of sequencing and the development of new sequencing technologies, e.g., resequencing, pyrosequencing, and micro- or nanochannel capillary electhrophoresis, will facilitate the sequencing of other whole vibrio genomes. The whole-genome sequencing of nonclinical vibrios abundantly found in the marine environment will help us to understand their ecology and better define their taxonomy and species boundaries. Comparative genomics of vibrios through the application of microarrays should facilitate the investigation of the gene repertoire at the species level (101) and may be an important tool for studying emerging pathogens of marine animals (333). Based on such new genomic information, the taxonomy of and species definition for vibrios will be reviewed in the next few years.
Acknowledgments
F.L.T. acknowledges a Ph.D. scholarship (no. 2008361/98-6) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and a postdoctoral fellowship from BCCM/LMG Bacteria Collection. J.S. acknowledges grants from of the Fund for Scientific Research (FWO), Belgium.
REFERENCES
- 1.Abbott, S. L., and J. M. Janda. 1994. Severe gastroenteritis associated with Vibrio hollisae infection: report of 2 cases and review. Clin. Infect. Dis. 18:310-312. [DOI] [PubMed] [Google Scholar]
- 2.Aiyar, S. E., T. Gaal, and R. L. Gourse. 2002. rRNA promoter activity in the fast-growing bacterium Vibrio natriegens. J. Bacteriol. 184:1349-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, R. D., and P. Baumann. 1971. Structure and arrangement of flagella in species of the genus Beneckea and Photobacterium fischeri. J. Bacteriol. 107:295-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsina, M., and A. R. Blanch. 1994. A set of keys for biochemical identification of environmental Vibrio species. J. Appl. Bacteriol. 76:79-85. [DOI] [PubMed] [Google Scholar]
- 5.Alsina, M., and A. Blanch. 1994. Improvement and update of a set of keys for biochemical identification of Vibrio species. J. Appl. Bacteriol. 77:719-21. [DOI] [PubMed] [Google Scholar]
- 6.Arias, C. R., E. Garay, and R. Aznar. 1995. Nested PCR method for rapid and sensitive detection of Vibrio vulnificus in fish, sediments, and water. Appl. Environ. Microbiol. 61:3476-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arias, C. R., L. Verdonck, J. Swings, R. Aznar, and E. Garay. 1997. Intraspecific differentiation of Vibrio vulnificus biotypes by amplified fragment length polymorphism and ribotyping. Appl. Environ. Microbiol. 63:2600-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias, C. R., L. Verdonck, J. Swings, R. Aznar, and E. Garay. 1997. A polyphasic approach to study the intraspecific diversity amongst Vibrio vulnificus isolates. Syst. Appl. Microbiol. 20:622-633. [Google Scholar]
- 9.Austin, B., and J. V. Lee. 1992. Aeromonadaceae and Vibrionaceae, p. 163-182. In R. G. Board, D. Jones, and F. A. Skinner (ed.), Identification methods in applied and environmental microbiology. Blackwell Scientific Publications, Oxford, United Kingdom.
- 10.Austin, B., L. F. Stuckey, P. A. W. Robertson, I. Effendi, and D. R. W. Griffith. 1995. A probiotic strain of Vibrio alginolyticus effective in reducing diseases caused by Aeromonas salmonicida, Vibrio anguilarum and Vibrio ordalli. J. Fish Dis. 18:93-96. [Google Scholar]
- 11.Austin, B., M. Alsina, D. A. Austin, A. R. Blanch, P. A. D. Grimont, J. Jofre, S. Koblavi, J. L. Larsen, K. Pedersen, T. Tiainen, L. Verdonck, and J. Swings. 1995. Identification and typing of Vibrio anguillarum: a comparison of methods. Syst. Appl. Microbiol. 18:285-302. [Google Scholar]
- 12.Austin, B., D. A. Austin, V. M. Falconer, K. Pedersen, J. L. Larsen, J. Swings, and L. Verdonck. 1996. Dissociation of Vibrio anguillarum and V. ordalii cultures into two or three discrete colony types. Bull. Eur. Fish Pathol. 16:101-103. [Google Scholar]
- 13.Austin, B., D. A. Austin, A. R. Blanch, M. Cerda, P. A. D. Grimont, J. Jofre, S. Koblavi, J. L. Larsen, K. Pedersen, T. Tiainen, L. Verdonck, and J. Swings. 1997. A comparison of methods for the typing of fish-pathogenic Vibrio spp. Syst. Appl. Microbiol. 20:89-101. [Google Scholar]
- 14.Austin, B., and D. A. Austin. 1999. Bacterial fish pathogens: disease of farmed and wild fish, 3rd ed. Springer-Verlag KG, Berlin, Germany.
- 15.Austin, B., A. C. Pride, and G. A. Rhodie. 2003. Association of a bacteriophage with virulence in Vibrio harveyi. J. Fish Dis. 26:55-58. [DOI] [PubMed] [Google Scholar]
- 16.Azam, F. 2001. Introduction, history, and overview: the methods to our madness. Methods Microbiol. 30:1-12. [Google Scholar]
- 17.Bang, S. S., P. Baumann, and K. H. Nealson. 1978. Phenotypic characterization of Photobacterium logei (sp. nov.), a species related to P. fischeri. Curr. Microbiol. 1:285-288. [Google Scholar]
- 18.Banin, E., Y. Israel, T. Kushmaro, A. Loya, Y. Orr, and E. Rosenberg. 2000. Penetration of the coral-bleaching bacterium Vibrio shiloi into Oculina patagonica. Appl. Environ. Microbiol. 66:3031-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banin, E., S. K. Khare, F. Naider, and E. Rosenberg. 2001. Proline-rich peptide from the coral pathogen Vibrio shiloi that inhibits photosynthesis of zooxanthellae. Appl. Environ. Microbiol. 67:1536-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbieri, E., L. Falzano, C. Fiorentini, A. Pianetti, W. Baffone, A. Fabbri, P. Matarrese, A. Casiere, M. Katouli, I. Kuhn, R. Mollby, F. Bruscolini, and G. Donelli. 1999. Occurrence, diversity, and pathogenicity of halophilic Vibrio spp. and non-O1 Vibrio cholerae from estuarine waters along the Italian Adriatic coast. Appl. Environ. Microbiol. 65:2748-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumann, L., and P. Baumann. 1973. Regulation of aspartokinase activity in the genus Beneckea and marine, luminous bacteria. Arch. Mikrobiol. 90:171-188. [DOI] [PubMed] [Google Scholar]
- 22.Baumann, P., L. Baumann, and M. Mandel. 1971. Taxonomy of marine bacteria: the genus Beneckea. J. Bacteriol. 107:268-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baumann, P., L. Baumann, and J. L. Reichelt. 1973. Taxonomy of marine bacteria: Beneckea parahaemolytica and Beneckea alginolytica. J. Bacteriol. 113:1144-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann, P., and L. Baumann. 1977. Biology of the marine enterobacteria: genera Beneckea and Photobacterium. Annu. Rev. Microbiol. 31:39-61. [DOI] [PubMed] [Google Scholar]
- 25.Baumann, P., L. Baumann, S. S. Bang, and M. J. Woolkalis. 1980. Reevaluation of the taxonomy of Vibrio, Beneckea, and Photobacterium: abolition of the genus Beneckea. Curr. Microbiol. 4:127-132. [Google Scholar]
- 26.Baumann, P., L. Baumann, M. J. Woolkalis, and S. S. Bang. 1983. Evolutionary relationships in Vibrio and Photobacterium: a basis for a natural classification. Annu. Rev. Microbiol. 37:369-398. [DOI] [PubMed] [Google Scholar]
- 27.Baumann, P., and R. H. W. Schubert. 1984. Vibrionaceae, p. 516-550. In N. R. Krieg and G. J. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. The Williams & Wilkins Co., Baltimore, Md.
- 28.Baumann, P., A. L. Furniss, and J. L. Lee. 1984. Genus I: Vibrio (Pacini, 1854), p. 518-538. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1, The Williams & Wilkins, Co., Baltimore, Md.
- 29.Beardsley, C., J. Pernthaler, W. Wosniok, and R. Amann. 2003. Are readily culturable bacteria in coastal North Sea waters suppressed by selective grazing mortality? Appl. Environ. Microbiol. 69:2624-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beltrán, P., G. Delgado, A. Navarro, F. Trujillo, R. K. Selander, and A. Cravioto. 1999. Genetic diversity and population structure of Vibrio cholerae. J. Clin. Microbiol. 37:581-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benediktsdóttir, E., L. Verdonck, C. Sproer, S. Helgason, and J. Swings. 2000. Characterization of Vibrio viscosus and Vibrio wodanis isolated at different geographical locations: a proposal for reclassification of Vibrio viscosus as Moritella viscosa comb. nov. Int. J. Syst. Evol. Microbiol. 50:479-488. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Haim, Y., E. Banim, A. Kushmaro, Y. Loya, and E. Rosenberg. 1997. Inhibition of photosynthesis and bleaching of zooxanthellae by the coral pathogen Vibrio shiloi. Environ. Microbiol. 1:223-229. [DOI] [PubMed] [Google Scholar]
- 33.Ben-Haim, Y. and E. Rosenberg. 2002. A novel Vibrio sp. pathogen of the coral Pocillopora damicornis. Mar. Biol. 141:47-55. [Google Scholar]
- 34.Ben-Haim, Y., F. L. Thompson, C. C. Thompson, M. C. Cnockaert, B. Hoste, J. Swings, and Rosenberg. 2003. Vibrio coralliilyticus sp. nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int. J. Syst. Evol. Microbiol. 53:309-315. [DOI] [PubMed] [Google Scholar]
- 35.Bergh, O., F. Nilsen, and O. B. Samuelsen. 2001. Diseases, prophylaxis and treatment of the Atlantic halibut Hippoglossus hippoglossus: a review. Dis. Aquat. Org. 48:57-74. [DOI] [PubMed] [Google Scholar]
- 36.Bertone, S., M. Giacomini, C. Ruggiero, C. Piccarolo, and L. Calegari. 1996. Automated systems for identification of heterotrophic marine bacteria on the basis of their fatty acid composition. Appl. Environ. Microbiol. 62:2122-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bina, J., J. Zhu, M. Dziejman, S. Faruque, S. Calderwood, and J. Mekalanos. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. USA 100:2801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blake, P. A. 1994. Historical perspectives on pandemic cholera, p. 293-296. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera. Molecular to global perspectives. American Society for Microbiology, Washington, D.C.
- 39.Blanch, A. R., M. Alsina, M. Simón, and J. Jofre. 1997. Determination of bacteria associated with reared turbot (Scophthalmus maximus) larvae. J. Appl. Microbiol. 82:729-734. [Google Scholar]
- 40.Blum, G., M. Ott, A. Lischewski, A. Ritter, H. Imrich, H. Tschape, and J. Hacker. 1994. Excision of large DNA regions termed pathogenicity island from tRNA-specific loci in the chromosome of an Escherichia coli wild- type pathogen. Infect. Immun. 62:606-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bodrossy, L. N., J. C. Stralis-Pavese, S. Murrell, S. Radajewski, A. Weilharter, and A. Sessitsch. 2003. Development and validation of a diagnostic microbial microarray for methanotrophs. Environ. Microbiol. 5:566-582. [DOI] [PubMed] [Google Scholar]
- 42.Borrego, J. J., D. Castro, A. Luque, C. Paillard, P. Maes, M. T. Garcia, and A. Ventosa. 1996. Vibrio tapetis sp. nov., the causative agent of the brown ring disease affecting cultured clams. Int. J. Syst. Bacteriol. 46:480-484. [Google Scholar]
- 43.Boyd, E. F., K. E. Moyer, L. Shi, and M. K. Waldor. 2000. Infectious CTXφ and the vibrio pathogenicity island prophage in Vibrio mimicus: evidence for recent horizontal transfer between V. mimicus and V. cholerae. Infect. Immun. 68:1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brazil, J. M., R. M. Alves, I. N. G. Rivera, D. P. Rodrigues, D. K. R. Karaolis, and L. C. Campos. 2002. Prevalence of virulence-associated genes in clinical and environmental Vibrio cholerae strains isolated in Brazil between 1991 and 1999. FEMS Microbiol. Lett. 215:15-21. [DOI] [PubMed] [Google Scholar]
- 45.Breed, R. S., E. G. D. Murray, and N. R. Smith. 1957. Bergey's manual of determinative bacteriology, 7th ed. p. 229-249. The Williams & Wilkins Co., Baltimore, Md.
- 46.Brenner, D. J., F. W. Hickman-Brenner, J. V. Lee, A. G. Steigerwalt, G. R. Fanning, D. G. Hollis, J. J. Farmer III, R. E. Weaver, S. W. Joseph, and R. J. Seidler. 1983. Vibrio furnissii (formerly aerogenic biogroup of Vibrio fluvialis), a new species isolated from human feces and the environment. J. Clin. Microbiol. 18:816-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brock, T. D. 1999. Robert Koch. A life in medicine and bacteriology. ASM Press, Washington, D.C.
- 48.Brown, B., R. Dunne, M. Goodson, and A. Douglas. 2002. Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs 21:19-126. [Google Scholar]
- 49.Buchmann, K., J. L. Larsen, and B. Therkildsen. 2001. Improved recapture rate of vaccinated sea-ranched Atlantic salmon, Salmo salar L. J. Fish Dis. 24:245-248. [Google Scholar]
- 50.Buchanan, R. E., and N. E. Gibbons. 1974. Bergey's manual of determinative bacteriology, 8th ed., p. 340-351. The Williams & Wilkins Co., Baltimore, Md.
- 51.Butler, S. M., and A. Camilli. 2004. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 101:5018-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byun, R., L. D. Elbourne, R. Lan, and P. R. Reeves. 1999. Evolutionary relationships of pathogenic clones of Vibrio cholerae by sequence analysis of four housekeeping genes. Infect. Immun. 67:1116-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camara, M., A. Hardman, P. Williams, and D. Milton. 2002. Quorum sensing in Vibrio cholerae. Nat. Genet. 32:217-218. [DOI] [PubMed] [Google Scholar]
- 54.Campbell, M. S., and A. C. Wright. 2003. Real-time PCR analysis of Vibrio vulnificus from oysters. Appl. Environ. Microbiol. 69:7137-7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campos, J., E. Martinez, E. Suzarte, B. L. Rodriguez, K. Marrero, Y. Silva, T. Ledon, R. del Sol, and R. Fando. 2003. VGJφ, a novel filamentous phage of Vibrio cholerae, integrates into the same chromosomal site as CTXφ. J. Bacteriol. 185:5685-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carnahan, A. M., J. Harding, D. Watsky, and S. Hansman. 1994. Identification of Vibrio hollisae associated with severe gastroenteritis after consumption of raw oysters. J. Clin. Microbiol. 32:1805-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Caugant, D. A. 2001. From multilocus enzyme electrophoresis to multilocus sequence typing, p. 299-349. In L. Dijkshoorn, K. J. Towner and M. Struelens (ed.), New approaches for the generation and analysis of microbial typing data. Elsevier, Amsterdam, The Netherlands.
- 58.Centers for Disease Control and Prevention. 1998. Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters—Pacific Northwest, 1997. Morb. Mortal. Wkly. Rep. 47:457-462. [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention. 1999. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York, 1998. Morb. Mortal. Wkly. Rep. 48:48-51. [PubMed] [Google Scholar]
- 59a.Center for Disease Control and Prevention. 1996. Vibrio vulnificus infections associated with eating raw oysters—Los Angeles, 1996. Morb. Mortal. Wkly. Rep. 45:621-624. [PubMed] [Google Scholar]
- 60.Cerda-Cuéllar, M., R. A. Rosselló-Mora, J. Lalucat, J. Jofre, and A. Blanch. 1997. Vibrio scophthalmi sp. nov., a new species from turbot (Scophthalmus maximus). Int. J. Syst. Bacteriol. 47:58-61. [DOI] [PubMed] [Google Scholar]
- 61.Cerda-Cuéllar, M., J. Jofre, and A. R. Blanch. 2000. A selective medium and a specific probe for detection of Vibrio vulnificus. Appl. Environ. Microbiol. 66:855-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cerda-Cuéllar, M., L. Permin, J. L. Larsen, and A. R. Blanch. 2001. Comparison of selective media for the detection of Vibrio vulnificus in environmental samples. J. Appl. Microbiol. 91:322-327. [DOI] [PubMed] [Google Scholar]
- 63.Cerda-Cuéllar M and A. R. Blanch. 2002. Detection and identification of Vibrio scophthalmi in the intestinal microbiota of fish and evaluation of host specificity. J. Appl. Microbiol. 93:261-268. [DOI] [PubMed] [Google Scholar]
- 64.Cerveny, K. E., A. DePaola, D. H. Duckworth, and P. A. Gulig. 2002. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 70:6251-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakraborty, S., A. K. Mukhopadhyay, R. K. Bhadra, A. N. Ghosh, R. Mitra, T. Shimada, S. Yamasaki, S. M. Faruque, Y. Takeda, R. R. Colwell, and G. B. Nair. 2000. Virulence genes in environmental strains of Vibrio cholerae. Appl. Environ. Microbiol. 66:4022-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang, B., H. Taniguchi, H. Miyamoto, and S. Yoshida. 1998. Filamentous bacteriophages of Vibrio parahaemolyticus as a possible clue to genetic transmission. J. Bacteriol. 180:5094-5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen, C., K. Wu, Y. Chang, Y. Chang, H. Tsai, T. Liao, Y. Liu, H. Chen, A. Shen, J. Li, T. Su, C. Shao, C. Lee, L. Hor, and T. Shih-Feng. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genet. Res. 13:2577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cho, J. C., and J. M. Tiedje. 2001. Bacterial species determination from DNA-DNA hybridization by using genome fragments and DNA microarrays. Appl. Environ. Microbiol. 67:3677-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chowdhury, N. R., S. Chakraborty, T. Ramamurthy, M. Nishibuchi, S. Yamasaki, Y. Takeda, and G. B. Nair. 2000. Molecular evidence of clonal Vibrio parahaemolyticus pandemic strains. Emerg. Infect. Dis. 6:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chowdhury, N. R., O. C. Stine, J. G. Morris, and G. B. Nair. 2004. Assessment of evolution of pandemic Vibrio parahaemolyticus by multilocus sequence typing. J. Clin. Microbiol. 42:1280-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chun, J., A. Huq, and R. R. Colwell. 1999. Analysis of 16S-23S rRNA intergenic spacer regions of Vibrio cholerae and Vibrio mimicus. Appl. Environ. Microbiol. 65:2202-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chun, J., I. N. Rivera, and R. R. Colwell. 2002. Analysis of 16S-23S rRNA intergenic spacer of Vibrio cholerae and Vibrio mimicus for detection of these species. Methods Mol. Biol. 179:171-178. [DOI] [PubMed] [Google Scholar]
- 73.Clark, C. A., L. Purins, P. Kaewrakon, and P. A. Manning. 1997. VCR repetitive sequence elements in the Vibrio cholerae chromosome constitute a mega-integron. Mol. Microbiol. 26:1137-1138. [DOI] [PubMed] [Google Scholar]
- 74.Citarella, R. V. and R. R. Colwell. 1970. Polyphasic taxonomy of the genus Vibro: polynucleotide sequence relationships among selected Vibrio species. J. Bacteriol. 104:434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coelho, A., J. R. C. Andrade, A. C. P. Vicente, and C. A. Salles. 1995. New variant of Vibrio cholerae O1 from clinical isolates in Amazonia. J. Clin. Microbiol. 33:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coenye, T., L. M. Schouls, J. R. W. Govan, K. Kersters, and P. Vandamme. 1999. Identification of Burkholderia species and genomovars from cystic fibrosis patients by AFLP fingerprinting. Int. J. Syst. Bacteriol. 49:1657-1666. [DOI] [PubMed] [Google Scholar]
- 77.Reference deleted.
- 78.Cohan, F. M. 2001. Bacterial species and speciation. Syst. Biol. 50:513-524. [DOI] [PubMed] [Google Scholar]
- 79.Cohan, F. M. 2002. What are bacterial species? Annu. Rev. Microbiol. 56:457-487. [DOI] [PubMed] [Google Scholar]
- 80.Colwell, R. R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274:2025-2031. [DOI] [PubMed] [Google Scholar]
- 81.Colwell, R. R. 1970. Polyphasic taxonomy of the genus Vibrio: numerical taxonomy of Vibrio cholerae, Vibrio parahaemolyticus, and related Vibrio species. J. Bacteriol. 104:410-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colwell, R. R., and D. J. Grimes. 2000. Nonculturable microorganisms. ASM Press, Washington, D.C.
- 83.Cortés, J. 2003. Latin American Coral Reefs. Elsevier, Amsterdam, The Netherlands.
- 84.Cotter, P. A., and V. J. DiRita. 2000. Bacterial virulence gene regulation: an evolutionary perspective. Annu. Rev. Microbiol. 54:519-565. [DOI] [PubMed] [Google Scholar]
- 85.Cottrell, M. T., and D. L. Kirchman. 2000. Community composition of marine bacterioplankton determined by 16S rRNA gene clone libraries and fluorescence in situ hybridization. Appl. Environ. Microbiol. 66:5116-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cottrell, M. T., and D. L. Kirchman. 2000. Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl. Environ. Microbiol. 66:1692-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cottrell, M. T., D. N. Wood, L. Yu, and D. L. Kirchman. 2000. Selected chitinase genes in cultured and uncultured marine bacteria in the alpha- and gamma-subclasses of the proteobacteria. Appl. Environ. Microbiol. 66:1195-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cottrell, M. T., and D. L. Kirchman. 2003. Contribution of major bacterial groups to bacterial biomass production (thymidine and leucine incorporation) in the Delaware estuary. Limnol. Oceanogr. 48:168-178. [Google Scholar]
- 89.Davis, B. R., G. R. Fanning, J. M. Madden, A. G. Steigerwalt, H. B. Bradford, H. L. Smith, and D. J. Brenner. 1981. Characterization of biochemically atypical Vibrio cholerae strains and designation of a new pathogenic species, Vibrio mimicus. J. Clin. Microbiol. 14:631-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis, G. H. G., and W. A. Park. 1962. A taxonomic study of certain bacteria currently classified as Vibrio species. J. Gen. Microbiol. 27:101-119. [DOI] [PubMed] [Google Scholar]
- 91.Daniels, N. A., L. MacKinnon, R. Bishop, S. Altekruse, B. Ray, R. M. Hammond, S. Thompson, S. Wilson, N. H. Bean, P. M. Griffin, and L. Slutsker. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J. Infect. Dis. 181:1661-1666. [DOI] [PubMed] [Google Scholar]
- 92.Daubin, V., N. A. Moran, and H. Ochman. 2003. Phylogenetics and the cohesion of bacterial genomes. Science 301:829-832. [DOI] [PubMed] [Google Scholar]
- 93.Deming, J. W. 1998. Deep ocean environmental biotechnology. Cur. Opin. Biotechnol. 9:283-287. [DOI] [PubMed] [Google Scholar]
- 94.DeLoney-Marino, C. R., A. J. Wolfe, and K. L. Visick. 2003. Chemoattraction of Vibrio fischeri to serine, nucleosides, and N-acetylneuraminic acid, a component of squid light-organ mucus. Appl. Environ. Microbiol. 69:7527-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Denner, E. B. M., D. Vybiral, U. R. Fischer, B. Velimirov, and H. J. Busse. 2002. Vibrio calviensis sp. nov., a halophilic, facultatively oligotrophic 0.2 micron-filterable marine bacterium. Int. J. Syst. Evol. Microbiol. 52:549-553. [DOI] [PubMed] [Google Scholar]
- 96.Diggles, B. K., J. Carson, P. M. Hine, R. W. Hickman, and M. J. Tait. 2000. Vibrio species associated with mortalities in hatchery-reared turbot (Colistium nudipinnis) and brill (C. guntheri) in New Zealand. Aquaculture 183:1-12. [Google Scholar]
- 97.Dijkshoorn, L., K. J. Towner, and M. Struelens. 2001. New approaches for the generation and analysis of microbial typing data. Elsevier, Amsterdam, The Netherlands.
- 98.Dorsch, M., D. Lane, and E. Stackebrandt. 1992. Towards a phylogeny of the genus Vibrio based on 16S rRNA sequences. Int. J. Syst. Bacteriol. 42:58-63. [DOI] [PubMed] [Google Scholar]
- 99.Douillet, P. A. 2000. Bacterial additives that consistently enhance rotifer growth under synxenic culture conditions 2. Use of single and multiple bacterial probiotics. Aquaculture 182:241-248. [Google Scholar]
- 100.Drews, G. 2000. The roots of microbiology and the influence of Ferdinand Cohn on microbiology of the 19th century. FEMS Microbiol. Rev. 24:225-249. [DOI] [PubMed] [Google Scholar]
- 101.Dziejman, M., E. Balon, D. Boyd, C. M. Fraser, J. F. Heidelberg, and J. J. Mekalanos. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. USA 99:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Egan, E. S., and M. K. Waldor. 2003. Distinct replication requirements for the two Vibrio chromosomes. Cell 114:521-530. [DOI] [PubMed] [Google Scholar]
- 103.Eilers, H., J. Pernthaler, F. O. Glockner, and R. Amann. 2000. Culturability and In situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66:3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eilers, H., J. Pernthaler, and R. Amann. 2000. Succession of pelagic marine bacteria during enrichment: a close look at cultivation-induced shifts. Appl. Environ. Microbiol. 66:4634-4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eisen, J. A., J. F. Heidelberg, O. White, and S. L. Salzberg. 2000. Evidence for symmetric chromosomal inversions around the replication origin in bacteria. Gen. Biol. 1:research0011.1-0011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Euzéby, J. P. 1997. List of bacterial names with standing in nomenclature: a folder available on the Internet. Int. J. Syst. Bacteriol. 47:590-592. [DOI] [PubMed] [Google Scholar]
- 107.Ezaki, T., Y. Hashimoto, N. Takeuchi, H. Yamamoto, S. L. Liu, H. Miura, K. Matsui, and E. Yabuuchi. 1988. Simple genetic method to identify viridans group streptococci by colorimetric dot hybridization and fluorometric hybridization in microdilution wells. J. Clin. Microbiol. 26:1708-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ezaki, T., Y. Hashimoto, and E. Yabuuchi. 1989. Fluorometric deoxyribonucleic acid- deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int. J. Syst. Bacteriol. 39:224-229. [Google Scholar]
- 109.Farfán, M., D. Minana, M. C. Fuste, and J. G. Loren. 2000. Genetic relationships between clinical and environmental Vibrio cholerae isolates based on multilocus enzyme electrophoresis. Microbiology 146:2613-2626. [DOI] [PubMed] [Google Scholar]
- 110.Farfán, M., D. Minana-Galbis, M. C. Fuste, and J. G. Loren. 2002. Allelic diversity and population structure in Vibrio cholerae O139 Bengal based on nucleotide sequence analysis. J. Bacteriol. 184:1304-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Farmer, J. J., III. 1980. Revival of the name Vibrio vulnificus. Int. J. Syst. Bacteriol. 30:656. [Google Scholar]
- 112.Farmer, J. J., III. 1986. International Committee on Systematic Bacteriology. Subcommittee on the Taxonomy of Vibrionaceae. Minutes of the meetings. Int. J. Syst. Bacteriol. 39:210-212. [Google Scholar]
- 113.Farmer, J. J., III. 1992. The Family Vibrionaceae, p. 2938-2951. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, and applications, 2nd ed. Springer-Verlag, KG, Berlin, Germany.
- 114.Farmer, J. J., III, and F. W. Hickman-Brenner. 1992. The genera Vibrio and Photobacterium, p. 2952-3011. In A. Balows, H. G. Trüper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, and applications, 2nd ed. Springer-Verlag KG, Berlin, Germany.
- 115.Faruque, S. M., M. J. Albert, and J. J. Mekalanos. 1998. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 62:1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Faruque, S. M., and J. J. Mekalanos. 2003. Pathogenicity islands and phages in Vibrio cholerae evolution. Trends Microbiol. 11:505-510. [DOI] [PubMed] [Google Scholar]
- 117.Faury, N., D. Saulnier, F. L. Thompson, M. Gay, J. Swings, and F. Le Roux. 2004. Vibrio crassostreae sp. nov. isolated from the hemolymph of oysters (Crassostrea gigas). Int. J. Syst. Evol. Microbiol., published online 21 May 2004. DOI 10.1099/ijs. 0.63232-0. [DOI] [PubMed]
- 118.Feil, E. J., and B. G. Spratt. 2001. Recombination and the population structures of bacterial pathogens. Annu. Rev. Microbiol. 55:561-590. [DOI] [PubMed] [Google Scholar]
- 119.Feil, E. J. 2004. Small change: keeping pace with microevolution. Nat. Rev. Microbiol. 12:483-495. [DOI] [PubMed] [Google Scholar]
- 120.Fidopiastis, P. M., S. von Boletzky, and E. G. Ruby. 1998. A new niche for Vibrio logei, the predominant light organ symbiont of squids in the genus Sepiola. J. Bacteriol. 180:59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fidopiastis, P. M., C. M. Miyamoto, M. G. Jobling, E. A. Meighen, and E. G. Ruby. 2002. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 45:131-143. [DOI] [PubMed] [Google Scholar]
- 122.Finkelstein, R., S. Edelstein, and G. Mahamid. 2002. Fulminant wound infections due to Vibrio vulnificus. Isr. Med. Assoc. J. 4:654-655. [PubMed] [Google Scholar]
- 123.Foale, S., and W. R. Day. 1992. Recognizability of algae ingested by abalone. Aust. J. Mar. Freshwater Res. 43:1331-1338. [Google Scholar]
- 124.Fouz, B., M. D. Esteve-Gassent, R. Barrera, J. L. Larsen, M. E. Nielsen, and C. Amaro. 2001. Field testing of a vaccine against eel diseases caused by Vibrio vulnificus. Dis. Aquat. Org. 45:183-189. [DOI] [PubMed] [Google Scholar]
- 125.Fujino, T., R. Sakazaki, and K. Tamura. 1974. Designation of the type strains of Vibrio parahaemolyticus and description of 200 strains of the species. Int. J. Syst. Bacteriol. 24:447-449. [Google Scholar]
- 126.Fukasawa, S., and P. Dunlap. 1986. Identification of luminous bacteria isolated from the light organ of the squid Doryteuths kensaki. Agric. Biol. Chem. 50:1645-1646. [Google Scholar]
- 127.Fukushima, H., Y. Tsunomori, and R. Seki. 2003. duplex real-time SYBR Green PCR assays for dectetion of 17 species of food- or waterborne pathogens in stools. J. Clin. Microbiol. 41:5134-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garg, P., A. Aydanian, D. Smith, J. G. Morris, G. B. Nair, and O. C. Stine. 2003. Molecular epidemiology of O139 Vibrio cholerae: mutation, lateral gene transfer, and founder flush. Emerg. Infect. Dis. 9:810-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Garrity, G. M., and J. H. Holt. 2000. A road map to the manual, p. 119-166. In D. R. Boone, R. W. Castenholz, and G. M. Garrity (ed.), Bergey's manual of systematic bacteriology, 2nd ed., vol. 1. Springer-Verlag KG, Berlin, Germany.
- 130.Gevers, D., K. Vandepoele, C. Simillion, and Y. Van de Peer. 2004. Gene duplication and biased functional retention of paralogs in bacterial genomes. Trends Microbiol. 12:148-154. [DOI] [PubMed] [Google Scholar]
- 131.Giovannoni, S. and M. Rappé. 2000. Evolution, diversity, and molecular ecology of marine prokaryotes, p. 47-84. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, London, United Kingdom.
- 132.Goldburg, R. J., M. S. Elliott, and R. L. Nayor. 2003. Marine aquaculture in The United States. Environmental impacts and policy options. Pew Oceans Commission report. Pan Ocean Commission, Arlington, Va.
- 133.Gomez-Gil, B., A. Roque, J. F. Turnbull, and L. Tron-Mayen. 1998. Species of Vibrio isolated from hepatopancreas, haemolymph and digestive tract of a population of healthy juvenile Penaeus vannamei. Aquaculture 163:1-9. [Google Scholar]
- 134.Gomez-Gil, B., A. Roque, and J. F. Turnbull. 2000. The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture 191:259-270. [Google Scholar]
- 135.Gomez-Gil, B., A. Roque, and G. Velasco-Blanco. 2002. Culture of Vibrio alginolyticus C7b, a potential probiotic bacterium, with the microalga Chaetoceros muelleri. Aquaculture 211:43-48. [Google Scholar]
- 136.Gomez-Gil, B., F. L. Thompson, C. C. Thompson, and J. Swings. 2003. Vibrio rotiferianus sp. nov., isolated from cultures of the rotifer Brachionus plicatilis. Int. J. Syst. Evol. Microbiol. 53:239-243. [DOI] [PubMed] [Google Scholar]
- 137.Gomez-Gil, B., F. L. Thompson, C. C. Thompson, and J. Swings. 2003. Vibrio pacinii sp nov., from cultured aquatic organisms. Int. J. Syst. Evol. Microbiol. 53:1569-1573. [DOI] [PubMed] [Google Scholar]
- 138.Gomez-Gil, B., F. L. Thompson, C. C. Thompson, A. Garcia-Gasca, A. Roque, and J. Swings. 2004. Vibrio hispanicus sp. nov., isolated from Artemia sp. and sea water in Spain. Int. J. Syst. Evol. Microbiol. 54:261-265. [DOI] [PubMed] [Google Scholar]
- 139.Gomez-Gil, B., S. Soto-Rodríguez, A. García-Gasca, A. Roque, R. Vazquez-Juarez, F. L. Thompson, and J. Swings. 2004. Molecular identification of Vibrio harveyi-related isolates associated with diseased aquatic organisms. Microbiology 150:1769-1777. [DOI] [PubMed] [Google Scholar]
- 140.Goodfellow, M. 2000. Microbiol systematics: background and uses, p. 1-18. In F. G. Priest and M. Goodfellow (ed.), Applied microbial systematics. Kluwer Academic Publishers. Dordrecht, The Netherlands.
- 141.Gogarten, J. P., W. F. Doolittle, and J. G. Lawrence. 2002. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 19:2226-2238. [DOI] [PubMed] [Google Scholar]
- 142.Goris, J., K. Suzuki, P. De Vos, T. Nakase, and K. Kersters. 1998. Evaluation of a microplate DNA-DNA hybridization method compared with the initial renaturation method. Can. J. Microbiol. 44:1148-1153. [Google Scholar]
- 143.Grimont, F., and P. A. D. Grimont. 1986. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann. Inst. Pasteur. Microbiol. 137B:165-175. [DOI] [PubMed] [Google Scholar]
- 144.Grimont, P. A. D., and F. Grimont. 2001. rRNA gene restriction pattern determination (Ribotyping) and computer interpretation, p. 107-133. In L. Dijkshoorn, K. J. Towner, and M. Struelens (ed.). New approaches for the generation and analysis of microbial typing data. Elsevier, Amsterdam, The Netherlands.
- 145.Grisez, L., M. Chair, P. Sorgeloos, and F. Ollevier. 1996. Mode of infection and spread of Vibrio anguillarum in turbot Scophthalmus maximus larvae after oral challenge through live feed. Dis. Aquat. Org. 26:181-187. [Google Scholar]
- 146.Grisez, L., J. Reyniers, L. Verdonck, J. Swings, and F. Ollevier. 1997. Dominant intestinal microflora of sea bream and sea bass larvae from two hatcheries, during larval development. Aquaculture 155:387-399. [Google Scholar]
- 147.Groisman, E. A., and H. Ochman. 1996. Pathogenicity islands: bacterial evolution in quantum leaps. Cell 87:791-794. [DOI] [PubMed] [Google Scholar]
- 148.Gurtler, V. and B. C. Mayall. 2001. Genomic approaches to typing, taxonomy and evolution of bacterial isolates. Int. J. Syst. Evol. Microbiol. 51:3-16. [DOI] [PubMed] [Google Scholar]
- 149.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 150.Hacker, J., and E. Carniel. 2001. Ecological fitness, genomic islands and bacterial pathogenicity. EMBO Rep. 2:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hacker, J., U. Hentschel, and U. Dobrindt. 2003. Prokaryotic chromosomes and disease. Science 301:790-793. [DOI] [PubMed] [Google Scholar]
- 152.Hada, H. S., P. A. West, J. V. Lee, J. Stemmler, and R. R. Colwell. 1984. Vibrio tubiashii sp. nov., a pathogen of bivalve mollusks. Int. J. Syst. Bacteriol. 34:1-4. [Google Scholar]
- 153.Hagström, A. T. Pommier, F. Rohwer, K. Simu, W. Stolte, D. Svensson, and U. Zweifel. 2002. Use of 16S ribosomal DNA for delineation of marine bacterioplankton species. Appl. Env. Microbiol. 68:3628-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hameed, A. S. S., and G. Balasubramanian. 2000. Antibiotic resistance in bacteria isolated from Artemia nauplii and efficacy of formaldehyde. Aquaculture 183:195-205. [Google Scholar]
- 155.Hammer, B. K. and B. L. Bassler. 2003. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50:101-114. [DOI] [PubMed] [Google Scholar]
- 156.Hang, L., M. John, M. Asaduzzaman, E. A. Bridges, C. Vanderspurt, T. J. Kim, R. K. Taylor, J. D. Hillman, A. Progulske-Fox, M. Handfield, E. T. Ryan, and S. B. Calderwood. 2003. Use of in vivo-induced antigen technology (IVIAT) to identify genes uniquely expressed during human infection with Vibrio cholerae. Proc. Natl. Acad. Sci. USA 100:8508-8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hansen, G. H., and J. A. Olafsen. 1999. Bacterial interactions in early life stages of marine cold water fish. Microb. Ecol. 38:1-26. [DOI] [PubMed] [Google Scholar]
- 158.Harris, L., L. Owens, and S. Smith. 1996. A selective and differential medium for Vibrio harveyi. Appl. Environ. Microbiol. 62:3548-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hauben, L., L. Vauterin, E. R. B. Moore, B. Hoste, and J. Swings. 1999. Genomic diversity of the genus Stenotrophomonas. Int. J. Syst. Bacteriol. 49:1749-1760. [DOI] [PubMed] [Google Scholar]
- 160.Hayashi, K., J. Moriwaki, T. Sawabe, F. L. Thompson, J. Swings, N. Gudkovs, R. Christen, and Y. Ezura. 2003. Vibrio superstes sp nov., isolated from the gut of Australian abalones Haliotis laevigata and Haliotis rubra. Int. J. Syst. Evol. Microbiol. 53:1813-1817. [DOI] [PubMed] [Google Scholar]
- 161.Hedlund, B. P. and J. T. Staley. 2001. Vibrio cyclotrophicus sp. nov., a polycyclic aromatic hydrocarbon (PAH)-degrading marine bacterium. Int. J. Syst. Evol. Microbiol. 51:61-66. [DOI] [PubMed] [Google Scholar]
- 162.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I, Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Bacteria of the gamma-subclass Proteobacteria associated with zooplankton in Chesapeake Bay. Appl. Environ. Microbiol. 68:5498-5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Heidelberg, J. F., K. B. Heidelberg, and R. R. Colwell. 2002. Seasonality of Chesapeake Bay bacterioplankton species. Appl. Environ. Microbiol. 68:5488-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Henke, J. M., and B. L. Bassler. 2004. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 186:3794-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hickman, F. W., J. J. Farmer III, D. G. Hollis, G. R. Fanning, A. G. Steigerwalt, R. E. Weaver, and D. J. Brenner. 1982. Identification of Vibrio hollisae sp. nov. from patients with diarrhea. J. Clin. Microbiol. 15:395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hidaka, T., and M. Sakai. 1968. Comparative observation of inorganic salt requirement of the marine and terrestrial bacteria. In H. Kadota and N. Taga (ed.), Proceedings of U.S.A. and Japan Seminar on Marine Microbiology. Bull. Misaki Mar. Biol. Inst. Kyoto Univ. 12:125-149. [Google Scholar]
- 168.Hjeltnes, B., and R. J. Roberts. 1993. Vibriosis, p. 109-121. In R. J. Roberts, N. R. Bromage, and V. Inglis (ed.). Bacterial diseases of fish. Blackwell Scientific, Oxford, United Kingdom.
- 169.Hoegh-Guldberg, O. 1999. Climate change, coral bleaching and the future of the world's coral reefs. Mar. Freshwater Res. 50:839-866. [Google Scholar]
- 170.Holt. J. G., and N. R. Krieg. 1994. Bergey's manual of determinative microbiology, 9th ed. p. 190-274. The Williams & Wilkins Co., Baltimore, Md.
- 171.Honda, T., and T. Iida. 1993. The pathogenicity of Vibrio parahaemolyticus and the role of the thermostable direct haemolysin and related haemolysins. Rev. Med. Microbiol. 4:106-113. [Google Scholar]
- 172.Honma, Y., M. Ikema, C. Toma, M. Ehara, and M. Iwanaga. 1997. Molecular analysis of a filamentous phage (fs1) of Vibrio cholerae O139. Biochim. Biophys. Acta 1362:109-115. [DOI] [PubMed] [Google Scholar]
- 173.Hooper, L. K., and J. I. Gordon. 2001. Commensal host-bacterial relationships in the gut. Science 292:1115-1118. [DOI] [PubMed] [Google Scholar]
- 174.Huber, K. E., and M. K. Waldor. 2002. Filamentous phage integration requires the host recombinases XerC and XerD. Nature 417:656-659. [DOI] [PubMed] [Google Scholar]
- 175.Hueck, C. J. 1998. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev. 62:379-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Huys, G., R. Coopman, P. Janssen, and K. Kersters. 1996. High-resolution genotypic analysis of the genus Aeromonas by AFLP fingerprinting. Int. J. Syst. Bacteriol. 46:572-580. [DOI] [PubMed] [Google Scholar]
- 177.Huys, G., and J. Swings. 1999. Evaluation of a fluorescent amplified fragment length polymorphism methodology for the genotypic discrimination of Aeromonas taxa. FEMS Microbiol. Lett. 177:83-92. [Google Scholar]
- 178.Huys, G., D. Gevers, R. Temmerman, M. Cnockaert, R. Denys, G. Rhodes, R. Pickup, P. McGann, M. Hiney, P. Smith, and J. Swings. 2001. Comparison of the antimicrobial tolerance of oxytetracycline-resistant heterotrophic bacteria isolated from hospital sewage and freshwater fishfarm water in Belgium. Syst. Appl. Microbiol. 24:122-130. [DOI] [PubMed] [Google Scholar]
- 179.Huys, L., P. Dhert, R. Robles, F. Ollevier, P. Sorgeloos, and J. Swings. 2001. Search for beneficial bacterial strains for turbot (Scophthalmus maximus L.) larviculture. Aquaculture 193:25-37. [Google Scholar]
- 180.Iida, T., K. Makino, H. Nasu, K. Yokoyama, K. Tagomori, A. Hattori, T. Okuno, H. Shinagawa, and T. Honda. 2002. Filamentous bacteriophages of vibrios are integrated into the dif-like site of the host chromosome. J. Bacteriol. 184:4933-4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Iida, T., K. S. Park, O. Suthienkul, J. Kozawa, Y. Yamaichi, K. Yamamoto, and T. Honda. 1998. Close proximity of the tdh, trh and ure genes on the chromosome of Vibrio parahaemolyticus. Microbiology 144:2517-2523. [DOI] [PubMed] [Google Scholar]
- 182.Iida, T., O. Suthienkul, K. S. Park, G. Q. Tang, R. K. Yamamoto, M. Ishibashi, K. Yamamoto, and T. Honda. 1997. Evidence for genetic linkage between the ure and trh genes in Vibrio parahaemolyticus. J. Med. Microbiol. 46:639-645. [DOI] [PubMed] [Google Scholar]
- 183.Ikema, M., and Y. Honma. 1998. A novel filamentous phage, fs2, of Vibrio cholerae O139. Microbiology 144:1901-1906. [DOI] [PubMed] [Google Scholar]
- 184.Ishimaru, K., M. Akagawa-Matsushita, and K. Muroga. 1995. Vibrio penaeicida sp. nov., a pathogen of the kuruma prawns (Penaeus japonicus) Int. J. Syst. Bacteriol. 45:134-138. [Google Scholar]
- 185.Ishimaru, K., M. Akagawa-Matsushita, and K. Muroga. 1996. Vibrio ichthyoenteri sp. nov., a pathogen of Japanese flounder (Paralichthys olivaceus) larvae. Int. J. Syst. Bacteriol. 46:155-159. [Google Scholar]
- 186.Islam, M. S., S. Mahmuda, M. G. Morshed, H. B. M. Bakht, M. N. H. Khan, R. B. Sack, and D. A. Sack. 2004. Role of cyanobacteria in the persistence of Vibrio choterae O139 in saline microcosms. Can. J. Microbiol. 50:127-131. [DOI] [PubMed] [Google Scholar]
- 187.Iwamoto, Y., Y. Suzukki, A. Kurita, Y. Watanabe, T. Shimizu, H. Ohgami, and Y. Yanagihara. 1995. Vibrio trachuri sp. nov., a new species isolated from diseased Japanese horse mackerel. Microbiol. Immunol. 39:831-837. [DOI] [PubMed] [Google Scholar]
- 188.Janssen, P., R. Coopman, G. Huys, J. Swings, M. Bleeker, P. Vos, M. Zabeau, and K. Kersters. 1996. Evaluation of the DNA fingerprinting method AFLP as an new tool in bacterial taxonomy. Microbiology 142:1881-1893. [DOI] [PubMed] [Google Scholar]
- 189.Janssen, P., K. Maquelin, R. Coopman, I. Tjernberg, P. Bouvet, K. Kersters, and L. Dijkshoorn. 1997. Discrimination of Acinetobacter genomic species by AFLP fingerprinting. Int. J. Syst. Bacteriol. 47:1179-1187. [DOI] [PubMed] [Google Scholar]
- 190.Janssen, P. J. D. 2001. Selective restriction fragment amplification by AFLP, p. 177-210. In L. Dijkshoorn, K. J. Towner and M. Struelens (ed.). New approaches for the generation and analysis of microbial typing data. Elsevier, Amsterdam, The Netherlands.
- 191.Jiang, S. C., V. Louis, N. Choopun, A. Sharma, A. Huq, and R. R. Colwell. 2000. Genetic diversity of Vibrio cholerae in Chesapeake Bay determined by amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 66:140-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jiang, S. C., M. Matte, G. Matte, A, Huq, and R. R. Colwell. 2000. Genetic diversity of clinical and environmental isolates of Vibrio cholerae determined by amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 66:148-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jobson, J. D. 1996. Applied multivariate data analysis: categorical and multivariate methods, vol. 1. Springer-Verlag KG, Berlin, Germany.
- 194.Johnson, F. H. and I. V. Shunk. 1936. An interesting new species of luminous bacteria. J. Bacteriol. 31:585-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Joseph, S. W., R. R. Colwell, and J. B. Kaper. 1982. Vibrio parahaemolyticus and related halophilic vibrios. Crit. Rev. Microbiol. 10:77-124. [DOI] [PubMed] [Google Scholar]
- 196.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. H. Munro (ed.), Mammalian protein metabolism. Academic Press Ltd., London, United Kingdom.
- 197.Jumas-Bilak, E., S. Michaux-Charachon, G. Bourg, D. O'Callaghan, and M. Ramuz. 1998. Differences in chromosome number and genome rearrangements in the genus Brucella. Mol. Microbiol. 27:99-106. [DOI] [PubMed] [Google Scholar]
- 198.Kachlany, S. C., P. J. Planet, M. K. Bhattacharjee, E. Kollia, R. DeSalle, D. H. Fine, and D. H. Figurski. 2000. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in bacteria and archaea. J. Bacteriol. 182:6169-6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kalina, G. P., A. S. Antonov, T. P. Turova, and T. I. Grafova. 1984. Allomonas enterica gen. nov., sp. nov., deoxyribonucleic-acid homology between Allomonas and some other members of the Vibrionaceae. Int. J. Syst. Bacteriol. 34:150-154. [Google Scholar]
- 200.Kaper, J. B., and J. Hacker. 1999. Pathogenicity islands and other mobile virulence elements. ASM Press, Washington D.C.
- 201.Kandler, O. 1985. Evolution of systematics of bacteria, p. 335-361. In K. H. Schleifer, and E. E. Stackebrandt (ed.), Evolution of prokaryotes. Academic Press, London, United Kingdom.
- 202.Kar, S., R. K. Ghosh, A. N. Ghosh, and A. Ghosh. 1996. Integration of the DNA of a novel filamentous bacteriophage VSK from Vibrio cholerae O139 into the host chromosomal DNA. FEMS Microbiol. Lett. 145:17-22. [DOI] [PubMed] [Google Scholar]
- 203.Karaolis, D. K., R. Lan, and P. R. Reeves. 1994. Molecular evolution of the seventh-pandemic clone of Vibrio cholerae and its relationship to other pandemic and epidemic V. cholerae isolates. J. Bacteriol. 176:6199-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Karaolis, D. K., R. Lan, and P. R. Reeves. 1995. The sixth and seventh cholera pandemics are due to independent clones separately derived from environmental, nontoxigenic, non-O1 Vibrio cholerae. J. Bacteriol. 177:3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Karaolis, D. K. R., J. A. Johnson, C. C. Bailey, E. C. Boedeker, J. B. Kaper, and P. R. Reeves. 1998. A Vibrio cholerae pathogenicity island associated with epidemic and pandemic strains. Proc. Natl. Acad. Sci. USA 95:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kersters, K., P. Vos, M. Gillis, J. Swings, P. Vandamme, and E. Stackebrandt. 2003. Introduction to the Proteobacteria. In A. Balows, M. Dworkin, W. Hardes, K. H. Schleifer, and H. G. Trüper (ed.), The prokaryotes. An electronic handbook on the biology of bacteria: ecophysiology, isolation, identification, and applications. Springer-Verlag KG, Berlin, Germany.
- 207.Kirkpatrick, B. D., W. K. Alston. 2003. Current immunizations for travel. Curr. Opin. Infect. Dis. 16:369-374. [DOI] [PubMed] [Google Scholar]
- 208.Kita-Tsukamoto, K., H. Oyaizu, K. Nanba, and U. Simidu. 1993. Phylogenetic relationships of marine bacteria, mainly members of the family Vibrionaceae, determined on the basis of 16S rRNA sequences. Int. J. Syst. Bacteriol. 43:8-19. [DOI] [PubMed] [Google Scholar]
- 209.Klingler, J. M., R. P. Stowe, D. C. Obenhuber, T. O. Groves, S. K. Mishra and D. L. Pierson. 1992. Evaluation of the Biolog automated microbial identification system. Appl. Environ. Microbiol. 58:2089-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Krieg, N. R., and J. G. Holt. 1984. Bergey's manual of systematic bacteriology, p. 516-548. The Williams & Wilkins Co., Baltimore, Md.
- 211.Kurland, C. G., B. Canback, and O. G. Berg. 2003. Horizontal gene transfer: a critical view. Proc. Natl. Acad. Sci. USA 100:9658-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kushmaro, A., Y. Loya, and E. Rosenberg. 1996. Bacterial infection and coral bleaching. Nature 380:396. [Google Scholar]
- 213.Kushmaro, A., E. Rosenberg, M. Fine, and Y. Loya. 1997. Bleaching of the coral Oculina patagonica by Vibrio AK-1. Mar. Ecol. Prog. Ser. 147:159-165. [Google Scholar]
- 214.Kushmaro, A., E. Rosenberg, M. Fine, Y. Ben-Haim, and Y. Loya. 1998. Effect of temperature on bleaching of the coral Oculina patagonica by Vibrio AK-1. Mar. Ecol. Prog. Ser. 171:131-137. [Google Scholar]
- 215.Kushmaro, A., E. Banin, Y. Loya, E. Stackebrandt, and E. Rosenberg. 2001. Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int. J. Syst. Evol. Microbiol. 51:1-6. [DOI] [PubMed] [Google Scholar]
- 216.Lambert, M. A., F. W. Hickman-Brenner, J. J. Farmer III, and W. Moss. 1983. Differentiation of Vibrionaceae species by their cellular fatty acid composition. Int. J. Syst. Bacteriol. 33:777-792. [Google Scholar]
- 217.Lambert, C., J. L. Nicolas, V. Cilia, and S. Corre. 1998. Vibrio pectenicida sp. nov., a pathogen of scallop (Pecten maximus) larvae. Int. J. Syst. Bacteriol. 48:481-487. [DOI] [PubMed] [Google Scholar]
- 218.Lan, R., and P. R. Reeves. 1998. Recombination between rRNA operons created most of the ribotype variation observed in the seventh pandemic clone of Vibrio cholerae. Microbiology 144:1213-1221. [DOI] [PubMed] [Google Scholar]
- 219.Lan, R., and P. R. Reeves. 2000. Intraspecies variation in bacterial genomes: the need for a species genome concept. Trends Microbiol. 8:396-401. [DOI] [PubMed] [Google Scholar]
- 220.Lan, R., and P. R. Reeves. 2001. When does a clone deserve a name? A perspective on bacterial species based on population genetics. Trends Microbiol. 9:419-424. [DOI] [PubMed] [Google Scholar]
- 221.Lan, R., and P. R. Reeves. 2002. Pandemic spread of cholera: genetic diversity and relationships within the seventh pandemic clone of Vibrio cholerae determined by amplified fragment length polymorphism. J. Clin. Microbiol. 40:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lanyi, B. 1987. Classical and rapid identification methods for medically important bacteria, p. 1-67. In R. R. Colwell and R. Grigorova (ed.), Current methods for classification and identification of microorganisms. Academic Press, Ltd., London, United Kingdom.
- 223.Larsen, M. H., and H. T. Boesen. 2001. Role of flagellum and chemotactic motility of Vibrio anguillarum for phagocytosis by and intracellular survival in fish macrophages. FEMS Microbiol. Lett. 203:149-152. [DOI] [PubMed] [Google Scholar]
- 224.Larsen, M. H., J. L. Larsen, and J. E. Olsen. 2001. Chemotaxis of Vibrio anguillarum to fish mucus: role of the origin of the fish mucus, the fish species and the serogroup of the pathogen. FEMS Microbiol. Ecol. 38:77-80. [Google Scholar]
- 225.Lavilla-Pitogo, C. R., and L. D. de la Pena. 1998. Mortalities of pond-cultured juvenile shrimp, Penaeus monodon, associated with dominance of luminescent vibrios in the rearing environment. Aquaculture 164:337-349. [Google Scholar]
- 226.Lawrence, J. G. 2002. Gene transfer in bacteria: speciation without species? Theor. Popul. Biol. 61:449-460. [DOI] [PubMed] [Google Scholar]
- 227.Leano, E. M., C. R. Lavilla-Pitogo, and M. G. Paner. 1998. Bacterial flora in the hepatopancreas of pond-reared Penaeus monodon juveniles with luminous vibriosis. Aquaculture 164:367-374. [Google Scholar]
- 228.Leão, Z. M.A. N., R. K. Kikuchi, and V. Testa. 2003. Corals and coral reefs of Brazil, p. 9-52. In J. Cortés (ed.) Latin American coral reefs. Elsevier, Amsterdam, The Netherlands.
- 229.Lee, J. V., P. Shread, A. L. Furniss, and T. N. Bryant. 1981. Taxonomy and description of Vibrio fluvialis sp. nov. (synonym group F vibrios, group EF6). J. Appl. Bacteriol. 50:73-94. [DOI] [PubMed] [Google Scholar]
- 230.Lee, K. K., S. R. Yu, F. R. Chen, T. I. Yang, and P. C. Liu. 1996. Virulence of Vibrio alginolyticus isolated from diseased tiger prawn, Penaeus monodon. Curr. Microbiol. 32:229-231. [DOI] [PubMed] [Google Scholar]
- 231.Lee, S. H., and A. Camilli. 2000. Novel approaches to monitor bacterial gene expression in infected tissue and host. Curr. Opin. Microbiol. 3:97-101. [DOI] [PubMed] [Google Scholar]
- 232.Lerat, E., V. Daubin, and N. A. Moran. 2003. From gene trees to organismal phylogeny in prokaryotes: the case of the γ-Proteobacteria. PLOS Biol. 1:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Le Roux, F., M. Gay, C. Lambert, M. Waechter, S. Poubalanne, B. Chollet, J. L. Nicolas, and F. Berthe. 2002. Comparative analysis of Vibrio splendidus-related strains isolated during Crassostrea gigas mortality events. Aquat. Living Res. 15:251-258. [Google Scholar]
- 234.Li, M., T. Shimada, J. G. Morris, A. Sulakvelidze, and S. Sozhamannan. 2002. Evidence for the emergence of non-O1 and non-O139 Vibrio cholerae strains with pathogenic potential by exchange of O-antigen biosynthesis regions. J. Clin. Microbiol. 70:2441-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li, Y., W. T. Yie-Jun, M. L. Foo-Rita, L. Julia, H. Xu, and Y. S. Woo-Norman. 1999. Antibiotic resistance and plasmid profiles of vibrio isolates from cultured Sparus sarba. Mar. Poll. Bull. 39:245-249. [PubMed] [Google Scholar]
- 236.Lightner, D. V., and R. M. Redman. 1998. Shrimp diseases and current diagnostic methods. Aquaculture 164:201-220. [Google Scholar]
- 237.Lilley, B. N., and B. L. Bassler. 2000. Regulation of quorum sensing in Vibrio harveyi by LuxO and sigma-54. Mol. Microbiol. 36:940-954. [DOI] [PubMed] [Google Scholar]
- 238.Lipp, E. K., A. Huq, and R. R. Colwell. 2002. Effects of global climate on infectious disease: the cholera model. Clin. Microbiol. Rev. 15:757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Liu, S. L., and K. E. Sanderson. 1996. Highly plastic chromosomal organization in Salmonella typhi. Proc. Natl. Acad. Sci. USA 93:10303-10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Long, R. A., and F. Azam. 2001. Antagonistic interactions among marine pelagic bacteria. Appl. Environ. Microbiol. 67:4975-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Loya, Y., K. Sakai, K. Yamazato, Y. Nakano, H. Sambali, and R. van Woesik. 2001. Coral bleaching: the winners and the losers. Ecol. Lett. 4:122-131. [Google Scholar]
- 242.Lyon, W. J. 2001. TaqMan PCR for detection of V. cholerae O1, O139, non-O1, and non- O139 in pure cultures, raw oysters, and synthetic seawater. Appl. Environ. Microbiol. 67:4685-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.MacDonell, M. T., and R. R. Colwell. 1985. Phylogeny of the Vibrionaceae, and recommendation of two new genera, Listonella and Shewanella. Syst. Appl. Microbiol. 6:171-182. [Google Scholar]
- 244.Macián, M. C., E. Garay, and M. J. Pujalte. 1996. The arginine dihydrolase (ADH) system in the identification of some marine Vibrio species. Syst. Appl. Microbiol. 19:451-456. [Google Scholar]
- 245.Macián, M. C., E. Garay, F. Gonzalez-Candelas, M. J. Pujalte, and R. Aznar. 2000. Ribotyping of Vibrio populations associated with cultured oysters (Ostrea edulis). Syst. Appl. Microbiol. 23:409-417. [DOI] [PubMed] [Google Scholar]
- 246.Macián, M. C., W. Ludwig, K. H. Schleifer, E. Garay, and M J. Pujalte. 2000. Vibrio pelagius: differences of the type strain deposited at various culture collections. Syst. Appl. Microbiol. 23:373-375. [DOI] [PubMed] [Google Scholar]
- 247.Macián, M. C., W. Ludwig, R. Aznar, P. D. A. Grimont, K. H. Schleifer, E. Garay, and M. J. Pujalte. 2001. Vibrio lentus sp. nov., isolated from Mediterranean oysters. Int. J. Syst. Evol. Microbiol. 51:1449-1456. [DOI] [PubMed] [Google Scholar]
- 248.Macián, M. C., W. Ludwig, K. H. Schleifer, M. J. Pujalte, and E. Garay. 2001. Vibrio agarivorans sp nov., a novel agarolytic marine bacterium. Int. J. Syst. Evol. Microbiol. 51:2031-2036. [DOI] [PubMed] [Google Scholar]
- 249.Maidak, B. L., J. R. Cole, C. T. Parker, G. M. Garrity, N. Larsen, B. Li, T. G. Lilburn, M. J. McCaughey, G. J. Olsen, R. Overbeek, S. Pramanik, T. M. Schmidt, J. M. Tiedje, and C. R. Woese. 1999. A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res. 27:171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Maiden, M. C., J. A. Bygraves, E. Feil, G. Morelli, J. E. Russell, R. Urwin, Q. Zhang, J. Zhou, K. Zurth, D. A. Caugant, I. M. Feavers, M. Achtman, and B. G. Spratt. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. USA 95:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori., and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 252.Manefield, M., L. Harris, S. A. Rice, R. de Nys, and S. Kjelleberg. 2000. Inhibition of luminescence and virulence in the black tiger prawn (Penaeus monodon) pathogen Vibrio harveyi by intercellular signal antagonists. Appl. Environ. Microbiol. 66:2079-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Martin, Y., J. L. Bonnefont, and L. Chancerelle. 2002. Gorgonians mass mortality during the 1999 late summer in French Mediterranean coastal waters: the bacterial hypothesis. Water Res. 36:779-782. [DOI] [PubMed] [Google Scholar]
- 254.Martínez-Picado, J., M. Alsina, A. R. Blanch, M. Cerda, and J. Jofre. 1996. Species-specific detection of Vibrio anguillarum in marine aquaculture environments by selective culture and DNA hybridization. Appl. Environ. Microbiol. 62:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Maynard Smith, J. M., N. H. Smith, M. O'Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Maynard Smith, J. M., E. J. Feil, and N. H. Smith. 2000. Population structure and evolutionary dynamics of pathogenic bacteria. Bioessays 22:1115-1122. [DOI] [PubMed] [Google Scholar]
- 257.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 1998. A distinctive class of integron in the Vibrio cholerae genome. Science 280:605-608. [DOI] [PubMed] [Google Scholar]
- 258.McDougald, D., S. A. Rice, D. Weichart, and S. Kjelleberg. 1998. Nonculturability: adaptation or debilitation? FEMS Microbiol. Ecol. 25:1-9. [Google Scholar]
- 259.McDougald, D., S. A. Rice, and S. Kjelleberg. 2000. The marine pathogen Vibrio vulnificus encodes a putative homologue of the Vibrio harveyi regulatory gene, luxR: a genetic and phylogenetic comparison. Gene 248:213-221. [DOI] [PubMed] [Google Scholar]
- 260.McFall-Ngai, M. 1999. Consequences of evolving with bacterial symbionts: insights from the squid-Vibrio associations. Annu. Rev. Ecol. Syst. 30:235-256. [Google Scholar]
- 261.McFall-Ngai, M. J. 2002. Unseen forces: the influence of bacteria on animal development. Devel. Biol. 242:1-14. [DOI] [PubMed] [Google Scholar]
- 262.Mellado, E., E. R. Moore, J. J. Nieto, and A. Ventosa. 1996. Analysis of 16S rRNA gene sequences of Vibrio costicola strains: description of Salinivibrio costicola gen. nov., comb. nov. Int. J. Syst. Bacteriol. 46:817-821. [DOI] [PubMed] [Google Scholar]
- 263.Merkel, S. M., S. Alexander, E. Zufall, J. D. Oliver, and Y. M. Huet-Hudson. 2001. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect. Immun. 69:6119-6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Merrell, D. S., S. M. Butler, F. Qadri, N. A. Dolganov, A. Alam, M. B. Cohen, S. B. Calderwood, G. K. Schoolnik, and A. Camilli. 2002. Host-induced epidemic spread of the cholera bacterium. Nature 417:642-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Millikan, D. S. and E. G. Ruby. 2002. Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects. Appl. Environ. Microbiol. 68:2519-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Miller, J. M., and D. L. Rhoden. 1991. Preliminary evaluation of Biolog, a carbon source utilization method for bacterial identification. J. Clin. Microbiol. 29:1143-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 268.Milton, D. L., A. Hardman, M. Camara, S. R. Chhabra, B. W. Bycroft, G. S. Stewart, and P. Williams. 1997. Quorum sensing in Vibrio anguillarum: characterization of the vanI/vanR locus and identification of the autoinducer N-(3-oxodecanoyl)-l-homoserine lactone. J. Bacteriol. 179:3004-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mintz, E. D., T. Popovic, and P. A. Blake. 1994. Transmission of Vibrio cholerae O1, p. 345-356. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera. Molecular to global perspectivas. ASM Press, Washington, D.C.
- 270.Miyoshi, S. I., A. Morita, T. Teranishi, K. I. Tomochika, S. Yamamoto, and S. Shinoda. 2004. An exocellular cytolysin produced by Vibrio vulnificus CDC B3547, a clinical isolate in biotype 2 (Serovar E). J. Toxicol. Toxin Rev. 23:111-121. [Google Scholar]
- 271.Molina-Aja, A., A. Garcia-Gasca, A. Abreu-Grobois, C. Bolan-Mejia, A. Roque, and B. Gomez-Gil. 2002. Plasmid profiling and antibiotic resistance of Vibrio strains isolated from cultured penaeid shrimp. FEMS Microbiol. Lett. 213:7-12. [DOI] [PubMed] [Google Scholar]
- 272.Mollet, C., M. Drancourt, and D. Raoult. 1997. rpoB sequence analysis as a novel basis for bacterial identification. Mol. Microbiol. 26:1005-1011. [DOI] [PubMed] [Google Scholar]
- 273.Moorthy, S., and P. I. Watnick. 2004. Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol. Microbiol. 52:573-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Moss, S. M., B. R. LeaMaster, and J.N. Sweeney. 2000. Relative abundance and species composition of gram-negative, aerobic bacteria associated with the gut of juvenile white shrimp Litopenaeus vannamei reared in oligotrophic well water and eutrophic pond water. J. World Aquacult. Soc. 31:255-263. [Google Scholar]
- 275.Mougel, C., J. Thioulouse, G. Perriere, and X. Nesme. 2002. A mathematical method for determining genome divergence and species delineation using AFLP. Int. J. Syst. Evol. Microbiol. 52:573-586. [DOI] [PubMed] [Google Scholar]
- 276.Munro, P. D., A. Barbour, and T. H. Birkbeck. 1994. Comparison of gut bacterial flora of start-feeding larval turbot under different conditions. J. Appl. Bacteriol. 77:560-566. [Google Scholar]
- 277.Muniesa-Pérez, M., J. Jofre, and A. R. Blanch. 1996. Identification of Vibrio proteolyticus with a differential medium and a specific probe. Appl. Environ. Microbiol. 62:2673-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Nasu, H., T. Iida, T. Sugahara, Y. Yamaichi, K. S. Park, K. Yokoyama, K. Makino, H. Shinagawa, and T. Honda. 2000. A filamentous phage associated with recent pandemic Vibrio parahaemolyticus O3:K6 strains. J. Clin. Microbiol. 38:2156-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Naylor, R. L., R. J. Goldburg, H. Mooney, M. Beveridge, J. Clay, C. Folke, N. Kautsky, J. Lubchenco, J. Primavera, and M. Williams. 1998. Nature's subsidies to shrimp and salmon farming. Nature 282:883-884. [Google Scholar]
- 280.Naylor, R. L., R. J. Goldburg, J. H. Primavera, N. Kautsky, M. C. Beveridge, J. Clay, C. Folke, J. Lubchenco, H. Mooney, and M. Troell. 2000. Effect of aquaculture on world fish supplies. Nature 405:1017-1024. [DOI] [PubMed] [Google Scholar]
- 281.Nedoluha, P. C, and D. Westhoff. 1997. Microbiology of striped bass grown in three aquaculture systems. Food Microbiol. 14:255-264. [Google Scholar]
- 282.Nichols, D. S. 2003. Prokaryotes and the input of polyunsaturated fatty acids to the marine food web. FEMS Microbiol. Lett. 219:1-7. [DOI] [PubMed] [Google Scholar]
- 283.Nicolas, J. L., S. Corre, G. Gauthier, R. Robert, and D. Ansquer. 1996. Bacterial problems associated with scallop Pecten maximus larval culture. Dis. Aquat. Org. 27:67-76. [Google Scholar]
- 284.Nishibuchi, M., and J. B. Kaper. 1995. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect. Immun. 63:2093-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Nishiguchi, M. K. 2000. Temperature affects species distribution in symbiotic populations of Vibrio spp. Appl. Environ. Microbiol. 66:3550-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Nivens, D. E., T. E. McKnight, S. A. Moser, S. J. Osbourn, M. L. Simpson, and G. S. Sayler. 2004. Bioluminescent bioreporter integrated circuits: potentially small, rugged and inexpensive whole-cell biosensors for remote environmental monitoring. J. Appl. Microbiol. 96:33-46. [DOI] [PubMed] [Google Scholar]
- 287.Novoa, B., A. Luque, D. Castro, J. J. Borrego, and A. Figueras. 1998. Characterization and infectivity of four bacterial strains isolated from brown ring disease-affected clams. J. Invertebr. Pathol. 71:34-41. [DOI] [PubMed] [Google Scholar]
- 288.Nunes-Halldorson, V. D., and N. L. Duran. 2003. Bioluminescent bacteria: lux genes as environmental biosensors. Braz. J. Microbiol. 34:91-96. [Google Scholar]
- 289.Nyholm, S. V., E. V. Stabb, E. G. Ruby, and M. J. McFall-Ngai. 2000. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc. Natl. Acad. Sci. USA 97:10231-10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Nyholm, S. V., and M. J. McFall-Ngai. 2003. Dominance of Vibrio fischeri in secreted mucus outside the light organ of Euprymna scolopes: the first site of symbiont specificity. Appl. Environ. Microbiol. 69:3932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Oakey, H. J., B. R. Cullen, and L. Owens. 2002. The compete nucleotide sequence of the Vibrio harveyi bacteriophage VHML. J. Appl. Microbiol. 93:1089-1098. [DOI] [PubMed] [Google Scholar]
- 292.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 293.Ochman, H., and N. A. Moran. 2001. Genes lost and genes found: evolution of bacterial pathogenesis and symbiosis. Science 292:1096-1098. [DOI] [PubMed] [Google Scholar]
- 294.Ohnishi, M., K. Kurokawa, and T. Hayashi. 2001. Diversification of Escherichia coli genomes: are bacteriophages the major contributors? Trends Microbiol. 9:481-485. [DOI] [PubMed] [Google Scholar]
- 295.Olafsen, J. A. 2001. Interactions between fish larvae and bacteria in marine aquaculture. Aquaculture 200:223-247. [Google Scholar]
- 296.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ortigosa, M., C. Esteve, and M. J. Pujalte. 1989. Vibrio species in seawater and mussels: abundance and numerical taxonomy. Syst. Appl. Microbiol. 12:316-325. [Google Scholar]
- 298.Ortigosa, M., E. Garay, and M. J. Pujalte. 1994. Numerical taxonomy of Vibrionaceae isolated from oysters and seawater along an annual cycle. Syst. Appl. Microbiol. 17:216-225. [Google Scholar]
- 299.Osterhout, G. J., V. H. Hull, and J. D. Dick. 1991. Identification of clinical isolates of gram- negative nonfermentative bacteria by an automated cellular fatty acid identification system. J. Clin. Microbiol. 29:1822-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ouverney, C. C., and J. A. Fuhrman. 1999. Combined microautoradiography-16S rRNA probe technique for determination of radioisotope uptake by specific microbial cell types in situ. Appl. Environ. Microbiol. 65:1746-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Oxley, A. P., W. Shipton, L. Owens, and D. McKay. 2002. Bacterial flora from the gut of the wild and cultured banana prawn, Penaeus merguiensis. J. Appl. Microbiol. 93:214-223. [DOI] [PubMed] [Google Scholar]
- 302.Panicker, G., M. L. Myers, and A. K. Bej. 2004. Rapid detection of Vibrio vulnificus in shellfish and Gulf of Mexico water by real-time PCR. Appl. Environ. Microbiol. 70:498-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Park, K.-S., T. Iida, Y. Yamaichi, T. Oyagi, K. Yamamoto, and T. Honda. 2000. Genetic characterization of DNA region containing the trh and ure genes of Vibrio parahaemolyticus. Infect. Immun. 68:5742-5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Reference deleted.
- 305.Park, R. W. A. 1961. A note on the systematic position of Vibrio fetus. J. Appl. Bacteriol 24:23-26. [Google Scholar]
- 306.Pearson, W. R. and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Pedersen, K., L. Verdonck, B. Austin, D. A. Austin, A. R. Blanch, P. A. D. Grimont, J. Jofre, S. Koblavi, J. L. Larsen, T. Tiainen, Vigneulle M., and J. Swings. 1998. Taxonomic evidence that Vibrio carchariae Grimes et al. 1985 is a junior synonym of Vibrio harveyi (Johnson and Shunk 1936) Baumann et al. 1981. Int. J. Syst. Bacteriol. 48:749-758. [Google Scholar]
- 308.Peters, E. C. 1997. Diseases of coral-reef organisms, p. 114-139. In C. Birkeland (ed.), Life and death of coral reefs. Chapman & Hall, New York, N.Y.
- 309.Popovic, T., C. Bopp, O. Olsvik, and K. Wachsmuth. 1993. Epidemiologic application of a standardized ribotype scheme for Vibrio cholerae O1. J. Clin. Microbiol. 31:2474-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Pourshafie, M. R., F. Grimont, M. Saifi, and P. A. Grimont. 2000. Molecular epidemiological study of Vibrio cholerae isolates from infected patients in Teheran, Iran. J. Med. Microbiol. 49:1085-1090. [DOI] [PubMed] [Google Scholar]
- 311.Pourshafie, M., F. Grimont, S. Kohestani, and P. A. Grimont. 2002. A molecular and phenotypic study of Vibrio cholerae in Iran. J. Med. Microbiol. 51:392-398. [DOI] [PubMed] [Google Scholar]
- 312.Pujalte, M. J., B. A. Ortiz-Conde, S. E. Steven, C. Esteve, E. Garay, and R. R. Colwell. 1992. Numerical taxonomy and nucleic acid studies of Vibrio mediterranei. Syst. Appl. Microbiol. 15:82-91. [Google Scholar]
- 313.Pujalte, M. J., M. Ortigosa, M. C. Macián, and E. Garay. 1999. Aerobic and facultative anaerobic heterotrophic bacteria associated to Mediterranean oysters and seawater. Int. Microbiol. 2:259-266. [PubMed] [Google Scholar]
- 314.Rademaker, J. L. W., F. J. Louws, and F. J. de Brujin. 1998. Characterization of the diversity of ecologically important microbes by rep-PCR genomic fingerprinting, p. 1-27. In J. D. Van Elsas et al. (ed.), Molecular microbial ecology manual, vol. 3.4.3. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 315.Rademaker, J. L., B. Hoste, F. J. Louws, K. Kersters, J. Swings, L. Vauterin, P. Vauterin and F. J. de Bruijn. 2000. Comparison of AFLP and rep-PCR genomic fingerprinting with DNA- DNA homology studies: Xanthomonas as a model system. Int. J. Syst. Evol. Microbiol. 50:665-677. [DOI] [PubMed] [Google Scholar]
- 316.Raguénès, G., R. Christen, J. Guezennec, P. Pignet, and G. Barbier. 1997. Vibrio diabolicus sp. nov., a new polysaccharide-secreting organism isolated from a deep-sea hydrothermal vent polychaete annelid, Alvinella pompejana. Int. J. Syst. Bacteriol. 47:989-995. [DOI] [PubMed] [Google Scholar]
- 317.Raimondi, F., J. P. Kao, C. Fiorentini, A. Fabbri, G. Donelli, N. Gasparini, A. Rubino, and A. Fasano. 2000. Enterotoxicity and cytotoxicity of Vibrio parahaemolyticus thermostable direct hemolysin in in vitro systems. Infect. Immun. 68:3180-3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Rappé, M. S., and S. J. Giovannoni. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57:369-394. [DOI] [PubMed] [Google Scholar]
- 319.Reichelt, J. L., P. Baumann, and L. Baumann. 1976. Study of genetic relationships among marine species of the genera Beneckea and Photobacterium by means of in vitro DNA/DNA hybridization. Arch. Microbiol. 110:101-120. [DOI] [PubMed] [Google Scholar]
- 320.Reidl, J., and K. E. Klose. 2002. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol. Rev. 26:125-139. [DOI] [PubMed] [Google Scholar]
- 321.Rehnstam, A. S., S. Backman, D. C. Smith, F. Azam, and A. Hagstrom. 1993. Blooms of sequence-specific culturable bacteria in the sea. FEMS Microbiol. Ecol. 102:161-166. [Google Scholar]
- 322.Richardson, L. L. 1998. Coral diseases: what is really known? Tree 13:438-443. [DOI] [PubMed] [Google Scholar]
- 323.Richardson, L. L., W. M. Goldberg, and K. G. Kuta. 1998. Florida's mystery coral-killer identified. Nature 392:557-558. [Google Scholar]
- 324.Riemann, L., and F. Azam. 2002. Widespread N-acetyl-d-glucosamine uptake among pelagic marine bacteria and its ecological implications. Appl. Environ. Microbiol. 68:5554-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ringo, E., and T. H. Birkbeck. 1999. Intestinal microflora of fish larvae and fry. Aquacult. Res. 30:73-93. [Google Scholar]
- 326.Ringo, E., J. B. Lodemel, R. Myklebust, T. Kaino, T. M. Mayhew, and R. E. Olsen. 2001. Epithelium-associated bacteria in the gastrointestinal tract of Arctic charr (Salvelinus alpinus L.). An electron microscopical study. J. Appl. Microbiol. 90:294-300. [DOI] [PubMed] [Google Scholar]
- 327.Riquelme, C. E., M. A. Jorquera, A. I. Rojas, R. E. Avendano, and N. Reyes. 2001. Addition of inhibitor-producing bacteria to mass cultures of Argopecten purpuratus larvae (Lamarck, 1819). Aquaculture 192:111-119. [Google Scholar]
- 328.Rivera, I. G., M. A. Chowdhury, A. Huq, D. Jacobs, M. T. Martins, and R. R. Colwell. 1995. Enterobacterial repetitive intergenic consensus sequences and the PCR to generate fingerprints of genomic DNAs from Vibrio cholerae O1, O139, and non-O1 strains. Appl. Env. Microbiol. 61:2898-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Rivera, I. N., J. Chun, A. Huq, R. B. Sack, and R. R. Colwell. 2001. Genotypes associated with virulence in environmental isolates of Vibrio cholerae. Appl. Environ. Microbiol. 67:2421-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Roque, A., A. Molina-Aja, C. Bolan-Mejia, and B. Gomez-Gil. 2001. In vitro susceptibility to 15 antibiotics of vibrios isolated from penaeid shrimps in Northwestern Mexico. Int. J. Antimicrobial Agents 17:383-387. [DOI] [PubMed] [Google Scholar]
- 331.Rosenberg, E., and Y. Ben-Haim. 2002. Microbial diseases of corals and global warming. Environ. Microbiol. 4:318-326. [DOI] [PubMed] [Google Scholar]
- 332.Rosenberg, E., and Y. Loya. 2004. Coral health and disease. Springer-Verlag KG, Berlin, Germany.
- 333.Rosenberg, E., and L. Falkovitz. The Vibrio shiloi/Oculina patagonica model system of coral bleaching. Annu. Rev. Microbiol., in press. [DOI] [PubMed]
- 334.Rossello-Mora, R., and R. Amann. 2001. The species concept for prokaryotes. FEMS Microbiol. Rev. 25:39-67. [DOI] [PubMed] [Google Scholar]
- 335.Rowe-Magnus, D. A., A.-M. Guetout, P. Ploncard, B. Dychinco, J. Davies, and D. Mazel. 2001. The evolutionary history of chromosomal super-integrons provides an ancestry for multiresistant integrons. Proc. Natl. Acad. Sci. USA 98:652-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Rowe-Magnus, D. A., J. Davies, and D. Mazel. 2002. Impact of integrons and transposons on the evolution of resistance and virulence. Curr. Top. Microbiol. Immunol. 264:167-188. [PubMed] [Google Scholar]
- 337.Rowe-Magnus, D. A., A.-M. Guetout, L. Biskri, P. Bouige, and D. Mazel. 2002. Comparative analysis of superintegrons: engineering extensive genetic diversity in the Vibrionaceae. Genet. Res. 13:428-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rowe-Magnus, D. A., A. M. Guerout, and D. Mazel. 2002. Bacterial resistance evolution by recruitment of super-integron gene cassettes. Mol. Microbiol. 43:1657-1669. [DOI] [PubMed] [Google Scholar]
- 339.Ruby, E. G. 1996. Lessons from a cooperative, bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis. Annu. Rev. Microbiol. 50:591-624. [DOI] [PubMed] [Google Scholar]
- 340.Ruimy, R., V. Breittmayer, P. Elbaze, B. Lafay, O. Boussemart, M. Gauthier, and R. Christen. 1994. Phylogenetic analysis and assessment of the genera Vibrio, Photobacterium, Aeromonas, and Plesiomonas deduced from small-subunit rRNA sequences. Int. J. Syst. Bacteriol. 44:416-426. [DOI] [PubMed] [Google Scholar]
- 341.Ruppert, E., R. S. Fox, and R. D. Barnes. 2003. Invertebrate zoology: a functional evolutionary approach, 7th ed. Brooks Cole, Florence, Kg.
- 342.Savelkoul, P. H., H. J. Aarts, J. de Haas, L. Dijkshoorn, B. Duim, M. Otsen, J. L. Rademaker, L. Schouls, and J. A. Lenstra. 1999. Amplified-fragment length polymorphism analysis: the state of an art. J. Clin. Microbiol. 37:3083-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sawabe, T., Y. Oda, Y. Shiomi, and Y. Ezura. 1995. Alginate degradation by bacteria isolated from the gut of sea urchins and abalones. Microb. Ecol. 30:192-202. [DOI] [PubMed] [Google Scholar]
- 344.Sawabe, T., F. L. Thompson, J. Heyrman, M. Cnockaert, K. Hayashi, R. Tanaka, M. Yoshimizu, B. Hoste, J. Swings, and Y. Ezura. 2002. Fluorescent amplified fragment length polymorphism and repetitive extragenic palindrome-PCR fingerprinting reveal host-specific genetic diversity of Vibrio halioticoli-like strains isolated from the gut of japanese abalone. Appl. Environ. Microbiol. 68:4140-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Sawabe, T., N. Setogushi, S. Inoue, R. Tanaka, M. Ootsubo, M. Yoshimizu, and Y. Ezura. 2003. Acetic acid production of Vibrio halioticoli from alginate: a possible role for establishment of abalone-V. halioticoli association. Aquaculture 219:671-679. [Google Scholar]
- 346.Sawabe, T. K. Hayashi, J. Moriwaki, F. L. Thompson, J. Swings, P. Potin, R. Christen, and Y. Ezura. 2004. Vibrio gallicus sp. nov., isolated from the gut of the French abalone Haliotis tuberculata. Int. J. Syst. Evol. Microbiol. 54:843-846. [DOI] [PubMed] [Google Scholar]
- 347.Sawabe, T., K. Hayashi, J. Moriwaki, F. L. Thompson, J. Swings, and R. Christen. 2004. Vibrio neonatus sp. nov. and Vibrio ezurae sp. nov. isolated from the gut of Japanese abalones. Syst. Appl. Microbiol. 27:527-534. [DOI] [PubMed] [Google Scholar]
- 348.Schena, M., D. Shalon, R. W. Davis, and P. O. Brown. 1995. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270:467-470. [DOI] [PubMed] [Google Scholar]
- 349.Schoolnik, G. K., and F. H. Yildiz. 2000. The complete genome sequence of Vibrio cholerae: a tale of two chromosomes and of two lifestyles. Gen. Biol. 1:1016.1-1016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sechi, L. A., I. Dupre, A. Deriu, G. Fadda, and S. Zanetti. 2000. Distribution of Vibrio cholerae virulence genes among different Vibrio species isolated in Sardinia, Italy. J. Appl. Microbiol. 88:475-481. [DOI] [PubMed] [Google Scholar]
- 351.Selander, R. K., and B. R. Levin. 1980. Genetic diversity and structure in Escherichia coli populations. Science 210:545-547. [DOI] [PubMed] [Google Scholar]
- 352.Sherr, E., and B. Sherr. 2000. Marine microbes: an overview, p. 13-46. In D. L. Kirchman (ed.), Microbial ecology of the oceans. Wiley-Liss, London, United Kingdom.
- 353.Sherr, E. B., and B. F. Sherr. 2002. Significance of predation by protists in aquatic microbial food webs. Antonie Leeuwenhoek 81:293-308. [DOI] [PubMed] [Google Scholar]
- 354.Singh, D. V., M. H. Matte, G. R. Matte, S. Jiang, F. Sabeena, B. N. Shukla, S. C. Sanyal, A. Huq, and R. R. Colwell. 2001. Molecular analysis of Vibrio cholerae O1, O139, non-O1, and non-O139 strains: clonal relationships between clinical and environmental isolates. Appl. Environ. Microbiol. 67:910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Skerman, V. B. D., V. McGowan, and P. H. A. Sneath. 1980. Approved lists of bacterial names. Int. J. Syst. Bacteriol. 30:225-420. [Google Scholar]
- 356.Sloan, E., M. O'Neill, C. Kaysner, A. DePaola, J. L. Nordstrom, and J. Sofosi. 2003. Evaluation of two nonradioactive gene probes for the enumeration of Vibrio parahaemolyticus in crabmeat. J. Rapid Metods Autom. Microbiol. 11:297-311. [Google Scholar]
- 357.Smith, A. B., and R. J. Siebeling. 2003. Identification of genetic loci required for capsular expression in Vibrio vulnificus. Infect. Immun. 71:1091-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Smith, S. K., D. C. Sutton, J. A. Fuerst, and J. L. Reichelt. 1991. Evaluation of the genus Listonella and reassignment of Listonella damsela (Love et al.) MacDonell and Colwell to the genus Photobacterium as Photobacterium damsela comb. nov. with an emended description. Int. J. Syst. Bacteriol. 41:529-534. [DOI] [PubMed] [Google Scholar]
- 359.Snow, J. 1855. On the mode of communication of cholera, 2nd ed. John Churchill, London, United Kingdom.
- 360.Stackebrandt, E., R. G. E. Murray, and H. G. Trüper. 1988. Proteobacteria classis nov., a name for the phylogenetic taxon that includes the “purple bacteria and their relatives“. Int. J. Syst. Bacteriol. 38:321-325. [Google Scholar]
- 361.Stackebrandt, E., W. Frederiksen, G. M. Garrity, P. A. Grimont, P. Kampfer, M. C. Maiden, X. Nesme, R. Rossello-Mora, J. Swings, H. G. Trüper, L. Vauterin, A. C. Ward, and W. B. Whitman. 2002. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 52:1043-1047. [DOI] [PubMed] [Google Scholar]
- 362.Stackebrandt, E. 2003. The richness of prokaryotic diversity: there must be a species somewhere. Food Technol. Biotechnol. 41:17-22. [Google Scholar]
- 363.Staley, J. T., R. L. Irgens, and D. J. Brenner. 1987. Enhydrobacter aerosaccus gen. nov., sp. nov., a gas-vacuolated, facultatively anaerobic, heterotrophic rod. Int. J. Syst. Bacteriol. 37:289-291. [Google Scholar]
- 364.Stibitz, S., and M. S. Yang. 1997. Genomic fluidity of Bordetella pertussis assessed by a new method for chromosomal mapping. J. Bacteriol. 179:5820-5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Suantika, G., P. Dhert, G. Rombaut, J. Vandenberghe, T. De Wolf, and P. Sorgeloos. 2001. The use of ozone in a high density recirculation system for rotifers. Aquaculture 201:35-49. [Google Scholar]
- 366.Sudheesh, P. S., K. Jie, and H. Xu. 2002. Random amplified polymorphism DNA-PCR typing of Vibrio parahaemolyticus and V. alginolyticus isolated from cultured shrimps. Aquaculture 207:11-17. [Google Scholar]
- 367.Suginta, W., A. Vongsuwan, C. Songsiriritthigul, H. Prinz, P. Estibeiro, R. R. Duncan, J. Svasti, and L. A. Fothergill-Gilmore. 2004. An endochitinase A from Vibrio carchariae: cloning, expression, mass and sequence analyses, and chitin hydrolysis. Arch. Biochem. Biophys. 424:171-180. [DOI] [PubMed] [Google Scholar]
- 368.Sugumar, G., T. Nakai, Y. Hirata, D. Matsubara, and K. Muroga. 1998. Vibrio splendidus biovar II as the causative agent of bacillary necrosis of japanese oyster Crassostrea gigas larvae. Dis. Aquat. Org. 33:111-118. [DOI] [PubMed] [Google Scholar]
- 369.Sussman, M., Y. Loya, M. Fine, and E. Rosenberg. 2003. The marine fireworm Hermodice carunculata is a winter reservoir and spring-summer vector for the coral-bleaching pathogen Vibrio shiloi. Environ. Microbiol. 5:250-255. [DOI] [PubMed] [Google Scholar]
- 370.Sutherland, K. P., J. W. Porter, and C. Torres. 2004. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266:273-302. [Google Scholar]
- 371.Suthienkul, O., M. Ishibashi, T. Iida, N. Nettip, S. Supavej, B. Eampokalap, M. Makino, and T. Honda. 1995. Urease production correlates with possession of the trh gene in Vibrio parahaemolyticus strains isolated in Thailand. J. Infect. Dis. 172:1405-1408. [DOI] [PubMed] [Google Scholar]
- 372.Svitil, A. L., S. M. N. Chadhain, J. A. Moore, and D. L. Kirchman. 1997. Chitin degradation proteins produced by the marine bacterium Vibrio harveyi growing on different forms of chitin. Appl. Environ. Microbiol. 63:408-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Taga, M. E., and B. L. Bassler. 2003. Chemical communication among bacteria. Proc. Natl. Acad. Sci. USA 100:14549-14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Tagomori, K., T. Iida, and T. Honda. 2002. Comparison of genome structures of vibrios, bacteria possessing two chromosomes. J. Bacteriol. 184:4351-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Tanaka, R., T. Sawabe, K. Tajima, J. Vandenberghe, and Y. Ezura. 2001. Identification of Vibrio halioticoli using 16S rDNA PCR/RFLP (restriction fragment length polymorphism) analysis. Fish Sci. (Tokyo) 67:185-187. [Google Scholar]
- 376.Tanaka, R., M. Ootsubo, T. Sawabe, K. Tajima, J. Vandenberghe, and Y. Ezura. 2002. Identification of Vibrio halioticoli by colony hybridization with non-radioisotope labeled genomic DNA. Fish Sci. (Tokyo) 68:227-229. [Google Scholar]
- 377.Tannock, G. W. 2002. Probiotics and prebiotics: where are we going? Caister Academic Press, Norwich, United Kingdom.
- 378.Thompson, C. C., F. L. Thompson, K. Vandemeulebroecke, B. Hoste, P. Dawyndt, and J. Swings. 2004. Use of recA as an alternative phylogenetic marker in the family Vibrionaceae. Int. J. Syst. Evol. Microbiol. 54:919-924. [DOI] [PubMed] [Google Scholar]
- 379.Thompson, F., B. Hoste, C. C. Thompson, J. Goris, B. Gomez-Gil, L. Huys, P. De Vos, and J. Swings. 2002. Entervibrio norvegicus gen. nov., sp. nov., isolated from the gut of turbot (Scophthalmus maximus) larvae: a new member of the family Vibrionaceae. Int. J. Syst. Evol. Microbiol. 52:2015-2022. [DOI] [PubMed] [Google Scholar]
- 380.Thompson, F. L., B. Hoste, C. C. Thompson, Huys, G., and J. Swings. 2001. The coral bleaching Vibrio shiloi Kushmaro et al. 2001 is a later synonym of Vibrio mediterranei Pujalte and Garay 1986. Syst. Appl. Microbiol. 24:516-519. [DOI] [PubMed] [Google Scholar]
- 381.Thompson, F. L., B. Hoste, K. Vendemeulebroecke, and J. Swings. 2001. Genomic diversity amongst Vibrio isolates from different sources determined by fluorescent amplified fragment length polymorphism. Syst. Appl. Microbiol. 24:520-538. [DOI] [PubMed] [Google Scholar]
- 382.Thompson, F. L., B. Hoste, K. Vandemeulebroecke, K. Engelbeen, R. Denys, and J. Swings. 2002. Vibrio trachuri Iwamoto et al. 1996 is a junior synonym of Vibrio harveyi (Johnson and Shunk 1936) Baumann et al. 1981. Int. J. Syst. Evol. Microbiol 52:973-976. [DOI] [PubMed] [Google Scholar]
- 383.Thompson, F. L., P. C. Abreu, and W. Wasielesky. 2002. Importance of biofilm for water quality and nourishment in intensive shrimp culture. Aquaculture 203:263-278. [Google Scholar]
- 384.Thompson, F. L., Y. Li, B. Gomez-Gil, C. C. Thompson, B. Hoste, K. Vandemeulebroucke, G. S. Rupp, A. Pereira, M. M. De Bem, P. Sorgeloos, and J. Swings. 2003. Vibrio neptunius sp. nov., V. brasiliensis sp. nov. and V. xuii sp. nov., isolated from the marine aquaculture environment (bivalves, fish, rotifers and shrimps). Int. J. Syst. Evol. Microbiol. 53:245-252. [DOI] [PubMed] [Google Scholar]
- 385.Thompson, F. L., C. C. Thompson, Y. Li, B. Gomez-Gil, J. Vandenberghe, B. Hoste, and J. Swings. 2003. Description of Vibrio kanaloae sp. nov, Vibrio pomeroyi sp. nov. and Vibrio chagasii sp. nov., from sea water and marine animals. Int. J. Syst. Evol. Microbiol. 53:753-759. [DOI] [PubMed] [Google Scholar]
- 386.Thompson, F. L., C. C. Thompson, and J. Swings. 2003. Vibrio tasmaniensis sp. nov., isolated from Atlantic salmon (Salmo salar L.). Syst. Appl. Microbiol. 26:65-69. [DOI] [PubMed] [Google Scholar]
- 387.Thompson, F. L., C. C. Thompson, B. Hoste, K. Vandemeulebroecke, M. Gullian, and J. Swings. 2003. Description of Vibrio fortis sp. nov., and V. hepatarius sp. nov., isolated from aquatic animals and the marine environment. Int. J. Syst. Microbiol. 53:1495-1501. [DOI] [PubMed] [Google Scholar]
- 388.Thompson, F. L. 2003. Improved taxonomy of the family Vibrionaceae. Ph.D. thesis. Ghent University, Ghent, Belgium.
- 389.Reference deleted.
- 390.Reference deleted.
- 391.Thyssen, A., S. Van Eygen, L. Hauben, J. Goris, J. Swings, and F. Ollevier. 2000. Application of AFLP for taxonomic and epidemiological studies of Photobacterium damselae subsp. piscicida. Int. J. Syst. Evol. Microbiol. 50:1013-1019. [DOI] [PubMed] [Google Scholar]
- 392.Trucksis, M., J. Michalski, Y. K. Deng, and J. B. Kaper. 1998. The Vibrio cholerae genome contains two unique circular chromosomes. Proc. Natl. Acad. Sci. USA 95:14464-14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Tubiash, H. S., R. R. Colwell, and R. Sakazaki. 1970. Marine vibrios associated with bacillary necrosis, a disease of larval and juvenile bivalve mollusks. J. Bacteriol. 103:271-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Urakawa, H., K. Kita-Tsukamoto, and K. Ohwada. 1997. 16S rDNA genotyping using PCR/RFLP (restriction fragment length polymorphism) analysis among the family Vibrionaceae. FEMS Microbiol. Lett. 152:125-132. [DOI] [PubMed] [Google Scholar]
- 395.Urakawa, H., K. Kita-Tsukamoto, and K. Ohwada. 1998. A new approach to separate the genus Photobacterium from Vibrio with RFLP patterns by HhaI digestion of PCR-amplified 16S rDNA. Curr. Microbiol. 36:171-174. [DOI] [PubMed] [Google Scholar]
- 396.Urakawa, H., K. Kita-Tsukamoto, S. E. Steven, K. Ohwada, and R. R. Colwell. 1998. A proposal to transfer Vibrio marinus (Russell 1891) to a new genus Moritella gen. nov. as Moritella marina comb. nov. FEMS Microbiol. Lett. 165:373-378. [DOI] [PubMed] [Google Scholar]
- 397.Urakawa, H., K. Kita-Tsukamoto, and K. Ohwada. 1999. 16S rDNA restriction fragment length polymorphism analysis of psychrotrophic vibrios from Japanese coastal water. Can. J. Microbiol. 45:1001-1007. [PubMed] [Google Scholar]
- 398.Urakawa, H., K. Kita-Tsukamoto, and K. Ohwada. 1999. Reassessment of the taxonomic position of Vibrio iliopiscarius (Onarheim et al. 1994) and proposal for Photobacterium iliopiscarium comb. nov. Int. J. Syst. Bacteriol. 49:257-260. [DOI] [PubMed] [Google Scholar]
- 399.Urakawa, H., T. Yoshida, M. Nishimura, and K. Ohwada. 2000. Characterization of depth- related population variation in microbial communities of a coastal marine sediment using 16S rDNA-based approaches and quinone profiling. Environ. Microbiol. 2:542-554. [DOI] [PubMed] [Google Scholar]
- 400.Urwin, R., and M. C. Maiden. 2003. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 10:479-487. [DOI] [PubMed] [Google Scholar]
- 401.van Beelen, P. 2003. A review on the application of microbial toxicity tests for deriving sediment quality guidelines. Chemosphere 53:795-808. [DOI] [PubMed] [Google Scholar]
- 402.van Belkum, A., M. Struelens, A. de Visser, H. Verbrugh, and M. Tibayrenc. 2001. Role of genomic typing in taxonomy, evolutionary genetics, and microbial epidemiology. Clin. Microbiol. Rev. 14:547-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Vandamme, P., B. Pot, M. Gillis, P. de Vos, K. Kersters, and J. Swings. 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 60:407-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Vandenberghe, J., Y. Li, L. Verdonck, J. Li, P. Sorgeloos, H. S. Xu, and J. Swings. 1998. Vibrios associated with Penaeus chinensis (Crustacea: Decapoda) larvae in Chinese shrimp hatcheries. Aquaculture 169:121-132. [Google Scholar]
- 405.Vandenberghe, J., L. Verdonck, R. Robles-Arozarena, G. Rivera, A. Bolland, M. Balladares, B. Gomez-Gil, J. Calderon, P. Sorgeloos, and J. Swings. 1999. Vibrios associated with Litopenaeus vannamei larvae, postlarvae, broodstock, and hatchery probionts. Appl. Environ. Microbiol. 65:2592-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Vandenberghe, J., F. L. Thompson, B. Gomez-Gil, and J. Swings. 2003. Phenotypic diversity amongst Vibrio isolates from marine aquaculture systems. Aquaculture 219:9-20. [Google Scholar]
- 407.Venter, J. C., K. Remington, J. F. Heidelberg, A. L. Halpern, D. Rusch, J. A. Eisen, D. Y. Wu, I. Paulsen, K. E. Nelson, W. Nelson, D. E. Fouts, S. Levy, A. H. Knap, M. W. Lomas, K. Nealson, O. White, J. Peterson, J. Hoffman, R. Parsons R, H. Baden-Tillson, C. Pfannkoch, Y. H. Rogers, and H. O. Smith. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66-74. [DOI] [PubMed] [Google Scholar]
- 408.Verdonck, L. J. Swings, K. Kersters, M. Dehasque, P. Sorgeloos, and P. Leger. 1994. Variability of the microbial environment of rotifer Brachionis plicatilis and Artemia production systems. J. World. Aquacult. Soc. 25:55-59. [Google Scholar]
- 409.Verdonck, L., L. Grisez, E. Sweetman, G. Minkoff, P. Sorgeloos, F. Ollevier, and J. Swings. 1997. Vibrios associated with routine productions of Brachionus plicatilis. Aquaculture 149:203-214. [Google Scholar]
- 410.Verschuere, L., H. Heang, G. Criel, P. Sorgeloos, and W. Verstraete. 2000. Selected bacterial strains protect Artemia spp. from the pathogenic effects of Vibrio proteolyticus CW8T2. Appl. Environ. Microbiol. 66:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Verschuere, L., G. Rombaut, P. Sorgeloos, and W. Verstraete. 2000. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 64:655-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Vieira, V. V., L. F. Teixeira, A. C. Vicente, H. Momen, and C. A. Salles. 2001. Differentiation of environmental and clinical isolates of Vibrio mimicus from Vibrio cholerae by multilocus enzyme electrophoresis. Appl. Environ. Microbiol. 67:2360-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Vos, P., R. Hogers, M. Bleeker, M. Reijans, T. Vandelee, M. Hornes, A. Frijters, J. Pot, J. Peleman, M. Kuiper, and M. Zabeau. 1995. AFLP: a new technique for dna-fingerprinting. Nucleic Acids Res. 23:4407-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Vuddhakul, V., T. Nakai, C. Matsumoto, T. Oh, T. Nishino, C. H. Chen, M. Nishibuchi, and J. Okuda. 2000. Analysis of gyrB and toxR gene sequences of Vibrio hollisae and development of gyrB- and toxR-targeted PCR methods for isolation of V. hollisae from the environment and its identification. Appl. Environ. Microbiol. 66:3506-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Wachsmuth, I. K., P. A. Blake, and O. Olsvik. 1994. Vibrio cholerae and cholera. Molecular to global perspectives. ASM Press, Washington, D.C.
- 416.Wai, S. N., Y. Mizunoe, and S. Yoshida. 1999. How Vibrio cholerae survive during starvation. FEMS Microbiol. Lett. 180:123-131. [DOI] [PubMed] [Google Scholar]
- 417.Waldor, M. K., and J. J. Mekalanos. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910-1914. [DOI] [PubMed] [Google Scholar]
- 418.Waldor, M. K., and D. RayChaudhuri. 2000. Treasure trove for cholera research. Nature 406:469-470. [DOI] [PubMed] [Google Scholar]
- 419.Walsh, C. 2003. Antibiotics. Actions, origins, resistance. ASM Press, Washington, D.C.
- 420.Ward, D. M. 1998. A natural species concept for prokaryotes. Curr. Opin. Microbiol. 1:271-277. [DOI] [PubMed] [Google Scholar]
- 421.Warner, J. M., and J. D. Oliver. 1999. Randomly amplified polymorphic DNA analysis of clinical and environmental isolates of Vibrio vulnificus and other Vibrio species. Appl. Environ. Microbiol. 65:1141-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Watanabe, H., S. Miyoshi, T. Kawase, K. Tomochika, and S. Shinoda. 2004. High growing ability of Vibrio vulnificus biotype 1 is essential for production of a toxic metalloprotease causing systemic diseases in humans. Microb. Pathog. 36:117-123. [DOI] [PubMed] [Google Scholar]
- 423.Watnick, P. I., and R. Kolter. 1999. Steps in the development of a Vibrio cholerae E1 Tor biofilm. Mol. Microbiol. 34:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Watnick, P., and R. Kolter. 2000. Biofilm, city of microbes. J. Bacteriol. 182:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Watnick, P. I., C. M. Lauriano, K. E. Klose, L. Croal, and R. Kolter. 2001. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol. Microbiol. 39:223-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. L. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Starr, and H. G. Trüper. 1987. Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37:463-464. [Google Scholar]
- 427.West, P. A., J. V. Lee, and T. N. Bryant. 1983. A numerical taxonomic study of species of Vibrio isolated from the aquatic environment and birds in Kent, England. J. Appl. Bacteriol. 55:263-282. [DOI] [PubMed] [Google Scholar]
- 428.Wiik, R., E. Stackebrandt, O. Valle, F. L. Daae, O. M. Rodseth, and K. Andersen. 1995. Classification of fish-pathogenic vibrios based on comparative 16S rRNA analysis. Int. J. Syst. Bacteriol. 45:421-428. [DOI] [PubMed] [Google Scholar]
- 429.Willems, A., F. Doignon-Boucier, R. Coopman, B. Hoste, P. de Lajudie, and M. Gillis. 2000. AFLP fingerprinting analysis of Bradyrhizobium strains isolated from Faidherbia albida and Aeschynomene species. Syst. Appl. Microbiol. 23:137-147. [DOI] [PubMed] [Google Scholar]
- 430.Willems, A., F. Doignon-Bourcier, J. Goris, R. Coopman, P. Lajudie, P. De Vos, and M. Gillis. 2001. DNA-DNA hybridization study of Bradyrhizobium strains. Int. J. Syst. Evol. Microbiol. 51:1315-1322. [DOI] [PubMed] [Google Scholar]
- 431.Williams, M. J., J. D. Bell, M. V. Gupta, M. Dey, M. Ahmed, M. Prein, S. Child, P. R. Gardiner, R. Brummett, and D. Jamu. 2000. Responsible aquaculture can aid food problems. Nature 406:673. [DOI] [PubMed] [Google Scholar]
- 432.Wilson, K. H., W. J. Wilson, J. L. Radosevich, T. Z. DeSantis, V. S. Viswanathan, T. A. Kuczmarski, and G. L. Andersen. 2002. High-density microarray of small-subunit ribosomal DNA probes. Appl. Environ. Microbiol. 68:2535-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wilson, B. A., and A. A. Salyers. 2003. Is the evolution of bacterial pathogens an out-of-body experience? Trends Microbiol. 11:347-350. [DOI] [PubMed] [Google Scholar]
- 434.Wittwer, C. 2001. Rapid cycle real-time PCR: methods and applications, p. 1-10. In S. Meuer, C. Wittwer, and K. Nakagawara (ed.), Rapid cycle real-time PCR. Methods and Applications. Springer-Verlag KG, Berlin, Germany.
- 435.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Wolfe, A. J., D. S. Millikan, J. M. Campbell, and K. L. Visick. 2004. Vibrio fischeri sigma(54) controls motility, biofilm formation, luminescence, and colonization. Appl. Environ. Microbiol. 70:2520-2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Wong, H. C., and C. H. Lin. 2001. Evaluation of typing of Vibrio parahaemolyticus by three PCR methods using specific primers. J. Clin. Microbiol. 39:4233-4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Woolkalis, M. J., and P. Baumann. 1981. Evolution of alkaline phosphatase in marine species of Vibrio. J. Bacteriol. 147:36-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.World Health Organization. 2001. Cholera, 2000. Wkly. Epidemiol. Rec. 76:233-240. [PubMed] [Google Scholar]
- 440.World Health Organization. 2002. Cholera, 2001. Wkly. Epidemiol. Rec. 77:257-268. [PubMed] [Google Scholar]
- 441.Wright, A. C., J. L. Powell, J. B. Kaper, and J. G. Morris, Jr. 2001. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 69:6893-6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Yamaichi, Y., T. Iida, K. S. Park, K. Yamamoto, and T. Honda. 1999. Physical and genetic map of the genome of Vibrio parahaemolyticus: presence of two chromosomes in Vibrio species. Mol. Microbiol. 31:1513-1521. [DOI] [PubMed] [Google Scholar]
- 443.Yamane, K., J. Asato, N. Kawade, H. Takahashi, B. Kimura, and Y. Arakawa. 2004. Two cases of fatal necrotizing fasciitis caused by Photobacterium damsela in Japan. J. Clin. Microbiol. 42:1370-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Young, J. M. 2001. Implications of alternative classifications and horizontal gene transfer for bacterial taxonomy. Int. J. Syst. Evol. Microbiol. 51:945-953. [DOI] [PubMed] [Google Scholar]
- 445.Yu, R. R., and V. J. DiRita. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119-134. [DOI] [PubMed] [Google Scholar]
- 446.Yumoto, I., H. Iwata, T. Sawabe, K. Ueno, N. Ichise, H. Matsuyama, H. Okuyama, and K. Kawasaki. 1999. Characterization of a facultatively psychrophilic bacterium, Vibrio rumoiensis sp. nov., that exhibits high catalase activity. Appl. Environ. Microbiol. 60:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Xu, Q., M. Dziejman, and J. J. Mekalanos. 2003. Determination of the transcriptome of Vibrio cholerae during intraintestinal growth and midexponential phase in vitro. Proc. Natl. Acad. Sci. USA 100:1286-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Zeigler, D. R. 2003. Gene sequences useful for predicting relatedness of whole genomes in bacteria. Int. J. Syst. Evol. Microbiol. 53:1893-1900. [DOI] [PubMed] [Google Scholar]
- 449.Zo, Y. G., I. N. Rivera, E. Russek-Cohen, M. S. Islam, A. K. Siddique, M. Yunus, R. B. Sack, A. Huq, and R. R. Colwell. 2002. Genomic profiles of clinical and environmental isolates of Vibrio cholerae O1 in cholera-endemic areas of Bangladesh. Proc. Natl. Acad. Sci. USA 99:12409-124014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Zorrilla, I., M. Chabrillón, S. Arijo, P. Diaz-Rosales, E. Martinez-Manzanares, M. C. Balebona, and M. A. Moriñigo. 2003. Bacteria recovered from diseased cultured gilthead sea bream (Sparus aurata L.) in southwestern Spain. Aquaculture 218:11-20. [Google Scholar]
- 451.Zhu, J., and J. J. Mekalanos. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5:647-656. [DOI] [PubMed] [Google Scholar]