Abstract
Metastatic disease ultimately occurs in approximately 50–70% of patients presenting with colorectal cancer. In patients with advanced disease, there is significant variability in individual patient outcomes. To improve understanding of tumor behavior, markers such as KRAS and BRAF mutation status are increasingly utilized. Additionally, newer surrogates of tumor biology, such as telomerase activity and the prevalence of circulating tumor cells and circulating tumor DNA, have generated increasing interest due to clinical potential. While the extent to which these newer markers can predict outcome and guide therapy is yet to be determined, KRAS mutation status is currently used to guide systemic therapy in selected patients. Furthermore, advances in our understanding of various tumorigenic pathways (such as the mitogen activated protein kinase pathway) have enabled newer targeted agents, including BRAF inhibitors. Interestingly, although inhibition of BRAF in patients has not translated into improved outcomes, characterization of BRAF mutations led to an association with microsatellite instability. A unique histologic characteristic of certain tumors in patients with microsatellite instability is the infiltration by lymphocytes at the tumor-stromal interface. This feature highlights the biology of the tumor in its microenvironment and underlies the efficacy of the programmed-death inhibitor, pembrolizumab, in patients with microsatellite unstable metastatic colorectal cancer. With an increasing number of prognostic markers and therapeutic options in metastatic colorectal cancer, the multidisciplinary approach becomes critical for appropriate treatment decisions.
Keywords: Colorectal, Targeted therapy, Metastatic colorectal cancer, Molecular markers, Biologic therapy
1. Introduction
Primary colorectal cancer (CRC) is one of the most common cancers in Western society with up to 50–70% of patients developing metastatic disease [1,2]. Overall survival (OS) for patients with unresectable metastatic colorectal cancer (mCRC) is poor, with a median survival of approximately 24–27 months and 5 year survival of 10–15% [3]. While surgical resection represents the best chance at cure, only a subset of patients is eligible for curativeintent surgery. In addition, among patients who undergo curative-intent surgical resection, median survival is 40–55 months; however, long-term 10-year survival is only about 15–25% when surgery is combined with multimodal systemic therapy [1,2]. In fact, even in the setting of a microscopically complete (R0) resection, approximately 50–75% of patients who undergo a curative-intent resection will experience disease recurrence by 5 years [4–6].
Given the high incidence of recurrence following resection, there has been an interest in the risk stratification of patients following surgery, as well as the selection of patients for adjuvant multimodal therapy. Risk stratification of patients with mCRC has historically been guided by evaluation of various clinical and pathologic features. For example, Fong and colleagues proposed the “Clinical Risk Score” (CRS) to stratify patients into low versus high risk groups (i.e. OS high CRS, 32 months vs. low CRS, 46 months; p < 0.05) [6]. More recently, radiographic and pathologic response to chemotherapy has been proposed as a more useful and clinically meaningful tool to assess risk of recurrence and stratify patients with regard to long-term survival [7–9]. For example, the Response Evaluation Criteria in Solid Tumors (RECIST) guideline uses cross-sectional imaging to measure tumor size before and after chemotherapy in order to provide an estimate of response to therapy [10]. Additional radiographic features such as morphologic response criteria (i.e. decreased attenuation, increased homogeneity, and loss of enhancement at tumor-liver interface after treatment) have also been combined with the RECIST criteria in an effort to improve prediction of patient-specific long-term survival [11,12]. Post-treatment pathologic tumor response can provide important information regarding the efficacy of treatment and long-term prognosis; unfortunately, this information can only be obtained after surgical extirpation [8,13].
The combination of clinical, radiographic, and pathologic measures provides a basis for the characterization of prognosis among patients with resected mCRC. These factors remain fairly nonspecific, however, and have a relatively limited capacity to direct personalized therapy. In fact, with increasing targeted therapeutic options, there is an increased interest in better characterizing and defining underlying mCRC tumor biology in an effort to individualize treatment. Specifically, indicators of tumor biology may be valuable to guide appropriate therapies and to provide accurate prognostic data for patients and providers. Furthermore, identification of molecular markers and specific molecular pathways that are involved in mCRC may allow providers to better target the use of novel therapeutics. We herein review the key molecular markers and molecular pathways involved in the treatment of patients with mCRC.
2. Molecular markers
2.1. Prognostic markers in metastatic colorectal cancer
Currently, CRC has relatively few established biomarkers to predict patient outcomes. Molecular markers include microsatellite instability (MSI), KRAS and BRAF [14,15]. More recently, other investigations have identified hTERT, circulating tumor cells (CTC), and circulating tumor DNA (ctDNA), as potential predictors of outcome [16–18]. Fewer studies have reported on PI-3 Kinase, thymidylate synthase, TP53, Ki67 and hypoxia-inducible factor–1 alpha; the association of these markers with outcomes are less well established, and therefore will not be discussed [19–21].
2.1.1. DNA microsatellite instability
Microsatellites consist of repetitive units within DNA. The integrity of these regions is maintained by the mismatch repair (MMR) system. When deficiencies in the MMR system occur, the resultant MSI predisposes to genomic instability and consequent tumor formation [22]. The inability to repair single nucleotide DNA mismatches can occur from germline mutations in specific genes of the MMR system (MLH1, MSH2, MSH6, PMS2 or TACSTD1) or can arise sporadically as a result of MLH1 promoter hypermethylation (associated with CpG island methylation phenotype (CIMP)) [15]. Sporadic MSI tumors are more commonly encountered (10–20% of patients with CRC) than tumors arising from hereditary germline mutations (Lynch Syndrome: 0.8–5%) in CRC [22].
Genomic instability is divided into two genotypic groups, MSI-high (MSI-H) and MSI-low (MSI-L), based on immunohistochemical analysis of MMR protein expression or quantification of microsatellite markers in the tumor [23]. MSI-H is defined as instability in greater than 30% of microsatellite loci or absence of expression of any MMR proteins. Instability in less than 30% of loci (generally one marker in the standard 5 marker panel) is indicative of MSI-low (Table 1) [23]. MSI-H is present in 15–20% of CRC overall and has a higher prevalence in stage II versus stage III or IV CRC (approximately 20% v 12% v 4%, respectively) [23]. MSI-H tumors are more commonly located in the right colon and are histologically typified by poor differentiation, mucinous features and lymphocytic invasion. MSI-H CRCs are also associated with a decreased risk of distant recurrence, which translates into an improved long-term prognosis in stage II and stage III CRC compared with microsatellite stable (MSS) tumors [23,24]. The favorable prognosis in stage II and III disease is not present in stage IV disease, possibly related to the strong correlation with BRAF mutations [15,22]. In addition to the associated high BRAF mutation rate, further prognostic (and therapeutic) considerations for MSI-H mCRC include the infrequent occurrence of KRAS mutations [23].
Table 1.
Colorectal cancer molecular subcategorization [23].
| CRC type | Subcategory | Characteristics | Prevalence |
|---|---|---|---|
| Microsatellite instability |
MSI-H: >30% of marker loci with instability (Bethesda panel of 5 markers or alternate panel) OR lack of MMR protein on IHC |
Germline: MLH1, MSH2, MSH6, PMS2 Sporadic: Hypermethylation MLH1 Hypermutation profile but stable karyotype; strong correlation with BRAF mutations (40–45%) Right-sided lesions with poor diff, mucinous features and lymphocytic invasion Associated with CpG-Island methylation phenotype-high (CIMP-H) |
5% 10% |
| Chromosomal instability |
Includes both MSI-L (<30% of marker loci with instability) and MSS (No evidence instability) tumors: |
Unstable karyotype, demonstrates chromosome gains and losses KRAS, TP53, APC, PIK3CA, SMAD4, CTNNB1 mutations More commonly associated with CIMP-low or negative |
80–85% |
MSI = microsatellite instability, MMR = mismatch repair, IHC = immunohistochemistry, MSS = microsatellite stable.
The disparate tumor biology seen in stage IV disease compared with stage II/III disease is also supported by the varying efficacy of some chemotherapeutics. For example, although sporadic MSI-H tumors (stage II/III) tend to exhibit chemoresistance to 5-fluorouracil (5-FU), a recent retrospective analysis demonstrated preserved efficacy of 5-FU in MSI-H stage IV CRC [15]. Therefore, among patients with mCRC, 5-FU is still considered the mainstay of systemic chemotherapy regardless of MSI-H status [15,23]. Recent evidence also suggests an important role for immunotherapy in these patients (discussed in 2.4.4 below) [25].
2.1.2. KRAS
Perhaps a more robust and clinically useful biologic marker among patients with mCRC is KRAS mutational status. KRAS has been shown to be predictive of response to biologic therapy, and to correlate with long-term outcomes in patients with metastatic disease. KRAS is a membrane bound proto-oncogene that functions downstream of the epidermal growth factor receptor (EGFR); activation of KRAS promotes cell growth, cell survival, and invasion [26]. Certain KRAS mutations result in a constitutively active protein with subsequent activation of proteins in the mitogen activated protein kinase (MAPK) pathway (RAS/RAF/MAPK kinase (MEK)/ extracellular signal-regulated kinase (ERK)); in turn, tumors with mutant KRAS tend to be unresponsive to anti-EGFR therapy [26]. KRAS mutational status does not, however, confer resistance to other biologic therapies as patients may still respond to vascular endothelial growth factor (VEGF) pathway inhibitors (bevacizumab) [27,28].
KRAS mutations occur in approximately 30–50% of patients with mCRC and are associated with an increased risk of relapse and death versus patients who have tumors characterized by wild-type KRAS (wtKRAS) status [29,30]. For example, Karagkounis and colleagues reported a significantly worse median OS (45.2 months vs 71.9 months) and 5-year OS (49.8% vs 57.4%, p = 0.007) among patients who harbored tumors with KRAS mutations (versus wtKRAS) undergoing hepatic resection for mCRC [31]. In a separate study, Yaeger et al. reported similar results when evaluating 918 patients with mCRC [29]. In this study, patients with wtKRAS experienced improved survival after surgical resection compared with patients who had mutant KRAS tumors (81 months v 47 months, respectively; p < 0.001). The association of KRAS status with outcome persisted when only patients treated with systemic therapy were analyzed (35.2 months wtKRAS v 28 months mutant KRAS; p = 0.005) [29].
More recent data have noted that the prognostic implications of KRAS status are still applicable in the modern era of multimodal therapy. For example, Vauthey et al. evaluated patients who had received neoadjuvant oxaliplatin or irinotecan-based chemotherapy (including bevacizumab) prior to curative hepatectomy for mCRC [30]. In this study, 22.3% of patients had KRAS mutated tumors and KRAS mutational status was independently associated with worse progression-free survival (PFS) and OS (hazard ratio (HR) = 1.9; p = 0.005 and HR = 2.3; p = 0.002, respectively). Five-year OS was 65.4% among patients with wtKRAS versus 44.7% among patients harboring KRAS-mutations (p = 0.002) [30]. Interestingly, KRAS mutation status was also associated with an increased incidence of lung recurrence (HR = 2.01, p = 0.01) but not liver recurrence, suggesting a variable pattern of disease recurrence for mCRC predicated on a tumor’s KRAS profile [30]. Yaeger et al. had observed a similar trend among patients with mCRC whereby KRAS mutation was associated with a higher incidence of lung metastases (HR 1.52, p < 0.01), bone metastases (HR 1.62, p = 0.012) and brain metastases (HR 3.7, p < 0.01) versus patients who had wtKRAS mCRC [29]. The association of KRAS status on biological pattern of recurrence remains equivocal, however, as other studies have not found an association. Specifically, in a study by Margonis et al., while the prognostic impact of KRAS mutation was validated for OS (KRAS mutation was associated with worse OS, HR = 1.65; p = 0.02), there was no difference in recurrence pattern among patients undergoing hepatectomy with curative intent for mCRC who had KRAS mutated versus wtKRAS tumors (liver recurrence: 39% v 52.1%, respectively; lung recurrence: 55.6% v 64.3%, respectively; both p > 0.05) [32].
These discrepant results among various studies may be attributable to the different biologic characteristics associated with specific KRAS mutations as not all KRAS mutations appear to have equal impact on outcome [33]. To this end, Andreyev et al. reported that patients with KRAS glycine to valine (G12V) and glycine to serine (G12S) codon 12 mutations had a worse recurrence-free survival (RFS) across all stages (I-IV) of CRC. Furthermore, those patients with G12V had a worse OS versus patients who had wtKRAS tumors [34,35]. In a separate study by Margonis and colleagues, the outcomes of patients undergoing hepatectomy for mCRC were stratified into the six most common KRAS mutations [33]. Patients with G12V and G12S mutations had an increased mortality compared with patients who had wtKRAS tumors (HR 1.78, p = 0.05; HR 3.33, p = 0.02; respectively) [33]. Among patients experiencing recurrence after resection, G12V (HR 2.96, p = 0.01), G12C (HR 6.74, p = 0.002) and G12S (HR = 4.91, p = 0.01) KRAS mutations had an even larger impact on patient OS [33]. Collectively, data from these studies demonstrate the significance of KRAS mutational status on PFS and OS among patients with mCRC. Whether patients possessing tumors with KRAS mutations develop specific recurrence patterns remains unclear. However, KRAS mutation status remains a strong biologic marker and continued investigation into distinct KRAS mutations may allow further tailoring of therapy.
2.1.3. BRAF
Within the mitogen activated protein kinase (MAPK) pathway (RAS/RAF/MEK/ERK), BRAF gene mutation has been the focus of much investigation. Conceptually, BRAF activation follows KRAS activation, suggesting that functional mutations in each of these genes may have similar phenotypic and therapeutic implications. However, resistance to anti-EGFR therapy has not been reliably demonstrated among patients with BRAF mutations and therefore BRAF mutational status does not preclude treatment with standard biologic agents [28,36].
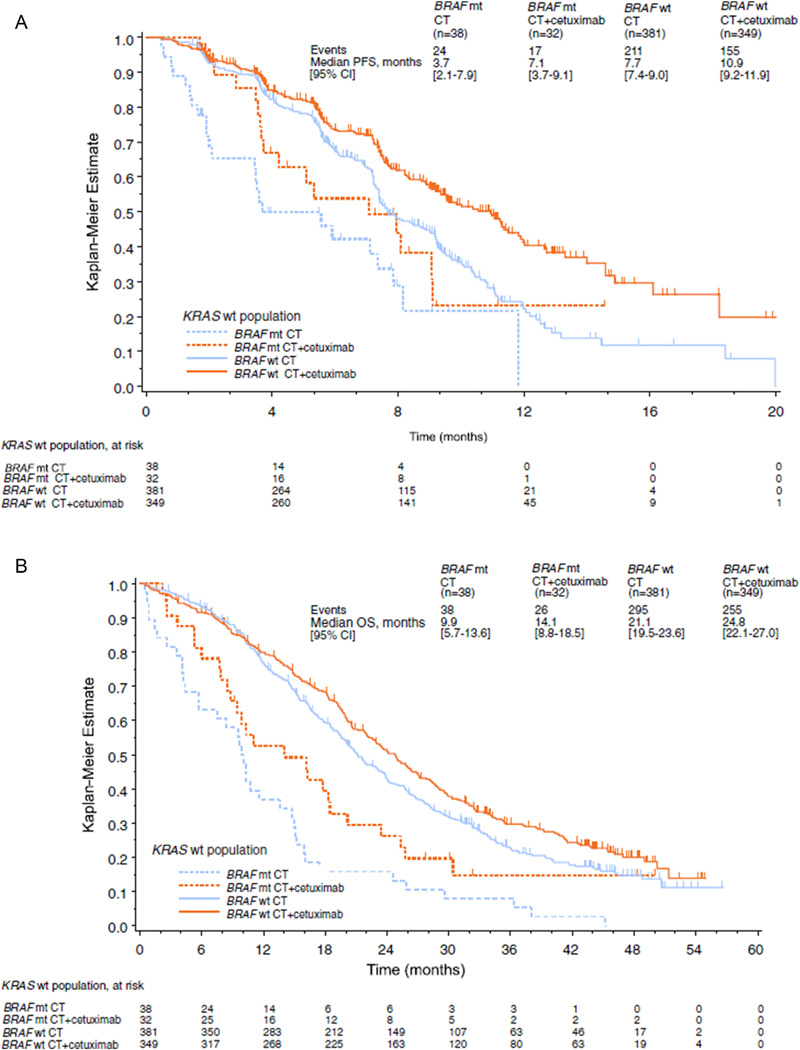
Interestingly, although BRAF mutations (BRAFV600E) are only identified in 5–10% of patients with MSS CRC, mutations are found in approximately 40–60% of patients with sporadic MSI (hypermethylation) CRC [37]. This correlation has implications for novel therapeutic targets (discussed in 2.2.4 below). BRAF mutation has also consistently been identified as a marker of worse prognosis. Specifically, BRAF mutation is associated with increased cancer mortality among patients with unresectable mCRC, as well as patients undergoing curative-intent hepatic resection [31,36,37]. For example, Tran and colleagues reported that patients with mCRC containing BRAF mutations had a significantly worse median OS (10.4 months) compared with patients who had a tumor that was wild-type BRAF (34.7 months, p < 0.01) [38]. Of note, the authors identified a propensity for peritoneal dissemination among patients with BRAF mutated tumors (46% vs 24%, p < 0.01), which may have contributed to the worse prognosis [38]. In a separate study, Bokemeyer et al. reported on 845 patients who had wtKRAS mCRC tumors and noted a markedly inferior OS among patients possessing BRAF-mutations versus patients who had wild-type BRAF tumors, irrespective of chemotherapy regimen [36]. Specifically, while the addition of an EGFR-inhibitor (cetuximab) to cytotoxic therapy improved OS among patients with a BRAF mutation versus cytotoxic therapy alone (14 months vs. 9 months, respectively), patients with BRAF-mutated tumors still experienced worse OS (9–14 months) compared with patients who had wild-type BRAF tumors (21–25 months, p < 0.01) [36]. The improved OS with the use of cetuximab indicated that BRAF mutational status did not mitigate the tumor’s response to anti-EGFR therapy (see Fig. 1). However, BRAF mutational status did remain a marker of poor prognosis.
Fig. 1.
Progression free survival and overall survival in patients with wtKRAS CRLM harboring BRAF mutations. Kaplan-Meier survival plots demonstrating impact of BRAF mutations on PFS (A) and OS (B). Although BRAF mutation status is associated with worse outcomes in patients with wtKRAS CRLM, patients with BRAF mutated tumors did demonstrate benefit to anti-EGFR therapy. CT = chemotherapy. (Figures used with permission from Bokemeyer et al. Eur J Cancer 2012).
Schirripa and colleagues recently sought to clarify the prognostic impact of BRAF mutations relative to KRAS mutations among patients with mCRC undergoing curative intent hepatectomy [14]. While confirming that BRAF mutational status was associated with a decreased OS compared with wild-type tumors (HR = 2.73, p = 0.012), patients harboring BRAF mutations demonstrated an even worse OS compared with patients who had KRAS mutations. In fact, median OS was 22.6 months, 42.0 months, and 63.3 months, for BRAF mutated, KRAS mutated, and wild-type tumors, respectively [14]. Given the strong implications of the presence or absence of BRAF mutation for patients with mCRC, current guidelines recommend evaluation of BRAF mutational status for all patients with mCRC [39]. Furthermore, in select patients with mCRC, BRAF status has become an increasing focus for targeted therapy (discussed in 2.2.3 below).
2.1.4. Telomerase
While MSI likely comprises a minority of patients with CRC, chromosome instability (CIN) is probably responsible for the remainder of patients with CRC [23]. The breakdown of the cellular capacity for maintenance of telomeres, regions protecting the end of each chromosome, is a potential driving factor behind CIN. Normally when telomeres reach a critical shortened length, cells undergo senescence [16]. In the setting of loss of protective proteins (i.e. TP53), erosion of telomeres may result in CIN favoring subsequent tumor formation [40]. The capacity of cells to avoid senescence is supported by the expression of a protein complex named Telomerase. Telomerase is composed of a telomere specific reverse transcriptase (hTERT), with an internal RNA template, and functions to elongate telomeres [16]. Telomerase is generally present only in immortalized cells (i.e. germ-line cells and cancer cells), and its acquisition in malignant cells has been substantiated as a hallmark of cancer [41].
Bertorelle and colleagues have postulated that overexpression of hTERT (as a surrogate for telomerase activity) increases replicative potential in CRC and that overexpression is associated with increased cancer recurrence [16]. In a study evaluating hTERT expression in tumor specimens from patients with stage I-IV CRC following resection, patients with elevated expression of hTERT had a significantly worse median OS compared with patients who had tumors characterized by low expression of hTERT (37 months vs. not reached, p < 0.0001) regardless of stage or systemic therapy administered [16]. Interestingly, patients with elevated hTERT expression were more likely to have advanced disease. However, even among patients with mCRC, the hazard ratio for death was about 15 times higher among patients with elevated hTERT versus low hTERT expression [16]. Similar results were obtained in several separate studies, thereby supporting the hypothesis that elevated hTERT expression (or telomerase activity) correlates with worse DFS and OS [40,42]. For example, Terrin et al. evaluated patients with CRC who underwent resection and similarly found hTERT expression to correlate with more advanced CRC disease stage [42]. Interestingly, in this study hTERT mRNA was also measured in the plasma of patients with CRC. Plasma levels of hTERT mRNA were concordant with tumor expression levels and increasing quantity of mRNA were detectable as the tumor stage advanced (2500 copies/ mL stage I and II vs. 19,600 copies/mL stage III and IV; p < 0.001). Among patients who were tumor-free there was a relative absence of circulating hTERT mRNA [42]. Circulating hTERT mRNA expression has also been found to be associated with response to therapy. For example, patients who experienced a complete pathologic response after neoadjuvant treatment have been noted to have a substantial decrease in circulating hTERT mRNA levels [43].
Collectively, results from several studies indicate a putative role for hTERT expression analysis in patients with mCRC. Use of hTERT as a biomarker may also have a role for patients who have both undergone curative-intent resection (i.e. monitor for recurrence), as well as for patients with more widespread disease whose tumor burden precludes surgical therapy (i.e. response to systemic treatment). While it appears that hTERT activity correlates with stage and prognosis, prospective studies confirming its utility are needed to better define the role of hTERT as a biologic marker.
2.1.5. Circulating tumor cells
Circulating tumor cells and disseminated tumor cells, which can be measured in the blood and bone marrow, respectively, are clinically associated with poor prognosis [44–46]. In a meta-analysis of data based on patients with mCRC, Koerkamp and colleagues reported that the presence of CTCs in blood samples correlated with worse PFS (HR 2.07, p = 0.0001) and OS (HR = 2.47, p = 0.01) compared with patients without detectable CTCs [44]. Koch and colleagues similarly reported that patients who had CTCs detected in intraoperative blood samples had a significantly shorter DFS after hepatic resection, relapsing at a median of 13 months versus 25 months for patients without CTCs [46]. In another study, Barbazan et al. examined a cohort of patients with liver-predominant metastatic disease who were receiving systemic treatment and evaluated a multi-marker CTC detection panel [17]. Patients with low-CTC (<3 markers) had a better PFS (12.7 months vs. 6.3 months; p = 0.0003) and OS (24.2 months vs. 12.7 months; p = 0.002) versus patients with high-CTC (3 or more markers) [17]. CTC biomarker analysis was again performed at 4 weeks to determine response to therapy; patients who converted from high-CTC to low-CTC, as well as those patients who remained at low-CTC, were considered responders while transition from low-CTC to high-CTC and those remaining at high-CTC were considered non-responders. In turn, categorization of CTC biomarker analysis into responders versus non-responders strongly correlated with tumor response on CT imaging, as well as provided additional prognostic information. All patients categorized as responders by CTC also demonstrated radiographic regression (RECIST partial response), with these patients experiencing the longest median OS (24.4 months). In contrast, patients who had only a partial response by RECIST and were non-responders by CTC fared significantly worse (14.4 months); furthermore, those patients who were non-responders both by imaging and CTC status had the worse median OS (5.1 months) (p < 0.0001) [17].
As such, CTC biomarker analysis may have utility as an indictor of therapeutic response, which may be used to direct therapy for patients with mCRC as well as help identify patients at highest risk of recurrence. Although emerging data on CTC are promising, compared with data on biologic markers such as KRAS and BRAF, the body of evidence to support routine use of CTC is lacking. Currently, a number of different clinical trials are investigating the impact of CTCs in patients with advanced stage CRC (NCT01596790, NCT01722903, NCT01163305, NCT01640444, NCT01640405). These trials should help to clarify the utility of CTCs in patients with mCRC.
2.1.6. Circulating tumor DNA
Another recently recognized biomarker with potential clinical implications is circulating tumor DNA (ctDNA). ctDNA differs from CTCs in that the identified fragments of nucleic acid are not associated with cells or cell fragments [47]. In a study by Bettegowda et al. that compared CTC with ctDNA, ctDNA was noted to be more sensitive for detecting tumor-specific DNA mutations and were more often identified in the circulation compared to CTCs in patients with malignancy [47]. Subsequently, Tie and colleagues have evaluated the role of ctDNA as a marker of response to systemic therapy among patients with mCRC [18]. In this study, ctDNA was identified in 92% of patients with metastatic disease; additionally, ctDNA was a superior marker of response to therapy compared with traditional carcinoembryonic antigen (CEA) levels. ctDNA levels were associated with response to therapy as 74% of patients who experienced a 10-fold decrease in ctDNA (measured at 3 weeks post-treatment after cycle 1 of systemic chemotherapy) demonstrated a radiographic RECIST response to therapy when measured at 8 weeks [18]. Due to its recent inception as a putative biomarker for mCRC, the role of ctDNA in determining outcomes for patients remains somewhat limited. However, emerging data on ctDNA suggest that it may allow more precise prediction of response (or resistance) to therapy compared to existing clinical and pathologic markers [18,47].
2.2. Therapeutic targets
The examination of the underlying biologic mechanisms of CRC has revealed the heterogeneous nature of the disease. Additionally, characterization of the various pathways involved in CRC has provided an opportunity to help direct therapy. While some attempts at targeted therapy have been unsuccessful, other endeavors have resulted in improved patient outcomes.
2.2.1. EGFR inhibitors
Perhaps the best-studied molecular therapies employed in patients with mCRC are the EGFR inhibitors, which include cetuximab and panitumumab, both of which are approved by the FDA for use in advanced CRC [48]. Ligand binding to EGFR (a receptor tyrosine kinase) triggers activation of KRAS and several downstream effectors, as well as the PI3K-AKT pathway, resulting in cell growth, proliferation, migration and angiogenesis [49]. Targeting these pathways through the use of anti-EGFR antibodies has demonstrated a benefit in specific subsets of patients with mCRC [48]. For instance, Bokemeyer and colleagues performed a randomized study evaluating 337 patients with unresectable mCRC treated in the first-line setting with cetuximab plus folinic acid (leucovorin, LV), 5-FU and oxaliplatin (FOLFOX4) versus FOLFOX4 alone (OPUS Trial – Oxaliplatin and Cetuximab in First-Line Treatment of Metastatic Colorectal Cancer) [50]. KRAS mutation status was identified in 233 patients; those with wtKRAS tumors experienced superior overall response (61% v 37%, p = 0.011) and PFS (7.7 months v 7.2 months, p = 0.0163) when cetuximab was added to FOLFOX4 compared with FOLFOX4 [50]. Similarly, in the Cetuximab Combined with Irinotecan in First-Line Therapy for Metastatic Colorectal Cancer (CRYSTAL) study, 1198 patients with unresectable mCRC were randomized to first-line cetuximab combined with folinic acid, 5-FU and irinotecan (FOLFIRI) (n = 599) or FOLFIRI (n = 599) alone [51]. Among all patients, 540 patients had data on KRAS mutation status. In the subgroup analysis of patients with wtKRAS tumors, there was a benefit in PFS (9.9 months v 8.7 months, p = 0.02) and trend towards a benefit in OS (24.9 months v 21 months, p > 0.05) in the cetuximab versus FOLFIRI alone group [51]. Furthermore, in the follow-up study, which identified KRAS mutation status in 1063 patients, the addition of cetuximab to FOLFIRI resulted in a modest increase in PFS (9.9 months v 8.4 months, p = 0.0012) and OS (23.5 months v 20 months, p = 0.0093) compared with FOLFIRI alone in the wtKRAS population [52].
A subsequent pooled analysis of the CRYSTAL and OPUS studies was performed to examine the efficacy of cetuximab encompassing a larger wtKRAS mCRC patient population (n = 845) [36]. Congruent with the individual studies, the addition of cetuximab to standard chemotherapy resulted in significant improvements in best overall response (57.3% v 38.5%, p < 0.0001), PFS (9.6 months v 7.6 months, p < 0.0001) and OS (23.5 months v 19.5 months, p = 0.0062) versus cytotoxic chemotherapy alone [36]. The impetus for these studies arose from the demonstration of cetuximab efficacy (PFS) in heavily pretreated and chemotherapy refractory patients with mCRC [53,54]. Similarly, initial studies evaluating panitumumab compared with best supportive care for patients progressing on standard chemotherapy demonstrated improved PFS, which encouraged subsequent examination of panitumumab in a first-line setting [55]. The Panitumumab Randomized Trial in Combination With Chemotherapy for Metastatic Colorectal Cancer to Determine Efficacy (PRIME trial) assessed patients with previously untreated mCRC [56]. In this study, Douillard et al. observed that patients who had wtKRAS tumors and were treated with panitumumab in conjunction with FOLFOX4 experienced improved PFS (9.6 months v 8.0 months, p < 0.05) with a trend towards improved OS versus FOLFOX4 alone. The final results from the PRIME trial reported a longer follow-up for 656 patients characterized as wtKRAS mCRC [57]. Patients receiving panitumumab plus FOLFOX4 exhibited significantly increased PFS (10 months v 8.6 months, p = 0.01) and OS (23.8 months v 19.4 months, p = 0.03) compared with FOLFOX4 alone [57].
In addition to first-line and non first-line therapy, anti-EGFR therapy may also be implemented as a means to improve the opportunity for resection in patients presenting with initially unresectable disease. Ye and colleagues investigated the resection rate among patients with initially unresectable wtKRAS mCRC after receiving mFOLFOX6 or FOLFIRI with or without cetuximab [58]. Cytotoxic chemotherapy alone resulted in an R0 hepatic resection in 7.4% of patients versus 25.7% among patients who received cytotoxic chemotherapy combined with cetuximab. When an R0 resection was achieved, median OS was extended to 46 months among patients who had received chemotherapy plus cetuximab versus 36 months for those patients who received chemotherapy alone [58].
Not all patients, however, derive a therapeutic benefit from anti-EGFR treatment. Numerous studies have noted the lack of clinical effectiveness from EGFR-inhibitor therapy among patients with RAS mutations [51,52,56,59]. One of the earliest phase III randomized studies to evaluate cetuximab in combination with cytotoxic chemotherapy was the CRYSTAL trial performed by Van Cutsem and colleagues [51]. Patients with mutated KRAS tumors did not derive benefit from addition of cetuximab, both in PFS (7.6 months v 8.1 months, p = 0.75) and OS (17.5 months v 17.7 months), compared with FOLFIRI alone [51]. Comparable findings were identified in the OPUS and PRIME trials, where patients harboring KRAS mutated mCRC demonstrated a propensity for lack of improvement with EGFR-inhibitor therapy [50,56,57,60]. In the OPUS trial (cetuximab plus FOLFOX4 v FOLFOX4), patients possessing KRAS mutated tumors (n = 99) trended towards decreased overall response (33% v 49%, p = 0.106) when receiving cetuximab and appeared to experience worse PFS (5.5 months v 8.6 months, p = 0.0192) compared with patients treated with FOLFOX4 alone [50]. These outcomes were also evident in the subsequent updated analysis (n = 136), with worse PFS in the EGFR-inhibitor group (5.5 months v 8.6 months, p = 0.0153) [60]. In the original PRIME trial (panitumumab plus FOLFOX4 v FOLFOX4), patients with KRAS mutated tumors (n = 440) receiving panitumumab experienced worse PFS (7.3 months v 8.8 months, p = 0.02) with no difference in OS (15.5 months v 19.3 months, p = 0.068) versus chemotherapy alone; the updated results were quite similar (PFS: 7.4 months v 9.2 months, p = 0.02; OS 15.5 months v 19.2 months, p = 0.16) [56,57].
Collectively, these studies establish that EGFR inhibitors as an adjunct to FOLFOX or FOLFIRI in patients with mCRC are ineffective in patients with KRAS mutated mCRC and these agents should only be utilized in patients with wtKRAS tumors. This concept was substantiated in a recent meta-analysis of trials evaluating the efficacy of EGFR inhibitors for patients with advanced CRC [48]. EGFR inhibitors demonstrated no benefit in PFS (HR = 0.99, p = 0.93) or OS (HR = 1.00, p = 0.99) compared with cytotoxic chemotherapy alone when KRAS mutations were present. Importantly, this holds true regardless of line of therapy in patients with unresectable disease [48]. For this reason, KRAS (exon 2 – codon 12/13, exon 3, exon4) and NRAS (exon 2, 3, 4) mutation status is currently a standard biomarker, which if present precludes the use of EGFR inhibitors [39,49].
Although KRAS mutation status is an important indicator for therapeutic efficacy, in patients with wtKRAS tumors, there remain subsets of patients who do not respond to EGFR-inhibitor therapy, indicating alternate mechanisms of resistance. Evidence suggesting additional mechanisms influencing response to therapy has been highlighted in several trials [61,62]. For instance, Maughan and colleagues investigated the effects of cetuximab when added to a fluoropyramidine and oxaliplatin regimen as first-line therapy in patients with advanced CRC [61]. Patients with wtKRAS tumors demonstrated no difference in risk of progression (8.6 months v 8.6 months, p = 0.6) or OS (17.0 months v 17.9 months) whether treated with cetuximab in addition to chemotherapy (n = 362) or chemotherapy alone (n = 367). Although patients with wtKRAS tumors demonstrated superior overall survival versus patients with KRAS mutations, NRAS mutations or BRAF mutations (17.5 months v 13.8 months v 14.4 months v 8.8 months, p < 0.0001), outcome was independent of EGFR therapy [61]. These results indicate that in patients possessing unresectable wtKRAS mCRC, additional biologic predictors may help identify the discrete patient population that responds to anti-EGFR therapy.
In contrast to studies evaluating unresectable disease, Primrose and colleagues sought to investigate the effect of cetuximab in patients with wtKRAS mCRC undergoing liver resection [62]. These authors reported that patients treated with cetuximab in addition to chemotherapy (12 weeks prior and 12 weeks following surgery) experienced reduced OS versus patients receiving chemotherapy alone. Specifically, patients treated with hepatectomy who received perioperative chemotherapy that included cetuximab had a median PFS of 14.1 months compared with 20.5 months among those patients who received perioperative cytotoxic chemotherapy alone (p = 0.03). The reason for these findings remains unclear and may be related to selection bias in the two study arms. However, judicious use of cetuximab among patients with resectable liver metastases should be considered in light of these data [39,62].
In the trials demonstrating that anti-EGFR therapy was ineffective for patients with wtKRAS tumors, proposed underlying etiologies included mutations in BRAF or PI3K [61,62]. Although BRAF testing is currently recommended for mCRC and is a strong negative prognostic marker, data supporting the use of BRAF mutation as a predictive marker for EGFR-inhibitor resistance is equivocal [36,52,61]. Studies evaluating mutations in additional genes such as PI3K (exon 9 or 20), PTEN, MAPK and MEK, whose protein products function downstream of EGFR, have not proven effective as biologic predictors of response to EGFR-inhibition [49,63]. Therefore, while the use of EGFR-inhibitor therapy can potentially provide a therapeutic benefit, an understanding of the complete underlying tumor biology that portends a good response remains elusive.
2.2.2. VEGF inhibitors
Vascular endothelial growth factor signaling is important for angiogenesis, a requisite characteristic for solid malignancy progression [41]. The targeting of this pathway has led to the development of VEGF pathway inhibitors as a strategy to inhibit tumor angiogenesis. Currently, bevacizumab, a humanized monoclonal antibody against VEGF-A, is approved for use in mCRC as first-line or non-first-line therapy due to modest improvements in PFS and OS, which has been demonstrated in various trials [64–66].
Following a phase 2 trial that reported increased response rate and PFS in patients with mCRC when bevacizumab was added to 5-FU/LV compared with 5-FU/LV alone, Hurwitz and colleagues sought to examine the effect of bevacizumab when combined with a more clinically active regimen [64,67]. In this pivotal study, 813 patients with unresectable mCRC were randomly assigned to irinotecan, 5-FU and leucovorin (IFL) with placebo (n = 411) or bevacizumab (n = 403). Patients administered bevacizumab demonstrated improved PFS (10.6 months v 6.2 months, p < 0.001) and OS (20.3 months v 15.6 months) compared to patients receiving only IFL. When examining the safety profile associated with bevacizumab, there was an increased rate of overall complications (84.9% v 74%, p < 0.01) compared with IFL alone [64]. Despite this, the favorable increase in PFS and OS with addition of bevacizumab to IFL established its use as first-line treatment of mCRC and provided a foundation for subsequent studies in mCRC. One of the ensuing studies by Saltz et al. investigated the effect of bevacizumab (n = 699) versus placebo (n = 701) in addition to FOLFOX4 or capecitabine plus oxaliplatin (XELOX) in patients with unresectable mCRC [66]. Duration of PFS was significantly increased in the bevacizumab arm compared with chemotherapy alone (9.4 months v 8.0 months, p = 0.0023); however, there was no difference in OS (21.3 months v 19.9 months, p = 0.077). Furthermore, there was no difference in complications between the two groups (80% v 75%) [66].
Due to the efficacy of bevacizumab in patients with unresectable mCRC, its use in the perioperative setting has also been investigated. Gruenberger and colleagues performed a single-center, nonrandomized, phase II study to investigate the safety and efficacy of neoadjuvant (first-line) bevacizumab in combination with XELOX in patients undergoing curative intent resection of mCRC [68]. Enrolled patients were considered high risk for recurrence (according to Fong clinical risk score) and received a median number of 6 cycles prior to surgery; bevacizumab was discontinued 5 weeks prior to surgery [6]. Potentially curative surgery (R0 resection) was accomplished in 52 of 56 patients and complete or partial pathologic response was seen in 73.2%. Median OS was not reported. Patients tolerated the treatment with low incidence of adverse events including thromboembolic events (7%), hypertension (3%) and gastrointestinal perforation (2%). Furthermore, there was no detriment to liver regeneration, or increased wound healing or bleeding complications. The authors reported bevacizumab was efficacious (pathologic response) and well tolerated as neoadjuvant therapy with cessation 6 weeks prior to surgery [68]. The safety and efficacy of neoadjuvant bevacizumab was also assessed in the Bevacizumab Expanded Access Trial (BEAT) (subgroup analysis) [69]. In this study, there were 1914 patients with mCRC (initially unsuitable for resection) who received bevacizumab as an adjunct to standard first-line chemotherapy including FOLFOX (29%), FOL-FIRI (26%) and XELOX (18%), among others. Curative-intent resection was performed in 225 patients (11.8%) and R0 resection achieved in 173 (76.9%). Median OS was 21.4 months overall in the 1769 patients not undergoing metastectomy and had not been reached at the time of the study in patients undergoing curative resection (HR = 0.24, p < 0.001) [70]. Hypertension occurred in 5%, wound-healing complications in 4%, GI perforation in 4% of patients with unresected primary tumor, and bleeding events were infrequent (<0.5%) [69]. Although bevacizumab appeared to be well tolerated as neoadjuvant therapy, the efficacy, including ability of bevacizumab to ‘downstage’ to resectable disease, proved difficult to determine (lack of specific criteria for inoperable disease pretherapy). Therefore, because of the insufficient data in favor of bevacizumab therapy for the purposes of conversion to resectable disease or neoadjuvant treatment, there are currently no strong recommendations for use in this regard [39]. Rather, guidelines suggest an active systemic chemotherapy regimen for a total of 6 months with frequent monitoring to determine timing of resection (if possible); if bevacizumab is being administered prior to resection of mCRC, it should be discontinued at least 6 weeks prior to surgery [39].
There have been no randomized trials directly addressing the use of bevacizumab for patients with mCRC following surgical resection of metastases. However, there is data demonstrating no benefit when used in the adjuvant setting for stage II and III colorectal cancer [71]. The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-08 phase III randomized trial was designed to evaluate the putative benefit to PFS and OS when bevacizumab (1-year duration) was added to mFOLFOX6 (6 months duration) in the adjuvant setting for stage III and high-risk stage II colorectal cancer [71]. The study population was comprised of a bevacizumab plus mFOLFOX6 arm (n = 1334) and control arm (mFOLFOX6 only, n = 1338). There was no benefit in DFS with addition of bevacizumab compared to chemotherapy alone in stage III (74.2% v 72.4%, p = 025) or stage II (87.4% v 84.7%) CRC [71]. For this reason, bevacizumab should not be used for stage II or stage III CRC, and likewise, bevacizumab is not recommended following resection of metastatic disease unless response was noted pre-operatively [39].
Aflibercept (recombinant protein with VEGF receptor 1 and VEGF receptor 2 components fused to IgG1) and ramucirumab (antibody directed against VEGF receptor 2) are newer VEGF pathway inhibitors used as second and third line agents to treat mCRC. One study demonstrated that for patients who had received prior oxaliplatin based treatment for mCRC, aflibercept plus FOLFIRI provided a small increase in median OS compared with FOLFIRI alone (13.5 months v 12.1 months, p = 0.003) [72]. Similarly, in a different study that evaluated patients with mCRC who progressed on first-line bevacizumab, oxaliplatin and 5-FU, patients receiving FOLFIRI plus ramucirumab experienced an increased median OS versus patients receiving FOLFIRI alone (13.3 months v 11.7 months, p = 0.022) [73]. The role of these newer agents outside of second-line therapy in patients who are FOLFIRI naive has not been well characterized. Therefore, aflibercept and ramucirumab are only recommended in this setting [39]. Presently, there are no bio-markers predicting response to VEGF inhibitors.
2.2.3. BRAF inhibitors
A potential utility of identifying MSI-H colorectal tumors is the propensity for these tumors to have concomitant BRAF mutations [23]. As noted, BRAF mutations are identified in approximately 40–60% of patients possessing sporadic MSI-H tumors, but are relatively infrequent among patients with MSS colorectal cancer (10% of patients). Although BRAF-inhibitors have not proven particularly effective (response rate of approximately 5%) for patients with stage IV CRC with the MSI-H genotype, there are numerous studies examining treatment approaches to overcome the resistance to BRAF inhibition [74].
BRAF mutants result in constitutively activated BRAF with ensuing downstream activation of MEK and ERK. This subsequently leads to increased expression of growth-promoting gene products [37]. Tumor resistance to BRAF inhibitors may arise from alterations of proteins in this pathway such as KRAS (and downstream PI3K), RAF, MEK and ERK. Another proposed mechanism of action includes feedback regulation of EGFR (CD25c) [37,74]. Therefore, effective therapy will likely require inhibition of numerous redundant components of the MAP kinase pathway. Targeting these proteins (EGFR, MAPK, MEK) in combination with BRAF inhibition has been reported to be associated with a modest (12–40%) response rate and is currently the focus of ongoing investigations [23,74–76].
2.2.4. PD-1 inhibitors
MSI-H tumors possess a significant concentration of infiltrating tumor lymphocytes at the tumor-stromal interface that are suppressed by immune-inhibitory signals such as the programmed death-1 (PD-1) and PD ligand (PD-L1) complexes [25]. In turn, Le and colleagues have proposed directed immunotherapy to target PD-1 in an attempt to induce tumor regression among patients with MSI-H tumors [25]. Mechanistically, the PD-1 pathway is involved in suppression of the Th1 cytotoxic immune response, and by inhibiting PD-1, a targeted immune response can promote tumor regression.
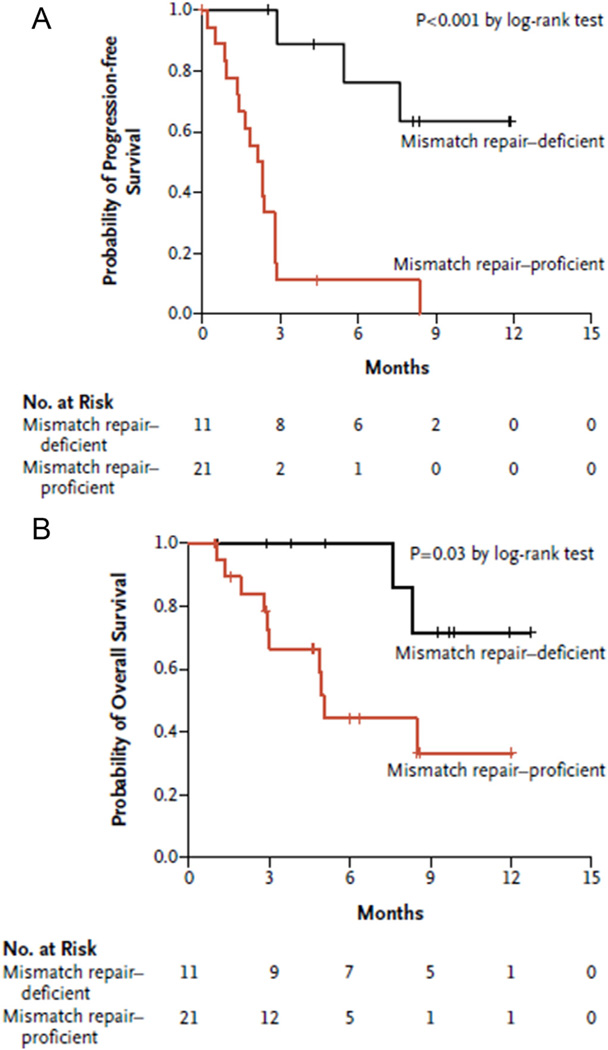
Le et al. have evaluated MSI-H and MSS patients with PD-1 directed treatment in the setting of refractory metastatic cancer [25]. Patients with MMR deficient (i.e. MSI-H) CRC had a significantly increased response to pembrolizumab (anti-PD-1 antibody) compared with patients who had MMR proficient tumors (see Fig. 2). Remarkably, among patients with MSI-H tumors, 40% had a partial response and an additional 50% experienced stable disease (90% disease control rate); in comparison, only 11% of patients with MSS tumors experienced stable disease [25]. The largest benefit of anti-PD-1 therapy was noted among patients who had the sporadic form of MSI. Specifically, among this subset of patients, 100% experienced objective response versus only 27% of patients with CRC arising in the setting of Lynch Syndrome [25]. Importantly, data from this study demonstrated that a population of patients with advanced CRC could be identified, selected and targeted for anti-PD-1 therapy. Such an approach is a prime example of using biologic pathways that may be unique to a malignant process to optimize and individualize patient care for CRC.
Fig. 2.
Progression free survival and overall survival in patients with mismatch repair deficient (MSI-H) and mismatch repair proficient colorectal cancer. Kaplan-Meier curves demonstrate PFS (A) and OS (B) in patients with colorectal cancer treated with the anti-PD1 antibody pembrolizumab. Shown are patients with mismatch repair deficient (MSI-H) tumors compared to mismatch repair proficient tumors. (Figures used with permission from Le et al. NEJM 2015).
3. Conclusion
Metastatic colorectal cancer encompasses a variable spectrum of disease phenotypes. Over the last several decades, there has been an increased understanding of the underlying tumor biology that differentiates patients with mCRC. The expanding number of identified pathways that contribute to CRC tumorigenesis have enabled discernment of putative biologic markers and therapeutic targets. In turn, novel markers in conjunction with clinical and pathologic features have allowed for more individualized approaches to patients with mCRC. Despite this solid foundation, continued investigative efforts are clearly required in order to better understand the varied and intricate molecular pathways involved in CRC tumorigenesis, which can then facilitate further refinement of individualized therapy for patients with metastatic disease. Additionally, as treatment decisions for patients with mCRC become more complex, the fundamental role of a multidisciplinary team participating in the management of these patients cannot be overlooked.
Abbreviations
- mCRC
metastatic colorectal cancer
- CRC
colorectal cancer
- OS
overall survival
- DFI
disease free interval
- MSI
microsatellite instability
- CTC
circulating tumor cells
- ctDNA
circulating tumor DNA
- MMR
mismatch repair
- MSS
microsatellite stable
- EGFR
epidermal growth factor receptor
- VEGF
vacular endothelial growth factor
- wtKRAS
wild type KRAS
- PFS
progression free survival
- RFS
recurrence free survival
- CIN
chromosomal instability
- DFS
disease free survival
Footnotes
Conflicts of interest
None.
Author disclosures
The authors have nothing to disclose.
References
- 1.Wanebo HJ, LeGolvan M, Paty PB, Saha S, Zuber M, D’Angelica MI, et al. Meeting the biologic challenge of colorectal metastases. Clin. Exp. Metastasis. 2012;29(7):821–839. doi: 10.1007/s10585-012-9517-x. [DOI] [PubMed] [Google Scholar]
- 2.Chua TC, Saxena A, Liauw W, Kokandi A, Morris DL. Systematic review of randomized and nonrandomized trials of the clinical response and outcomes of neoadjuvant systemic chemotherapy for resectable colorectal liver metastases. Ann. Surg. Oncol. 2010;17(2):492–501. doi: 10.1245/s10434-009-0781-1. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Colorectal Cancer Facts and Figures 2014–2016. American Cancer Society. 2014 [Google Scholar]
- 4.Bredt LC, Rachid AF. Predictors of recurrence after a first hepatectomy for colorectal cancer liver metastases: a retrospective analysis. World J. Surg. Oncol. 2014;12:391. doi: 10.1186/1477-7819-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: the evolution of determining prognosis. World J. Gastrointest. Oncol. 2013;5(12):207–221. doi: 10.4251/wjgo.v5.i12.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann. Surg. 1999;230(3):18–21. doi: 10.1097/00000658-199909000-00004. 309–18; discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung WS, Park MS, Shin SJ, Baek SE, Kim YE, Choi JY, et al. Response evaluation in patients with colorectal liver metastases: RECIST version 1.1 versus modified CT criteria. AJR Am. J. Roentgenol. 2012;199(4):809–815. doi: 10.2214/AJR.11.7910. [DOI] [PubMed] [Google Scholar]
- 8.Blazer 3rd DG, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J. Clin. Oncol. 2008;26(33):5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 9.Gruenberger B, Scheithauer W, Punzengruber R, Zielinski C, Tamandl D, Gruenberger T. Importance of response to neoadjuvant chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120. doi: 10.1186/1471-2407-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Boonsirikamchai P, Asran MA, Maru DM, Vauthey JN, Kaur H, Kopetz S, et al. CT findings of response and recurrence, independent of change in tumor size, in colorectal liver metastasis treated with bevacizumab. AJR Am. J. Roentgenol. 2011;197(6):W1060–W1066. doi: 10.2214/AJR.11.6459. [DOI] [PubMed] [Google Scholar]
- 12.Chun YS, Vauthey JN, Boonsirikamchai P, Maru DM, Kopetz S, Palavecino M, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302(21):2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubbia-Brandt L, Giostra E, Brezault C, Roth AD, Andres A, Audard V, et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neoadjuvant chemotherapy followed by liver surgery. Ann. Oncol. 2007;18(2):299–304. doi: 10.1093/annonc/mdl386. [DOI] [PubMed] [Google Scholar]
- 14.Schirripa M, Bergamo F, Cremolini C, Casagrande M, Lonardi S, Aprile G, et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br. J. Cancer. 2015;112(12):1921–1928. doi: 10.1038/bjc.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, et al. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H) Ann. Oncol. 2014;25(5):1032–1038. doi: 10.1093/annonc/mdu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertorelle R, Briarava M, Rampazzo E, Biasini L, Agostini M, Maretto I, et al. Telomerase is an independent prognostic marker of overall survival in patients with colorectal cancer. Br. J. Cancer. 2013;108(2):278–284. doi: 10.1038/bjc.2012.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbazan J, Muinelo-Romay L, Vieito M, Candamio S, Diaz-Lopez A, Cano A, et al. A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. Int. J. Cancer. 2014;135(11):2633–2643. doi: 10.1002/ijc.28910. [DOI] [PubMed] [Google Scholar]
- 18.Tie J, Kinde I, Wang Y, L Wong H, Roebert J, Christie M, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann. Oncol. 2015;26(8):1715–1722. doi: 10.1093/annonc/mdv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanecz A, Kavalar R, Palfy M, Pivec V, Sremec M, Horvat M, et al. Can we improve the clinical risk score? the prognostic value of p53, Ki-67 and thy-midylate synthase in patients undergoing radical resection of colorectal liver metastases. HPB Oxf. 2014;16(3):235–242. doi: 10.1111/hpb.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maithel SK, Gonen M, Ito H, Dematteo RP, Allen PJ, Fong Y, et al. Improving the clinical risk score: an analysis of molecular biomarkers in the era of modern chemotherapy for resectable hepatic colorectal cancer metastases. Surgery. 2012;151(2):162–170. doi: 10.1016/j.surg.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Neal CP, Garcea G, Doucas H, Manson MM, Sutton CD, Dennison AR, et al. Molecular prognostic markers in resectable colorectal liver metastases: a systematic review. Eur. J. Cancer. 2006;42(12):1728–1743. doi: 10.1016/j.ejca.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 22.Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer. 2009;100(2):266–73. doi: 10.1038/sj.bjc.6604867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3(5):502–511. doi: 10.1158/2159-8290.CD-12-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J. Natl. Cancer Inst. 2011;103(11):863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J. Gastroenterol. 2012;18(37):5171–5180. doi: 10.3748/wjg.v18.i37.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price TJ, Bruhn MA, Lee CK, Hardingham JE, Townsend AR, Mann KP, et al. Correlation of extended RAS and PIK3CA gene mutation status with outcomes from the phase III AGITG MAX STUDY involving capecitabine alone or in combination with bevacizumab plus or minus mitomycin C in advanced colorectal cancer. Br. J. Cancer. 2015;112(6):963–970. doi: 10.1038/bjc.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.L Hurwitz H, Yi J, Ince W, Novotny WF, Rosen O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist. 2009;14(1):22–28. doi: 10.1634/theoncologist.2008-0213. [DOI] [PubMed] [Google Scholar]
- 29.Yaeger R, Cowell E, Chou JF, Gewirtz AN, Borsu L, Vakiani E, et al. RAS mutations affect pattern of metastatic spread and increase propensity for brain metastasis in colorectal cancer. Cancer. 2015;121(8):1195–1203. doi: 10.1002/cncr.29196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vauthey JN, Zimmitti G, Kopetz SE, Shindoh J, Chen SS, Andreou A, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann. Surg. 2013;258(4):26–27. doi: 10.1097/SLA.0b013e3182a5025a. 619–26; discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karagkounis G, Torbenson MS, Daniel HD, Azad NS, Diaz LA, Jr, Donehower RC, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer. 2013;119(23):4137–4144. doi: 10.1002/cncr.28347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margonis GA, Spolverato G, Kim Y, Karagkounis G, Choti MA, Pawlik TM. Effect of KRAS mutation on long-term outcomes of patients undergoing hepatic resection for colorectal liver metastases. Ann. Surg. Oncol. 2015;22(13):4158–4165. doi: 10.1245/s10434-015-4587-z. [DOI] [PubMed] [Google Scholar]
- 33.Margonis GA, Kim Y, Spolverato G, Ejaz A, Gupta R, Cosgrove D, et al. Association between specific mutations in KRAS Codon 12 and colorectal liver metastasis. JAMA Surg. 2015;150(8):722–729. doi: 10.1001/jamasurg.2015.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ study. Br. J. Cancer. 2001;85(5):692–696. doi: 10.1054/bjoc.2001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter “RASCAL” study. J. Natl. Cancer Inst. 1998;90(9):675–684. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- 36.Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer. 2012;48(10):1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 37.Tie J, Desai J. Targeting BRAF mutant metastatic colorectal cancer: clinical implications and emerging therapeutic strategies. Target Oncol. 2015;10(2):179–188. doi: 10.1007/s11523-014-0330-0. [DOI] [PubMed] [Google Scholar]
- 38.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117(20):4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benson 3rd AB, Venook AP, Bekaii-Saab T, Chan E, Chen YJ, Cooper HS, et al. Colon cancer, version 3.2014. J. Natl. Compr. Cane Netw. 2014;12(7):1028–1059. doi: 10.6004/jnccn.2014.0099. [DOI] [PubMed] [Google Scholar]
- 40.Bertorelle R, Rampazzo E, Pucciarelli S, Nitti D, De Rossi A. Telomeres, telomerase and colorectal cancer. World J. Gastroenterol. 2014;20(8):1940–1950. doi: 10.3748/wjg.v20.i8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Terrin L, Rampazzo E, Pucciarelli S, Agostini M, Bertorelle R, Esposito G, et al. Relationship between tumor and plasma levels of hTERT mRNA in patients with colorectal cancer: implications for monitoring of neoplastic disease. Clin. Cancer Res. 2008;14(22):7444–7451. doi: 10.1158/1078-0432.CCR-08-0478. [DOI] [PubMed] [Google Scholar]
- 43.Pucciarelli S, Rampazzo E, Briarava M, Maretto I, Agostini M, Digito M, et al. Telomere-specific reverse transcriptase (hTERT) and cell-free RNA in plasma as predictors of pathologic tumor response in rectal cancer patients receiving neoadjuvant chemoradiotherapy. Ann. Surg. Oncol. 2012;19(9):3089–3096. doi: 10.1245/s10434-012-2272-z. [DOI] [PubMed] [Google Scholar]
- 44.Groot Koerkamp B, Rahbari NN, Buchler MW, Koch M, Weitz J. Circulating tumor cells and prognosis of patients with resectable colorectal liver metastases or widespread metastatic colorectal cancer: a meta-analysis. Ann. Surg. Oncol. 2013;20(7):2156–2165. doi: 10.1245/s10434-013-2907-8. [DOI] [PubMed] [Google Scholar]
- 45.Vogelaar FJ, Mesker WE, Rijken AM, van Pelt GW, van Leeuwen AM, Tanke HJ, et al. Clinical impact of different detection methods for disseminated tumor cells in bone marrow of patients undergoing surgical resection of colorectal liver metastases: a prospective follow-up study. BMC Cancer. 2010;10:153. doi: 10.1186/1471-2407-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koch M, Kienle P, Hinz U, Antolovic D, Schmidt J, Herfarth C, et al. Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann. Surg. 2005;241(2):199–205. doi: 10.1097/01.sla.0000151795.15068.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.L Vale C, Tierney JF, Fisher D, Adams RA, Kaplan R, Maughan TS, et al. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat. Rev. 2012;38(6):618–625. doi: 10.1016/j.ctrv.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Shaib W, Mahajan R, El-Rayes B. Markers of resistance to anti-EGFR therapy in colorectal cancer. J. Gastrointest. Oncol. 2013;4(3):308–318. doi: 10.3978/j.issn.2078-6891.2013.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2009;27(5):663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 51.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 2009;360(14):1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 52.Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011;29(15):2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351(4):337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 54.Wilke H, Glynne-Jones R, Thaler J, Adenis A, Preusser P, Aguilar EA, et al. Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL Study. J. Clin. Oncol. 2008;26(33):5335–5343. doi: 10.1200/JCO.2008.16.3758. [DOI] [PubMed] [Google Scholar]
- 55.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 2007;25(13):1658–1664. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 56.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol. 2010;28(31):4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 57.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014;25(7):1346–1355. doi: 10.1093/annonc/mdu141. [DOI] [PubMed] [Google Scholar]
- 58.Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J. Clin. Oncol. 2013;31(16):1931–1938. doi: 10.1200/JCO.2012.44.8308. [DOI] [PubMed] [Google Scholar]
- 59.Peeters M, Oliner KS, Price TJ, Cervantes A, Sobrero AF, Ducreux M, et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal Cancer. Clin. Cancer Res. 2015;21(24):5469–5479. doi: 10.1158/1078-0432.CCR-15-0526. [DOI] [PubMed] [Google Scholar]
- 60.Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann. Oncol. 2011;22(7):1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 61.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–2114. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Primrose J, Falk S, Finch-Jones M, Valle J, O’Reilly D, Siriwardena A, et al. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 2014;15(6):601–611. doi: 10.1016/S1470-2045(14)70105-6. [DOI] [PubMed] [Google Scholar]
- 63.Mao C, Yang ZY, Hu XF, Chen Q, Tang JL. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann. Oncol. 2012;23(6):1518–1525. doi: 10.1093/annonc/mdr464. [DOI] [PubMed] [Google Scholar]
- 64.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 65.Macedo LT, da Costa Lima AB, Sasse AD. Addition of bevacizumab to first-line chemotherapy in advanced colorectal cancer: a systematic review and meta-analysis, with emphasis on chemotherapy subgroups. BMC Cancer. 2012;12:89. doi: 10.1186/1471-2407-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 67.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 2003;21(1):60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 68.Gruenberger B, Tamandl D, Schueller J, Scheithauer W, Zielinski C, Herbst F, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer. J. Clin. Oncol. 2008;26(11):1830–1835. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 69.Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann. Oncol. 2009;20(11):1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 70.Okines A, Puerto OD, Cunningham D, Chau I, Van Cutsem E, Saltz L, et al. Surgery with curative-intent in patients treated with first-line chemotherapy plus bevacizumab for metastatic colorectal cancer First BEAT and the randomised phase-Ill N016966 trial. Br. J. Cancer. 2009;101(7):1033–1038. doi: 10.1038/sj.bjc.6605259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allegra CJ, Yothers G, O’Connell MJ, Sharif S, Petrelli NJ, Colangelo LH, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J. Clin. Oncol. 2011;29(1):11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012;30(28):3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 73.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 74.Ahronian LG, Sennott EM, Van Allen EM, Wagle N, Kwak EL, Faris JE, et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal Cancer through MAPK pathway alterations. Cancer Discov. 2015;5(4):358–367. doi: 10.1158/2159-8290.CD-14-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Corcoran RB, Atreya CE, Falchook GS, L Kwak E, Ryan DP, Bendell JC, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J. Clin. Oncol. 2015;1(33):4023–4031. doi: 10.1200/JCO.2015.63.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bendell JCAC, Andre T, Tabernero J, Gordon MS, Bernards R, Van Cutsem E, Tejpar S, Sidhu R, Go WY, Allred A, Motwani M, Suttle BB, Wu Y, Hoos A, Orford KW, Corcoran RB, Schellens JHM. Efficacy and tolerability in an open-label phas I/II study of MEK inhibitor trametinib (T), BRAF inhibitor dabrafenib (D) and anti-EGFR antibody panitumumab (P) in combination in patients with BRAF V600E mutated colorectal cancer (CRC) J. Clin. Oncol. 2014;(5s):32. [Google Scholar]