Abstract
Patients with cystic fibrosis (CF) exhibit an excessive host inflammatory response. The aim of this study was to determine (i) whether interleukin 8 (IL-8) secretion is increased from monocytes from subjects heterozygous as well as homozygous for cystic fibrosis transmembrane conductance regulator (CFTR) mutations and (ii) whether this is due to increased cell surface lipopolysaccharide (LPS) receptors or, alternatively, increased activation of mitogen-activated protein kinases (MAPK). The basal level of IL-8 secretion was higher from monocytes from CF patients than from monocytes from healthy controls (P = 0.02) and obligate heterozygotes (parents of the CF patients). The 50% effective concentrations for LPS-induced IL-8 production for monocytes from both CF patients and obligate heterozygotes were 100-fold lower than those for monocytes from healthy controls (P < 0.05). No differences in the levels of IL-1β production were seen between these groups. Expression of the LPS surface receptors CD14 and Toll-like receptor 4 were not different between CF patients and healthy controls. In contrast, phosphorylation of the MAPKs p38 and ERK occurred at lower doses of LPS in monocytes from patients heterozygous and homozygous for CFTR mutations. These results indicate that a single allelic CFTR mutation is sufficient to augment IL-8 secretion in response to LPS. This is not a result of increased LPS receptor expression but, rather, is associated with alterations in MAPK signaling.
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) (16). Patients with CF express a typical phenotype characterized by recurrent pulmonary infections that ultimately lead to pulmonary failure and death. It has become evident that in addition to poor clearance of bacteria, pulmonary disease in CF patients is characterized by an excessive inflammatory response to infection by Pseudomonas aeruginosa. Even in the absence of detectable lung infection, the bronchoalveolar lavage (BAL) fluid of CF patients contains increased levels of proinflammatory cytokines and neutrophils (10, 13). In addition, interleukin 8 (IL-8) levels in BAL fluids from children with CF are significantly higher than those in BAL fluids from non-CF children with bacterial infection of the lower airways (14). These data suggest that CFTR mutations may lead to an excessive inflammatory response in the lung. This appears to be a constitutive abnormality, since CF bronchial submucosal glands cultured from CF patients demonstrate increases in basal IL-6 and IL-8 levels compared with those cultured from non-CF control cells (9, 21). This increase in IL-8 levels can be explained by significant amounts of constitutively activated NF-κB in these CF cell lines (5) as well as low levels of cytosolic factor IκB in CF bronchial gland cells (19). In addition to the increases in the levels of these proinflammatory mediators, the levels of the anti-inflammatory interleukin, IL-10, have been reported to be markedly decreased both in BAL fluid (2) and in isolated bronchial epithelial cells from CF patients (1).
In an effort to determine whether inflammatory cells are involved in the genesis of this altered inflammatory response, macrophages from CF patients, differentiated both in vitro from monocytes (15) and from BAL fluid (2, 10), have been shown to secrete increased levels of IL-8 as well as to show increased tumor necrosis factor alpha (TNF-α) responses to LPS compared to those of macrophages from healthy controls. A similar hypersensitivity to LPS-induced induction of TNF-α mRNA has been shown in cultured bone marrow-derived macrophages from G551D CF mice (22). This altered monocyte/macrophage response in CF is consistent with the fact that monocytes and macrophages express CFTR RNA, based on reverse transcription-PCR and Southern analysis (22, 28).
Although monocytes/macrophages from CF patients secrete increased amounts of proinflammatory cytokines, this could be explained by the chronic state of infection of the host. In order to determine whether CFTR dysfunction directly leads to enhanced cytokine secretion from peripheral blood monocytes, we examined both basal and stimulated IL-8 secretion from the monocytes of CF patients as well as those of healthy obligate heterozygotes and compared these results to those observed for healthy control subjects. Our data indicate that the 50% effective concentration (EC50) for LPS-induced IL-8 secretion for monocytes from humans homozygous or heterozygous for CFTR mutations is decreased 100-fold. We also show that this enhanced IL-8 secretion is not due to changes in LPS receptors but, rather, is associated with increased phosphorylation of the mitogen-activated protein kinases (MAPK) P38 and ERK.
MATERIALS AND METHODS
Reagents and antibodies.
Histopaque and Escherichia coli O55:B5 LPS were purchased from Sigma Diagnostics (St. Louis, Mo.); RPMI 1640 medium, penicillin-streptomycin, and fetal bovine serum (FBS) were purchased from Life Technologies (Gaithersburg, Md.). Antibodies and cytokines were purchased from the following sources: recombinant human IL-8, anti-human IL-8, and IL-1β from R&D Systems (Minneapolis, Minn.); mouse anti-human CD14 from Biosource International (Camarillo, Calif.); mouse anti-human TLR4 from Serotec Inc. (Raleigh, N.C.); and purified mouse immunoglobulin G1 (IgG1) and mouse IgG2a from BD Biosciences (San Jose, Calif.). Goat serum and goat anti-mouse fluorescein isothiocyanate were from Jackson ImmunoResearch (West Grove, Pa.), and all MAPK antibodies were obtained from Cell Signaling Technologies (Beverly, Mass.).
Study population.
In this prospective study, 11 CF patients (6 males and 5 females; mean age, 25 ± 6.8 years), 10 obligate heterozygotes (1 male and 9 females; mean age, 44.4 ± 7.3 years), and 9 healthy controls (4 males and 5 females; mean age, 31 ± 6.4 years) were enrolled for analysis of IL-8 secretion.
An additional five CF patients (four males and one female; mean age, 25.4 ± 6.9 years) and five healthy controls (four males and one female; mean age, 32 ± 5.4 years) were enrolled for fluorescence-activated cell sorter (FACS) analysis of CD14 and Toll-like receptor 4 receptors.
For MAPK immunoblot assays, an additional six CF patients (four males and two females; mean age, 19.61 ± 8.9 years), six heterozygotes (six females; mean age, 49.3 ± 11.0 years), and six healthy controls (five males and one female; mean age, 30.3 ± 5.9 years) were enrolled.
The CF patients were clinically stable at the time of the study and had mild to moderate disease with a mean forced expiratory volume in 1 s of 62% ± 21% (mean ± standard deviation) of the predicted value. All CF subjects had two mutations causing CF that included ΔF508 on at least one allele. Although the majority of CF subjects were homozygous for ΔF508, mutations on the other allele (compound heterozygotes) included 621 + 1G-T, N1303K, C276X, W1282X, and 1717-1G>A. All obligate heterozygotes (carriers) had previously been genotyped and were found to have a single ΔF508 allele and had no known recent infections. The obligate heterozygotes were older because they were the parents of the CF patients. The healthy controls did not have a family history of CF or features associated with CF, including nasal polyps, sinusitis, recurrent lung infections, male infertility, or pancreatitis, or any chronic illness or recent infection. These studies were approved by the Institutional Review Boards at Beth Israel Deaconess Medical Center and the University of Massachusetts Memorial Health Center.
Cell isolation.
Human monocytes were obtained from peripheral blood by density gradient centrifugation with Histopaque, as described previously (26). Briefly, 40 ml of heparinized blood was diluted 1:1 (by volume) in phosphate-buffered saline (PBS). Twenty milliliters of the blood-PBS mixture was layered on top of 15 ml of Histopaque, and the mixture was centrifuged at 900 × g for 30 min at room temperature. Mononuclear cells were collected at the interface and washed once by centrifugation at 400 × g and twice by centrifugation at 200 × g with washing buffer (1× PBS with 0.5 mM EDTA, 10 mM HEPES, 0.2% [vol/vol] FBS). The cells were resuspended in RPMI 1640 medium containing 10% FBS (R-10). Monocytes were purified by adherence to plastic tissue culture plates (Falcon; Becton Dickinson, Franklin Lakes, N.J.) for 30 min at 37°C in 5% CO2 and were then used for all subsequent assays. By this method, monocytes represent 89% of total adherent cells by flow cytometry. The viabilities of the cells were >92%, as determined by trypan blue exclusion.
IL-8 cytokine assay.
Since we found no difference between CF patients, obligate heterozygotes, and healthy controls in the percentage of lymphocytes in the monocyte population after adherence by flow cytometry, we plated 500,000 of mononuclear cells per ml by standard methods. After overnight incubation, the monocytes were stimulated with increasing concentrations of LPS (0.0001 to 10,000 ng/ml) and IL-1β (0.000001 to 100 ng/ml) for 6 h at 37°C. The supernatants were collected and frozen at −20°C. The IL-8 levels in the supernatants were measured by a double-ligand enzyme-linked immunosorbent assay as described previously (11). Briefly, the wells of a 96-well plate (Fisher Scientific, Pittsburgh, Pa.) were coated with anti-human IL-8 at a concentration of 5 μg/ml in carbonate coating buffer (pH 9.6). After overnight incubation, the plate was washed with PBS with 0.05% Tween 20 (PBS-T; pH 7.4). Nonspecific binding was blocked with 2% bovine serum albumin in PBS-T for 1 h at room temperature. The plates were washed, 50 μl of the recombinant human IL-8 standard or samples was added to the wells, and the plates were incubated for 30 min at room temperature. Anti-human IL-8 antiserum biotinylated with ImmunoPure (Pierce, Rockford, Ill.), according to the directions of the manufacturer, was used as the secondary antibody. The biotinylated anti-IL-8 was added after the plates were washed with PBS-T, and then the plates were incubated for 15 min at room temperature. The plates were washed, streptavidin-horseradish peroxidase complex (Amersham, Arlington Heights, Ill.) was added, and the plates were incubated for 10 min at room temperature. After the plates were carefully washed with PBS, 50 μl of tetramethylbenzidine substrate solution (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was added to each well and the reaction was stopped with 50 μl of 1 M o-phosphoric acid. The optical density at 450 nm was read with an automated microplate photometer (Dynatech), and the concentrations of IL-8 were determined by comparison with a standard curve. The EC50 was calculated from the dose-response curve. The sensitivity of this method was 10 pg/ml.
FACS analysis.
Mononuclear cells were incubated for 15 min at 37°C either with 100 ng of LPS per ml in R-10 or with R-10 alone. The cells were fixed by suspending them in 1% (vol/vol) paraformaldehyde in FACS buffer (Hank's balanced salt solution without calcium or magnesium and with 2.5% goat and human serum) for 20 min on ice. Antibody solutions were made by using 10 μg of antibody per ml in FACS buffer, and all the following steps were carried out at 4°C. Mononuclear cells (10 × 106/ml) from the LPS- and non-LPS-stimulated groups were then resuspended for 30 min in either anti-human CD14, anti-human Toll-like receptor 4, or their appropriate isotype-matched control antibodies for background staining (mouse IgG1 and mouse IgG2a, respectively). After the cells were washed with FACS buffer, the cells were incubated with a goat anti-mouse IgG-fluorescein isothiocyanate F(ab′)2 fragment for 30 min. A FACScan instrument with CELLQuest software (version 1.0; Becton Dickinson) was used for data acquisition and analysis. The monocyte population was gated, and data from 10,000 events were stored and analyzed.
MAPK phosphorylation assay.
Mononuclear cells were plated at a concentration of 3 × 106/ml. After overnight incubation at 37°C with 5% CO2, the adherent monocytes were incubated with LPS for 15 min at 37°C. The cells were washed with ice-cold PBS and lysed in lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EGTA, 1% Nonidet P-40, 0.25% sodium deoxycholate) containing protease inhibitor cocktail (Sigma Chemical Co., St. Louis, Mo.), 1 mM phenylmethylsulfonyl fluoride, and protein phosphatase inhibitors (1 mM NaF, 1 mM Na3VO4). The protein concentration in the cell extracts was measured by a bicinchoninic acid-based assay. Equal amount of proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% polyacrylamide gel, electrotransferred onto an Immobilon-P membrane (Millipore), and then immunoblotted for phosphorylated or control ERK1/2 and p38 by using specific antibodies. Densitometric analysis was performed with the National Institutes of Health Image (version 1.62) program.
Statistics.
The levels of IL-8 secretion from monocytes were summarized as the median, range (minimum and maximum), and interquartile range (25th and 75th percentiles) by subject group. A Kruskal-Wallis test was used to compare the three groups, and then Wilcoxon rank-sum tests were used for pairwise comparison of the subject groups if the Kruskal-Wallis test was statistically significant. MAPK levels were summarized as mean ± standard deviation by subject group and LPS concentration and were analyzed by a mixed-model analysis of variance, with factors for the group LPS concentration and the group-by-LPS interaction; if the interaction was significant, the model contrasts were used to make pairwise comparisons of interest. All reported P values are two sided. Data were analyzed with SAS (version 8.0) software (SAS Institute Inc., Cary, N.C.).
RESULTS
Basal and stimulated IL-8 secretion from peripheral blood monocytes.
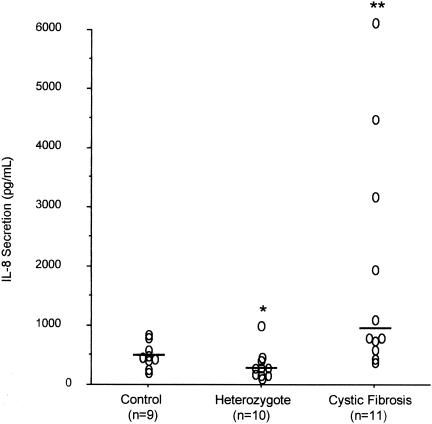
In order to determine if basal or stimulated IL-8 secretion is altered in the setting of CFTR dysfunction, peripheral blood monocytes were isolated from healthy control subjects, CF patients, and obligate heterozygotes. As shown in Fig. 1, the basal levels of IL-8 secretion from monocytes incubated for 6 h at 37°C differed significantly among the three groups (P = 0.002). The basal levels of IL-8 secretion were increased for CF patients (median, 800 pg/ml; range, 385 to 7,383 pg/ml) compared to those for the healthy controls (median, 450 pg/ml; range, 200 to 834 pg/ml) (P = 0.02). In contrast, the levels of secretion from heterozygotes (median, 219 pg/ml; range 110 to 1,000 pg/ml) were slightly less than those from the healthy controls (P = 0.04).
FIG. 1.
Basal levels of IL-8 secretion. Monocyte preparations from healthy controls, heterozygotes, and CF patients were incubated for 6 h at 37°C; and the levels of IL-8 secretion were measured. Individual datum points are shown. The bars indicate median values. *, P = 0.04 compared to the results for the healthy controls; **, P = 0.02 compared to the results for the healthy controls.
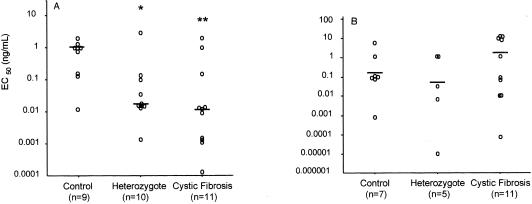
For assessment of stimulated IL-8 secretion, monocytes from the same subject were incubated with concentrations of LPS or IL-1β ranging from 0.0001 ng/ml to 10 μg/ml for 6 h, and the results were expressed as the EC50, which represents the concentration required for 50% maximal secretion of IL-8. E. coli LPS was used since it resulted in increased levels of IL-8 secretion compared to those achieved with Pseudomonas LPS. The EC50 for IL-8 secretion in response to E. coli LPS stimulation (Fig. 2A) differed significantly among all three groups (P = 0.014). In response to E. coli LPS, the EC50s for monocytes from both CF patients (median, 0.012 ng/ml) and obligate heterozygotes (median, 0.017 ng/ml) were approximately 100-fold lower than those for monocytes from healthy controls (median, 1.0 ng/ml) (P = 0.01 and 0.03, respectively). In contrast, there were no significant differences in the EC50s between these three groups following IL-1β stimulation (Fig. 2B), with mean EC50s between 0.1 and 1.0 ng/ml. Stimulation with TNF-α resulted in minimal secretion over basal levels (data not shown).
FIG. 2.
Stimulated IL-8 secretion. Monocyte preparations from healthy controls, heterozygotes, and CF subjects were incubated with increasing concentrations of LPS (A) or IL-1β (B) for 6 h at 37°C to determine the EC50s for stimulation of IL-8 secretion. Individual EC50s are shown. The bars indicate median values. *, P = 0.03 compared to the results for the healthy controls; **, P = 0.01 compared to the results for the healthy controls. No statistically significant differences were observed between the groups after stimulation with IL-1β.
Analysis of LPS cell surface binding proteins.
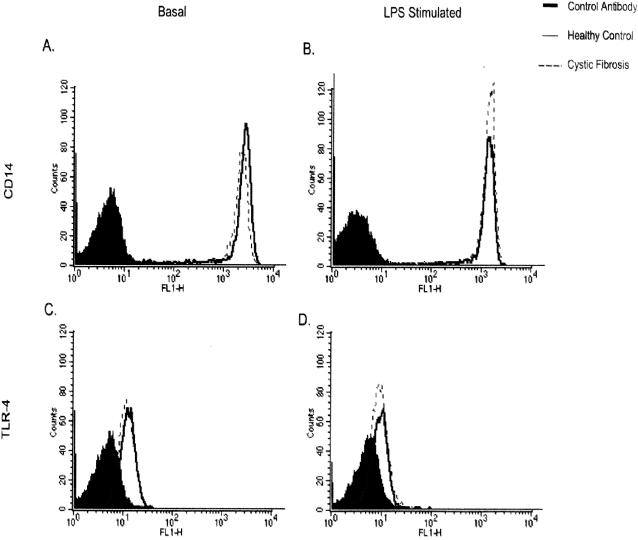
In order to explore the mechanism by which LPS-induced IL-8 secretion is enhanced, the surface expression of receptors for LPS (CD14 and Toll-like receptor 4) were examined. Monocyte cell populations were gated by FACS analysis, and data from 10,000 events were stored and analyzed. There was no difference in the expression of CD14 by monocytes from CF patients and those from healthy controls under basal and LPS-stimulated conditions (Fig. 3A and B). Similarly, there was no difference in the expression of Toll-like receptor 4 either in the presence or in the absence of LPS (Fig. 3C and D). After stimulation with LPS, the differences in mean fluorescences between CF patients and healthy controls were 8% for CD14 expression and 6% for Toll-like receptor 4 expression.
FIG. 3.
FACS analysis of monocyte CD14 and Toll-like receptor 4 (TLR-4) expression. The levels of expression of both CD14 (A and B) and Toll-like receptor 4 (C and D) were similar in CF and healthy control monocytes under both basal (A and C) and LPS-stimulated (B and D) conditions.
Analysis of MAPKs.
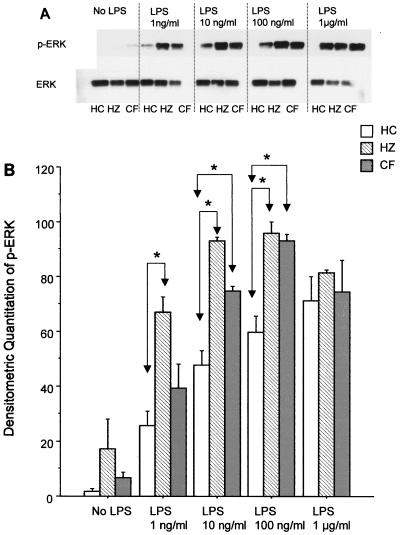
Since monocytes from CF patients and healthy controls showed no difference in LPS receptor expression, the downstream signaling MAPKs were examined. Total levels of ERK as well as the phosphorylated (active) form were analyzed by Western blotting (Fig. 4). The total levels of ERK were not significantly different between the groups. In contrast, with lower concentrations of LPS the level of phosphorylation of ERK in monocyte preparations from both CF patients and heterozygotes was increased compared to that for monocyte preparations from healthy controls. Only at an LPS concentration of 1 μg/ml were similar levels of phosphorylation observed between all groups. At LPS concentrations of 10 and 100 ng/ml, the level of phosphorylation of ERK was significantly increased in monocytes from both CF patients and heterozygous subjects compared to that in monocytes from healthy controls (P < 0.005). The difference was also significant at 1 ng/ml when the levels of phosphorylation of ERK in monocytes from heterozygous subjects and healthy controls were compared.
FIG. 4.
Effect of LPS on ERK phosphorylation. (A) Western blots of phosphorylated ERK (p-ERK) and total ERK (ERK) in monocytes from healthy controls (HC), heterozygotes (HZ), and CF patients stimulated with various concentrations of LPS; (B) quantitation of the Western blot analyses by densitometric scanning, in which the maximum value was set at 100 and all other values are expressed relative to that value. The results are for six subjects per group and are expressed as the mean ± standard deviation. *, P < 0.005.
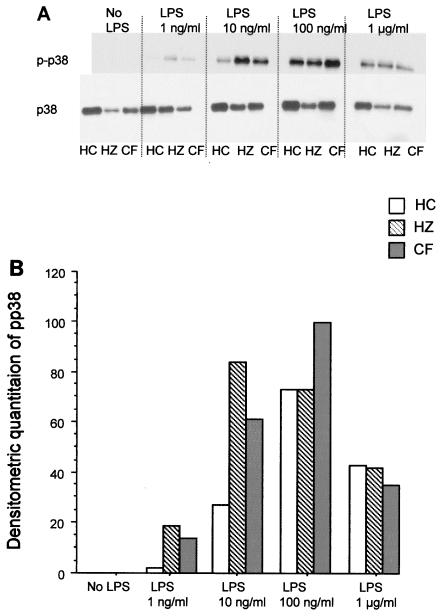
Similar analyses were performed for p38. The total levels of p38 were similar between the groups. Increased levels of phosphorylation of p38 occurred with lower concentrations of LPS for preparations of monocytes from CF patients and heterozygous subjects compared to those achieved for monocyte preparations from healthy controls (Fig. 5), similar to that seen for ERK, although the differences were not statistically different when all groups were compared. The findings from similar approaches to the study of JNK were inconclusive due to low levels of detectable product by Western blot analysis.
FIG. 5.
Effect of LPS on p38 phosphorylation. The analyses were conducted as described in the legend to Fig. 4, except that phosphorylated p38 (p-p38) and total p38 (p38) were analyzed by Western blotting (A) and quantitated by densitometric scanning (B). The results are for four subjects per group and are expressed as the mean ± standard deviation.
DISCUSSION
The data presented here demonstrate that the EC50s in response to LPS for peripheral blood monocytes from subjects either heterozygous or homozygous for mutations in CFTR were 100-fold lower compared to those observed for monocytes from healthy control subjects. The increased levels of basal IL-8 secretion seen only from monocytes from CF patients and not those from obligate heterozygotes or healthy controls may be due to the chronic infectious state of the CF patients. Although this could also explain the lower EC50 for monocytes from CF patients in response to LPS, the fact that monocytes from healthy obligate heterozygotes, who had no recent history of infections, show similar results would imply that the enhanced LPS response is a result of CFTR dysfunction. Although the healthy controls did not undergo CFTR genotyping, only 1 in 29 would be expected to be a carrier, and the presence of a carrier among the healthy controls would have minimized the differences that were observed.
It is interesting that this response of monocytes from heterozygotes was of a magnitude similar to that observed for monocytes from CF patients, even though the amount of functional CFTR is decreased by ≤50% in heterozygotes. This is in contrast to the finding that cholera toxin-induced intestinal secretion is 50% less in mice heterozygous for CFTR mutations than in mice homozygous for CFTR mutations (7) and suggests that CFTR may have more of a threshold effect and not a graded response on IL-8-induced secretion in human monocytes.
Since the difference in EC50s was seen only with LPS and was not observed in response to IL-1β, the priming of monocyte function would have to be due to either increased numbers of LPS receptors or, alternatively, upregulation of intracellular signaling pathways activated by LPS. LPS binds to serum binding protein and is transferred to CD14 on the cell surface (for a review, see reference 8). This complex interacts with Toll-like receptor 4 and the accessory protein MD-2, which leads to activation of the MAPKs ERK, p38, and JNK. In addition, LPS activates the I kappa B kinase pathway. The result is activation of multiple transcription factors, including NF-κB. Our data demonstrate that the expression of both CD14 and Toll-like receptor 4 on monocytes did not differ between healthy controls and CF patients. Hence, the lack of difference between CF patients and healthy control subjects in response to IL-1β implies that in the setting of CFTR alleles there is an alteration either in MD-2 or in downstream factors that lead to the activation of NF-κB-inducing kinase. It is at this point that the LPS and IL-1β signaling pathways converge (8, 18).
The phosphorylation of ERK in monocytes from patients either homozygous or heterozygous for CFTR mutations occurred with lower doses of LPS than that seen in healthy control monocytes. It should be noted that 1 ng/ml was the dose found to cause 50% of maximal IL-8 secretion (EC50), as shown in Fig. 2A. Hence, the changes in MAPK activity seen at this dose and higher doses parallel the alterations in IL-8 secretion. Although similar trends were observed for p38, the differences were not statistically significant. How CFTR may regulate MAPKs in monocytes to modulate IL-8 levels is unknown. Whether MD-2 or other components of cell membrane signaling are altered in the setting of CFTR dysfunction remains to be determined. It has been shown in epithelial cells expressing defective CFTR that, following stimulation with P. aeruginosa, IL-8 expression is increased whether one examines cell lines (5, 27), naïve human CF airway grafts (23), or CF knockout mice (24). In addition, constitutive activation of NF-κB leading to IL-8 synthesis occurs in the setting of either an impairment in CFTR chloride channel function or the accumulation of ΔF508 CFTR protein in the endoplasmic reticulum, which leads to cell stress (27). In addition, the levels of cytosolic IκBα, which inhibits the activation of NF-κB, are decreased in ΔF508 CF bronchial tissues as well as cultured CF bronchial gland cells (20). Taken together these data indicate that CFTR dysfunction is associated with activation of NF-κB in epithelial cells, although the precise mechanism remains unknown. Although a similar effect on NF-κB activation may be expected in monocytes/macrophages, this remains to be tested.
The fact that peripheral blood monocytes from healthy obligate heterozygotes demonstrate enhanced LPS-induced production of IL-8 has two implications. First, these data would suggest that a single allelic mutation is sufficient to modulate LPS-induced cytokine secretion. CFTR dysfunction has been shown to lead to other abnormalities in obligate heterozygotes, as evidenced by elevated sweat chloride concentrations (6) and diminished levels of pancreatic juice bicarbonate and chloride secretion as well as diminished water flow in infants (12); resistance to cholera toxin-induced intestinal fluid secretion in CF knockout mice (7); and the association of the CFTR dysfunction with the development of CF-related diseases in humans, such as sinusitis (25), the congenital bilateral absence of the vas deferens (3), and chronic pancreatitis (4, 17). The second implication of these data may relate to the high frequency of single allelic mutations in the general population. The enhanced response to LPS may be advantageous in priming the innate immune response, which leads to the rapid eradication of bacteria. In the setting of overt CF, persistent bacterial infections due to viscous secretions and poor clearance would result in continued excessive secretion of cytokines by these primed monocytes and would explain, at least in part, the excessive host inflammatory response in patients with CF.
Acknowledgments
We thank I. Ghiran for insightful discussions and valuable technical advice.
This work was funded by a grant (FREEDM00Z0) from the Cystic Fibrosis Foundation and in part by grant RR 01032 to the Beth Israel Deaconess Medical Center General Clinical Research Center from the National Institutes of Health.
REFERENCES
- 1.Bonfield, T. L., M. W. Konstan, and M. Berger. 1999. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J. Allergy Clin. Immunol. 104:72-78. [DOI] [PubMed] [Google Scholar]
- 2.Bonfield, T. L., J. R. Panuska, M. W. Konstan, K. A. Hilliard, J. B. Hilliard, H. Ghnaim, and M. Berger. 1995. Inflammatory cytokines in cystic fibrosis lungs. Am. J. Respir. Crit. Care Med. 152:2111-2118. [DOI] [PubMed] [Google Scholar]
- 3.Casals, T., L. Bassas, S. Egozcue, M. D. Ramos, J. Gimenez, A. Segura, F. Garcia, M. Carrera, S. Larriba, J. Sarquella, and X. Estivill. 2000. Heterogeneity for mutations in the CFTR gene and clinical correlations in patients with congenital absence of the vas deferens. Hum. Reprod. 15:1476-1483. [DOI] [PubMed] [Google Scholar]
- 4.Cohn, J. A., K. J. Friedman, P. G. Noone, M. R. Knowles, L. M. Silverman, and P. S. Jowell. 1998. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N. Engl. J. Med. 339:653-658. [DOI] [PubMed] [Google Scholar]
- 5.DiMango, E., A. J. Ratner, R. Bryan, S. Tabibi, and A. Prince. 1998. Activation of NF-κB by adherent Psuedomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J. Clin. Investig. 101:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell, P. M., and R. E. Koscik. 1996. Sweat chloride concentrations in infants homozygous and heterozygous for F508 cystic fibrosis. Pediatrics 97:524-528. [PubMed] [Google Scholar]
- 7.Gabriel, S. E., K. N. Brigman, B. H. Killer, R. C. Boucher, and M. J. Stutts. 1994. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science 266:107-109. [DOI] [PubMed] [Google Scholar]
- 8.Guha, M., and N. Mackman. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85-94. [DOI] [PubMed] [Google Scholar]
- 9.Kammouni, W., C. Figarella, S. Marchand, and M. Merten. 1997. Altered cytokine production by cystic fibrosis tracheal gland serous cells. Infect. Immun. 65:5176-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan, T. Z., J. S. Wagener, T. Bost, J. Martinez, F. J. Accurso, and D. W. Riches. 1995. Early pulmonary inflammation in infants with cystic fibrosis. Am. J. Respir. Crit. Care Med. 151:1075-1082. [DOI] [PubMed] [Google Scholar]
- 11.Linevsky, J. K., C. Pothoulakis, S. Keates, M. Warny, A. C. Keates, J. T. LaMont, and C. P. Kelly. 1997. IL-8 release and neutrophil activation by Clostridium difficile toxin-exposed human monocytes. Am. J. Physiol. 273:G1333-G1340. [DOI] [PubMed] [Google Scholar]
- 12.Massie, R. J., B. Wilcken, P. Van Aseren, S. Dorney, M. Gurca, V. Wiley, and K. Gastrin. 2000. Pancreatic function and extended mutation analysis in delta F508 heterozygous infants with an elevated immunoreactive trypsinogen but normal sweat electrolyte levels. J. Pediatr. 137:214-220. [DOI] [PubMed] [Google Scholar]
- 13.Muhlebach, M. S., P. W. Stewart, M. W. Leigh, and T. L. Noah. 1999. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am. J. Respir. Crit. Care Med. 160:186-191. [DOI] [PubMed] [Google Scholar]
- 14.Noah, T. L., H. R. Black, P. W. Cheng, R. E. Wood, and M. W. Leigh. 1997. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J. Infect. Dis. 175:638-647. [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer, K. D., T. P. Huecksteadt, and J. R. Hoidal. 1993. Expression and regulation of tumor necrosis factor in macrophages from cystic fibrosis patients. Am. J. Respir. Cell Mol. Biol. 9:511-519. [DOI] [PubMed] [Google Scholar]
- 16.Riordan, J. R., J. M. Rommens, B. S. Kerem, N. Alon, R. Rozmahel, Z. Grzelczak, J. Zielenski, S. Lok, N. Plavsic, J. Chou, M. L. Drum, M. C. Iannuzzi, F. S. Collins, and L.-C. Tsui. 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245:1066-1073. [DOI] [PubMed] [Google Scholar]
- 17.Sharer, N., M. Schwarz, G. Malone, A. Howarth, J. Painter, M. Super, and J. Braganza. 1998. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N. Engl. J. Med. 339:645-652. [DOI] [PubMed] [Google Scholar]
- 18.Stancovski, I., and D. Baltimore. 1997. NF-κB activation: the IκB kinase revealed? Cell 91:299-302. [DOI] [PubMed] [Google Scholar]
- 19.Tabary, O., S. Escotte, J. P. Couetil, D. Hbert, D. Dusser, E. Puchelle, and J. Jacquot. 1999. Genistein inhibits constitutive and inducible NFκB activation and decreases IL-8 production by cystic bronchial gland cells. Am. J. Pathol. 155:473-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabary, O., S. Escotte, J. P. Couetil, D. Hubert, D. Dusser, E. Puchelle, and J. Jacquot. 2002. Relationship between IkappaBalpha deficiency, NFkappaB activity and interleukin-8 production in CF human airway epithelial cells. Pflugers Arch. 443(Suppl. 1):S40-S44. [DOI] [PubMed] [Google Scholar]
- 21.Tabary, O., J. M. Zahm, J. Hinnrasky, J. P. Couetil, P. Cornillet, M. Guenounou, D. Gaillard, E. Puchelle, and J. Jacquot. 1998. Selective up-regulation of chemokine IL-8 expression in cystic fibrosis bronchial gland cells in vivo and in vitro. Am. J. Pathol. 153:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas, G. R., E. A. Costelloe, D. P. Lunn, K. J. Stacey, S. J. Delaney, R. Passey, E. C. McGlinn, B. J. McMorran, A. Ahadizadeh, C. L. Geczy, B. J. Wainwright, and D. A. Hume. 2000. G551D cystic fibrosis mice exhibit abnormal regulation of inflammation in lungs and macrophages. J. Immunol. 164:3870-3877. [DOI] [PubMed] [Google Scholar]
- 23.Tirouvanziam, R., S. de Bentzmann, C. Hubeau, J. Hinnrasky, J. Jacquot, B. Peault, and E. Puchelle. 2000. Inflammation and infection in naive human cystic fibrosis airway grafts. Am. J. Respir. Cell Mol. Biol. 23:121-127. [DOI] [PubMed] [Google Scholar]
- 24.Van Heekeren, A., R. Walenga, M. W. Konstan, T. Bonfield, P. B. Davis, and T. Ferkol. 1997. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J. Clin. Investig. 100:2810-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, X., B. Moylan, D. A. Leopold, J. Kim, R. C. Rubenstein, A. Togias, D. Proud, P. L. Zeitlin, and G. R. Cutting. 2000. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. JAMA 284:1814-1819. [DOI] [PubMed] [Google Scholar]
- 26.Warny, M., A. C. Keates, S. Keates, I. Catagliuolo, J. K. Zacks, S. Aboudola, A. Qamar, C. Pothoulakis, J. T. LaMont, and C. P. Kelly. 2000. p38 MAPK activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production, and enteritis. J. Clin. Investig. 105:1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber, A. J., G. Soong, R. Bryan, S. Saba, and A. Prince. 2001. Activation of NF-kB in airway epithelial cells is dependent on CFTR trafficking and Cl− channel function. Am. J. Physiol. Lung Cell Mol. Physiol. 281:L71-L78. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura, K., H. Nakamura, B. C. Trapnell, C. S. Chu, W. Dalemans, A. Pavirani, J. P. Lecocq, and R. G. Crystal. 1991. Expression of the cystic fibrosis transmembrane conductance regulator gene in cells of non-epithelial origin. Nucleic Acids Res. 19:5417-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]