To the Editor
Continuous renal replacement therapy (CRRT) was introduced to enable dialytic treatment of hemodynamically unstable patients for whom intermittent hemodialysis could be difficult to administer.1 CRRT has potentially harmful unintended effects, including excessive removal of amino acids, trace elements, and electrolytes such as phosphate.2 Phosphate is the most abundant intracellular anion and is essential for multiple biological functions. Because hypophosphatemia is a complication reported in >10% of patients undergoing CRRT,3–5 we investigated phosphate balance during continuous venovenous hemofiltration (CVVH), hypothesizing that CRRT leads to a negative phosphate balance despite protocol-driven phosphate repletion strategies and normalization of serum phosphate levels.
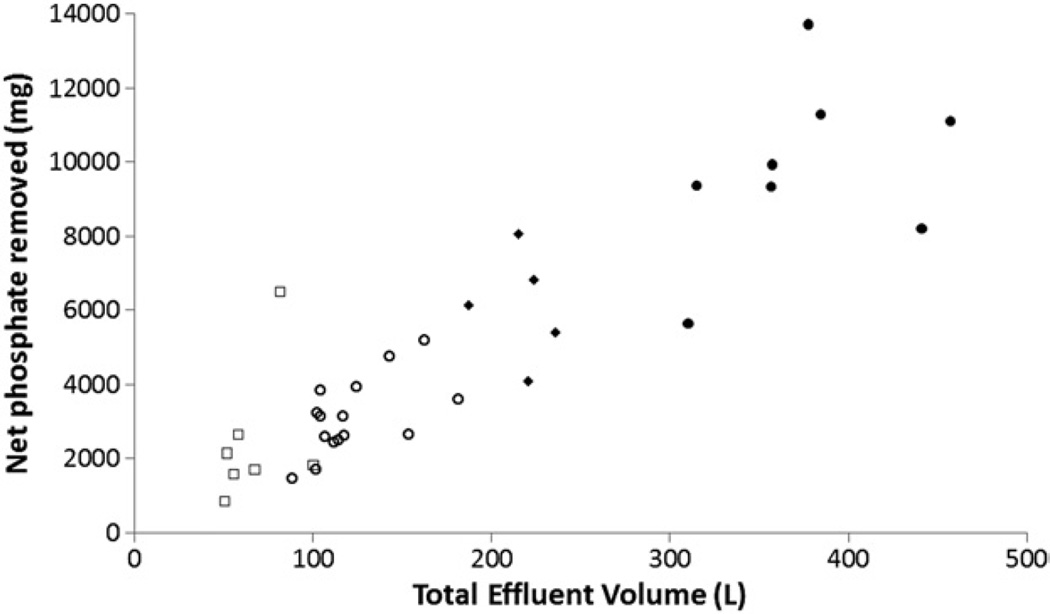
We studied 35 patients with acute kidney injury who underwent CVVH by using a partial effluent collection device that diverted ~1% of the total effluent volume to a collection bag. CVVH was performed using biocompatible polyethersulfone membranes, bicarbonate or citrate (phosphate-free) replacement solution delivered prefilter at rates of 1,600–4,000 mL/h, and blood flow rates of 200–250 mL/min. We calculated phosphate balance by subtracting urinary and CVVH losses from dietary (enteral or parenteral) intake. Baseline characteristics are described in Tables 1 and S1. We found a significant correlation between total effluent volume and net phosphate removal (Fig 1; r = 0.86; P < 0.001). The lowest recorded serum phosphate concentration during the study was 2 mg/dL (median, 2.6 [range, 2–8.6] mg/dL). According to reference laboratory values (2.5–4.3 mg/dL), 34.2% of patients had overt hypophosphatemia. All patients were in negative phosphate balance during CVVH despite protocol-driven phosphate repletion strategies (Table S2 and Fig S1). Our estimates of negative phosphate balance are slight underestimates because we did not collect stool, which may contain ~500 mg/d of phosphate.6 Using univariate regression analyses, the predictors of natural log–transformed net phosphate balance were age (β coefficient, −0.02; P = 0.05), medical versus surgical intensive care unit (β = −0.60; P = 0.02), pre-CVVH serum phosphate level (β = 0.19; P = 0.004), effluent volume (β = 0.0047 [per 1 L]; P < 0.001), and days of CVVH (β = 0.26; P < 0.001). We found no association between phosphate balance and in-hospital mortality (P = 0.1; OR, 2.26; 95% CI, 0.76–6.70). Of the 15 patients for whom balance studies were performed for at least 3 days, 87% required intravenous phosphate repletion even though they were hyperphosphatemic at CVVH initiation. Negative phosphate balance was highest at CVVH initiation, reflecting phosphate excess. Although daily phosphate balance became less negative over time as steady state was achieved and nutrition was introduced, it remained persistently negative even after a week of CVVH (Table S3). In the 6 patients who had serial CVVH effluent collections for 7 consecutive days, cumulative net phosphate balance ranged between −6.1 and −13.7 (median, −8.9) g. We found a persistent net negative phosphate balance even on day 7 of treatment with CVVH (range, −449 to −1,259 mg). Our calculations of negative phosphate balance are underestimates because we did not measure phosphate losses in gastrointestinal secretions or surgical drains due to concerns over feasibility.
Table 1.
Baseline Characteristics of Study Participants and Delivered CRRT
| Parameter | Value |
|---|---|
| Demographics | |
| Age (y) | 60 (25–80) |
| Male sex (%) | 72 |
| Weight (kg) | 92.4 ± 19 |
| Laboratory parameters at CRRT onset | |
| Serum urea nitrogen (mg/dL) | 77 (23–242) |
| Creatinine (mg/dL) | 3.9 ± 1.5 |
| Phosphate (mg/dL) | 6.2 ± 1.8 |
| Delivered CRRT | |
| Treatment mode | CVVH |
| Treatment duration (d) | 6 (5–9) |
| CRRT dose (mL/kg/h) | 23.2 ± 6 |
| Effluent collections per patient (d) | 2 (1–7) |
| Phosphate kinetics | |
| Net nutritional supplementation (mg) | 143 (0–2,355) |
| Net intravenous supplementation (mg) | 310 (0–1,550) |
| Net phosphate removal (g) | 1.4 (0.7–6.5) |
| Daily phosphate balance (g) | −1.2 (−0.2 to −6.5) |
| Outcomes | |
| In-hospital mortality (%) | 71 |
| RRT dependence at discharge (%) | 23 |
| Recovery (%) | 6 |
Note: Values for continuous variables given as mean ± SD or median (range), as appropriate.
Figure 1.
Relationship between effluent volume and net phosphate removal during CVVH. Each point represents data from an individual patient; symbol type identifies the day of CVVH: 1, open squares; 2–3, open circles; 4–5, closed diamonds; 6–7, closed circles. Y axis, total amount of phosphate measured in effluent during the course of CVVH; x axis, total effluent volume during CVVH.
In maintenance hemodialysis, the initial decrease in serum phosphate level during the first phase of dialysis is followed by a stable plateau phase and then a postdialysis rebound as phosphate moves from the intracellular to extracellular compartments.7 Continuous modalities, by contrast, are able to remove phosphate more efficiently because of the extended time of therapy, which allows removal of phosphate as it moves transcompartmentally.8 In the VA/NIH Acute Renal Failure Trial Network, which examined the intensity of renal support in acute kidney injury, hypophosphatemia was more common in patients randomly assigned to higher intensity CRRT and hemodialysis (17.6% vs 10.9%).9 Negative phosphate balance may be desired early in the course of treatment, but it may be detrimental during prolonged courses of CVVH. Because the sieving coefficient of small solutes such as phosphate is close to 1.0,3 clearance is nearly the ultrafiltration rate. Since phosphate is primarily intracellular and orchestrates several vital enzymatic steps, obligate CRRT losses from the intracellular pool may have important clinical consequences, perhaps even in the absence of overt serum hypophosphatemia. Whether phosphate is mobilized from bone, muscle, or red blood cell stores during CRRT-induced phosphate depletion is unknown and may underlie potential complications. Strategies to avoid excessive phosphate depletion during prolonged CRRT may be prudent. In addition, the potential clinical implications of such depletion in the critically ill—including effects on diaphragmatic or cardiac muscle function, red blood cells 2,3-diphosphoglycerate levels, and the affinity of hemoglobin for oxygen—deserve further study.
Supplementary Material
Acknowledgments
We thank Dr Venkata Sabbisetti for assistance with laboratory assays.
Support: This study was funded in part by an investigator-initiated grant from NxStage Medical Inc, which had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Other funding was from DK093574, DK085660, and DK075941 (DrWaikar).
Financial Disclosure: Dr Waikar has served as a consultant to CVS Caremark, BioTrends Research Group, and Takeda; provided expert testimony for GE Healthcare, Northstar Rx, and Salix; and received grants from the NIDDK, Otsuka, Merck, Genzyme, and Satellite Healthcare.
Footnotes
This work was presented as an abstract at the ASN Kidney Week, November 10, 2011, Philadelphia, PA.
Dr Sharma declares that she has no relevant financial interests.
Supplementary Material
Table S1: Demographics and clinical characteristics at CVVH initiation.
Table S2: Serial effluent collections in patients on CVVH.
Table S3: Daily phosphate balances in patients on CVVH.
Figure S1: Infused and removed phosphate during CVVH.
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2012.12.026) is available at www.ajkd.org.
References
- 1.Davenport A, Will EJ, Davidson AM. Improved cardiovascular stability during continuous modes of renal replacement therapy in critically ill patients with acute hepatic and renal failure. Crit Care Med. 1993;21(3):328–338. doi: 10.1097/00003246-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Himmelfarb J. Continuous renal replacement therapy in the treatment of acute renal failure: critical assessment is required. Clin J Am Soc Nephrol. 2007;2(2):385–389. doi: 10.2215/CJN.02890806. [DOI] [PubMed] [Google Scholar]
- 3.Troyanov S, Cardinal J, Geadah D, et al. Solute clearances during continuous venovenous haemofiltration at various ultrafiltration flow rates using Multiflow-100 and HF1000 filters. Nephrol Dial Transplant. 2003;18(5):961–966. doi: 10.1093/ndt/gfg055. [DOI] [PubMed] [Google Scholar]
- 4.Broman M, Carlsson O, Friberg H, Wieslander A, Godaly G. Phosphate-containing dialysis solution prevents hypophosphatemia during continuous renal replacement therapy. Acta Anaesthesiol Scand. 2011;55(1):39–45. doi: 10.1111/j.1399-6576.2010.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santiago MJ, Lopez-Herce J, Urbano J, Bellon JM, del Castillo J, Carrillo A. Hypophosphatemia and phosphate supplementation during continuous renal replacement therapy in children. Kidney Int. 2009;75(3):312–316. doi: 10.1038/ki.2008.570. [DOI] [PubMed] [Google Scholar]
- 6.Amanzadeh J, Reilly RF., Jr Hypophosphatemia: an evidence-based approach to its clinical consequences and management. Nat Clin Pract Nephrol. 2006;2(3):136–148. doi: 10.1038/ncpneph0124. [DOI] [PubMed] [Google Scholar]
- 7.Ratanarat R, Brendolan A, Volker G, et al. Phosphate kinetics during different dialysis modalities. Blood Purif. 2005;23(1):83–90. doi: 10.1159/000082016. [DOI] [PubMed] [Google Scholar]
- 8.Tan HK, Bellomo R, M’Pis DA, Ronco C. Phosphatemic control during acute renal failure: intermittent hemodialysis versus continuous hemodiafiltration. Int J Artif Organs. 2001;24(4):186–191. [PubMed] [Google Scholar]
- 9.Network VNARFT. Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.