Figure 3.
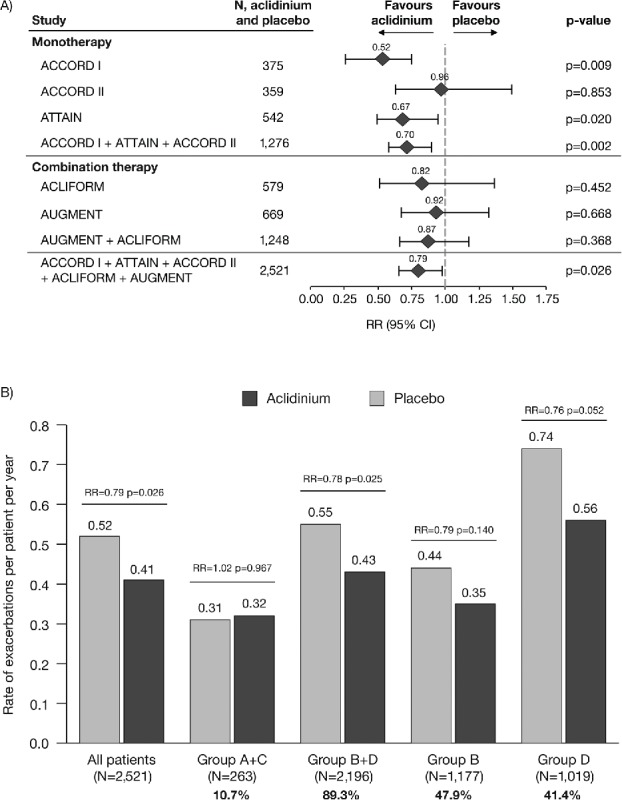
Rate of COPD exacerbations of any severity (A) overall (individual studies and pooled analyses)a and (B) stratified by GOLD group (pooled analysis). aExacerbations analyses from the individual ACLIFORM and AUGMENT studies are reported for the ITT-exacerbations populations of the aclidinium monotherapy and placebo arms only (579 and 669 patients, respectively; all patients who received at least one dose of the study medication), as specified in the study protocols and published previously (20,32); pooled analyses include data from the ITT populations (577 and 668 patients, respectively; all patients who received at least one dose of the study medication and had a baseline and ≥1 post-baseline FEV1 assessment) for consistency with the ACCORD I, ACCORD II and ATTAIN study populations. Percentages are the proportion of all GOLD-classified patients (N = 2,459) in each GOLD group. CI, confidence interval; COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for chronic Obstructive Lung Disease; ITT, intent-to-treat; RR, rate ratio; N, number of patients in aclidinium 400 μg and placebo groups.
