Abstract
In recent years photopheresis has been claimed to be an effective form of immunomodulation. It has also been shown to have an effect on the disease process at the onset of type 1 diabetes. In a double-blind, placebo-controlled randomized study, we analyzed if the effect of photopheresis in children with newly diagnosed diabetes is related to changes in the balance of lymhocyte populations. We also analyzed if lymphocyte subsets were related to recent infection, mild or aggressive disease manifestations, heredity, or gender. Nineteen children received active treatment with photopheresis, while 21 children received sham pheresis (placebo group). No influence of a history of previous infection, heredity, or certain clinical parameters on lymphocyte subsets was found. At the onset of type 1 diabetes, girls showed a higher proportion and a larger number of T cells (CD3+) and T-helper cells (CD4+) and a higher proportion of naïve CD4+CD45RA+ cells. In the placebo group, an increase in the number of subsets with the activated phenotype in both the CD4 (CD29+) and the CD8 (CD11a+) compartments was noted during the course of the study. These changes did not occur in the photopheresis group. No relation between lymphocyte subsets and clinical outcome was found 1 year after the treatment with photopheresis. In conclusion, we found no major effect of photopheresis on lymphocyte populations in a group of children with newly diagnosed type 1 diabetes. However, in the placebo group the proportions of activated CD4 and CD8 cells increased over time. Since these changes did not occur in the actively treated group, our findings suggest that photopheresis may have some suppressive effects.
Type 1 diabetes is regarded as an autoimmune disease, with genetic and environmental factors involved in its pathogenesis (1, 8). Several studies have reported that physical, psychological, or chemical stress can produce imbalances in the proportions of T-cell subsets, immunoglobulin levels, and lymphocyte reactivity (6, 25, 32). Since it became evident that type 1 diabetes may be caused by an autoimmune process, several types of immune interventions have been tried, mostly with minor or transient effects (4, 15, 19, 22, 27, 33). Photopheresis has been claimed to be an effective form of immunomodulation (37), and it has been used with positive results for the treatment of several immunological diseases, such as rheumatoid arthritis (29), systemic lupus erythematosus (21), psoriatic arthritis (34), and cutaneous T-cell lymphoma (7). Photopheresis has also been proposed to be effective in graft-versus-host disease (12) and to prevent rejection in cardiac transplantation (2).
Photopheresis is an extracorporeal form of photochemotherapy that uses psoralen and UV type A (UVA) radiation. However, the mechanisms of action are not fully understood, but it is known that on activation by UVA light, 8-methoxypsoralen interacts with bases of DNA and binds to sites on the cell surface and/or in the cytoplasm of the target cells (3, 26). It is suggested that reinfused treated cells may stimulate an autologous suppressor response toward T cells of similar clones not reached by active photopheresis treatment (37). Repeated photopheresis treatment may have a booster effect.
In a double-blind, placebo-controlled (placebo tablets and sham pheresis) randomized study, we have shown that photopheresis has a weak but significant effect on the disease process at the onset of type 1 diabetes (28). The aim of the present study was to analyze if the effect of photopheresis is related to changes in the balance of lymphocyte populations, in particular, subsets of T cells. In addition, we examined lymphocyte populations in samples before treatment to see if lymphocyte subsets were related to recent infection, mild or aggressive disease manifestations, heredity, or gender.
MATERIALS AND METHODS
Subjects.
Details regarding the clinical study have recently been published (28). In short, three pediatric departments in southeastern Sweden participated in the study. A blood sample was taken at the time of diagnosis, before the first insulin injection. The patients were subsequently treated with intravenous insulin infusion until the blood glucose concentration was stable at normal concentrations and the patients were free of ketonuria and had normal acid-base balances. To be eligible for the study the patients had to be from 10 to 18 years of age. The patients and their parents were given careful oral and written information about the study, in compliance with the guidelines of the Research Ethics Committee of the Faculty of Health Sciences at Linköping University. Patients who participated in the study received traditional treatment with multiple insulin therapy, diet, regular exercise, and self-control and were then randomly allocated either to active treatment or to placebo treatment. The randomization code was not broken until all patients had been monitored for at least 2 years. Only the staff of the apheresis unit knew whether the patient was actively treated or not.
A total of 49 children with newly diagnosed (first insulin injection) diabetes were included after informed consent was obtain from the children and their parents. Nine children, three from the placebo group and six from the actively treated group, withdrew from the study for various reasons after zero to five photopheresis procedures (28). The 40 children who fulfilled the study inclusion criteria were between 10 and 16.5 years of age at the time of diagnosis (mean age, 13.6 ± 1.8 years).
Nineteen children received active photopheresis treatment, consisting of an oral dose of 8-methoxypsoralen and a subsequent apheresis procedure. The buffy coat cells were then exposed to UVA light (2 J/cm2) for 90 min and returned to the patient's circulation. The control group (21 children) received placebo tablets and sham treatment. The sham procedure included venipuncture and a slow saline infusion, but the patients were not connected to the running photopheresis machine, i.e., no blood was drawn from the patients, and thus, the sham treatment by itself should not influence the blood cell activity. The apheresis procedure was administrated on two consecutive days (double treatment). The aim was for the first double treatment to be given at days 5 to 6 after diagnosis and then repeated after 2, 4, 8, and 12 weeks so that every patient should have received five double treatments in a 3-month period. Blood samples were drawn before each of these treatments. In this study we analyzed blood samples taken before treatment and after 4 weeks, 12 weeks, and approximately 1 year (Fig. 1).
FIG. 1.
Treatment protocol and sampling for lymphocyte populations. Each treatment consisted of one photopheresis procedure on two consecutive days (double treatment).
In the analyses we subgrouped the children according to whether they seemed to have a mild disease process or an aggressive disease process. Children with a mild disease process needed the lowest doses of insulin per kilogram of body weight during monitoring (3 years) to keep good metabolic control. Children with an aggressive disease process needed the highest doses to keep good metabolic control. For instance, at 3 months after diagnosis, children in the mild disease process group needed <0.25 E/kg of body weight/24 h, whereas children in the aggressive disease process group needed a mean of 0.88 E/kg. We also subgrouped the children according to aggressive onset or mild onset. Children with aggressive onset had at least two of the following findings at onset: pH <7.30, +++ ketonuria, and a blood glucose level of >30 mmol/liter. Children with mild onset had none of these.
The study was approved by the Research Ethics Committee of the Faculty of Health Sciences at Linköping University.
Flow cytometric analysis of lymphocyte populations.
Peripheral venous blood was collected in tubes containing EDTA. Fifty microliters of the blood was incubated for 10 min at room temperature with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies (MAbs) to various lymphocyte cell surface markers (see below). After treatment with Q-prep (Coulter Corp., Hialeah, Fla.) for hemolysis of blood cells, stabilization, and fixation with paraformaldehyde, a two-color fluorescence analysis (Epics Profile I; Coulter Corp.) was performed. Lymphocytes were identified by their characteristic light scatter (low forward scatter and low sideways scatter). At least 5,000 cells were analyzed for each antibody combination. By using standardized volumes, the absolute numbers were calculated by using a previously calibrated cell counter instrument. MAbs against the following cell surface molecules (explained in Table 1) were used: CD19 (B4-FITC), CD3 (T3-PE and FITC), CD4 (T4-FITC), CD8 (T8-FITC), CD45RA (2H4-PE), CD29 (4B4-PE), CD11a (S6F1-PE), HLA-DR (I3-PE), and CD3γ/δ (IΜΜU 510-PE) (all MAbs were from Coulter Corp.). An appropriate FITC- or PE-conjugated mouse isotypic control was used for each MAb. NK cells were not included at the time of this study. The major lymphocyte populations (B cells [CD19+], T cells [CD3+], T-helper cells [CD4+], and T cytolytic cells [CD8+]) are presented as the total numbers of cells and as a percentage of lymphocytes. Subsets are presented as a percentage of their mother population (CD4+CD45RA+ and CD4+CD29+ as a percentage of CD4+, CD8+CD11a+ as a percentage of CD8, CD3+HLA-DR+ as a percentage of CD3+, and T cells with the γ/δ receptor as a percentage of CD3+).
TABLE 1.
Lymphocyte populations before treatment in relation to some background parametersa
| Major lymphocyte population, ratio, or subset | Mean for the whole study group (SD) | Value for population or subset, with comparison by:
|
|||||
|---|---|---|---|---|---|---|---|
| Genderb
|
Disease processc
|
Onset of symptomsd
|
|||||
| Boys | Girls | Mild | Aggressive | Mild | Aggressive | ||
| Major lymphocyte populations | |||||||
| B cells | |||||||
| No. of CD19 cells/μl | 382 (140) | NS | NS | NS | NS | NS | NS |
| % of lymphocytes | 18 (5.7) | 15.2 (3.6)e | 19.3 (6.1) | 12.9 (2.4)e | 20.8 (5.9) | 18.8 (5.3)f | 11.6(2.9) |
| T cells | |||||||
| No. of CD3 cells/μl | 1,491 (542) | 1,819 (569)f | 1,293 (425) | NS | NS | NS | NS |
| % of lymphocytes | 68 (13) | 74.1 (7.1)f | 63.6 (14.2) | NS | NS | NS | NS |
| T-helper cells | |||||||
| No. of CD4 cells/μl | 918 (286) | 1,075 (313)f | 823 (227) | NS | NS | NS | NS |
| % of lymphocytes | 42 (6.6) | 44.5 (7.7)e | 40.2 (5.3) | NS | NS | NS | NS |
| T-cytolyic cells | |||||||
| No. of CD8 cells/μl | 625 (244) | NS | NS | NS | NS | NS | NS |
| % of lymphocytes | 27 (5.3) | NS | NS | NS | NS | NS | NS |
| CD4/CD8 ratio | 1.58 (0.43) | NS | NS | NS | NS | NS | NS |
| Subsets (% of mother populationg) | |||||||
| CD4+ CD45RA+ | 58 (11) | 63.8 (9.4)f | 54.8 (9.8) | NS | NS | NS | NS |
| CD4+ CD29+ | 50 (17) | NS | NS | NS | NS | NS | NS |
| CD8+ CD11a+ | 45 (22) | NS | NS | NS | NS | NS | NS |
| CD3+ HLA-DR+ | 3.5 (2.6) | NS | NS | NS | NS | NS | NS |
| CD3γ/δ | 11 (6.9) | NS | NS | NS | NS | NS | NS |
Mild and aggressive disease processes and onset of symptoms are described in Materials and Methods. NS, not significant.
Fourteen girls and 25 boys were studied.
There were 6 patients with mild disease progression and 5 patients with aggressive disease progression.
There were 13 patients with mild onset of symptoms and 7 patients with aggressive onset of symptoms.
P < 0.05.
P < 0.01.
See Materials and Methods for explanation of subset designations.
Antibodies to GAD.
Antibodies to glutamic acid decarboxylase (GAD) were measured as described by Grubin et al. (13). Our laboratory participated in the second International GAD Antibodies Proficiency Test in 1996 and reached a sensitivity and a specificity of 100% each. Validity and consistency were also 100% each.
Statistical analysis.
The results were analyzed by the Mann-Whitney U test for comparisons between groups and the Wilcoxon rank-sum test for intragroup comparisons. Differences were considered significant at P values of <0.05. The results are expressed as means ± standard deviations.
RESULTS
Lymphocyte populations before treatment in relation to other parameters.
Samples were taken before treatment from the whole group of children with newly diagnosed diabetes, irrespective of further treatment. In this cohort we found no influence of a history of previous infection, heredity, or the occurrence of antibodies to GAD on lymphocyte subsets. Clinical parameters also had no clear-cut associations with lymphocyte populations, with the only significant change being a higher proportion of B cells in children with a mild disease onset and a lower proportion in children with a more mild disease process (Table 1). However, a difference in the distribution of lymphocyte populations was found between boys and girls (Table 1): girls showed a higher proportion and number of T cells (CD3+) and T-helper cells (CD4+) and a higher proportion of naïve CD4 cells (CD4+CD45RA+).
Longitudinal changes in lymphocyte populations during treatment.
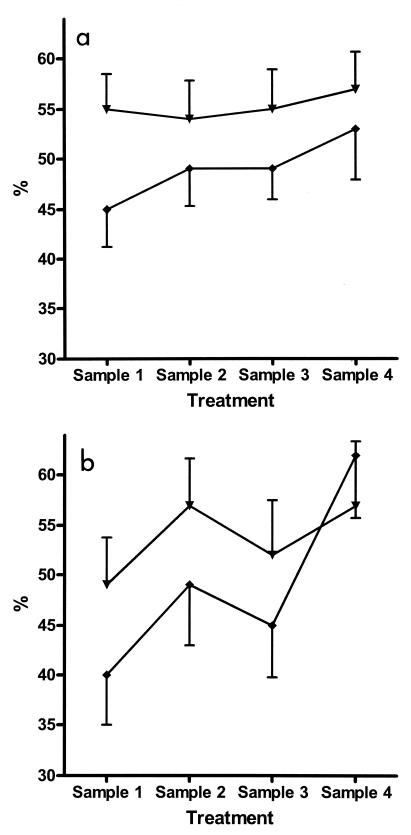
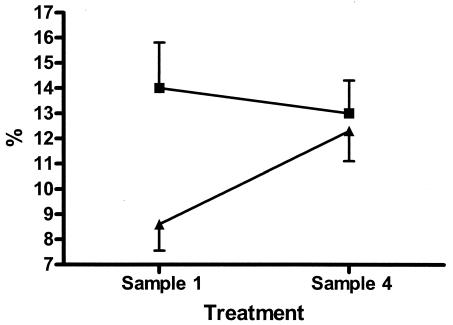
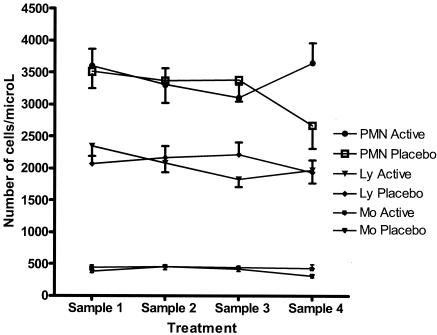
When longitudinal changes possibly achieved by photopheresis were considered, it is also important to consider changes in the placebo-treated group, since these changes should mirror the course of disease. Thus, in the placebo-treated group we found posttreatment increases in the proportions of CD4+CD29+ cells and, despite a marked decrease for sample 3, increases in the proportions of CD8+CD11a+ cells compared with the pretreatment levels (P < 0.05 and P < 0.01, respectively) (Fig. 2). The proportion of CD3γ/δ cells was increased after the last treatment compared with the levels at diagnosis (P < 0.01) (Fig. 3). None of these changes except for an increase in the proportion of CD8+CD11a+ cells after the first treatment compared to the pretreatment level occurred in the group that received active treatment. The number of lymphocytes decreased significantly during treatment (P < 0.02) in the group that received active treatment (Fig. 4), resulting in decreased numbers of both B cells and T cells. However, the distribution of these cells did not change significantly.
FIG. 2.
Proportion (mean ± standard error) of lymphocyte subsets during treatment with photopheresis or sham pheresis. (a) CD4+CD29+ cells represent an activated, memory population of T-helper (CD4) cells. (b) CD8+CD11a+ represent an activated, effective population of T-cytotoxic (CD8) cells. These subpopulations are given as a percentage of the mother population (CD4 and CD8, respectively). ▾, active treatment group; ♦, placebo treatment group.
FIG. 3.
Proportion (mean ± standard error) of lymphocytes with γ/δ receptor before and after treatment with photopheresis (▪) or sham pheresis (▴).
FIG. 4.
Number of cells per liter (mean ± standard error). PMN, polymorphonuclear granulocytes; Ly, lymphocytes; Mo, monocytes; filled symbols, photopheresis group; open symbols, sham pheresis group.
Comparison of lymphocyte populations between study groups before and after treatment.
Before treatment the proportion of CD4+ cells was lower in the group that subsequently received active treatment (Table 2). This difference was identically preserved after five treatments, indicating that the mode of treatment did not affect the proportion of this subset. The other statistically significant difference between the groups was that the proportion of T cells with γ/δ was higher before treatment in the group randomized to the active treatment (Table 2 and Fig. 3). After 1 year no difference was found between the groups, indicating indirectly that this subset was affected by photopheresis, since this cell population did not increase in the actively treated group as it did in the placebo group. Although the difference was not statistically significant, there was a trend for the CD8+CD11a+ subpopulation to be present at higher levels in the active treatment group before treatment, while the reverse pattern was noted after the fourth treatment (sample 4). After 1 year the number of polymorphonuclear granulocytes was significantly lower (P < 0.05) in the placebo-treated group than in the actively treated group (Fig. 4).
TABLE 2.
Lymphocyte populations with significant differences between the two groups before treatment or after the last treatmenta
| Cluster designation | Mean (SD)
|
|||
|---|---|---|---|---|
| Sample 1
|
Sample 4
|
|||
| Active group (n = 19) | Placebo group (n = 21) | Active group (n = 12) | Placebo group (n = 12) | |
| CD4 (%) | 39 (5.1)b | 45 (6.4)b | 39 (3.5)b | 44 (4.4)b |
| CD4/CD8 ratio | 1.38 (0.38)c | 1.76 (0.52)c | 1.43 (0.28)c | 1.78 (0.38)c |
| CD3γ/δ | 14 (8.0) | 8.6 (4.6)b | 13 (4.0) | 12.3 (4.2) |
The statistical analysis compares the results for the sample obtained before treatment (sample 1) for the actively treated group with the results obtained for the sample obtained before treatment for the placebo group, and the corresponding comparison is done for the samples obtained from each group after the last treatment (sample 4).
P < 0.01.
P < 0.02.
Lymphocyte subsets in relation to response and clinical outcome.
The group of responders in the actively treated group who were defined by their low doses of insulin during monitoring (Table 1, mild disease process) had a pretreatment distribution of lymphocyte populations similar to that in the group with an aggressive disease process (data not shown). Also, the distribution of lymphocyte populations in the group defined to have a mild clinical onset, irrespective of the subsequent treatment, did not differ in comparison with that either in the remaining subjects (data not shown) or in the group defined to have an aggressive disease onset (Table 1).
DISCUSSION
No major change in lymphocyte populations was found during the course of photopheresis treatment in children with newly diagnosed diabetes. However, in the placebo group the proportions of activated T cells, both CD4 cells expressing CD29 and CD8 cells expressing CD11a, increased. These changes might reflect the natural course during the first year after diagnosis, which is line with previous findings of major alterations in cytokine secretion patterns during the same period (18). A factor that could influence differences between the groups is the occurrence of more pronounced symptoms in the treated group (28), which, theoretically, could imply that the groups were at different time points in the natural course of their disease. However, the duration of symptoms before the first sampling occasion did not differ, indicating that this was not the case. Therefore, the placebo group displayed the natural changes that occur in type 1 diabetes. Since the changes recorded in the placebo group did not occur in the group receiving active treatment, this might indicate that photopheresis induces some suppressive effect.
Aberrant activation of CD8 cells in patients with newly diagnosed insulin-dependent diabetes mellitus was reported previously (16). It has also been shown that type 1 diabetes of long standing is associated with activation of circulating T cells (9), corroborating our findings of increased activated T-cell phenotypes during the natural course of the disease, as shown in the placebo group. Thus, an important effect of photopheresis may be in the direction of the creation of a suppressive phenotype. This is in agreement with the effects on cytokine patterns induced by photopheresis in children with insulin-dependent diabetes mellitus (M. Faresjö et al., unpublished data), patients with chronic graft-versus-host disease (11), and healthy blood mononuclear cells (20).
Another change was related to the CD3γ/δ population, which increased in the placebo group but not in the photopheresis group. T cells with γ/δ can recognize nonpeptide antigens and belong to the innate immune system, constituting a link to the adaptive response. A role for T cells with γ/δ has also been suggested in autoimmunity, and increased levels were reported in patients with type 1 diabetes compared with those in controls (14, 24). Interestingly, it has been proposed that T cells with γ/δ could be induced by the delivery of exogenous insulin (23), which would fit well with our finding of an increasing proportion of T cells with γ/δ in the control group (those who received insulin but no photopheresis). It is therefore of particular interest that children treated with photopheresis did not encounter any increase in this subset, suggesting that this effect could be pathogenetically relevant.
In patients with cutaneous T-cell lymphoma, a certain lymphocyte profile was associated with a response to photopheresis (37), and this was also suggested in patients with psoriatic arthritis, among whom we found that patients with high CD4/CD8 ratios had beneficial outcomes following photopheresis treatment (34). In the present study on type 1 diabetes, however, no such predictions could be made. Regarding the number of cells, we found that photopheresis significantly reduced the numbers of lymphocytes, leading to lower numbers of both T cells and B cells in the group that received active treatment. It is unclear if this change could play a role in the immunological effects induced by photopheresis. Although the focus in the study described in this paper was lymphocytes, we also noted that the number of polymorphonuclear granulocytes decreased in the placebo group compared to the number in the photopheresis group. The significance of this finding remains to be clarified.
We also looked at correlations in the whole group before treatment, i.e., a large group of children with recently onset of type 1 diabetes. However, we found just a few associations with clinical parameters. A history of previous infection did not even seem to affect lymphocyte populations, possibly because the time interval that had passed allowed the lymphocyte populations to normalize. The major difference found was related to gender: girls showed lower proportions of B cells and higher proportions of T cells, in particular, CD4 cells of the naïve phenotype. To our knowledge, this difference has not been described previously. The reason remains unclear, although hormonal influences might play a role.
Although some significant and relevant changes occurred in the material evaluated in the present study, it should be kept in mind that, on the whole, many lymphocyte populations were not dramatically affected. This does not reflect methodological errors, since the same methodology and the same markers proved to be able to define suppressive lymphocyte phenotypes during normal pregnancy (30) and in patients with Guillain-Barré syndrome (5) and active phenotypes in patients with recurrent spontaneous abortions (17), polyneuropathy associated with monoclonal gammopathy (35), multiple sclerosis (36), and preeclampsia (31). Thus, the results of the present study support the notion that the effects of photopheresis should also be investigated by determination of functional properties like cytokine secretion patterns (20) as well as dendritic cell function and dendritic cell-T cell interactions (10, 11).
In conclusion, we found no major effects of photopheresis on the lymphocyte populations in a group of children with newly diagnosed type 1 diabetes. However, the placebo group showed increased proportions of activated CD4 and CD8 cells, probably reflecting the course of disease. Since these changes did not occur in the treated group, our findings suggest that photopheresis may have some suppressive effects.
Acknowledgments
We are very grateful to the children who gladly and with enthusiasm participated in this extensive study. We thank Karin Backteman, Ingegerd Fälth Gustavsson, and Joacim Nilson for excellent technical assistance with flow cytometry. We also thank Lena Berglert, Sonja Hellström, and Eva Isacsson for technical and administrative assistance.
The study was supported by Barndiabetesfonden (Child Diabetes Foundation), Nove Nordisk Foundation, Lundström Foundation, Söderberg Foundation, Swedish Diabetes Association, JDF-Wallenberg Foundation (grant K98-99JD-12813-01A), County Council of Östergötland, and Swedish Medical Research Council (K99-72X-11242-05A). The study was also sponsored in part by Therakos Inc., which provided the disposables for the photopheresis treatments, and Tika, Lund, Sweden, which provided the Puvament (8-methoxy-psoralen) tablets.
REFERENCES
- 1.Atkinson, M. A., and N. K. Maclaren. 1994. The pathogenesis of insulin-dependent diabetes mellitus. N. Engl. J. Med. 331:1428-1436. [DOI] [PubMed] [Google Scholar]
- 2.Barr, M. L., B. M. Meiser, H. J. Eisen, R. F. Roberts, U. Livi, R. Dall'Amico, R. Dorent, J. G. Rogers, B. Radovanovic, B. O. Taylor, and K. Jecvanandam. 1998. Photopheresis for the prevention of rejection in cardiac transplantation. N. Engl. J. Med. 339:1744-1751. [DOI] [PubMed] [Google Scholar]
- 3.Cadet, J., C. Anselmino, T. Douki, and L. Voituriez. 1992. New trends in photobiology, photochemistry of nucleic acids in cells. J. Photochem. Photobiol. 15:277-298. [DOI] [PubMed] [Google Scholar]
- 4.Coutant, R., P. Landais, M. Rosilio, C. Johnsen, N. Lahlou, P. Chatelain, J. C. Carel, J. Ludvigsson, C. Boitard, and P. F. Bougneres. 1998. Low dose linomide in type 1 juvenile diabetes of recent onset: a randomized placebo-controlled double blind trial. Diabetologia 41:1040-1046. [DOI] [PubMed] [Google Scholar]
- 5.Dahle, L., M. Vrethem, and J. Ernerudh. 1994. T-lymphocyte subset abnormalities in peripheral blood from patients with Guillain-Barré syndrome. J. Neuroimmunol. 53:219-225. [DOI] [PubMed] [Google Scholar]
- 6.Dobbin, J. P., M. Harth, G. A. McCain, R. A. Martin, and K. Couisin. 1991. Cytokine production and lymphocyte transformation during stress. Brain Behav. Immun. 5:339-348. [DOI] [PubMed] [Google Scholar]
- 7.Edelson, R. L., C. Berger, and F. Gasparro. 1987. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy: preliminary results. N. Engl. J. Med. 316:297-303. [DOI] [PubMed] [Google Scholar]
- 8.Eisenbarth, G. S. 1986. Type 1 diabetes a chronic autoimmune disease. N. Engl. J. Med. 22:360-368. [DOI] [PubMed] [Google Scholar]
- 9.Gessl, A., and W. Waldhäusl. 1998. Increased CD69 and human leukocyte antigen-DR expression on T-lymphocytes in insulin-dependent diabetes mellitus of long standing. J. Clin. Endocrinol. Metab. 83:2204-2209. [DOI] [PubMed] [Google Scholar]
- 10.Giradi, M., J. Schechner, E. Glusac, C. Berger, and R. Edelson. 2002. Transimmunization and the evolution of extracorporeal photochemotherapy. Transfusion Apheresis Sci. 26:181-190. [DOI] [PubMed] [Google Scholar]
- 11.Gorgun, G., K. B. Miller, and F. M. Foss. 2002. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood 100:941-947. [DOI] [PubMed] [Google Scholar]
- 12.Greinix, H. T., B. Volc-Platzer, W. Rabitsch, B. Gmeinhart, C. Guevara-Pineda, P. Kahls, J. Krutman, H. Honigsman, M. Coivica, and R. M. Knobler. 1998. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood 92:3098-3104. [PubMed] [Google Scholar]
- 13.Grubin, C. E., T. Daniels, B. Toivola, M. Landin-Olsson, W. A. Hagopian, L. Li, A. E. Karlsen, E. Boel, B. Michelsen, and A. Lernmark. 1994. A novel radioligand binding assay to determine diagnostic accuracy of isoform-specific glutamic acid decarboxylase antibodies in childhood IDDM. Diabetologia 37:344-350. [DOI] [PubMed] [Google Scholar]
- 14.Gyarmati, J., J. Szekeres-Bartho, B. Fischer, and G. Soltesz. 1999. Fetal type lymphocytes in insulin dependent diabetes mellitus. Autoimmunity 30:63-69. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, L. C., P. G. Coleman, B. Dean, R. Baxter, and F. I. R. Martin. 1985. Increase of remission rate in newly diagnosed type 1 diabetes subjects treated with azathioprine. Diabetes 34:1306-1308. [DOI] [PubMed] [Google Scholar]
- 16.Hehmke, B., D. Michaelis, E. Gens, F. Laube, and K. D. Kohnert. 1995. Aberrant activations of CD8+ T-cell and CD8+ T-cell subsets in patients with newly diagnosed IDDM. Diabetes 44:1414-1419. [DOI] [PubMed] [Google Scholar]
- 17.Jablonowska. J., M. Palfi, L. Matthiesen, A. Selbing, S. Kjellberg, and J. Ernerudh. 2001. Lymphocyte subsets in patients with unexplained recurrent spontaneous abortions treated with intravenous immunoglobulin. Am. J. Reprod. Immunol. 45:226-231.11327549 [Google Scholar]
- 18.Karlsson Faresjö, M. G., J. Ernerudh, and J. Ludvigsson. 2004. Cytokine profile in children during the first 3 months after diagnosis of type 1 diabetes. Scand. J. Immunol. 59:517-526. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa, T., and J. Ludvigsson. 1988. Cyclosporin in diabetes mellitus. J. Pediatr. 112:500-501. [DOI] [PubMed] [Google Scholar]
- 20.Klosner, G., F. Trautinger, R. Knobler, and P. Neuner. 2001. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from Th1 to a Th2 response. J. Investig. Dermatol. 116:459-462. [DOI] [PubMed] [Google Scholar]
- 21.Knobler, R. M., W. Graninger, A. Lindmaier, F. Trautinger, and J. S. Smolen. 1992. Extracorporeal photochemotherapy for the treatment of systemic lupus erythematosus: a pilot study. Arthritis Rheum. 35:319-324. [DOI] [PubMed] [Google Scholar]
- 22.Kohnert, K. D., B. Hehnmke, H. Keilacker, M. Zigler, F. Emmrich, F. Laube, and D Michaelis. 1996. Antibody response to islet antigens in anti-CD4/prednisolone immune intervention of type 1 diabetes. Int. J. Clin. Lab. Res. 26:55-59. [DOI] [PubMed] [Google Scholar]
- 23.Kretowski, A., J. Mysliwiec, and I. Kinalska. 2000. Abnormal distribution of gamma delta T lymphocytes in Graves' disease and insulin-dependent diabetes type 1. Arch. Immunol. Ther. Exp. 48:39-42. [PubMed] [Google Scholar]
- 24.Kretowski, A., J. Mysliwiec, M. Szelachowska, D. Turowski, J. Wysocka, I. Kowalska, and I. Kinalska. 1999. Gamma delta T-cells alterations in the peripheral blood of high risk diabetes type 1 subjects with subclinical pancreatic B-cells impairment. Immunol. Lett. 68:289-293. [DOI] [PubMed] [Google Scholar]
- 25.Lacey, K., M. D. Zaharia, J. Griffiths, A. V. Ravindran, Z. Merali, and H. Anisma. 2000. A prospective study of neuroendocrine and immune alterations associated with the stress of an oral academic examination among graduate students. Psychoneuroendocrinology 25:339-356. [DOI] [PubMed] [Google Scholar]
- 26.Laskin, J. D., E. Lee, E. J. Yurkow, D. L Laskin, and M. A. Gallo. 1985. A possible mechanism of phototoxicity not involving direct interaction with DNA. Proc. Natl. Acad. Sci. USA 82:6158-6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludvigsson, J., L. Heding, G. Lieden, B. Marner, and Å. Lernmark. 1983. Plasmapheresis in the initial treatment of insulin-dependent diabetes mellitus. BMJ 286:176-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludvigsson, J., U. Samuelsson, J. Ernerudh, C. Johansson, L. Stenhammar, and G. Berlin. 2001. Photopheresis at onset of type 1 diabetes: a randomised, double blind, placebo controlled trial. Arch. Dis. Child. 85:149-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malawista, S. E., D. H. Trock, and R. L. Edelson. 1991. Treatment of rheumatoid arthritis by extracorporeal photochemotherapy. Arthritis Rheum. 34:646-654. [DOI] [PubMed] [Google Scholar]
- 30.Matthiesen, L., G. Berg, J. Ernerudh, and L. Håkansson. 1996. Lymphocyte subsets and mitogen stimulation of blood lymphocytes in normal pregnancy. Am. J. Reprod. Immunol. 35:70-79. [DOI] [PubMed] [Google Scholar]
- 31.Matthiesen, L., G. Berg, J. Ernerudh, and L. Håkansson. 1999. Lymphocyte subsets and mitogen stimuation of blood lymphocytes in preeclampsia. Am. J. Reprod. Immunol. 41:192-203. [DOI] [PubMed] [Google Scholar]
- 32.Miller, H. 1988. Neuroendocrine and immune system interactions in stress and depression. Psychiatr. Clin. N. Am. 21:443-463. [DOI] [PubMed] [Google Scholar]
- 33.Stiller, C. R., J. Dupre, and M. Gent. 1984. Effects of cyclosporin immuno-suppression in insulin-dependent diabetes of recent onset. Science 223:1362-1367. [DOI] [PubMed] [Google Scholar]
- 34.Vahlquist, C., M. Larsson, J. Ernerudh, G. Berlin, T. Skogh, and A. Vahlquist. 1996. Treatment of psoriatic arthritis with extracorporeal photochemotherapy and conventional psoralen-ultraviolet A irradiation. Arthritis Rheum. 39:19-23. [DOI] [PubMed] [Google Scholar]
- 35.Vrethem, M., L. Dahle, C. Ekerfelt, J. Nilsson, B. Ekstedt, and J. Ernerudh. 1994. Abnormalities in T-lymphocyte populations in blood from patients with demyelinating polyneuropathy associated with monoclonal gammopathy. J. Neurol. Sci. 122:171-178. [DOI] [PubMed] [Google Scholar]
- 36.Vrethem, M., L. Dahle, C. Ekerfelt, P. Forsberg, O. Danielsson, and J. Ernerudh. 1998. CD4 and CD8 lymphocyte subsets in cerebrospinal fluid and peripheral blood from patients with multiple sclerosis, meningitis and normal controls. Acta Neurol. Scand. 97:215-220. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe, J. T., R. S. R. Lessin, A. H. Singh, and A. H. Rook. 1994. Review of immunomodulation by photopheresis: treatment of cutaneous T-cell lymphoma, autoimmune disease, and allograft rejection. Artificial Organs 18:888-897. [DOI] [PubMed] [Google Scholar]