Abstract
Background
Unicuspid aortic valve is an important subset of bicuspid aortic valve, and knowledge regarding its aortopathy pattern and surgical outcomes is limited. Our objectives were to characterize unicuspid aortic valve patients, associated aortopathy, and surgical outcomes.
Methods
From January 1990 to May 2013, 149 adult unicuspid aortic valve patients underwent aortic valve replacement or repair for aortic stenosis (n = 13), regurgitation (n = 13), or both (n = 123), and in 91 (61%) the aortic valve operation was combined with aortic repair. Data were obtained from the Cardiovascular Information Registry and medical record review. Three-dimensional imaging analysis was performed from preoperative computed tomography and magnetic resonance imaging scans. The Kaplan-Meier method was used for survival analysis.
Results
Patients had a mean maximum aortic diameter of 44 ± 8 mm and variably involved the aortic root, ascending, or arch, or both. Patients with valve operations alone were more likely to be hypertensive (p = 0.01) and to have severe aortic stenosis (p = 0.07) than those who underwent concurrent aortic operations. There were no operative deaths, strokes, or myocardial infarctions. Patients undergoing aortic repair had better long-term survival. Estimated survival at 1, 5, and 10 years was 100%, 100%, and 100% after combined operations and was 100%, 88%, and 88% after valve operations alone (p = 0.01).
Conclusions
Patients with a dysfunctional unicuspid aortic valve frequently present with an ascending aneurysm that requires repair. Combined aortic valve operations and aortic repair was associated with significantly better long-term survival than a valve operation alone. Further study of this association may direct decisions about timing of surgical intervention.
Unicuspid aortic valve (UAV) has been described as an important morphologic subset of bicuspid aortic valve (BAV), the most common congenital cardiac anomaly commonly associated with aortic valve stenosis, regurgitation, and resulting heart failure [1–5]. The different types of BAV morphology are thought to represent a continuous pathologic spectrum [1, 5–8]. Studies have shown that UAV patients presented with predominantly aortic stenosis at a younger age than other BAV patients [9–11].
Ascending aortic dilatation, also known as aortopathy, is frequent in patients with BAV [1–4]. However, current guidelines for surgical repair of ascending aortic aneurysm do not consider the types of BAV morphology or aortopathy observed [12–16]. A small number of studies have investigated UAV patients and showed ascending aortic dilatation with mild histologic changes of the media [9–11]. Detailed knowledge regarding the pattern of aortic dilatation is limited. Clinical outcomes after aortic valve replacement (AVR) or repair, with or without ascending aortic repair, are also unclear. The objectives of this study were to characterize UAV patients and to describe associated aortopathy and surgical outcomes after AVR.
Patients and Methods
Patients
From January 1990 to May 2013, 149 adult UAV patients underwent AVR at Cleveland Clinic for aortic stenosis (AS; n = 13), regurgitation (n = 13), or both (n 123). The presence of UAV was determined by direct intraoperative inspection. Mean age at the operation was 44 ± 12 years, and 65% were women. At the time of presentation, 104 patients (70%) had an aortic diameter of 4 cm or more, and 91 patients (61%) underwent concurrent aortic operations at the time of AVR. None had descending aortic repair. Ten patients (6.7%) underwent concomitant coronary artery bypass. Patients were actively followed up at 2-year intervals. Mean follow-up was 4 ± 2.8 years. Death data were augmented with information from the Social Security Death Index.
Data Sources
Patients and clinical data were extracted from the Cleveland Clinic Cardiovascular Information Registry, a prospectively maintained registry. Additional data were gathered by retrospective record review. Use of the registry data for research and this study were approved by the Institutional Review Board, with patient consent waived.
Cross-Sectional Imaging Measurements
Preoperative cross-sectional imaging was available for 102 patients (68%). Detailed imaging analyses were made from computed tomography and magnetic resonance imaging using three-dimensional reconstruction software (TeraRecon, Foster City, CA). The observer was blinded to surgical procedures and outcomes until after the imaging data were collected. Aortic cross-sectional diameters were collected in the orthonormal plane to the centerline of the aorta at nine different landmarks (Fig 1). These landmarks are based on the American College of Cardiology–American Heart Association consensus guidelines [17] and Mendoza and colleagues [18] (Fig 2). Long-axis and short-axis diameters were measured at aortic anulus. The cross-sectional area was also measured at each location to account for the asymmetrical shape and for calculation of the maximum area/height ratio [14]. Calcium volume was quantified at the level of the aortic valve, and arch configurations with regard to the pattern of vessel branching were also noted [15].
Fig 1.
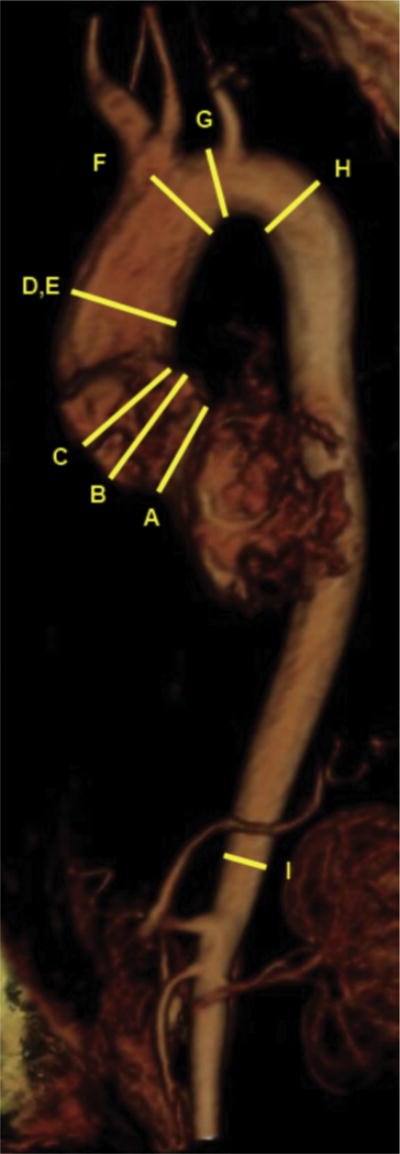
Measurement landmarks on volume-rendered image are the (A) aortic anulus, (B) sinuses of Valsalva, (C) sinotubular junction, (D) tubular ascending aorta, (E) maximum ascending aorta, (F) aorta at innominate takeoff, (G) mid arch, (H) proximal descending aorta, and (I) distal descending aorta.
Fig 2.
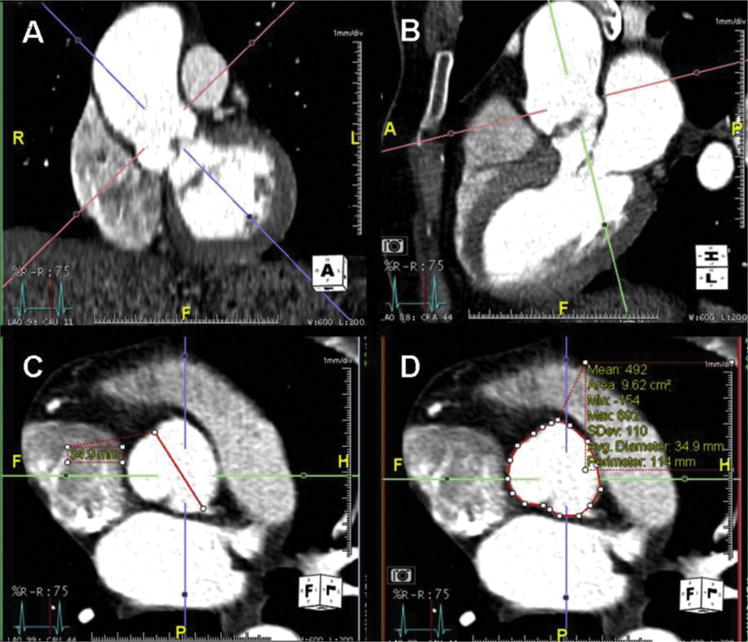
(A) Coronal and (B) sagittal views of the root and ascending thoracic aorta demonstrate representative landmarks: sinotubular junction and anulus. Orthogonal imaging planes show the (C) sinus to commisure diameter (diagonal line) and (D) area (circular line) quantification methods of the level of the sinuses of Valsalva.
Interobserver and Intraobserver Variability
Intraobserver variability was determined by comparing measurements at individual landmarks taken by the same blinded observer in 10 randomly selected patients. Interobserver variability was determined by comparing measurements at individual landmarks made by 2 independent blinded observers in the same 10 randomly chosen patients.
Statistical Analysis
Multilogistic and multilinear regression models were used to further analyze variables associated with preoperative patient characteristics and postoperative complications to adjust for potential confounders and effect modifiers. Continuous variables are presented as mean ± standard deviation (15-50-85 percentiles), and categoric variables are presented as percentages. Bland-Altman analysis was computed to assess measurement reproducibility. Survival analysis was performed using Kaplan-Meier survival curves. A two-sided p value of less than 0.05 was considered indicative of statistical significance. Statistical calculations were performed using JMP 7 software (SAS Institute Inc, Cary, NC).
Results
Patient Characteristics
Preoperative patient characteristics are reported in Table 1. Most patients presented with severe AS (58%) and 3+ aortic regurgitation (33%). Peak and mean aortic gradients were 77.8 ± 37.1 mm Hg and 44 ± 21.5 mm Hg, respectively. The mean aortic orifice area was 0.9 ± 0.44 cm2. Aortic valve calcification was present in 102 patients (68%), and the average calcification volume was 2.11 ± 2.1 cm3.
Table 1.
Preoperative Patient Characteristics
| Variable | AVR + Aortic Operation (n = 91)
|
AVR Alone (n = 58)
|
Total (N = 149)
|
p Value | |||
|---|---|---|---|---|---|---|---|
| No. | No. (%) or Mean ± SD | No. | No. (%) or Mean ± SD | No. | No. (%) or Mean ± SD | ||
| Demographics | |||||||
| Female | 91 | 61 (67) | 58 | 36 (62) | 149 | 97 (65) | 0.6 |
| Age, y | 91 | 43.8 ± 11.1 | 58 | 43.6 ± 12.6 | 149 | 43.8 ± 11.7 | 0.91 |
| Height, cm | 91 | 174 ± 11.3 | 58 | 171 ± 17 | 149 | 173 ± 13.8 | 0.37 |
| Pre-op comorbidities | |||||||
| Hypertension | 87 | 15 (17) | 58 | 21 (36) | 145 | 36 (25) | .01a |
| Diabetes | 87 | 3 (3.4) | 57 | 3 (5.3) | 144 | 6 (4.2) | 0.68 |
| Carotid disease | 91 | 1 (1.1) | 58 | 3 (5.2) | 149 | 4 (2.7) | 0.3 |
| Myocardial infarction | 91 | 4 (4.3) | 58 | 3 (5.2) | 149 | 7 (4.7) | 1 |
| Cerebrovascular accident | 91 | 0 (0) | 58 | 4 (6.9) | 149 | 4 (2.7) | .02a |
| Congestive heart failure | 87 | 4 (4.6) | 58 | 8 (14) | 145 | 12 (8.3) | 0.07 |
| COPD | 87 | 1 (1.1) | 58 | 5 (8.6) | 145 | 6 (4.1) | .04a |
| Prior cardiac operation | 91 | 13 (14) | 58 | 8 (14) | 149 | 21 (14) | 0.95 |
| Coronary artery disease | 91 | 2 (2.2) | 58 | 8 (14) | 149 | 10 (6.7) | .006a |
| Pre-op aortic valve diagnosis | |||||||
| Aortic stenosis | 91 | 84 (92) | 58 | 52 (90) | 149 | 136 (91) | .03a |
| Mild | 87 | 15 (17) | 52 | 3 (5.8) | 139 | 18 (13) | 0.25 |
| Moderate | 20 (23) | 8 (15) | 28 (20) | ||||
| Severe | 45 (52) | 35 (67) | 80 (58) | ||||
| Aortic regurgitation | 91 | 87 (96) | 58 | 49 (85) | 149 | 136 (91) | 0.57 |
| 1+ | 88 | 28 (32) | 53 | 12 (23) | 141 | 40 (28) | 0.7 |
| 2+ | 19 (22) | 8 (15) | 27 (19) | ||||
| 3+ | 27 (31) | 20 (38) | 47 (33) | ||||
| 4+ | 10 (11) | 4 (7.5) | 14 (10) | ||||
| Stenosis + regurgitation | 91 | 80 (88) | 58 | 43 (74) | 149 | 123 (83) | .04a |
| Orifice area, cm2 | 74 | 0.94 ± 0.42 | 46 | 0.83 ± 0.47 | 120 | 0.9 ± 0.44 | 0.18 |
| Peak gradient, mm Hg | 87 | 74.7 ± 37.3 | 52 | 83 ± 36.5 | 139 | 77.8 ± 37.1 | 0.17 |
| Mean gradient, mm Hg | 86 | 43.4 ± 22.8 | 50 | 45.1 ± 19.2 | 136 | 44 ± 21.5 | 0.58 |
| Aortic valve calcification | 91 | 61 (67) | 58 | 41 (71) | 149 | 102 (68) | 0.74 |
| Calcification volume, cm3 | 43 | 2.16 ± 2.3 | 20 | 1.99 ± 1.63 | 63 | 2.11 ± 2.1 | 0.87 |
| Left ventricular function | |||||||
| End-diastolic diameter, cm | 88 | 5 ± 0.9 | 52 | 5 ± 0.91 | 140 | 5 ± 0.9 | 0.96 |
| End-systolic diameter, cm | 88 | 3.2 ± 0.91 | 52 | 3.1 ± 0.86 | 140 | 3.2 ± 0.89 | 0.94 |
| Ejection fraction | 88 | 0.564 ± 0.075 | 53 | 0.548 ± 0.0989 | 141 | 0.558 ± 0.085 | 0.26 |
Statistically significant (p < 0.05).
AVR = aortic valve replacement; COPD = chronic obstructive pulmonary disease; SD = standard deviation.
Patients were further stratified by whether they underwent a concurrent aortic operation. In those who had AVR alone, 35 (67%) had severe AS compared with 45 (52%) who underwent a concurrent aortic operation (p = 0.07). Patients who underwent AVR alone had more hypertension (p = 0.01), chronic obstructive pulmonary disease (p = 0.04), coronary artery disease (p = 0.006), and a history of cerebral vascular accident (p =0.02) compared with those who had AVR and an aortic operation. Only the difference in hypertension was independent of patient age (p = 0.01). Four patients (4%) had aortic valve repair. They were younger (mean age, 36 ± 6 years) and all underwent a concurrent aortic operation.
Aortic Morphology
Aortic diameters were most notably dilated at the proximal levels: aortic anulus, sinuses of Valsalva, sinotubular junction, ascending aorta, and innominate artery (Table 2). Diameters at these proximal levels, except at the aortic anulus, were statistically significantly greater in patients who had an aortic operation compared with those who had AVR alone (p < 0.01). This difference was also noted when aortic size was indexed to patient height [14]. Patients undergoing an aortic operation had significantly higher maximum aortic area/height ratios at these proximal levels than those who had AVR alone (p < 0.05). Interobserver and intraobserver errors were computed, and reproducibility was excellent at all levels (Supplementary Tables S1 and S2).
Table 2.
Aortic Measurements
| Variable | AVR + Aortic Operation (n = 91)
|
AVR Alone (n = 58)
|
Total (N = 149)
|
p Value | |||
|---|---|---|---|---|---|---|---|
| No. | Mean ± SD or No. (%) | No. | Mean ± SD or No. (%) | No. | Mean ± SD or No. (%) | ||
| Diameter, mm | |||||||
| Anulus long axis | 68 | 31.1 ± 4.8 | 33 | 31 ± 4.1 | 101 | 31.1 ± 4.6 | 0.98 |
| Anulus short axis | 68 | 21.5 ± 3.9 | 33 | 21.5 ± 4 | 101 | 21.5 ± 3.9 | 0.97 |
| Sinuses of Valsalva | 68 | 37.5 ± 6 | 34 | 33.6 ± 4.3 | 102 | 36.2 ± 5.8 | .0003a |
| Sinotubular junction | 68 | 36.7 ± 6.1 | 34 | 30.3 ± 4.4 | 102 | 34.6 ± 6.4 | <.0001a |
| Tubular ascending | 67 | 46.4 ± 6.1 | 33 | 37 ± 5.8 | 100 | 43.3 ± 7.4 | <.0001a |
| Maximum ascending | 67 | 46.8 ± 6.3 | 31 | 37.2 ± 5.8 | 98 | 43.8 ± 7.6 | <.0001a |
| Innominate | 65 | 33.6 ± 3.9 | 30 | 30.5 ± 4.9 | 95 | 32.6 ± 4.4 | .004a |
| Mid arch | 65 | 27.3 ± 3.7 | 30 | 26 ± 3.6 | 95 | 26.9 ± 3.7 | 0.11 |
| Proximal descending | 65 | 24.5 ± 3.6 | 30 | 23.9 ± 4.4 | 95 | 24.3 ± 3.8 | 0.51 |
| Distal descending | 65 | 20.7 ± 3 | 29 | 20 ± 2.9 | 94 | 20.5 ± 3 | 0.32 |
| Area/height ratio, cm2/m | |||||||
| Anulus | 68 | 3.4 ± 1 | 33 | 3.4 ± 1.1 | 101 | 3.4 ± 1 | 0.98 |
| Sinuses of Valsalva | 68 | 6.7 ± 1.9 | 34 | 5.5 ± 1.8 | 102 | 6.3 ± 1.9 | .004a |
| Sinotubular junction | 66 | 6.3 ± 2.3 | 34 | 4.4 ± 1.3 | 100 | 5.6 ± 2.2 | <.0001a |
| Tubular ascending | 67 | 9.9 ± 2.6 | 33 | 6.5 ± 2.1 | 100 | 8.8 ± 2.9 | <.0001a |
| Maximum ascending | 67 | 10 ± 2.8 | 31 | 6.6 ± 2.1 | 98 | 9 ± 3 | <.0001a |
| Innominate | 65 | 5.1 ± 1.2 | 30 | 4.4 ± 1.5 | 95 | 4.9 ± 1.3 | 0.03a |
| Mid arch | 65 | 3.4 ± 1 | 30 | 3.2 ± 1 | 95 | 3.4 ± 1 | 0.38 |
| Proximal descending | 65 | 2.7 ± 0.8 | 30 | 2.8 ± 1.3 | 95 | 2.7 ± 0.9 | 0.91 |
| Distal Descending | 65 | 2 ± 0.5 | 29 | 1.9 ± 0.8 | 94 | 1.9 ± 0.6 | 0.88 |
| Arch configuration | |||||||
| Normal | 68 | 55 (80) | 34 | 25 (7) | 102 | 80 (78) | 0.25 |
| Bovine | 11 (16) | 4 (1) | 15 (15) | ||||
| Variant | 3 (4) | 5 (1) | 8 (8) | ||||
Statistically significant (p < 0.05).
AVR = aortic valve replacement; SD = standard deviation.
Of the patients who underwent a concurrent aortic operation, 28 (31%) had a maximum ascending aorta diameter of less than 4.5 cm, and only 9 (10%) had a ratio of less than 8 cm2/m [14]. Of those 28 patients, 17 (61%) had graft replacement and 11 (39%) had aortoplasty. In some patients who were planning a pregnancy, the threshold for aortic intervention was lowered. Two patients underwent prior aortoplasty at other institutions and experienced further aortic dilatation requiring aortic graft replacement at the Cleveland Clinic.
Aortic arch configuration anomalies were present in 22% of UAV patients, with 14% being bovine arch and 8% being other variants. The arch configuration was not associated with the decision to perform aortic repair.
Surgical Outcomes
Surgical details are reported in Table 3. No hospital deaths, myocardial infarctions (MIs), or strokes occurred. Surgical safety was similar between those who did and did not have an aortic operation. This was true for unadjusted and adjusted analyses. For patients who underwent aortic valve operations alone, 3 required reoperative AVR due to degenerated valve diseases at 3, 7, and 11 years. One patient required reoperation for valve dysfunction 8 days after the initial operation due to technical failure of an attempted mitral valve repair. Mean follow-up was 4 ± 2.8 years.
Table 3.
Operative Details and Outcomes
| Variable | AVR + Aortic Operation (n = 91)
|
AVR Alone (n = 58)
|
Total (N = 149)
|
p Value | |||
|---|---|---|---|---|---|---|---|
| No. | No. (%) or Mean ± SD | No. | No. (%) or Mean ± SD | No. | No. (%) or Mean ± SD | ||
| Operative details | |||||||
| Bioprosthetic valve | 88 | 47 (53.4) | 55 | 37 (67.3) | 146 | 84 (57.5) | .3 |
| Mechanical valve | 88 | 32 (36.4) | 55 | 18 (32.7) | 146 | 50 (34.2) | .48 |
| Homograft | 88 | 3 (3.4) | 55 | 3 (5.5) | 146 | 6 (4.1) | .62 |
| Composite root | 88 | 8 (9.1) | 146 | 8 (5.5) | N/A | ||
| Ascending repair | 88 | 81 (92) | 146 | 81 (55.5) | N/A | ||
| Hemiarch repair | 88 | 17 (19.3) | 146 | 17 (11.6) | N/A | ||
| Complete arch repair | 88 | 2 (2.3) | 146 | 2 (1.4) | N/A | ||
| Circulatory arrest | 88 | 18 (20.5) | 146 | 18 (12.3) | N/A | ||
| Surgical safety (operative, hospital, or 30 days) | |||||||
| Hospital death | 91 | 0 (0) | 58 | 0 (0) | 149 | 0 (0) | N/A |
| Stroke | 91 | 0 (0) | 58 | 0 (0) | 149 | 0 (0) | N/A |
| Peri-op myocardial infarction | 91 | 0 (0) | 58 | 0 (0) | 149 | 0 (0) | N/A |
| Dialysis | 91 | 1 (1.1) | 58 | 0 (0) | 149 | 1 (0.7) | 1 |
| Prolonged ventilation | 89 | 3 (3.3) | 57 | 5 (8.8) | 146 | 8 (5.5) | .26 |
| Length of stay | |||||||
| Intensive care unit, h | 91 | 40.7 ± 49.3 | 58 | 56 ± 84.6 | 149 | 46.6 ± 65.4 | .21 |
| Hospital, d | 91 | 7.5 ± 5.4 | 58 | 8.5 ± 6.5 | 149 | 7.9 ± 5.83 | .34 |
| Atrial fibrillation | 91 | 20 (22) | 58 | 7 (12) | 149 | 27 (18) | .13 |
| Bleeding | 91 | 4 (4.3) | 58 | 2 (3.4) | 149 | 6 (4) | 1 |
AVR = aortic valve replacement; N/A = not applicable; SD = standard deviation.
There were 6 late deaths. Cause of death included MI at the age of 64 at 1.1 years postoperatively, MI at the age of 53 and 1.5 years later, anoxic brain injury at the age of 44 and 3.5 years later, leukemia at the age of 66 and 3.8 years later, Burkitt lymphoma at the age of 31 and 11.7 years later, and one unknown at the age of 71 and 1 year later.
Kaplan-Meier estimated survival at 1, 5, and 10 years was 100%, 95%, and 95% for the entire population. Estimated survival at 1, 5, and 10 years was 100% at all three time points for those who had a concurrent aortic operation and 100%, 88%, and 88%, respectively, for those who had AVR alone. Late survival was better for those who underwent combined procedures (p = 0.01) than for those who had AVR alone (Fig 3).
Fig 3.
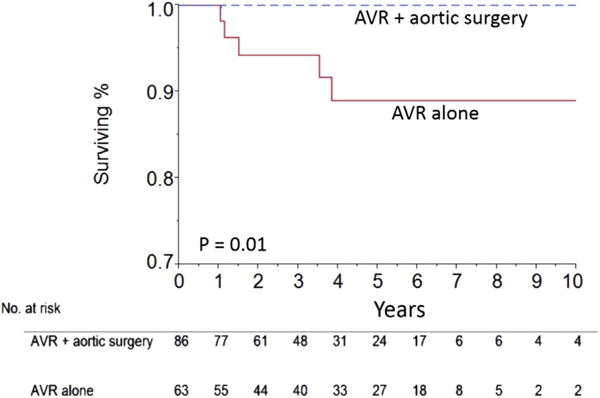
Kaplan-Meier curves show survival for patients undergoing aortic valve replacement (AVR) and an aortic operation (blue line) and those undergoing AVR alone (red line).
Comment
Principal Findings
This study demonstrated that most patients with UAV had coexisting aortopathy and thoracic aortic dilatation. This finding is very similar to what is seen in patients with BAV, suggesting that a similar disease process affects these patients. UAV patients were an average age of 44 ± 12 years at the operation, which is younger than when other BAV patients typically present. Overall surgical outcomes were excellent after AVR, with and without aortic operations, but long-term survival was better in patients who underwent combined valve and aortic operations.
UAV and Aortopathy
We indexed aortic size to height because the cross-sectional area/height ratio takes into account the greater risk of dissection in shorter patients [14, 19, 20]. We found that UAV patients demonstrated thoracic aortic dilatation from the aortic anulus to the proximal arch. Aortic anulus dilatation is another common feature of UAV, independent of the diameter of ascending aorta, suggesting that aortic anulus dilatation and the development of ascending aorta aneurysm are likely due to different pathologic processes. We also noted that there was no descending aorta dilatation, in accordance with previous studies [13, 21, 22]. This may be at least partially explained by the difference in embryologic origin of the descending aorta, the dorsal aorta, and that of the ascending aorta, the truncus arteriosus [23, 24].
Furthermore, one might expect that patients with aortic dilatation would have more uncontrolled hypertension and more severe AS leading to increased turbulent flow in the aorta if hydrodynamic stresses were the etiology of this association. Interestingly, we found the opposite in our population, and none of those who underwent isolated AVR required aortic repair later within the study period. Most likely, the aortic disease process associated with UAV has a mixed etiology, partially influenced by genetically triggered mechanisms.
Surgical Approach and Outcomes
Four patients had aortic valve repair and the rest had AVR. The 4 patients were much younger than the average UAV patient, and the need for later valve replacement is anticipated. Two had mild aortic regurgitation, and an aneurysm was the primary indication for the operation.
A BAV aorta can be safely resected without added penalty if the maximum diameter exceeds 4.5 cm or the ratio is at least 8 cm2/m when associated with symptomatic BAV disease [14]. Some patients in this study underwent aortic operations with diameters less than 4.5 cm or a maximum area/height ratio of less than 8 cm2/m. This subset all had symptomatic aortic valve disease and other risk factors, such as plans for pregnancy, that were considered in that decision. In several of these patients, we found that echocardiography overestimated the ascending aorta diameters compared with computed tomography. Accuracy of aortic measurements has been shown to be dependent on the imaging modality [18].
With an aggressive treatment approach, there was no increased risk of surgical complications or long-term death when aortic repair was included. In fact, long-term survival was better in patients who had a concurrent aortic operation than those who had AVR alone, in accordance with a previous BAV study [14]. Both late cardiac deaths were in the AVR-alone group and were attributable to MI, but the exact details of those events are unclear. We theorize that the difference in survival for AVR-alone patients may be because they had more severe myocardial remodeling but admit that this is impossible to determine in a study of this size with so few events. We have previously shown that markers of myocardial remodeling are a powerful predictor of survival in patients treated for AS [25–28].
Patients undergoing an aortic intervention as the primary indication may have had earlier operations in terms of the extent of myocardial remodeling that had occurred, and so the aortic operation may have provided a protective effect by improving fluid dynamics and forcing early aortic valve intervention. At the same time, the primary surgical indication in patients who had AVR alone was for late-stage aortic valve disease probably associated with myocardial remodeling.
Aortic Valve and Arch Configuration
Aortic arch configuration anomalies in the UAV population were present in 22% compared with 15% of the general population [29]. This suggests a possible contribution of abnormal cardiac embryogenesis to the development of UAV. The aortic valve, proximal ascending aorta, and pulmonary trunk all derive from the same neural crest cell lines [30, 31]. Future embryologic study is needed to understand the pathophysiology of UAV disease.
Limitations
This was a retrospective clinical cohort study, and the patient population was drawn from a surgical patient registry, which is a selection bias. Cross-sectional imaging data before 2000 and for some studies that were performed at other institutions were not available for review. The limited number of events meant we were not able to adjust for every potential confounder in evaluating long-term survival. Follow-up data were valid for up to 10 years after the operation, and in such a young population, end points such as death may not be expected to be seen until beyond this time frame. However, this is by far the largest study on UAV patients with aortic characteristics and surgical outcomes.
Conclusions
Patients with a dysfunctional UAV frequently present with an ascending aneurysm that requires repair. Combined aortic valve operations and aortic repair was associated with significantly better long-term survival than a valve operation alone. Further study of this association may direct decisions about timing of surgical intervention.
Supplementary Material
Footnotes
The Supplementary Tables can be viewed in the online version of this article [http://dx.doi.org/10.1016/j.athoracsur.2015.07.058] on http://www.annalsthoracicsurgery.org.
References
- 1.Kari FA, Beyersdorf F, Siepe M. Pathophysiological implications of different bicuspid aortic valve configurations. Cardiol Res Pract. 2012;2012:735829. doi: 10.1155/2012/735829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borger MA, Preston M, Ivanov J, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128:677–83. doi: 10.1016/j.jtcvs.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Sundt TM, 3rd, Mora BN, Moon MR, et al. Options for repair of a bicuspid aortic valve and ascending aortic aneurysm. Ann Thorac Surg. 2000;69:1333–7. doi: 10.1016/s0003-4975(00)01220-0. [DOI] [PubMed] [Google Scholar]
- 4.Braverman AC, Güven H, Beardslee MA, et al. The bicuspid aortic valve. Curr Probl Cardiol. 2005;30:470–522. doi: 10.1016/j.cpcardiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–33. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Edwards JE. Pathology of left ventricular outflow tract obstruction. Circulation. 1965;31:586–99. doi: 10.1161/01.cir.31.4.586. [DOI] [PubMed] [Google Scholar]
- 7.McKay R, Smith A, Leung MP, et al. Morphology of the ventriculoaortic junction in critical aortic stenosis. J Thorac Cardiovasc Surg. 1992;104:434–42. [PubMed] [Google Scholar]
- 8.Falcone MW, Roberts WC, Morrow AG, Perloff JK. Congenital aortic stenosis resulting from a unicommissural valve. Circulation. 1971;44:272–80. doi: 10.1161/01.cir.44.2.272. [DOI] [PubMed] [Google Scholar]
- 9.Mookadam F, Thota VR, Garcia-Lopez AM, et al. Unicuspid aortic valve in adults: a systematic review. J Heart Valve Dis. 2010;19:79–85. [PubMed] [Google Scholar]
- 10.Novaro GM, Micky M, Brian PG. Incidence and echocar-diographic features of congenital unicuspid aortic valve in an adult population. J Heart Valve Dis. 2003;12:674–8. [PubMed] [Google Scholar]
- 11.Butany J, Vaideeswar P, Dixit V, et al. Ascending aortic aneurysms in unicommissural aortic valve disease. Cardiovasc Pathol. 2009;18:11–8. doi: 10.1016/j.carpath.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Park CB, Greason KL, Suri RM, et al. Fate of nonreplaced sinuses of Valsalva in bicuspid aortic valve disease. J Thorac Cardiovasc Surg. 2011;142:278–84. doi: 10.1016/j.jtcvs.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 13.Fazel SS, Mallidi HR, Lee RS, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg. 2008;135:901–7. doi: 10.1016/j.jtcvs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 14.Svensson LG, Kim KH, Blackstone EH, et al. Bicuspid aortic valve surgery with proactive ascending aortic surgery. J Thorac Cardiovasc Surg. 2011;142:622–9. doi: 10.1016/j.jtcvs.2010.10.050. [DOI] [PubMed] [Google Scholar]
- 15.Budoff MJ, Takasu J, Katz R, et al. Reproducibility of CT measurements of aortic valve calcification, mitral annulus calcification, and aortic wall calcification in the multi-ethnic study of atherosclerosis. Acad Radiol. 2006;13:166–72. doi: 10.1016/j.acra.2005.09.090. [DOI] [PubMed] [Google Scholar]
- 16.Svensson LG, Al Kindi AH, Vivacqua A, et al. Long-term durability of bicuspid aortic valve repair. Ann Thorac Surg. 2014;97:1539–48. doi: 10.1016/j.athoracsur.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 18.Mendoza DD, Kochar M, Devereux RB, et al. Impact of image analysis methodology on diagnostic and surgical classification of patients with thoracic aortic aneurysms. Ann Thorac Surg. 2011;92:904–13. doi: 10.1016/j.athoracsur.2011.03.130. [DOI] [PubMed] [Google Scholar]
- 19.Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2003;126:892–3. doi: 10.1016/s0022-5223(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 20.Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2002;123:360–1. doi: 10.1067/mtc.2002.118497. [DOI] [PubMed] [Google Scholar]
- 21.Cecconi M, Manfrin M, Moraca A, et al. Aortic dimensions in patients with bicuspid aortic valve without significant valve dysfunction. Am J Cardiol. 2005;95:292–4. doi: 10.1016/j.amjcard.2004.08.098. [DOI] [PubMed] [Google Scholar]
- 22.Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–90. doi: 10.1161/CIRCULATIONAHA.108.795401. [DOI] [PubMed] [Google Scholar]
- 23.van Meurs-Van Woezik H, Krediet P. Measurements of the descending aorta in infants and children: comparison with other aortic dimensions. J Anat. 1982;135:273–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Stojanovska J, Cascade PN, Chong S, et al. Embryology and imaging review of aortic arch anomalies. J Thorac Imag. 2012;27:73–84. doi: 10.1097/RTI.0b013e318218923c. [DOI] [PubMed] [Google Scholar]
- 25.Mihaljevic T, Nowicki ER, Rajeswaran J, et al. Survival after valve replacement for aortic stenosis: Implications for decision making. J Thorac Cardiovasc Surg. 2008;135:1270–9. doi: 10.1016/j.jtcvs.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 26.Gjertsson P, Caidahl K, Bech-Hanssen O. Left ventricular diastolic dysfunction late after aortic valve replacement in patients with aortic stenosis. Am J Cardiol. 2005;96:722–7. doi: 10.1016/j.amjcard.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 27.Beach JM, Mihaljevic T, Rajeswaran J, et al. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2013;147:362–9. doi: 10.1016/j.jtcvs.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Roselli EE, Abdel Azim A, Houghtaling PL, et al. Pulmonary hypertension is associated with worse early and late outcomes after aortic valve replacement: implications for transcatheter aortic valve replacement. J Thorac Cardiovasc Surg. 2012;144:1067–74. doi: 10.1016/j.jtcvs.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Wanamaker KM, Amadi CC, Mueller JS, Moraca RJ. Incidence of aortic arch anomalies in patients with thoracic aortic dissections. J Cardiac Surg. 2013;28:151–4. doi: 10.1111/jocs.12072. [DOI] [PubMed] [Google Scholar]
- 30.Stoller JZ, Epstein JA. Cardiac neural crest. Semin Cell Dev Biol. 2005;16:704–15. doi: 10.1016/j.semcdb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Nomura-Kitabayashi A, Phoon CK, Kishigami S, et al. Outflow tract cushions perform a critical valve-like function in the early embryonic heart requiring BMPRIA-mediated signaling in cardiac neural crest. Am J Physiol Heart Circ Physiol. 2009;297:1617–28. doi: 10.1152/ajpheart.00304.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


