Abstract
At high levels, inorganic arsenic exposure is linked to peripheral arterial disease (PAD) and cardiovascular disease. To our knowledge, no prior study has evaluated the association between low-to-moderate arsenic exposure and incident PAD by ankle brachial index (ABI). We evaluated this relationship in the Strong Heart Study, a large population-based cohort study of American Indian communities. A total of 2,977 and 2,966 PAD-free participants who were aged 45–74 years in 1989–1991 were reexamined in 1993–1995 and 1997–1999, respectively, for incident PAD defined as either ABI <0.9 or ABI >1.4. A total of 286 and 206 incident PAD cases were identified for ABI <0.9 and ABI >1.4, respectively. The sum of inorganic and methylated urinary arsenic species (∑As) at baseline was used as a biomarker of long-term exposure. Comparing the highest tertile of ∑As with the lowest, the adjusted hazard ratios were 0.57 (95% confidence interval (CI): 0.32, 1.01) for ABI <0.9 and 2.24 (95% CI: 1.01, 4.32) for ABI >1.4. Increased arsenic methylation (as percent dimethylarsinate) was associated with a 2-fold increased risk of ABI >1.4 (hazard ratio = 2.04, 95% CI: 1.02, 3.41). Long-term low-to-moderate ∑As and increased arsenic methylation were associated with ABI >1.4 but not with ABI <0.9. Further studies are needed to clarify whether diabetes and enhanced arsenic metabolism increase susceptibility to the vasculotoxic effects of arsenic exposure.
Keywords: arsenic, metabolism, peripheral vascular disease
Exposure to inorganic arsenic in food and water is a significant global health problem (1, 2). At chronically high levels, inorganic arsenic exposure is vasculotoxic and has been associated with ischemic heart disease (1, 3), cerebrovascular disease (1, 4), and carotid atherosclerosis (5). In the historically high-arsenic area of southwestern Taiwan, high-level arsenic exposure is linked to the development of blackfoot disease, a severe form of peripheral arterial disease (PAD) leading to gangrene and spontaneous amputation (6–8). PAD and lower-extremity amputations were also common among German vintners exposed to high levels of arsenic through application of arsenical pesticides and intake of arsenic-contaminated wine from 1930 to 1950 (6). Pathological studies of PAD in these patients demonstrated vascular features consistent with thromboangitis obliterans and arteriosclerosis obliterans (7, 8).
In contrast to the vasculotoxic effects of high-level arsenic exposure, studies on the association between low-level arsenic exposure in the United States and PAD have been inconsistent and characterized by ecological designs and PAD definitions based on death certificates (1). To our knowledge, no prior study has evaluated the association of low-to-moderate arsenic exposure levels with incident PAD. In the Strong Heart Study (SHS), a population-based cohort study conducted in American Indian communities from Arizona, Oklahoma, and North and South Dakota (9), and the San Luis Valley Diabetes Study, conducted in rural white and Hispanic communities in Colorado (10), exposure to low-to-moderate levels of arsenic in drinking water was found to be associated with increased cardiovascular disease (CVD) incidence and mortality. Furthermore, while epidemiologic studies from Taiwan and Bangladesh have shown differential associations of arsenic metabolism with CVD and other health outcomes (11, 12), the relationship between arsenic metabolism at low-to-moderate exposure levels and risk of PAD has not been investigated.
Conventionally, an ankle brachial index (ABI) less than 0.9 has been considered occlusive PAD related to atherosclerosis, while an ABI greater than 1.4 has been related to arterial wall calcification and noncompressible vessels, which are frequently associated with diabetes and chronic kidney disease (13). In the US population aged 40 years or more, the prevalence of ABI >1.4 is relatively low at 1.4% (14). In contrast, in the SHS (15, 16), the prevalence of ABI >1.4 is nearly 9-fold higher than in a US population of similar age range (17). This may in part be due to the higher burden of comorbid type 2 diabetes in the American Indian population as compared with the overall US population (16, 18), although the reasons are not completely understood.
Our hypothesis in this investigation was that greater arsenic exposure would be associated with increased PAD incidence in the SHS. Because the association between arsenic and CVD in the SHS was stronger among participants with type 2 diabetes (9), we hypothesized that there would also be a stronger association between arsenic and PAD among participants with type 2 diabetes. Therefore, we evaluated the prospective associations of arsenic exposure and arsenic metabolite patterns with PAD incidence, defined as either ABI <0.9 or ABI >1.4 in at least 1 leg, over a 10-year period among SHS participants who were free of PAD at baseline.
METHODS
Study population
The SHS is a large, population-based prospective cohort study of CVD and its risk factors among American Indian communities in Arizona, Oklahoma, and North and South Dakota (15, 16). Conducted in a population with high burdens of CVD and diabetes and funded by the National Heart, Lung, and Blood Institute, the SHS has served as a model for the evaluation of physiological and environmental risk factors for CVD and PAD in populations with a high burden of CVD and type 2 diabetes (9, 15, 19). From 1989 to 1991, men and women aged 45–75 years from 13 American Indian communities in Arizona, Oklahoma, and North and South Dakota were invited to participate in the SHS. In Arizona and Oklahoma, every eligible person was invited; in North Dakota and South Dakota, a cluster sampling technique was used (15, 20). The overall participation rate was 62% (15).
Comparisons between participants and nonparticipants in the SHS have been published; all information on nonparticipant characteristics was self-reported (20). Overall, included participants were similar to nonparticipants in age, body mass index, prevalence of diabetes, and current smoking (20). Participants were more likely than nonparticipants to be women. There were also significant differences between participants and nonparticipants with respect to self-reported hypertension for both sexes in Arizona and for females in North and South Dakota; sex-specific hypertension rates in Oklahoma did not differ significantly between participants and nonparticipants (20).
As previously described (9, 21), 3,974 participants had frozen urine specimens available for measurement of urinary metal levels. We excluded 224 participants who were missing data on total urinary arsenic or arsenic metabolites, 5 participants missing information on smoking, 13 participants missing data on diabetes status, and 114 participants missing data on other variables of interest. Prevalent cases of PAD (376 participants with either ABI <0.9 or ABI >1.4 in at least 1 leg at the baseline visit) were excluded. Stenotic PAD and vascular calcification are generally long-term and progressive (22). Therefore, consistent with prior SHS analyses (21), participants missing ABI measurements at visit 2 (1993–1995) but free of PAD at visit 3 (1997–1999) (n = 149 and n = 158 for ABI <0.9 and ABI >1.4, respectively) were considered to not have PAD at visit 2. We also excluded 367 and 369 participants from analyses of ABI <0.9 and ABI >1.4, respectively, because of missing ABI follow-up measurements at both visit 2 and visit 3. In summary, the final study population comprised 2,875 and 2,873 participants at risk for incident ABI <0.9 and ABI >1.4, respectively.
The study protocol was approved by the institutional and Indian Health Service review boards and the participating American Indian communities. All participants provided oral and written informed consent.
Baseline data collection
Data on sociodemographic factors (age, sex, geographical location, education), menopausal status, smoking history (smoking status and pack-years), history of diabetes mellitus, and medication use were obtained through the baseline SHS questionnaire, administered by trained and certified interviewers (9, 15, 21). Body mass index was calculated as measured weight in kilograms divided by the square of measured height in meters. At each examination, 3 consecutive blood pressure readings were taken from the right arm in the seated position by trained nurses and medical assistants after 5 minutes of rest using a Baum mercury sphygmomanometer (W. A. Baum Company, Copiague, New York) (15), and the last 2 determinations were averaged. Hypertension was defined as mean systolic blood pressure ≥140 mm Hg, mean diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication.
Total and high-density lipoprotein cholesterol, plasma glucose, and plasma creatinine concentrations were measured in fasting serum samples using enzymatic methods as previously described (9, 15, 21). The proportion of low-density lipoprotein (LDL) cholesterol was estimated by means of the Friedewald equation (15, 16, 23). A high LDL cholesterol level was defined as estimated LDL cholesterol ≥130 mg/dL. Hemoglobin A1c level was measured by high-performance liquid chromatography (9, 19, 24). Diabetes mellitus was defined as fasting glucose concentration ≥126 mg/dL, nonfasting glucose concentration ≥200 mg/dL, hemoglobin A1c level ≥6.5%, or use of insulin or oral hypoglycemic agents. Estimated glomerular filtration rate was calculated from calibrated creatinine level, age, and sex using the Modification of Diet in Renal Disease Study formula (15, 20, 25). The ethnicity adjustment for the formula was dropped for all participants (15, 21).
Urinary arsenic determinations
For assessment of long-term arsenic exposure, urinary arsenic measurements were performed in thawed urine samples, using up to 1 mL of urine, in 2007 (Trace Element Laboratory, Graz University, Graz, Austria). The analytical methods and associated quality control criteria for arsenic analysis have been described in detail elsewhere (9, 21, 26). Concentrations of arsenic species were determined by high-performance liquid chromatography linked with inductively coupled mass spectrometry, as described previously (9, 26). Arsenic speciation distinguishes arsenic species that are directly related to inorganic arsenic exposure (arsenite, arsenate, monomethylarsonous acid (MMA), and dimethylarsinous acid (DMA)) from those related to organic arsenicals in seafood (arsenobetaine as an overall marker of seafood arsenicals), which are generally considered nontoxic (2, 9). We used the sum of urinary inorganic arsenic (arsenite and arsenate) and the methylated species (DMA and MMA) as a biomarker integrating inorganic arsenic exposure from multiple sources (2, 9, 27). To account for dilution in urine, urinary arsenic concentrations were divided by urinary creatinine levels and expressed as µg/g creatinine. To assess arsenic metabolism, we estimated the proportions of inorganic arsenic, MMA, and DMA by dividing the concentration of each species by the sum of all 3 species and multiplying by 100, yielding percentage of inorganic arsenic (iAs%), MMA%, and DMA%, respectively. Low urinary concentrations of arsenobetaine confirmed that seafood intake was low in the SHS (9, 28), supporting the fact that measured methylated species reflect inorganic arsenic exposure. The intraclass correlation coefficients for 3 repeated arsenic measurements taken over a 10-year period were 0.80 for the sum of inorganic and methylated urinary arsenic species (∑As) and 0.64, 0.80, and 0.77 for iAs%, MMA%, and DMA%, respectively (9, 28).
Incident PAD follow-up
At baseline and follow-up at the second (1993–1995) and third (1997–1999) SHS examinations, right arm blood pressure and right and left posterior tibial artery pressure were measured twice, with the subject supine, using a hand-held Doppler device (Imex Medical Systems, Golden, Colorado) as previously described (9, 15–17, 21). The ABI for each leg was computed as the mean of 2 measurements of systolic blood pressure in the posterior tibial artery divided by the mean systolic blood pressure in the right brachial artery, and the worser of the 2 values from either leg (i.e., the lower value for ABI <0.9 and the higher value for ABI >1.4) was used to categorize the corresponding ABI definition for each individual (16, 17). Incident cases of PAD were defined as either 1) ABI <0.9 in at least 1 leg or 2) ABI >1.4 in at least 1 leg at one or more of the 2 follow-up clinic visits. Time to event was calculated from the date of baseline examination in 1989–1991 to the date of follow-up examination with the first diagnosis of PAD for participants with incident PAD and to the date of the last examination available or the date of death, whichever came first, for participants without incident PAD. The mean follow-up times for participants who did and did not develop an ABI less than 0.9 were 4.4 years and 6.8 years, respectively. The mean follow-up times were similar for participants who did and did not develop an ABI greater than 1.4 (4.4 years and 6.9 years, respectively).
Statistical methods
Baseline data are presented as mean (standard error) and median (interquartile range) values (Table 1). The prospective association of urinary arsenic concentration with PAD incidence was evaluated using Cox proportional hazards models for interval-censored data with the R package Intcox (R Foundation for Statistical Computing, Vienna, Austria), using years since baseline examination as the time scale. The assumption of hazards proportionality was visually assessed based on the smoothed association between time and log(−log[S(t)]), with S(t) being survival at time t, by category of urinary arsenic concentration; there were no major departures from proportionality. Urinary arsenic (∑As) was introduced into the models both as tertiles (categorical variable) and as log-transformed urinary arsenic concentrations (continuous variable) to compare the 80th and 20th percentiles (interquintile range). We also modeled nonlinear relationships between urinary arsenic levels and incident PAD by using restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles of creatinine-corrected urinary arsenic concentration (corresponding to 4.1 µg/g, 9.8 µg/g, and 24.0 µg/g, respectively). The corresponding percentiles were 8.1%, 13.8%, and 21.4% for MMA% and 65.8%, 77.9%, and 86.6% for DMA%. Bootstrap confidence intervals for the hazard ratios were estimated as bias-corrected and accelerated percentile intervals based on 10,000 resamplings (29, 30). Probability values for linear and nonlinear trend and probability values for interaction were estimated using Wald tests based on the bootstrap standard errors.
Table 1.
Baseline Characteristics of Participants by Incident Peripheral Arterial Disease Status in the Strong Heart Study, 1989–1999
| Characteristic | Incident PAD Statusa | |||||
|---|---|---|---|---|---|---|
| ABI <0.9 (n = 280) | ABI >1.4 (n = 206) | No PAD (n = 2,405) | ||||
| Mean (SE) | Median (IQR) | Mean (SE) | Median (IQR) | Mean (SE) | Median (IQR) | |
| Age, years | 58.7 (0.5) | 55.7 (0.6) | 55.6 (0.2) | |||
| % women | 70.4 (2.7) | 48.8 (3.5) | 59.9 (1.0) | |||
| Postmenopausal women,b % | 84.8 (2.6) | 75.5 (4.3) | 74.3 (1.2) | |||
| Location, % | ||||||
| Arizona | 41.8 (2.9) | 35.8 (3.3) | 32.2 (1.0) | |||
| Oklahoma | 25.7 (2.6) | 33.3 (3.3) | 33.5 (1.0) | |||
| North or South Dakota | 32.5 (2.8) | 30.8 (3.3) | 34.3 (1.0) | |||
| Less than high school education, % | 52.5 (3.0) | 48.8 (3.5) | 44.9 (1.0) | |||
| Smoking, % | ||||||
| Former smoker | 27.5 (2.7) | 42.8 (3.4) | 33.3 (1.0) | |||
| Current smoker | 41.1 (2.9) | 28.9 (3.2) | 34.5 (1.0) | |||
| Cumulative smoking, pack-years | 13.1 (1.1) | 10.3 (1.3) | 10.6 (0.4) | |||
| Body mass indexc | 30.6 (0.4) | 32.3 (0.4) | 30.8 (0.1) | |||
| Total cholesterol, mg/dL | 198.2 (2.3) | 187.0 (2.7) | 190.5 (0.8) | |||
| LDL cholesterol, mg/dL | 121.9 (2.0) | 110.7 (2.3) | 115.9 (0.7) | |||
| Hypertension, % | 49.6 (3.0) | 38.8 (3.4) | 35.4 (1.0) | |||
| Diabetes, % | 63.9 (2.9) | 58.7 (3.4) | 43.6 (1.0) | |||
| eGFR <60 mL/minute/1.73 m2, % | 14.3 (2.1) | 7.0 (1.8) | 7.6 (0.5) | |||
| ∑As, μg/g creatinined | 10.9 (10.0, 11.8) | 10.1 (9.2, 11.1) | 9.8 (9.5, 10.0) | |||
| iAs% | 8.0 (5.6–11.0) | 8.1 (5.8–11.0) | 7.9 (5.6–11.0) | |||
| MMA% | 14.4 (10.9–17.1) | 13.6 (10.5–17.1) | 13.7 (10.6–17.3) | |||
| DMA% | 77.4 (72.1–82.5) | 78.0 (71.9–83.3) | 78.1 (71.9–82.9) | |||
Abbreviations: ABI, ankle brachial index; DMA, dimethylarsinous acid; eGFR, estimated glomerular filtration rate; iAs, inorganic arsenic; IQR, interquartile range; LDL, low-density lipoprotein; MMA, monomethylarsonous acid; PAD, peripheral arterial disease; ∑As, sum of inorganic and methylated urinary arsenic species; SE, standard error.
a Incident PAD was defined as ABI <0.9 or ABI >1.4 in at least 1 leg.
b Subsample of women (ABI <0.9: n = 197; ABI 0.9–1.4: n = 1,441; ABI >1.4: n = 98).
c Weight (kg)/height (m)2.
d Values are presented as geometric mean (95% confidence interval).
We used 3 statistical models with progressive adjustment. In model 1, we adjusted for sex and age at baseline (continuous as restricted cubic splines with knots at 50 and 60 years). In model 2, we further adjusted for education (≥12 years of education completed vs. <12 years), smoking status (never, former, or current smoker), low-density lipoprotein cholesterol (mg/dL; continuous), body mass index (continuous), hypertension (no, yes), diabetes mellitus (no, yes), and estimated glomerular filtration rate (mL/minute/1.73 m2; continuous). In model 3, we additionally adjusted for geographic location (Arizona, the Dakotas, or Oklahoma).
Patterns of arsenic methylation reflecting individual differences in arsenic metabolism have been related to differences in cardiovascular endpoints, including PAD, in populations from Taiwan (31, 32). To evaluate the potential role of arsenic metabolism in PAD risk, we examined the relationship between the relative proportions of arsenic species in urine (logit-transformed proportions of inorganic arsenic, MMA, and DMA) and incident PAD using both conventional and “leave-one-out” approaches. In the conventional approach, each arsenic metabolism biomarker alone was entered into the regression model together with ∑As to adjust for arsenic exposure. Findings from the conventional approach are challenging to interpret. For instance, the increase in iAs% could be related to a decrease in either MMA% or DMA%, as the sum of arsenic metabolism markers always equals 1. To address this issue, we used a “leave-one-out” approach. In this method, 2 metabolism biomarkers are entered at a time, together with ∑As. For example, iAs% and MMA% are entered together, leaving out DMA%, while adjusting for urinary inorganic arsenic concentration. The regression coefficients thus estimate the hazard ratios associated with an increase in iAs% by a corresponding decrease in DMA% and with an increase in MMA% by a corresponding decrease in DMA%, respectively. This modeling strategy has been used in nutrition and hematology literature (33, 34) and was recently used to model the relationship between arsenic metabolism and incident diabetes (35).
Finally, we performed subgroup analyses to evaluate effect modification in adjustment models by including terms for the interaction of log-transformed urinary arsenic concentration or DMA% with indicator variables for age group, sex, smoking status, and diabetes status. For models of urinary arsenic, we added an analysis of interaction with DMA% (above and below the median). For models of DMA%, we added an analysis of interaction with ∑As (above and below the median).
RESULTS
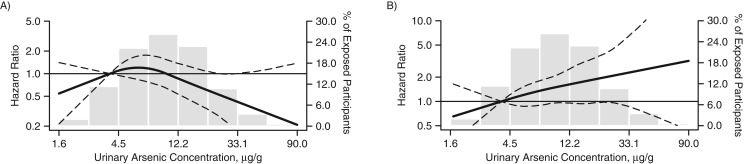
The overall median urinary concentration of ∑As was 9.9 µg/g creatinine (interquartile range, 6.0–15.7 µg/g creatinine). Urinary arsenic concentrations varied by study region; the median values were 14.1 µg/g creatinine, 5.8 µg/g creatinine, and 10.6 µg/g creatinine in Arizona, Oklahoma, and North/South Dakota, respectively (9). A total of 280 and 206 incident PAD cases were identified through 1999 for ABI <0.9 and ABI >1.4, respectively. Incident ABI <0.9 and ABI >1.4 were associated with lower levels of education and with the presence of type 2 diabetes (Table 1). Incident ABI <0.9 was more common among women, and it was associated with higher age, current smoking, cumulative smoking, higher lipid levels, hypertension, and reduced estimated glomerular filtration rate. Incident ABI >1.4 was associated with higher body mass index. The adjusted hazard ratios for PAD, comparing the highest tertile of ∑As concentration with the lowest tertile, were 0.59 (95% confidence interval (CI): 0.33, 1.04) and 2.40 (95% CI: 1.11, 4.95) for ABI <0.9 and ABI >1.4, respectively (Table 2, model 3).
Table 2.
Hazard Ratios for Incident Peripheral Arterial Disease (Defined as Either ABI <0.9 or ABI >1.4) According to Tertilea of Inorganic and Methylated Urinary Arsenic Concentration in the Strong Heart Study, 1989–1999
| Model and Tertile of ∑As | Incident PAD Status | |||
|---|---|---|---|---|
| ABI <0.9 | ABI >1.4 | |||
| HR | 95% CI | HR | 95% CI | |
| Model 1b | ||||
| 1 (lowest) | 1.00 | Referent | 1.00 | Referent |
| 2 | 1.06 | 0.65, 1.55 | 1.41 | 0.90, 2.26 |
| 3 (highest) | 0.63 | 0.39, 0.92 | 1.63 | 0.99, 2.67 |
| Model 2c | ||||
| 1 (lowest) | 1.00 | Referent | 1.00 | Referent |
| 2 | 1.06 | 0.62, 1.56 | 1.40 | 0.80, 2.50 |
| 3 (highest) | 0.57 | 0.32, 0.89 | 1.71 | 0.96, 3.29 |
| Model 3d | ||||
| 1 (lowest) | 1.00 | Referent | 1.00 | Referent |
| 2 | 1.10 | 0.66, 1.72 | 1.80 | 0.93, 3.35 |
| 3 (highest) | 0.59 | 0.33, 1.04 | 2.40 | 1.11, 4.95 |
Abbreviations: ABI, ankle brachial index; CI, confidence interval; HR, hazard ratio; PAD, peripheral arterial disease; ∑As, sum of inorganic and methylated urinary arsenic species.
a Tertiles 1, 2, and 3 included participants with ∑As values of ≤7.07 μg/g, 7.07–13.3 μg/g, and >13.3 μg/g, respectively.
b Model 1 adjusted for sex and age.
c Model 2 adjusted for sex and age and further adjusted for education, smoking, body mass index, low-density lipoprotein cholesterol, diabetes, hypertension, and estimated glomerular filtration rate.
d Model 3 adjusted for all of the variables in model 2 and further adjusted for study center.
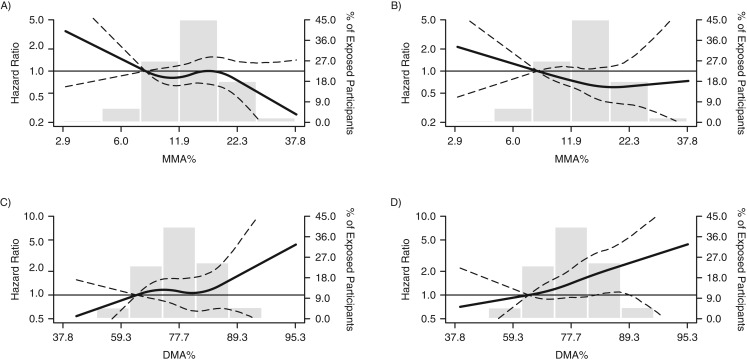
There was no clear relationship between urinary arsenic concentrations and PAD defined as ABI <0.9 (Figure 1A). In contrast, the association with PAD defined as ABI >1.4 was nearly linear throughout the range of urinary arsenic concentrations (Figure 1B). Throughout the range of DMA%, there was a nearly linear relationship with PAD for both ABI <0.9 and ABI >1.4 (Figure 2).
Figure 1.
Hazard ratios (HRs) for incident peripheral arterial disease (PAD) according to ankle brachial index (ABI) and urinary arsenic concentration (sum of inorganic arsenic levels) in the Strong Heart Study, 1989–1999. A) PAD defined as ABI <0.9; B) PAD defined as ABI >1.4. The curves represent adjusted HRs based on restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles (4.06 μg/g, 9.84 μg/g, and 24.0 μg/g, respectively) of the distribution of the sum of urinary inorganic and methylated arsenic species (∑As). The reference value (HR = 1) was set at the 10th percentile of the ∑As distribution (4.06 µg/g). The models adjusted for sex, age, education, smoking, body mass index, low-density lipoprotein cholesterol, diabetes, hypertension, estimated glomerular filtration rate, and study center. The histograms (gray columns) represent the frequency distribution of urinary arsenic concentrations in the Strong Heart Study sample for 1989–1999. Dashed lines, 95% confidence intervals.
Figure 2.
Hazard ratios (HRs) for incident peripheral arterial disease (PAD) according to ankle brachial index (ABI), by arsenic metabolite level, in the Strong Heart Study, 1989–1999. The curves represent adjusted HRs for incident PAD defined as ABI <0.9 (panels A and C) or ABI >1.4 (panels B and D) based on restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles of the arsenic species distribution, corresponding to 8.1%, 13.8%, and 21.4%, respectively, for percent monomethylarsonous acid (MMA%) and 65.8%, 77.9%, and 86.6%, respectively, for percent dimethylarsinous acid (DMA%). The reference value (HR = 1) was set at the 10th percentile of the arsenic species distribution (8.1% for MMA% and 65.8% for DMA%). The models adjusted for sex, age, education, smoking, body mass index, low-density lipoprotein cholesterol, diabetes, hypertension, estimated glomerular filtration rate, study center, and the sum of inorganic arsenic species. The histograms (gray columns) represent the frequency distribution of urinary arsenic concentrations in the Strong Heart Study sample for 1989–1999. Dashed lines, 95% confidence intervals.
Table 3 presents the hazard ratios for ABI <0.9 or ABI >1.4, comparing the 80th and 20th percentiles, by biomarkers of arsenic metabolism. Using either standard linear regression or a leave-one-out approach, there were no significant associations between biomarkers of arsenic metabolism and PAD defined as ABI <0.9. For ABI >1.4, the association with DMA% using the standard approach was of borderline statistical significance (hazard ratio = 1.46, 95% CI: 0.99, 1.97; Table 3). After leaving out MMA% in the leave-one-out approach, the corresponding association for DMA% after adjustment for urinary arsenic concentrations and iAs% was 2.23 (95% CI: 1.10, 3.72; Table 3). The interpretation of the leave-one-out approach to these results is that for a given fixed percentage of iAs%, an interquintile-range increase in DMA% accompanied by a corresponding decrease in MMA% is associated with a 2-fold increase in risk of PAD defined as ABI >1.4.
Table 3.
Hazard Ratiosa for Incident Peripheral Arterial Disease (Defined as Either ABI <0.9 or ABI >1.4) Among Participants in the Strong Heart Study, Comparing the 80th Percentiles of Arsenic Metabolism Biomarkers With the 20th Percentiles, 1989–1999
| Modeling Approach and Biomarker | iAs% | MMA% | DMA% | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| ABI <0.9 | ||||||
| Standard approach | 0.97 | 0.73, 1.21 | 0.78 | 0.60, 1.08 | 1.19 | 0.81, 1.63 |
| Leave-one-out approach | ||||||
| iAs%b | 0.69 | 0.42, 1.28 | 0.85 | 0.49, 1.54 | ||
| MMA%c | 1.16 | 0.76, 1.56 | 1.35 | 0.79, 1.98 | ||
| DMA%d | 1.03 | 0.76, 1.31 | 0.76 | 0.57, 1.07 | ||
| ABI >1.4 | ||||||
| Standard approach | 0.89 | 0.67, 1.19 | 0.65 | 0.49, 0.93 | 1.46 | 0.99, 1.97 |
| Leave-one-out approach | ||||||
| iAs% | 0.59 | 0.34, 1.10 | 0.93 | 0.50, 1.70 | ||
| MMA% | 1.54 | 0.83, 2.50 | 2.23 | 1.10, 3.72 | ||
| DMA% | 1.06 | 0.74, 1.46 | 0.62 | 0.45, 0.92 | ||
Abbreviations: ABI, ankle brachial index; CI, confidence interval; DMA, dimethylarsinous acid; HR, hazard ratio; iAs, inorganic arsenic, MMA, monomethylarsonous acid.
a The model adjusted for sex, age, education, smoking, body mass index, low-density lipoprotein cholesterol, diabetes, hypertension, estimated glomerular filtration rate, study center, and the sum of inorganic and methylated arsenic species.
b The model further adjusted for DMA% or MMA%.
c The model further adjusted for DMA% or iAs%.
d The model further adjusted for MMA% or iAs%.
There were no statistically significant interactions by sex, study site, or diabetes status subgroup for the hazard ratios for ABI >1.4 comparing the 80th and 20th percentiles of urinary arsenic concentration or for DMA% (Table 4). By diabetes status, the hazard ratio for ABI >1.4 was 1.88 (95% CI: 1.09, 3.57) among patients with type 2 diabetes as compared with 1.12 (95% CI: 0.66, 2.00) among patients without type 2 diabetes. For DMA%, there were no differences by type 2 diabetes status. The association between total inorganic arsenic and ABI >1.4 was stronger in patients with DMA% at or above the median than in those with DMA% below the median: Hazard ratios were 2.23 (95% CI: 1.10, 4.63) and 1.05 (95% CI: 0.58, 1.90), respectively. Similarly, the corresponding association with DMA% was stronger in participants with total inorganic arsenic at or above the median than in those with total inorganic arsenic below the median: Hazard ratios were 2.68 (95% CI: 1.30, 4.99) and 1.71 (95% CI: 0.70, 3.58), respectively. However, neither of these interactions reached statistical significance (P = 0.09 and P = 0.24, respectively).
Table 4.
Hazard Ratiosa for Ankle Brachial Index >1.4 Among Selected Subgroups of Participants in the Strong Heart Study, Comparing the 80th Percentiles of Inorganic and Methylated Urinary Arsenic Concentrations and DMA% With the 20th Percentiles, 1989–1999
| Subgroup | No. of Cases | No. of Noncases | ∑As Concentration | DMA%b | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | Pinteraction | HR | 95% CI | Pinteraction | |||
| Overall | 201 | 2,672 | 1.37 | 0.87, 2.21 | 2.23 | 1.10, 3.72 | ||
| Sex | ||||||||
| Male | 103 | 1,043 | 1.30 | 0.75, 2.16 | 0.44 | 2.51 | 1.20, 4.33 | 0.39 |
| Female | 98 | 1,629 | 1.66 | 0.93, 3.36 | 1.85 | 0.76, 3.71 | ||
| Location | ||||||||
| North or South Dakota | 62 | 912 | 1.18 | 0.63, 1.88 | 0.32 | 2.03 | 0.92, 3.71 | 0.38 |
| Oklahoma | 67 | 875 | 2.46 | 0.95, 5.01 | 1.95 | 0.75, 3.93 | ||
| Arizona | 72 | 885 | 1.17 | 0.55, 2.74 | 3.53 | 1.41, 7.71 | ||
| Type 2 diabetes | 0.90 | |||||||
| No | 83 | 1,456 | 1.12 | 0.66, 2.00 | 0.11 | 2.20 | 1.01, 4.41 | |
| Yes | 118 | 1,216 | 1.88 | 1.09, 3.57 | 2.42 | 1.04, 4.24 | ||
| DMA% | ||||||||
| Less than median | 98 | 1,338 | 1.05 | 0.58, 1.90 | 0.09 | |||
| Median or higher | 103 | 1,334 | 2.23 | 1.10, 4.63 | ||||
| Arsenic, µg/g | 0.24 | |||||||
| Less than median | 94 | 1,341 | 1.71 | 0.70, 3.58 | ||||
| Median or higher | 107 | 1,331 | 2.68 | 1.30, 4.99 | ||||
Abbreviations: CI, confidence interval; DMA, dimethylarsinous acid; HR, hazard ratio; ∑As, sum of inorganic and methylated arsenic species.
a The model adjusted for sex, age, education, smoking, body mass index, low-density lipoprotein cholesterol, diabetes, hypertension, estimated glomerular filtration rate, and study center.
b The model further adjusted for ∑As and percent inorganic arsenic.
DISCUSSION
In this study, increasing levels of inorganic arsenic exposure and DMA%, as measured in urine, were associated with an ABI greater than 1.4. This association remained significant after adjustment for etiological factors important in the development of a high ABI, including age, diabetes, and renal function. This finding supports the possibility of an independent effect of inorganic arsenic and DMA% on the development of ABI >1.4 in the SHS cohort. The association with increasing inorganic arsenic and DMA% was also stronger for participants with both higher arsenic exposure and higher methylation efficiency (higher DMA%). Arsenic exposure and arsenic metabolism, however, were not related to ABI <0.9. These findings support a role for both arsenic exposure and arsenic metabolism in the vasculotoxic effects of low-to-moderate exposure to inorganic arsenic. Our results add prospectively gathered evidence on the role of inorganic arsenic exposure and metabolism as risk factors for the development of ABI >1.4, particularly in a population with increased arsenic methylation capacity and one of the highest burdens of comorbid type 2 diabetes worldwide.
The adverse cardiovascular and vasculotoxic effects of long-term exposure to high levels of inorganic arsenic (>100 µg/L) in drinking water have been well characterized (1, 32). High-level arsenic exposure has been consistently associated with severe forms of PAD in southwestern Taiwan and among German vintners (32, 36). In an updated systematic review of the health effects of inorganic arsenic exposure, comparing the highest categories of arsenic exposure with the lowest among populations with high levels of mean arsenic in drinking water (>50 µg/L), the pooled relative risk of PAD was 2.17 (95% CI: 1.47, 3.20) (1), and the findings were consistent for the 8 individual studies available. In contrast, the data on the association between arsenic and PAD in populations exposed to low-to-moderate levels of arsenic (mean arsenic concentration in drinking water <50 µg/L) have been largely inconsistent and assessed in only a limited number of studies, all of them ecological and based on death certificates (36, 37). In US communities with drinking-water arsenic concentrations greater than 20 µg/L as compared with <10 µg/L, the relative risk of PAD mortality was 1.58 (95% CI: 1.34, 1.88) (36, 38). In contrast, ecological studies in Utah (comparing communities with drinking-water arsenic concentrations less than 200 µg/L with the general US population) (38) and Michigan (standardized mortality ratios for drinking-water arsenic of 10–100 µg/L compared with the overall Michigan population) (37) found no association between arsenic exposure and PAD-associated mortality. By measuring individual markers of low-to-moderate levels of inorganic arsenic exposure and metabolism and by using robust standardized clinical assessments of PAD obtained during a physical examination by trained and certified nurses, the current study has advanced our evaluation of the vascular effects of low-to-moderate arsenic exposure and metabolism.
Experimental studies support the role of arsenic in CVD, including PAD. Potential mechanisms for the vasculotoxic effects of inorganic arsenic include endothelial dysfunction, smooth-muscle cell proliferation, angiogenesis, vascular injury, and enhanced platelet aggregation (39). Direct endothelial toxicity is one of the most likely mechanisms of arsenic-associated PAD risk (39). High-level arsenic exposure increases arterial stiffness and pulse pressure (40), which in turn is associated with PAD risk, especially in patients with comorbid type 2 diabetes (41). Animal studies reveal that arsenic exposure, even at low-to-moderate levels (42), leads to enhancement of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) (39). Enhanced NOX leads to impaired endothelial nitric oxide synthase (eNOS) activity (39, 43), impairments in vasomotor tone, and arterial relaxation (43) and may increase PAD risk. There is also experimental evidence to support the differential effects of arsenic metabolites on health outcomes, including vasculotoxic effects of arsenic metabolites on model systems of endothelial cell function (44, 45). Other potential molecular mechanisms for arsenic toxicity include arsenic-related epigenetic changes, including histone modification (46, 47).
Humans metabolize inorganic arsenic (arsenate and arsenite) to methylated compounds, predominantly MMA and DMA, which are largely excreted in urine together with inorganic arsenic (48, 49). In human populations, the average distribution of arsenic metabolites in urine is approximately 10%–30% inorganic arsenic, approximately 10%–20% MMA, and approximately 60%–80% DMA (48). However, substantial interindividual variation is found in the distribution of urinary arsenic metabolites, and these differences in arsenic methylation have been associated with differential risks of skin lesions, cancer, and CVD in several exposed populations (11, 12). The first studies on arsenic metabolism and cardiovascular outcomes, most of them conducted in Taiwan, showed adverse health effects of incomplete methylation and increased levels of MMA (31, 32). Recent data, however, show that higher DMA%, a metabolic profile related to higher methylation efficiency, can also be related to adverse health effects. Specifically, higher DMA% has been related to higher body mass index and obesity in several studies (19, 50), as well as to incident diabetes in the SHS (35).
Many of the studies showing increased CVD risk with incomplete methylation and increased MMA% were conducted in populations with very high levels of inorganic arsenic exposure (51). In contrast, at low-to-moderate levels of exposure in the current study, we observed a stronger relationship between DMA% and ABI >1.4. Consistent with our findings, a stronger association between DMA and CVD mortality was observed in a recent analysis from the SHS. In this study, however, MMA also displayed a statistically significant positive association with CVD (9). Because that study used only a traditional approach to evaluate arsenic metabolism and not the leave-one-out approach, it is difficult to interpret the stronger association of both higher MMA% and DMA% with CVD (9). Evidence from the Strong Heart Family Study (an ancillary study to the SHS) and other populations has shown that arsenic metabolic patterns are related to variants in the arsenite methyltransferase gene (AS3MT), which codes for arsenic (III) methyltransferase, the main enzyme methylating inorganic arsenic (52, 53), and perhaps genetic variants in other genes (52). It is possible that our interaction with arsenic metabolism reflects increased susceptibility to the health effects of arsenic exposure, particularly ABI >1.4 in our study population, possibly for genetic reasons. Unfortunately, we currently do not have genotyping information for AS3MT in the initial SHS cohort, and the possibility of an effect of gene-arsenic interactions on PAD risk cannot be evaluated at this time. Given the high burden of ABI >1.4 in our study population and the higher arsenic exposure levels in SHS communities compared with other US populations, the interplay between genetics, metabolism, and arsenic exposure for disease development, particularly for ABI >1.4, warrants evaluation.
Although the interaction with type 2 diabetes was not significant, in the current analysis and in an earlier paper from the SHS (9), inorganic arsenic exposure had a stronger association with CVD in participants with type 2 diabetes than in those without it. Patients with type 2 diabetes appear more susceptible to the effects of some environmental exposures, including cadmium (54) and particulate emissions (55, 56), and may represent an important high-risk population in which to examine the CVD risks of environmental exposures. Recent epidemiologic evidence suggests that conditions associated with increased risk of type 2 diabetes, such as obesity and higher body mass index, are also associated with higher levels of urinary DMA% (50), which in the current analysis was more strongly associated with risk of ABI >1.40 than was total inorganic arsenic or MMA%. However, because the interactions in the current study were not significant, these comments should be considered cautiously.
In the current study, the associations between arsenic exposure and incident PAD differed for ABI <0.9 versus ABI >1.4. Specifically, we observed an inverse (though not significant) and somewhat J-shaped relationship between ∑As and PAD defined as ABI <0.9. In comparison, throughout the range of DMA%, there was a nearly linear relationship with PAD for both ABI <0.9 and ABI >1.4 (Figure 2). The inconsistency of the association between inorganic arsenic and PAD defined as ABI <0.9 was an unexpected finding given the pathology of PAD among highly exposed persons in southwestern Taiwan (6, 7).
It is known that occlusive PAD defined as ABI <0.9 is highly prevalent among patients with medial arterial calcinosis defined as ABI >1.4 (22). However, measures for diagnosing concomitant occlusive PAD (e.g., toe-brachial index or Doppler waveform analyses) among participants with ABI >1.4 were not available. Therefore, we cannot exclude the possibility that an association exists between low-to-moderate arsenic exposure and occlusive PAD that we were unable to detect because of concomitant medial arterial calcinosis.
Other potential explanations include competing risk of other adverse health outcomes for participants with ABI <0.9 or the potential for differential effects of inorganic arsenic by exposure level. It is also possible that the near-significant finding of a protective effect for low ABI may reflect vascular stiffening masking underlying PAD, leading to a falsely normal ABI. Some persons with a normal ABI may have abnormal Doppler waveform patterns and a CVD risk similar to those with ABI <0.9 (57), which could not be evaluated in this study because Doppler waveform analyses were not available. However, prior studies have shown that ABI <0.9 or ABI >1.4 poses a greater risk of incident CVD than a normal ABI between 0.9 and 1.4 (22, 58), and this suggests that undetected PAD among participants with a normal ABI is unlikely to significantly alter our results. It is also possible that the difference in association between exposure to inorganic arsenic and ABI <0.9 versus ABI >1.4 is due to nonatherosclerotic vascular effects of arsenic that alter arterial function but do not cause vessel narrowing. Earlier reports suggested that high levels of inorganic arsenic were associated with nonatherosclerotic vascular diseases such as blackfoot disease and thromboangitis obliterans (7, 8). While these findings may not be applicable to the evaluated endpoints and low-to-moderate arsenic exposure levels examined in this study, they provide evidence of the potential nonatherosclerotic vascular effects of arsenic exposure. Murine models have demonstrated disorganized collagen formation and decreased elastin production in the arteriolar walls of mice treated with environmentally relevant concentrations of inorganic arsenic (59). It is unknown whether these pathological changes in a model system are analogous to the incompressible distal arteries with ABI >1.4 from inorganic arsenic exposure and DMA% observed in this study. These findings suggest that further study of the atherosclerotic and nonatherosclerotic vascular effects of low-to-moderate arsenic exposure is needed.
Our study had some limitations. The SHS used right brachial artery systolic blood pressure to calculate ABI (16), whereas recent scientific statements on PAD ascertainment have recommended measurement of bilateral brachial artery pressure (22). While the use of unilateral brachial artery blood pressure may have introduced measurement error in the assessment of PAD in the SHS, this error probably would have biased the relationship between arsenic and PAD towards the null and potentially obscured our ability to detect an association. Moreover, the use of right-arm brachial artery systolic pressure alone is well established, and this method has been used for all measurements of PAD in the National Health and Nutrition Examination Survey (60, 61). Additionally, the coefficients of variation reported in the literature for ABI calculated using the right arm versus ABI calculated using bilateral brachial artery systolic pressure are similar (22). Therefore, it is unlikely that the difference in methodology between the brachial artery pressure assessment used in the SHS and recent scientific statements would significantly alter our findings.
Additionally, we lacked data on the intra- and interobserver validity of brachial and ankle blood pressure measurement for ABI calculation in the SHS. However, the SHS cohort is a well-described and well-tested cohort (15, 62), with multiple published articles on the assessment of blood pressure (62, 63), CVD risk (63), and PAD (17, 21). PAD in the SHS is associated with CVD risk factors of the expected magnitude and direction, which makes it less likely that questions of observer validity would undermine the additional findings from this prospective cohort study.
As noted, occlusive PAD defined as ABI <0.90 is highly prevalent among patients with medial arterial calcinosis defined as ABI >1.40, and additional measurements to detect concomitant occlusive PAD among patients with ABI >1.4, as recommended in current guidelines (22), were not collected in the SHS. However, the ascertainment of ABI >1.4 in the SHS is consistent with the methodology from another large observational population study (64). Together, these studies have suggested an increased risk of mortality and CVD for ABI >1.4 (17, 64). We measured urinary arsenic levels in a single sample at baseline, and individual levels in drinking water were unavailable (9). However, arsenic levels in public and private water supplies have been shown in multiple studies to be stable over time (28, 65). Our results were robust to adjustment for education, smoking, and other CVD risk factors, although we cannot discard the possibility of unmeasured confounding. We also demonstrated good temporal stability of markers of arsenic exposure in our study population (9). The high burden of diabetes mellitus and obesity, as well as mortality due to cardiovascular and noncardiovascular causes in the American Indian population studied, needs to be considered (16, 66, 67). Generalizability to other populations with different CVD risk factor profiles, such as a lower burden of type 2 diabetes, is unknown, although arsenic toxicity pathways appear to be common across human populations.
Strengths of the current study include the use of high-quality data collection methods and standardized surveillance for incident PAD outcomes over a long follow-up period (15) and the use of rigorous laboratory methods for measuring concentrations of urinary arsenic species (26). Urinary arsenic measurements integrate all sources of exposure at the individual level, including water and food, and are an excellent biomarker of internal dose (9, 68). The SHS uses a specialized model for compositional data to assess the health effects of different arsenic metabolites that takes into account their interrelatedness (35). Other methods to evaluate arsenic metabolism—for instance, based on the analysis of compositional data (69, 70)—could be developed for future studies. Furthermore, our study contributes to characterization of the association between inorganic arsenic exposure, a common environmental exposure, and PAD risk in American Indian populations, an understudied ethnic group.
In summary, we found a nearly linear relationship of low-to-moderate inorganic arsenic exposure, as measured in urine, with incident ABI >1.4 but not with ABI <0.9. These findings support the vasculotoxic effects of arsenic on the lower extremities at low-to-moderate levels of inorganic arsenic exposure. Our results suggest that a metabolic profile characterized by increased methylation efficiency may increase the vascular toxicity of arsenic exposure, but additional studies of interindividual differences in the relationship between arsenic methylation and vascular outcomes are needed in other populations. Our findings of increased risk of an ABI greater than 1.4 for low-to-moderate inorganic arsenic exposure, together with previous findings from our research group and others on cardiovascular and cerebrovascular effects of arsenic exposure, highlight an unmet need for studies of strategies and novel treatment pathways to reduce the risk of CVD due to inorganic arsenic exposure and other environmental exposures.
ACKNOWLEDGMENTS
Author affiliations: Division of Cardiology and Center for the Prevention of Cardiovascular Disease, Department of Medicine, New York University Medical Center, New York, New York (Jonathan D. Newman); Department of Environmental Health Sciences, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Ana Navas-Acien, Chin-Chi Kuo, Maria Tellez-Plaza); Department of Epidemiology and Welch Center for Prevention, Epidemiology, and Clinical Research, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Ana Navas-Acien, Chin-Chi Kuo, Eliseo Guallar); Kidney Institute and Division of Nephrology, Department of Internal Medicine, China Medical University Hospital and College of Medicine, China Medical University, Taichung, Taiwan (Chin-Chi Kuo); Department of Medicine, Johns Hopkins Medical Institutions, Baltimore, Maryland (Eliseo Guallar); MedStar Health Research Institute and Georgetown University, Hyattsville, Maryland (Barbara V. Howard, Jason G. Umans); Epidemiology Branch, National Heart, Lung, and Blood Institute, Bethesda, Maryland (Richard R. Fabsitz); Division of Cardiology, Department of Medicine, Weill Cornell Medical College, Cornell University, New York, New York (Richard B. Devereux); Institute of Chemistry–Analytical Chemistry, Karl-Franzens University Graz, Graz, Austria (Kevin A. Francesconi, Walter Goessler); Missouri Breaks Industries Research, Inc., Timber Lake, South Dakota (Lyle T. Best); and INCLIVA Biomedical Research Institute, Hospital Clinic of Valencia, Valencia, Spain (Maria Tellez-Plaza).
This work was supported by grants from the National Heart, Lung, and Blood Institute (grants HL090863, K23HL125991, and 5T32HL007024; Strong Heart Study grants HL41642, HL41652, HL41654, and HL65521), the National Institute of Environmental Health Sciences (grants P42ES10349, R01ES021367, and R01ES025216), the American Heart Association (grant 15MCPRP24480132), and the National Institute of Diabetes and Digestive and Kidney Diseases (grant U24DK076169-09, subcontract 25732-60). M.T.-P. was supported by the Strategic Action for Research in Health Sciences Program (grant CP12/03080) from the Spanish Ministry of Economy and Competitiveness and the European Funds for Regional Development.
Conflict of interest: none declared.
REFERENCES
- 1.Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: an updated systematic review. Curr Atheroscler Rep. 2012;14(6):542–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Subcommittee on Arsenic in Drinking Water, National Research Council, National Academy of Sciences Arsenic in Drinking Water. Washington, DC: National Academies Press; 1999. [Google Scholar]
- 3.Chen CJ, Chiou HY, Chiang MH, et al. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16(4):504–510. [DOI] [PubMed] [Google Scholar]
- 4.Cheng T-J, Ke D-S, Guo H-R. The association between arsenic exposure from drinking water and cerebrovascular disease mortality in Taiwan. Water Res. 2010;44(19):5770–5776. [DOI] [PubMed] [Google Scholar]
- 5.Wang C-H, Jeng J-S, Yip P-K, et al. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105(15):1804–1809. [DOI] [PubMed] [Google Scholar]
- 6.Navas-Acien A, Sharrett AR, Silbergeld EK, et al. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162(11):1037–1049. [DOI] [PubMed] [Google Scholar]
- 7.Chen CJ. Blackfoot disease [letter]. Lancet. 1990;336(8712):442. [DOI] [PubMed] [Google Scholar]
- 8.Chen CJ, Wu MM, Lee SS, et al. Atherogenicity and carcinogenicity of high-arsenic artesian well water. Multiple risk factors and related malignant neoplasms of blackfoot disease. Arteriosclerosis. 1988;8(5):452–460. [DOI] [PubMed] [Google Scholar]
- 9.Moon KA, Guallar E, Umans JG, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. A prospective cohort study. Ann Intern Med. 2013;159(10):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James KA, Marshall JA, Hokanson JE, et al. A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ Res. 2013;123:33–38. [DOI] [PubMed] [Google Scholar]
- 11.Wu M-M, Chiou H-Y, Hsueh Y-M, et al. Effect of plasma homocysteine level and urinary monomethylarsonic acid on the risk of arsenic-associated carotid atherosclerosis. Toxicol Appl Pharmacol. 2006;216(1):168–175. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y-C, Su H-JJ, Guo Y-LL, et al. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control. 2003;14(4):303–310. [DOI] [PubMed] [Google Scholar]
- 13.Aboyans V, Criqui MH, McClelland RL, et al. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA). J Vasc Surg. 2007;45(2):319–327. [DOI] [PubMed] [Google Scholar]
- 14.Resnick HE, Foster GL. Prevalence of elevated ankle-brachial index in the United States 1999 to 2002. Am J Med. 2005;118(6):676–679. [DOI] [PubMed] [Google Scholar]
- 15.Lee ET, Welty TK, Fabsitz R, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–1155. [DOI] [PubMed] [Google Scholar]
- 16.Fabsitz RR, Sidawy AN, Go O, et al. Prevalence of peripheral arterial disease and associated risk factors in American Indians: the Strong Heart Study. Am J Epidemiol. 1999;149(4):330–338. [DOI] [PubMed] [Google Scholar]
- 17.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109(6):733–739. [DOI] [PubMed] [Google Scholar]
- 18.Lee ET, Welty TK, Cowan LD, et al. Incidence of diabetes in American Indians of three geographic areas: the Strong Heart Study. Diabetes Care. 2002;25(1):49–54. [DOI] [PubMed] [Google Scholar]
- 19.Gribble MO, Howard BV, Umans JG, et al. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epidemiol. 2012;176(10):865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoddart ML, Jarvis B, Blake B, et al. Recruitment of American Indians in epidemiologic research: the Strong Heart Study. Am Indian Alsk Native Ment Health Res. 2000;9(3):20–37. [DOI] [PubMed] [Google Scholar]
- 21.Tellez-Plaza M, Guallar E, Fabsitz RR, et al. Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes. 2013;6(6):626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. [DOI] [PubMed] [Google Scholar]
- 23.Krauss RM, Burke DJ. Identification of multiple subclasses of plasma low density lipoproteins in normal humans. J Lipid Res. 1982;23(1):97–104. [PubMed] [Google Scholar]
- 24.Little RR, England JD, Wiedmeyer HM, et al. Interlaboratory standardization of glycated hemoglobin determinations. Clin Chem. 1986;32(2):358–360. [PubMed] [Google Scholar]
- 25.Shara NM, Resnick HE, Lu L, et al. Decreased GFR estimated by MDRD or Cockcroft-Gault equation predicts incident CVD: the Strong Heart Study. J Nephrol. 2009;22(3):373–380. [PMC free article] [PubMed] [Google Scholar]
- 26.Scheer J, Findenig S, Goessler W, et al. Arsenic species and selected metals in human urine: validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Methods. 2012;4(2):406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes MF. Biomarkers of exposure: a case study with inorganic arsenic. Environ Health Perspect. 2006;114(11):1790–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navas-Acien A, Umans JG, Howard BV, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environ Health Perspect. 2009;117(9):1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Efron B. Bootstrap methods: another look at the jackknife. Ann Stat. 1979;7(1):1–26. [Google Scholar]
- 30.Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82(397):171–185. [Google Scholar]
- 31.Huang Y-L, Hsueh Y-M, Huang Y-K, et al. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis-hyperendemic areas in southwestern Taiwan. Sci Total Environ. 2009;407(8):2608–2614. [DOI] [PubMed] [Google Scholar]
- 32.Tseng C-H, Huang Y-K, Huang Y-L, et al. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol Appl Pharmacol. 2005;206(3):299–308. [DOI] [PubMed] [Google Scholar]
- 33.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 suppl):1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 34.Donahue JG, Nelson KE, Muñoz A, et al. Transmission of HIV by transfusion of screened blood [letter]. N Engl J Med. 1990;323(24):1709. [DOI] [PubMed] [Google Scholar]
- 35.Kuo C-C, Howard BV, Umans JG, et al. Arsenic exposure, arsenic metabolism, and incident diabetes in the Strong Heart Study. Diabetes Care. 2015;38(4):620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engel RR, Smith AH. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Arch Environ Health. 1994;49(5):418–427. [DOI] [PubMed] [Google Scholar]
- 37.Meliker JR, Wahl RL, Cameron LL, et al. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environ Health. 2007;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis DR, Southwick JW, Ouellet-Hellstrom R, et al. Drinking water arsenic in Utah: a cohort mortality study. Environ Health Perspect. 1999;107(5):359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.States JC, Srivastava S, Chen Y, et al. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107(2):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Factor-Litvak P, Howe GR, et al. Arsenic exposure from drinking water, dietary intakes of B vitamins and folate, and risk of high blood pressure in Bangladesh: a population-based, cross-sectional study. Am J Epidemiol. 2007;165(5):541–552. [DOI] [PubMed] [Google Scholar]
- 41.Tseng C-H. Pulse pressure as a risk factor for peripheral vascular disease in type 2 diabetic patients. Clin Exp Hypertens. 2003;25(8):475–485. [DOI] [PubMed] [Google Scholar]
- 42.Barchowsky A, Klei LR, Dudek EJ, et al. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med. 1999;27(11-12):1405–1412. [DOI] [PubMed] [Google Scholar]
- 43.Lee M-Y, Jung B-I, Chung S-M, et al. Arsenic-induced dysfunction in relaxation of blood vessels. Environ Health Perspect. 2003;111(4):513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirano S, Kobayashi Y, Cui X, et al. The accumulation and toxicity of methylated arsenicals in endothelial cells: important roles of thiol compounds. Toxicol Appl Pharmacol. 2004;198(3):458–467. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe T, Hirano S. Metabolism of arsenic and its toxicological relevance. Arch Toxicol. 2013;87(6):969–979. [DOI] [PubMed] [Google Scholar]
- 46.Dao T, Cheng RYS, Revelo MP, et al. Hydroxymethylation as a novel environmental biosensor. Curr Environ Health Rep. 2014;1(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chervona Y, Costa M. The control of histone methylation and gene expression by oxidative stress, hypoxia, and metals. Free Radic Biol Med. 2012;53(5):1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tellez-Plaza M, Gribble MO, Voruganti VS, et al. Heritability and preliminary genome-wide linkage analysis of arsenic metabolites in urine. Environ Health Perspect. 2013;121(3):345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Concha G, Vogler G, Nermell B, et al. Intra-individual variation in the metabolism of inorganic arsenic. Int Arch Occup Environ Health. 2002;75(8):576–580. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Rubio P, Roberge J, Arendell L, et al. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol. 2011;252(2):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Wu F, Liu M, et al. A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect. 2013;121(7):832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tellez-Plaza M, Tang W-Y, Shang Y, et al. Association of global DNA methylation and global DNA hydroxymethylation with metals and other exposures in human blood DNA samples. Environ Health Perspect. 2014;122(9):946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pierce BL, Kibriya MG, Tong L, et al. Genome-wide association study identifies chromosome 10q24.32 variants associated with arsenic metabolism and toxicity phenotypes in Bangladesh. PLoS Genet. 2012;8(2):e1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tellez-Plaza M, Guallar E, Howard BV, et al. Cadmium exposure and incident cardiovascular disease. Epidemiology. 2013;24(3):421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible. Epidemiology. 2002;13(5):588–592. [DOI] [PubMed] [Google Scholar]
- 56.O'Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111(22):2913–2920. [DOI] [PubMed] [Google Scholar]
- 57.Aboyans V, Lacroix P, Tran M-H, et al. The prognosis of diabetic patients with high ankle-brachial index depends on the coexistence of occlusive peripheral artery disease. J Vasc Surg. 2011;53(4):984–991. [DOI] [PubMed] [Google Scholar]
- 58.Allison MA, Hiatt WR, Hirsch AT, et al. A high ankle-brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol. 2008;51(13):1292–1298. [DOI] [PubMed] [Google Scholar]
- 59.Hays AM, Lantz RC, Rodgers LS, et al. Arsenic-induced decreases in the vascular matrix. Toxicol Pathol. 2008;36(6):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect. 2012;120(9):1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–743. [DOI] [PubMed] [Google Scholar]
- 62.Howard BV, Lee ET, Yeh JL, et al. Hypertension in adult American Indians. The Strong Heart Study. Hypertension. 1996;28(2):256–264. [DOI] [PubMed] [Google Scholar]
- 63.Howard BV, Lee ET, Cowan LD, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99(18):2389–2395. [DOI] [PubMed] [Google Scholar]
- 64.Criqui MH, McClelland RL, McDermott MM, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2010;56(18):1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinmaus CM, Yuan Y, Smith AH. The temporal stability of arsenic concentrations in well water in western Nevada. Environ Res. 2005;99(2):164–168. [DOI] [PubMed] [Google Scholar]
- 66.Yancy CW, Benjamin EJ, Fabunmi RP, et al. Discovering the full spectrum of cardiovascular disease: Minority Health Summit 2003: executive summary. Circulation. 2005;111(10):1339–1349. [DOI] [PubMed] [Google Scholar]
- 67.Welty TK, Rhoades DA, Yeh F, et al. Changes in cardiovascular disease risk factors among American Indians. The Strong Heart Study. Ann Epidemiol. 2002;12(2):97–106. [DOI] [PubMed] [Google Scholar]
- 68.Marchiset-Ferlay N, Savanovitch C, Sauvant-Rochat M-P. What is the best biomarker to assess arsenic exposure via drinking water. Environ Int. 2012;39(1):150–171. [DOI] [PubMed] [Google Scholar]
- 69.Aitchison J. A new approach to null correlations of proportions. Math Geol. 1981;13(2):175–189. [Google Scholar]
- 70.Aitchison J. The statistical analysis of compositional data. J R Stat Soc Series B Methodol. 1982;44(2):139–177. [Google Scholar]