Abstract
Antisocial Personality Disorder (ASPD), one of whose characteristics is high impulsivity, is of great interest in the field of brain structure and function. However, little is known about possible impairments in the cortical anatomy in ASPD, in terms of cortical thickness and surface area, as well as their possible relationship with impulsivity. In this neuroimaging study, we first investigated the changes of cortical thickness and surface area in ASPD patients, in comparison to those of healthy controls, and then performed correlation analyses between these measures and the ability of impulse control. We found that ASPD patients showed thinner cortex while larger surface area in several specific brain regions, i.e., bilateral superior frontal gyrus, orbitofrontal and triangularis, insula cortex, precuneus, middle frontal gyrus, middle temporal gyrus, and left bank of superior temporal sulcus. In addition, we also found that the ability of impulse control was positively correlated with cortical thickness in the superior frontal gyrus, middle frontal gyrus, orbitofrontal cortex, pars triangularis, superior temporal gyrus, and insula cortex. To our knowledge, this study is the first to reveal simultaneous changes in cortical thickness and surface area in ASPD, as well as their relationship with impulsivity. These cortical structural changes may introduce uncontrolled and callous behavioral characteristic in ASPD patients, and these potential biomarkers may be very helpful in understanding the pathomechanism of ASPD.
Keywords: Cortical anatomy, impulsivity, response inhibition, MRI, cortical thickness, surface area
Introduction
Antisocial personality disorder (ASPD) describes patterns of antagonism and impulsively aggressive behaviors that begin in childhood and remain stable throughout the lifespan according to Diagnostic and Statistical Manual of Mental Disorders (DSM-V). Epidemiological studies report a prevalence of 2–3% in the general population, with estimates of approximately 3% in men and 1% in women (Gibbon et al., 2010). Furthermore, 47% of male prisoners in worldwide prison systems were diagnosed with ASPD (Fazel and Danesh, 2002), showing a very close link between ASPD and criminal behavior.
Abnormal brain structures are found to correlate with ASPD, based on morphological MRI studies. Raine et al. found that the prefrontal gray matter volume in ASPD was reduced by about 11% in comparison to that of the control group (Raine et al., 2000). The reduced gray matter volume in the prefrontal cortex was replicated in many other studies of ASPD (Yang and Raine, 2009). Reduced gray matter volume of temporal regions was also revealed in ASPD (Bassarath, 2001, Barkataki et al., 2006). Additionally, the relationship between impulsive aggression and the reduced volume of the frontal lobe was also found in ASPD patients (Raine et al., 2000, Laakso et al., 2002). These studies of ASPD mainly focused on gray matter volume. However, the cortical gray mater volume is essentially jointly determined by biologically distinct cortical attributes of cortical thickness (CTh) and surface area (SA), each having its own cellular mechanism and genetic underpinning, thus providing unique and complementary information of the cortex (Chen et al., 2013, Li et al., 2016, Lyall et al., 2015). More importantly, CTh and SA have been found distinctively correlated with cognitive functions, but also differentially affected in various brain disorders (Lyall et al., 2015). Hence, studying CTh and SA can better capture subtle, but important, cortical changes associated with ASPD. Ly et al. found that psychopathy patients showed cortical thinning in a number of regions, specially the left insula, bilateral anterior temporal cortices and right inferior frontal gyrus (Ly et al., 2012). Though psychopathy shares behavioral overlap with the clinical diagnosis of ASPD, ASPD is not synonymous of psychopathy (Blair, 2012). Currently, the study of cortical anatomy on ASPD population is still scarce. The changes in cortical thickness and surface area in ASPD still remain unclear.
Impulsivity is a central and important characteristic of ASPD according to DSM-V. Response inhibition, i.e., impulse control is the main impulsivity variable (Dougherty et al., 2005). Swann et al. found that ASPD was characterized more by increased rapid-response impulsivity, while aspects of impulsivity about reward-delay or attention appear relatively intact (Swann et al., 2009). GoStop impulsivity paradigm developed by NRLC-group can measure the level of impulse control or rapid-response impulsivity precisely (Dougherty et al., 2005). This paradigm has been used in all kinds of people, e.g., borderline personality disorder (BPD) (Cackowski et al., 2014), internet addiction (Li et al., 2014), and substance abuse (Coffey et al., 2011). But to date, there is no study on the correlation between CTh/SA changes and the levels of impulse control in ASPD.
In this study, we hypothesized that there were distinct alterations in CTh and SA in ASPD, and there also were potential relationships between these structural changes and the ability of impulse control. This study will provide valuable information regarding to the abnormal neuroanatomy of ASPD, while also highlighting the potential relations between structural changes and impulsivity in ASPD patients.
Methods and Materials
Participants
The School for Youth Offender of Hunan Province performed “Enclosed-style” management and reformatory education for those offenders under 18 years of age, when committing certain crimes, e.g., robbery and violent attacks. We recruited volunteers at legal age (18 years of age at scan) from this school. The participants were diagnosed whether with ASPD or not, according to the following steps. In the first step, all of the volunteers in groups were tested by a professional using the Personality Diagnostic Questionaire-4+ (PDQ-4+). For those ASPD scores equal to or above 4 score, two senior psychiatrists then tested whether they had Axis 1 disorders of major mental illness and excluded those with Axis 1 disorders. The remaining volunteers were continuously tested using the structured clinical interview for DSM-IV (SCID-II) by the same psychiatrists. The SCID-II is a diagnostic exam to determine personality disorders. Finally, 27 subjects were diagnosed with only ASPD, i.e., all ASPD disorders met both PDQ-4 criteria and SCID-II criteria for ASPD. We also chose 25 healthy control subjects, who met neither PDQ-4 criteria nor SCID-II criteria for ASPD. All the controls were tested using the same methods as ASPD disorders by the same psychiatrists. The control subjects were matched to the ASPD subjects in age, education, and IQ (Table 1). IQ score of each subject was obtained using Wechsler Adult Intelligence Scale. One-way ANOVAs were performed on the demographics of the groups to test whether the groups were well matched.
Table 1.
Characteristics of the participants in this study.
| ASPD (Mean±SD) | Controls (Mean±SD) | p-value | |
|---|---|---|---|
| Number | 27 | 25 | - |
| Gender | 27 males | 25 males | - |
| Age (Years) | 20.30±3.01 | 21.13±3.16 | 0.385 |
| Education (Years) | 9.54±3.42 | 10.26±2.33 | 0.753 |
| IQ | 94.55±8.62 | 95.30±10.5 | 0.797 |
ASPD: Offenders with antisocial personality disorder.
All subjects were right-handed native Chinese speakers, with no history or current diagnosis of drugs abuse, and they were kept away from alcohol at least 6 months before the experiment. They were accompanied by three instructors individually during the experiment. This study was approved by the Ethical Committee of the Third Xiangya Hospital of Central South University and also the Ethical Committee of the School for Youth Offender of Hunan Province. All volunteers signed on the written informed consent after they understood the study.
Impulsivity Measure
In the task, we used GoStop impulsivity paradigm to measure the level of impulse control. Black five-digit numbers are presented successively on the computer screen (with each 500 ms following by 1-second blank screen). When the present number is same as the previous number, the participant should press a mouse button as quickly as possible (‘no-stop trials’). When the target’s color changes from black to red (‘stop signal’, occurring from 50, 150, 250 to 350 ms after stimulus onset), the response needs to be withheld (‘stop trials’). If the five-digit number does not exactly match the previous number, the participant must withhold responding (‘novel trials’). In our study, no-stop trials were 25% of the trials, stop trials 25%, and novel trials 50%. The total number of all trails was 160 and the task lasted 240 s. Before the normal task, the participants received a detailed description of the task and underwent a training session of 60 s so as to be familiar with the task. To ensure cooperation, the response accuracy of each subject during “novel trials” and “stop trials” were calculated. The subjects whose response accuracies in the “novel trials” and “stop trials” conditions were lower than 80% were excluded. The level of impulse control is calculated as the percentage of successful inhibition. Lower percentages of successful inhibitions (during ‘stop trials’) indicate more difficulties with response inhibition.
To investigate whether there were significant differences between groups under different delay tasks, independent-sample t-tests were employed.
Image Acquisition
T1-weighted structural magnetic resonance images were acquired at the Third Xiangya Hospital of Central South University on a 3T Philips scanner. The principal sequence of this study was an ultrafast gradient echo 3D sequence (T1W_3D_TFE_ref). Scans were acquired in the sagittal plane with the following parameters: TR = 7.4 ms, TE = 3.5 ms, TI = 900 ms, gap = 0.6 mm, flip angle = 8°, BW = 210 Hz/pixel, FOV = 228 × 227 mm, matrix = 240 × 240; 301 slices with resolution = 1.04*1.04*0.6 mm3, acquisition time = 4 min 58 s. Images were inspected for motion artifact at the time of acquisition and scans were repeated as necessary. Images were also reviewed and discarded, if there were any pathological findings.
Image Processing
All MR images were processed on the same workstation using FreeSurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu/). FreeSurfer morphometric procedures have good test-retest reliability across different scanner manufacturers or field strengths (Cannon et al., 2015), and measuring methods of CTh and SA have also been extensively validated in normal subjects or patients with various brain disorders (McLaughlin et al., 2014, Zielinski et al., 2014, Cannon et al., 2015).
Cortical thickness and surface areas measures at each vertex were calculated based on cortical surface reconstruction (Fischl et al., 2004). First, several important processing steps were performed, including skull stripping, spatial transformation, and atlas registration. Then, both gray matter/white matter and gray matter/cerebrospinal fluid (CSF) surfaces were reconstructed and parcellated into different regions based on the folding structures of gyri and sulci (Desikan et al., 2006). Cortical thickness was calculated as the shortest distance between the gray/white surface and the pial surface (Fischl and Dale, 2000). More technical details of these procedures were described previously (Fischl et al., 2004, Desikan et al., 2006). The results of the automated segmentation, surface reconstruction, and parcellation process were manually inspected for all subjects, with no abnormality found.
Statistical Analyses
As a first step, cortical thickness and surface area maps were smoothed using a 20-mm Gaussian kernel (Karama et al., 2014). We also smoothed the map using other three Gaussian kernels, i.e., 10mm, 15mm and 25mm and found there were similar results. So we would only report the results smoothed using the 20-mm Gaussian kernel. Then, a general linear model (GLM) implemented in Freesurfer was used to investigate whether there were significant differences in measures of cortical thickness and surface area at each vertex (separately) between ASPD patients and control subjects. The false-discovery rate (FDR) of 0.05, corrected for multiple comparisons at the vertex level, was used in this study. We also modeled the age, IQ, and whole brain volume as covariates in order to minimize any confounding effects of these variables on the findings, i.e., parameter estimates for yij (CTh/SA) and the main effect of group Gj were estimated by regression of a general linear model (GLM) at each vertex i and subject j, with the age, IQ, and total brain volume as covariates:
where ε is the residual error.
Secondary analyses were conducted to determine the correlation of the ability of impulse control, i.e., the percentage of successful inhibition during different task, and the regional measures showing evidence of difference between two groups. First, each cluster identified at the first step was extracted. Second, average cortical thickness and total surface area in each cluster were calculated for each subject. Finally, the Pearson correlation coefficient was calculated between the percentage of successful inhibition and average CTh or total SA of each cluster (P < 0.05, uncorrected).
Results
Behavioral Performance
The ASPD patients showed impaired response inhibition during the Go-Stop task (Figure 1 and Table 2). The percentage of successfully inhibited responses was significantly lower in the ASPD patients, compared with the controls, during a 50 ms, 150 ms and 250 ms delay task (p < 0.05). When the task became harder (from 50ms delay to 150ms delay, to 250ms delay, and to 350ms delay), both groups demonstrated more and more difficulty in withholding their responses, as their percentage of successful inhibition was lower and lower. However, it was observed that the ASPD patients had more difficulties in response inhibition compared to healthy controls under the same task. Although the differences did not reach significance (p > 0.05) during the 350 ms delay tasks, the control group noticeably performed better.
Figure 1.
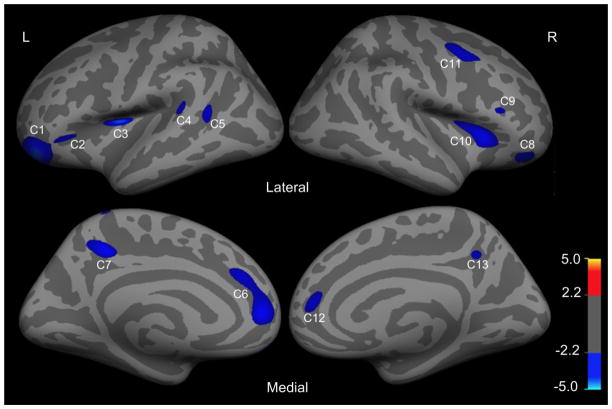
Regions in the left hemisphere (L) and right hemisphere (R) with significant reductions in cortical thickness (CTh) in ASPD patients relative to control subjects (false discovery rate correction (FDR), p < 0.05). The colorbar indicates t value.
Table 2.
The behavioral performance of ASPD patients and healthy controls (HC) during the Go-Stop task.
| Delay | ASPD (Mean±SD) | HC (Mean±SD) | t-value | p-value |
|---|---|---|---|---|
| 50 msec | 80.0%±20% | 93.5%±16% | 2.382 | 0.021 |
| 150 msec | 62.5%±30% | 83.8%±16% | 3.153 | 0.003 |
| 250 msec | 43.2%±29% | 70.0%±28% | 3.420 | 0.001 |
| 350 msec | 30.0%±25% | 45.4%±34% | 1.386 | 0.072 |
Reduced Cortical Thickness in ASPD Patients
On the left hemisphere, statistical analyses revealed 7 clusters (p < 0.05, FDR corrected) that differed significantly in thickness between ASPD patients and healthy controls (Figure 2 and Table 3). Importantly, all of the 7 clusters had reduced thickness in ASPD patients, compared with healthy controls. Specifically, they were located in the superior frontal gyrus (SFG)/rostral anterior cingulate cortex (ACC), precuneus, pars orbitalis/orbitofrontal cortex (OFC), pars triangularis, insula cortex, superior temporal sulcus (SFS) and superior temporal gyrus (STG). The average cortical thickness in each of the 7 clusters was calculated for each subject.
Figure 2.
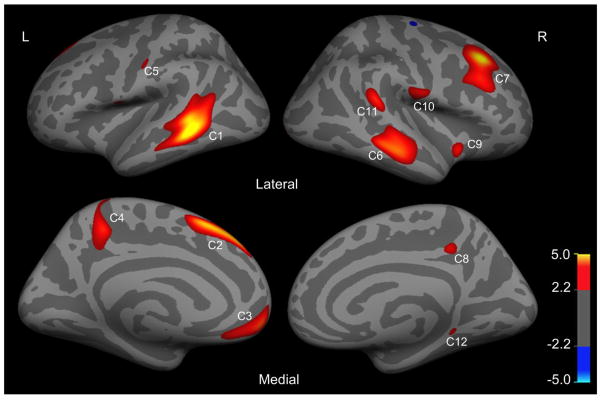
Regions in left hemisphere (L) and right hemisphere (R) with significant increase in surface area (SA) in ASPD patients relative to control subjects (false discovery rate correction (FDR), p < 0.05). The colorbar indicates t value.
Table 3.
Significant alterations in cortical thickness and surface area in ASPD patients relative to control subjects.
| Cluster | Hemisphere | Brain region | Peak Vertex Talairach | t value | p value (FDR corrected) | Size (mm2) | Percentage of Change | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Cortical Thickness (mm) | |||||||||
| C1 | Left | Orbitofrontal/pars orbitalis | −29.8 | 44.2 | −12.0 | −3.674 | 0.000 | 1483.31 | −8.30% |
| C2 | Left | Pars triangularis | −38.7 | 31.7 | −2.9 | −2.429 | 0.018 | 108.34 | −7.43% |
| C3 | Left | Insula/pars triangularis | −34.2 | −2.2 | 14.0 | −3.811 | 0.000 | 194.99 | −6.60% |
| C4 | Left | Bank of superior temporal sulcus | −47.7 | −45.5 | 13.2 | −2.533 | 0.014 | 139.65 | −3.70% |
| C5 | Left | Superior temporal gyrus | −50.4 | −37.5 | 13.8 | −2.553 | 0.013 | 104.75 | −7.09% |
| C6 | Left | Superior frontal gyrus/rostral anterior cingulate cortex | −11.1 | 43.9 | 2.7 | −2.989 | 0.004 | 650.26 | −7.75% |
| C7 | Left | Precuneus | −7.9 | −40.5 | 41.5 | −2.995 | 0.004 | 290.19 | −6.25% |
| C8 | Right | Orbitofrontal/pars orbitalis | 30.2 | 38.1 | −10.1 | −2.636 | 0.011 | 265.12 | −6.14% |
| C9 | Right | Pars triangularis | 48.1 | 26.1 | 13.2 | −2.379 | 0.021 | 60.04 | −9.29% |
| C10 | Right | Insula/pars triangularis/orbitofrontal | 29.5 | 23.1 | 5.3 | −3.069 | 0.003 | 433.06 | −7.59% |
| C11 | Right | Caudal middle frontal gyrus | 39.8 | 6.7 | 46.5 | −2.761 | 0.008 | 543.18 | −9.89% |
| C12 | Right | Superior frontal gyrus/rostral anterior cingulate cortex | 15.5 | 41.2 | 7.9 | −2.508 | 0.015 | 110.92 | −9.09% |
| C13 | Right | Precuneus | 14.1 | −47.3 | 38.5 | −2.426 | 0.018 | 49.34 | −6.58% |
| Surface Area (mm2) | |||||||||
| C1 | Left | Middle temporal gyrus/superior temporal sulcus | −61.4 | −42.8 | −0.7 | 5.645 | 0.000 | 2227.48 | 10.70% |
| C2 | Left | Superior frontal gyrus | −7.0 | 22.3 | 49.7 | 4.665 | 0.000 | 1075.83 | 5.11% |
| C3 | Left | Medial orbitofrontal | −8.3 | 53.6 | −10.3 | 3.783 | 0.000 | 816.17 | 6.79% |
| C4 | Left | Precuneus | −12.4 | −41.7 | 46.4 | 3.534 | 0.001 | 501.69 | 9.22% |
| C5 | Left | Postcentral gyrus | −54.0 | −13.5 | 36.8 | 2.751 | 0.008 | 79.24 | 8.70% |
| C6 | Right | Middle temporal/superior_temporal | 59.0 | −23.9 | −10.2 | 4.058 | 0.000 | 1570.22 | 8.44% |
| C7 | Right | Middle frontal/superior frontal | 30.2 | 26.2 | 38.2 | 5.206 | 0.000 | 1728.3 | 6.25% |
| C8 | Right | Precuneus | 6.9 | −38.0 | 40.8 | 3.038 | 0.004 | 89.47 | 9.89% |
| C9 | Right | Insula | 36.4 | 6.2 | −7.4 | 3.373 | 0.000 | 100.19 | 3.23% |
| C10 | Right | Precentral | 62.5 | −10.8 | 16.3 | 3.244 | 0.002 | 262.28 | 2.49% |
| C11 | Right | Supramarginal | 20.8 | −96.6 | −1.1 | 2.669 | 0.010 | 145.46 | 9.94% |
| C12 | Right | Parahippocampal | 18.2 | −35.7 | −6.9 | 2.825 | 0.007 | 30.03 | 9.80% |
On the right hemisphere, statistical analyses revealed 6 clusters (p < 0.05, FDR corrected) that differed significantly between the two groups, with ASPD exhibiting reduced cortical thickness in all clusters, when compared with control subjects (Figure 2 and Table 3). These 6 clusters were located at the superior frontal gyrus (SFG)/rostral anterior cingulate cortex (ACC), precuneus, caudal middle frontal gyrus (MFG), pars orbitalis/orbitofrontal cortex (OFC), pars triangularis and insula/pars triangularis/orbitofrontal cortex. Similarly, the average cortical thickness in each of the 6 clusters was calculated for each subject.
Increased Surface Area in ASPD Patients
On the left hemisphere, statistical analyses revealed 5 clusters (p < 0.05, FDR corrected) that differed significantly in surface area between ASPD patients and healthy controls (Figure 3 and Table 3). Interestingly, all these 5 clusters had increased surface area in ASPD patients compared with healthy controls. Specifically, they were located in the superior temporal gyrus (STG)/bank of superior temporal sulcus, superior frontal gyrus (SFG), medial orbitofrontal cortex, precuneus and postcentral gyrus. The total surface area of each cluster was calculated for each subject.
Figure 3.
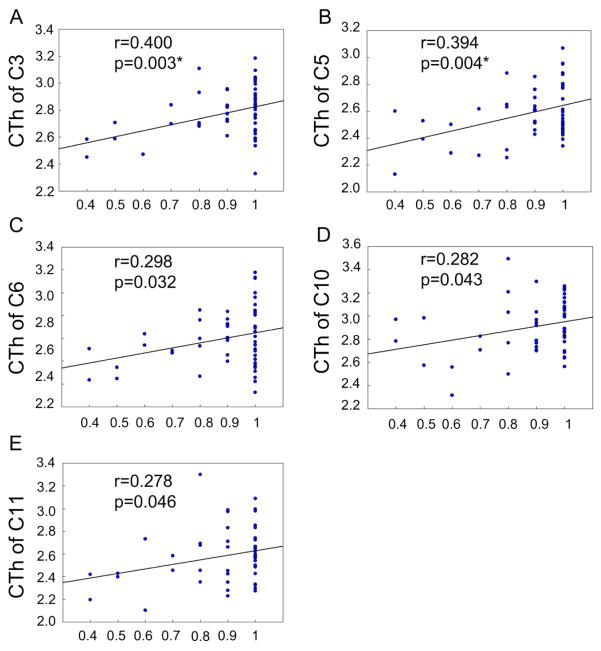
Significant correlation between behavioral performance and cortical thickness (CTh, mm) in clusters under 50ms delay task. The horizontal axis shows the percentage of successfully inhibited response. C denotes Cluster. * p < 0.05 under false discovery rate correction.
On the right hemisphere, statistical analyses revealed 7 clusters (p < 0.05, FDR corrected) that differed significantly between the two groups, with ASPD exhibiting increased surface area compared with control subjects in all clusters (Figure 3 and Table 3). These 7 clusters were located at the middle temporal/superior temporal gyrus, superior frontal gyrus (SFG), middle frontal gyrus (MFG), precuneus, insula cortex, precentral gyrus, supramarginal gyrus and parahippocampal gyrus. The total surface area of each of the 7 clusters was calculated for each subject.
Correlation between Cortical Measures and the Ability of Impulse Control
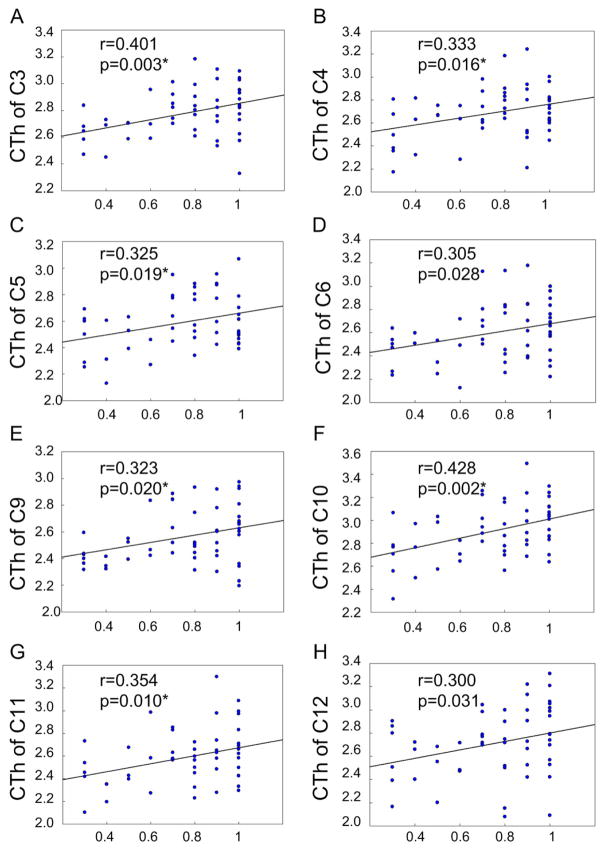
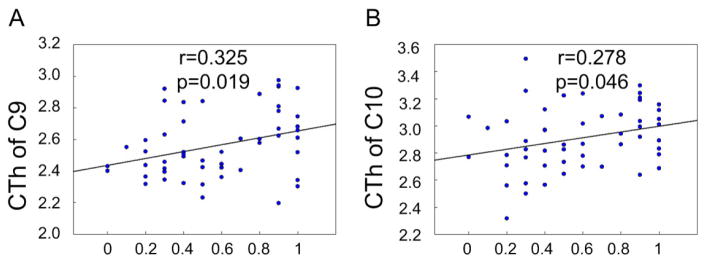
For average cortical thickness in each cluster, we performed correlation analysis with the percentage of successful inhibition. During 50ms delay, cortical thickness was correlated positively and significantly with the percentage of successful inhibition in the following clusters (P < 0.05, uncorrected): C3 (left insula cortex, Figure 3A), C5 (left superior temporal gyrus, Figure 3B), C6 (left superior frontal gyrus, Figure 3C), C10 (right insula cortex, Figure 3D), and C11 (right caudal middle frontal cortex, Figure 3E). During 150ms delay, cortical thickness was correlated positively and significantly with the percentage of successful inhibition in the following clusters (P < 0.05, uncorrected): C3 (left insula cortex, Figure 4A), C4 (left bank of superior temporal gyrus, Figure 4B), C5 (left superior temporal gyrus, Figure 4C), C6 (left superior frontal gyrus, Figure 4D), C9 (right pars triangularis, Figure 4E), C10 (right insula cortex, Figure 4F), C11 (right caudal middle frontal gyrus, Figure 4G), and C12 (right superior frontal gyrus, Figure 4H). During 250ms delay, cortical thickness in the C9 (right pars triangularis, Figure 5A) and C10 (right insula, Figure 5B) was correlated positively and significantly with the percentage of successful inhibition (P < 0.05, uncorrected). In other clusters, there existed also positive correlational trends, although not significant. These results demonstrate that the ability of impulse control is, in fact, correlated with cortical thickness, i.e., response inhibition is more difficult, if cortical thickness is thinner.
Figure 4.
Significant correlation between behavioral performance and cortical thickness (CTh, mm) in clusters under 150ms delay task. The horizontal axis shows the percentage of successfully inhibited response under different delay tasks. C denotes Cluster. * p < 0.05 under false discovery rate correction.
Figure 5.
Significant correlation between behavioral performance and cortical thickness (CTh, mm) in clusters under 250ms delay task. The horizontal axis shows the percentage of successfully inhibited response. C denotes Cluster.
We also performed correlation analysis between the percentage of successful inhibition and the surface area of each cluster, but no significant results were obtained.
Discussion
In this study, we investigated the ability of impulse control in ASPD patients, the changes of cortical thickness (CTh) and surface area (SA) in ASPD patients, and the relationship between CTh/SA and impulsivity. We found that the percentage of successfully inhibited responses was significantly lower in ASPD patients, which suggests impaired response inhibition in ASPD patients. Though this result is in line with DSM-V and previous studies that had reported poor impulse control in ASPD patients (Verona et al., 2012, Swann et al., 2013), it well quantified the ability of response inhibition. A more important finding is that ASPD patients present reduced CTh, but increased SA, when compared with healthy controls, while the traditional cortical volume could obscure information about CTh and SA, which are the two biologically distinct determinants of cortical volume (Raznahan et al., 2011).
Abnormalities in the prefrontal cortex (PFC) have been widely found in many previous imaging studies of ASPD (Raine et al., 2011, Yang and Raine, 2009). PFC is believed to be responsible for executive functions (EF) of ASPD (Morgan and Lilienfeld, 2000, Dolan, 2002), and neuropsychological deficits in executive function may introduce severe antisocial and aggressive behavior (Ogilvie et al., 2011). A damage study found that the injured PFC resulted in an increase in impulsivity, a decrease of judgment ability, and a lack of decision-making ability (Damasio et al., 1994). Specially, the superior frontal gyrus (SFG) is believed to be involved in self-awareness (Goldberg et al., 2006) and acted as functional operators when loading, maintaining, and switching between distinct stimulus-response tasks (Cutini et al., 2008). Abnormalities of SFG may be associated with disturbances of executive functioning, cognition, and judgment (Loveland et al., 2001). Rajah et al. also suggested that the middle frontal gyrus might mediate response selection and monitoring processes (Rajah et al., 2008).
Notably, the orbitofrontal cortex (OFC) extending to pars orbitalis and pars triangularis showed significantly reduced CTh in ASPD in our results. Our prior study also found that there were abnormal functional connections within OFC in ASPD patients (Tang et al., 2013). Major functions ascribed to OFC are the regulation of emotional responses, especially under threaten or risky situations (Buchheim et al., 2013) and behavioral inhibition (Christopoulos et al., 2009, Lopez-Caneda et al., 2012). The lateral OFC plays an important role in conflict resolution and encodes new expectations about punishment and social reprisal (Campbell-Meiklejohn et al., 2012). The mesial PFC is highly involved in emotional processing and is more regularly activated by emotional tasks than by cognitive tasks (Steele and Lawrie, 2004). OFC injures may lead to severe antisocial behaviors with the symptoms of antisocial disorders (Seguin, 2004). Disruption of this area’s activity by transcranial magnetic stimulation results in changes in risk attitudes in decision-making behaviors (Fecteau et al., 2007). Individuals with OFC lesions generally presented certain behavioral patterns of social inappropriate, misinterpreting others’ moods, impulsivity and risk (Tranel et al., 2002, Seguin, 2004).
Our evidence of thinner CTh and increased SA in PFC, amalgamated those prior converging lines of evidence, further implicates that prefrontal regions contribute to the neuropathology of ASPD. If PFC cannot function properly, a person may act impulsively and inappropriately, producing antisocial emotion. Hence, the changes of CTh and SA in PFC are significantly compromised and may serve as structural biomarkers for ASPD.
In our research, the left superior temporal gyrus (STG) showed reduced CTh, while bilateral middle temporal gyrus (MTG) and STG showed increased SA. Jastorff et al. found that the main cognitive function of MTG was to evaluate action rationality (Jastorff et al., 2011). The temporal lobe takes part in sensory, affective, and higher cognitive processing (Kolb and Whishaw, 1990), and is also involved in language comprehension and emotion association (Arfken, 2009). The impairment in emotional comprehension and contagion may introduce changes in comportment and social behavior (Zahn et al., 2009, Rascovsky et al., 2011).
The abnormal temporal and frontal lobes were occurred together in ASPD offenders in this study, which may increase the risk of anger and aggression, compared with each area independently (Potegal, 2012). Frontal-temporal joint dysfunction is believed to predispose to a patient’s antisocial behavior (Miller et al., 1997), and the frontal-temporal hypoactivity is suggested to be a trait of severe violent crimes (Anckarsater et al., 2007). Psychopathy has multiple overlaps with ASPD in diagnostic criteria. Prior studies demonstrated co-occurring lesions in frontal-temporal regions in psychopathy (de Oliveira-Souza et al., 2008, Muller et al., 2008). Especially, psychopaths were also found to have thinner cortex in the left insula, bilateral anterior temporal cortices and right inferior frontal gyrus, compared to healthy controls (Ly et al., 2012).
In our study, bilateral anterior insula cortex and precuneus represented significantly reduced CT but increased SA in ASPD. The anterior insular cortex is believed to be responsible for emotional feelings, including anger, fear, sadness, social exclusion, and empathy (Craig, 2009, Vilares et al., 2012), and be important for motor impulsivity and reactive aggression (Dambacher et al., 2015). Especially, it is believed to be involved in the processing of norm violations (Sanfey et al., 2003). As for the precuneus, it plays an essential role in conscious information processing, specifically involved in self-reflection processes (Cavanna, 2007, Cavanna and Trimble, 2006). In brief, the functions of the regions (i.e., PFC, MTG/STG, Insula and Precuneus) in our study are important for selection, control and performance of socially relevant behavior. Injury to these cortical regions may cause violent and aggressive behavior, and cause a feature of callousness in ASPD.
Interestingly, these main brain regions with cortical thinning in ASPD offenders were also found in subjects with Conduct Disorder (CD). ASPD individuals have evident conduct problems by the age of 15 according to DSM-5’s criteria for ASPD, and about 25–40% of youths with conduct disorder will develop into ASPD in adulthood (Zoccolillo et al., 1992). Fahim et al. observed reduced cortical thickness in STG, insula, and OFC in children with CD (Fahim et al., 2011). The thinner STG was also found in CD adolescents and showed a negative correlation with callous-unemotional traits (Wallace et al., 2014). More recently, male youths with CD showed abnormal cortical measurements in the STG, OFC and the insula relative to healthy controls (Fairchild et al., 2015). A meta-analysis study had also demonstrated gray matter reductions in youths with conduct problems, mainly within the insula, amygdala, frontal and temporal regions (Rogers and De Brito, 2016). So the thinner cortex in some brain regions in ASPD may be the sustainment from their childhood to adolescent then to adult.
The CTh and SA reflect different cellular mechanisms, i.e., CTh is mainly determined by the horizontal layers in the cortical columns including neurons and neuropil, whereas SA reflects the number of radial columns perpendicular to the pial surface (Rakic, 2009, Raznahan et al., 2011). In our study, the ASPD showed decreased CTh but increased SA, which may imply that ASPD subjects had less horizontal layers in the cortical columns but larger number of radial columns. The increased SA in the current study may occur due to the reduced CTh and result in “inefficient” or compensatory of cognitive control functions.
Finally, our study, for the first time, found the ability of impulse control is correlated with CTh in the superior frontal gyrus, middle frontal gyrus, superior temporal gyrus, pars orbitofrontal and triangularis, and insula cortex. From the discussion above, we know that these regions are correlated with impulsivity. Response inhibition may be more difficult when CTh in these regions is thinner. Of note, the relationship between impulsive aggression and the reduced volume of the frontal lobe was also found in ASPD patients (Raine et al., 2000, Laakso et al., 2002). SA didn’t show significant correlation with impulse control, which could imply that impulse control may not be strongly affected by SA.
The trials under different conditions (the 50/150/250/350ms delay) were limited, and we will add more trails to our studies in the future. In our study, though the surface area showed no significant correlation with impulse control, it may be correlated with other features of ASPD, which will be investigated in future. In addition, our subject number should be increased. Currently, our subjects are all males and thus these findings cannot be directly generalized to the female subjects. Also, our correlation finding should be considered exploratory.
To our knowledge, this study is the first to reveal simultaneous changes of cortical thickness and surface area in ASPD, as well as the relationship between these structural changes and impulsivity. These specific indicators in specific brain regions could provide structural biomarkers for ASPD and potentially be helpful for understanding the pathomechanism of ASPD.
Highlights.
ASPD patients showed thinner cortex in several specific brain regions
ASPD patients showed larger surface area in several specific brain regions.
The ability of impulse control was positively correlated with cortical thickness.
Acknowledgments
We thank all the volunteers for their participation in the study. The Funding Project of Education Ministry for the Development of Liberal Arts and Social Sciences (13YJCZH068), the China Postdoctoral Science Foundation (2015M582879), Key Laboratory of Basic Education Information Technology of Hunan Province (2015TP1017), and Research Foundation of Education Bureau of Hunan Province (NO.16A043) helped support this work. Additionally, this study was partially supported by the National Natural Science Foundation of China (61420106001, 61375111, 81571298) and, in part, supported by NIH grants (AG041721, MH107815, MH108914, EB006733, EB008374, EB009634).
All authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anckarsater H, Piechnik S, Tullberg M, Ziegelitz D, Sorman M, Bjellvi J, Karlsson E, Femandez NV, Wikkelso C, Forsman A. Persistent regional frontotemporal hypoactivity in violent offenders at follow-up. Psychiat Res-Neuroim. 2007;156:87–90. doi: 10.1016/j.pscychresns.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Arfken M. Brain, Mind, and Human Behavior in Contemporary Cognitive Science: Critical Assessments of the Philosophy of Psychology. Theor Psychol. 2009;19:860–862. [Google Scholar]
- Barkataki I, Kumari V, Das M, Taylor P, Sharma T. Volumetric structural brain abnormalities in men with schizophrenia or antisocial personality disorder. Behav Brain Res. 2006;169:239–247. doi: 10.1016/j.bbr.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Bassarath L. Neuroimaging studies of antisocial behaviour. Can J Psychiat. 2001;46:728–732. doi: 10.1177/070674370104600805. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Cortical thinning and functional connectivity in psychopathy. Am J Psychiat. 2012;169:684–687. doi: 10.1176/appi.ajp.2012.12030396. [DOI] [PubMed] [Google Scholar]
- Buchheim A, Labek K, Walter S, Viviani R. A clinical case study of a psychoanalytic psychotherapy monitored with functional neuroimaging. Front Hum Neurosci. 2013:7. doi: 10.3389/fnhum.2013.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cackowski S, Reitz AC, Ende G, Kleindienst N, Bohus M, Schmahl C, Krause-Utz A. Impact of stress on different components of impulsivity in borderline personality disorder. Psychol Med. 2014;44:3329–3340. doi: 10.1017/S0033291714000427. [DOI] [PubMed] [Google Scholar]
- Campbell-Meiklejohn DK, Kanai R, Bahrami B, Bach DR, Dolan RJ, Roepstorff A, Frith CD. Structure of orbitofrontal cortex predicts social influence. Curr Biol. 2012;22:123–124. doi: 10.1016/j.cub.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun DQ, Jacobson A, van Erp TGM, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R, SNAPL Progressive Reduction in Cortical Thickness as Psychosis Develops: A Multisite Longitudinal Neuroimaging Study of Youth at Elevated Clinical Risk. Biol Psychiat. 2015;77:147–157. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE. The precuneus and consciousness. CNS spectrums. 2007;12:545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen CH, Fiecas M, Gutierrez ED, Panizzon MS, Eyler LT, Vuoksimaa E, Thompson WK, Fennema-Notestine C, Hagler DJ, Jernigan TL, Neale MC, Franz CE, Lyons MJ, Fischl B, Tsuang MT, Dale AM, Kremen WS. Genetic topography of brain morphology. P Natl Acad Sci USA. 2013;110:17089–17094. doi: 10.1073/pnas.1308091110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos GI, Tobler PN, Bossaerts P, Dolan RJ, Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J Neurosci. 2009;29:12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Schumacher JA, Baschnagel JS, Hawk LW, Holloman G. Impulsivity and Risk-Taking in Borderline Personality Disorder With and Without Substance Use Disorders. Personal Disord. 2011;2:128–141. doi: 10.1037/a0020574. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Cutini S, Scatturin P, Menon E, Bisiacchi PS, Gamberini L, Zorzi M, Dell’Acqua R. Selective activation of the superior frontal gyrus in task-switching: an event-related fNIRS study. NeuroImage. 2008;42:945–955. doi: 10.1016/j.neuroimage.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The Return of Gage, Phineas - Clues About the Brain from the Skull of a Famous Patient. Science. 1994;264:1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, Schuhmann T. Out of control: Evidence for anterior insula involvement in motor impulsivity and reactive aggression. Soc Cogn Affect Neur. 2015;10:508–516. doi: 10.1093/scan/nsu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Ignacio FA, Tovar-Moll F, Moll J. Psychopathy as a disorder of the moral brain: Fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. NeuroImage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dolan M. What neuroimaging tells us about psychopathic disorders. Hosp Med. 2002;63:337–340. doi: 10.12968/hosp.2002.63.6.2003. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behav Res Methods. 2005;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Fahim C, He Y, Yoon U, Chen J, Evans A, Perusse D. Neuroanatomy of childhood disruptive behavior disorders. Aggress Behav. 2011;37:326–337. doi: 10.1002/ab.20396. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Toschi N, Hagan CC, Goodyer IM, Calder AJ, Passamonti L. Cortical thickness, surface area, and folding alterations in male youths with conduct disorder and varying levels of callous-unemotional traits. Neuroimage-Clin. 2015;8:253–260. doi: 10.1016/j.nicl.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel S, Danesh J. Serious mental disorder in 23000 prisoners: a systematic review of 62 surveys. Lancet. 2002;359:545–550. doi: 10.1016/S0140-6736(02)07740-1. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald DH, Liguori P, Theoret H, Boggio PS, Fregni F. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. J Neurosci. 2007;27:6212–6218. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. P Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gibbon S, Duggan C, Stoffers J, Huband N, Völlm BA, Ferriter M, Lieb K. Psychological interventions for antisocial personality disorder. Cochrane Database Syst Rev. 2010:16. doi: 10.1002/14651858.CD007668.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Jastorff J, Clavagnier S, Gergely G, Orban GA. Neural mechanisms of understanding rational actions: middle temporal gyrus activation by contextual violation. Cereb Cortex. 2011;21:318–329. doi: 10.1093/cercor/bhq098. [DOI] [PubMed] [Google Scholar]
- Karama S, Bastin ME, Murray C, Royle NA, Penke L, Munoz Maniega S, Gow AJ, Corley J, Hernandez MV, Lewis JD, Rousseau ME, Lepage C, Fonov V, Collins DL, Booth T, Rioux P, Sherif T, Adalat R, Starr JM, Evans AC, Wardlaw JM, Deary IJ. Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Mol Psychiat. 2014;19:555–559. doi: 10.1038/mp.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Whishaw I. Fundamentals of Human Neuropsychology. WH Freeman and Co; New York: 1990. [Google Scholar]
- Laakso MP, Gunning-Dixon F, Vaurio O, Repo-Tiihonen E, Soininen H, Tiihonen J. Prefrontal volumes in habitually violent subjects with antisocial personality disorder and type 2 alcoholism. Psychiat Res-Neuroim. 2002;114:95–102. doi: 10.1016/s0925-4927(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Li BJ, Friston KJ, Liu J, Liu Y, Zhang GP, Cao FL, Su LY, Yao SQ, Lu HB, Hu DW. Impaired Frontal-Basal Ganglia Connectivity in Adolescents with Internet Addiction. Sci Rep-Uk. 2014a:4. doi: 10.1038/srep05027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang L, Shi F, Lyall AE, Ahn M, Peng Z, Zhu H, Lin W, Gilmore JH, Shen D. Cortical thickness and surface area in neonates at high risk for schizophrenia. Brain Struct Funct. 2016;221:447–461. doi: 10.1007/s00429-014-0917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Caneda E, Cadaveira F, Crego A, Gomez-Suarez A, Corral M, Parada M, Caamano-Isorna F, Rodriguez Holguin S. Hyperactivation of right inferior frontal cortex in young binge drinkers during response inhibition: a follow-up study. Addiction. 2012;107:1796–1808. doi: 10.1111/j.1360-0443.2012.03908.x. [DOI] [PubMed] [Google Scholar]
- Loveland KA, Pearson DA, Tunali-Kotoski B, Ortegon J, Gibbs MC. Judgments of social appropriateness by children and adolescents with autism. J Autism Dev Disord. 2001;31:367–376. doi: 10.1023/a:1010608518060. [DOI] [PubMed] [Google Scholar]
- Ly M, Motzkin JC, Philippi CL, Kirk GR, Newman JP, Kiehl KA, Koenigs M. Cortical thinning in psychopathy. Am J Psychiat. 2012;169:743–749. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng XJ, Woolson S, Li G, Wang L, Hamer RM, Shen DG, Gilmore JH. Dynamic Development of Regional Cortical Thickness and Surface Area in Early Childhood. Cereb Cortex. 2015;25:2204–2212. doi: 10.1093/cercor/bhu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Ab. 2013;39:424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Berman SA, Scheibel RS, Hayman A. Case-Report - Acquired Antisocial Personality-Disorder Associated with Unilateral Left Orbital Frontal-Lobe Damage. J Psychiatr Neurosci. 1992;17:121–125. [PMC free article] [PubMed] [Google Scholar]
- Miller BL, Darby A, Benson D, Cummings J, Miller M. Aggressive, socially disruptive and antisocial behaviour associated with fronto-temporal dementia. Brit J Psychiat. 1997;170:150–154. doi: 10.1192/bjp.170.2.150. [DOI] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clin Psychol Rev. 2000;20:113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Muller JL, Ganssbauer S, Sommer M, Dohnel K, Weber T, Schmidt-Wilcke T, Hajak G. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiat Res-Neuroim. 2008;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Ogilvie JM, Stewart AL, Chan RCK, Shum DHK. Neuropsychological Measures of Executive Function and Antisocial Behavior: A Meta-Analysis*. Criminology. 2011;49:1063–1107. [Google Scholar]
- Potegal M. Temporal and frontal lobe initiation and regulation of the top-down escalation of anger and aggression. Behav Brain Res. 2012;231:386–395. doi: 10.1016/j.bbr.2011.10.049. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiat. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Raine A, Yang Y, Narr KL, Toga AW. Sex differences in orbitofrontal gray as a partial explanation for sex differences in antisocial personality. Mol Psychiat. 2011;16:227–236. doi: 10.1038/mp.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, Ames B, D’Esposito M. Prefrontal contributions to domain-general executive control processes during temporal context retrieval. Neuropsychologia. 2008;46:1088–1103. doi: 10.1016/j.neuropsychologia.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. How Does Your Cortex Grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JC, De Brito SA. Cortical and Subcortical Gray Matter Volume in Youths With Conduct Problems A Meta-analysis. JAMA psychiatry. 2016;73:64–72. doi: 10.1001/jamapsychiatry.2015.2423. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Seguin JR. Neurocognitive elements of antisocial behavior: Relevance of an orbitofrontal cortex account. Brain Cognition. 2004;55:185–197. doi: 10.1016/S0278-2626(03)00273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele JD, Lawrie SM. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. NeuroImage. 2004;21:868–875. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Trait impulsivity and response inhibition in antisocial personality disorder. J Psychiat Res. 2009;43:1057–1063. doi: 10.1016/j.jpsychires.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG. Antisocial personality disorder and borderline symptoms are differentially related to impulsivity and course of illness in bipolar disorder. J Affect Disorders. 2013;148:384–390. doi: 10.1016/j.jad.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Jiang WX, Liao J, Wang W, Luo AJ. Identifying Individuals with Antisocial Personality Disorder Using Resting-State fMRI. Plos One. 2013:8. doi: 10.1371/journal.pone.0060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Verona E, Sprague J, Sadeh N. Inhibitory Control and Negative Emotional Processing in Psychopathy and Antisocial Personality Disorder. J Abnorm Psychol. 2012;121:498–510. doi: 10.1037/a0025308. [DOI] [PubMed] [Google Scholar]
- Vilares I, Howard JD, Fernandes HL, Gottfried JA, Kording KP. Differential Representations of Prior and Likelihood Uncertainty in the Human Brain. Curr Biol. 2012;22:1641–1648. doi: 10.1016/j.cub.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, White SF, Robustelli B, Sinclair S, Hwang S, Martin A, Blair RJR. Cortical and Subcortical Abnormalities in Youths With Conduct Disorder and Elevated Callous-Unemotional Traits. J Am Acad Child Psy. 2014;53:456–465. doi: 10.1016/j.jaac.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiat Res-Neuroim. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Iyengar V, Huey ED, Tierney M, Krueger F, Grafman J. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. 2009;132:604–616. doi: 10.1093/brain/awn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MB, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, Fletcher PT, Zygmunt KM, Travers BG, Lange N, Alexander AL, Bigler ED, Lainhart JE. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137:1799–1812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolillo M, Pickles A, Quinton D, Rutter M. The Outcome of Childhood Conduct Disorder - Implications for Defining Adult Personality-Disorder and Conduct Disorder. Psychol Med. 1992;22:971–986. doi: 10.1017/s003329170003854x. [DOI] [PubMed] [Google Scholar]