Abstract
Antibody levels rise during treatment of tuberculosis. This study examined when this rise occurred, whether there was recognition of new antigen binding sites (epitopes) on the same or different antigens, and how long specific antibody persisted. Forty patients with smear-positive pulmonary tuberculosis provided serum before and during treatment. Antibody levels were measured using a monoclonal antibody competition assay to epitopes restricted to the Mycobacterium tuberculosis complex and an enzyme-linked immunosorbent assay for lipoarabinomannan. Significant increases in antibody levels were apparent after 7 days of treatment. Five samples (12.5%) had positive titers to all epitopes at the start of treatment, and this increased to 23 (58%) during treatment. Antibody to epitopes with the poorest sensitivity (the TB23 epitope of the 19-kDa antigen and the TB78 epitope of hsp65) showed the greatest increases after treatment. Antibody to these two epitopes was also absent in some patients with relapsed tuberculosis until after treatment. Antibody titers showed a biphasic response, with a fall at 2 to 3 months of treatment. Sera from two patients showed changes in the affinity of epitope-specific antibody during treatment, whereas the majority did not. Those infected with isoniazid-resistant strains of M. tuberculosis showed a late rise in antibody. Antibody to the TB68 epitope of the 16-kDa α-crystallin homolog was short-lived, but it recurred with bacteriological relapse during treatment. Positive antibody titers persisted for at least 3 to 18 months after treatment. Diagnostic tests for tuberculosis should be evaluated using only pretreatment sera. Delayed antigenic recognition could be due to active suppression and/or failure to engage internal antigens of M. tuberculosis.
Tuberculosis is one of the most important diseases in the world today. Approximately one-third of the world's population has been infected, infection lasts a lifetime, and the number of deaths is the largest attributable to a single bacterial species. A typical immune response to a self or foreign antigen is usually focused on one or two epitopes, which are termed “immunodominant” (27). In cancer immunotherapy, epitope spreading is associated with tumor regression (25). A focused immune response in a chronic infectious disease such as tuberculosis might be pathogenic, acting as a smoke screen to divert attention from an effective immune response. Treatment of tuberculosis should reveal antigens that are recognized when bacilli are killed. An immune response to these antigens could be protective. This study was undertaken to document epitope spreading after treatment of tuberculosis as part of a program to discover potentially protective antigens.
Kaplan and Chase were among the first to note a rise in the levels of antibodies to mycobacterial antigens following treatment (18). In their study, most sera recognized only one or two antigens. Treatment resulted in either intensification or appearance of one or two additional lines detected by an immunodiffusion test. Increases in antibody levels to purified P32, the 38-kDa antigen (also known as antigen 5), and lipoarabinomannan following treatment of tuberculosis have also been documented (12, 16, 29, 30). The subsidiary aim of the present study was to answer the following questions pertinent to the clinical utility of serologic tests for tuberculosis. When do antibody levels rise after treatment, and how does this affect the number of patients with titers above a cutoff point defined by control sera? Is the rise due to an increase in antibody levels of a single specificity and/or recognition of additional antibody binding sites (epitopes) on the same antigen? Do the levels of antibodies of different specificities rise concurrently or at different times during treatment? How long do raised antibody levels persist, and can they be used to predict cure or relapse?
The monoclonal antibody competition test used in this study is especially useful in addressing the question whether treatment affects antibody levels or recognition of antibody binding determinants, since it can pinpoint the measured antibody specificity to an individual epitope. The test does not measure antibody directly but, rather, measures it by the inhibition of binding of the monoclonal antibody to a soluble extract of Mycobacterium tuberculosis (MTSE) by various dilutions of human sera. The advantages of this test over a standard enzyme-linked immunosorbent assay (ELISA) are that there is no need to purify the specified antigen, antibodies of all classes (immunoglobulin M [IgM], IgG, IgA, and IgE) are detected simultaneously, and the levels of antibodies to individual antibody-binding sites (epitopes) can be inferred (15).
MATERIALS AND METHODS
Patients.
Patients starting to receive treatment for pulmonary tuberculosis were invited to join the study, approved by the local ethics committee, and all gave informed consent. Of the patients, 40 had smear- and culture-positive tuberculosis, 7 had smear-negative but culture-positive disease, and 7 were both smear and culture negative but showed a good response to antituberculosis chemotherapy with no alternative diagnosis. All except seven were treated with a standard 6-month course of rifampin and isoniazid, with pyrazinamide in the initial phase. Those from sub-Saharan Africa (three smear-positive patients and one smear- and culture-negative patient) and those with previous tuberculosis (three smear-positive patients and two smear-negative and culture-positive patients) were given a four-drug regimen including ethambutol because of possible drug resistance. These six, along with one other patient with smear-positive pulmonary tuberculosis, proved to be infected with strains of M. tuberculosis resistant to isoniazid alone; their treatment was modified accordingly to a 9-month regimen of rifampin and ethambutol, with pyrazinamide for the first 2 months. Testing for the human immunodeficiency viruses was routinely offered to all patients with tuberculosis; anonymous testing was also being undertaken at that time, and the batch of 1,000 sera that included aliquots of sera from the present series gave no positive results, i.e., no patient had concurrent human immunodeficiency virus infection.
Monoclonal antibody competition test.
Sera were taken before the start of treatment, on days 7 (22 sera) and 14 (25 sera) and months 1, 2, 3, 4, 6, and 9 (25, 25, 15, 23, 15, and 18 sera, respectively), and at any subsequent follow-up appointments (12 months [8 sera] and 18 and 24 months [1 serum sample each]). Sera were aliquoted to avoid freezing and thawing. Monoclonal antibodies used in this study were denoted TB23 (which binds to the 19-kDa secreted antigen), TB68 (16-kDa α-crystallin homolog), TB71 and TB72 (38-kDa antigen, also known as antigen 5 in serologic studies), TB78 (65-kDa heat shock protein), and ML34 (lipoarabinomannan). TB23 shows limited binding to a soluble extract of M. kansasii and M. marinum, and TB78 shows limited binding to M. paratuberculosis. ML34 binds to all mycobacteria. TB68, TB71, and TB72 are restricted to the M. tuberculosis complex (13). The soluble extract of M. tuberculosis H37Rv (MTSE) was prepared by disruption of irradiated bacilli (10 g of bacilli suspended in 10 ml of phosphate-buffered saline [PBS] with 2 μmol of phenylmethylsulfonyl fluoride [Sigma]) with glass beads (50 g of beads, 0.1 to 0.11 mm in diameter) in a Braun MSK cell homogenizer followed by centrifugation of the homogenate at 47,000 × g for 60 min, yielding a supernatant with a protein content of 2.7 mg/ml. Aliquots were prepared and stored at −20°C until use. Different preparations have been shown to give the same results (3a), although a single preparation was used for this study.
Sera were initially analyzed using the radiolabeled monoclonal antibody shortly after collection. Polyvinyl flexible U-shaped 96-well plates (Dynatech) were coated with 50 μl of 30-μg/ml MTSE per well overnight at 4°C in a humidified atmosphere. The plates were washed once with PBS, blocked with 3% bovine serum albumin in PBS for 1 h at 20°C, and then washed twice with PBS. Sera were diluted at 1/5, 1/25, 1/125, and 1/625 in 3% bovine serum albumin, and 25-μl aliquots were added to duplicate wells and incubated for 4 h at 20°C. Without washing the plates, 25 μl of 125I-labeled monoclonal antibody, diluted to give 1,000 to 2,000 cpm in wells without competing serum (high control), was added to each well. The plates were shaken on a Dynatech Microtiter Varishaker for 30 s and then incubated overnight at 4°C. They were then washed four times with PBS and dried, and individual wells were counted with an LKB 1260 Multigamma II counter. Counts were corrected for binding to uncoated wells. Antibody titers (50% inhibitory doses [ID50]) were calculated as that dilution of serum reducing the binding of the monoclonal antibody by 50% of the corrected high control. Titers were calculated by interpolation between the two serum dilutions on either side of the 50% inhibition value or by extrapolation when the ID50 was <5 or >625. Consistency between assays was maintained using both standardized high and low control sera.
The combined series of sera from an individual patient were analyzed simultaneously using an ELISA (33). Microtiter plates (96 wells) were coated with 50 μl of MTSE diluted in PBS and incubated for 20 h at 4°C in a humidified atmosphere. After a single wash with PBS-Tween 20 (0.05%, wt/vol) (PBST), the wells were blocked for 1 h at 37°C with 200 μl of 3% dried milk diluted in PBST (PBSTM). The PBSTM was tipped off, and four fivefold dilutions of human sera in PBSTM (1/5 to 1/625; 25 μl/well) were added to duplicate wells, which were then incubated for 1 h at 37°C. Without washing, 25 μl of monoclonal antibody diluted in PBSTM was added to give a final concentration previously determined to generate 90% of the maximal binding of the monoclonal antibody to MTSE (TB23, 50 ng/ml; TB68, 50 μg/ml; TB71, 500 ng/ml; TB72, 5 μg/ml; TB78, 2.5 μg/ml; L4 [an IgG monoclonal antibody that binds to the same epitope as ML34], 5 μg/ml). The plates were shaken as before and incubated at 37°C for 2 h. The wells were then washed thoroughly five times with PBST and patted dry. Goat anti-mouse IgG antibody-peroxidase conjugate was diluted in PBSTM as specified by the manufacturer (Sigma), 50 μl was added to each well, and the plates were incubated for 1 h at 37°C. Following a further five washes with PBST, the plates were dried and 75 μl of TMB/H2O2 (0.1 mg of tetramethylbenzidine per ml in combination with 0.01% hydrogen peroxide in citrate buffer [pH 5.1]) was added to each well. The reaction was stopped after 10 min by addition of 50 μl of 0.5 M sulfuric acid, and the absorbance (optical density [OD]) was read on a Titertek Multiskan Spectrophotometer at 450 nm. Antibody titers (ID50) were calculated again as that dilution of serum inhibiting the binding of the monoclonal antibody by 50%.
ELISA.
Microtiter plates were coated with lipoarabinomannan or the recombinant 16-kDa antigen at 1 μg/liter in PBS or with PBS for subsequent blank correction, 50 μl per well, overnight at 4°C. After being washed with PBST, the plates were blocked with 150 μl of 1% dried milk in PBST per well for 1 h at 37°C. After the blocking solution was tipped off, 50 μl of serum diluted at 1:40, 1:200, and 1:1,000 in duplicate was added to each well, and the wells were incubated for 1 h at 37°C. The plates were washed four times with PBST, and then 50 μl of goat anti-human IgG-peroxidase conjugate diluted 1:1,000 was incubated for 1 h at 37°C. After six washes with PBST, bound peroxidase conjugate was measured as in the competition ELISA and the OD was corrected by subtraction of binding to PBS-coated plates. Antibody titers (ABT50) were calculated as the dilution of serum giving 50% of maximal binding as given by a standard high control used on each plate.
Analysis.
A positive titer was defined as greater than the mean plus two standard deviations (SD) of values from control sera in previous studies (145 patients with suspected pulmonary tuberculosis for whom an alternative diagnosis was obtained, 480 M. bovis BCG-vaccinated controls admitted to hospital for another reason, and 39 healthy BCG-vaccinated controls [6, 7]), i.e., TB23, ≥20; TB68, ≥15; TB71, ≥5; TB71, ≥10; TB78, ≥4, and ML34, ≥35. Differences in positive rating were compared using the χ2 test. To compare patients with different starting titers during treatment, subsequent antibody titers (ID50) were divided by the titer before treatment to give a relative ID50. A log scale was used in preparing the figures so that reductions in antibody titer would be given the same weight as increases. Changes in relative antibody titer were compared using Student's t test.
The law of mass action can be used to describe the interaction between antigen and antibody in an ELISA. Microtiter plates are coated with an antigen, and different dilutions of serum are allowed to bind to the antigen. As the concentration of antibody increases, the absorbance (a measure of the bound antibody) will describe a simple rectilinear hyperbola. The equilibrium constant is equal to the concentration of antibody that causes 50% of the maximal absorbance. A double-reciprocal or Lineweaver-Burk plot of the absorbance and serum dilution will produce a straight line whose intersection on the x-axis and on the y-axis is the reciprocal of the equilibrium constant (or affinity of the antibody for the antigen) and the reciprocal of the maximal absorbance, respectively. Competitive antagonism can also be described by the law of mass action to show that the proportion of antibody sites occupied by antibody A (pA) is related to the concentrations of A and its antagonist B and their equilibrium constants KA and KB for binding to the same epitope by the equation
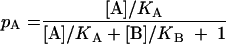 |
where the brackets indicate the concentration of the antibodies. In our assay, antibody sites occupied by the monoclonal antibody are measured as the absorbance. The quantity of monoclonal antibody and its affinity are fixed (let [A]/KA = k1). pA is the OD divided by the maximum ODmax. The concentration of the serum antibody is calculated as the ID50, i.e.,
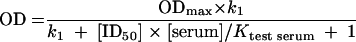 |
Plotting the reciprocal of the absorbance × ID50 against dilutions of the test serum will then reveal changes in the relative affinity of the competing epitope-specific antibody:
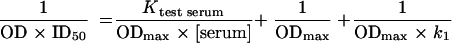 |
where the slope will vary according to the affinity of the competing antibody, all other factors being constant. Using these graphs, the “affinity” of the antibody for its binding sites was calculated. By plotting the calculated affinity and plotting this against the calculated antibody titer, whether ID50 or ABT50, a change in affinity could be demonstrated. The correlation of these values was assessed by multiple-regression analysis.
RESULTS
Increase in antibody titer and diagnostic sensitivity.
Levels of antibody to the TB72 epitope gave the greatest diagnostic sensitivity (67.5%) in pretreatment samples. After only 7 days of treatment of patients with smear-positive pulmonary tuberculosis, the number of positive titers had risen for all probes (Table 1). The increases were most marked for probes with a poor initial sensitivity, which improved by 125% for TB23, 107% for TB68, and 74% for TB78 during the period of chemotherapy. Samples were taken from four patients between days 0 and 7 of treatment: one showed a new positive titer to the TB68 epitope on day 5 of treatment. New positive titers were not seen in those who were smear negative but culture positive until after 2 months of treatment. Of those who were sputum smear and culture negative, three gave a positive titer after 14 days of treatment, one gave a positive titer at 1 month, two gave a positive titer at 2 months, and one gave a positive titer at 4 months. Three additional patients with smear- and culture-negative pulmonary tuberculosis failed to provide a pretreatment sample. Each was positive for antibody to more than a single monoclonal antibody-defined epitope after treatment for 7 days and became positive to between three and five probes at either the 2- or 3-month sample.
TABLE 1.
Frequency of positive titers of antibodies to six epitopes restricted to M. tuberculosis during treatment
| Epitope | Antigen | No. of positive titersa
|
||||||
|---|---|---|---|---|---|---|---|---|
| S+C+
|
S−C+
|
S−C−
|
||||||
| Day 0 | Day 7 | >Day 7 | Day 0 | >Day 7 | Day 0 | >Day 7 | ||
| TB23 | 19 kDa | 13 | 16 | 27 | 0 | 0 | 0 | 1 |
| TB68 | 16 kDa | 14 | 19 | 29 | 1 | 3 | 1 | 3 |
| TB71 | 38 kDa | 22 | 25 | 35 | 1 | 2 | 1 | 4 |
| TB72 | 38 kDa | 27 | 28 | 35 | 1 | 4 | 1 | 4 |
| TB78 | hsp65 | 19 | 20 | 33 | 1 | 1 | 2 | 4 |
| ML34 | LAMb | 19 | 22 | 32 | 2 | 2 | 2 | 2 |
| Any | 35 | 36 | 38 | 2 | 4 | 3 | 6 | |
S+C+, smear- and culture-positive pulmonary tuberculosis (n = 40); S−C+, smear-negative, culture-positive pulmonary tuberculosis (n = 7); S−C−, smear- and culture-negative pulmonary tuberculosis with response to treatment (n = 7).
LAM, lipoarabinomannan.
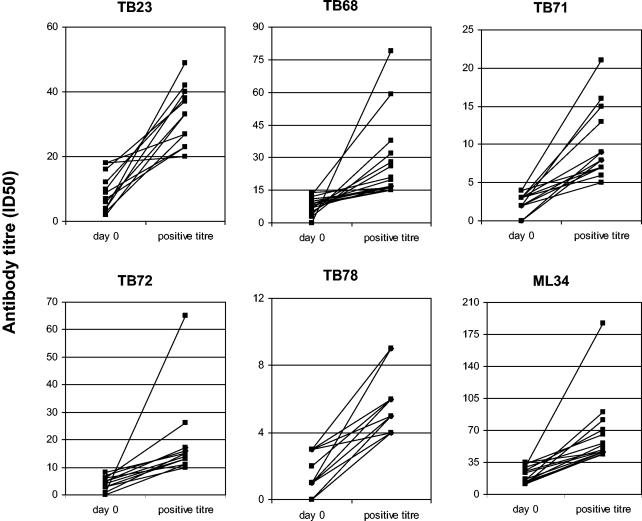
Individual antibody levels for those who were negative at the start of treatment and became positive during treatment are given in Fig. 1. There were 50 instances where the antibody titer increased such that the ID50 was found in the next dilution and 7 where the ID50 was between 5 and 25 times the pretreatment antibody titer. A few increases were of exceptional magnitude. Two patients with smear-negative, culture-positive tuberculosis accounted for the top two positive titers to the TB68 epitope and were individually represented as the top titer for levels of antibody to the TB71 and TB72 epitopes, respectively. The outlier for increases in titers of antibody to the ML34 epitope of lipoarabinomannan was in serum taken from a patient with isoniazid-resistant tuberculosis.
FIG. 1.
Increase in antibody levels during treatment. Only samples with an antibody titer (ID50) below the cutoff, defined as greater than the mean plus two SD of values from control sera, are shown. Gridlines indicate multiples of the cutoff titer for each monoclonal antibody probe. Antibody levels are defined by extrapolation as the dilution that reduces the maximal binding of the monoclonal antibody by 50%. The second value indicates the first positive titer obtained during treatment for tuberculosis.
Recognition of additional antibody binding sites on the same antigen.
The TB71 and TB72 monoclonal antibodies both bind to nonoverlapping epitopes of the 38-kDa antigen. At the start of chemotherapy, 22 patients were positive for antibody to both epitopes: 14 had higher titers of antibody to the TB72 epitope, 7 had higher titers of antibody to the TB71 epitope, and 1 had almost identical titers of antibody to the two epitopes. Despite a general rise in antibody titers, the ratio between levels of antibody to the two epitopes in 18 of these 22 patients did not change significantly; 2 patients showed an increase in the antibody ratio, and 2 showed a fall. Five patients with positive titers of antibody to only the TB72 epitope became positive for the TB71 epitope during treatment. There were no patients with positive titers of antibody to the TB71 epitope who later developed positive titers of antibody to the TB72 epitope. Thirteen patients had no antibody to either of the two epitopes at the start of treatment, but six became positive to both epitopes simultaneously and by 3 months. Those who developed antibody to only one of the epitopes did so later in treatment (two developed antibody to the TB71 epitope at 4 and 8 months, and 1 developed antibody to the TB72 epitope at 8 months).
Do titers of antibodies of different specificities rise concurrently or at different times during treatment?
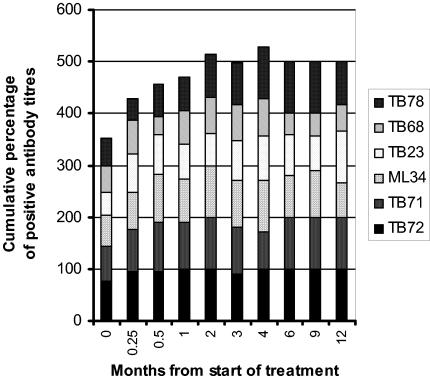
There was an increase in positive antibody titers with treatment (Fig. 1). As noted above, the number of patients who recognized the TB23 epitope at any stage more than doubled. Figure 2 shows that the rise in the positive rating occurred later for the antibody to the TB78 epitope than for the other epitopes. Antibody to the TB68 epitope showed a variability lacking in positive titers of antibodies to the other epitopes, with both new positive and new negative titers affecting its sensitivity at any particular time point. A fall in antibody titer from a positive to a negative value during treatment occurred most frequently for antibody to the TB68 epitope (43% of all occurrences). Positive titers of antibody to the ML34 epitope of lipoarabinomannan showed a rise and fall during treatment. More patients (4/5) who had previously had tuberculosis recognized five or six epitopes before the start of treatment compared to those experiencing their first episode of tuberculosis (6 of 49; χ2 = 9.7 with Yates' correction; P < 0.05). In the five with previous tuberculosis, antibody to the TB78 epitope was initially absent in two with smear-positive disease and antibody to the TB23 epitope was initially absent in one patient with smear-negative, culture-positive pulmonary tuberculosis. A majority (23/40) recognized all six epitopes at some point during their treatment. Patients infected with isoniazid-resistant strains of M. tuberculosis showed a later rise in positive ratings, with 8 of 11 instances occurring at 2 months or later in treatment, compared to 32 of 72 new positive titers in those with fully sensitive strains.
FIG. 2.
Recognition of different epitopes during treatment for tuberculosis. Cumulative percentages of positive titers of antibodies to the six epitopes are plotted against time from the start of treatment. This figure includes sera from patients with smear-positive pulmonary tuberculosis who were ever positive for antibody to the epitopes defined by the monoclonal antibodies TB72 and TB71 (38-kDa secreted antigen), ML34 (lipoarabinomannan), TB23 (19-kDa secreted antigen), TB68 (16-kDa α-crystallin stress protein homologue), and TB78 (stress protein hsp65). Those with isoniazid-resistant or previous tuberculosis have been excluded, as have those with smear-negative pulmonary disease. A positive titer was defined as greater than the mean plus two SD of control sera.
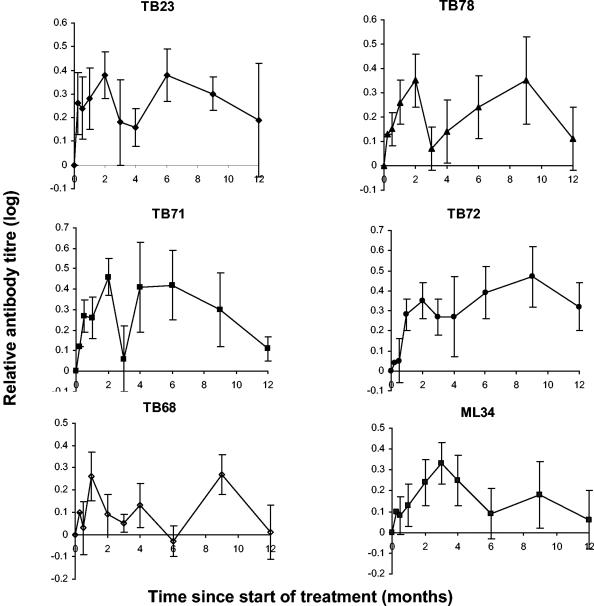
In patients who were ever positive for antibody to a particular epitope, the pattern of the rise in antibody titer was further examined by comparing antibody levels during treatment to the pretreatment levels (Fig. 3). Mean antibody levels doubled during treatment (log10 2 ≈ 0.3). Relative levels of antibodies to the protein antigens appeared to fall at between 2 and 3 months before rising again for antibody to the TB23, TB71, and TB78 epitopes. This finding was reexamined by inspection of sera from individual patients, reasoning that a late response to some epitopes might lead to a false interpretation of the data as suggesting a biphasic response. In fact, 66% of individual sets of data showed a biphasic response, 21% showed a late response, 11% showed a single peak, and 2% showed no change in antibody titer with treatment. All those with a late peak in the titer of antibody to one or more epitopes also showed a biphasic response to at least one other epitope. Levels of antibody to the TB68 epitope varied within individual patients with time, precluding speculation about a general trend. Relative levels of antibody to the ML34 epitope of lipoarabinomannan showed a rise to a peak at 3 months followed by a gradual decline.
FIG. 3.
Change in antibody titers during treatment of tuberculosis. These graphs include data from only those patients with smear-positive pulmonary tuberculosis who had a positive antibody titer at some point during their treatment but exclude those with isoniazid-resistant tuberculosis (see Fig. 4). Antibody levels obtained during treatment were each compared to the patient's pretreatment level to give a relative antibody titer. The relative antibody titers were then combined to give a mean ± standard error. A logarithmic scale has been used to treat rises and falls in antibody titer equally. Antibody levels were measured by competition with monoclonal antibodies TB23 (which binds to a secreted 19-kDa antigen), TB78 (which binds to the stress protein hsp65), TB71 and TB72 (which bind to a secreted 38-kDa antigen), TB68 (which binds to a 16-kDa α-crystallin stress protein homolog), and ML34 (which binds to lipoarabinomannan).
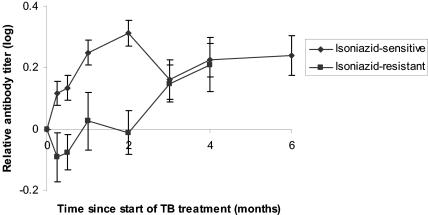
Examination of the relative titers of antibodies to all the epitopes combined showed no early rise in antibody titers in patients with isoniazid-resistant tuberculosis (Fig. 4).
Fig. 4. .
Differences in the change in antibody levels in sera from patients infected with isoniazid-sensitive and isoniazid-monoresistant strains of M. tuberculosis. These graphs include data from only those patients with smear-positive pulmonary tuberculosis who had a positive antibody titer at some point during their treatment (i.e., 207 of 240, being 40 patients multiplied by 6 antibody measurements of different specificity). Seven patients with isoniazid-resistant tuberculosis are compared to 33 patients infected with fully sensitive strains of M. tuberculosis. Antibody levels obtained during treatment were each compared to the patient's pretreatment level to give a relative antibody titer. The relative antibody titers to all six epitopes were then combined to give a mean ± standard error. A logarithmic scale has been used to treat rises and falls in antibody titer equally. TB, tuberculosis.
Is the rise in antibody level due to a change in antibody titer or maturation of the antibody response to higher affinity?
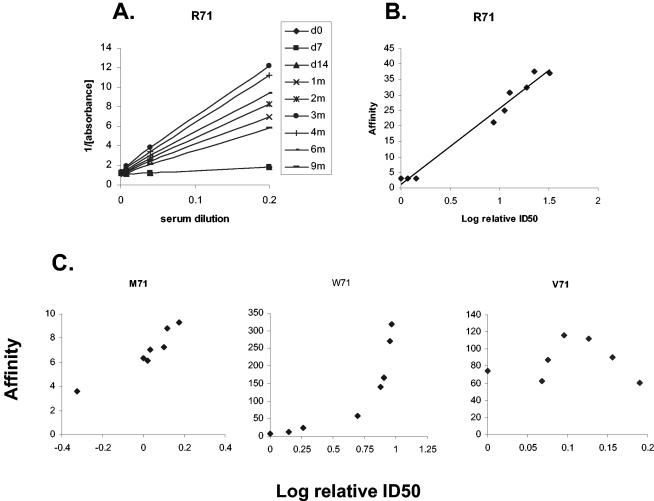
By plotting the reciprocal of the OD against the serum dilution (a modified Lineweaver-Burk plot) for each serum sample at different time points, we were able to show that the slopes changed at different times during chemotherapy (Fig. 5A). The lines intersect the y-axis at the same point, confirming that the maximal number of binding sites remained constant. The slope of the graph gives an estimate of the affinity. To examine how the affinity changed with ID50, we plotted the slope of the graph for each serum sample against its ID50 for the same individual (Fig. 5B). For those who had positive titers at the start of treatment, no inflexion point to suggest a change in the affinity of epitope-specific antibodies during treatment was noted, except for two subjects (Table 2; Fig. 5C). Special attention was paid to those whose antibody levels were initially within the range defined by controls but who later developed a positive titer. Changes in affinity in the transition from a negative to a positive titer could not be reliably calculated for cases when the second and all subsequent samples were positive, since there were insufficient data to establish the initial affinity. The cutoff titer for antibody to the TB78 epitope was at the first dilution of serum, making measurement of the changes in affinity from a positive to a negative titer impossible. There were two patients with several negative titers before a positive titer, one of whom showed an increase in affinity while the other did not (Table 2).
FIG. 5.
Affinity of epitope-specific antibody and its change with time. (A) Different slopes of the reciprocal of the OD for the antibody to the TB71 epitope with time. The point of intersection on the y-axis indicates that the maximal number of binding sites remained constant in the different assays. (B) The gradients from graph A have been used to calculate the affinity of the antibody, which has then been plotted against the log of the relative ID50. (C) Most subjects gave a linear relationship between affinity and ID50, but one showed an exponential relationship and one showed a bell-shaped curve. All antibody levels are those of antibody to the TB71 epitope.
TABLE 2.
Relationship between affinity and antibody titer (ID50)
| Subject | Epitope | Relationship between relative affinity and ID50a | No. of sera examined | r2 |
|---|---|---|---|---|
| R | TB71 | Log-linearb | 9 | 0.978 |
| W | TB71 | Exponentialc | 8 | 0.970 |
| TB72 | Log-linear | 8 | 0.709 | |
| M | TB71 | Log-linearc | 7 | 0.891 |
| TB72 | Log-linear | 7 | 0.934 | |
| F | TB23 | Log-lineard | 7 | 0.885 |
| Wi | TB23 | Exponentiald | 7 | 0.865 |
| TB71 | Log-linear | 7 | 0.747 | |
| S | TB71 | Log-linear | 7 | 0.515 |
| A | TB68 | Log-linear | 7 | 0.676 |
| Ve | TB23 | Log-linear | 6 | 0.893 |
| C | TB78 | Log-linear | 6 | 0.603 |
| TB71 | Log-linear | 5 | 0.669 | |
| H | L4f | Log-linear | 5 | 0.845 |
A log-linear relationship indicates no change in affinity of the antibody, whereas an exponential relationship indicates affinity maturation during treatment.
As shown in Fig. 5B.
As shown in Fig. 5C.
These two subjects had antibody levels within the range defined by healthy controls for three or four initial samples and then positive titers for later samples.
Same individual as V71 in Fig. 5C.
Monoclonal antibody L4 binds to the same epitope as ML34 but is an IgG antibody, and therefore analysis of the serum could be carried out at the same time as for the other IgG monoclonal antibodies.
How do epitope-specific antibody levels relate to antibody binding to the purified antigen?
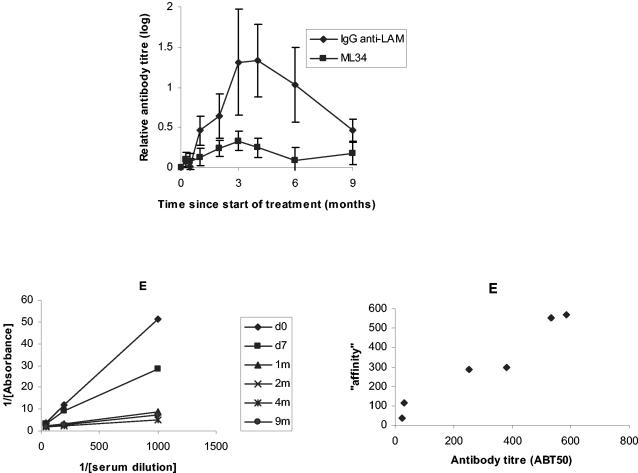
The many monoclonal antibodies raised against lipoarabinomannan (LAM) of M. tuberculosis bind to a single epitope (14). We therefore predicted that changes in the IgG antibody to purified lipoarabinomannan would follow the pattern observed with antibody to the ML34 epitope. This prediction was confirmed (Fig. 6). Interestingly, the increase in antibody levels to the purified antigen was significantly higher than in the monoclonal antibody-based assay. The data from these assays allowed a more certain estimation of the affinity of the competing antibody by using antibody detection of a single class. In most cases, there was a linear relationship between the calculated antibody titer and the slope of the graph, suggesting that there had been no change in antibody affinity (Table 3). One patient appeared to show a change in affinity between 1 and 2 months, and another showed significant correlation between the slope of the graph of the reciprocal of OD plotted against the reciprocal of the serum dilution and antibody titer in only posttreatment sera.
FIG. 6.
Antibody to lipoarabinomannan during treatment of tuberculosis. The relative increase in antibody titer appeared greater for the purified lipoarabinomannan than for antibody, as determined by the competition assay. An example of plotting the reciprocal of the absorbance against the serum dilution is given, from which the affinity of the serum was calculated. The third graph suggests that antibody titers follow the calculated “affinity” in a linear fashion, consistent with an absence of affinity maturation during treatment.
TABLE 3.
Relationship between ABT50 to purified antigens and the relative affinity of the serum antibody
| Purified antigen | Relationship between relative affinity and ABT50a | No. of patients examined |
|---|---|---|
| LAMb | Linear | 6 |
| No relationship | 2 | |
| 16 kDa | Linear | 3 |
| No relationship | 12 |
A linear relationship indicates no change in affinity of the antibody.
LAM, lipoarabinomannan.
We have previously shown that levels of antibodies to the TB71 and TB72 epitopes correlate with those of antibody to the purified 38-kDa antigen, whereas the level of antibody to the TB68 epitope does not correlate with that of IgG antibody to its parent antigen (17). Titers of antibody to the 16-kDa antigen showed no change with treatment in 10 of 19 subjects tested (Table 3). Of the nine that showed a definite increase, three showed increases of more than 20-fold and the remainder showed smaller increases of up to 3.6-fold with respect to the initial antibody titer. In one of the three, a 23-fold change in antibody titer was coincident with bacteriological relapse during treatment. As noted with antibody to the TB68 epitope, there was no consistent pattern of increase in antibody titer.
Can antibody levels be used to predict cure or relapse?
Five patients developed a positive sputum culture after being negative on smear and culture (not included in Fig. 2 to 4 after relapse). At the time sputum had been taken which later proved positive on culture, four gave a renewed positive titer to the TB68 epitope, three gave a renewed positive titer to the TB78 epitope, and two gave a renewed positive titer to the TB23 and TB71 epitopes. Only one patient showed a change in affinity such that a linear relationship, noted before relapse between affinity and the ID50 to the TB71 epitope, was lost after relapse (data not shown).
Sixteen patients infected with fully sensitive strains of M. tuberculosis attended for follow-up between 2 and 3 months after completing treatment successfully, i.e., at 8 to 9 months after the start of treatment. Positive antibody titers were still present in the majority, but 9 of 16 were no longer positive for the TB68 epitope. A single patient was seen 18 months after successfully completing treatment, and antibody titers had fallen to within the normal range for all except the TB71 epitope.
DISCUSSION
This study has shown that titers of antibodies to mycobacterial antigens rose during the treatment of tuberculosis and that a significant change in positive rating occurred as early as 7 days (Table 1). The increase included a rise in antibody titer as well as the recognition of new epitopes, i.e., epitope spreading. Antibody to epitopes on protein antigens gave a biphasic pattern, with a fall in antibody titer at 2 to 3 months, whereas antibody to lipoarabinomannan showed a single peak. The affinity of antibody to individual epitopes rarely changed during treatment. Positive titers of antibodies to these epitopes persisted in some patients for up to 18 months after the start of treatment, i.e., 1 year after successful completion of antituberculosis chemotherapy, although there was a trend to pretreatment levels, except perhaps for antibody to the TB72 epitope (Fig. 3). The fluctuation from positive to negative titers of antibody to the TB68 epitope and later positive titers concurrent with relapse might be of clinical value in monitoring treatment. The hypothesis that increases in titers of antibody to the 19-kDa antigen, hsp65, and the distinction between the TB71 and TB72 epitopes of the 38-kDa antigen indicate protective immune responses will be explored with reference to the current literature.
The rise in antibody levels occurred early, with a documented increase in patients with smear-positive pulmonary tuberculosis within days of starting treatment for tuberculosis. Looking at a few recent reports of serologic tests, some used exclusively pretreatment sera (11, 19, 20), one used sera “from almost all patients before initiation of antituberculosis treatment” (24), and another used sera collected “within 4 to 24 weeks after the initiation of therapy” (26). The last two were interested in the repertoire of human antibody specificity in tuberculosis. This study has shown that treatment permits epitope spreading, with recognition of new antigens and epitopes. Pretreatment sera should therefore be used in the evaluation of serodiagnostic tests for tuberculosis.
Kaplan and Chase argued that the scarcity of antibody before treatment might be due either to the mycobacterial lipid cell coat insulating internal proteins from the attention of the immune system or to active suppression of the immune response to other antigens (18). The former would be supported by evidence that killing of tubercle bacilli permitted epitope spreading, especially to internal antigens. Isoniazid kills tubercle bacilli in vivo rapidly, and the combination of rifampin and isoniazid also kills more rapidly than rifampin alone does (21). Thus, the delayed rise in positive rating and in relative antibody titers in serum from patients harboring an isoniazid-resistant strain of M. tuberculosis would favor the model in which killing of tubercle bacilli releases additional antigens (Fig. 4). The possibility of immune suppression is considered below.
Mitchison proposed the existence of different populations of tubercle bacilli based on their response to isoniazid, rifampin, and pyrazinamide (21). The rapidly dividing population was sensitive to isoniazid, whereas persister populations were killed in the later stages of treatment. Such a distinction might account for the apparently biphasic nature of the antibody response. The absence of an initial peak in those infected with an isoniazid-resistant strain would be consistent with this hypothesis (Fig. 4). The fall in antibody levels might have been due to immune complex formation but might have occurred at a later time than reported by other authors (29). The monoclonal antibody TB78 describes a species-restricted epitope on the stress protein hsp65. The late rise in positive rating and relative titer of antibody to this epitope would be consistent with expression of stress proteins in the persister but not the rapidly dividing populations (10). The absence of antibody to this epitope in pretreatment sera from patients with previous tuberculosis might then be explained by the lack of significant quantities of this internal antigen for presentation to the immune system.
Antibody to the ML34 epitope of lipoarabinomannan consistently showed a single peak in antibody levels at 3 to 4 months. Polysaccharide antigens frequently stimulate a T-independent B-cell response (28). The number of persister tubercle bacilli present after 2 months of treatment will be <1% of the initial bacterial population. Without T-cell help to accentuate the second phase in the release of antigen, the kinetics of the antibody response might merely reflect the relative persistence of this antigen compared to protein antigens and the availability of the polysaccharide to stimulate B cells.
Pereira Arias-Bouda et al. reported a fall in avidity with an increase in the levels of IgG antibody to a sonicate of M. tuberculosis during treatment (22). This is consistent with the release of antigens early in treatment and the recognition of new specificities. In this study, changes in affinity for an antibody of a single specificity were rare. For patients with smear-positive pulmonary tuberculosis, this observation implies that maximal affinity had been achieved before the start of treatment and that the same clone continued to secrete antibody during treatment.
Active suppression to explain low pretreatment antibody levels would predict a difference in repertoire between those with previous tuberculosis who had relapsed and those with a first episode of the disease. Those with previous episodes of tuberculosis were more likely to recognize at least five epitopes before treatment than were those infected with tuberculosis for the first time. However, in the five who had had tuberculosis before, two had no pretreatment antibody to the stress protein hsp65 but did develop antibody of this specificity during treatment and another had no antibody to the TB23 epitope until later during treatment. Additional support for suppression comes from the lack of change in affinity despite antibody titers changing from within to above the range defined by healthy controls.
Heat shock proteins are increasingly considered to play a role in the regulation of immune responses via the induction of adhesion molecules, proinflammatory cytokines, CD14, and Toll-like receptors (23). In mice, fusion of mycobacterial hsp65 with the nucleoprotein of influenza virus promoted CD8+ cytotoxic T-cell development (2). T-cell suppression of antibody to the species-restricted epitope of hsp65 might prevent loss of this adjuvant by immune complex formation and thereby may be a protective response. Alternatively, as previously noted, stress proteins may characterize the persister population of tubercle bacilli (10), and therefore late presentation to the immune system might be responsible for the later development of antibody to the TB78 epitope.
Antibody to the TB23 epitope was least likely to be positive and showed the greatest increase in positive rating during treatment. We have previously shown that antibody to the 19-kDa antigen, but not its TB23 epitope, is found especially in paucibacillary forms of tuberculosis (3, 17). If the ratio of antibody to the TB23 epitope and IgG antibody to the 19-kDa antigen is a valid measure, there may be genetic restriction by HLA-DQ of antibody to the non-TB23 epitopes (9). The allele associated with the development of smear-positive tuberculosis, DQ5 (8), was associated with low ratios of anti-19-kDa antibody to anti-TB23 antibody. The 19-kDa antigen is also actively secreted by tubercle bacilli (32). The absence of antibody to the TB23 epitope until after treatment is therefore surprising and might support the possibility of T-cell suppression, which influences the clinical form of disease.
Antibody to the TB72 epitope of the 38-kDa antigen was immunodominant, with TB71 mapping a subdominant epitope, as noted in previous studies (15,17). This antigen is therefore a candidate to focus the immune response in pathogenesis, with T-cell help for the subdominant TB71 epitope potentially providing a protective response. However, both epitopes were recognized concurrently in most patients. HLA-DR2 was associated with disease and antibody to both epitopes (5, 8). The later appearance of antibody to the TB71 epitope might merely indicate that degradation of the 38-kDa antigen is required since the TB72 monoclonal antibody requires much of the complete protein whereas the TB71 monoclonal antibody can bind to a shorter amino acid sequence (1).
The level of antibody to the TB68 epitope of the 16-kDa stress protein showed significant fluctuations within individual patients and increased with relapse. Considering the persistence of antibody to the other antigens, it remains unclear why the antibody of this specificity should be so short-lived in patients being treated for smear-positive tuberculosis. Antibody to the TB68 epitope has been noted after occupational exposure to tuberculosis (4) and to its parent antigen to be associated with a good prognosis (7). Failure to mature an IgM response to an IgG response due to the lack of T-cell help in patients with smear-positive tuberculosis might explain these findings and suggest a protective role for a T-cell response to this antigen. T-cell responses to a peptide (p91-110) from the 16-kDa antigen and the recombinant antigen were significantly lower in patients with smear-positive pulmonary tuberculosis than in those with pleural or lymph node disease and were highest in BCG-vaccinated controls (35). The same study showed a significant increase in gamma interferon levels in response to the 16-kDa antigen with treatment, consistent with an enhanced Th1 response, i.e., for cell-mediated immunity rather than B-cell help in antibody production. Lymphocyte proliferation in response to two different peptides (p21-40 and p111-130) from the 16-kDa antigen showed an increase with treatment (34). These data would be consistent with a protective effect of a T-cell response to the 16-kDa antigen, which permits B-cell help only after prolonged stimulation.
The presence of antibody to M. tuberculosis-restricted epitopes in patients with smear- and culture-negative pulmonary tuberculosis could be used to justify empirical treatment of patients in whom a positive diagnosis by mycobacterial culture is never obtained. The marked increase in antibody levels in patients with smear-negative, culture-positive pulmonary tuberculosis is noted. Its clinical value would apply only where access to tests based on PCR is not readily available.
This study confirms the importance of evaluating serodiagnostic tests for tuberculosis by using samples obtained before starting treatment. Epitope spreading of the antibody response with treatment indicates a method by which potentially protective immune responses might be uncovered.
Acknowledgments
I thank Juraj Ivanyi for the soluble extract of M. tuberculosis, monoclonal antibodies, 16-kDa antigen and helpful advice; Patrick Brennan for purified lipoarabinomannan and L4; and Geoff Laurent for use of laboratory space and reagents.
I thank the NHS Culyer allocation to the Homerton University Hospital for supporting this research.
REFERENCES
- 1.Andersen, A. B., and E. B. Hansen. 1989. Structure and mapping of antigenic domains of protein antigen b, a 38,000-molecular-weight protein of Mycobacterium tuberculosis. Infect. Immun. 57:2481-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anthony, L. S., H. Wu, H. Sweet, C. Turnmir, L. J. Boux, and L. A. Mizzen. 1999. Priming of CD8+ CTL effector cells in mice by immunization with a stress protein-influenza virus nucleoprotein fusion molecule. Vaccine 17:378-383. [DOI] [PubMed] [Google Scholar]
- 3.Bothamley, G., H. Batra, V. Ramesh, A. Chandramuki, and J. Ivanyi. 1992. Serodiagnostic value of the 19 kilodalton antigen of Mycobacterium tuberculosis in Indian patients. Eur. J. Clin. Microbiol. Infect. Dis. 11:912-915. [DOI] [PubMed] [Google Scholar]
- 3a.Bothamley, G. H. 1990. Analysis of epitope-specific antibody levels in tuberculosis. Ph.D. thesis. University of London, London, United Kingdom.
- 4.Bothamley, G. H., J. S. Beck, R. C. Potts, J. M. Grange, T. Kardjito, and J. Ivanyi. 1992. Specificity of antibodies and tuberculin response after occupational exposure to tuberculosis. J. Infect. Dis. 166:182-186. [DOI] [PubMed] [Google Scholar]
- 5.Bothamley, G. H., J. S. Beck, G. M. T. Schreuder, J. D'Amaro, R. R. P. de Vries, T. Kardjito, and J. Ivanyi. 1989. Association of tuberculosis and M. tuberculosis-specific antibody levels with HLA. J. Infect. Dis. 159:549-555. [DOI] [PubMed] [Google Scholar]
- 6.Bothamley, G. H., and R. M. Rudd. 1994. Clinical evaluation of a serological assay using a monoclonal antibody (TB72) to the 38 kDa antigen of Mycobacterium tuberculosis. Eur. Respir. J. 7:240-246. [DOI] [PubMed] [Google Scholar]
- 7.Bothamley, G. H., R. Rudd, F. Festenstein, and J. Ivanyi. 1992. Clinical value of the measurement of Mycobacterium tuberculosis specific antibody in pulmonary tuberculosis. Thorax 47:270-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bothamley, G. H., and G. M. T. Schreuder. 1992. Human leukocyte antigen, tuberculosis and Mycobacterium-tuberculosis-specific antibody. J. Infect. Dis. 165:598. [DOI] [PubMed] [Google Scholar]
- 9.Bothamley, G. H., G. M. T. Schreuder, R. R. P. de Vries, and J. Ivanyi. 1993. Association of antibody responses to the 19kDa antigen of Mycobacterium tuberculosis and the HLA-DQ locus. J. Infect. Dis. 167:992-993. [DOI] [PubMed] [Google Scholar]
- 10.Dahl, J. L., C. N. Kraus, H. I. M. Boshoff, B. Doan, K. Foley, D. Avarbock, G. Kaplan, V. Mizrahi, H. Rubin, and C. E. Barry III. 2003. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA 100:10026-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. W. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drouwert, A., K. Huygen, J. de Bruyn, J.-C. Yernault, C.-M. Farber, and J.- P. van Vooren. 1991. Antibody levels to whole culture filtrate antigens and to purified P32 during treatment of smear-positive tuberculosis. Chest 100:685-687. [DOI] [PubMed] [Google Scholar]
- 13.Engers, H. D., V. Houba, J. Bennedsen, T. M. Buchanan, S. D. Chaparas, G. Kadival, O. Closs, J. R. David, J. D. A. van Embden, T. Godal, S. A. Mustafa, J. Ivanyi, D. B. Young, S. H. E. Kaufmann, A. G. Khomenko, A. H. J. Kolk, M. Kubin, J. A. Louis, P. Minden, T. M. Shinnick, L. Truka, and R. A. Young. 1986. Results of a World Health Organization sponsored workshop to characterize antigens recognized by mycobacterium-specific monoclonal antibodies. Infect. Immun. 51:718-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaylord, H., P. J. Brennan, D. B. Young, and T. M. Buchanan. 1987. Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect. Immun. 55:2860-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt, J., A. R. M. Coates, D. A. Mitchison, and J. Ivanyi. 1982. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J. Immunol. Methods 55:205-211. [DOI] [PubMed] [Google Scholar]
- 16.Ivanyi, J., E. Krambovitis, and M. Keen. 1983. Evaluation of a monoclonal antibody (TB72) based serological test for tuberculosis. Clin. Exp. Immunol. 54:337-345. [PMC free article] [PubMed] [Google Scholar]
- 17.Jackett, P. S., G. H. Bothamley, H. V. Batra, A. Mistry, D. B. Young, and J. Ivanyi. 1988. Specificity of antibodies to immunodominant myobacterial antigens in tuberculosis. J. Clin. Microbiol. 26:2313-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan, M. H., and M. W. Chase. 1980. Antibodies to mycobacteria in human tuberculosis. I. Development of antibodies before and after antimicrobial therapy. J. Infect. Dis. 142:825-834. [DOI] [PubMed] [Google Scholar]
- 19.Lodes, M. J., D. C. Dillon, R. Mohamath, C. H. Day, D. R. Benson, L. D. Reynolds, P. McNeill, D. Pedral Sampaio, Y. A. W. Skeiky, R. Badaro, D. H. Persing, S. G. Reed, and R. L. Houghton. 2001. Serological expression cloning and immunological evaluation of MTB48, a novel Mycobacterium tuberculosis antigen. J. Clin. Microbiol. 39:2485-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maekura, R., Y. Okuda, M. Nakagawa, T. Hiraga, S. Yokota, M. Ito, I. Yano, H. Kohno, M. Wada, C. Abe, T. Toyoda, T. Kishimoto, and T. Ogura. 2001. Clinical evaluation of anti-tuberculous glycolipid immunoglobulin G antibody assay for rapid serodiagnosis of pulmonary tuberculosis. J. Clin. Microbiol. 39:3603-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchison, D. A. 1979. Basic mechanisms of chemotherapy. Chest 76:771-781. [DOI] [PubMed] [Google Scholar]
- 22.Pereira Arias-Bouda, L. M., S. Kuijper, A. van der Werf, L. N. Nguyen, H. M. Jansen, and A. H. J. Kolk. 2003. Changes in avidity and level of immunoglobulin G antibodies to Mycobacterium tuberculosis in sera of patients undergoing treatment for pulmonary tuberculosis. Clin. Diagn. Lab. Immunol. 10:702-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pockley, A. G. 2003. Heat shock proteins as regulators of the immune response. Lancet 362:469-476. [DOI] [PubMed] [Google Scholar]
- 24.Pottumarthy, S., V. C. Wells, and A. J. Morris. 2000. A comparison of seven tests for serological diagnosis of tuberculosis. J. Clin. Microbiol. 38:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribas, A., J. M. Timmerman, L. H. Butterfield, and J. S. Economou. 2003. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. Trends Immunol. 24:58-61. [DOI] [PubMed] [Google Scholar]
- 26.Samanich, K., J. T. Belisle, and S. Laal. 2001. Homogeneity of antibody responses in tuberculosis patients. Infect. Immun. 69:4600-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sercarz, E. E. 2000. Driver clones and determinant spreading. J. Autoimmun. 14:275-277. [DOI] [PubMed] [Google Scholar]
- 28.Snapper, C. M., and J. J. Mond. 1996. A model for induction of T-cell- independent humoral immunity in response to polysaccharide antigens. J. Immunol. 157:2229-2232. [PubMed] [Google Scholar]
- 29.Sousa, A. O., A. Wargnier, Y. Poinsignon, N. Simmoney, F. Gerber, F. Lavergne, J. L. Herrmann, and P. H. Lagrange. 2000. Kinetics of circulating antibodies, immune complex and specific antibody-secreting cells in tuberculosis patients during 6 months of antimicrobial therapy. Tubercle Lung Dis. 80:27-33. [DOI] [PubMed] [Google Scholar]
- 30.Turneer, M., J.-P. van Vooren, J. de Bruyn, E. Serruys, P. Dierckx, and J.-C. Yernault. 1988. Humoral immune response to human tuberculosis: immunoglobulin G, A, and M directed against the purified P32 protein antigen of Mycobacterium bovis bacillus Calmette-Guérin. J. Clin. Microbiol. 26:1714-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiker, H. G., and M. Harboe. 1992. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol. Rev. 56:648-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiker, H. G., M. Harboe, and S. Nagai. 1991. A localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J. Gen. Microbiol. 137:875-884. [DOI] [PubMed] [Google Scholar]
- 33.Wilkins, E., G. Bothamley, and P. Jackett. 1991. A rapid simple enzyme immunoassay for detection of antibody to individual epitopes in the serodiagnosis of tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 10:559-563. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson, R. J., H. M. Vordermeier, K. A. Wilkinson, A. Sjolund, C. Moreno, G. Pasvol, and J. Ivanyi. 1998. Peptide specific T cell responses to Mycobacterium tuberculosis: clinical spectrum, compartmentalization and effect of chemotherapy. J. Infect. Dis. 178:760-768. [DOI] [PubMed] [Google Scholar]
- 35.Wilkinson, R. J., K. A. Wilkinson, K. A. L. de Smet, K. Haslov, G. Pasvol, M. Singh, I. Svarcova, and J. Ivanyi. 1998. Human T- and B-cell reactivity to the 16kDa α-crystallin protein of Mycobacterium tuberculosis. Scand. J. Immunol. 48:403-409. [DOI] [PubMed] [Google Scholar]